HAL Id: hal-02798228
https://hal.inrae.fr/hal-02798228
Submitted on 5 Jun 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Co-exposition effects on honey bee Apis meliffera
olfactory learning performance
Anna Papach
To cite this version:
Anna Papach. Co-exposition effects on honey bee Apis meliffera olfactory learning performance. Biodiversity and Ecology. 2015. �hal-02798228�
Master 20 Année Biologie, Ecologie, Evolution 2014-2015 Université de Poitiers
U.F.R. Sciences Fondamentales et Appliquées 15 Rue de l’Hôtel Dieu
86000 Poitiers
Papach Anna
Supervisor: Richard Freddie-Jeanne University of Poitiers
freddie.jeanne.richard@univ-poitiers.fr Co-supervisor: Aupinel Pierrick
INRA, Le Magneraud
pierrick.aupinel@magneraud.inra.fr
Co-exposition effects on honey bee Apis meliffera
olfactory learning performance
Acknowledgment
I would like to thank Freddie-Jeanne Richard for suggesting me an interesting topic and Pierrick Aupinel for welcoming me in his team, but also for their advices and supervision. Many thanks to Dominique Fortini and Carole Moreau-Vauzelle for sharing with me their knowledge, experience and for being patience and supportive. Also I would like to thank Stéphane Grateau for providing me with beautiful brood frames and for showing a small, but an interesting part of beekeepers’ job. I am very grateful for all the employees and all the interns from the experimental entomological unit of INRA, Le Magneraud for positive and encouraging working atmosphere. And at last, but not least I would like to thank the IMAE program that gave me the opportunity to conduct this work, but also for meeting all inspiring and wonderful IMAE people.
Table of content
Introduction ... 1
Materials and Methods ... 5
Larval rearing and exposure ... 5
Olfactory Proboscis Extension Response conditioning ... 7
Absolute conditioning procedure ... 7
Middle-term memory ... 8
Long-term memory... 9
Data analysis ... 9
Results ... 10
Honey bee larvae survival and emergence ... 10
Acquisition, middle-term memory ... 12
Acquisition, long-term memory ... 13
Middle-term memory ... 15
Long-term memory ... 15
Discussion ... 17
Effect of exposition and co-exposition on honey bee larval survival and emergence ... 17
Olfactory learning performance ... 19
Memory retention ... 21
Conclusion ... 22
References ... 24
Annex 1 ... 29
Introduction
Pollination is an essential ecosystem service that sustains biodiversity and contributes to the global food security. More than 70% of all flowering plants depend on animal pollination; this value varies depending on the region and reaches its maximum of 94% in the tropical communities (Ollerton et al. 2011). Besides sustaining the biodiversity, pollinators support crop yields; around 35% of global crop production relies on insect pollination (Klein et al. 2007) and around 84% of commercial crops in EU are pollinated by insects, mostly by honey bees (Carreck, Williams, 1998). Still, insect pollination is an essential for one third of global food market, but this one third provides us with the majority of nutrients as mostly fruits and vegetables depend on animal pollination. In 2005 the estimated economic value of pollination worldwide was about €153 billion (Gallai et al. 2009).
However, in last decades there was a decline in populations of wild and managed pollinators (honey bees in particular), while the demand for their services has significantly increased (Breeze et al. 2011; Calderone 2012). Starting from 2006 beekeepers in USA reported great losses of adult bees in their colonies (vanEngelsdorp et al. 2009). The same decline of domesticated honey bee populations was observed in Europe (Neumann, Carreck, 2010; Potts et al. 2010) which is characterized with high winter mortality. Unexplained losses in bee colonies were also observed in China and in some places in Africa (Peduzzi et al. 2010). Decline of pollinators has a negative impact on agriculture but also on landscapes (Ricketts et al. 2008) and plant diversity.
There are several factors that contribute to pollinator losses. Among the main pressures on pollinators are the land-use intensification that also includes habitat fragmentation and heavily usage of pesticides; climate change and also spread of alien species and diseases (Potts et al. 2010; Vanbergen 2013). Population growth leads to the increase of food production that in turns leads to the land-use intensification. Therefore, natural habitats are being transformed into the monotonous crops and are being fragmented. This implies that less food sources are available to pollinators and monotonous crops can’t provide them with all the necessary nutrients. Additionally, land-use intensification leads to the application of pesticides that sometimes also can affect non-target species. Climate change in turn leads to the decrease of
floral resource availability to pollinators; models done showed that this reduction can reach about 17-50% (Memmott et al. 2007). Another problem is introduction of alien species and spread of diseases. New species out-compete native pollinators but also pests and pathogens are causing a big danger. Varroa destructor mite is a well-known pest that is spread across the world and besides feeding on honey bee hemolymph it is also the primary vector of many viruses (Le Conte et al. 2010). Another widespread pest is microsporidian gut parasite Nosema
apis and Nosema ceranae that can also lead to the colony losses (Higes et al. 2008; Wolf et al.
2014). One of the most detrimental honey bee diseases is American Foulbrood. This disease is the only highly virulent disease that occurs in honey bees. It is caused by the spore-forming
Paenibacillus larvae that infect honey bee larvae; larvae that are older than 53 hours after the
egg has hatched cannot be infected (Crailsheim & Riessberger-Gallé 2001). American Foulbrood occurs throughout the world and can cause serious threats to the colonies; it is very hard to treat it. One of the available treatments is application of antibiotics; however they don’t kill spores and just suppress clinical symptoms. In Europe use of antibiotics is prohibited as the residues can be stored in honey, they may affect the longevity of the bees and also
Paenibacillus larvae gets more resistant after several applications (Genersch 2010). In many
countries infected colonies should be destroyed, the bees are killed with poisonous gas and hives are burned along with the contaminated equipment; this method causes a big economic damage (Ritter & Akratanakul 2006). Another method is artificial swarm method, but it is not always efficient and effective just during the early stages of disease (Fries, Camazine, 2001; Genersch 2010).
As previously mentioned, application of pesticides is another factor that can lead to the decrease of honey bee colonies. Nowadays the most well-known and widely used group of pesticides is neonicotinoids. This group of chemicals in 2012 shared 26% of the global insecticide market (Van der Sluijs et al. 2013). Although this group of chemicals also affects the non-target species, honey bees in particular. A lot of studies have been done showing their negative impact on honey bees and other pollinators (Peduzzi et al. 2010; Hopwood et al. 2013; Vanbergen 2013). Neonicotinoids reduce adult longevity and cause delayed development (Desneux et al. 2007; Wu et al. 2011), they impair the brain functions and have negative influence on the foraging behavior (Decourtye, Devillers, et al. 2004; Potts et al. 2010;
Armengaud, et al. 2004; Decourtye et al. 2005; Aliouane et al. 2009; Blacquière et al. 2012; Tan et al. 2015). Previous studies have shown that neonicotinoids like imidacloprid also act on hypopharyngeal gland of honey bee nurses and shift their nest activities to the field activities (Hatjina et al. 2013).
Thiamethoxam along with other pesticides of this group causes higher mortality (Laurino et al. 2011), impairment of learning, behavioral disturbances and also evokes problems with orientation (Aliouane et al. 2009; Williamson et al. 2014). Sub-lethal doses of thiamethoxam also induce homing failure in honey bees (Henry et al. 2012). Further, in Europe in April 2013 application of thiamethoxam along with imidacloprid and clotianidin was forbidden on crops that attract pollinators (EFSA 2013). This ban came into force in December 2013 and was based on the conclusion of the European Food Safety Authority (Food & Authority 2013). This year this ban comes to an end and reopens the debates about its acceptance again.
