Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
ACS Applied Nano Materials, 3, 1, pp. 294-302, 2019-12-04
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=c1e24e91-8a23-4d7d-9a02-2db2faa58331 https://publications-cnrc.canada.ca/fra/voir/objet/?id=c1e24e91-8a23-4d7d-9a02-2db2faa58331
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1021/acsanm.9b01952
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
In-flight plasma functionalization of boron nitride nanotubes with
ammonia for composite applications
Iannitto, Robyn; Shin, Homin; Martinez-Rubi, Yadienka; Simard, Benoit;
Coulombe, Sylvain
In-Flight Plasma Functionalization of Boron Nitride Nanotubes with
Ammonia for Composite Applications
Robyn Iannitto,
*
,†,‡Homin Shin,
‡Yadienka Martinez Rubi,
‡Benoit Simard,
*
,‡and Sylvain Coulombe
††
Plasma Processing Laboratory, Department of Chemical Engineering, McGill University, Montréal, Québec H3A 0C5, Canada
‡
Security and Disruptive Technologies Research Centre, Emerging Technologies Division, National Research Council of Canada, Ottawa, Ontario K1A 0R6, Canada
*
S Supporting InformationABSTRACT: Surface functionalization is an essential step to successfully harness the properties of boron nitride nanotubes (BNNTs) in several applications. Currently available function-alization methods are prohibitively costly for commercial use and have significant environmental impacts. Here, we show that a low-pressure, capacitively coupled radio-frequency (RF, 13.56 MHz) glow discharge plasma reactor can be used to effectively carry out surface chemical functionalization of BNNTs. This low-pressure vacuum and gas handling system incorporates a solenoid valve to periodically fluidize the free-standing BNNTs, which provides for functionalizing BNNTs in-flight. Ammonia, which generates ·H, ·NH, and ·NH2 radicals by energetic electron impact, was employed as the
functionalization gas. The functionalization begins by anchoring the ·H, ·NH, and ·NH2radicals to the surface of BNNTs, which is promoted by the presence of free electrons forming reduced BNNTs. On the basis of density functional theory (DFT) calculations, we propose a mechanism wherein BNNTs are functionalized to the final product of BNNT−NH3, a weakly bonded complex, through subsequent cascade reactions with H radicals abundant in the plasma reactor. BNNT surface functionalization with ammonia was confirmed by tandem thermogravimetric analysis−infrared spectroscopy (TGA-IR), Fourier transform infrared (FTIR) spectroscopy, dynamic vapor sorption (DVS), our recently reported BNNT quality assessment method based on the formation of regiorandom poly(3-hexylthiophene) (rra-P3HT) aggregates on BNNTs, and solubility in water. This solvent-free and environmentally friendly approach can be applied by using other functionalizing gases, and the in-flight functionalization of fluidized BNNT powder is also advantageous in terms of scalability. These features offer the potential to dramatically advance BNNT composites research efforts and the development of BNNT applications. KEYWORDS: boron nitride nanotubes, amine functionalization, solvent-free process, plasma functionalization,
capacitively coupled radio-frequency plasma
■
INTRODUCTIONBoron nitride nanotubes (BNNTs) are structural analogues of carbon nanotubes (CNTs) but differ in composition where BNNTs can be viewed as rolled-up cylinders of hexagonal boron nitride (h-BN) sheets.1 Their extraordinary properties include high mechanical strength, wide bandgap semiconduct-ing capabilities,2optical and infrared transparency, biocompat-ibility,3−6 and thermal and chemical stability.7 These
proper-ties make BNNTs an attractive option for many applications, such as for mechanical reinforcement,8,9 transparent compo-sites,8−11
high-temperature materials,1,8,11drug delivery,5,12−14
boron neutron capture therapy,5,12,15,16 and radiation shield-ing.1,17 However, as is the case for CNTs, pristine, small diameter BNNTs agglomerate into large bundles and have little compatibility with many industrially relevant polymer, ceramic, or metal matrices. To alleviate these issues, chemical
functionalization is required;18 however, chemical functional-ization of BNNTs still remains challenging due to BNNT’s chemical inertness.19
Chemical functionalization methods, specifically covalent functionalization, for BNNTs have only recently been developed, and successful functionalization without causing damage to their surface is difficult to achieve.20,21Nevertheless, successful covalent functionalization can tremendously im-prove the stability and solubility of BNNTs in various solvents.18−24 As with CNTs, covalent functionalization of
BNNTs is typically done by using wet chemistry methods.25−29
However, there are significant drawbacks associated with this
Received: October 9, 2019
Accepted: December 4, 2019
Published: December 4, 2019
Article
www.acsanm.org
Cite This:ACS Appl. Nano Mater. 2020, 3, 294−302
Read License, which permits copying and redistribution of the article for non-commercial scholarly purposes.
Downloaded via NATL RESEARCH COUNCIL CANADA on June 22, 2020 at 19:20:39 (UTC).
approach, such as the large production of chemical waste, which is detrimental to the environment, and scale-up limitations due to time and cost-efficiency issues.25Moreover, processing such functionalized BNNTs afterward is challenging due to the reagglomeration and densification of BNNTs when the solvent is removed.
Plasma chemistry in a dry and solvent-free environment has the potential to overcome the difficulties associated with BNNT’s chemical inertness and solvent-based covalent functionalization methods. Chemical species such as radicals, ions, and electrons are produced in plasmas and can bind to the surface of unreactive materials, such as BNNTs, with little to no activation barrier.18,20,30,31 Plasma chemistry has previously been employed to achieve both amine and amide functionalization of BNNTs.18,20Ikuno et al. have shown that ammonia plasma treatment can be used to graft amino groups onto the BNNTs; however, surface damage also occurred.18In another study, Dai et al. added amide functional groups to the surface of BNNTs using a multistep functionalization approach.20 First, oxygen plasma was used to create nitrogen vacancies on the surface of BNNTs; subsequently, oxygen atoms were substituted onto the surface. Amide groups were then created by using a N2+ H2plasma.20In these two studies, the BNNT samples were synthesized on substrates and then subjected to plasma treatments, thus limiting functionalization to the fraction of the BNNTs’ surface directly exposed to the plasma. In addition, the scale of these functionalization methods is restricted to the scale of the BNNT production method.
