Publisher’s version / Version de l'éditeur:
The Journal of Nutrition, 139, 8, pp. 1487-1494, 2009-06-17
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.3945/jn.109.107920
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Dietary Ascophyllum nodosum increases urinary excretion of
tricarboxylic acid Cycle intermediates in male Sprague-Dawley rats
Simmons-Boyce, Joanne L.; Purcell, Sara L.; Nelson, Carolanne M.;
Mackinnon, Shawna L.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=52936ee4-fb35-4bfb-9d9f-948cb2c84b3f
https://publications-cnrc.canada.ca/fra/voir/objet/?id=52936ee4-fb35-4bfb-9d9f-948cb2c84b3f
The Journal of Nutrition Nutrient Physiology, Metabolism, and Nutrient-Nutrient Interactions
Dietary Ascophyllum nodosum Increases
Urinary Excretion of Tricarboxylic Acid Cycle
Intermediates in Male Sprague-Dawley Rats
1–3
Joanne L. Simmons-Boyce,4Sara L. Purcell,5,6Carolanne M. Nelson,5and Shawna L. MacKinnon4*
4National Research Council of Canada, Institute for Marine Biosciences, Halifax, NS, Canada B3H 3Z1 and5Department of Family and Nutritional Sciences, University of Prince Edward Island, Charlottetown, PE, Canada C1A 4P3
Abstract
We used a1H NMR-based metabonomics approach to examine the physiological effects of the seaweed Ascophyllum
nodosumin a mammalian model, assess the dosage level required to elicit a response in the urinary profile, and identify potential toxic effects. Male Sprague-Dawley rats (n = 6/group) were fed a control or 5, 10, or 15% freeze-dried, ground A. nodosumdiet for 4 wk. Urine samples were collected 3 times daily (0–4, 4–8, and 8–24 h) prior to feeding experimental diets and, at the end of the study, were profiled using1H NMR spectroscopy. Food intake, weight gain, and serum enzyme
(alanine transaminase and aspartate transaminase) levels indicated that seaweed diets were well tolerated. The spectral data and principal component analysis (PCA) revealed that rats fed 5, 10, and 15% seaweed diets had increased urinary excretion of citrate, 2-oxoglutarate, succinate, trimethylamine (TMA), TMA-N-oxide, and malonate and decreased excretion of taurine, creatinine, and acetate compared with the controls. In addition, mannitol was detected in the 8- to 24-h urine samples from seaweed-fed rats. Metabolic responses related to ingestion of seaweed polyphenolics and fiber were not observed in the spectral profiles. Increased seaweed concentration in the diet did not increase the magnitude of the rats’ response as detected by1H NMR. Visual analysis and PCA of the spectral data for serum samples collected at the
end of the study did not show diet-related clustering. The lack of toxicity at 15% seaweed incorporation allows the use of this concentration in future A. nodosum intervention studies. J. Nutr. 139: 1487–1494, 2009.
Introduction
Of the 3.5 billion kg of seaweed harvested for commercial use yearly, 2 billion kg is for human consumption, and most of that is used in the Far East (1). Even though dietary consumption of seaweed in Western countries is gaining popularity, its primary use remains the production of hydrocolloids such as alginate, agar, and carrageenan, which find applications in various industries (2). Numerous bioactive components have been identified in seaweeds over the past 3 decades. These include a structurally diverse set of natural products, such as carotenoids, terpenoids, polyphenols, sulfated polysaccharides, peptides, and fiber that exhibit a range of biological properties, including antibiotic, antiinflammatory, antioxidant, cytotoxic, and anti-tumor activities (2–5).
Ascophyllum nodosum is an edible brown seaweed abundant along the Atlantic coastline of Canada. It is a commercially important source of alginate, fertilizer, and seaweed meal products for human and animal consumption (6). Chemical and nutritional analyses indicate that it contains mannitol; fiber components such as laminaran and fucoidan; polyphenolic compounds formed by polymerization of phloroglucinol units and with molecular weights from ,1 to 100 kDa; vitamins such as thiamin, folic acid, and vitamin C; and amino acids and minerals such as sodium, potassium, calcium, iron, and iodine (7–9). Supplementing the diet of cattle with A. nodosum resulted in enhanced animal health and meat quality, improved immune cell function, and increased circulating antioxidant levels (8). Even though direct human consumption of the seaweed is limited, it is a constituent of health food tablets (10).
Instead of measuring specific biomarkers, as is the classical approach, a metabonomic strategy was employed to explore the complexity of the metabolic response to a chemically diverse dietary ingredient such as A. nodosum. Metabonomics has found widespread use in pharmacology and toxicology but remains relatively new in nutrition research (11). The approach is defined as “the quantitative measurement of the multi-parametric metabolic response in a living system to pathophys-iological stimuli or genetic modification” (12). It provides information on in vivo multi-organ responses in real time (12)
1Supported by an Atlantic Innovation Fund via the Atlantic Canada Opportunities Agency.
2Author disclosures: J. L. Simmons-Boyce, S. L. Purcell, C. M. Nelson, and S. L. MacKinnon, no conflicts of interest.
3Supplemental Figures 1–8 are available with the online posting of this paper at jn.nutrition.org.
6Current address: Pathology and Microbiology, Atlantic Veterinary College, University of Prince Edward Island, 550 University Avenue, Charlottetown, PE, Canada C1A 4P3.
* To whom correspondence should be addressed. E-mail: shawna.mackinnon@ nrc-cnrc.gc.ca.
0022-3166/08 $8.00 ã 2009 American Society for Nutrition.
