Studies of the enzymes that convert steroid hormones
in the human adipose tissue
Thèse
Mohamed Mansour
Doctorat en biologie cellulaire et moléculaire
Philosophiæ doctor (Ph.D.)
Québec, Canada
Studies of the enzymes that convert steroid hormones
in the human adipose tissue
Thèse
Mohamed Mansour
Sous la direction de :
André Tchernof, directrice de recherche
Katherine Cianflone, codirectrice de recherche
iii
RÉSUMÉ
Les tissus adipeux ont été reconnus il y a longtemps comme des sites importants de transformation et d’action des hormones stéroïdiennes. Parmi ces hormones, les androgènes et les oestrogènes jouent un rôle important dans la régulation des fonctions du tissu adipeux comme l'accumulation de triglycérides, la lipolyse, la différenciation des préadipocytes et la prolifération cellulaire. La disponibilité de ces hormones est modulée par un groupe d’enzymes de conversion des stéroïdes qui n’ont pas été entièrement caractérisées dans les tissus adipeux humains. Objectif: Notre objectif était de caractériser les isoenzymes de la 5α-réductase de même que la 17β-hydroxystéroïd déshydrogénase (17β-HSD) de type 2 et leur association avec les mesures anthropométriques et/ou les marqueurs d'adiposité.
Méthodes: Des tissus adipeux omental et sous-cutané ont été obtenus auprès d’hommes
et/ou de femmes non-obèses et/ou obèses. L'expression des 5α-réductases et 17β-HSD type 2 ont été mesurées dans différents modèles de tissus adipeux. Des techniques d’immunohistochimie et d'imagerie confocale ont été utilisées pour localiser la 17β-HSD type 2 dans les tissus adipeux. Nous avons utilisé des inhibiteurs spécifiques à ces enzymes dans nos expériences. De plus, des cultures de cellules HEK-293 ont été utilisées pour tester les inhibiteurs des 5α-réductases. Résultats: Nous avons démontré que la dihydrotestostérone est formée principalement à partir de l'androsténedione (4-dione) et est responsable de la grande majorité de l’effet inhibiteur du 4-dione et de la testostérone sur l'adipogenèse. Les isoenzymes de la 5α-réductase jouent donc un rôle important dans la régulation de la différenciation des préadipocytes. Nos résultats indiquent également que la conversion de la testostérone et de l'estradiol en stéroïdes moins actifs tels que le 4-dione et l'estrone, respectivement, est effectuée par la 17β-HSD type 2 qui est localisée dans les vaisseaux sanguins des tissus adipeux des hommes et des femmes. Conclusion: Les 5α-réductases et la 17β-HSD type 2 modulent la disponibilité des hormones stéroïdiennes actives dans les tissus adipeux humains. Leur activité et/ou leur expression est associée aux mesures d’adiposité. Ceci supporte la notion d’un rôle possible de ces enzymes dans l'altération des dépôts graisseux via une modulation de la disponibilité des hormones stéroïdiennes actives.
iv
ABSTRACT
Adipose tissue has long been recognized as a significant site for steroid hormone transformation and action. These hormones include androgens and estrogens, which play a pivotal role in the regulation of many adipose tissue functions including triglyceride accumulation, lipolysis, preadipocyte differentiation and proliferation. The availability of these hormones is regulated through a group of steroid hormone-converting enzymes that have not been fully characterized in adipose tissue. Our objective was to characterize steroid hormone-converting enzymes 5α-reductase and 17β-hydroxysteroid dehydrogenase (17β-HSD) type 2 and their involvement in the regulation of androgen and estrogen availability in abdominal adipose tissues of men and/or women, and define their association with anthropometric measurements or other adiposity markers. Methods: Omental (OM) and subcutaneous (SC) adipose tissues were obtained from non-obese and/or obese men and/or women. The expression of 5α-reductase and 17β-HSD type 2 isoenzyme was measured in various OM and SC tissue models. Immunohistochemistry and confocal imaging techniques were used to localize 17β-HSD type 2 in adiposes tissues. We used specific enzyme inhibitors in our experiments. In addition, HEK-293 cell cultures were used to test the 5α-reductase isoenzymes inhibitors. We also measured glycerol-3-phosphate dehydrogenase (G3PDH) activity with or without 5α-reductase inhibitors to assess the extent of preadipocyte differentiation. Results: Dihydrotestosterone is formed mainly through 4-androst-4-ene-3,17-dione (4-dione) and it is responsible for the vast majority of the inhibitory effect of 4-dione and testosterone on adipogenesis. 5α-reductase isoenzymes play an important role in the regulation of preadipocyte differentiation through modulation of androgenic activity. Our results also indicated that the conversion of testosterone and estradiol into less active steroids such as 4-dione and estrone, respectively, is caused by 17β-HSD type 2, which is localized in the blood vessels of adipose tissue in both men and women. No sex difference was detected in HSD17B2 mRNA expression. However, opposite correlations were found between 17β-HSD type 2/HSD17B2 mRNA expression and/or activity with age or adiposity measurements in both sexes. Conclusion: 5α-reductases and 17β-HSD type 2 have opposite actions on the availability of active steroid hormones in human OM and SC adipose tissues. The activity and/or the expression
v
of these enzymes is associated with adiposity measurements. This supports a possible role of these enzymes in altering fat deposition through the modulation of active steroid hormone availability in adipose tissue.