Here, as shown inScheme 1, we introduce a novel approach to plasma functionalization that addresses these drawbacks by exploiting the low density of BNNTs to make them easily fluidized in a gas flow. By pulsing the carrier gases, a periodic
fluidization of BNNTs and exposure to a reactive plasma can be achieved, thus, for the first time, chemically functionalizing free-standing BNNTs in-flight. To demonstrate the applic-ability of this new method, we focus on an ammonia plasma because amine functional groups are very important for the chemical and pharmaceutical industries.32,33The
functionaliza-tion was verified through characterizafunctionaliza-tion by tandem thermogravimetric analysis−infrared spectroscopy (TGA-IR), Fourier transform infrared (FTIR) spectroscopy, dynamic vapor sorption (DVS), regiorandom poly(3-hexylthiophene) (rra-P3HT) quality assessment methodology, and solubility in water along with density functional theory (DFT) calculations.
■
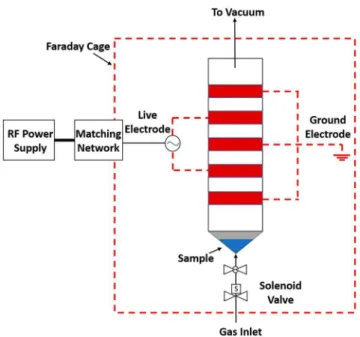
EXPERIMENTAL SECTIONReactor Design.Unless described below, all other experimental details are in theSupporting Information.Figure 1shows a schematic of the custom-built, continuous-wave, capacitively coupled RF plasma reactor specifically designed to sustain a large volume and uniform glow discharge at low pressure and operate with pulsed injection of the plasma-forming gases. An actual image of the reactor can be found in Figure S2 of theSupporting Information. The plasma-confinement chamber consists of a glass tube (i.d. = 3.8 cm, length = 35 cm) terminated at both ends with standard (NW25) stainless steel flanges. The whole plasma reactor assembly is enclosed in a Faraday cage to provide electromagnetic shielding. Five copper straps (width = 3.8 cm, spacing = 0.64 cm) wrapped around the glass tube act as electrodes for the radio-frequency (RF) power supply, alternating in polarity between live and ground electrodes. The live electrodes are powered by a continuous-wave RF power supply (Advanced Energy RFX600A, 13.56 MHz, VM-100 automatic matching network). BNNT powders were initially loaded at the bottom of the reactor, in the conical section resting immediately above the solenoid valve. The three-way solenoid air directional control valve, which acts as a rapid ON−OFF valve, was used to periodically pulse-inject the reactive gases causing temporary fluidization of the BNNTs in the Scheme 1. Schematic Outlining the Steps in Plasma Functionalization Processa
aInitially, the BNNTs remain in the conical reducer at the base of the reactor. After the 100 W plasma is initiated, the BNNTs are pulsed into the
plasma zone, and the cascading functionalization process is initiated to obtain the final BNNT−NH3product.
ACS Applied Nano Materials Article
DOI:10.1021/acsanm.9b01952
ACS Appl. Nano Mater. 2020, 3, 294−302 295
area where the plasma is sustained (defined by the copper straps assembly). The valve cycle time was 1 s with a duty cycle of 5%, thus implying that the valve was fully open during the first 50 ms of the cycle and closed during 950 ms. Various ratios of the Ar/NH3 gas mixtures were investigated. The individual gas flow rates were regulated by using two MKS mass flow controllers (N2, 100 sccm, 15 pin analogue, N.C. Viton, LCEA12CR1BV20 and Cl2, 100 sccm, 15 pin analogue, N.C. Teflon, LCMA22CR1BM20) calibrated for Ar (Megs Specialty Gases and Equipment, liquid, >99%) and NH3 (Praxair, anhydrous, >99%), respectively, and a CCR four-channel power supply and digital readout. The whole system was evacuated with a dual-stage rotary vane vacuum pump (Alcatel, Pascal Series 2005 SD) to a base pressure of 25 mTorr (3.3 Pa). The pressure was monitored by using an absolute capacitance manometer (MKS, AA01A11TBAS3B00000) pressure gauge coupled with a digital power supply and readout (MKS, Type 660B) mounted on the downstream end of the glass tube. Under typical processing conditions, the periodic pressure oscillations stabilized to an average pressure of 2 Torr (267 Pa). A video showing the pulsing operation with BNNTs can be found in Video S1 of theSupporting Information.
BNNT Functionalization. The BNNTs used in this work were produced at the NRC’s Nanotube Production Research Facility by using the hydrogen-assisted BNNT synthesis (HABS) process10from a hexagonal boron nitride (h-BN, MK-hBN-N70 from MK Impex Corp.) feedstock. The elemental boron impurities were removed by using chlorine gas, as described recently,34in three steps: first at 750
°C, then at 850 °C, and finally at 950 °C. These pristine, boron-free BNNTs were used for all plasma functionalization experiments. The samples consisted of very thin (veil-like) and small sheets of BNNTs (maximum 1 cm × 1 cm). Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) images of the BNNTs are shown inFigures S3 and S4, respectively. The TEM images of the BNNTs confirm that they have between 2 and 5 highly crystalline walls, diameters between 3 and 5 nm, and hollow tubular structure. Also, as shown in the TEM images, there is little amorphous B present on the surface of the BNNTs. The SEM images of the purified, nonfunctionalized BNNTs at different magnifications show many strands of boron-free, fibrous BNNTs (Figure S3a,b).
Moisture present in the BNNT samples was removed by baking at 110 °C for a minimum of 3 h before transferring BNNTs to the conical reducer mounted at the base of the reactor. Once added to the conical reducer, the reactor was evacuated to its base pressure (∼25 mTorr) for 1 h to ensure the maximum amount of moisture was
removed. The plasma chamber was subsequently backfilled by using an Ar/NH3gas mixture at the desired gas injection cycle. To stabilize the surface before exposure to air after an experiment,20the samples were held under vacuum for 20 min and then exposed to a 10 min Ar purge.
