The structure of the di-zinc subclass B2 metallo--lactamase CphA reveals
that the second inhibitory zinc ion binds in the “histidine” site.
Carine Bebrone1,3,*,§, Heinrich Delbrück2,*, Michaël B. Kupper2, Philipp Schlömer2, Charlotte Willmann2, Jean-Marie Frère1, Rainer Fischer2, Moreno Galleni3 and Kurt M. V. Hoffmann2
1 Centre for Protein Engineering, University of Liège, Allée du 6 Août B6, Sart-Tilman 4000
Liège, Belgium
2 Institute of Molecular Biotechnology, RWTH-Aachen University, c/o Fraunhofer IME,
Forckenbeckstrasse 6, 52074 Aachen, Germany
3 Biological macromolecules, University of Liège, Allée du 6 Août B6, Sart-Tilman, 4000 Liège,
Belgium
* both authors contributed equally to this work
Running title: The inhibitory zinc ion binds in the “histidine” site of CphA
§ Corresponding author: Carine Bebrone, Centre for Protein Engineering, University of Liège, Allée du 6 Août B6, Sart-Tilman, 4000 Liège, Belgium, Tel.: 003243663315, Fax: 003243663364, e-mail: carine.bebrone@ulg.ac.be 1 2 3 4 5 6 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22
Abstract
Bacteria can defend themselves against -lactam antibiotics through the expression of class B -lactamases, which cleave the -lactam amide bond and render the molecule harmless. There are three subclasses of class B -lactamases (B1, B2 and B3), all of which require Zn2+ for
activity and can bind either one or two zinc ions. Whereas the B1 and B3 metallo--lactamases are most active as di-zinc enzymes, subclass B2 enzymes such as Aeromonas hydrophila CphA are inhibited by the binding of a second zinc ion. We crystallized A. hydrophila CphA in order to determine the binding site of the inhibitory zinc ion. X-ray data from zinc-saturated crystals allowed us to solve the crystal structures of the di-zinc forms of the wild-type enzyme and N220G mutant. The first zinc ion binds in the “cysteine” site, as previously determined for the mono-zinc form of the enzyme. The second zinc ion occupies a slightly modified “histidine” site, where the conserved His118 and His196 residues act as metal ligands. This atypical coordination sphere probably explains the rather high dissociation constant for the second zinc ion compared to those observed in enzymes of subclasses B1 and B3. Inhibition by the second zinc ion results from immobilization of the catalytically-important His118 and His196 residues, as well as the folding of the Gly232–Asn233 loop into a position that covers the active site.
23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40
Introduction
Class B -lactamases (also called zinc -lactamases or metallo--lactamases) play a key role in bacterial resistance to -lactam antibiotics by efficiently catalyzing the hydrolysis of the -lactam amide bond. The existence of such enzymes is a particular concern because they are effective against most -lactam antibiotics (including the carbapenems), the corresponding genes are easily transferred between bacteria and there are no clinically useful inhibitors. On the basis of the known sequences,three different lineages, identified as subclasses B1, B2, andB3, can be characterized (12, 13). All class B -lactamases possess two potential zinc-binding sites and share a small number of conserved motifs bearing some of the residues that coordinate the zinc ion(s), notably His/Asn/Gln116-Xaa-His118-Xaa-Asp120 and Gly/Ala195-His196-Ser/Thr197 (13). Structural analysis of subclass B1 enzymes shows that one zinc ion has a tetrahedral coordination sphere involving His116, His118, His196 and a water molecule or OH- ion
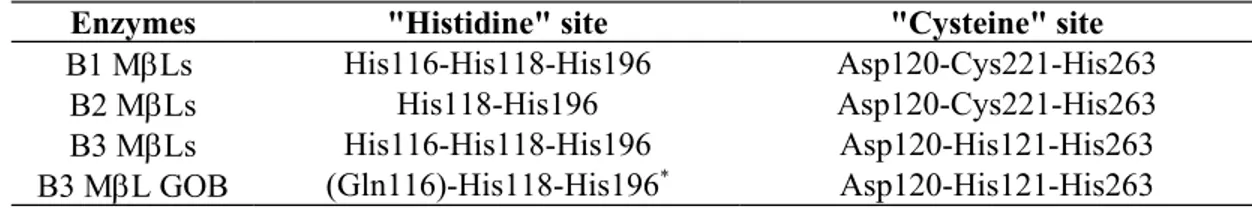
(“histidine” site), whereas the other has a trigonal-pyramidal coordination sphere involving Asp120, Cys221, His263 and two water molecules (“cysteine” site) (Table 1). One water/hydroxide serves as a ligand for both metal ions (3). In the mononuclear structures of B1 enzymes (BcII, VIM-2, SPM-1 and VIM-4), the sole metal ion was found to be located in the “histidine” site (5, 15, 25 and P. Lassaux, unpublished data). In some monozinc B1 structures, the Cys221 residue is found under an oxidized form. In subclass B3, the “histidine” site is similar to that found in subclass B1, but Cys221 is replaced by a serine residue and the second zinc ion is coordinated by Asp120, His121, His263 and the nucleophilic water molecule which again forms a bridge between the two metal ions (Table 1) (33).
Aeromonas hydrophila CphA is a subclass B2 metallo--lactamase characterized by a
uniquely narrow specificity. CphA efficiently hydrolyses only carbapenems and shows very poor 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63
activity against penicillins and cephalosporins, in contrast to subclass B1 and B3 enzymes which hydrolyse nearly all -lactam compounds, with the exception of monobactams (10, 29). In contrast to subclass B1 and B3 enzymes which are more active as di-zinc species, subclass B2 is inhibited in a non-competitive manner by the binding of a second zinc ion. For CphA, the dissociation constant of the second zinc ion (KD2) is 46 µM at pH 6.5 (16). In agreement with
EXAFS studies (17) and site-directed mutagenesis (34), the crystallographic structure of CphA shows that the first zinc ion is in the “cysteine” site (14). However, the second binding site in subclass B2 enzymes remains to be determined because Garau and colleagues could not produce crystals of the di-zinc form despite presence of zinc concentration well above KD2 (14). The
structure of Sfh-1, a subclass B2 enzyme from Serratia fonticola, has been solved recently, once again with only one zinc ion in the active site (11). In subclass B2, the His116 residue found throughout the metallo--lactamase superfamily (with the exception of the B3 GOB enzymes where Gln is present) is replaced by an asparagine (8, 12, 13). It has been previously shown that the Asn116 residue has no role in the binding of the zinc ions in CphA (34). On the basis of spectroscopic studies with the Aeromonas veronii cobalt-substituted ImiS enzyme, Crawford and co-workers postulated that the second metal binding site was not the traditional “histidine” site but a site, remote from the active site, which involves both His118 and Met146 as zinc ligands (6, 7). However, a recent study of potential zinc ligand mutants contradicts this hypothesis and indicates that the position of the second zinc ion in CphA is probably equivalent to the “histidine” site observed in subclasses B1 and B3 enzymes, with His118 and His 196 involved in the binding of this second zinc perhaps with Cys221 (or Asn116 or Asp120) as the third ligand (4).