All mentioned above leads to the decrease of pollinators, doubting the food security and preservation of the ecosystem services. Nevertheless, none of these factors alone were identified as responsible for the observed great colony losses. Honey bees can be infected with different pathogens and parasites; on the same time they are exposed to pesticides and residues of different chemical treatments applied by beekeepers and their metabolites that are stored in pollen, nectar, wax (Mullin et al. 2010). However, the interaction between those factors is unknown and may have different effects. Therefore, it is believed that the current decrease of wild and managed pollinators has a multifactorial nature (vanEngelsdorp et al. 2009; Potts et al. 2010; Vanbergen 2013; Food & Authority 2014). In scientific literature several studies have been done on co-exposition effects of pesticides and pathogen on honey bee and bumblebee survival (Alaux et al. 2010; Vidau et al. 2011; Aufauvre et al. 2012; Locke et al. 2012; Pettis et al. 2012; Di Prisco et al. 2013; Aufauvre et al. 2014; Baron et al. 2014; Doublet et al. 2014; Fauser-Misslin et al. 2014; Retschnig et al. 2014). It was shown that honey bees that are exposed to clotianidin have lower immune defenses and are more susceptible to viruses (Di Prisco et al. 2013) and exposure to sub-lethal concentrations of imidacloprid during the larval stage makes workers more sensitive to the gut parasite Nosema ceranae (Pettis et al. 2012). In general, exposure to neonicotinoids (imidacloprid, thiacloprid and fipronil) and to Nosema leads to the increased mortality; pathogen and pesticide can have synergistic effect (Alaux et al.
2010; Vidau et al. 2011; Aufauvre et al. 2012; Pettis et al. 2012; Di Prisco et al. 2013; Doublet et al. 2014; Retschnig et al. 2014).
Honey bees exposure to neonicotinoids leads to the impairment of their olfactory learning and memory (Decourtye, Armengaud, et al. 2004; Decourtye et al. 2005; Aliouane et al. 2009; Blacquière et al. 2012; Tan et al. 2015). Though, olfactory learning is one of the key conditions of successful foraging. Honey bees learn to associate the floral odor with a nectar reward and afterwards share this information with newly recruited foragers (von Frisch, 1967). Learned odors can be retrieved several days and weeks after (Menzel 1999; Beekman 2005). Therefore olfactory learning and memory are very important for the colony success and survival. One of the classical methods that is used for studying learning and memory processes in honey bees is olfactory proboscis extension response (PER) conditioning. During the conditioning the honey bee worker learns to associate the odorant with sucrose reward and therefore it learns to extend its proboscis after the presentation of the odor. The first well-known protocol belongs to Takeda (Takeda, 1961) and lately it was improved by Bitterman (Bitterman et al., 1983) and in 2012 it was revised by Sandoz and Giurfa (Giurfa & Sandoz 2012).
There is a gap of knowledge in co-exposition studies between pesticide and pathogen; moreover, yet all the studies done on impact of pesticides on honey bees were done on workers and none of them looked at the impact that it might have after early exposure of the honey bee larvae.
Here we decided to test whether exposure of honey bee larvae to sub-lethal doses of thiamethoxam and American Foulbrood have an effect and also if the co-exposition modify the effect. To that end, we studied the following aspects, the effect of exposition and co-exposition on: 1) honey bee larvae survival and emergence; 2) impairment of learning and memory abilities.
Figure 1. Key drivers of honey bee loss and interaction between them. Red arrows show direct pressures; green arrows represent interaction between drivers and blue within drivers (Potts et al. 2010 a).
Materials and Methods
We studied the effect of co-exposition between neonicotinoid and pathogen on honey bee survival and learning performances. The experiments were conducted in the entomological experimental unit of INRA, le Magneraud, France between March and August, 2015.
Larval rearing and exposure
The honey bee larvae were obtained from three different colonies of Apis mellifera
ligustica. In our work we followed the method of artificial larval rearing that was improved by
Aupinel and colleagues (Aupinel et al. 2005). To get the comb with first instar larvae, the queen was confined in the empty comb by means of the excluder cage and left there for less than 30 hours; the queen was confined four days before grafting. After 24-30 hours the queen was removed from the cage to prevent further egg laying in the comb; workers were able to freely move between the encaged comb and the rest of the hive. This method allowed us to obtain enough first instar larvae for the grafts. With the help of a thin paintbrush larvae were transferred from the comb to the artificial cells earlier filled with the special diet that consisted of royal jelly and aqueous solution of D-glucose, D-fructose and yeast extract (Annex 1). In total we performed two grafts per colony and we had 96 larvae per treatment for each graft. After grafting plates with larvae were transferred into desiccator ensuring relative humidity (RH) of about 95% that was placed in the incubator with the temperature of 34,50 C (Fig. 2).
In our work we used five different treatments to study the co-exposition impact on honey bee survival and learning performances. The first group was the control group and it had no treatment. The second group was subjected to sub-lethal dose of Paenibacillus larvae that is four spores per larva. The pretesting of determining the number of spores that doesn’t induce larval mortality was done earlier in February and INRA, le Magneraud (unpublished data). The third group received sub-lethal dose of thiamethoxam that is four ppb. Again, the sub-lethal concentrations of thiamethoxam were determined earlier with another test in the INRA, le Magneraud (unpublished data). The next two groups were subjected simultaneously to
Paenibacillus larvae and thiamethoxam. The fourth group received four spores of Paenibacillus larvae and four ppb of thiamethoxam and the last group was subjected to the half dose used in
the fourth group, making it two spores of Paenibacillus larvae and two ppb of thiamethoxam per cell. For the 2nd, 4th and 5th group honey bee larvae were exposed to spores of Paenibacillus
larvae that was added to the diet on day 1. We used a strain of Paenibacillus larvae that was
obtained from the apiaries in Magneraud in 2010.
Figure 2. Schematic representation of the main steps of the larval rearing and exposure.
Thiamethoxam was obtained from the Cluzeau Info Labo with purity of 99 %. Stock solutions of this pesticide were prepared in water; to get the solutions of the desired concentrations we prepared several aliquots from the stock solution. We performed chronic exposure to thiamethoxam, therefore starting from day three and until day six, larvae were fed with thiamethoxam that was added to their diet. In total each larvae received the full amount of 0.628 ng of thiamethoxam in treatment with 4 ppb and 0.314ng in the treatment with 2 ppb. The mortality was controlled every day before feeding larvae.
On the 8th day all larvae were transferred into the desiccator with a lower humidity of 80% (Fig. 2). At the end of the pupation stage larvae plates were transferred into the alimentary crystal polypropylene boxes with aerated lids. Each box contained the Queen Mandibular Pheromone and a piece of wax. The boxes were kept in incubator at 34.50 C and RH around 30-40%. After the emergence honey bees workers were provided with pollen powder and 50% sugar solution ad libitum. Pollen was changed every two days. Adults were kept in these conditions for two weeks until the olfactory learning tests.
Olfactory Proboscis Extension Response conditioning
We used the classical olfactory proboscis extension response (PER) conditioning procedure for testing the exposition and co-exposition effect on honey bee workers’ learning and memory abilities.
We used honey bee workers that were reared in vitro and exposed them to different treatments during the larval stage. For the conditioning we used workers that were 13-14 days old as at this age they show better responses to odour and better learning abilities compare to the younger bees (Laloi et al. 2000; Wang et al. 2005). We followed the revised olfactory classical conditioning of the proboscis extension response protocol by Matsumoto and colleagues(Matsumoto et al. 2012). In order to prepare the honey bee workers for the PER test they were anesthetised by cooling on ice until they became motionless (Felsenberg et al. 2011; Frost et al. 2012). Every testing day we had five groups of ten individuals. Bees were individually harnessed with the adhesive tape in the metal tubes and tubes were marked for individual identification. Every honey bee worker was restrained in the tube in a way that the worker was able to extend its proboscis and freely move its mouth parts. After harnessing bees were left for 3-4 hours to recover in a dark place with the room temperature (Giurfa & Sandoz 2012).
