Any correspondence concerning this service should be sent to the repository administrator:
staff-oatao@inp-toulouse.fr
To link to this article:
DOI:10.1016/j.porgcoat.2012.06.005
Official URL:
http://dx.doi.org/10.1016/j.porgcoat.2012.06.005
This is an author-deposited version published in:
http://oatao.univ-toulouse.fr/
Eprints ID: 8743
To cite this version:
Cambon, Jean-Baptiste and Ansart, Florence and Bonino, Jean-Pierre and Turq,
Viviane
Effect of cerium concentration on corrosion resistance and
polymerization of hybrid sol–gel coating on martensitic stainless steel. (2012)
Progress in Organic Coatings, pp. 75 (n° 4). pp. 486-493. ISSN 0300-9440
O
pen
A
rchive
T
oulouse
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
Effect
of
cerium
concentration
on
corrosion
resistance
and
polymerization
of
hybrid
sol–gel
coating
on
martensitic
stainless
steel
Jean-Baptiste
Cambon
∗,
Florence
Ansart,
Jean-Pierre
Bonino,
Viviane
Turq
InstitutCarnotCIRIMAT,UniversitédeToulouse,UMRCNRS5085,118RoutedeNarbonne,31062ToulouseCedex9,France
Keywords: Stainlesssteel Sol–gelcoating Corrosioninhibitor EIS NMRspectroscopy
a
b
s
t
r
a
c
t
Stainlesssteelsareincreasinglyusedintheaeronauticsfieldforthemanufactureofstructuralparts.One ofthem,theX13VDmartensiticstainlesssteel(X12CrNiMoV12-3),knownforitsgoodmechanical prop-erties,hasapoorcorrosionresistanceinconfinedorsevereenvironments.Inthepastyears,Cr(VI)based pre-treatmentshavebeencurrentlyusedforcorrosionprotectionofdifferentmetals,however,theyare toxicandduetoenvironmentalregulations,theywillbedefinitelybannedinanearfuture.Alternatives toreplaceCr(VI)showadvantagesanddrawbacksconsideringkeypropertiessuchas:corrosion resis-tance,adhesionofcoatings,fatigueresistance,durabilityandreliability.However,someoftheirpossible alternativesshowhighpotential.
Inthispaper,aprocesswasdevelopedtoimprovethecorrosionresistanceofthemartensitic stain-lesssteel.Organic–inorganichybridcoatingswithdifferentceriumconcentrationsweredepositedonto stainlesssteelbysol–gelprocess.Corrosionresistanceofthecoatingswasevaluatedby electrochemi-calimpedancemeasurementsandithasbeenprovedthatceriumconcentrationof0.01Mintohybrid coatingwasanoptimalcontent.Adhesiontestswerealsocarriedoutby“nanoscratchtest”to character-izethecoatingsmechanicalpropertiesasafunctionofceriumconcentrationbutresultsdonotclearly showtheinfluenceofceriumforthecoatingadhesiontowardthesubstrate.Totrytocorrelatewiththe electrochemicalproperties,liquid29SiNMRspectroscopywasthenperformedtoinvestigatehydrolysis
andcondensationreactionsofsol–gelprocess,andbythismethod,itwasdemonstratedthatforhigher ceriumconcentration(>0.01M)thereisamodificationofthechemicalstructureofthesol–gelnetwork.
1. Introduction
Metallic corrosion occurs as a result of chemical reactions betweenthemetalsurfaceandtheenvironment,whichconverts themetalintoitsoriginalore.Inthecaseofsteelandaluminum, Cl−,O
2andH2Ospecies,inadditiontoelectrontransport,playkey rolesinthecorrosionprocess[1,2].Generally,corrosion preven-tiontechnologyusesoneormoreofthefollowingmethods.The firstoneistheadditionofelements(Cr,Ni,etc.)inthemetalsto enrich thesurface witha corrosion-resistantcomponentduring thecorrosionprocess. Thiscan beassociatedtotheadditionof aqueousinhibitors,whichcanbestronglyadsorbedbychemical conversiontreatmentonthemetalsurfacetopreventthereaction withtheoxidizingagent.Chromates(Cr(VI))areusedasthemost commoncompoundsduetotheirefficiencyinsevereatmosphere andtheirlowcost.Butchromiumbasedcompoundsareextremely toxicadditives,carcinogen,mutagenandreprotoxic.Sotheuseof chromateswillbestrictlyprohibitedinthenextyears[3].
∗ Correspondingauthor.
E-mailaddress:cambon@chimie.ups-tlse.fr(J.-B.Cambon).