Since crystals of the wild-type CphA protein could not be obtained directly, single-site mutants were engineered by site-directed mutagenesis and overproduced. These mutants were 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
selected in order to introduce residues that are conserved in either subclass B1 or B3, or both (2). Among these mutants, the N220G mutant (2) easily yielded crystals which were used as seeds to grow wild-type CphA crystals (14). The kinetic parameters of the wild-type and N220G mutant CphA enzymes were similar, indicating strong conservation of enzymatic properties in the mutant (2). However, the N220G mutation results in a slightly higher KD2 value (86 µM) (2). We
therefore set out to solve the crystallographic structures of the di-zinc forms of the wild-type CphA enzyme and its N220G mutant. We confirmed that the second zinc ion occupies a slightly modified “histidine” site, where the conserved His118 and His196 residues act as metal ligands. The implication of this discovery on what is known about the coordination of zinc ions by metallo--lactamases is discussed. We propose an explanation for the inhibition by the second zinc ion. Moreover, the role of the Asn233 residue in this inhibition phenomenon is also apprehended based on a site-directed mutagenesis study. In this work, a structural role for the zinc ions is also investigated.
87 88 89 90 91 92 93 94 95 96 97 98 99
Materials and Methods
Protein purification and crystallization
Site-directed mutagenesis, protein expression and purification of the proteins were carried out as described previously (2), with the addition of a third purification step consisting of a size exclusion chromatography (Hiload 16/60 Superdex 75 prep grade column, Pharmacia Biotech). The purified enzyme solution was dialyzed against 15 mM sodium cacodylate (pH 6.5). Monodispersity of the protein solutions and the hydrodynamic radii of the dissolved protein molecules were checked by Dynamic Light Scattering (DLS).The crystallisation conditions used previously (14) were not easy to reproduce because the crystallisation drop comprised two phases. Therefore, a screen was carried out to find new conditions. Initial screens were carried out at 8°C using commercial Hampton Crystal Screens 1 and 2, Grid Screen Ammonium Sulphate and Grid Screen PEG 6000 (Hampton Research, California, USA). N220G CphA (10 mg/ml) was crystallized from 2.2–2.4 M ammonium sulphate and 0.1 M MES (pH 6.0–6.5), using the sitting drop method with new crystallisation plates designed by Taorad GmbH (Aachen, Germany). The (1 µl) reservoir solution was mixed with the protein solution (1 µl) and the mixture was left to equilibrate against the solution reservoir. Typically, orthorhombic crystals of the N220G mutant grew within a few days to dimensions of 80 x 100 x 100 µm and belong to space group C2221 (a=42.68, b=101.06 and c=116.82). Crystals of the wild-type CphA enzyme
were obtained under similar conditions using mutant micro-crystals as starting seeds and showed similar orthorhombic symmetry in space group C2221 (a=42.80, b=101.50 and c=116.49). Before
data collection, 1µl of 100 mM ZnCl2 was added to the drops containing the wild-type and the
mutant crystals and soaked for one day. 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122
Data collection and processing
For data collection, wild-type and mutant crystals were transferred to a cryoprotectant solution containing reservoir solution supplemented with 30% (v/v) glycerol. The mounted crystals were flash-frozen in a liquid nitrogen stream. Near-complete X-ray data sets were collected using a Bruker FR591 rotating anode X-ray generator and a Mar345dtb detector. Diffraction data were processed with XDS (20) and scaled with SCALA from the CCP4-Suite (32).
Structure determination and refinement
The structures of di-zinc variants from the wild-type and N220G mutant enzymes were solved using a molecular replacement approach with Molrep (32), and the mono-zinc structure of CphA (PDB file 1X8G) as starting model. The resulting model was rebuilt with ARP/wARP (28). The structures were refined in a cyclic process including manual inspection of the electron density with Coot (9) and refinement with Refmac (26). The refinement to convergence was carried out with isotropic B-values and using TLS parameter (27). The positions of the zinc, sulphate and chloride ions were verified by calculating anomalous maps and the occupancies of the ions were determined by disappearing of difference electron density. Alternative conformations were modelled for a number of side chains and occupancies were adjusted to yield similar B-values for the disordered conformations.
Circular dichroism (CD) spectroscopy
Circular dichroism (CD) spectra for the apo-, mono-and di-zinc forms of the wild-type and N220G enzymes (0.3 mg/ml) were obtained using a JASCO J-810 spectropolarimeter. The 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145
apoenzymes were obtained in the presence of 10 mM EDTA. The di-zinc forms were obtained in the presence of 500 µM Zn2+. The spectra were scanned at 25°C with 1 nm steps from 200 to 250
nm (far UV). Thermal stability was assessed for the apo-, mono- and di-zinc forms of the wild-type and N220G proteins using CD spectroscopy at 220 nm with increasing temperature (25°C– 85°C). Urea denaturation studies of the wild-type and N220G enzymes were also carried out in the presence and absence of 500 µM Zn2+. The mono- and di-zinc enzymes were incubated for
18h in increasing concentrations of urea (0–8 M) at 4°C. Stability was assessed for the mono- and di-zinc forms of both proteins using CD spectroscopy at 220 nm with increasing concentrations of urea.
Thermal shift assay
Thermal shift assays were carried out using a LightCycler real-time PCR instruments (Roche Diagnostics). Briefly, 10 µl of wild-type CphA enzyme or the N220G mutant (0.3 mg/ml) was mixed with 10 µl of 5000 x Sypro Orange (Molecular Probes) diluted 1:500 in 15 mM cacodylate (pH 6.5). Samples were heat-denatured from 25 to 90°C at a rate of 0.5°C per minute. Protein thermal unfolding curves were monitored by detecting changes in Sypro Orange fluorescence. The inflection point of the fluorescence vs temperature curves was identified by plotting the first derivative over the temperature and the minima were referred to as the melting temperature (Tm). Buffer fluorescence was used as the control.
Differential Scanning Calorimetry (DSC)
Thermal denaturation was investigated using a NDSC 6100 differential scanning calorimeter (Calorimetry Sciences Corp., Lindon, USA) after dialysis of the N220G enzyme 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168
sample (1 mg/ml) against 15 mM cacodylate (pH 6.5). The dialysis buffer was used as reference. The data were collected and analysed with a MCS Observer software package assuming a two-state folding mechanism.
