HAL Id: hal-03003339
https://hal.archives-ouvertes.fr/hal-03003339
Submitted on 18 Nov 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Synthesis of heterocyclic enamine-zinc complexes as
precursors of stereocontrolled substitution of nitrogen
α-position
H.-V. Tran, H.-D. Vu, J. Graton, D. Jacquemin, J. Renault, P. Uriac
To cite this version:
H.-V. Tran, H.-D. Vu, J. Graton, D. Jacquemin, J. Renault, et al.. Synthesis of heterocyclic
enamine-zinc complexes as precursors of stereocontrolled substitution of nitrogen α-position. Tetrahedron
Letters, Elsevier, 2020, 61 (44), pp.152405. �10.1016/j.tetlet.2020.152405�. �hal-03003339�
N O Boc n Z ZnCl21M (5 eq.) N Z x(ZnCl2)y * * N Z X * * R R R n n n = 1; R = H, OCH3; Z H X = H, Allyl de : 50% n = 1, 2; R = H; Z = H X = Allyl de : 0% Z = Allyl, C C
alkaloids
C C n-C3H7, X Et2O, RT, 12h1
Synthesis of heterocyclic enamine-zinc complexes as precursors of
stereocontrolled substitution of nitrogen α-position
Hoang-Van Tran
a, Huy-Dinh Vu
b, Jérôme Graton
c, Denis Jacquemin
c, Jacques Renault
a,
Philippe Uriac
a,*———
*Corresponding author. Fax: +33 2 23234425. E-mail: philippe.uriac@univ-rennes1.fr ORCID: 0000-0002-2526-5967
aUniversité de Rennes I, Institut des Sciences Chimiques de Rennes - UMR CNRS 6226, F-35000 Rennes, France bDepartment of Chemistry, Vietnam National University of Forestry, Hanoi, Vietnam
cLaboratoire CEISAM - UMR CNRS 6230, Université de Nantes, 44322 Nantes, France
Introduction
Stereocontrolled functionalization of the carbon in α-position of the nitrogen of heterocycles has been often a key-step in natural product synthesis.1 Efficient strategies have been
proposed for the monosubstitution of various heterocycles2-4but
the quaternization of the carbon in nitrogen α-position remains poorly explored.2a, 5 In some recent publications, we have shown
that 1M ZnCl2 in solution in diethyl ether was a useful reactant to
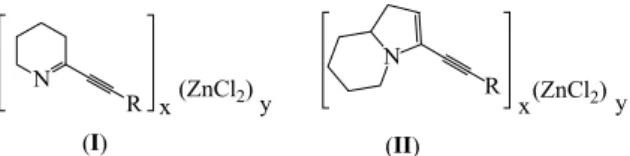
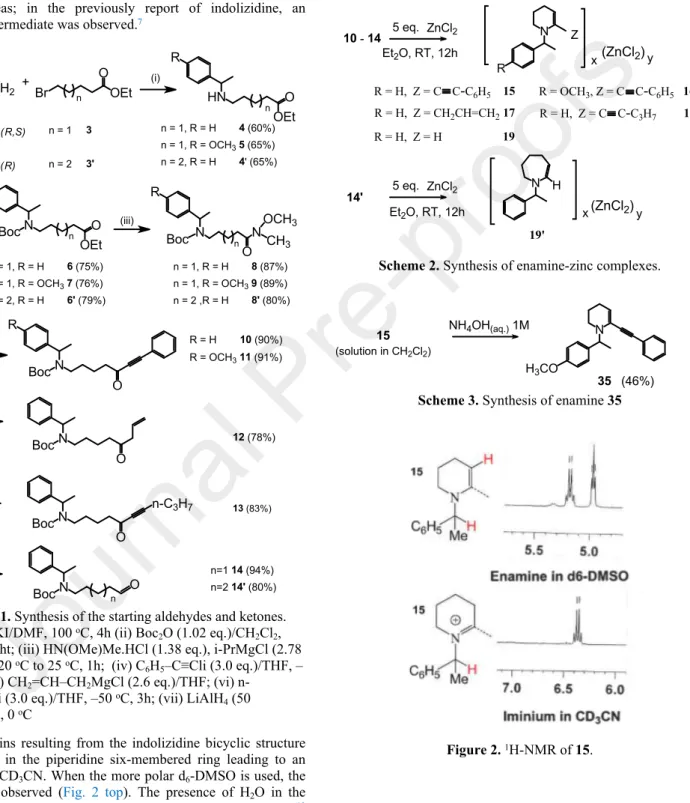
prepare iminocomplexes I6 or enaminocomplexes II7 which were
very stable gums or solids (Fig. 1). The neutralization of the iminocomplexes I yielded to heterocyclic imines that revealed excellent ligands of Zn(II), Pd(II) and Au(III)8 whereas the
reduction of the enaminocomplexes II using NaBH4 in EtOH
allowed obtaining of 2-substituted indolizidine with low diastereomeric excess (de = 20) (Fig. 1).7
N R N R (ZnCl2) x y x(ZnCl2)y (I) (II)
Figure 1. Structure of iminocomplexes (I) and
enaminocomplexes (II).