The 50% sucrose solution was used as the unconditioned stimulus (US). In most studies 30% sugar solution is used (Morgan et al. 1998; Laloi et al. 2000; Decourtye et al. 2005; Matsumoto et al. 2012), but considering the fact that our bees were reared in vitro and fed with the 50% sugar solution during their life for our experiment we decided to use 50% sugar solution. For odor delivery we used 20 ml plastic syringe, containing a piece of a filter paper (10mm*30mm) soaked with 5 µl of odorant. 1-nonanol was used as a conditioning stimulus (CS) during the conditioning trials and two odors (1-nonanol and 2-hexanol) were used for the memory retention test that workers are able to distinguish from each other (Guerrieri et al. 2005). The odours were purchased from Sigma Aldrich.
Absolute conditioning procedure
In the present study we used the absolute olfactory conditioning when the bee learns that the CS predicts the US and it associates odour with the sucrose reward. We tested the exposition and co-exposition effects on worker’s middle and long term memory. To determine the impact on the middle term memory we performed three conditioning trials (Decourtye,
Armengaud, et al. 2004). Before the experiment all the bees were checked for the PER to sucrose and those individuals that didn’t extend their proboscis were discarded from the test. The conditioning site had a ventilation hood for the odour removal and workers were placed in front of it so it was at their back.
Middle-term memory
All bees were conditioned in a following way: a bee was placed to the conditioning site and left for 15 sec to habituate to the experiment conditions; after 15 sec we presented the odour for 4 sec with subsequent sucrose stimulation for 4 sec and 2 sec overlap. Therefore the inter-stimulus (ISI) interval was 2 sec (Fig.3). After the CS-US pairing the bee was left in the conditioning place for another 15 sec. When the bee was extending it proboscis it was allowed to drink. We used 10 min Inter Trial Interval (ITI) (Matsumoto et al. 2012) as ITI that are less than 10 min show lower acquisition and retention levels (Menzel et al. 2001). In total we performed three conditional trials and at the end we had 50-54 individuals per treatment.
Figure 2. Experimental procedure of absolute conditioning
Memory retention test was performed 1 hour after the last conditioning trial. As was mentioned above we used two odours for this test; one odour was the novel one – 2-hexanol and the second one was the conditioned odour – 1-nonanol. The trial time was the same as for the conditioning experiment; the only difference was the absence of the sugar reward. To avoid the sequential effect the odours were presented in a random way.
After the retention test we checked all the bees for the PER to sucrose, we stimulate the antennae with the sucrose solution and individuals that didn’t show the response were discarded from the test.
Long-term memory
To test the influence of different treatments on the long-term memory we used the same olfactory PER conditioning procedure as described above. Bees were harnessed in the morning and the conditioning trials started four hours after. It was shown that five conditioning trials are enough for the formation of a robust long-term memory (Menzel 1999; Matsumoto et al. 2012) therefore we performed five conditioning trials instead of three that were done for the testing of the middle-term memory. After the last conditioning trial each worker was fed with sucrose solution to avoid excessive starvation and mortality. Bees were kept in the dark place with a room temperature overnight and the memory retention test was performed 24 hours after. At the end we had around 38-42 individuals per treatment.
Data analysis
Cumulative larval mortality was compared between each treatment and control by multiple two-by-two x2 with 1 df. The same test was using to compare the adults’ emergence rate between treatments and control. To compare the effect of our treatments on mortality between larval stage and pupation we also used multiple two-by-two x2 with 1 df between each treatment and control.
In the experiment with conditioning of PER, we compared the number of reflex responses with the number of conditioned responses at each trial between the group that received treatment and the control group by multiple two-by-two x2 with 1 df.
Results
Honey bee larvae survival and emergence
In total we grafted 576 first instar larvae per treatment. The mortality was controlled six times on day: three, four, five, eight, fifteen and twenty two. We observed mortality during the larval stage, pupation and until the emergence of adults (Annex 2). Fig. 4 shows the cumulative mortality rates for each treatment during the experiment. We observed an increase in mortality after day five in the treatment with 4 spores of Paenibacillus larvae and both treatments with co-exposition.
Figure 4. Evolution of mortality after application of treatments from day 3 to day 22. Error bars represent the 95% confidence interval.
Our results showed that at day 3 and at day 4 the difference between control and four treatments was not significant. However, at day 5 we observed significant differences of cumulative honey bee larvae mortality between control and Paenibacillus larvae 4 spores (x2=9.3, df:1, P<0.05) and also between control and Paenibacillus larvae 4 spores + thiamethoxam 4 ppb (x2=6.46, df:1, P<0.05). 0 10 20 30 40 50 60 70 80 90 100 D3 D4 D5 D8 D15 D22 M o rtali ty %
Day after grafting
Control Paenibacillus larvae 4 sp
Thiamethoxam 4 ppb Paenibacillus larvae 4 sp + Thiamethoxam 4 ppb Paenibacillus larvae 2 sp + Thiamethoxam 2 ppb
Start of the pupation stage
To see the influence of our treatments on the honey bee larvae survival we compared each cumulative mortality rate with the control at the end of the larval stage, at day 8. We found significant differences between control and treatments with Paenibacillus larvae 4 spores (x2= 27.2, df:1, P<0.05) , Paenibacillus larvae 4 spores + thiamethoxam 4ppb (x2=30.4, df:1, P<0.05) ,
Paenibacillus larvae 2 spores + thiamethoxam 2 ppb (x2 =17.8, df:1, P<0.05).
Another tested aspect was the level of mortality between two stages, larval and pupation. We calculated the mortality rates for each treatment during both stages and compared number of dead larvae (D3-D8) with the number of dead pupae (D15) relative to the number of individuals at the beginning of the respective stage. We didn’t detect significant difference in mortality between stages in any treatment, however we can see that in all the treatment that contained spores of Paenibacillus larvae the percentage of dead individuals was higher during the pupation (Fig.5) while for control and group treated with thiamethoxam it is opposite.
Figure 5. Percentage of mortality during the larval stage and pupation. The number of dead larvae was compared with the number of dead pupae for each treatment using a x2 test with 1 df (*P<0.05).
We also analysed the impact of applied treatments on the adult emergence rate. There was no effect of thiamethoxam 4 ppb alone on the workers’ emergence rate (x2=1.75, df:1, P>0.05). Significant decrease in adults’ emergence rate in groups that received Paenibacillus larvae 4
0 5 10 15 20 25 Control Paenibacillus larvae 4 sp Thiamethoxam 4 ppb Paenibacillus larvae 4 sp + Thiamethoxam 4 ppb Paenibacillus larvae 2 sp + Thiamethoxam 2 ppb M o rtali ty (% ) Treatment Larval stage Pupation
spores (x2=48.8, df:1, P<0.05), Paenibacillus larvae 4spores + thiamethoxam 4 ppb (x2=77.2, df:1, P<0.05) and Paenibacillus larvae 2 spores + thiamethoxam 2 ppb (x2=37.3, df:1, P<0.05).
Figure 6. Effects of treatments applied during the larval stage on adults’ emergence; n=number of alive honey bee workers at day 22. The number of emerged workers was compared with the control group using x2 test with 1 df (*P<0.05). Error bars represent the 95% confidence interval.