Process Optimization. The Ar/NH3 ratio, RF power, and treatment time were varied to achieve a significant level of chemical functionalization. Optical emission spectroscopy (OES) and ther-mogravimetric analysis (TGA) were used for process optimization. A modular OES (Ocean Optics, JAZ-EL200-XR1, 1.7 nm fwhm resolution) provided for monitoring of optical emission from the Ar/NH3plasma and especially the NH radical emission. An optical fiber (Gamble Technologies, OCF-107054, Custom fiber, 5 m, 400 μm core size) positioned outside of the glass tube in the region between the second and third electrodes from the top was used to collect and direct the light emission (200−1100 nm acquisition range) to the spectrometer. The spectra were normalized with respect to the integration time. TGA (Netzsch STG 449 F1) was used to detect the presence of addends on the functionalized BNNTs.
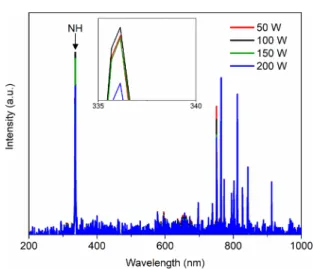
NH and NH2radicals are produced in an ammonia plasma35,36and have also been proposed to anchor on both BNNTs and h-BN.24,37 Hence, the gas composition was optimized to produce the highest concentration of these species. When electronically excited in the plasma, the A3Π−X3Σsystem of NH emits with a prominent peak at 336 nm ((0, 0) band). Although the NH2radical does not emit in the wavelength range covered by our spectrometer, its concentration is known to correlate with that of the NH radical in an Ar/NH3 plasma.35,36Figure 2presents typical optical emission spectra for an
Ar/NH3plasma with varying gas flow rate ratios. The other species present in the spectra are Hαand Hβat 656 and 486 nm, respectively, and molecular N2bands near 359, 376, and 382 nm. The negative system of N2+bands is present at 328.9, 388.5, 421.6, and 431.3 nm.38 Such emission bands were present only when operating with a 2 vol % NH3concentration. The peaks in the 700−900 nm range are atomic Ar lines. The inset inFigure 2shows that the intensity of the peaks, specifically the NH molecular emission peak, increases as the concentration of ammonia in the plasma decreases. Therefore, operating with 2 vol % NH3results in the maximum concentration of NH radicals. Interestingly, as shown inFigure S5, the TGA data indicate that there are only signs of chemical functionalization (mass loss near 250 °C) when using a 2 vol % NH3gas composition. This confirms that radicals (either ·NH, ·NH2, or ·H) are required to initialize BNNT functionalization.
OES was also used to determine the operating power that resulted in the highest concentration of the NH radical produced in the plasma. As seen inFigure 3, the NH band at 336 nm is maximized at 100 W presumably because of competing reactions that consume the Figure 1. Schematic of the custom-built plasma reactor used for
functionalization of BNNTs.
Figure 2.Normalized optical emission spectra for various plasma gas compositions.
NH radical. Consequently, 100 W was set as the operating condition for the subsequent experiments.
To determine an optimal reaction time to achieve the highest degree of surface chemical functionalization, experiments were performed with varying plasma treatment times at 2 vol % NH3 and 100 W. The results are reported in the derivative thermogravi-metric (DTG) data inFigure 4, which shows that the highest degree
of surface chemical functionalization, as indicated by the highest mass loss at 250 °C, occurs for 100 min. At plasma treatment times >100 min, the degree of functionalization drastically decreases, as seen from the DTG trace for 120 min. This is attributed to the temperature increase in the reactor with increased plasma treatment times, measured by using irreversible temperature strips. At 120 min treatment times, the temperature in the reactor approached 250 °C, which is approximately the desorption temperature of the addend on the BNNTs.23
Having established the conditions for maximum surface chemical functionalization of the BNNTs at 2 vol % NH3, RF plasma power of 100 W, and 100 min treatment, a batch of 2 g of functionalized BNNTs was prepared for various characterizations to determine the identities of the addends and characteristics of the material. BNNTs functionalized by using these optimized conditions are henceforth termed F-BNNTs.
■
RESULTSTandem TGA-IR spectroscopy (Netzsch STG 449 F1 instrument coupled to a Bruker Tensor 27 FTIR spectrometer) was used to characterize the F-BNNT material. The DTG result is shown inFigure 4by the black trace. Two mass loss events occurred: one at 90 °C and the other near 250 °C. IR absorption spectra are recorded simultaneously during the TGA acquisition; the red and blue traces shown in the in-line FTIR spectra (Figure 5) are the spectra for the species released at 90 and 250 °C, respectively. The spectroscopic signatures are unmistakably those of water and ammonia, respectively.
To verify that the water release is attributable to the physical adsorption onto the F-BNNTs’ surface after exposure to air, a two-step TGA experiment was performed. In the first step, water was desorbed by heating the sample to 140 °C in air for 10 min until all the water was removed and then letting the sample return to room temperature in the TGA. The next step was to expose the sample to air again for 1 h and then repeat the TGA operation (heating at 10 °C/min in air until reaching 950 °C). The results, which are shown in Figure S6, clearly indicate that the water released at 90 °C is from physical adsorption onto the surface of F-BNNTs after exposure to air. This drastic increase in water adsorption does not occur with the untreated BNNTs, suggesting that the surface of the F-BNNTs changed from mildly hydrophobic (hydrophobic with partial hydrophilic character) to hydrophilic upon plasma treatment. To confirm this assertion, water adsorption and desorption isotherms were measured by using DVS for both untreated and plasma-treated BNNTs. The results are shown Figure 3.Normalized optical emission spectra for various RF plasma
power levels.
Figure 4.DTG graph of BNNTs exposed to varying reaction times.
Figure 5.(a) In-line FTIR spectrum of the released species at 90 °C. (b) In-line FTIR spectrum of the released species at 250 °C.