Manuscript received March 30, 2009. Initial review completed April 27, 2009. Revision accepted May 24, 2009. 1487
Downloaded from ht tps: //academic. oup. com/ jn/ art icle-abst ract /139/ 8/ 1487/ 4670502 by Nat ional Research Council Canada user on 12 December 2018
and in response to nutritional interventions (13–15). One- and 2-dimensional NMR spectroscopy, GC-MS, and HPLC are analytical methods used to profile urine, serum, and tissues. NMR has the advantages of being relatively inexpensive, nondestructive, and applicable to intact biomaterials with minimal sample preparation required and the ability to simul-taneously detect hundreds of metabolites (metabolome) of varying molecular weights (12,16). Multivariate statistical analysis of the data facilitates the isolation of metabolites influenced by the nutritional intervention (13). The current study utilized a nontargeted 1H NMR-based metabonomic
approach to first investigate the physiological effects of a diet containing A. nodosum, second, to assess the dosage level that can be administered to elicit a response in the urinary profile, and third, to identify potential toxic effects of the seaweed. The information obtained could be of use in future targeted studies. A similar approach has been used to unravel new physiological effects of foods, dietary ingredients, and phytochemicals such as whole-grain flour (13), green and black teas (14), chamomile (17), and polyphenols (15,18–20) on the metabolite profiles of urine and plasma in rats and humans.
Materials and Methods
Preparation of the seaweed.A. nodosum, in its vegetative state, was collected from the National Research Council of Canada-Institute for Marine Biosciences (NRC-IMB),7 Marine Research Station (Ketch Harbor, NS, Canada), in July 2005. The seaweed was washed with distilled water, drained, and frozen at 2808C before being freeze-dried and ground to a powder. It was stored at 2808C until incorporation into the diet.
Dosing and sample collection.Animal maintenance and experimental treatment were conducted in accordance with the Canadian Council of Animal Care guidelines and approved by the University of Prince Edward Island Animal Care Committee. Twenty-four male Sprague-Dawley rats (Charles River Labs) weighing 135–154 g were used in this study. To eliminate variation due to individual dietary behavior, all rats were meal trained and pair fed with an isocaloric, isonitrogenous AIN-93G diet (21) prepared at the University of Prince Edward Island. Water was consumed ad libitum. Initially, the rats were housed in individual cages and meal training commenced 1 wk prior to the experimental diet. During this period, the rats were fed for 4 h each day at the beginning of the 12-h dark period. Pair feeding commenced on d 4; each pair-fed group included 1 rat from each diet group. On d 7, the rats were transferred and allowed to acclimatize in metabolic cages for 24 h. The room temperature was maintained at 22 6 18C, the relative humidity at 55 6 10%, and the light cycle consisted of 12 h of light and 12 h of dark. Throughout the study, the control group (n = 6) received the AIN-93G diet. After the acclimatization period, the remaining rats were random-ized into 3 groups of 6 and fed isocaloric, isonitrogenous diets containing 5, 10, or 15% (wt:wt) freeze-dried, ground A. nodosum. This was achieved by adjusting the cellulose content in the diet. The cellulose: seaweed ratio in control and 5, 10, and 15% A. nodosum diets was 150:0, 100:50, 50:100, and 0:150 g/kg diet, respectively. Fat content (70 g/kg diet) for all 4 diets was the same. Identical amounts of mineral (AIN-93G-MX, 35 g/kg diet) and vitamin (AIN-93-VX, 10 g/kg diet) mixes were added to each diet. Urine samples were collected from each rat during the 24-h acclimatization period at the beginning of the first
week and at the end of wk 4 at 0–4, 4–8, and 8–24 h after being fed. Sodium azide (1%, wt:v) was added to the urine samples at the time of collection to minimize bacterial contamination and samples were stored at 2808C pending 1H NMR analysis. Food intake was monitored throughout the study and body weights were determined once per week. At the end of the study, the rats were anesthetized with 65 mg/kg euthanyl (sodium pentobarbital) then killed by exsanguination. The blood was allowed to clot at room temperature for 1 h and then centrifuged at 2500 3 g; 10 min. Serum samples were stored at 2808C prior to1H NMR analysis. Liver damage was assessed by analyzing a
combined serum sample from each group for alanine transaminase (ALT) and aspartate transaminase (AST) using commercially available kits (ALT-SL and AST-SL assays; Diagnostics Chemicals).
1H NMR spectroscopic analysis.
All samples were thawed at room temperature before analysis. Urine samples (400 mL) were added to 0.2 mol/L sodium phosphate buffer (200 mL, pH 7.4) in a polypropylene microcentrifuge tube. Samples were left to stand for 10 min then centrifuged at 11,000 3 g; 10 min to remove any precipitates. Buffered urine (500 mL) was added to (3-trimethylsilyl)propionic-(2,2,3,3-d4
)-acid sodium salt (TSP; 50 mL;) in D2O (1 mmol/L final concentration) in
a 5-mm (o.d.) NMR tube. Serum samples (200 mL) were diluted with saline (400 mL; 0.9% NaCl in water: D2O, 80:20) and 10-mL aliquots
were placed in 1-mm (o.d.) NMR tubes.1H NMR spectra were acquired
on a Bruker Avance-500 spectrometer operating at 500.13 MHz and 208C. The spectrometer was equipped with a TBI gradient probe for the urine analysis and a 1-mm (o.d.) TXI (1H:13C:15N) microliter probe with
z-gradient for the serum analysis. In all cases, a standard 908 pulse sequence was used with a relaxation delay of 2 s, during which the water resonance was selectively irradiated. Each spectrum was acquired with 128 scans collected into 32K data points using a spectral width of 15 ppm and an acquisition time of 2.18 s. Datasets were zero-filled to 64 k and exponential line broadenings of 0.3 and 1 Hz were applied to the FID of the urine and serum samples, respectively, prior to Fourier transforma-tion. The spectra were corrected for phase and baseline distortions using XWINNMR (version 2.6, Bruker Analytische). Urine samples were calibrated to TSP (d 0.0) and serum samples to lactate (d 1.33). In preparation for1H NMR analysis, mannitol (25 mg) was dissolved in 0.2 mol/L sodium phosphate buffer (500 mL, pH 7.4) and TSP in D2O (50
mL) was added. Assignment of metabolite resonances was accomplished by comparison with published data (22,23) and using Chenomx NMR suite (version 4.6 Professional, Chenomx).