This latter result prompted us to design enamine-zinc complexes with α-methylbenzylamine acting as a chiral inductor
in order to obtain heterocycles mono- or disubstituted in α-position of the heterocyclic nitrogen atom.
Results and discussion
Access to the N-Boc protected aminoketones and
aminoaldehydes was realized as follows (Scheme 1). The
nucleophilic substitution of either the (R, S)
α-methylbenzylamine 1 or the (R) 4-methoxy-α-benzylamine 2 with the ethyl 5-bromopentanoate 3 (n=1)9 led to the aminoesters
4 and 5 which were protected using (Boc)2O to give the
compounds 6-7. Then, the ester function was transformed into
Weinreb amide 8-910 in the presence of lithium phenylacetylide,
lithium propylacetylide, allylmagnesium chloride or LiAlH4,6, 11
which allowed the formation of the desired N-Boc protected aminoketones 10-13 and aminoaldehyde 14. As indicated in the Scheme 2, yields were generally good. One can notice that the use of other bromoesters could allow introduction of diversity. Thus, with the ethyl 6-bromohexanoate 3’ (n=2), the N-Boc protected aminoaldehyde 14’ was obtained.
Then, we carried out the formation of the zinc complexes
(Scheme 2). The aminoketones 10-13 and the aminoaldehydes 14
and 14’ were allowed to react with 5 equivalents of a 1M ZnCl2
solution in diethyl ether, at room temperature for 12h. Six zinc-complexes 15-19 and 19’ were obtained as gums or solids after work-up.6, 7 The neutralization of the zinc-complex 15 with 1M
NH4OH (aq.) allowed obtaining the corresponding enamine 35
A B S T R A C T
In the presence of ZnCl2,chiral protected amino-ketones and amino-aldehydes gave zinc enamino-complexes. Both enamine and iminium structures
of these complexes were observed in 1H and 13C NMR spectra depending on the solvent. Introduction of either an allyl or a hydrogen substituent was
performed using allylmagnesium chloride or NaBH4 in excess leading to various heterocycles. With the amino-ketones diastereoselectivity (de = 50)
methylbenzylamine and the imine carbon was observed near 160 ppm whereas; in the previously report of indolizidine, an
enamine intermediate was observed.7
NH2 OEt O (i) + Br HN OEt O N O Boc N O Boc n = 1 3 n = 2 3' n = 1, R = H 4 (60%) n = 1, R = OCH35 (65%) n = 2, R = H 4' (65%) n=1 14 (94%) n=2 14' (80%) R R = H 1 (R,S) R = OCH32 (R) R N O Boc 12 (78%) N OEt O R Boc n = 1, R = H 6 (75%) n = 1, R = OCH37 (76%) n = 2, R = H 6' (79%) R R = H 10 (90%) R = OCH311 (91%) n n n N O N Boc n = 1, R = H 8 (87%) n = 1, R = OCH39 (89%) n = 2 ,R = H 8' (80%) n OCH3 CH3 8, 8' n 8, 9 8 N O Boc n-C3H7 13 (83%) (iv) (ii) (iii) (vii) (v) 8 (vi) R
Scheme 1. Synthesis of the starting aldehydes and ketones.