We also found in the group that received 4 spores of Paenibacillus larvae + 4 ppb of thiamethoxam adults’ emergence rate was lower compare to the group that was co-exposed to 2 spores of Paenibacillus larvae + 2 ppb of thiamethoxam (x2=7.35, df:1, P<0.05). The lowest adult emergence rate of 58% was observed in the group that was exposed to 4 spores of
Paenibacillus larvae + 4 ppb of thiamethoxam while in the control group this value was 82%
(Fig.6). In groups that were treated with 4 spores of Paenibacillus larvae and group with 2 spores of Paenibacillus larvae + 2 ppb of thiamethoxam adults’ emergence rate was 64% and 66% respectively.
Acquisition, middle-term memory
We subjected 13-14 days old honey bee workers that were exposed to different treatments during larval stage to the absolute olfactory conditioning of PER (N=53 control, N=53
Paenibacillus larvae 4 spores, N=52 thiamethoxam 4, N=54 Paenibacillus larvae 4 spores +
thiamethoxam 4 ppb and N=50 Paenibacillus larvae 2 spores + thiamethoxam 2 ppb). All
n=471 n=366 n=491 n=335 n=380 0 10 20 30 40 50 60 70 80 90 100
Control Paenibacillus larvae 4 sp Thiamethoxam 4 ppb Paenibacillus larvae 4 sp + Thiamethoxam 4 ppb Paenibacillus larvae 2 sp + Thiamethoxam 2 ppb A d u lt e m e rg e n ce (% ) Treatment * * *
the end of the third trial the lowest response was observed in the group that was subjected to thiamethoxam 4 ppb; the % of PER for this group was 48%, while for control group it was 66% (x2=3.46, df:1, P>0.05).
Figure 7. Olfactory learning performance of restrained honey bee workers during the absolute olfactory conditioning of PER.
However, we observed a tendency that workers from the treated groups had lower acquisition level compare to control (Fig.7), especially during trial 2 we can see that in control group the response to the conditioned stimulus (CS) was about 58%, while in treated groups it lays within the range of 36-43%. We found significantly lower response rate compare to control in the groups that received 4 ppb of thiamethoxam (trial2: x 2=4.22, df:1, P<0.05) and the group that was exposed to 2 spores of Paenibacillus larvae and 2 ppb of thiamethoxam (trial2: x2=5.22, df:1, P<0.05).
Acquisition, long-term memory
The groups of honey bee workers that were trained for testing the long-term memory performance received five conditional trials (Fig. 8). We can see that the learning in all tested groups was rapid during the first three trials and after the level of PER to CS stayed almost the same (Fig.8). 0 10 20 30 40 50 60 70
Trial 1 Trial 2 Trial 3
% o f c o n d ition e d PE R Conditioning trials Control, N=53 Paenibacillus larvae 4sp, N=53 Thiamethoxam 4ppb, N=52 Paenibacillus larvae 4sp + Thiamethoxam 4ppb, N=54 Paenibacillus larvae 2sp + Thiamethoxam 2ppb, N=50
Figure 8. Olfactory learning performance of restrained honey bee workers during the absolute olfactory conditioning of PER.
We compared the level of acquisition at the end of the fifths and found significantly reduced level of responses compare to control in the group subjected to 4 ppb of thiamethoxam (x2=4.22, df:1, P<0.05), showing the negative effect of thiamethoxam at concentration of 4 ppb on the olfactory learning performance.
As we used the same conditioning procedure we decided to pull the data that we obtained for the third trial from both sets of groups (groups that were trained during three trials for testing middle-term memory and groups that were trained during five trials for testing the long-term memory) together. We found the reduction of olfactory learning performance in the group that was treated with 4 ppb of thiamethoxam (trial 3: x2=13.9, df:1, P<0.05) and in the group that received 2 spores of Paenibacillus larvae and 2 ppb of thiamethoxam (trial 3: x2=4.45, df:1, P<0.05). No significant difference were found between control and Paenibacillus
larvae 4 spores (trial 3: x2=0.54, df:1, P>0.05), between control and Paenibacillus larvae 4 spores + thiamethoxam 4ppb (trial 3: x2=1.73, df:1, P<0.05).
0 10 20 30 40 50 60
Trial 1 Trial 2 Trial 3 Trial 4 Trial 5
% o f c o n d ition e d PE R Conditioning trials Control, N=42 Paenibacillus larvae 4sp, N=35 Thiamethoxam 4ppb, N=38 Paenibacillus larvae 4sp + Thiamethoxam 4ppb, N=41 Paenibacillus larvae 2sp + Thiamethoxam 2ppb, N=44
Middle-term memory
All groups showed significantly higher responses to the CS than to the novel odour (NOd) (control: x2 =5.5, P<0.05; Paenibacillus larvae 4spores: x2 = 7.26, P<0.05; thiamethoxam 4 ppb: x2 =25.29, P<0.05; Paenibacillus larvae 4 spores + thiamethoxam 4 ppb: x2 =12.03, P<0.05;
Paenibacillus larvae 2 spore + thiamethoxam 2 ppb: x2 =7.26, P<0.05). Our results confirmed that honey bee workers had a CS-specific memory and could distinguish between odours. We compared the number of PER to the CS one hour after conditioning between the control group and our treatments and we detected reduced level of responses in the group treated with thiamethoxam 4 ppb (x2=7.51, df:1, P<0.05) showing that thiamethoxam impairs the middle-term memory (Fig.9).
Figure 9. Memory retention performance of honey bee workers subjected to olfactory conditioning of PER one hour after the conditioning. Bars represent % of PER for the. The number of conditioned responses was compared with the control group using a x2 test with 1 df (*P<0.05).
Long-term memory
We also tested the influence of exposition and co-exposition on the long-term memory corresponding to 24 hours after the conditioning (Fig. 10).
We used two odours (same as with the middle-term memory for the memory retention test, CS and NOd) and found significant higher response level to the CS compare to NOd for all the groups (control: x2 =12.2, P<0.05; Paenibacillus larvae 4 spores: x2 = 18.4, P<0.05; thiamethoxam 4 ppb: x2 =21.3, P<0.05; Paenibacillus larvae 4 spores + thiamethoxam 4 ppb: x2 =20.9, P<0.05; Paenibacillus larvae 2 spores + thiamethoxam 2 ppb: x2 =28.3, P<0.05).
0 10 20 30 40 50 60 70 80 90 100 CS % o f c o n d ition e d PE R Treatment Control Paenibacillus larvae 4sp Thiamethoxam 4ppb Paenibacillus larvae 4sp + Thiamethoxam 4ppb Paenibacillus larvae 2sp + Thiamethoxam 2ppb *
We found lower response rate (Fig.10) in groups that were exposed to Paenibacillus larvae and thiamethoxam at different concentrations (Paenibacillus larvae 2 spores+ thiamethoxam 2 ppb: x2 = 6.96, df:1, P<0.05; Paenibacillus larvae 4 spores + thiamethoxam 4 ppb: x2 =4.49, P<0.05) and the group that received 4 ppb of thiamethoxam (x2 =4.36, P<0.05). The treatment with 4 spores of Paenibacillus larvae doesn’t show impairment of the long-term memory (x2 = 3.23, P>0.05).
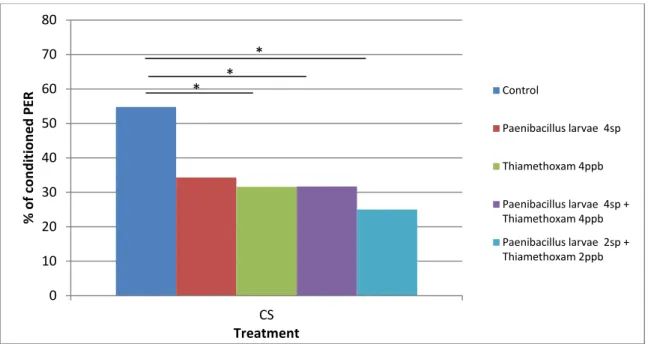
Figure 10. Memory retention performance of honey bee workers subjected to olfactory conditioning of PER 24 hours after the conditioning. Bars represent % of PER to the CS. The number of conditioned responses was compared with the control group using a x2 test with 1 df (*P<0.05).