Analysis of the N233A mutant
The Quik Change Site-directed mutagenesis kit (Stratagene Inc., La Jolla, Calif.) was used to introduce the N233A mutation into CphA, using pET9a-CphA WT as the template, forward primer GGAGAAGCTGGGCGCCCTGAGCTTTGCCG-3′ and reverse primer 5′-CGGCAAAGCTCAGGGCGCCCAGCTTCTCC-3′. The vector was then introduced into E. coli strain BL21 (DE3) pLysS Star. Overexpression and purification of the mutant protein, CD spectroscopy, electrospray ionization-mass spectrometry (ESI-MS) and the determination of metal content, kinetic parameters and residual activity in the presence of increasing concentrations of zinc were carried out as previously described (2, 4, 34).
Protein Data Bank accession codes
Coordinates and structure factors were deposited in the Protein Data Bank using accession codes 3F90 (di- zinc form of wild-type CphA) and 3FAI (di-zinc form of the N220G mutant). 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185
Results
Structure determination.
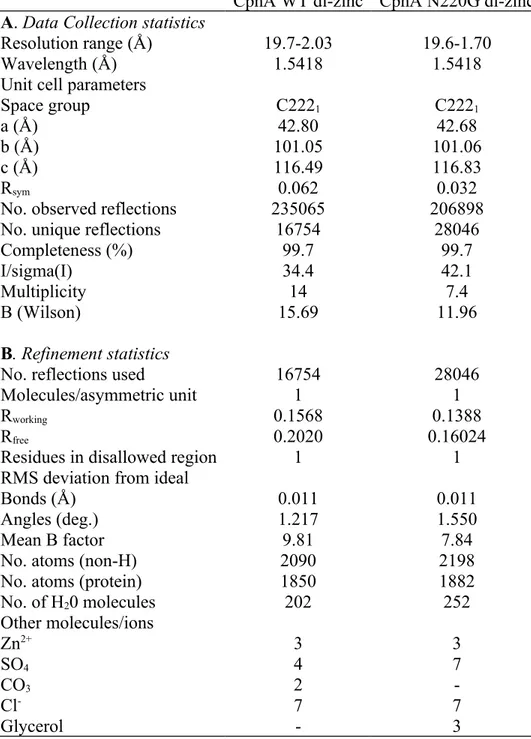
The crystal structures of the zinc saturated wild-type and N220G CphA enzymes were solved. Stereo-chemical parameters were calculated by PROCHECK (21) and WHAT_CHECK (18) and fell within the range expected for a structure with similar resolution. The crystallographic and model statistics for the di-zinc form of the wild-type and N220G mutant structures are shown in Table 2.
Di-zinc form of the wild-type CphA metallo--lactamase
The crystal structure of the di-zinc form of the wild-type CphA enzyme was solved by molecular replacement using the structure of the mono-zinc form (PDB code 1X8G) as the starting model. The structure was refined to a resolution of 2.03 Å. The Rwork and Rfree values for
the refined structure were 0.1589 and 0.2020, respectively. The crystals adopted a C2221 space
group with one molecule in the asymmetric unit. Only one residue (Ala195) was found in a disallowed region of the Ramachandran plot. Ala195 (= 105°, = 155°) is located on the loop between strands 8 and 9, with His196 at its apex.
The model of the wild-type di-zinc structure includes 226 amino acid residues (41-312, according to the BBL numbering (13)). The last serine residue at the C-terminus is missing. The electron density was good enough to identify three zinc ions (with two in the active site and one on the surface) and a sulphate ion in the active site. The composition of the visible solvent sphere is mentioned in table 2. The Gly232–Phe236 mobile loop region had a low electron density and the main chain for the residues was modelled in two conformations. The Phe236 side chain was 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207
not visible in the electron density map and other side chains were modelled with alternative conformations.
CphA shows the typical fold of the metallo--lactamase superfamily. The overall fold is not altered by the presence of the second zinc. The di-zinc form has an average RMSD value of 0.272 Å for backbone atoms with respect to the mono-zinc form.
The positions of the zinc ions were verified at the level of the electron density and by using anomalous signals. Both zinc ions were refined to a 100 % occupancy and show an increased mobility. This is indicated by the B-factors (17.68 and 20.63 for the first and the second zinc ions, respectively) lying over the mean B-factor (9.81). The first zinc ion is found in the Asp120-Cys221-His263 site or “cysteine” site as previously observed in the mono-zinc form (14) (Figure 1). The distances between the zinc ion and the Asp120 carboxyl oxygen atom, the Cys221 sulphur atom, and the His263 side-chain nitrogen atom are 2.00, 2.26 and 2.09 Å, respectively. In the mono-zinc form, these distances were 1.96, 2.20 and 2.05 Å, respectively (14). The tetrahedral coordination sphere is completed by a sulphate ion from the crystallization solution at a distance of 2.04 Å.
The second zinc ion is coordinated by the His118 and His196 residues. Both histidine residues belong to the conserved “histidine” site in metallo--lactamases. The distances between the zinc ion and the His118 ND1 and the His196 NE2 are 2.01 and 2.03 Å, respectively. In CphA, the third ligand His116 is not conserved and is replaced by an asparagine residue, which is not involved in the binding of the second zinc (the distance is more than 4 Å). A sulphate ion (1.95 Å) and a water molecule (2.01Å) complete the vacant coordination positions of the zinc ion in the “histidine” site, forming a tetrahedral coordination sphere. The sulphate ion acts as a bridging agent between the zinc ions (Figure 1). The distances between the sulphate ion and zinc 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230
in the “cysteine” site and zinc in the “histidine” site are 2.04 and 1.95 Å, respectively. The distance between both zinc ions is 4.08 Å.
The third observed zinc ion was found on the surface, away from the active site (about 17 Å). It is coordinated by His289 (2.09 Å from His NE) and the coordination sphere is completed by three chloride ions (2.19, 2.25 and 3.17 Å).
Di-zinc form of the N220G mutant
The crystal structure of the di-zinc form of N220G was solved by molecular replacement based on the mono-zinc structure (PDB code 1X8G). The structure was refined to 1.70 Å with Rwork and Rfree values of 0.1308 of 0.1602, respectively.
The model of the N220G di-zinc structure included all the 227 residues of the protein (41-313 according to the BBL numbering (13)), three zinc ions (two in the active site and one on the surface) and one sulphate ion located in the active site. The complete content of the solvent sphere is described in table 2. The C-terminal residues were highly flexible. Ala195 was found in a disallowed region of the Ramachandran plot. The electron density for the Gly232–Phe236 mobile loop region allowed a well-defined model to be constructed.