(i) K2CO3, KI/DMF, 100 oC, 4h (ii) Boc2O (1.02 eq.)/CH2Cl2,
RT, overnight; (iii) HN(OMe)Me.HCl (1.38 eq.), i-PrMgCl (2.78 eq.)/THF, –20 oC to 25 oC, 1h; (iv) C
6H5‒C≡Cli (3.0 eq.)/THF, –
50 oC, 3h (v) CH
2=CH‒CH2MgCl (2.6 eq.)/THF; (vi)
n-C3H7‒C≡Cli (3.0 eq.)/THF, –50 oC, 3h; (vii) LiAlH4 (50
mol%)/THF, 0 oC
The strains resulting from the indolizidine bicyclic structure disappeared in the piperidine six-membered ring leading to an
iminium in CD3CN. When the more polar d6-DMSO is used, the
enamine is observed (Fig. 2 top). The presence of H2O in the
zinc-complex should allow the enamine-iminium tautomerism.12
In the case of 17, the iminium formation resulted in an allylic transposition giving an aza-dienic iminium (Fig. 3) probably due to the medium acidity. The structure of 17 was clearly attested in
1H NMR by the presence of an ABX system (J
trans = 17 Hz). N Z ZnCl2 10 - 14 5 eq. x(ZnCl2)y R R = H, Z = C C-C6H5 15 R = OCH3, Z = C C-C6H5 16 R = H, Z = CH2CH=CH217 R = H, Z = H 19 N 14' x H R = H, Z = C C-C3H7 18 (ZnCl2)y 19' Et2O, RT, 12h ZnCl2 5 eq. Et2O, RT, 12h
Scheme 2. Synthesis of enamine-zinc complexes.
15
(solution in CH2Cl2)
NH4OH(aq.)1M N
H3CO
35 (46%)
Scheme 3. Synthesis of enamine 35
3
17 N Me C6H5 Figure 3. 1H-NMR of 17 (CD 3CN). 15,16 N R N 19 a,b de = 50% 19' N R = H, 20a (R*R*), 20b (R*S*), 47% from 10 22a (R*S*), 22b (R*R*), 31% from 14; a,b de = 0% 23a (R*S*), 23b (R*R*), H H N H Me H3CO R allyl favoured Re attack (i) (i) (i)R = OCH3, 21a (RR), 21b (RS), 36% from 11
39% from 14'; a,b de = 0%
Re attack Si attack
Scheme 4. Reactivity of the zinc complexes with allyl
magnesium chloride. (i) CH2=CH‒CH2MgCl 1M (10 eq.)/THF, 20 oC, 2h -6 -4 -2 0 2 4 6 225 250 275 300 325 RR RS Delt a Eps ilon Wavelength (nm)
Figure 4. TD-DFT-simulated (left) and experimental (right)
electronic circular dichroism spectra of 19a-b.
All these piperidine derivatives were obtained as a mixture of
a (major) and b (minor) diastereomers. A diastereoselectivity (de
= 50) was only observed when the starting complex was substituted in position 2. The diastereomers were separated using column chromatography. The configurations of 21a and 21b (and then of 22) have been determined by electronic circular dichroism. Thus, comparing the calculated (see the ESI section
for details) and experimental ECD spectra of 21a and 21b (Fig. 4) led us to attribute the RR configuration to the major isomer
21a.
The configurations of 22a and 22b (and then of 23 and 24) were attributed using the literature data.4a These configurations
were in accordance with a classical transition state postulated for nucleophilic attack involving piperidinium intermediates14 and
conformational analysis of 21 (see the ESI). Optical purity was checked with 21a (RR) and 20 (RR + SS) by formation of salts
with the (R)-(-)-tert-butylphenylphosphanylthioic acid,15,4a
because use of various chiral columns in HPLC has failed (see
the ESI).