0 10 20 30 40 50 60 70 80 CS % o f co n d itio n e d PE R Treatment Control Paenibacillus larvae 4sp Thiamethoxam 4ppb Paenibacillus larvae 4sp + Thiamethoxam 4ppb Paenibacillus larvae 2sp + Thiamethoxam 2ppb * * *
Discussion
Effect of exposition and co-exposition on honey bee larval survival and emergence
First of all in our experiment honey bee larvae were reared in vitro and even after the emergence until we performed our olfactory conditioning adults were kept in the emergence boxes in the incubator. Our experimental conditions differ from the one in hives; in hives larvae are fed by nurse bees, they may receive different amount and quality of food. We didn’t perform the study in the field as we used infectious disease. Moreover, laboratory conditions allowed us to control all the variables and rear all larvae in the same conditions.
There are several acceptance criteria’s that must be fulfilled in order to validate the results of a toxicity test. As we performed chronic exposure to thiamethoxam we referred to these criteria’s. According to them, in the control group, cumulative mortality at day 8 should be ≤15% and the adults’ emergence rate in this group at day 22 should be ≥70% (OECD, 2014). In our experiment the observed cumulative mortality in the control group at day 8 was 6% and the adults’ emergence rate at day 22 was 82%.
We didn’t reveal differences in larval mortality and adults’ emergence rate between the control group and the one that was subjected to the chronic exposure with thiamethoxam at concentration of 4 ppb. Therefore we confirmed the results of pretesting that were done before at INRA, le Magneraud (unpublished data) that at this concentration thiamethoxam doesn’t induce honey bee larvae mortality and this dose is a sub-lethal dose for Apis mellifera
ligustica larvae’s. Up to now, there is just one study that showed the effect of thiamethoxam on
the development of Africanized honey bee larvae’s (Tavares et al. 2015). They determined the oral LC50 (concentration of a chemical at which 50% of individuals die) of thiamethoxam (14.34
ng/µL of diet) for the larvae of Africanized honey bees and after they calculated the sub-lethal concentration as 1/10 of LC50 that was 1.43 ng/µL of diet. They showed that at sub-lethal
concentrations thiamethoxam induces alteration in the optic lobes of Africanized honey bee larvae. However, the concentration that were used in their study is much higher compare to one used in our experiment. The sub-lethal concentration they used was 42.9 ng/larvae by acute exposure while in our study we used 0.628 ng/larvae by chronic exposure. It suggests that sensitivity of honey bee larvae to thiamethoxam may depend on subspecies, strain, laboratory conditions.
Regarding treatments with Paenibacillus larvae, our results showed significant increase in mortality at day five in the treatments that contained 4 spores of Paenibacillus larvae. We infected first instar larvae that were less than 24 hours old as in this age they are the most susceptible to Paenibacillus larvae (Crailsheim & Riessberger-Gallé 2001). The cumulative mortality rate at the end of the larval stage (D8) in the group that received 4 spores of
Paenibacillus larvae was 16% and the adults’ emergence rate in this group was 64%,that was
significantly lower compare to control. Hence, the amount of spores that we used in our test was not a sub-lethal as it induced increased level of mortality. The virulence of Paenibacillus
larvae depends on the strain and sometimes it is genotype specific (Brodsgaard et al. 1998;
Genersch et al. 2005; Genersch 2010). The number of spores that is considered as sub-lethal varies and should be determined for each strain. Another factor that determines the larval mortality is the genetic constitution of the exposed honey bees as some strains are more resistant compare to others and it depends on the innate larval tolerance (Genersch 2010). For our experiment the pretesting on determining the number of spores that doesn’t induce mortality was done in February at INRA, Le Magneraud. Results of the pretesting showed that 4 spores of Paenibacillus larvae didn’t cause significant increase in larval mortality compare to the control group. However, the tests were performed on “winter” larvae collected from the indoor hives, but with the same Apis mellifera ligustica strain. In our experiment we used larvae from the outdoor hives and they were collected between April and July, 2015. Possibly, the honey bee larvae that were used in our experiment were more susceptible to the pathogen. Season may be one of the factors that influence larvae’s susceptibility to a pathogen or a pesticide (Crailsheim et al. 2013). Comparison of mortality between larval stage and pupation didn’t show any significant difference, however we had more dead individuals during the pupation stage in all the treatments with Paenibacillus larvae. In treatments with 4 spores of
Paenibacillus larvae, 4 spores of Paenibacillus larvae + 4 ppb of thiamethoxam and 2 spores of Paenibacillus larvae + 2 ppb of thiamethoxam most of the individuals died between day 5 and
day 15. This can be explained with the fact that American Foulbrood kills honey bee larvae within 6 to 13 days post-infection, depending on the strain of Paenibacillus larvae used because some strains act rapidly while others slower (Genersch et al. 2005). For this reason, we can say that in groups that received spores of Paenibacillus larvae mortality was caused by the pathogen. And as we didn’t find significant difference between the treatments that contained
we can conclude that co-exposition of honey bee larvae to this pesticide and pathogen has no additive effect on the larval mortality and adults’ emergence.
Similar study was done by Brodsgaard and colleagues (Brødsgaard et al. 2000) where they examined the interaction between Paenibacillus larvae and Varroa jacobsoni along with acute paralysis virus on honey bee larval mortality. They conclude that American Foulbrood is severe infection and other viruses have no additive effect. Hence, our results also showed that mortality during the larval stage and pupation is caused by Paenibacillus larvae and thus we didn’t find a synergistic effect with thiamethoxam.
Olfactory learning performance
In our work we grafted first instar larvae and exposed them to pesticide and pathogen during the larval stage. After emergence, we still reared them in the incubators until adult were 13-14 days old. We used classical olfactory proboscis extension response (PER) conditioning to study the influence of our treatments on their learning performances. PER conditioning was shown to be a useful tool for studying effects of pesticides on behavioral essays (Laloi et al. 2000; Pham-Delegue et al. 2002; Decourtye, Devillers, et al. 2004). The available data from previous studies confirmed that results obtained during the PER laboratory tests is consistent with the data obtained during the semi field experiments, therefore the individual effects of pesticides on learning and memory that are observed during the PER conditioning can be extrapolated on the colony level (Decourtye, Devillers, et al. 2004).
Olfactory learning performance showed that in the control group bees were learning fast and at the end of the third conditioning trial the percentage of PER was almost 70% . As we were using a syringe for odor delivery we had to use two odors for memory retention test in order to check whether tested bees learned the odor or the air puff. The results we obtained showed that tested bees (in all treatments) had an odor-specific memory and therefore they were really learning the odor. Considering all that, we affirm that our control group was correct.
The group that received 4 spores of Paenibacillus larvae did not differ from the control one; consequently we assume that this pathogen does not influence the adults’ learning performance.