ACS Applied Nano Materials Article
DOI:10.1021/acsanm.9b01952
ACS Appl. Nano Mater. 2020, 3, 294−302 297
inFigure 6. The untreated BNNT isotherm can be classified as Type V in the IUPAC classification.39,40The isotherm shows
that there was a low uptake of water at low relative humidity (under 50% RH), which suggests a weak interaction between the surface and water molecules (i.e., some hydrophobic character40) and that there was a hysteresis loop at higher relative humidity. It has been shown that water adsorption and the corresponding isotherms can change behavior with the addition of functional groups,41,42which is observed with the present F-BNNTs. The F-BNNT isotherm is similar to Type II or IV in the IUPAC classification,40with a dramatic increase in water uptake at low RH, indicating enhanced interactions between the surface of the F-BNNTs and water molecules. Moreover, the unclosed adsorption−desorption hysteresis suggests irreversible adsorption of water at 25 °C. These observations explain the large release of water seen from the DTG data for the F-BNNTs.
The release of ammonia at 250 °C suggests that amine functional groups were present on the surface of F-BNNTs because this desorption event did not occur with the untreated, pristine BNNTs. The functional groups could be either NH3, NH2, NH, or H. If the functional groups are H, NH, or NH2, the detection of ammonia would arise from complex reactions between the fragments to produce ammonia before it reaches the IR detector. All four addends are capable of binding to the surface of BNNTs; however, NH3 is the one with lowest binding energy,24and the release at 250 °C is indicative of a weakly bound addend. Typically, strongly bound addends are released in the 350−500 °C range.24,43,44It will be conjectured later that the plasma conditions are favorable for the production of BNNT−NH3as the final product. The presence of amine functional groups is also consistent with the hydrophilic character of F-BNNTs because water molecules solvate the amine groups through the formation of hydrogen-bonding networks. The enhanced hydrophilicity of F-BNNTs provides a handle on how to disperse/individualize BNNTs in water as shown inFigure S7.
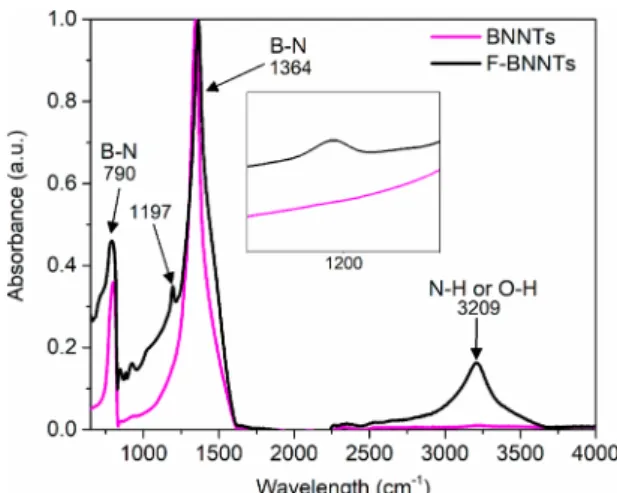
FTIR analysis of the solid F-BNNT samples was also performed to further ascertain the presence of amine groups. The spectra for untreated BNNTs and F-BNNTs are shown in
Figure 7. The peaks at 805 and 1346 cm−1are characteristic
peaks of BNNTs and are a result of the out-of-plane B−N−B bending and in-plane B−N stretching, respectively.24 Three
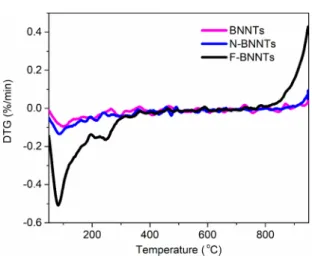
new peaks appear for the F-BNNTs at 900, 1197, and 3209 cm−1. Because of the overlapping of the N−H and the O−H stretching regions, it is difficult to identify the broad absorption band at 3209 cm−1. To help identify the peak, the F-BNNTs were heated at 115 °C overnight to remove any adsorbed water (see FTIR inFigure S8). In that case, the absorption band at 3209 cm−1 decreases but is still present, which suggests that this broad band is a result of both the N−H stretching of the amine groups present on the F-BNNTs surface and the O−H stretching due to adsorbed water. The small absorption bands that appear for the F-BNNTs at 1197 and 900 cm−1 are assigned to the in-plane bending and out-of-plane bending of B−OH,45,46respectively. It is speculated that these are formed after functionalization when the boron reacts with oxygen and water after exposure to air.45 As with the broad absorption band at 3209 cm−1, these small peaks significantly decreased after baking the sample overnight at 115 °C. This suggests that the OH groups are weakly bound to the B site of the F-BNNTs. As previously mentioned, the binding of H to form B−H bonds through the empty p-orbital of B is possible considering the presence of ·H in the plasma reactor. The FTIR signature for B−H should be in the vicinity of 2350 cm−1. As shown inFigure 7, there is no band in this region, indicating that a B−H bond is not present in the final product. To validate that ammonia groups are indeed present on the surface of F-BNNTs after exposure to an Ar/NH3 plasma, treatment was repeated on the BNNTs but without the presence of ammonia (100 vol % Ar) at 100 W for 100 min. This was done to ensure that the characterization results obtained for the F-BNNTs were a result of ammonia surface functionalization and not just surface modifications, such as nitrogen vacancies (VN), created by free electron bombard-ment from exposure to plasma. The 100 vol % Ar treated BNNTs will be termed N-BNNTs hereafter. As seen from the DTG results in Figure 8, there was negligible difference between the N-BNNTs and the untreated BNNTs. Addition-ally, the release of ammonia at 250 °C is not observed, which confirms that the observed released ammonia is due to chemical binding onto the F-BNNTs surface.
Dai et al. demonstrated that an Ar plasma can be used to activate and clean impurities from the surface of BNNTs.20 This was also confirmed in this study using the newly reported rra-P3HT characterization methodology for BNNTs39,47 to Figure 6.Water adsorption and desorption isotherms for BNNTs and
F-BNNTs.