Sol–gelcoatingsareoneofthemostpromisingalternative pre-treatments,completelychrome-freewithotheradvantagessuchas cost-effectiveness,lowlife-cycleenvironmentalimpactandsimple applicationprocedures.Thepotentialapplicationofsol–gel coat-ingsas a corrosion protectionsystemfor metalsubstrates was highlighted15yearsagobyGuglielmi[4].Thenseveralworkshave beenundertakentomake varioussol–gelbasedprotective coat-ings,asdescribedinnumerousrecentreviews.Submicronmetal oxidefilmsinhibitcorrosionbyprovidingachemicallyinertbarrier tothediffusionofcorrosivespecies,andtheprotective character-isticsofsol–gelfilmscontainingSiO2[5],TiO2 [6],Al2O3[7],and ZrO2[8],havebeenreportedinvariousstudies.Despiteadvantages ofsol–gelprocessing,ceramiccoatingssufferfromseveral draw-backs:(a)high-temperaturetreatments(>600◦C)arerequiredto decreaseporosityandstabilizethefilms;(b)cracksoccurduring temperaturefluctuationsduetothebrittlenatureofceramicfilms andincompatibilitybetweenthethermalexpansioncoefficientsof metalsandprotectivecoatings;(c)thick(>1mm)crack-freesol–gel coatingsaredifficulttoobtain.Theselimitationscanbeavoidedby thedevelopmentofhybridorganic–inorganicprotectivecoatings, whichcombinethehardness,scratchresistanceandthermal sta-bilityoftheceramiccomponentwiththeflexibility,transparency andtunableadhesionoftheorganicsubstances[9–12].
Table1
ChemicalcompositionofCX13VDstainlesssteel(Febalance).
Element C Cr Ni Mo Va
mol% 0.12 11.5 2.50 1.60 0.30
Fig.1. Semi-developedformulaofGPTMS(glycidoxyproil-trimethoxysilane)(a) andAl(OsBu)
3(aluminumsec-butoxide)(b).
Corrosioninhibitorsareincorporatedintothecoatinginorderto improveitscorrosionresistance.Rareearthelementsand particu-larlyceriumareamongthosecommonlyusedforthatpurposeand ceriumhasbeenfoundasanefficientcathodicinhibitor[13–16]. Theobjectiveofthisworkwastostudytheinfluenceofcerium con-centrationwithinasol–gelhybridcoatingappliedonmartensitic stainlesssteelanddevelopedfromthesystem: glycidoxypropyl-trimethoxysilane(GPTMS)andaluminumsec-butoxideAl(OsBu)
3. Aninvestigationofthesolformulationandchemicalmechanisms wasmade,kineticofhydrolysisandcondensationofalkoxysilanes hasbeenfollowedwithdifferentceriumconcentrationsbyliquid 29SiNMRspectroscopytoidentifysol–gelmechanisms. Hydropho-bic and adhesion properties of different coatingswere studied respectivelybycontactanglemeasurementand“nanoscratchtest” devicetoverifyceriuminfluence.Finally,theanti-corrosion prop-ertiesofceriumdopedorundopedhybridorganic–inorganicwere investigatedbyelectrochemicalconventionaltechniquesandan optimalceriumconcentrationwasfound:0.01Mandacorrelation with29SiNMRresultshasbeentakeninevidence.
2. Experimentalprocedure
2.1. Material
Metallicsubstrates,CX13VDmartensiticstainlesssteelSS (com-positiongiveninTable1)50mm×25mm×1mmsizessamples are cleaned and pre-treated using several steps, after alkaline degreasing,chemicalcleaningcorrespondsto:1minimmersionin fluonitricacid(pH=1)maintainedat60◦C.Sampleisfinallywashed inethanolanddriedinair.
2.2. Solandcoatingpreparation
Solsfromwhich coatingswereprocessed, werepreparedby mixingglycidoxypropyl-trimethoxysilane(GPTMS)andaluminum
tri-sec-butoxideAl(OsBu)
3(formulasinFig.1)anddistilledwater andpropanolinmolarratio5:1:1:10.ThenCe(NO3)3·6H2Owas addedto reach0.001,0.005,0.01,0.05 and 0.1M Ce3+.The sol wasstirredduring2hatroomtemperatureandmaturedfor24h beforedepositiononthesubstratetoinitiatethesol–gelreactions. Theycouldschematicallybedividedintotwosteps,thefirstisa hydrolysisreaction(1)whichaimstopromotereactivehydroxyl groupsreactiveM-OH.Thesecondstepisthecondensation reac-tioninvolvingthealkoxide(2)oronlythehydrolyzedspecies(3).
M(OR)n+nH2O→ M(OH)n+nROH
M(OH)n+M(OR)n→(HO)n−1M-O-M(OR)n−1+ROH M(OH)n→(HO)n−1M-O-M(OH)n−1+H2O
Thesol–gelfilmswereobtainedbydip-coatingprocedure,using animmersion followedby a withdrawalat a controlledrateof 20cmmin−1.Thiswithdrawalspeedhasbeenchosenintherange [1–50cmmin−1] becauseitcorrespondstothemoreconvenient coveringintermofthickness(LandauandLevichlaw)and homo-geneityofthefilm.Afterdeposition,coatedsamplesweredriedin anovenat100◦Cduring24htocompletepolymerizationofthe hybridfilm.