The active site of the N220G mutant with two bonded zinc ions had a conformation nearly identical to that of the wild-type di-zinc enzyme. The RMSD for all main chain atoms was 0.216 Å. In contrast to the mono-zinc form of the N220G mutant where the zinc ion occupied two sites 1.5 Å apart (14), in the di-zinc form of the enzyme described here, the zinc ion was in the classical “cysteine” site with distances to ligands identical to those in the wild-type structure. Also, a sulphate ion and a water molecule (both with 50 % occupancy) were included in the coordination sphere at distances of 2.04 and 2.27 Å, respectively. Under the new crystallisation 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253
conditions described above, a mono-zinc form of the N220G mutant was also obtained, in which a fully occupied zinc ion was present in the canonical “cysteine” site (data not shown).
The second zinc ion could not be modelled with an occupancy higher than 0.8. This second zinc ion shows a B-factor of 9.85, nearly the same as the mean B-factor (7.84) and in the same range as the B-factor of the zinc ion in the “cysteine” site (10.50). This zinc ion was coordinated by His118 and His196. The coordination sphere was completed by a water molecule (2.02 Å) and the sulphate ion (2.15 Å). An additional water molecule was visible at a distance of 2.54 Å.
The third zinc ion was found nearly in the same position as in the wild-type structure. Here, the zinc ion and the coordinating chloride ions exhibited 50 % occupancy. The coordination sphere was affected by a water molecule and a disordered side chain from Glu292.
Comparison between the mono- and di-zinc structures of CphA
The binding of the second zinc ion in the active site has no influence on the global structure of CphA. This is confirmed by the low RMSD values obtained by superposition of the mono- and di-zinc forms. The differences between the mono- and di-zinc structures are limited to the Gly232–Asn233 loop region (Figure 2). This loop, located at the entrance to the active site, is known to be stabilized upon the binding of the substrate (14) or inhibitors (19, 22). For the wild-type di-zinc structure, this loop can exist in two conformations (both half occupied), corresponding, respectively, to the open form (as in the mono-zinc structures 1X8G and 1X8H) or the closed form (as in the structures with substrate or inhibitor in the active site, 1X8I, 2QDS and 2GKL). In the di-zinc form of the N220G mutant, this loop is found in the closed form (Figure 2). The closed conformation is stabilized by additional H-bonds between the Asn233 side chain and the Ser235 side chain oxygen and backbone nitrogen.
254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277
The active site of the di-zinc forms can be superimposed nearly perfectly over all the previously available CphA structures (PDB codes 1X8G, 1X8H, 1X8I, 2QDS and 2GKL). For example, the RMSD is 0.56 Å for the superposition of the “cysteine” and “histidine” sites over their counterparts in 1X8G. The position of the zinc in the “cysteine” site is nearly identical, with the exception of the mono-zinc N220G mutant where a disordered zinc ion was observed (14). The coordinating His118 residue in the “histidine” site is not affected by the binding of the second zinc. In contrast, the His196 residue displays a slight flexibility. It is noticeable that the orientation of the imidazole ring of His196 in all X-ray structures is selected to achieve an optimal pattern of contacts in the coordination sphere (H-bonds, zinc binding). Therefore, in di-zinc structures, the His196 imidazole ring is rotated by 180° compared to its position in the mono-zinc structures (Figure 2).
Comparison with the active sites in B1 and B3 metallo--lactamases
The active sites of all three metallo-β-lactamase subclasses show high degree of correlation (RMSD values between 0.5 Å and 1.6 Å). In subclass B2, the coordination sphere of the zinc in the “histidine” site is altered by the substitution of His116 by an asparagine residue. In subclasses B1 and B3, the His116 residue is involved in the binding of the zinc ion in the “histidine” site (Figure 3). Removing one coordinating residue causes this zinc ion to shift by 1.12, 1.03, 1.03 and 0.92 Å compared to BcII (PDB code 1BVT) (Figure 3), VIM-2 (PDB code 1KO3), VIM-2 (PDB code 1KO2) and L1 (PDB code 2FM6) structures, respectively. The position of the zinc in the “cysteine” site is nearly unchanged when compared to the available structures of subclass B1 enzymes.
Stability study 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301
As already reported (16), the helical contents of the apo-, mono- and di-zinc forms of the wild-type CphA enzyme are similar. The apparent Tm values determined by CD spectroscopy
were 46.9, 58.7 and 61.6°C for the apo-, mono- and di-zinc forms, respectively. The mono-zinc wild-type CphA is also less stable than the di-zinc form with urea as a denaturing reagent. Transitions betweennative and denatured states occurred at 3.4 and 4.4 M urea for the mono- and di-zinc forms, respectively. (data not shown)
The far-UV CD spectra of the apo-, mono- and di-zinc forms of the N220G mutant exhibited small but significant differences (Figure 4a). However, as observed for the wild-type enzyme, the helical content did not vary significantly. Thermal denaturation was irreversible in all cases. The apparent Tm values determined by CD spectroscopy were 49.2, 61.7 and 62.9°C for
the apo-, mono- and di-zinc forms, respectively (Figure 4b). Also, the apparent Tm value
determined for the mono-zinc form of N220G using a thermal shift assay (Figure 4c) and by DSC was 62°C but decreased to 50°C in the presence of 10 mM EDTA (Figure 4c). The mono-zinc form of the N220G mutant was slightly less stable than the di-zinc form with urea as the denaturingreagent. Transitions betweennative and denatured states occurred at 4.1 and 4.7 M urea for the mono- and di-zinc forms, respectively (data not shown).