Data processing and statistical analyses. For urine and serum samples, the spectral region d 0.2–10.0 was reduced to 245 integrated regions of 0.04 ppm width, using AMIX (version 3.6.8, Analysis of MIXtures, Bruker). The region d 4.5–6.0 was excluded prior to the statistical analyses to remove variations in the presaturation of the water resonance and cross-relaxation alterations of the urea signal (due to solvent exchanging protons). Each spectral dataset was then normalized to the total sum of the integrals to compensate for concentration differences between individual samples. Datasets were exported to the SIMCA-P software (version 10.5, Umetrics) and mean-centered before principal component analysis (PCA), which was employed to reduce the dimensionality in the data and graphically display intersample and intervariable relationships in the data (24). Models were constructed using all the samples and predictions were within the 95% CI. Score plots provide a graphical depiction of groups, trends, and outliers in the observations (spectra). Loading plots indicate the magnitude and manner in which the measured variables (metabolites) are responsible for separation in the score plots (24). Spectra from samples that mapped separately from the main cluster in the score plot of principal component (PC)1 compared with PC2 or that showed a large distance to model in the outlier diagnostics plot were examined to ensure that they were indeed atypical to the normal population before being removed. The mean centered data were also analyzed by partial least squares for discriminate analysis (PLS-DA), a pattern recognition method that discriminates between samples on the basis of class membership. The quality of the PLS-DA models was evaluated by the goodness-of-fit parameter, R2, and the predictive ability parameter, Q2. These
param-7Abbreviations used: ALT, alanine transaminase; AST, aspartate transaminase; DMA, dimethylamine; NRC-IMB, National Research Council of Canada-Institute for Marine Biosciences; 2-OG, 2-oxoglutarate; PC, principal component; PCA, principal component analysis; PLS-DA, partial least squares for discriminant analysis; SCFA, short-chain fatty acids; TCA, tricarboxylic acid; TMA, trimethyl-amine; TMAO, trimethylamine-N-oxide; TSP, (3-trimethylsilyl)propionic-(2,2,3, 3-d4)-acid sodium salt.
1488 Simmons-Boyce et al. Downloaded from ht tps: //academic. oup. com/ jn/ art icle-abst ract /139/ 8/ 1487/ 4670502 by Nat ional Research Council Canada user on 12 December 2018
eters are calculated by a 7-round internal cross validation of the data and values $0.5 indicate good quality of the model (24). Food intake, weight gain, and urine output values are given as means 6 SEM. Statistical analyses were performed using two way ANOVA (SPSS software, version 11). Serum enzyme concentrations were evaluated using the t test. Values of P , 0.05 were considered significant.
Results
Food intake, weight gain, and serum enzyme levels.Rats in all groups consumed their diet within the 4-h feeding period. For the duration of the study, food intake, weight gain, and urinary output at the end of wk 4 did not differ significantly between control and A. nodosum-fed rats. As expected, food intake within a group varied over the course of the study. Levels of ALT and AST in serum did not differ among the groups at the end of the study (Table 1).
Metabolic variability in control urine samples. Visual comparison of 1H NMR spectra of urine samples obtained
from control rats revealed differences in the biochemical profiles associated with the week of the study the collections were conducted (wk 1 compared with 4) and the time of collection over a 24-h period (Supplemental Figs. 1 and 2). PCA of the spectral data of urine samples from control rats showed that PC1 described metabolic variations relating to the week urine samples were collected, i.e. wk 1 compared with 4 (Fig. 1). Separation in PC2 was associated with diurnal variation; urine samples collected 0–4 h and 4–8 h after feeding clustered together. The first 2 PC accounted for 57% of the variance in the data. Examination of the loading plot (Supplemental Fig. 3) revealed that the main variables responsible for clustering were associated with citrate, 2-oxoglutarate, tartaric acid, taurine, creatinine, acetate, succinate, and carbohydrates. Urine samples collected at the end of wk 4 contained higher concentrations of taurine, creatinine, and acetate and lower concentrations of citrate, 2-oxoglutarate (2-OG), and tartaric acid compared with those collected during the first week (Table 2). Inspection of the
loading plot (Supplemental Fig. 3) and the1H NMR spectra revealed that the diurnal separation was attributed to lower concentrations of tartaric acid and taurine and higher concen-trations of citrate, 2-OG, succinate, acetate, creatine, dimethyl-amine (DMA), and trimethyldimethyl-amine (TMA) in the 8–24 h samples. Diurnal differences and those related to the week of study in which the urine samples were collected are summarized in Table 2.