vi TABLE OF CONTENTS RÉSUMÉ ... iii ABSTRACT ... iv TABLE OF CONTENTS ... vi LIST OF TABLES ... ix LIST OF FIGURES ... x
LIST OF ABBREVIATIONS ... xii
AKNOWLEDGEMENT ... xv
FOREWORD ... xvi
INTRODUCTION ... 1
1. SEX DIMORPHISM IN BODY COMPOSITION AND FAT DISTRIBUTION ... 3
2. SEX DIFFERENCES IN ADIPOSE TISSUE CELLULARITY AND LIPOLYSIS ... 5
2.1 Adipose tissue cellularity ... 5
2.2 Lipolysis ... 6
3. PATHWAYS OF ANDROGEN DYNAMICS ... 9
3.1 HORMONAL THERAPY ... 9
3.2 MAJOR STEROID-CONVERTING ENZYMES INVOLVED IN THE SYNTHESIS OR INACTIVATION OF ANDROGENS ... 18
3.2.1 5α-reductases ... 18
3.2.2 17β-hydroxysteroid dehydrogenases (17-HSDs) ... 22
3.3 ANDROGEN-GLUCOCORTICOID INTERACTION ... 26
4. PATHWAYS OF ESTROGEN DYNAMICS ... 27
4.1 MAJOR STEROID-CONVERTING ENZYMES INVOLVED IN ESTROGEN SYNTHESIS OR INACTIVATION ... 28
4.1.1 Aromatase ... 28
4.1.2 17β-HSDs ... 29
5. CLINICAL IMPLICATION OF STEROID HORMONES, THEIR CONVERTING-ENZYMES AND WEIGHT LOSS ... 32
GENERAL OBJECTIVE ... 35
HYPOTHESIS ... 35
SPECIFIC OBJECTIVES AND HYPOTHESES ... 36
CHAPTER 1 ... 37
RÉSUMÉ ... 38
vii
1. INTRODUCTION ... 40
2. SUBJECTS AND METHODS ... 41
3. RESULTS ... 46 4. DISCUSSION ... 49 5. CONCLUSION ... 51 REFERENCES ... 53 FIGURE LEGENDS ... 55 CHAPTER 2 ... 65 RÉSUMÉ ... 66 ABSTRACT ... 67 1. INTRODUCTION ... 68
2. MATERIALS AND METHODS ... 70
3. RESULTS ... 76 4. DISCUSSION ... 79 REFERENCES ... 83 FIGURE LEGENDS ... 86 FIGURES ... 88 CHAPTER 3 ... 97 RÉSUMÉ ... 98 ABSTRACT ... 99 1. INTRODUCTION ... 100
2. MATERIALS AND METHODS ... 102
3. RESULTS ... 106 4. DISCUSSION ... 108 REFERENCES ... 113 FIGURE LEGENDS ... 116 FIGURES ... 118 CHAPTER 4 ... 124 RÉSUMÉ ... 125 ABSTRACT ... 126 1. INTRODUCTION ... 127
2. MATERIALS AND METHODS ... 128
3. RESULTS ... 131
viii
REFERENCES ... 136
FIGURE LEGENDS ... 137
FIGURES ... 140
CONCLUSION ... 142
SUMMARY OF THE MAIN FINDINGS ... 142
5α-reductases ... 142
17β-HSD type 2 in men ... 143
17β-HSD type 2 in women ... 145
Sex dimorphism in adipose tissue 17β-HSD type 2 ... 147
CLINICAL SIGNIFICANCE ... 148
STRENGTHS AND LIMITATIONS ... 149
FUTURE DIRECTIONS ... 150 GENERAL CONCLUSION ... 151 APPENDICES ... 153 І- Review article ... 153 ІІ- Book chapter ... 189 REFERENCES ... 242