2.3. Characterization
Theviscosityofeachsolwasdeterminedbyaviscometer Rheo-matRM100of typeTaylor–Couette flowat ashear in therange [600–966s−1].
Kineticsofhydrolysisandcondensationofalkoxysilaneswere studiedbyliquid29SiNMRspectroscopy.Themeasurementswere performedonaBrucker400MHzspectrometer.NMRspectrawere recordedwithbenzenelikeinternalchemicalshiftreference.The chemicalshiftsweregiveninppmrelativetotheinternalreference. MacrostructuralpropertiesofcoatingswereevaluatedbySEM microscopy,observationswereperformedonsampleswithaJEOL JSM-6510LVapparatus.
Thickness measurements were performed with a fisher dualscopeFMP20,thistechniqueiscommonlyusedforthe deter-minationofcoatingthicknessessuchaspaints.
Coatingroughnessisevaluatedbywhite-lightinterferometry (ZygoInstruments).
Contactanglemeasurementswereperformedwithwaterdrop methodusingcamera.Thentheimagewasprocessedbyasoftware package(windrop).
ScratchtestswereperformedusingaNanoScratchTesterCSM withadiamondindenterwithconicalgeometry.Thescratchtest wascarriedoutwithprogressiveloadingfrom0to60mN.After thescratchtest,thechangesinthemechanismofscratchandthe criticalloadswerevisualizedbyopticalmicroscopy.
The electrochemical behavior of coating was evaluated by electrochemical impedance spectrometry (EIS) in NaCl (0.1M)+Na2SO4(0.04M)solution.AKClsatelectrode,connectedto theworkingsolution,wasusedasreferenceelectrodeanda plat-inumtapeasauxiliaryelectrode.Allelectrochemicalexperiments wereperformedafterstabilization(1h)offreeopencircuit poten-tial.Electrochemicalimpedancespectra(EIS)rangingfrom65kHz to10mHzwerecarriedwitha80mVsinusoidalperturbation sig-naltothecorrosionpotentialwithSolartronInstrumentSI1286 ElectrochemicalInterfacepotentiostatandaSolartronInstrument SI1260Impedance/Gain-Phase-analyser.Alltheelectrochemical experimentswerecarriedouttoroomtemperatureand theEIS diagramswererecordedforimmersiontimesupto24h.
3. Resultsanddiscussion
Thescanningelectronmicrographsofsubstratebeforeandafter cleaningareshowninFig.2anditwasrevealedaftersurface prepa-rationofmetallicsubstrate,themartensiticmicrostructurewith thepresenceoflamellas.
Theviscosityofasolundergoinghydrolysisand polyconden-sationisstronglydependentintimeandisrelatedtothesizeof particles.Thelargertheformedmolecules,thehighertheviscosity [17].Fig.3showstheeffectofceriumconcentrationonthe viscos-ityofthesolagedfor24h(justbeforedeposition).Anincreaseof viscositycanbeobservedwiththeincreasingofcerium concen-tration.Thisisassociatedwiththehighhygroscopiccharacterof ceriumwhichabsorbsthelargeamountofwatertrappedinthesol network[18].
Fig.2. SEMimagesofsurfacemorphologiesofX13VDstainlesssteelbefore(a)andafter(b)cleaning.
Fig.3.Viscosityofsolswithdifferentconcentrationsofcerium.
Forabetterunderstandingofsol–gelmechanismsintothesols theworkwasonthebondinvolvedinthereactionsversuscerium concentration.
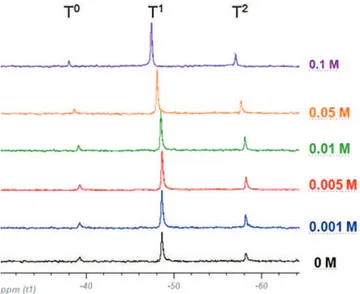
Liquid29SiNMRspectroscopywasusedtoinvestigatethe chem-icalstructureoftheinorganicnetworkjustafterthesynthesisof sols(0h)andafter(24h).Duringhydrolysisandcondensation reac-tions,thesiliconenvironmentchangesfromSi ORtoSi OHthento Si O Si.Then,eachcondensationreaction,withtheformationofa Si O Sioxobridgeinducesachemicalshiftof8–10ppminthehigh field[19].29SiNMRwascarriedouttofollowtheinorganicphase polymerizationofeachsol.The29Sipresentstheresonancesignals at−39,−49,−59and−67ppm,denotedT0,T1,T2andT3 respec-tively[20,21].TheTspeciescorrespondtothesilanescontaining0, 1,2or3hydrolysedgroupsasshowninFig.4.
Fig.5showsthe29SiNMR ofdifferentsampleswithvarious cerium concentrations atthe initialstate(0h). Atthis reaction stage,T0,T1 andT2,werethepresentspeciesinallsamples,no studiedsamplesshowedanysignalofT3.Eachpeakwhich corre-spondedtoT0,T1 and T2 respectivelyhadthesameareaforall samples,whichmeansthattheconcentrationofceriumhad no realinfluenceonthecondensationofsol–gelnetworkand each speciewaspresentinthesameconcentration.Howeveraslight
Fig.5. Liquid29SiNMRspectraofsolwithdifferentceriumconcentrations(0h).