Metabolic effects of A. nodosum supplementation. Anal-ysis of the1H NMR spectra acquired for urine samples collected
from control and A. nodosum-fed rats during wk 4 of the study revealed diet-related differences in the metabolite profiles (Fig. 2). To eliminate the influence of diurnal variation from subse-quent PCA models, the dataset was subdivided, with the
1H NMR spectra of urine samples collected 0–4 and 4–8 h after
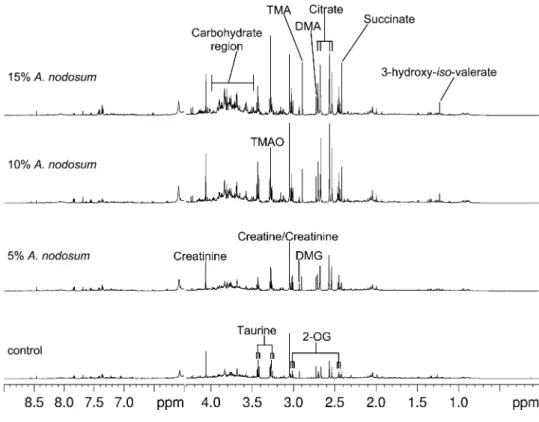
feeding being combined. For both the 0–8 h and 8–24 h urine samples, the PCA score plots showed clustering in relation to the diet consumed (Fig. 3). In the plot of PC1 compared with PC2 for urine samples collected up to 8 h after feeding, PC1 described the diet-related separation and accounted for 42% of the variance in the dataset (Fig. 3A). For the 8–24 h samples, the plot PC2 compared with PC3 showed diet-related separation (Fig. 3B) and accounted for 38% variation; PC1 accounted for intersample variation (data not shown). The fact that a lower PC accounted for the biochemical differences in the 8–24 h group suggests that the effect of the diet was less pronounced after 8 h of consumption. Examination of the corresponding loading plots for both groups (Supplemental Fig. 4), together with the1H NMR spectra, indicated that the discriminating metabolites in the 0–8 and 8–24 h groups were similar. Separation was attributed primarily to increased urinary excretion of citrate, 2-OG, succinate, TMA, and TMA-N-oxide (TMAO) and decreased excretion of taurine, creatinine, and acetate in the A. nodosum-fed rats. The magnitude of the contribution of acetate to separation between controls and seaweed-fed rats in the loadings was greater in urine samples collected 8–24 h after feeding than in those collected 0–8 h. Seaweed-fed rats also excreted higher concentrations of malonate, but the magnitude of this metabolite’s contribution to the loadings on the first 2 PC was smaller than that of citrate, 2-OG, and succinate
(Supple-TABLE 1 Food intake, weight gain, and serum ALT and AST concentrations in Sprague-Dawley rats consuming control and 5, 10, and 15% A. nodosum diets for 4 wk1
% A. nodosum 0 5 10 15 Food intake, g/d Wk 1 8.84 6 0.56 8.29 6 0.34 8.64 6 0.13 8.98 6 0.16 Wk 2 15.41 6 0.42 15.10 6 0.31 15.05 6 0.24 14.98 6 0.38 Wk 3 17.61 6 0.27 17.39 6 0.62 17.66 6 0.40 18.06 6 0.44 Wk 4 16.39 6 0.56 16.62 6 0.38 16.90 6 0.45 17.33 6 0.72 Weight gain, g/wk Wk 1 8.30 6 2.71 9.93 6 1.97 11.07 6 2.61 10.92 6 2.51 Wk 2 60.13 6 3.40 62.60 6 2.74 57.92 6 3.28 62.63 6 2.63 Wk 3 45.35 6 1.94 48.70 6 0.98 47.75 6 2.33 42.45 6 3.73 Wk 4 34.25 6 2.79 33.63 6 1.27 34.33 6 2.30 28.63 6 3.04 Urine volume,2mL/d 19.24 6 2.73 14.24 6 1.21 13.56 6 1.99 16.10 6 2.73 Liver enzymes,3U/L
ALT 10 10 6 10
AST 23 30 27 30
1
Values are means 6 SEM, n = 6.
2Measured during a 24-h period at the end of wk 4, n = 6.
3Measurement done on a combined serum sample collected at the end of wk 4, n = 6.
FIGURE 1 Score plot of PC1 compared with PC2 derived from1H NMR spectra of urine samples collected from Sprague-Dawley rats fed the control diet over a 24-h period during wk 1 and 4 of the study.
Downloaded from ht tps: //academic. oup. com/ jn/ art icle-abst ract /139/ 8/ 1487/ 4670502 by Nat ional Research Council Canada user on 12 December 2018
mental Fig. 4). Signals attributed to mannitol were identified in the 1H NMR spectra of urine samples collected 8–24 h after
feeding seaweed to the rats (Supplemental Fig. 5).
The overlap observed between urine samples from the 5, 10, and 15% A. nodosum groups in the score plots suggested that response to the seaweed was not proportional to the amount in the diet. A PCA model using only the spectral data from the 3 seaweed treatments (Fig. 4A) did not show separation related to the percentage of A. nodosum in the diet. PC1, which accounted for 29% of the variance, described diurnal variation. Individual
PCA models were also generated using the spectral data of controls and samples from each A. nodosum treatment group (Fig. 4B–D). The score plot for the 5% group showed that dietary separation was accounted for by PC2 and PC1 described diurnal variation. For the 10 and 15% treatments, dietary separation was described by PC1. These observations suggest a slightly less marked response to the seaweed at lower levels (i.e. 5%).
PLS-DA showed poor predictability (Q2
, 0.5) and did not identify new discriminating metabolites between the groups.
TABLE 2 Differences in the relative concentrations of urinary metabolites in relation to week of study, diurnal variation, and consumption of A. nodosum1
Chemical shift,2ppm Metabolite
Change in 8–24 h relative to 0–8 h3 Change in wk 4 relative to wk 13 Difference in A. nodosum-fed rats relative to controls4
0–8 h 8–24 h 1.94 (s) Acetate [ [ Y 2.53 (d), 2.67 (d) Citrate [ Y [ [ 3.06 (s), 3.94 (s) Creatine [ 3.06 (s), 4.06 (s) Creatinine [ Y Y 2.72 (s) DMA [ 3.15 (s) Malonate [ [ 3.69 (dd), 3.77 (m), 3.81 (d), 3.87 (dd) Mannitol [ 2.46 (t), 3.02 (t) 2-OG [ Y [ [ 2.42 (s) Succinate [ [ [ 4.34 (s) Tartrate Y Y 3.26 (t), 3.42 (t) Taurine Y [ Y Y 2.88 (s) TMA [ [ [ 3.26 (s) TMAO [ [ 1
[indicates a relative increase and Y a relative decrease in metabolite levels.