Figure 7.Normalized FTIR absorption spectra comparing BNNTs to F-BNNTs. New peaks such as the peak around 1197 cm−1appear with the F-BNNTs, as shown in the inset.
evaluate the relative quality of the different samples. The BNNT quality is defined as a mixture of the sample purity (i.e., fraction of nanotubes in a sample) and BNNT wall defect density, and it is determined from the measured absorption spectrum of rra-P3HT/BNNT hybrids with subtracted scattering background. The results for our samples are shown inFigure 9. The scattering background was calculated by using the process described by Martinez Rubi et al.39
The rra-P3HT to BNNT mass ratio at the maximum BNNT surface polymer coverage is defined as the saturation point (S), which corresponds to the color change observed (Figure S9). This S point can be used as a first estimation of the relative quality of a sample; a higher S value means the sample has a higher quality. Additionally, an rra-P3HT quality index has been developed by Martinez Rubi et al.,39 which can also be used to determine the quality of the three different BNNT samples: (1) untreated BNNTs, (2) N-BNNTs, and (3) F-BNNTs. The saturation point and quality index have been calculated for each sample by using the procedure outlined by Martinez Rubi et al.39and are shown inTable 1.
Both the saturation point and quality index imply that N-BNNTs had the highest BNNT available surface area for the formation of ordered rra-P3HT aggregates (BNNT quality) and that the F-BNNTs had the lowest BNNT available surface area, in agreement with the SBETresults. The results from the
rra-P3HT test confirm that the 100% Ar plasma cleans the surface of BNNTs,20 resulting in an improved purity and crystallinity (i.e., BNNT surface quality) compared to the untreated BNNTs. The low SBETobtained for the F-BNNTs also suggests that polar functional groups (NH3) have been added to the surface because octane, a nonpolar solvent, changed its adsorption behavior after surface modification. Similarly, for the F-BNNTs, Martinez Rubi et al. hypothesized that the addition of functional groups to the surface of BNNTs would hinder the interaction with rra-P3HT and create a less homogeneous orientation between the conjugated polymer chains and the BNNTs surface, resulting in a less intense and less resolved absorption band (i.e., lower saturation point and lower quality index).39 This phenomenon is observed here with the F-BNNTs and supports the claim that addends have been added to the surface of F-BNNTs.
■
DISCUSSIONWe have successfully demonstrated that BNNT powders can be chemically functionalized in-flight using a RF Ar/NH3glow discharge plasma. We found that ·H, ·NH, and ·NH2radicals are critically important to promote functionalization, which generates the final BNNT−NH3 product. Shin et al.24 calculated that all three addends, ·NH, ·NH2, and NH3, can form stable products with BNNTs. Both ·NH2 and NH3 anchor via a dative bond involving the transfer of the nitrogen lone pair into the empty p orbital of boron, which leaves the lattice structure of the BNNTs intact. In contrast, ·NH attachment involves breaking the B−N bond of the lattice structure. However, when the BNNTs are reduced (negatively charged), all three addends bond datively.24 One important question raised by our findings is why are the compounds BNNT−NH and BNNT−NH2not observed? To answer this question, the composition of the plasma must be examined.
In nonthermodynamic equilibrium low-pressure plasmas like the one used in this work, the electron temperature is much greater than the temperature of heavy particles (ions and neutrals): Te≫Ti= Tn. For a typical electron temperature of 1−2 eV, a very large fraction of the plasma electron population have low kinetic energies, and the ions and neutral species are at even lower energies (0.025 eV average energy at room temperature).48,49 The relatively low kinetic energy of the electrons is experimentally supported in our work through the characterization with rra-P3HT in conjunction with SEM analysis, which shows that the crystallinity of the BNNTs was not negatively affected by the plasma treatment (specifically, with 100 vol % Ar). It requires 7−8 eV to break the B−N bond in the h-BN lattice.20 For electrons with mean kinetic temperatures of 1 and 2 eV, the fractions of the Maxwell− Figure 8.DTG graph comparing BNNTs, N-BNNTs, and F-BNNTs.
Figure 9. Absorption spectra of rra-P3HT/BNNT hybrids after subtraction of the scattering background comparing three different BNNT samples.
Table 1. rra-P3HT Saturation Point (S), BET Surface Area (SBET), and rra-P3HT Quality Index Comparing Three Different BNNT Samples
sample saturationpoint (S)
SBETa (m2/g) rra-P3HTquality index Q[Q i i= I(A1−0)/I420]b BNNTs 0.12 280.2 1.7 N-BNNTs 0.14 314.6 1.9 F-BNNTs 0.06 69.9 1.4 aS
BETdetermined experimentally from octane adsorption isotherms in DVS experiments.bI(A
1−0) and I420: representative absorbance in the low-energy region and the high-energy region (at 420 nm), respectively.
ACS Applied Nano Materials Article
DOI:10.1021/acsanm.9b01952
ACS Appl. Nano Mater. 2020, 3, 294−302 299
Boltzmann electron population with kinetic energies under 7 eV are 99.7% and 92.8%, respectively. Because most of the electrons have kinetic energies <7 eV, production of ·NH, ·NH2, and ·H radicals from the dissociation of NH3is more probable than ionization, which requires energy above 10 eV.49 Thus, we expect that the abundant species in our Ar/NH3(98/ 2 v%/v%) plasma system were ·NH, ·NH2, ·H, NH3, and free electrons. As previously explained, theoretical EA calculations reported by Shin et al.24showed that free electron transfer to the surface of BNNTs is favorable, suggesting that reduced BNNT anions form stable species with excess electrons localized at the empty p orbital of boron sites. Such reduced BNNTs subsequently interact with the available ·NH, ·NH2, and ·H radicals in the current functionalization process. Although [BNNT−NH]−, [BNNT−NH
2]−, and [BNNT− H]−
are stable species, further subsequent reactions of these complexes likely occurred in the presence of H, NH, and NH2 radicals, as shown in Scheme 2. The calculation of reaction enthalpy using DFT (see the Supporting Information for details on the calculation method) shows that the cascade reactions with H radicals are always energetically favorable, implying that the reactions continue until a BNNT−NH3 complex is formed. The presence of radical species of ·H, ·NH, and ·NH2in the plasma creates the unique environment that makes the cascade reactions possible and eventually forms the BNNT−NH3 products, as observed in our experimental work. Our finding is entirely consistent with previous theoretical works, which show that ammonia chemisorb on small diameters BNNTs50−52 like the ones used here (3−5
nm). The calculated binding energies of ∼0.2 eV for these tubes suggests that the ammonia is weakly bound to the surface of F-BNNTs, which is consistent with the low ammonia desorption temperature of 250 °C seen in the DTG data.