We found decreased level of PER in the group treated with thiamethoxam 4 ppb. In this group we had the lowest rate of PER to the CS. It allows us to indicate that chronic exposure of honey bee larvae to thiamethoxam at concentration 4 ppb leads to the impairment of olfactory learning in adults. Results that we obtained are consistent with other studies that showed that neonicotinoids impair foraging behavior (Decourtye, Devillers, et al. 2004; Decourtye, Armengaud, et al. 2004; Decourtye et al. 2005; Aliouane et al. 2009; Potts et al. 2010; Blacquière et al. 2012; Vanbergen 2013; Tan et al. 2015). After acute exposure of honey bee workers to thiamethoxam at 1 ng/ bee no effect was detected on locomotor activity (El Hassani et al. 2008), but application of thiamethoxam at the amount of 0.1 ng/bee by contact reduced adults’ learning.
Study by Oliveira and colleagues (Oliveira et al. 2013) showed that sub-lethal doses of thiamethoxam applied on newly emerged Africanised bees cause impairment in the brain; they found changes in mushroom bodies and optical lobes. Similar study was done with imidacloprid (Yang et al. 2012) where they did chronic exposure to imidacloprid during the larval stage directly in the hive. They applied 1µL solution of imidacloprid with different concentrations to each larva and determined that imidacloprid at the concentration of 0,4 ng/larva doesn’t induce larval mortality. On day 15 they removed pupae from the hives and placed in incubator until the emergence. Afterwards, they colour-marked every emerged adult to distinguish between different treatments and released them back to their home colonies. The olfactory learning performance was conducted when adults were 15 days old. The results of their experiment showed that chronic exposure to imidacloprid (0.4 ng/larva) during the larval stage leads to the impairment of associative behaviour.
The group with co-exposition that had higher concentrations of Paenibacillus larvae and thiamethoxam had higher acquisition levels than the group that was receiving 4 ppb of thiamethoxam alone. As in the group that received just 4 spores of Paenibacillus larvae we didn’t detect any changes in the learning performance, but we showed impairment of learning in the group that received 4 ppb of thiamethoxam, we would have expect to see the same impairment of learning in the group that was subjected to 4 spores of Paenibacillus larvae + 4 ppb of thiamethoxam. However, even though we obtained lower acquisition levels for this group compare to control, the difference was not significant. We can explain it with the highest
larval mortality rates and lowest adults’ emergence in this group; under selection pressure just the stronger individuals had survived and therefore they have better performance.
In the group that was treated with 2 spores of Paenibacillus larvae + 2 ppb of thiamethoxam we found significantly lower response rate compare to control after the second trial, nonetheless there was no significant difference after trial three, four and five. But this allows us to suggest that this group tend to learn slower and we believe that it caused by thiamethoxam at concentration of 2 ppb alone. It shows that concentrations even lower than the sub-lethal one can negatively affect honey bees’ learning performance.
However, it was demonstrated that thiamethoxam doesn’t interact with the nicotinic receptors (Nauen et al. 2003) as all the other neonicotinoids, but it has the same biological effects. It is related to its rapid conversion to clotianidin, and this compound binds to insects’ nAChRs with high affinity and it is considered as full agonist of these receptors. Nauen and colleagues (2003) showed that thiamethoxam is likely to be a neonicotinoid precursor for clothianidin, which is highly active open-chain neonicotinoid and is highly toxic to bees.
Memory retention
Our results showed that thiamethoxam impairs middle-tem and long-term memory. Results obtained by Decourtye and colleagues (Decourtye, Armengaud, et al. 2004) showed that oral exposure to sub-lethal doses of imidacloprid, another pesticide from the group of neonicotinoids, impairs the honey bees’ middle-term memory. In their study they applied sub-lethal doses of imidacloprid (12 ng/bee) on honey bee workers and subjected them to the conditioning trials half an hour later. Their results suggested that imidacloprid at sub-lethal doses has a negative effect on memory formation processes; it affects the consolidation process that insures transfer of information from the short-term to midlle-term memory.
Another study was done by (Aliouane et al. 2009) that showed that chronic treatment of honey workers with thiamethoxam induces changes in behaviour. Oral treatment with thimathoxam at concentrations of 1 ng/bee and 0.1 ng/bee causes decrease in learning performance and retention test. Topical application caused significant decrease of learning at concentration of 1 ng/bee. They showed that at concentration of 0.1 ng/bee thiamethoxam causes impairment of long-term memory that was checked 24 hours after learning trials. The concentration they used is lower compare to one used in our experiment, but it was suggested
that honey bee larvae are more tolerant to some of neonicotinoids compare to adults (Yang et al. 2012; Tavares et al. 2015).
Neither middle-term nor long-term memory was impaired in the group treated with 4 spores of American Foulbrood. Thus, this pathogen doesn’t influence the memory performance.
As we didn’t find learning and memory impairment in the group that received 4 spores of
Paenibacillus larvae, we suggest that in both treatments that received Paenibacillus larvae +and
thiamethoxam, impairment of long-term memory was caused by thiamethoxam. However, neither group that received 4 spores of Paenibacillus larvae + 4 ppb of thiamethoxam, nor the group that received 2 spores of Paenibacillus larvae + 2 ppb of thiamethoxam showed any changes in the middle-term memory, but we found significantly lower response rate in this groups during the long-term memory testing.
It is known that neonicotinoids are widely used and persist in the environment and their residues can be found in the hives. Pohorecka and colleagues in 2012 showed that thiamethoxam was one of the most abundant neonicotinoids; it was present in 65% of nectar samples and 37% of pollen samples examined. They showed that in nectar collected from flowers, the concentration of thiamethoxam was about 3.2-12.9 ng/g. In the guttation fluid the concentration of thiamethoxam residues is about 11.9±3.32 mg/L (Blacquière et al. 2012). Mullin and colleagues (Mullin et al. 2010) found thiamethoxam in pollen and wax in Northern American apiaries, the mean amount of thiamethoxam found in wax was 53.3 ppb. These findings show that this pesticide abundant in nature and honey bee larvae can be exposed to it in the hives, hence thiamethoxam and its residues can be accumulated in larvae and have negative impact on their adult life. It reduced learning and memory consequently they are not effective in learning and memorizing food locations and homing routes and that can cause the homing failure that earlier was shown by Henry and his colleagues (Henry et al. 2012).
Conclusion
Our results confirmed the pretesting results that 4 ppb of thiamethoxam is the sub-lethal dose for larvae of Apis meliffera ligustica. We demonstrated that the co-exposition between American Foulbrood and thiamthoxam doesn’t have a synergistic effect on honey bee larvae mortality and adults’ emergence neither on their learning and memory performance. Our
and memory performances. We showed that the exposition to sub-lethal doses of thiamethoxam during the larval stage can lead to the impairment of olfactory learning and memory in adults. Moreover, even doses lower than sub-lethal can cause this impairment. The knowledge about sub-lethal effects of neonicotinoids on honey bee larvae is poor and our study contributed to a better knowledge. A successful learning and memory performance ensures successful foraging behaviour which is an important for colony survival and thiamethoxam impairs this performance doubting the colony survival. Therefore, application of this pesticide should be careful and alternatives should be found.
References
1. Alaux, C., Brunet, J-L., Dussaubat, C. et al., 2010. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environmental
Microbiology, 12(3), pp.774–782.
2. Aliouane, Y., Hassani, A. K., Gary, V. et al., 2009. Subchronic exposure of honeybees to sublethal doses of pesticides: effects on behavior. Environmental toxicology and
chemistry / SETAC, 28(1), pp.113–122.
3. Aufauvre, J., Biron, D., Vidau C. et al., 2012. Parasite-insecticide interactions: a case study of Nosema ceranae and fipronil synergy on honeybee. Scientific Reports, 2(326), pp. 1-7.
4. Aufauvre, J., Misme-Aucouturier, B., Viguès, B. et al., 2014. Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PloS one, 9(3),pp. 1-12. 5. Aupinel, P., Fortini, D., Dufour, H. et al., 2005. Improvement of artificial feeding in a
standard in vitro method for rearing Apis mellifera larvae. Bulletin of Insectology, 58(2), pp.107–111.