2Letters in parentheses indicate1H NMR peak multiplicities: s, singlet; d, doublet; dd, doublet of doublets; t, triplet; m, multiplet. 3Comparison between urine samples from control rats.
4Includes responses of rats on 5, 10 and 15% A. nodosum diets.
FIGURE 2 The 500-MHz 1H NMR spectra of urine samples collected at the end of the 4-h feeding period from control and A. nodosum-fed Sprague-Dawley rats during wk 4 of the study.
1490 Simmons-Boyce et al. Downloaded from ht tps: //academic. oup. com/ jn/ art icle-abst ract /139/ 8/ 1487/ 4670502 by Nat ional Research Council Canada user on 12 December 2018
Furthermore, PLS-DA models constructed to specifically inves-tigate influences of dietary polyphenol metabolism on the aromatic spectral region d 6.0–10.0 did not show clear-cut clustering of the urine samples on the basis of diet (Supplemental Fig. 6).
The1H NMR spectrum of a typical serum sample obtained
from a control Sprague-Dawley rat is illustrated in Supplemental Figure 7. Visual inspection of the1H NMR profiles of serum
collected from control and A. nodosum-fed rats did not show obvious differences. No diet-related separation was observed in the plot of PC1 compared with PC2 (Supplemental Fig. 8) or in the plots of lower PC.
Discussion
Metabolic differences related to week of study. Urine samples collected from control rats during wk 4 of the study had higher concentrations of taurine, creatinine, and acetate than the urine samples collected in wk 1. Taurine concentration in the body is regulated by the kidneys (25) and its excretion is affected by age and diet (26). Urinary increases in creatinine have been correlated to increased muscle mass and increased glomerular
filtration (26). Concentrations of citrate, 2-OG, and tartaric acid also changed as the rats aged, decreasing from wk 1 to 4. Citrate and 2-OG are intermediates in the tricarboxylic acid (TCA) cycle (26). Differences in the levels of intracellular electrolytes and, by extension, the pH that are associated with aging could account for lower citrate and 2-OG concentrations in the wk 4 samples. The patterns in this study were in agreement with those previously reported (26–28) and reemphasize the necessity of having age-matched controls in metabonomics studies. Diurnal metabolic differences. The metabonomic approach employed allowed for comparison of rat urine samples collected during the periods of high (0–4 and 4–8 h after feeding) and low (8–24 h after feeding) metabolic activity. Excretion of citrate, 2-OG, succinate, acetate, creatine, DMA, and TMA increased during the 8–24 h period after feeding, whereas excretion of taurine and tartaric acid decreased. Rats are nocturnal and previous studies have reported differences in urinary metabolites in response to diurnal variation (13,29,30). On this basis, spectral data were separated into 2 groups, 0–8 h and 8–24 h, before further multivariate data analyses were conducted. Diet-induced metabolic differences. Rats consuming A. nodosum excreted elevated concentrations of urinary citrate, 2-OG, and succinate up to 24 h after feeding. This suggests that the seaweed primarily affected metabolites associated with the TCA cycle, a key pathway in the metabolism of carbohydrates, fats, and proteins for the production of energy in the body. Nutritional analyses indicate that, like many other types of seaweed, A. nodosum is high in minerals, including potassium, magnesium, and calcium (9). Regulation of the TCA cycle is influenced by calcium and magnesium levels (31); thus, seaweed consumption may have increased the rate of production of these TCA intermediates. Another possibility is that seaweed con-sumption affected the acid-base homeostasis and decreased reabsorption of the TCA metabolites in the kidneys. The high potassium content of some foods is thought to increase the alkaline load in humans and inhibit tubular reabsorption of citrate (32). In addition, the role of magnesium in regulating citrate has been investigated; i.m. injections of magnesium sulfate reduced tubular reabsorption of citrate and increased urinary citrate excretion (33). Citrate excretion in humans is affected not only by alterations in the acid-base balance but also diet, hormones, vitamin D, calcium, and renal metabolites (34). Increasing urinary excretion of citrate is essential in preventing the formation of kidney stones. A previous study investigated the use of lemonade to increase urinary citrate levels in patients with recurrent kidney stone formation in hopes of finding an alternative to potassium citrate therapy (32). Because urinary concentration of citrate increased after consumption of A. nodosum, even when incorporation was only 5%, it is possible that with further research A. nodosum could be used in similar applications for patients with recurrent kidney stone formation. Consumption of A. nodosum also resulted in increased urinary excretion of TMA and TMAO. Experiments show that germfree mice do not excrete TMA or TMAO, indicating that methylamines are direct products of gut microflora metabolism (35). Red, green, and brown seaweeds contain TMA and other methylamines (36). However, it is unlikely that the seaweed contained excessive levels of TMA, because its disagreeable odor deters feeding (37). It is more feasible that the urinary TMA is a result of ingestion of dietary precursors. A. nodosum contains several quaternary ammonium compounds such as choline (38), betaine, and laminine (39) that could act as precursors to TMA. FIGURE 3 PCA score plots of 1H NMR spectral data of urine
samples collected during wk 4 of the study at 0–4 and 4–8 h (A) and 8– 24 h (B) after feeding Sprague-Dawley rats control and 5, 10, and 15% (wt:wt) A. nodosum diets. Downloaded from ht tps: //academic. oup. com/ jn/ art icle-abst ract /139/ 8/ 1487/ 4670502 by Nat ional Research Council Canada user on 12 December 2018
Metabolic studies with healthy rats showed that even though the capacity for TMA oxidation is very high, ~10% of the TMA produced in the gut is excreted intact in the urine (40). The presence of free choline and betaine in A. nodosum is of nutritional importance, because these compounds are readily bioavailable and function as osmolytes, methyl group donors, and lipotropes, thus maintaining heart, liver, and kidney health (41). Choline and betaine content in the dried A. nodosum is 0.09–0.32% and 0.04–0.12%, respectively (38,42).