A second important question is: why is the product not observed when subjecting the BNNTs to simply ammonia gas or an Ar/NH3plasma with a high concentration of NH3? Wu et al.37 reported an activation barrier of 0.1 eV for the direct adsorption of NH3to the BNNT surface and a binding energy of around 0.4 eV through DFT calculations, which suggests that such a reaction would be slow at room temperature even though the reaction is thermodynamically favorable. Increased temperature may accelerate the speed of the reaction overcoming the reaction barrier; however, high-temperature environments would favor the desorption of NH3 from
BNNTs due to the exothermic nature of the reaction (i.e., BNNT + NH3 → BNNT−NH3). This is supported by our TGA analysis where desorption of NH3starts at a relatively low temperature of ∼250 °C. This is the reason why direct amination with NH3is not observed in our study. The present plasma reactor configuration generates ·H, ·NH, and presumably ·NH2 radicals effectively through electron-medi-ated dissociation reactions. Radical reactions usually do not involve activation energy; thus, reactions proceed readily even at room temperature, and the final product, BNNT−NH3, is readily formed in the plasma reactor.
■
CONCLUSIONSA novel approach achieving in-flight functionalization of BNNT powders has been developed by adapting a vertical, nonthermal, Ar/NH3, capacitively coupled, RF glow discharge plasma reactor. A solenoid valve mounted at the bottom of the reactor allowed for periodically fluidizing the BNNT powders and exposing them to the reactive plasma environment. This approach is solvent-free, environmentally friendly, and attractive for industrial scalability. Although demonstrated here with ammonia, the process is applicable to several functionalities with the potential to significantly impact the field of nanocomposites as well as any fields requiring chemically functionalized BNNTs and other fluidizable powders.
The initial expectations were to anchor NH and NH2groups to the surface of the BNNTs; however, the chemical composition of the plasma and the binding characteristics of BNNTs led to the formation of BNNT−NH3as the product. The addition of NH3 transforms the BNNTs from hydro-phobic to hydrophilic, which may have utility anywhere requiring the preparation of stable aqueous suspensions of BNNTs and is favorable for interaction with epoxy and other matrices for BNNT−polymer composites. We are currently exploring the utility of this F-BNNT material in a range of applications.
■
ASSOCIATED CONTENT*
S Supporting InformationThe Supporting Information is available free of charge at
https://pubs.acs.org/doi/10.1021/acsanm.9b01952.
Scheme 2. Proposed Cascade Reaction Pathways toward the Formation of Ammonia-Functionalized F-BNNTs in the Plasma Reactor
Experimental details for BNNT synthesis and purifica-tion, BNNT morphology characterizapurifica-tion, DVS, rra-P3HT methodology for surface quality characterization and computation methods; photograph of the plasma reactor; TEM and SEM images of the purified BNNTs; additional DTG and FTIR data; a photograph comparing the dispersion of BNNTs and F-BNNTs in water; and a schematic of the rra-P3HT methodology for quality assessment of BNNTs (PDF)
Video S1 demonstrating the BNNT pulsing inside the reactor (MPEG)
■
AUTHOR INFORMATION Corresponding Authors *E-mail:robyn.iannitto@nrc-cnrc.gc.ca. *E-mail:benoit.simard@nrc-cnrc.gc.ca. ORCID Robyn Iannitto: 0000-0003-1446-5207 Homin Shin:0000-0001-9300-6898Yadienka Martinez Rubi:0000-0002-1548-6504
Benoit Simard:0000-0001-6689-4308
Sylvain Coulombe:0000-0001-9521-181X Notes
The authors declare no competing financial interest.
■
ACKNOWLEDGMENTSThis work was supported financially through the NRC Ideation Program, the Eugenie Ulmer Lamothe funds (Chemical Engineering, McGill University), the Natural Sciences and Engineering Research Council of Canada, and the Gerald Hatch Faculty Fellowship. The authors are grateful to M. Daroszewska for the TGA-IR and DVS measurements, S. Walker and H. Cho for the BNNT purification, and K. S. Kim, M. Plunkett, and D. Ruth for the BNNT synthesis. The authors also thank F. Caporuscio, L. Cusmich, and G. Lepkyj for their assistance during the construction of the plasma reactor as well as the NRC’s Nanocomposites Group for guidance and help with characterization analysis. H.S. acknowledges the Technology Innovation Program (20000479) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea). The authors thank Dr. Michael Jakubinek at the NRC for his proofreading of this paper and help with analysis of characterization results.
■
REFERENCES(1) Kim, K. S.; Kim, M. J.; Park, C.; Fay, C. C.; Chu, S.-H.; Kingston, C. T.; Simard, B. Scalable manufacturing of boron nitride nanotubes and their assemblies: a review. Semicond. Sci. Technol. 2017,
32, 013003.
(2) Wilder, J. W. G.; Venema, L. C.; Rinzler, A. G.; Smalley, R. E.; Dekker, C. Electronic structure of atomically resolved carbon nanotubes. Nature 1998, 391, 59−62.
(3) Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C. R. Boron Nitride Nanotubes Are Noncytotoxic and Can Be Functionalized for Interaction with Proteins and Cells. J. Am.
Chem. Soc. 2009, 131, 890−891.
(4) Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Cytocompat-ibility, interactions, and uptake of polyethyleneimine-coated boron nitride nanotubes by living cells: Confirmation of their potential for biomedical applications. Biotechnol. Bioeng. 2008, 101, 850−858.
(5) Ciofani, G.; Danti, S.; Genchi, G. G.; Mazzolai, B.; Mattoli, V. Boron Nitride Nanotubes: Biocompatibility and Potential Spill-Over in Nanomedicine. Small 2013, 9, 1672−1685.