6. Baron, G.L., Raine, N.E. & Brown, M.J.F., 2014. Impact of chronic exposure to a pyrethroid pesticide on bumblebees and interactions with a trypanosome parasite.
Journal of Applied Ecology, 51(2), pp.460–469.
7. Beekman, M., 2005. How long will honey bees (Apis mellifera L.) be stimulated by scent to revisit past-profitable forage sites? Journal of comparative physiology. A,
Neuroethology, sensory, neural, and behavioral physiology, 191(12), pp.1115–1120.
8. Bitterman,M. E., Menzel, R., Fietz, A. and Schafer, S. 1983. Classical Conditioning of Proboscis Extension in Honeybees (Apis mellifera). Journal of Comparative Physiology, 97(2), pp.107–119.
9. Blacquière, T., Smagghe, G., van Gestel, A.M., Mommaerts, V.2012. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology, 21(4), pp.973–992.
10. Breeze, T.D., Bailey, A. P., Balcombe, K.G., Potts, S.G. 2011. Pollination services in the UK: How important are honeybees? Agriculture, Ecosystems and Environment, 142(3-4), pp.137–143.
11. Brodsgaard, C.J., Ritter, W., Hansen, H. 1998. Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae larvae spores. Apidologie, 29(6), pp.569– 578.
12. Brødsgaard, C.J., Ritter, W., Hansen, H., Brodsgaard, H.F. 2000. Interactions among Varroa jacobsoni mites , acute paralysis virus , and Paenibacillus larvae larvae and their influence on mortality of larval honeybees in vitro. Apidologie, 31, pp.543–554.
13. Calderone, N.W., 2012. Insect pollinated crops, insect pollinators and US agriculture: Trend analysis of aggregate data for the period 1992-2009. PLoS ONE, 7(5), pp.24–28. 14. Carreck, N., Neumann, P., 2010. Honey bee colony losses. Journal of Apicultural
15. Carreck, N., Williams, I. 1998. The economic value of bees in the UK. Bee World, 79, pp.115–123.
16. Le Conte, Y., Ellis, M., Ritter, W. 2010. Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie, 41(3), pp.353–363.
17. Crailsheim, K., Brodschneider, R., Aupinel, P. et al. 2013. Standard methods for artificial rearing of Apis mellifera larvae. Journal of Apicultural Research, 52(1), pp.1–16.
Available at: http://www.ibra.org.uk/articles/The-COLOSS-BEEBOOK-artificial-rearing. 18. Crailsheim, K., Riessberger-Gallé, U. 2001. Honey bee age-dependent resistance against
American foulbrood. Apidologie, 32, pp.91–103.
19. Decourtye, A., Devillers, J., Geneeque, E. et al. 2005. Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera.
Archives of Environmental Contamination and Toxicology, 48(2), pp.242–250.
20. Decourtye, A., Devillers, J.,Cluzeau, S. et al. 2004. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicology and Environmental Safety, 57(3), pp.410–419.
21. Decourtye, A., Armengaud, C.,Renou, M. et al. 2004. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pesticide Biochemistry and
Physiology, 78(2), pp.83–92.
22. Desneux, N., Decourtye, A., Delpuech, J.-M. 2007. The sublethal effects of pesticides on beneficial arthropods. Annual review of entomology, 52, pp.81–106.
23. Doublet, V., Labarussias, M., de Miranda, J. R. et al. 2014. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environmental Microbiology,17(4),pp.969-983.
24. EFSA, 2013. Conclusion on the peer review of the pesticide risk assessment for bees for thiamethoxam. EFSA Journal, 11(1), pp.1–68. Available at:
www.efsa.europa.eu/efsajournal.
25. Fauser-Misslin, A., Sadd, B.M., Neumann, P., Sandrock, C. 2014. Influence of combined pesticide and parasite exposure on bumblebee colony traits in the laboratory. Journal of
Applied Ecology, 51(2), pp.450–459.
26. Felsenberg, J., Gehring, K., Antemann, V., Eisenhardt, D.2011. Behavioural pharmacology in classical conditioning of the proboscis extension response in honeybees (Apis mellifera). Journal of visualized experiments : JoVE, (47).
27. Food, E. & Authority, S., 2013. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance clothianidin. EFSA Journal, 11(1:(3067)), p.68.
28. Food, E. & Authority, S., 2014. Towards an integrated environmental risk assessment of multiple stressors on bees : review of research projects in Europe , knowledge gaps and. , 12(3), pp.1–102.
29. Frost, E.H., Shutler, D. ,Hillier, N.K. 2012. The proboscis extension reflex to evaluate learning and memory in honeybees (Apis mellifera): Some caveats.
30. Gallai, N., Salles, J.-M., Settele, J., Vaissière, B.E. 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecological
Economics, 68(3), pp.810–821.
31. Genersch, E., 2010. American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. Journal of Invertebrate Pathology, 103, pp. S10-S19.
32. Genersch, E., Ashiralieva, A., Fries, I. 2005. Strain- and genotype-specific differences in virulence of Paenibacillus larvae subsp. larvae, a bacterial pathogen causing American foulbrood disease in honeybees. Applied and Environmental Microbiology, 71(11), pp.7551–7555.
33. Giurfa, M., Sandoz, J.-C. 2012. Invertebrate learning and memory: Fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learning & Memory, 19(2), pp.54–66.
34. Guerrieri, F., Schubert, M., Sandoz, J.-C., Giurfa, M. 2005. Perceptual and neural olfactory similarity in honeybees. PLoS Biology, 3(4), pp.0718–0732.
35. El Hassani, A.K., Dacher, M., Gary, V. et al. 2008. Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera).
Archives of Environmental Contamination and Toxicology, 54(4), pp.653–661.
36. Hatjina, F., Papaefthimiou, C., Charistos, L. et al. 2013. Sublethal doses of imidacloprid decreased size of hypopharyngeal glands and respiratory rhythm of honeybees in vivo.
Apidologie, 44(4), pp.467–480.
37. Henry, M., Beguin, M., Requier, F. et al., 2012. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science, 336(6079), pp.348–350.
38. Higes, M., Martin-Hernandez, R., Botias, C. et al. 2008. How natural infection by Nosema ceranae causes honeybee colony collapse. Environmental Microbiology, 10(10),
pp.2659–2669.
39. Hopwood, J., Vaughan, M., Shepherd, M. et al. 2013. Are Neonicotinoids Killing Bees ?
Xerces Society, p.44.
40. Fries, I., Camazine, S. 2001. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie, 32, pp.199–214.
41. Takeda, K. 1961. Classical conditioned response in the honey bee. Insect physiology, 6, pp.168–179.
42. Klein, A.-M., Vaissière, B., Cane, J. H. et al. 2007. Importance of pollinators in changing landscapes for world crops. Proceedings. Biological sciences / The Royal Society, 274, pp.303–313.
43. Laloi, D., Bailez, O., Blight, M.M. et al., 2000. Recognition of complex odors by restrained and free-flying honeybees, Apis mellifera. Journal of Chemical Ecology, 26(10), pp.2307– 2319.
44. Laurino,D., Porporato,M., Patetta, A., Manino, A. 2011. Toxicity of neonicotinoid insecticides to honey bees: laboratory tests. Bulletin of Insectology, 64(1), pp.107–113.
45. Locke, B., Forsgren, E., Fries, I., de Miranda, J.R. 2012. Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Applied and Environmental Microbiology, 78(1), pp.227–235.