The possibility that A. nodosum might affect gut microflora metabolism is consistent with the presence of nondigestible carbohydrates. The seaweed consists of 48–52% nonstarch polysaccharides such as alginate, fucoidan, and laminaran (7,9,43). As shown with macroalgae, nondigestible carbohy-drates are fermented in the gut to produce short-chain fatty acids (SCFA), which have beneficial effects on gut health and function (43). In in vitro fermentations by mixed populations of intestinal bacteria, ~90% of the laminaran and ~50% of the alginate was susceptible to degradation after 24-h incubation, whereas fucoidan was resistant (43). Increased excretion of SCFA was not observed in the1H NMR profiles of urine from A.
nodosum-fed rats in this study, implying that the seaweed matrix affected the fermentation process of these compounds. Degradation products of laminaran promoted the growth of cecal Bifidobac-teria in rats (44). Furthermore, whole dried A. nodosum depressed the growth of Escherichia coli, streptococci, lactoba-cilli, and total anaerobes in in vitro simulations of pig small intestine and the production of SCFA in simulations of cecal fermentation (45). These findings indicate matrix effects on the fermentation of seaweed fiber components and may account for the decreased urinary acetate concentration in the A. nodosum-fed rats, which was more pronounced 8–24 h after feeding.
In this study, mannitol was one of the descriptors responsible for differentiating between urine samples of seaweed-fed and control rats 8–24 h after feeding. A. nodosum contains 8–10% mannitol (7). Although the mannitol in the urine samples was not quantitated, it is reasonable to expect that the administra-tion of mannitol in a complex biological matrix such as seaweed would alter its absorption, metabolism, and excretion. In a previous study, rats fed 20% mannitol excreted ~2% in their urine, suggesting that mannitol is not completely reabsorbed by the kidneys (46). These findings imply that the majority of the mannitol ingested was metabolized. Mannitol is passively absorbed from the digestive tract and thus enters the circulation at a slower rate than glucose and does not result in dramatic fluctuations in blood sugar levels (47). Metabolism is thought to proceed via fructose, which undergoes phosphorylation, before being shunted into the glycolytic pathway. However, mannitol
ingestion has the unexplained effect of increasing hepatic glycogen without altering body weight (46). Compared with the controls, weight gained by rats on the seaweed diets did not differ. Mannitol is also susceptible to degradation by the gut microflora (48).
Surprisingly, the typical response to ingestion of dietary polyphenolics was not observed in this study. Polyphenolics are degraded by the gut microflora to benzoate, which undergoes conjugation with glycine in the kidneys and liver and leads to increased urinary excretion of hippurate (49). A. nodosum contains 4–6% phloroglucinol-derived polyphenolic compo-nents (phlorotannins), but the proton NMR spectral profiles of the urine did not reflect the expected chemical shifts in the aromatic region. Explanations for this may be related to bioavailability and, possibly, enzyme inhibitory activity of the phlorotannins. Bioavailability in humans was greatly reduced, with polyphenols having a high degree of polymerization, such as the proanthocyanidins (50). It is therefore likely that the highly polymerized phlorotannins in the seaweed were not bioavailable to the rats. Alternatively, the complex seaweed matrix may have limited accessibility of the polyphenol-degrading enzymes to their substrates. Another possibility is that the phlorotannins in A. nodosum inhibited the digestive enzymes in the rats. Phlorotannins from the brown seaweed Eisenia bicyclis exhibited inhibitory activity against glycosidases from the viscera of the turban shell Turbo cornutus (51).
The relative concentrations of urinary taurine and creatinine decreased in the A. nodosum-fed rats compared with the controls. Elevated urinary concentrations of taurine and creat-inine are biomarkers of hepatoxicity (52). Elevated serum concentrations of creatinine are indicative of impaired renal function (28). Furthermore, ALT and AST concentrations in the serum did not differ significantly between the treatment groups. ALT and AST are enzymes found in the cytosol of the liver. Damage to the liver releases higher than normal concentrations of these enzymes in the blood and their measurement is considered to be one of the most reliable markers of hepatocel-lular injury and necrosis (53). These observations suggest that the incorporation of up to 15% A. nodosum in the diet did not have a toxic effect on the rats despite the anomalous increase in urinary malonate concentrations in seaweed-fed rats. Malonate is a well-known inhibitor of succinate dehydrogenase in the TCA cycle. It is involved in fatty acid and polyketide biosyntheses and there is evidence to show that free malonate accumulates in plants (54). Seaweed phlorotannins are end products of polyketide biosynthesis (55).
The increased incidence of chronic diseases worldwide has fueled greater interest in examining dietary ingredients for FIGURE 4 (A–D) Score plots of PC1 compared with PC2 derived from urinary1H NMR spectroscopic data comparing the metabolic response
of Sprague-Dawley rats fed diets containing varying concentrations of A. nodosum.
1492 Simmons-Boyce et al. Downloaded from ht tps: //academic. oup. com/ jn/ art icle-abst ract /139/ 8/ 1487/ 4670502 by Nat ional Research Council Canada user on 12 December 2018
potentially valuable roles in maintaining health. This study examined the role of whole, dried A. nodosum as a dietary ingredient. It demonstrates that incorporation of this seaweed in the diets of rats at concentrations of 5–15% resulted in differences in the metabolic processes occurring in the rats that can be observed in their urinary1H NMR profiles. Ingestion of the seaweed primarily increased urinary excretion of TCA cycle intermediates without producing a diuretic response. The results indicate that 15% A. nodosum could be incorporated into the diet of rats without producing toxic effects in the livers and kidneys, a finding that will be useful for other targeted studies of other seaweed species and specific components found in seaweeds such as A. nodosum. Lastly, despite limitations in compound identification, the study highlights the applicability of metabonomics techniques for evaluating the global metabolic response of an organism to a complex food such as seaweed.