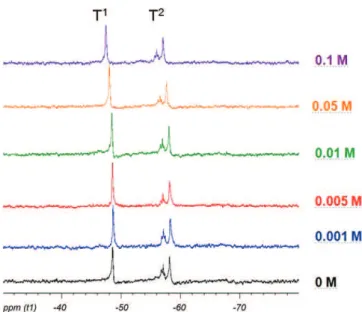
displacementofsignalsinthelowfieldwasobservedforsolswith higherceriumconcentration(0.05and0.1M).Fig.6showsthe29Si NMRofsamesamplesafterasol–gelreactionat24hjustbeforethe depositionstep.Nostudiedsampleshowedsignalofthefree tri-alkoxysilaneprecursorT0andpeakareacorrespondingtospecieT2 increasedforallsamples.Itmeansthatthecondensationincreased inthesamewayforthesolswithdifferentceriumconcentrations. Thisphenomenonisstillobservedforsolswhichcontainedcerium concentrationof0.05and0.1M.Forexample,thefocusontheshift ofT2specie(Fig.7),thiswasclearlyobservedforcerium concentra-tionhigherthan0.01M.Furthermore,onthiszoom,thepresence ofsatellitepeakscanbeunderlined.TheyalsocorrespondtoT2 bondsbutfordifferentspeciesinthesecondsphereof coordina-tion.Inallcases(meanpeakandsatellitepeaks)theshiftfrom0.01 to0.1ispreserved.Intheliterature[22,23],forothersilanebased matrixwithTEOSitcanbeduetotheelectronegativeeffectofCe(III) whichcanreducethelocaldiamagneticshieldinginthevicinityof
Fig.6. Liquid29SiNMRspectraofsolwithdifferentceriumconcentrations(24h).
Fig.7.Liquid29SiNMRspectraofsolwithdifferentconcentrationsofcerium(24h)
focuson10mm.
theattachedprotons.Ceriumhadnoinfluenceoncondensationof networksol–gelbutalargequantityofthisdopant(>0.01M)can changethechemicalstructureofsol–gelnetwork.
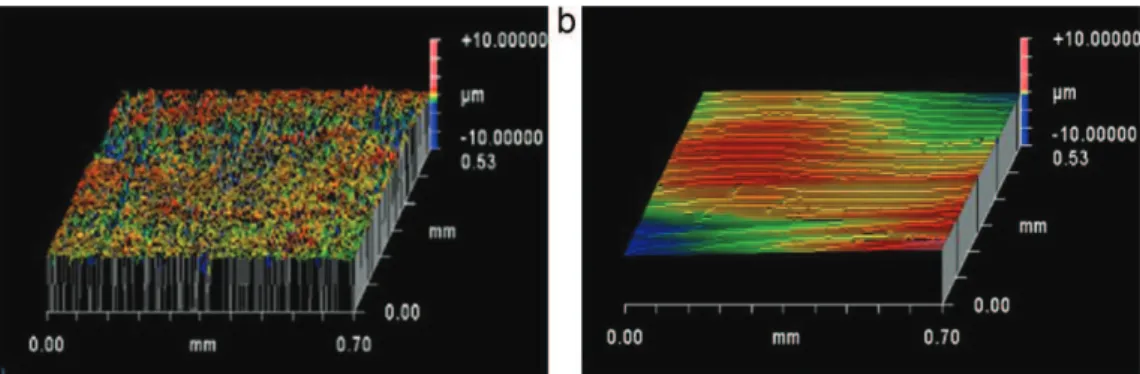
Nowitisinterestingtocharacterizethecoatingselaboratedby dip-coatingwiththesolsstudiedpreviously.Fig.8presentsaSEM micrographofasurfaceandcrosssectionofsol–gelcoating with-outceriumdepositedX13VDsubstrate.Atthisscale,thecoatingis homogeneous,covering,crack-freeandadherenttothesubstrate. Theaveragethicknessofcoating,estimatedbythecrosssection analysis(Fig.8b)isabout5–6mm.Thelevelingcharacterofthis filmcanbeunderlinedbywhitelightinterferometry(Fig.9)andthe surfaceroughnessRadecreases(0.42→0.17mm).
Thickness measurements performed by fisher dualscope for hybrid coatings with different cerium concentrations and are reportedinTable2.Thevaluesandtheiraccuracycannotshowa realinfluenceonthelayerthicknessalthoughtheceriumpresence hasincreasedthesolviscosity,parameterdirectlycorrelatedtothe layerthickness[24].
Thehydrophobicitywasevaluatedfromcontactangle measure-mentofawaterdroponhybridcoating.Theobtainedvaluesversus theceriumconcentrationaregiveninFig.10.Thisgraphshowsa slighttendencyofthecontactangleincreasewiththeincreaseof ceriumconcentration,from75◦foracoatingwithoutceriumuntil about80◦forcoatingswithhigherceriumconcentration.Thisvalue underlinesthehydrophobiccharacterofcoatingandimprovesthe tightnesstoward waterwhichisanimportantcharacteristic for anticorrosionproperties.