46. Matsumoto, Y., Menzel, R., Sandoz, J., Giurfa, M. 2012. Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: A step toward standardized procedures. Journal of Neuroscience Methods, 211(1), pp.159–167. 47. Memmott, J., Reynders, S., Boulet, J. et al. 2007. Global warming and the disruption of
plant-pollinator interactions. Ecology Letters, 10(8), pp.710–717.
48. Menzel, R., Manz, G., Menzel, Rebecca, Greggers, U. 2001. Massed and Spaced Learning in Honeybees: The Role of CS, US, the Intertrial Interval, and the Test Interval. Learning
& Memory, 8(4), pp.198–208.
49. Menzel, R. 1999. Memory dynamics in the honeybee. Journal of Comparative Physiology
- A Sensory, Neural, and Behavioral Physiology, 185(4), pp.323–340.
50. Morgan, S.M., Butz Huryn, V.M., Downes, S.R. et al. 1998. The effects of queenlessmess on the maturation of the honey bee olfactory system. Behavioural Brain Research, 91, pp.115–126.
51. Mullin, C.A., Frazier, M., Frazier, J. et al. 2010. High Levels of Miticides and
Agrochemicals in North American Apiaries: Implications for Honey Bee Health. PLoS
ONE, 5(3), pp.1-19.
52. Nauen, R., Ebbinghaus-Kintscher, U., Salgado, V.L., Kaussmann, M. 2003. Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants. Pesticide
Biochemistry and Physiology, 76(2), pp.55–69.
53. OECD guidance document: Honey bee (Apis mellifera) larval toxicity test, repeated exposure, 2014.
54. Oliveira, R.A., Roat, T.C., Carvalho, S.M., Malaspina, O. 2013. Side-effects of thiamethoxam on the brain andmidgut of the africanized honeybee Apis mellifera (Hymenopptera: Apidae). Environmental Toxicology, 13(4), pp.1122–1133.
55. Ollerton, J., Winfree, R. & Tarrant, S. 2011. How many flowering plants are pollinated by animals? Oikos, 120(3), pp.321–326.
56. Peduzzi, P. et al., 2010. Global Bee Colony Disorder and Threats insect pollinators. 57. Pettis, J.S., vanEngelsdorp, D., Johnson, J., Dively, G. 2012. Pesticide exposure in honey
bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften, 99(2), pp.153–158.
58. Pham-Delegue, M.H., Decourtye, A., Kaiser, L., Devillers, J.2002. Behavioural methods to assess the effects of pesticides on honey bees. Apidologie, 33(5), pp.425–432.
59. Pohorecka, K., Skubida P., Miszczak., A. et al., 2012. Residues of neonicotinoid
insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. JOURNAL OF APICULTURAL SCIENCE ,56(2),pp. 115-134.
60. Potts, S.G., Biesmeijer, J. C., Kremen, C. et al. 2010. Global pollinator declines: Trends, impacts and drivers. Trends in Ecology and Evolution, 25(6), pp.345–353.
61. Di Prisco, G., Cavaliere, V., Desiderato, A. et al. 2013. Neonicotinoid clothianidin
adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proceedings of the National Academy of Sciences of the United States of America, 110(46), pp.18466–18471.
62. Retschnig, G., Neumann, P., Williams, G.R., 2014. Thiacloprid-Nosema ceranae interactions in honey bees: host survivorship but not parasite reproduction is dependent on pesticide dose. Journal of invertebrate pathology, 118, pp.18–19.
63. Ricketts, T.H., Regetz, J., Dewenter, I.S. et al. 2008. Landscape effects on crop pollination services: Are there general patterns? Ecology Letters, 11(5), pp.499–515.
64. Ritter, W., Akratanakul, P. 2006. Honey bee diseases and pests: a practical guide. Available at: http://www.fao.org/3/a-a0849e.pdf, data accessed [August 16th, 2015]. 65. Van der Sluijs, J.P., Simon-Delso, N., Goulson, D. et al. 2013. Neonicotinoids, bee
disorders and the sustainability of pollinator services. Current Opinion in Environmental
Sustainability, 5(3-4), pp.293–305.
66. Tan, K., Chen, W., Dong, S. et al., 2015. A neonicotinoid impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Scientific Reports, 5. 67. Tavares, D.A., Roat, C.T., Carvalho, M. et al. 2015. In vitro effects of thiamethoxam on
larvae of Africanized honey bee Apis mellifera (Hymenoptera: Apidae). Chemosphere, 135, pp.370–378.
68. Vanbergen, A.J., 2013. Threats to an ecosystem service: Pressures on pollinators.
Frontiers in Ecology and the Environment, 11(5), pp.251–259.
69. vanEngelsdorp, D., Evans, J.D., Saegerman, C. et al. 2009. Colony collapse disorder: A descriptive study. PLoS ONE, 4(8), pp.1-17.
70. Vidau, C., Diogon, M., Aufauvre, J. et al.,2011. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by nosema ceranae. PLoS ONE, 6(6), pp.1-8.
71. von Frisch K. The dance language and orientation in honey bees. Harvard University Press, Cambridge, MA (1967).
72. Wang, S., Zhang, S., Sato, K., Srinivasan, M.V. 2005. Maturation of odor representation in the honeybee antennal lobe. Journal of Insect Physiology, 51(11), pp.1244–1254. 73. Williamson, S.M., Willis, S.J., Wright, G.A. 2014. Exposure to neonicotinoids influences
the motor function of adult worker honeybees. Ecotoxicology, 23, pp.1409-1418. 74. Wolf, S. et al., 2014. So near and yet so far: Harmonic radar reveals reduced homing
ability of nosema infected honeybees. PLoS ONE, 9(8).
75. Wu, J.Y., Anelli, C.M., Sheppard, W.S., 2011. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (apis mellifera) development and longevity. PLoS
ONE, 6(2).
76. Yang, E.C., Chang, H.C., Wu, W.Y., Chen, Y.W. 2012. Impaired Olfactory Associative Behavior of Honeybee Workers Due to Contamination of Imidacloprid in the Larval Stage. PLoS ONE, 7(11).
Annex 1
Composition of the diet fed to honey bee larvae during the experiment
Rearing day D1 D2 D3 D4 D5 D6
Diet name A No diet B C C C
Volume (µl) per larvae 20 - 20 30 40 50 % Royal jelly 50 - 50 50 50 50 % Glucose 6 - 7,5 9 9 9 % Fructose 6 - 7,5 9 9 9 % Yeast extract 1 - 1,5 2 2 2
Annex 2
Abstract
In the last ten years honey bee (Apis mellifera) colonies have been in decline. There are multiple causes that contribute to that rapid downtrend. Among them are pests and pathogens, land-use intensification, habitat loss and fragmentation. In most of the cases, the consequences of co-exposition are unknown as only few studies addressed these questions so far. Here we would like to investigate the impact of co-expositon between a pathogen and a neonicotinoid on honey bee survival and learning performances. Yet all the studies about the influence of pesticides on learning and memory abilities are conducted on honey bees that were exposed to pesticide after emergence. But it is known that neonicotinoids are also stored inside the hives in pollen, nectar and brood is exposed to it. In our work we exposed honey bee larvae to American Foulbrood and to sub-lethal doses of Thiamethoxam (chronic exposure). First we hypothesise that the application of two treatments may have synergistic effect and induce higher mortality rates on larvae. We also tested the impact of this co-exposition, during the larval stage, on impairment of learning and memory abilities as well as the social behaviour of the honey bee workers. We used the standard olfactory conditioning procedure that is based on Proboscis Extension Response (PER) to study the changes in learning and memory abilities caused by the co-exposition effect.
Key words: co-exposition, honey bee, proboscis extension response (PER), olfactory