Acknowledgments
We thank Robyn MacPhee, formerly of the University of Prince Edward Island, for assistance with the animal handling and sample collection; Ian Burton, NRC-IMB, for technical assis-tance with the NMR acquisition, and Drs. K. Vanya Ewart and Joy Roach, NRC-IMB, for their constructive comments on the manuscript.
Literature Cited
1. Jensen A. Present and future needs for algae and algal products. Hydrobiologia. 1993;260–1:15–23.
2. Smit AJ. Medicinal and pharmaceutical uses of seaweed natural products: a review. J Appl Phycol. 2004;16:245–62.
3. Ko¨nig GM, Wright AD. Algal secondary metabolites and their pharmaceutical potential. In: Kinghorn AD, Balandrin MF, editors. Human medicinal agents from plants. Washington (DC): American Chemical Society; 1993. p. 276–93.
4. Haugan JA, Liaaen-Jensen S. Algal carotenoids 54. Carotenoids of brown algae (Phaeophyceae). Biochem Syst Ecol. 1994;22:31–41. 5. Blunt JW, Copp BR, Hu W-P, Munro MHG, Northcote PT, Prinsep MR.
Marine natural products. Nat Prod Rep. 2008;25:35–94.
6. Rangeley RW. The effects of seaweed harvesting on fishes: a critique. Environ Biol Fishes. 1994;39:319–28.
7. Moen E, Horn S, Østgaard K. Biological degradation of Ascophyllum nodosum. J Appl Phycol. 1997;9:347–57.
8. Anderson MJ, Blanton JR Jr, Gleghorn J, Kim SW, Johnson JW. Ascophyllum nodosum supplementation strategies that improve overall carcass merit of implanted English crossbred cattle. Asian-australas J Anim Sci. 2006;19:1514–8.
9. MacArtain P, Gill CIR, Brooks M, Campbell R, Rowland IR. Nutri-tional value of edible seaweeds. Nutr Rev. 2007;65:535–43.
10. Sharp GJ. Ascophyllum nodosum and its harvesting in Eastern Canada. In: Case studies of seven commercial seaweed resources. FAO Technical Report. 1986;281:3–46.
11. Gibney MJ, Walsh M, Brennan L, Roche HM, German B, van Ommen B. Metabolomics in human nutrition: opportunities and challenges. Am J Clin Nutr. 2005;82:497–503.
12. Lindon JC, Nicholson JK, Holmes E, Everett JR. Metabonomics: metabolic processes studied by NMR spectroscopy of biofluids. Con-cepts Magn Reson. 2000;12:289–320.
13. Fardet A, Canlet C, Gottardi G, Lyan B, Llorach R, Re´me´sy C, Mazur A, Paris A, Scalbert A. Whole-grain and refined wheat flours show distinct metabolic profiles in rats as assessed by a 1H NMR-based
metabonomic approach. J Nutr. 2007;137:923–9.
14. van Dorsten FA, Daykin CA, Mulder TPJ, van Duynhoven JPM. Metabonomics approach to determine metabolic differences between green tea and black tea consumption. J Agric Food Chem. 2006; 54:6929–38.
15. Solanky KS, Bailey NJ, Beckwith-Hall BM, Bingham S, Davis A, Holmes E, Nicholson JK, Cassidy A. Biofluid 1H NMR-based
metabonomic techniques in nutrition research: metabolic effects of dietary isoflavones in humans. J Nutr Biochem. 2005;16:236–44. 16. Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a
platform for studying drug toxicity and gene function. Nat Rev Drug Discov. 2002;1:153–61.
17. Wang Y, Huiru T, Nicholson JK, Hylands PJ, Sampson J, Holmes E. A metabonomic strategy for the detection of the metabolic effects of chamomile (Matricaria recutita L.) ingestion. J Agric Food Chem. 2005;53:191–6.
18. Solanky KS, Bailey NJ, Holmes E, Lindon JC, Davis AL, Mulder TP, Van Duynhoven JPM, Nicholson JK. NMR-based metabonomic studies on the biochemical effects of epicatechin in rats. J Agric Food Chem. 2003;51:4139–45.
19. Daykin CA, Van Duynhoven JP, Groenewegen A, Dachtler M, Van Amelsvoort JM, Mulder TP. Nuclear magnetic resonance spectroscopic based studies of the metabolism of black tea polyphenols in humans. J Agric Food Chem. 2005;53:1428–34.
20. Fardet A, Llorach R, Orsoni A, Martin J, Pujos-Guillot E, Lapierre C, Scalbert A. Metabolomics provide new insight on the metabolism of dietary phytochemicals in rats. J Nutr. 2008;138:1282–7.
21. Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:S838–41.
22. Lindon JC, Nicholson JK, Everett JR. NMR spectroscopy of biofluids. Ann Rep NMR Spectrosc. 1999;38:1–88.
23. Nicholson JK, Foxall PJD, Spraul M, Farrant RD, Lindon JC. 750 MHz
1H and1H-13C NMR spectroscopy of human blood plasma. Anal
Chem. 1995;67:793–811.
24. Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikstro¨m C, Wold S. Multi- and megavariate data analysis: part 1. Basic principles and applications. Umea˚ (Sweden): Umetrics; 2006.