(6) Augustine, J.; Cheung, T.; Gies, V.; Boughton, J.; Chen, M.; Jakubek, Z. J.; Walker, S.; Martinez-Rubi, Y.; Simard, B.; Zou, S. Nanoscale Advances Assessing size-dependent cytotoxicity of boron nitride nanotubes using a novel cardiomyocyte AFM assay. Nanoscale
Adv. 2019, 1, 1914−1923.
(7) Blase, X.; Rubio, A.; Louie, S. G.; Cohen, M. Stability and band gap constancy of boron-nitride nanotubes. EPL-EUROPHYS LETT 1994, 28, 335−340.
(8) Jakubinek, M. B.; Ashrafi, B.; Martinez-Rubi, Y.; Guan, J.; Rahmat, M.; Kim, K. S.; Dénommée, S.; Kingston, C. T.; Simard, B. Boron Nitride Nanotube Composites and Applications. Nanotub.
Superfiber Mater. 2019, 91−111.
(9) Jakubinek, M. B.; Ashrafi, B.; Martinez-Rubi, Y.; Rahmat, M.; Yourdkhani, M.; Kim, K. S.; Laqua, K.; Yousefpour, A.; Simard, B. Nanoreinforced epoxy and adhesive joints incorporating boron nitride nanotubes. Int. J. Adhes. Adhes. 2018, 84, 194−201.
(10) Kim, K. S.; Kingston, C. T.; Hrdina, A.; Jakubinek, M. B.; Guan, J.; Plunkett, M.; Simard, B. Hydrogen-catalyzed, pilot-scale production of small-diameter boron nitride nanotubes and their macroscopic assemblies. ACS Nano 2014, 8, 6211−6220.
(11) Hamilton, T. Battle of the nanotubes. Chemical Institute of Canada, 2015.
(12) Kalay, S.; Yilmaz, Z.; Sen, O.; Emanet, M.; Kazanc, E.; Çulha, M. Synthesis of boron nitride nanotubes and their applications.
Beilstein J. Nanotechnol. 2015, 6, 84−102.
(13) Ferreira, T. H.; De Sousa, E. M. B. Chapter 6-Applications and
Perspectives of Boron Nitride Nanotubes in Cancer Therapy; William
Andrew Publishing: 2016.
(14) Raffa, V.; Ciofani, G.; Cuschieri, A. Enhanced low voltage cell electropermeabilization by boron nitride nanotubes. Nanotechnology 2009, 20, 075104.
(15) Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Folate Functionalized Boron Nitride Nanotubes and their Selective Uptake by Glioblastoma Multiforme Cells: Implications for their Use as Boron Carriers in Clinical Boron Neutron Capture Therapy.
Nanoscale Res. Lett. 2009, 4, 113−121.
(16) Ciofani, G.; Boni, A.; Calucci, L.; Forte, C.; Gozzi, A.; Mazzolai, B.; Mattoli, V. Gd-doped BNNTs as T2-weighted MRI contrast agents. Nanotechnology 2013, 24, 315101.
(17) Thibeault, S. A.; Kang, J. H.; Sauti, G.; Park, C.; Fay, C. C.; King, G. C. Nanomaterials for radiation shielding. MRS Bull. 2015, 40, 836−841.
(18) Ikuno, T.; Sainsbury, T.; Okawa, D.; Fréchet, J. M. J.; Zettl, A. Amine-functionalized boron nitride nanotubes. Solid State Commun. 2007, 142, 643−646.
(19) Lee, C. H.; Bhandari, S.; Tiwari, B.; Yapici, N.; Zhang, D.; Yap, Y. K. Boron nitride nanotubes: Recent advances in their synthesis, functionalization, and applications. Molecules 2016, 21, 922.
(20) Dai, X. J.; Chen, Y.; Chen, Z.; Lamb, P. R.; Li, L. H.; Du Plessis, J.; McCulloch, D. G.; Wang, X. Controlled surface modification of boron nitride nanotubes. Nanotechnology 2011, 22, 245301.
(21) Cohen, M. L.; Zettl, A. The physics of boron nitride nanotubes.
Phys. Today 2010, 63, 34.
(22) Le, V. T.; Ngo, C. L.; Le, Q. T.; Ngo, T. T.; Nguyen, D. N.; Vu, M. T. Surface modification and functionalization of carbon nanotube with some organic compounds. Adv. Nat. Sci.: Nanosci. Nanotechnol. 2013, 4, 035017.
(23) Guan, J.; Kim, K. S.; Jakubinek, M. B.; Simard, B. pH-Switchable Water-Soluble Boron Nitride Nanotubes. ChemistrySelect 2018, 3, 9308−9312.
(24) Shin, H.; Guan, J.; Zgierski, M. Z.; Kim, K. S.; Kingston, C. T.; Simard, B. Covalent Functionalization of Boron Nitride Nanotubes via Reduction Chemistry. ACS Nano 2015, 9, 12573−12582.
(25) Hordy, N.; Coulombe, S.; Meunier, J. Plasma Functionalization of Carbon Nanotubes for the Synthesis of Stable Aqueous Nanofluids and Poly (vinyl alcohol) Nanocomposites. Plasma Processes Polym. 2013, 10, 110−118.
(26) Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. In Micro
and Nano Technologies; Elsevier Inc.: 2016; Chapter 2.
ACS Applied Nano Materials Article
DOI:10.1021/acsanm.9b01952
ACS Appl. Nano Mater. 2020, 3, 294−302 301
(27) Zhi, C. Y.; Bando, Y.; Tang, C. C.; Huang, Q.; Golberg, D. Boron nitride nanotubes: functionalization and composites. J. Mater.
Chem. 2008, 18, 3900.
(28) Zhi, C.; Bando, Y.; Tang, C.; Golberg, D. Immobilization of proteins on boron nitride nanotubes. J. Am. Chem. Soc. 2005, 127, 17144−17145.
(29) Quiles-Díaz, S.; Martínez-Rubí, Y.; Guan, J.; Kim, K. S.; Couillard, M.; Salavagione, H. J.; Gómez-Fatou, M. A.; Simard, B. Enhanced Thermal Conductivity in Polymer Nanocomposites via Covalent Functionalization of Boron Nitride Nanotubes with Short Polyethylene Chains for Heat-Transfer Applications. ACS Appl. Nano
Mater. 2019, 2, 440−451.