Is the “closed” position of the Gly232-Asn233 loop important for the inhibition by the second zinc ion? - Analysis of the N233A mutant
An Asn233 residue is present in all metallo--lactamases with the exception of the L1, BlaB and IND-1 enzymes (13). In CphA, the binding of a carbapenem substrate or inhibitor modifies the Asn233 angle so that its side chain closes the entrance of the active site in a conformation stabilised by the formation of an H-bond between the oxygen of the Asn233 side-chain and the hydroxyl of Ser235 (14, 19, 22). In the structure of the di-zinc form of CphA, the 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325
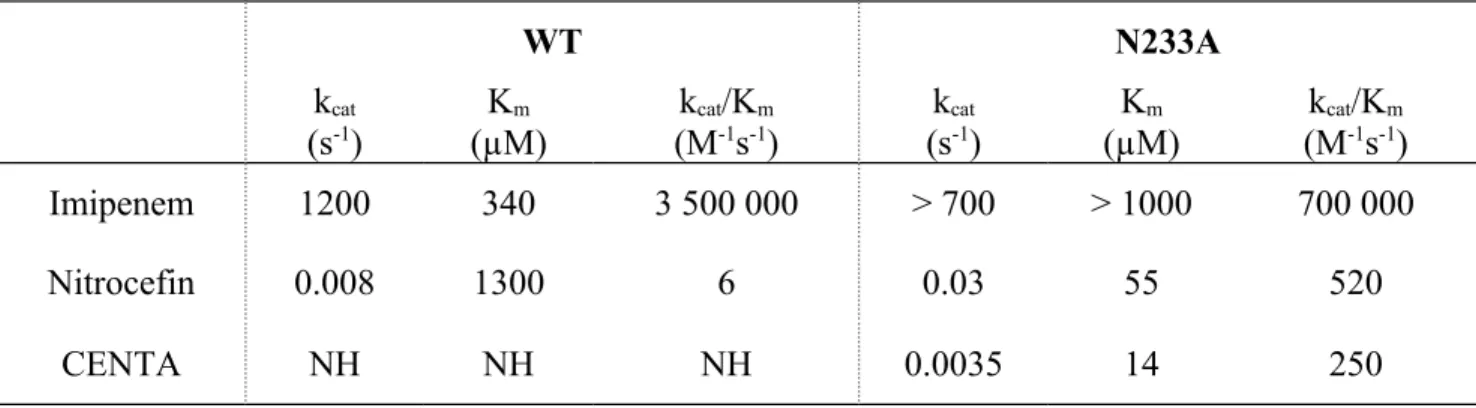
loop adopts the closed position even in the absence of substrate, thus hindering access to the active site (Figure 2). To explore the role of the Asn233 residue in the inhibition phenomenon, the N233A mutant was expressed in Escherichia coli BL21 (DE3) pLysS Star, purified to homogeneity and characterized. The mass of the protein was verified by electrospray ionisation mass spectrometry (ESI-MS). Within experimental error, the mutant was found to exhibit the expected mass (25144 Da measured vs. 25146 Da expected). The far UV CD spectrum of the mutant enzyme showed the same / ratio as the wild type. The enzyme was stored in 15mM sodium cacodylate (pH 6.5) with < 0.4 µM free zinc. Under these conditions, the N233A protein contained one zinc ion per molecule as reported for the wild-type -lactamase. The activity of the mutant was measured in the absence of added Zn2+ (< 0.4 µM) with imipenem, nitrocefin and
CENTA (Table 3). The N233A mutant showed an increased Km value for imipenem. In contrast
to the wild-type enzyme, the N233A mutant was able to hydrolyse (albeit rather poorly) both cephalosporins, with quite low Km values. The hydrolysis of imipenem by N233A was inhibited
by the binding of the second zinc ion, but the KD2 value decreased to 11 ± 2 µM compared to 46
µM for the wild-type enzyme. 326 327 328 329 330 331 332 333 334 335 336 337 338 339 340
Discussion
There are three subclasses of metallo--lactamases (B1, B2 and B3), all of which require Zn2+ for activity and can bind either one or two zinc ions (12, 13). Subclass B2
metallo--lactamases are active only in the mono-zinc form, because the binding of a second zinc ion inhibits the enzyme in a non-competitive manner (16). This behaviour contrasts with that of B1 and B3 subclasses, where the presence of a second zinc ion in the active site has either little effect or facilitates enzyme activity. Since it has thus far proven difficult to determine the structure of the di-zinc form of a subclass B2 -lactamase to investigate the basis of this inhibitory effect, we set out to crystallise the di-zinc form of Aeromonas hydrophila CphA and solve its structure.
Previous attempts to determine the crystal structure of di-zinc A. hydrophila CphA have not been successful, even with a high concentration of zinc in the mother liquor (14). It was proposed that this might reflect the conditions used to grow crystals and/or the presence of a carbonate ion in the active site. Also, it was suggested that the binding of the second zinc ion required a change of active site conformation impossible to obtain in the crystal. Indeed, nuclear magnetic resonance (NMR) measurements indicate major conformation changes upon binding of the second zinc ion (C. Damblon, personal communication). However, CD spectra showed that the secondary structures of the mono-zinc forms of the wild-type (16) and N220G CphA enzymes are only slightly different from those of the di-zinc forms. By modifying the crystallization conditions, we succeeded in obtaining CphA crystals with two zinc ions in the active site. Moreover, the overall conformation of the enzyme was not affected by the presence of a second zinc ion. The di-zinc form of CphA was obtained by soaking the crystal into a solution containing a zinc concentration well above the KD2 value. In the crystal, major movements
leading to the significant unfolding of the protein as observed by NMR are impossible. 341 342 343 344 345 346 347 348 349 350 351 352 353 354 355 356 357 358 359 360 361 362 363
In the mono-zinc form, a carbonate ion was present in the active site (14). In our structures, a sulphate ion from the crystallization solution was bound to both zinc ions.
As previously postulated (4, 34), the second inhibitory zinc ion is located in a slightly modified “histidine” binding site with the two conserved His118 and His196 residues as ligands. The Met146 residue, proposed as one of the inhibitory zinc ion ligands together with His118 in the ImiS metallo--lactamase (6), was 12.54 Å from this ion in CphA and the sulphur atom points in the opposite direction. Also, the space between the His118 and Met146 residues is occupied by the backbone of the Asn114-His118 loop, connecting strand 6 and helix 2, and thus does not allow the accommodation of a zinc ion. The sequence of ImiS is 96% identical to that of CphA, so it would be remarkable if these few substitutions produced such a radically different mechanism for the binding of the inhibitory zinc ion.
This “atypical” coordination sphere with only two of the three conserved histidine residues (figure 3) could probably explain the rather high value of the dissociation constant for the “histidine” site in CphA (46 µM) compared to that in subclasses B1 and B3. The value of the dissociation constant for the “histidine” site is 1.8 nM for BcII (a subclass B1 enzyme) and subclass B3 enzymes have high affinity constants for both binding sites (KD < 6 nM) (35).