Anticorrosionpropertiesdependonintrinsicpropertiesofthe coatingsbutalsoonbondsestablishedwiththesubstrateandas a consequenceontheadhesion.To investigatetheinfluence of ceriumonthecoatingsadhesiontostainlesssteelsubstratesscratch testswithincreasingloadwereperformedonthevarious sam-ples.Differentcriticalloads,indicatingchangesinthemechanism ofscratchweredefined[25–27]asitcanbeseeninFig.11: - CL0:Plasticdeformation
-CL1:Brittlefracture -CL2:Delamination
Thedifferentcriticalloadvalues(mN)ofthehybridsol–gel coat-ingwhichcontaindifferentinhibitorconcentrationswereshowed inFig.12.Itwasobservedthatthisconcentrationhadno significa-tiveinfluenceontheadhesionpropertiesofcoatings.Thisgraph showsthatforallceriumconcentrationsinthecoating,thetwo criticalloadsCL0,CL1stayedalmostunchangedconsideringthe accuracyofthemeasurementsbecausethecurveswhich repre-senteachcriticalloadarequitestable.ForthecriticalloadCL2,a slightdecreaseofthisloadcanbeunderlined,probablyduetoa lowerdelaminationresistanceforhighceriumcontent.Thentotry
Fig.9.3Dtopographicanalysisofthesurfacesof(a)X13VDstainlesssteeland(b)hybridcoatingonX13VD,fromawhitelightinterferometer.
Table2
Hybridcoatingthicknessobtainedfordifferentconcentrationsofcerium.
Ceriumconcentration(M) 0 0.001 0.005 0.01 0.05 0.1
Thickness(mm) 5.73±0.6 5.35±0.7 6.01±0.8 5.61±0.5 5.77±0.7 5.89±0.9
Fig.10.ContactanglevaluesofawaterdroponhybridcoatingonX13VDwith differentceriumconcentrations.
tounderstandwhyceriumcontentplaysaroleonsolproperties, itwasplannedtodeterminetheceriumcontentinfluenceonthe corrosionresistanceofcoatings.
Theelectrochemicalimpedancespectroscopy(EIS)isoneofthe mostcommonlytechniqueusedforinvestigationandpredictionof theanticorrosionprotection[28,29].Thispowerfulmethodallows notonlyacomparisonbetweenperformancesofdifferentsystems butalsocangivekeyinformationonkineticsmechanismsofthe coatingdamageandthecorrosionactivityduringimmersioninthe corrosionmedia.InthisworkEISwasusedtoinvestigatetheeffect ofcerium concentrationin solonthecorrosionbehaviorofthe
0 10 20 30 40 50 60 0,12 0,1 0,08 0,06 0,04 0,02 0 Ce(NO3)3 (M) Load (mN) CL 0 CL1 CL2
Fig.12.CriticalloadsforhybridcoatingonX13VDstainlesssteelwithdifferent ceriumconcentrations.
X13VDsubstratescoatedincorrosivesolution(NaCl0.1M+Na2SO4 0.04M).EISdiagramswithX13VDstainlesssteelandhybridcoated sampleswereobtainedafterstabilizationofthecorrosionpotential andfordifferentimmersiontimes.
Fig.13showstheEISplotsinBoderepresentationobtainedfrom thesol–gel hybridcoating withoutceriumcompared toX13VD substrateafter respectively1hand 24himmersion. ForX13VD substrate,theelectrochemicalimpedancediagramsobtainedafter differentimmersiontimesarecharacterizedbytwotimeconstants
Fig.13.Bodephase(a)andmodulusimpedance(b)plotsforX13VDstainlesssteeluncoatedandhybridcoatingwithoutceriumonX13VDstainlesssteelinNaCl (0.1M)+Na2SO4(0.04M)1hand24himmersion.
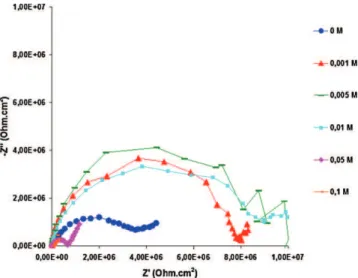
Fig.14.NyquistplotsforhybridcoatingonX13VDstainlesswithdifferent concen-trationsofceriuminNaCl(0.1M)+Na2SO4(0.04M)1himmersion.