(30) Pakdel, A.; Bando, Y.; Golberg, D. Plasma-assisted interface engineering of boron nitride nanostructure films. ACS Nano 2014, 8, 10631−10639.
(31) Achour, H.; Achour, A.; Solaymani, S.; Islam, M.; Vizireanu, S.; Arman, A.; Ahmadpourian, A.; Dinescu, G. Plasma surface functionalization of boron nitride nano-sheets. Diamond Relat.
Mater. 2017, 77, 110−115.
(32) Hermanson, G. T. The Reactions of Bioconjugation. In
Bioconjugate Techniques 2013, 229−258.
(33) Hara, O. Curing agents for epoxy resins. In Chemistry and
Technology of Epoxy Resins; Springer: Dordrecht, 1993; pp 37−71.
(34) Simard, B.; Ingold, K.; Walker, S.; Iannitto, R.; Cho, H.; Kim, K.; Kingston, C.; Dénommée, S.; Ruth, D.; Plunkett, M. Process and Apparatus for Purifying BNNT. US. Provisional No. 62/696,377, 2018.
(35) Nicholas, J. E.; Spiers, A. I. Kinetics and mechanism in the decomposition of CCl4in a radio-frequency pulse discharge. Plasma
Chem. Plasma Process. 1985, 5, 263−273.
(36) Jorge, L.; Coulombe, S.; Girard-Lauriault, P. L. Nanofluids Containing MWCNTs Coated with Nitrogen-Rich Plasma Polymer Films for CO2 Absorption in Aqueous Medium. Plasma Processes
Polym. 2015, 12, 1311−1321.
(37) Lei, W.; Mochalin, V. N.; Liu, D.; Qin, S.; Gogotsi, Y.; Chen, Y. Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization. Nat.
Commun. 2015, 6, 1−8.
(38) Kim, K. S.; Couillard, M.; Shin, H.; Plunkett, M.; Ruth, D.; Kingston, C. T.; Simard, B. Role of Hydrogen in High-Yield Growth of Boron Nitride Nanotubes at Atmospheric Pressure by Induction Thermal Plasma. ACS Nano 2018, 12, 884−893.
(39) Martinez Rubi, Y.; Jakubek, Z. J.; Chen, M.; Zou, S.; Simard, B. Quality Assessment of Bulk Boron Nitride Nanotubes for Advancing Research, Commercial, and Industrial Applications. ACS Appl. Nano
Mater. 2019, 2, 2054−2063.
(40) Thommes, M.; Kaneko, K.; Neimark, A. V.; Olivier, J. P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. S. W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015,
87, 1051−1069.
(41) Liu, L.; Tan, S.; Horikawa, T.; Do, D.D.; Nicholson, D.; Liu, J. Water adsorption on carbon - A review. Adv. Colloid Interface Sci. 2017, 250, 64−78.
(42) Lian, B.; De Luca, S.; You, Y.; Alwarappan, S.; Yoshimura, M.; Sahajwalla, V.; Smith, S. C.; Leslie, G.; Joshi, R. K. Extraordinary water adsorption characteristics of graphene oxide. Chem. Sci. 2018, 9, 5106−5111.
(43) Guan, J.; Martinez-Rubi, Y.; Dénommée, S.; Ruth, D.; Kingston, C. T.; Daroszewska, M.; Barnes, M.; Simard, B. About the solubility of reduced SWCNT in DMSO. Nanotechnology 2009,
20, 245701.
(44) Kingston, C. T.; Martínez-Rubí, Y.; Guan, J.; Barnes, M.; Scriver, C.; Sturgeon, R. E.; Simard, B. Coupled thermogravimetry, mass spectrometry, and infrared spectroscopy for quantification of surface functionality on single-walled carbon nanotubes. Anal. Bioanal.
Chem. 2010, 396, 1037−1044.
(45) Marincel, D. M.; Adnan, M.; Ma, J.; Bengio, E. A.; Trafford, M. A.; Kleinerman, O.; Kosynkin, D. V.; Chu, S. H.; Park, C.; Hocker, S.
J. A.; Fay, C. C.; Arepalli, S.; Martí, A. A.; Talmon, Y.; Pasquali, M. Scalable Purification of Boron Nitride Nanotubes via Wet Thermal Etching. Chem. Mater. 2019, 31, 1520−1527.
(46) Díez-Pascual, A. M.; Díez-Vicente, A. L. PEGylated boron nitride nanotube-reinforced poly(propylene fumarate) nanocomposite biomaterials. RSC Adv. 2016, 6, 79507−79519.
(47) Martinez-Rubi, Y.; Jakubek, Z. J.; Jakubinek, M. B.; Kim, K. S.; Cheng, F.; Couillard, M.; Kingston, C.; Simard, B. Self-Assembly and Visualization of Poly(3-hexyl-thiophene) Chain Alignment along Boron Nitride Nanotubes. J. Phys. Chem. C 2015, 119, 26605−26610. (48) Grill, A. Fundamentals of Plasma. In Cold Plasma Materials
Fabrication: From Fundamentals to Applications 1994, 21−22.
(49) Thiry, D.; Konstantinidis, S.; Cornil, J.; Snyders, R. Plasma diagnostics for the low-pressure plasma polymerization process: A critical review. Thin Solid Films 2016, 606, 19−44.
(50) Wu, X.; An, W.; Zeng, X. C. Chemical functionalization of boron-nitride nanotubes with NH3 and amino functional groups. J.
Am. Chem. Soc. 2006, 128, 12001−12006.
(51) Rimola, A.; Sodupe, M. Physisorption vs. chemisorption of probe molecules on boron nitride nanomaterials: The effect of surface curvature. Phys. Chem. Chem. Phys. 2013, 15, 13190−13198.
(52) Li, Y.; Zhou, Z.; Zhao, J. Transformation from chemisorption to physisorption with tube diameter and gas concentration: Computational studies on NH3 adsorption in BN nanotubes. J.