Vanhove et al. proposed an explanation for the inhibition of subclass B2 enzymes by the second zinc ion. The side-chain of the Cys221 which is essential for binding the first zinc ion would be displaced by the binding of the second zinc ion (34). However, a comparison between mono- and di-zinc structures showed that this residue was not displaced. On the basis of the results obtained here, we can confirm that the binding of the inhibitory zinc ion to the catalytically important His118 and His196 residues prevents them from playing their catalytic roles in the hydrolysis of carbapenems (4, 14) where His118 is the general base which activates 364 365 366 367 368 369 370 371 372 373 374 375 376 377 378 379 380 381 382 383 384 385 386
the hydrolytic water molecule and His196 contributes to the oxyanion hole by forming a H-bond with the carbonyl oxygen of the -lactam bond (14). This model is supported by the very weak activities of the H118A and H196A mutants (4). This conclusion reinforces the hypothesis that the nucleophile is not adequately metal activated in B2 -lactamases (30, 36). Superimposition of the structures of the dizinc forms of BcII (B1) and CphA shows that in the latter, the water molecule does not bridge the two zinc ions but is only in interaction with that in the “histidine” site (figure 5). It is possible that an interaction with this sole zinc ion does not sufficiently decrease the water pKa so that the concentration of OH- ions remains very low. Moreover,
His118 which now serves as a zinc ligand can non longer play the role of general base. The closure of the Gly232–Asn233 loop in the di-zinc form could also help to inhibit the enzyme, but Asn233 probably plays only a minor role since the N233A mutant is still inhibited by the binding of the second zinc ion. Finally, a third zinc ion is bound to a superficial histidine residue, but this is unlikely to be involved in the inhibition phenomenon. Its binding is probably due to the high zinc concentration utilized.
Whereas the N233S mutant of ImiS exhibits kinetic parameters similar to those of wild-type enzyme (30), the N233A mutant of CphA shows an increased Km value for imipenem, and
thus seems to play a role in the binding of this substrate. Two-thirds of all sequenced metallo-β-lactamases have an Asn at position 233 (12, 13), and this residue was shown to be involved in substrate binding and activation by interacting electrostatically with the substrate -lactam carbonyl (37). A same role for Asn233 residue in substrate binding by CphA was already predicted by computational modelling (36) and is in agreement with our results.
In contrast to the zinc ion located in the “cysteine” site, the second zinc ion does not play an important structural role. Indeed, the binding of the first zinc ion stabilizes the wild-type and 387 388 389 390 391 392 393 394 395 396 397 398 399 400 401 402 403 404 405 406 407 408 409
N220G CphA enzymes significantly, whereas the second zinc ion has a much more limited impact on stability. Both the mono- and di-zinc forms of the N220G mutant are somewhat more stable than their wild-type counterparts, perhaps explaining why crystals of the N220G mutant are obtained more easily than those of the wild-type enzyme.
In conclusion, the catalytic metal ion is located in the “cysteine” site of CphA and the second inhibitory zinc ion binds to a slightly modified “histidine” site. The “histidine” site has first been considered as the catalytic site in the mononuclear metallo--lactamases. Indeed, in the first reported monozinc structures (B1 enzymes such as BcII, VIM-2, VIM-4 and SPM-1), the single metal ion was shown to be located in the “histidine” site (5, 15, 25). In contrast, a B3 enzyme called GOB was shown to be active as a monozinc enzyme with the metal ion located in the “cysteine” site (24). Recently, Vila and co-workers proposed that B1 enzymes can be active in their mono- and dizinc forms and that in mono-cobalt BcII the metal ion is localized in the “cysteine” site (23, 31). Two mononuclear mutants of BcII in which each of the metal binding sites was selectively removed produced inactive variants. The authors concluded that the mononuclear form can be active only if assisted by residues at the other binding site (1). According to the model proposed by Tioni et al. (31), the metal ion in the “histidine” site would activate the hydrolytic water molecule in the dizinc enzyme. Vila and co-workers proposed that this is facilitated by a net of H-bond interactions in the mononuclear enzyme (23, 31) probably including His118 or Asp120, as already proposed for CphA (4, 14, 36). However, Asp120 appears unlikely because it already coordinates a zinc ion. In subclass B2, as previously postulated by Vanhove et al. (34), Asn116 is not involved in the binding of the second metal ion but the N116H (34) and N116H-N220G (2) mutants could have a reconstituted His116-His118-His196 site and behave similarly to B1 enzymes. Indeed, the N116H-N220G mutant has an extended substrate spectrum and its di-zinc form is active (2).
410 411 412 413 414 415 416 417 418 419 420 421 422 423 424 425 426 427 428 429 430 431 432 433
The crystal structure of the subclass B2 CphA -lactamase in its metal-inhibited form provides thus an answer to the structural characterization of the inhibitory site that has been elusive and controversial so far.
434 435 436
Acknowledgements
The work in Liège was supported by the Belgian Federal Government (PAI P5/33) and grants from the FNRS (Brussels, Belgium, FRFC grant n° 2.4511.06 and Lot. Nat. 9.4538.03). C.B. is a FRS/FNRS post-doctoral researcher.
437 438 439 440
References
1. Abriata, L. A., L. J Gonzalez, L. I. Llarrull, P. E. Tomatis, W. K. Myers, A. L. Costello, D. L Tierney, and A. J. Vila. 2008. Engineered mononuclear variants in Bacillus cereus metallo--lactamase are inactive. Biochemistry, 47:8590–8599.
2. Bebrone, C., C. Anne, K. De Vriendt, B. Devresse, J. Van Beeumen, J.M. Frère, and M. Galleni. 2005. Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo--lactamase by site-directed mutagenesis, J. Biol. Chem.,17:180-188.
3. Bebrone, C. 2007. Metallo--lactamases (classification, activity, genetic organization, structure, zinc coordination) and their Superfamily. Biochem. Pharma. 74:1686-1701.
4. Bebrone, C., C. Anne, F. Kerff, G. Garau, K. De Vriendt, R. Lantin, B. Devreese, J. Van Beeumen, O. Dideberg, J.M. Frère, and M. Galleni. 2008. Mutational analysis of the zinc and substrate binding sites in the CphA metallo--lactamase from Aeromonas hydrophila, Biochem. J, 114:151-159.
5. Carfi, A., S. Parès, E. Duée, M. Galleni, C. Duez, J.M. Frère, and O. Dideberg. 1995. The 3-D structure of a zinc metallo- -lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 14:4914-4921.
6. Costello, A.L., N.P. Sharma, K.W. Yang, M.W. Crowder, and D.L. Tierney. 2006. X-ray absorption spectroscopy of the zinc-binding sites in the class B2 metallo--lactamase ImiS from
Aeromonas veronii bv. sobria. Biochemistry. 45:13650-13658.
7. Crawford, P.A., K.W. Yang, N. Sharma, B. Bennett, and M.W. Crowder. 2005. Spectroscopic studies on cobalt(II)-substituted metallo--lactamase ImiS from Aeromonas
veronii bv. sobria. Biochemistry. 44:5168-5176.