(Fig.13a).Thefirsttimeconstant[0–1000Hz]isindependentofthe immersiontimeandthesecond[0.01–1Hz]oneisbetterdefined forincreasingimmersiontimes.Accordingtoliterature[30,31]if thebarsteelisconsidered,thetimeconstantathighfrequency isattributedtochargetransferprocessandthetimeconstantat thelowfrequencyiscorrelated withtheredoxprocessestaking placeintothepassivefilm.Theincrease ofimpedancemodulus in thelow frequency range (Fig.13b)when immersion time is longercanbeinterpretedbytheimprovementoftheprotection ofthepassivefilm.Forthehybridcoatingdepositedonthe sub-strate(Fig.14a)after1himmersion,twotimeconstantscanbethen observed,oneforthehighfrequencyarea(HF)[1000–65000Hz] whichcanbeattributedtothehybridcoatingproperties:barrier effectwhichlimitschargetransferprocessandanothertime con-stantatmediumfrequency(MF)[1–1000Hz]whereaninflexionof thecurveisobservedwhichcanbeassociatedtothehybridfilm permeability.On hybridcoatingafter24himmersion (10mHz), theappearanceofathirdtimeconstantwasobservedatlow fre-quency(LF).Itisprobablyduetotheformationofapassivation layerattheinterfacebetweenthehybridcoatingandthesubstrate. Thislayer can resultfrom the reactionbetween stainless steel andtheelectrolyte,whichdiffusesintothecoating.Itisthesame phenomenonthatforuncoatedsubstratebuthoweverthis contri-butionremainedrelativelylowduetothehybridbarriereffect.The
highmodulusimpedance(Fig.14b)ofcoatedstainlesssteelslightly decreasedafter24hofimmersionandindicatesthebeginningof thefilmdamage;howevertheperformancesarestillgreatly supe-riortotheseofthepassivatedsubstrate,sothisprovesthegood barriereffectofthesol–gelcoating.
Fig.14showsEISmeasurementswithNyquistplotforhybrid sol–gelcoatingscontainingdifferentconcentrationsofceriumafter 1himmersion.Whenceriumwasaddedincoatingto0.01M,itwas clearlyobservedanincreaseofthecapacitiveloop,itmeansthatthe barriereffectwasimprovedwithlowceriumcontentintherange [0–0.01M].Whenceriumcontentishigherthan0.01M,another behaviorwastakeninevidence:animportantdropofthebarrier effectwithadecreaseofcapacitiveloops,butthistimeitisnot duetoadecreaseofthecoatingthicknessbecausethesolswith ceriumcontentof0.05and0.1Mhaveaviscosityhigher(Fig.3). Thedeteriorationofbarrierpropertiesofthefilmcanbeattributed tothemodificationin thehybridfilmsnetworkby theCeions incorporation,asitwasmentionedbeforebyotherothers[32].
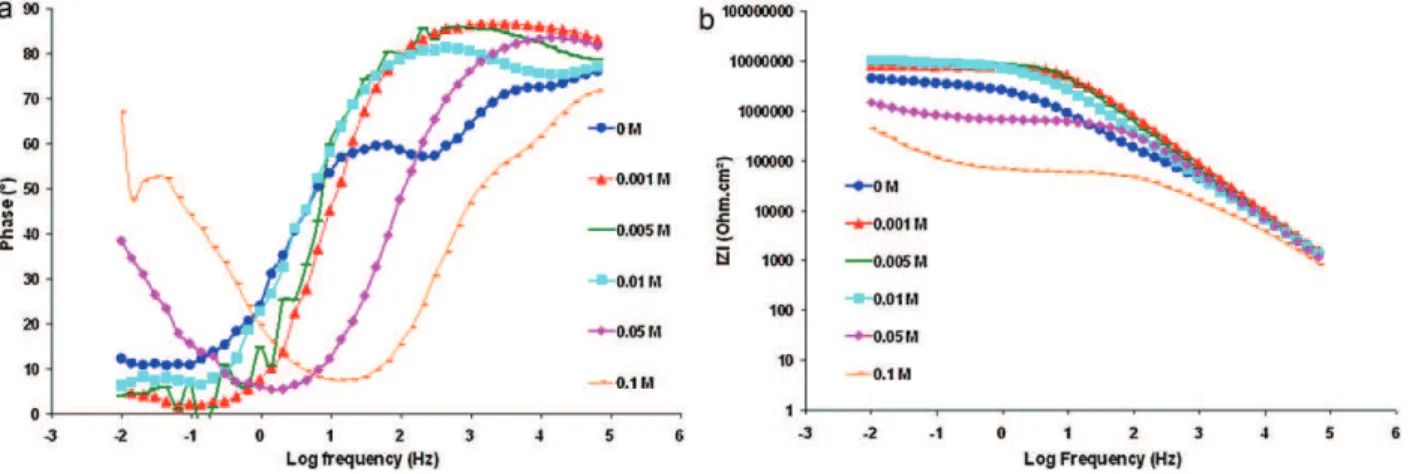
Fig.15 shows EISmeasurements in theBode representation (phaseandmodulus)inordertocomparecorrosionperformance forhybridsol–gelcoatingscontainingdifferentcerium concentra-tions after1himmersion.Whenceriumwasadded insol until aconcentrationreaching0.01M,itwasclearlyobserved onthe curves (Fig. 15a), an improvement of the barrier effect and a decreaseofthepermeabilityofhybridcoatingbecausethe inflec-tionofthecurveatmediumfrequency(MF)wasshiftedtolower frequency.Forhigherceriumconcentrations(0.05and0.1M),the corrosionperformancesofthesecoatingsclearlycollapsedandthe inflectionof thecurveatMFwasshiftedtohigher frequencies. These coatings are more permeable and the electrolyte pene-tratesthecoatings moreeasilyto reactwiththesubstrate and toformthepassivationlayerbecausetheconstanttime at low frequency,characteristicofthispassivationlayer,ismore significa-tive.Theimpedancemodulus(Fig.15b)increasesforlowcerium content(<0.01M)and sharplydecreasesforthesesame cerium concentrationsin thesol–gel coating.Fig.16 shows impedance modulusatlowfrequency(10MHz)versusceriumconcentration. Thismodulusischaracteristicofcorrosionprotectionofthewhole system:hybridcoatingand reactivitywiththesubstrate.Itwas alsoobservedthatthismodulusincreasedwhencerium concen-trationreachedavaluebetween0.005and0.01M.Then,forhigher inhibitorconcentrations, themodulus highlydecreased andthe coatingbecamelessprotectorthancoatingwithoutcerium.The sameremark maybemade for coatingwithdifferent inhibitor concentrationsafteranimmersionof24hinthecorrosive solu-tion. Bode representation withphase (Fig. 17) and impedance