Some attempts of reduction/deprotection of 15 using H2 (5
bar) in the presence of Pearlman’s catalyst gave a mixture of
compounds corresponding to both debenzylation and N-C2
cleavage. Then, other strategies must be developed to obtain α, α- disubstituted heterocycles. The reactivity of 17 with NaBH4 in
EtOH16 (Scheme 5) has been tested and interestingly, 22 was
obtained with a 60% yield and de = 50. This result can be probably explained by the basicity of the medium which did not allow allylic transposition. As the hydride attack occurred by the same face as the allyl one the major diastereomer 20a has the
R*S* absolute configuration. The same behaviour was observed
with 18 and 24a has also the R*S* absolute configuration.
N C3H7 N 17 NaBH4(excess) 18 22a (R*S*), 22b (R*R*) 24a (R*S*), 24b (R*R*) H H EtOH, RT, 3h NaBH4(excess) EtOH, RT, 3h
60% from 12; a,b de = 50% 31% from 13; a,b de = 50%
Scheme 5. Reactivity of 17, 18 with NaBH4
N N 26a N 25a N H.HCl rac- Homoconiine N 23a + 23b 22a rac-Coniine 22b 25b (85%) (i) (ii)* (ii)* (iii) (iii) (iii) N H (iv)* (90%) OH
Scheme 6. Alkaloids synthesis.
(i) 9-BBN (2.5 eq.)/THF, RT, overnight, then H2O2, NaOH, 3
min; (ii) H2 (3 bar)/Wilkinson’s catalyst (10%), EtOH, RT, 72h;
(iii)H2 (Pd/C); (iv)H2 (3 bar)/Pd(OH)2, EtOH, RT, 24h then HCl
(excess)/dioxane. *The quantitative reduction was checked by TLC.
We then carried out the synthesis to obtain natural alkaloids
(Scheme 6). The rac-homoconiine has been quantitatively
prepared from 23 in one step using H2 in the presence of
Pearlman’s catalyst at 3 bar.5a Addition of 1M HCl in dioxane
furnished the hydrochloride. Starting from 22a (R*S*) and 22b
(R*R*) the coniine precursors 25a (R*R*) and 25b (R*S*)5a were
prepared using H2 with Wilkinson’s catalyst at 3 bar whereas the
indolizidine precursor 26a (R*S*) was obtained with 9-BBN/H2O2.11 The NMR spectra of 25 and 26 are consistent with
the literature(see the ESI).5a
Conclusion
In conclusion, the proposed strategy for the 2-substituted heterocycles synthesis, involving formation and storage of iminium intermediates, is easy and can offer many possibilities. Then, diversity can be introduced by tuning the nature of the precursor (length, substitutions, cycle…) and/or changing the nature of the nucleophile. Finally, the use of enantiopure α-methylbenzylamine and the easy separation of the diastereomers by chromatography can allow obtaining enantiopure
1. (a) Daly J. W., Spande T. F., Garraffo H. M., J. Nat.
Prod. 2005, 68, 1556-1575. (b) Schneider M. J., In Alkaloids: Chemical and Biological Perspectives;
Pelletier S. W., Ed.; John Wiley & Sons, New York,
1996, vol.10, pp. 155. (c) Weintraub P. M., Sabol J. S.,
Kane J. M., Borcherding D. R., Tetrahedron 2003, 59, 2953-2989. (d) Comins D. L, Joseph S. P., In Advances
in Nitrogen Heterocycles; C. J Moody Ed.; 2JAI Press,
Greenwich, CT, 1996, pp. 251–294. (e) Joseph S., Comins D. L., Curr. Opin. Drug Discovery Dev. 2002,
5, 870–880. (f) Comins D. L., O’Connor S., Al-awar R.