441 442 443 444 445 446 447 448 449 450 451 452 453 454 455 456 457 458 459 460 461 462
8. Daiyasu, H., K. Osaka, Y. Ishino, and H. Toh. 2001. Expansion of the zinc metallo-hydrolase family of the -lactamase fold. FEBS Lett. 503:1-6.
9. Emsley, P., and K.Cowtan. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D60:2126-2132.
10. Felici, A., G. Amicosante, A. Oratore, R. Strom, P. Ledent, B. Joris, L. Fanuel, and J.M. Frère. 1993. An overview of the kinetic parameters of class B -lactamases. Biochem.J. 291:151-155.
11. Fonseca, F., A. Correia, and J. Spencer. 2008. Structural and kinetic characterisation of Sfh-1, the B2 metallo--lactamase of Serratia fonticola. p.46. Proceedings of the 10th -lactamase
meeting, Eretria, Greece.
12. Galleni, M., J. Lamotte-Brasseur, G.M. Rossolini, J. Spencer, O. Dideberg, J.M. Frère, and the Metallo--lactamases Working Group. 2001. Standard numbering scheme for class B -lactamases. Antimicrob.Agents Chemother., 45:660-663.
13. Garau, G., I. Garcia-Saez, C. Bebrone, C. Anne, P.S. Mercuri, M. Galleni, J.M. Frère, and O. Dideberg. 2004. Update of the standard numbering scheme for class B -lactamases. Antimicrob.Agents Chemother., 48:2347-2349.
14. Garau, G., C. Bebrone, C. Anne, M. Galleni, J.M. Frère, and O. Dideberg. 2005. A Metallo--lactamase Enzyme in Action: Crystal Structures of the Monozinc Carbapenemase CphA and its Complex with Biapenem. J. Mol. Biol. 345:785-795.
15. Garcia-Saez, I., J.D. Docquier, G.M. Rossolini, and O. Dideberg. 2008. The three-dimensional structure of VIM-2, a Zn--lactamase from Pseudomonas aeruginosa in its reduced and oxidised form. J. Mol. Biol. 375:604-611.
463 464 465 466 467 468 469 470 471 472 473 474 475 476 477 478 479 480 481 482 483 484
16. Hernandez-Valladares, M., A. Felici., G. Weber, H.W. Adolph, M. Zeppezauer, G.M. Rossolini, G. Amicosante, J.M. Frère, and M. Galleni. 1997 Zn(II) dependence of the
Aeromonas hydrophila AE036 metallo--lactamase activity and stability. Biochemistry. 36:11534-11541.
17. Hernandez-Valladares, M., M. Kiefer, U. Heinz, R. Paul Soto, W. Meyer-Klaucke, H. Friederich Nolting, M. Zeppezauer, M. Galleni, J.M. Frère, G.M. Rossolini, G. Amicosante and H.W. Adolph. 2000. Kinetic and spectroscopic characterization of native and metal-substituted -lactamase from Aeromonas hydrophila AE036. FEBS Lett. 467:221-225.
18. Hooft, R.W.W., G. Vriend, C. Sander, E.E. and Abola. 1996. Errors in protein structures. Nature. 381:272-272.
19. Horsfall, L.E., G. Garau, B.M. Liénard, O. Dideberg, C.J. Schofield, J.M. Frère, and M. Galleni. 2007 Competitive inhibitors of the CphA metallo--lactamase from Aeromonas
hydrophila. Antimicrob. Agents Chemother. 51:2136-2142.
20. Kabsch, W. 1993. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Cryst. 26:795-800.
21. Laskowski, R. A., M.W. MacArthur, D.S. Moss, and J.M. Thornton. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26:283-291. 22. Liénard, B.M.R., G. Garau, L. Horsfall, A.I. Karsisiotis, C. Damblon, P. Lassaux, C. Papamicael, G.C.K. Roberts, M. Galleni, O. Dideberg, J.M. Frère, and C.J. Schofield. 2008. Structural basis for the broad-spectrum inhibition of metallo--lactamases by thiols. Org. Biomol. Chem. 6:2282-2294.
23. Llarull, L.I., M.F. Tioni and A.J. Vila. 2008. Metal content and localization during turnover in Bacillus cereus metallo--lactamase. J. Am. Chem. Soc. 130:15842-15851.
485 486 487 488 489 490 491 492 493 494 495 496 497 498 499 500 501 502 503 504 505 506 507
24. Morán-Barrio, J., J.M. González, M.N. Lisa, A.L. Costello, M. Dal Peraro, P. Carloni, B. Bennett, D.L. Tierney, A.S. Limansky, A.M. Viale, and A.J. Vila. 2007. The metallo- -lactamase GOB is a mono-Zn(II) enzyme with a novel active site. J. Biol. Chem. 282:18286-18293.
25. Murphy, T.A., L.E. Catto, S.E. Halford, A.T. Hadfield, W. Minor, T.R. Walsh, and J. Spencer. 2006. Crystal structure of Pseudomonas aeruginosa SPM-1 provides insights into variable zinc affinity of metallo--lactamases. J.Mol.Biol. 357:890-903.
26. Murshudov, G.N., A.A. Vagin, and E.J. Dodson. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D53:240-255.
27. Painter, J., E.A. and Merritt. 2006. Optimal description of a protein structure in terms of multiple groups undergoing TLS motion.Acta Crystallogr. D62:439-450.
28. Perrakis, A., R. Morris, and V.S. Lamzin. 1999. Automated protein model building combined with iterative structure refinement. Nat Struct Biol. 6(5):458-463.
29. Segatore, B., O. Massida, G. Satta, D. Setacci, and G. Amicosante. 1993. High specificity of cphA-encoded metallo--lactamase from Aeromonas hydrophila AE036 for carbapenems and its contribution to -lactam resistance. Antimicrobial Agents and Chemother. 37:1324-1328. 30. Sharma, N.P., C. Hajdin, S. Chandrasekar, B. Bennett, K.W. Yang, and M.W. Crowder. 2006. Mechanistic studies on the mononuclear ZnII-containing metallo--lactamase ImiS from
Aeromonas sobria. Biochemistry. 45:10729-10738.
31. Tioni, M.F., L.I. Llarull, A.A. Poeylaut-Palena, M.A. Marti, M. Saggu, G.R. Periyannan, E.G. Mata, B. Bennett, D.H. Murgida, and A.J. Vila. 2008. Trapping and characterization of a reaction intermediate in carbapenem hydrolysis by Bacillus cereus metallo--lactamase. J. Am. Chem. Soc. 130:15852-15863.