Fig.15.Bodephase(a)andimpedancemodulus(b)plotsforhybridcoatingonX13VDstainlesswithdifferentconcentrationsofceriuminNaCl(0.1M)+Na2SO4(0.04M)1h
immersion.
Fig.16.Impedancemodulus(f=10mHz)withdifferentconcentrationsofceriumin NaCl(0.1M)+Na2SO4(0.04M)1himmersion.
modulus(Fig.18), showsthattheoptimalcerium concentration inthecoatingcorrespondstoavalueof0.01M.
Takenintoaccounttheseresults,onekeypointisconfirmed: theexistenceofathresholdconcentrationofcerium correspond-ingto0.01M.So,itispossibletotrytocorrelatethesecorrosion resultswithchemicaldisplacementsobtainedbyliquid29SiNMR spectroscopyforsolswithahighceriumconcentration.Theseshifts correspondtotheimportantcollapseinanti-corrosionproperties ofsol–gelhybridcoating.Ceriumhadnotanyinfluenceon conden-sationnetworksilanebutalargequantityofthisdopant(>0.01M) canchangethechemicalstructureofhybridnetwork.Thereisan
Fig.18.Impedancemodulus(f=10mHz)forhybridcoatingonX13VDstainlesswith differentceriumconcentrationsinNaCl(0.1M)+Na2SO4(0.04M)24himmersion.
abilitytocreatenewconnection(Ce–Al)withaluminumfromthe ASBofthematrixwhichcoulddestabilizethehybridnetworkand alsoreducetheresistancetowardcorrosionofcoatings.Itwillthen beinterestingtostudythechemicalstructureofsol–gelhybridsat solidstatewithtechniquessuchassolid29Siand13CNMR charac-terizationandRamanspectrometryinordertobetterunderstand nowtheinfluenceofceriumdirectlyoncoatingproperties(these experimentsarenowinprogress).
Fig.17. Bodephase(a)andimpedancemodulus(b)plotsforhybridcoatingonX13VDstainlesswithdifferentceriumconcentrationsinNaCl(0.1M)+Na2SO4(0.04M)24h
4. Conclusion
The influence of cerium concentration on behavior against corrosionofhybridsol–gel coatingonmartensiticX13VD stain-lesssteelhasbeeninvestigated.Theincrease ofceriumcontent improved hydrophobic character of coating and its tightness towardwater.Electrochemicalimpedancespectroscopyshowedan optimalceriumconcentrationof0.01Minthehybridlayer.Besides, theincreaseofceriumconcentrationabove0.01Mdecreasedthe barriereffectofsol–gelcoating.Thecoatingswereperformedby scratchtests,andnosignificativeceriuminfluencehasbeenproved onthe adhesion properties of coatingsto themartensitic sub-strate;soadirectcorrelationbetweenanticorrosionandadhesion performancesofsol–gelcoatingisnotpossible.Liquid29SiNMR spectroscopywasthencarriedouttodeterminatethecerium influ-enceonsolcondensation.Itwasshownthatanexcessofcerium (>0.01M)leadtoachemicalshiftofTspeciesandcanchangethe chemicalstructure of sol–gelnetwork. Thereforea reduction of corrosionresistanceofcoatingsupwasnotedtothesamecerium concentration(0.01M).
Acknowledgments
Thiswork wascarried out in theframework of the ARCAM project,withthefinancial supportof DGCIS,Regions Aquitaine, AuvergneandMidi-Pyrénées.Theauthorsthankfullyacknowledge thepartnersoftheproject:Ratier-Figeac,Aubert&Duval,Olympus, theMechanicalandEngineeringInstituteofBordeaux,the Materi-alsDepartmentofICAMandtheInstituteCARNOTCIRIMAT. References
[1]M.R.Ryan,D.E.Williams,R.J.Chater,B.M.Hutton,D.S.McPhail,Nature415 (2002)770.