25. Trachtman H, Sturman JA. Taurine: a therapeutic agent in experimental kidney disease. Amino Acids. 1996;11:1–13.
26. Bell JD, Sadler PJ, Morris VC, Levander OA. Effect of aging and diet on proton NMR spectra of rat urine. Magn Reson Med. 1991;17:414–22. 27. Williams RE, Lenz EM, Lowden JS, Rantalainen M, Wilson ID. The metabonomics of aging and development in the rat: an investigation in the effect of age on the profile of endogenous metabolites in the urine of male rats using1H NMR and HPLC-TOF MS. Mol Biosyst. 2005;
1:166–75.
28. Schnackenberg LK, Sun J, Espandiari P, Holland RD, Hanig J, Beger RD. Metabonomics evaluations of age-related changes in the urinary compositions of male Sprague Dawley rats and effects of data normalization methods on statistical and quantitative analysis. BMC Bioinformatics 2007 [cited 2008 Jan 28];8 Suppl 7:S3. Available from: http://www.biomedcentral.com/1471–2105–8-S7–S3.
29. Bollard ME, Holmes E, Lindon JC, Mitchell SC, Branstetter D, Zhang W, Nicholson JK. Investigations into biochemical changes due to diurnal variation and estrus cycle in female rats using high-resolution
1H NMR spectroscopy of urine and pattern recognition. Anal Biochem.
2001;295:194–202.
30. Gavaghan CL, Wilson ID, Nicholson JK. Physiological variation in metabolic phenotyping and functional genomic studies: use of orthog-onal signal correction and PLS-DA. FEBS Lett. 2002;530:191–6. 31. Denton RM, Randle PJ, Bridges BJ, Cooper RH, Kerbey AL, Pask HT,
Severson DL, Stransbie D, Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975;9:27–53.
32. Penniston KL, Steele TH, Nakada SY. Lemonade therapy increases urinary citrate and urine volumes in patients with recurrent calcium oxalate stone formation. Urology. 2007;70:856–60.
33. Rudman D, Dedonis JL, Fountain MT, Chandler JB, Gerron GG, Fleming GA, Kutner MH. Hypocitraturia in patients with gastrointes-tinal malabsorption. N Engl J Med. 1980;303:657–61.
34. Guneral F, Bachmann C. Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem. 1994;40:862–8.
35. Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511–6. 36. Bhakuni DS, Rawat DS. Bioactive marine natural products. New York:
Springer; 2005. p. 2–25.
37. Zhang AQ, Mitchell SC, Smith RL. Dietary precursors of trimethyla-mine in man: a pilot study. Food Chem Toxicol. 1999;37:515–20.
Downloaded from ht tps: //academic. oup. com/ jn/ art icle-abst ract /139/ 8/ 1487/ 4670502 by Nat ional Research Council Canada user on 12 December 2018
38. DaSilva E, Jensen A. Benthic marine and blue-green algal species as a source of choline. J Sci Food Agric. 1973;24:855–61.
39. Blunden G, Gordon SM, Smith BE, Fletcher RL. Quaternary ammo-nium compounds in species of the Fucaceae (Phaeophyceae) from Britain. Br. Eur Phycol J. 1985;20:105–8.
40. Zeisel SH, daCosta KA, Youssef M, Hensey S. Conversion of dietary choline to trimethylamine and dimethylamine in rats: dose-response relationship. J Nutr. 1989;119:800–4.
41. Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539–49. 42. Blunden G, Currie M, Mathe J, Hohmann J, Critchley A. Variations in betaine yields from marine algal species commonly used in the pre-paration of seaweed extracts used in agriculture. The Phycologist. 2009; 76:14.
43. Michel C, Macfarlane GT. Digestive fates of soluble polysaccharides from marine macroalgae: involvement of the colonic microflora and physio-logical consequences for the host. J Appl Bacteriol. 1996;80:349–69. 44. Kuda T, Yano T, Matsuda N, Nishizawa M. Inhibitory effects of
laminaran and low molecular alginate against the putrefactive com-pounds produced by intestinal microflora in vitro and in rats. Food Chem. 2005;91:745–9.
45. Dierick N, Ovyn A, De Smet S. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. J Sci Food Agric. 2009;89:584–94. 46. Ma¨kinen KK, Ha¨ma¨la¨inen M. Metabolic effects in rats of high oral
doses of galactitol, mannitol and xylitol. J Nutr. 1985;115:890–9.
47. Dills WL Jr. Sugar alcohols as bulk sweeteners. Annu Rev Nutr. 1989; 9:161–86.
48. Wiggins HS. Nutritional value of sugars and related compounds undigested in the small gut. Proc Nutr Soc. 1984;43:69–75.
49. Nicholson JK, Wilson ID. Understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov. 2003;2:668–76.
50. Manach C, Williamson G, Morand C, Scalbert A, Re´me´sy C. Bioavail-ability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81 Suppl:S230–42. 51. Shibata T, Yamaguchi K, Nagayama K, Kawaguchi S, Nakamura T.
Inhibitory activity of brown algal phlorotannins against glycosidases from the viscera of the turban shell Turbo cornutus. Eur J Phycol. 2002;37:493–500.
52. Waterfield CJ, Turton JA, Scales MD, Timbrell JA. Investigations into the effects of various hepatotoxic compounds on urinary and liver taurine levels in rats. Arch Toxicol. 1993;67:244–54.
53. Giboney PT. Mildly elevated liver transaminase levels in the asymp-tomatic patient. Am Fam Physician. 2005;71:1105–10.
54. Hatch MD, Stumpf PK. Fat metabolism in higher plants. XVII. Metabolism of malonic acid and its a-substituted derivatives in plants. Plant Physiol. 1962;37:121–6.
55. Targett NM, Arnold TM. Predicting the effects of brown algal phlorotannins on marine herbivores in trpical and temperate oceans. J Phycol. 1998;34:195–205. 1494 Simmons-Boyce et al. Downloaded from ht tps: //academic. oup. com/ jn/ art icle-abst ract /139/ 8/ 1487/ 4670502 by Nat ional Research Council Canada user on 12 December 2018