508 509 510 511 512 513 514 515 516 517 518 519 520 521 522 523 524 525 526 527 528 529 530
32. The CCP4. 1994. CCP4 suite: programs for protein crystallography. Acta Crystallog. Sect. D. 50:760-763.
33. Ullah, J.H., T.R. Walsh, I.A. Taylor, D.C. Emery, C.S. Verma, S.J. Gamblin, and J. Spencer. 1998. The crystal structure of the L1 metallo--lactamase from Stenotrophomonas
maltophilia at 1.7 Å resolution. J.Mol.Biol. 284:125-136.
34. Vanhove, M., M. Zakhem, B. Devreese, N. Franceschini, C. Anne, C. Bebrone, G. Amicosante, G.M. Rossolini, J. Van Beeumen., J.M. Frère, and M. Galleni. 2003. Role of Cys221 and Asn116 in the zinc-binding sites of the Aeromonas hydrophila metallo--lactamase. Cell Mol Life Sci. 60:2501-2509.
35. Wommer, S., S. Rival, U. Heinz, M. Galleni, J.M. Frère, N. Franceschini, G. Amicosante, B; Rasmussen, R. Bauer, and H.W. Adolph. 2002. Substrate-activated zinc binding of metallo- -lactamases: physiological importance of mononuclear enzymes. J. Biol. Chem. 277:24142-24147.
36. Xu, D., D. Xie, and H. Guo. 2006. Catalytic mechanism of class B2 metallo--lactamase. J. Biol. Chem. 281:8740-8747.
37. Yanchak, M.P., Taylor, R.A. and M.W. Crowder. 2000. Mutational analysis of metallo--lactamase CcrA from Bacteroides fragilis. Biochemistry. 39:11330-11339.
531 532 533 534 535 536 537 538 539 540 541 542 543 544 545 546 547
Table 1. Composition of the two metal-binding sites in the three metallo-
-lactamase subclasses. * This “histidine” site is still putative, since the 3D structure of the GOB enzyme isnot available.
Enzymes "Histidine" site "Cysteine" site
B1 MLs His116-His118-His196 Asp120-Cys221-His263
B2 MLs His118-His196 Asp120-Cys221-His263
B3 MLs His116-His118-His196 Asp120-His121-His263
B3 ML GOB (Gln116)-His118-His196* Asp120-His121-His263
548 549 550 551
Table 2. X-ray data collection and structure refinement
CphA WT di-zinc CphA N220G di-zinc A. Data Collection statistics
Resolution range (Å) 19.7-2.03 19.6-1.70
Wavelength (Å) 1.5418 1.5418
Unit cell parameters
Space group C2221 C2221
a (Å) 42.80 42.68
b (Å) 101.05 101.06
c (Å) 116.49 116.83
Rsym 0.062 0.032
No. observed reflections 235065 206898
No. unique reflections 16754 28046
Completeness (%) 99.7 99.7
I/sigma(I) 34.4 42.1
Multiplicity 14 7.4
B (Wilson) 15.69 11.96
B. Refinement statistics
No. reflections used 16754 28046
Molecules/asymmetric unit 1 1
Rworking 0.1568 0.1388
Rfree 0.2020 0.16024
Residues in disallowed region 1 1
RMS deviation from ideal
Bonds (Å) 0.011 0.011
Angles (deg.) 1.217 1.550
Mean B factor 9.81 7.84
No. atoms (non-H) 2090 2198
No. atoms (protein) 1850 1882
No. of H20 molecules 202 252 Other molecules/ions Zn2+ SO4 CO3 Cl -Glycerol 3 4 2 7 -3 7 -7 3 552 553
Table 3.
Kinetic parameters of the wild-type and N233A CphA enzymes. All the experiments were performed at 30°C in 15 mM cacodylate (pH 6.5), [Zn2+] < 0.4 µM. Standarderrors were below 10%. NH: no observed hydrolysis
WT N233A kcat (s-1) Km (µM) kcat/Km (M-1s-1) kcat (s-1) Km (µM) kcat/Km (M-1s-1) Imipenem 1200 340 3 500 000 > 700 > 1000 700 000 Nitrocefin 0.008 1300 6 0.03 55 520 CENTA NH NH NH 0.0035 14 250 554 555 556 557
Figure legends
Figure 1
Structure of the wild-type CphA metal-binding sites. Zinc ions and water molecule are shown as grey and red spheres, respectively. ZnHis is the zinc ion that binds in the “histidine” site while ZnCys is the zinc ion that binds in the “cysteine” site. The anomalous map at a contour level of 4 is shown in orange. Relevant distances between zinc ions and ligands are indicated.
Figure 2
Superposition of the mono- (in grey) and di-zinc (in yellow) forms of the N220G CphA enzyme. The Gly232–Asn233 loop region of the di-zinc form is represented in red to underline the conformational change. The positions of the His196 residue in the mono-form (H196mono) and in the di-zinc form (H196di) are represented. The zinc ions in the “histidine” site (ZnHis) and in the “cysteine” site (ZnCys) are shown as grey spheres. The distance between His196di and the zinc
ion located in the “histidine” site is indicated.
Figure 3
The “histidine” site in CphA (a) and in BcII (PDB code 1BVT) (b). Zinc ions and water molecules are represented as grey and red spheres, respectively.
Figure 4
Structural studies of the N220G mutant 558 559 560 561 562 563 564 565 566 567 568 569 570 571 572 573 574 575 576 577 578 579
a) Circular dichroism spectra of the apo-enzyme (dotted line), mono-zinc (dashed line) and di-zinc forms (solid line) of the N220G enzyme (0.3 mg/ml in both cases).
b) Thermal denaturation of the N220G mutant (0.5 mg/ml): fraction of native protein vs temperature (°C). Apo-N220G (dotted line), mono-zinc N220G (dashed line), di-zinc N220G (solid line).
c) Thermal shift assay for N220G (0.3 mg/ml). N220G + 10 mM EDTA (dotted line), mono-zinc N220G (dashed line).
Figure 5
Superimposition of the structures of the di-zinc forms of BcII (PDB code 2BFK) and CphA. Zinc ligands of BcII and CphA are represented as grey and green sticks, respectively. Zinc ions of BcII and CphA are shown as grey and green spheres, respectively. ZnHis is the zinc ion that binds in the “histidine” site while ZnCys is the zinc ion that binds in the “cysteine” site. Water molecules of BcII and CphA are shown as purple and red spheres, respectively. The sulphate ion that bridges the two zinc ions in the CphA structure (SO4CphA) is also represented, as well as
glycerol, the fifth ligand of ZnCys in BcII (glycerolBcII).
580 581 582 583 584 585 586 587 588 589 590 591 592 593 594 595 596 597