[2]I.Betova,M.Bojinov,T.Laitinen,K.Makela,P.Pohjanne,T.Saario,Corros.Sci. 44(2002)2675.
[3]ToxicologicalProfileforChromium,ATDSR/Tp-88/10,AgencyforToxic Sub-stances,USPublicService,Washington,DC,1989.
[4]M.Guglielmi,J.Sol–GelSci.Technol.8(1997)443.
[5]D.C.L.Vasconcelos,J.A.N.Carvalho,M.Mantel,W.L.Vasconcelos,J.Non-Cryst. Solids273(2000)135.
[6]A.Nazeri,P.P.TrzaskomaPaulette,D.Bauer,J.Sol–GelSci.Technol.10(1997) 317.
[7]J.Masalski,J.Gluszek,J.Zabrzeski,K.Nitsch,P.Gluszek,ThinSolidFilms349 (1999)186.
[8] F.Perdomo,P.D.Lima,M.A.Aegerter,L.A.Avaca,J.Sol–GelSci.Technol.15 (1999)87.
[9] V.H.V.Sarmento,M.G.Schiavetto,P.Hammer,A.V.Benedetti,C.S.Fugivara, P.H.Suegama,S.H.Pulcinelli,C.V.Santilli,Surf.Coat.Technol.204 (2010) 2689–3270.
[10]E.Roussi,A.Tsetsekou,D.Tsiourvas,A.Karantonis,Surf.Coat.Technol.205 (2011)3235–3244.
[11]R.T.Sakai,F.M.Di,L.daCruz,H.G.deMelo,A.V.Benedetti,C.V.Santilli,P.H. Suegama,Prog.Org.Coat.74(2012)288–301.
[12]T.P.Chou,C.Chandrasekaran,G.Z.Cao,J.Sol–GelSci.Technol.26(2003)321. [13] A.Pepe,M.Aparicio,S.Ceré,A.J.Durán,J.Non-Cryst.Solids348(2004)162–171. [14]M.A.Arenas,J.J.deDamborena,Electrochem.Acta48(2003)3693–3698. [15] F.Andreatta,L.Paussa,A.Lanzutti,N.C.RoseroNavarro,M.Aparicio,Y.Castro,
A.Duran,D.Ondratschek,L.Fedrizzi,Prog.Org.Coat.72(2011)3–14. [16]C.Motte,M.Poelman,A.Roobroeck,M.Fedel,F.Deflorian,M.-G.Olivier,Prog.
Org.Coat.74(2012)326–333.
[17]L.L.Hench,J.K.West,Chem.Rev.90(1990)33–72.
[18]X.Zhong,Q.Li,J.Hu,X.Yang,Prog.Org.Coat.69(2010)52–56.
[19]M.Feuillade,C.Croutxé-Barghorn,C.Carré,J.Non-Cryst.Solids352(2006) 34–341.
[20]L.B.Toung,W.S.Trahanovsky,J.Am.Chem.Soc.91(1969)5060. [21]F.L.S.Purgato,J.R.Romero,J.Catal.209(2000)394.
[22]D.L.Pavia,G.R.Lampman,G.S.Kriz,IntroductiontoSpectroscopy,2nded., Har-courtBrace,London,1996.
[23] S.Aubonnet,C.C.Perry,J.AlloysCompd.300–3001(2000)224–229. [24]L.D.Landau,B.G.Levich,ActaPhysiochim.U.R.S.S.17(1942)42–54. [25]A.Kupicka,Prog.Org.Coat.46(2003)32.
[26]F.Bondioli,R.Taurino,A.M.Ferrari,J.ColloidInterfaceSci.334(2009)195–201. [27] S.M.Noh,J.W.Lee,J.H.Nam,J.M.Park,H.W.Jung,Prog.Org.Coat.74(2012)
192–203.
[28]S.V.Lamaka,M.L.Zheludkevich,K.A.Yasakau,R.Serra,S.K.Poznyak,M.G.S. Ferreira,Prog.Org.Coat.58(2007)127–135.
[29] M.L.Zheludkevich,K.A.Yasakau,A.C.Bastos,O.V.Karavai,M.G.S.Ferreira, Elec-trochem.Commun.9(2007)2622–2628.
[30]L.Freire,M.J.Carmezin,M.G.S.Ferreira,M.F.Montemor,Electrochim.Acta55 (2010)6174–6181.
[31] C.M.Abreu,M.J.Cristóbal,R.Losada,X.R.Nóvoa,G.Pena,M.C.Pérez, Elec-trochim.Acta51(2006)1881–1890.
[32]C.F.Malfatti,T.L.Menezes,C.Radtke,J.Esteban,F.Ansart,J.P.Bonino,Mater. Corros.62(2011)9999.