S., In Comprehensive Heterocyclic Chemistry III, Elsevier: Oxford, UK, 2008, pp. 41–99.
2. (a) Coia N., Mokhtari N., Vasse J.-L., Szymoniak J.,
Org. Lett. 2011, 13, 6292-6295. (b) Adriaenssens L. V.,
Austin C. A., Gibson M., Smith D., Hartley R. C., Eur.
J. Org. Chem. 2006, 4998-5001.
3. (a) Beng T. K., Gawley R. E., J. Am. Chem. Soc. 2010,
132, 12216-12217. (b) Coldham I., Leonori D., J. Org.
Chem. 2010, 75, 4069-4077. (c) Cordier C. J., Lundgren
R. J., and Fu G. C., J. Am. Chem. Soc. 2013, 135, 10946-10949.
4. (a) Vu V.-H., Jouanno L.-A., Cheignon A., Roisnel T.,
Dorcet V., Sinbandhit S., Hurvois J.-P., Eur. J. Org.
Chem. 2013, 5464–5474. (b) Girard N., Pouchain L.,
J.-Hurvois P., and Moinet C., Synlett 2006, 11, 1679-1682.
5. (a) Pattenden L. C., Wybrow R. A. J., Smith S. A., and
Harrity J. P. A., Org. Letters 2006, 8(14), 3089-3091. (b) Li C., Ragab S. S., Liu G., and Tang W., Nat. Prod.
Rep., 2020, 37, 276-292. c) Body A. J., Affon D. P., Cordier C. J., Rivers E. L., Spivey A. C., and Bull J. A.,
Ang. Chem. Int. Ed. 2019, 58, 1458-1462. (d) Boisson
J., Thomasset A., Racine E., Cividino P., Sainte-Luce T. B., Poisson J.-F., Behr J.-B., and Py S., Org. Lett.
2015, 17, 3662-3665. (e) Roulland E., Cecchin F., and
Husson H.-P., J. Org. Chem. 2005, 70(11), 4474-4477. 6. (a) Vu H.-D., Renault J., Toupet L., Uriac P., Gouault
N., Eur. J. Org. Chem. 2013, 6677-6686. (b) Vu H.-D., Renault J., Roisnel T., Gouault N., Uriac P., Eur. J.
Org. Chem. 2014, 4506-4514. (c) Vu H. -D., Renault J.,
Roisnel T., Robert C., Jéhan P., Gouault N., Uriac P.,
Eur. J. Org. Chem. 2015, 4868-4875.
7. Vu H.-D., Renault J., Roisnel T., Gouault N., Uriac P.,
Tetrahedron Lett. 2016, 57, 3036-3038.
8. (a) Vu H.-D., Bouvry C., Renault J., Bondon A.,
Lambert F., Roisnel T. and Uriac P., J. Organomet.
Chem. 2019, 897, 228-235. (b) Vu H.-D., Renault J.,
Roisnel T., Uriac P., Tetrahedron Lett. 2020, DOI: 10.1016/j.tetlet.2020.152323
9. Gois P. M. P., Candeias N. R., Afonso C. A. M., J. Mol.
Cat. A Chemical, 2005, 227, 17-24.
10. (a) Nahm S., Weinreb S. M., Tetrahedron Lett. 1981,
22, 3815- 3818. (b) Williams J. M., Jobson R. B.,
15. Szawkało J., Zawadzka A., Wojtasiewicz K., Leniewski A., Drabowicz J., Czarnocki Z., Tetrahedron:
Asymmetry 2005, 16, 3619-3621.
16. (a) Bull S. D., Davies S. G., Fenton G., Mulraney A. W., Prasad R. S., Smith A. D., J. Chem. Soc., Perkin
Trans. 1, 2000, 3765-3774. (b) Davies S. G., Ichihara
O., Tetrahedron Lett., 1998, 39, 6045-6048. (c) Shih Y.-C., Wang J.-S., Hsu C.-C., Tsai P.-H., and Chien T.-C., Synlett., 2016, 27, 2841-2845.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at http:
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.