ix
LIST OF TABLES Introduction
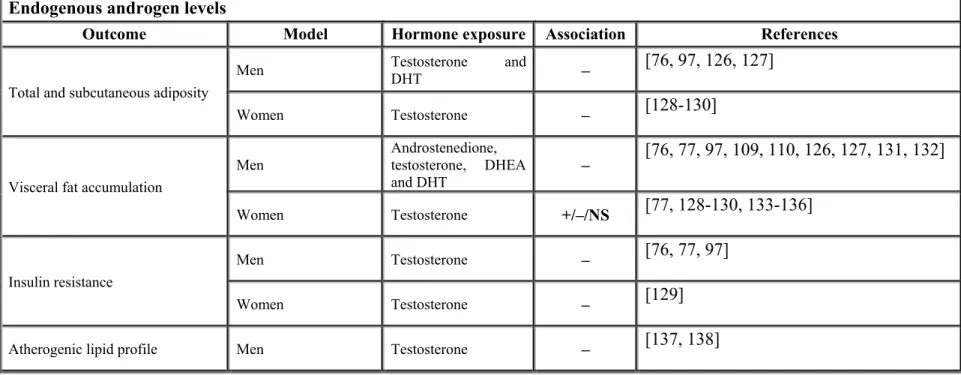
Table 1: Summary of androgen actions on fat accumulation, metabolic profile and adipose tissue metabolism ... 14
Table 2: Associations between endogenous androgen levels, fat accumulation, insulin resistance or atherogenic lipid profile ... 15
Table 3: Impact of androgens on adipose tissue LPL activity, catecholamine-stimulated lipolysis and adipogenesis ... 23
Table 4: Summary of estrogen actions on adipose tissue metabolism or adipocyte
proliferation and differentiation ... 33
Chapter 1
Table 1: Vector, subcloning sites, GenBank accession numbers and primer sequences used to generate HEK-293 cells stably expressing each 5α-reductase isoenzyme ... 57
Table 2: GenBank accession numbers, amplicon sizes and primer sequences used for
expression measurement in the present study ... 58
Chapter 4
Table 1: Characteristics of the samples of men and women . Mean ± SD are shown. ... 138
Table 2: Pearson correlations between 17β-HSD type 2 activity/mRNA and age or
anthropometric measurements. ... 139
Appendices ІІ-Book chapter
Table 1: Summary of androgen actions on fat accumulation, metabolic profile and adipose tissue metabolism ... 209
Table 2: Summary of estrogen actions on adipose tissue metabolism ... 213
Table 3: Summary of glucocorticoid actions on fat accumulation, metabolic profile and adipose tissue metabolism ... 214
x
LIST OF FIGURES Introduction
Figure 1: Axial computed tomography image obtained at the L4-L5 vertebrae level ... 2
Figure 2: pathways of andrgen and estrogen secretion and transformation in men ... 8
Figure 3: Androgen actions in various tissues of males ... 10
Figure 4: Summary of steroid hormones effects on adipose tissue function and metabolism ... 11
Figure 5: Effects of hyperandrogenemia in females ... 12
Figure 6: Pathways of steroid hormone metabolism in adipose tissue ... 18
Figure 7: Messenger RNA expression of 5α-reductase isoenzymes in various body tissues ... 21 Chapter 1 Figure 1 ... 59 Figure 2 ... 60 Figure 3 ... 61 Figure 4 ... 62 Figure 5 ... 63 Figure 6 ... 64 Chapter 2 Figure 1 ... 88 Figure 2 ... 89 Figure 3 ... 90 Figure 4 ... 91 Figure 5 ... 92 Figure 6 ... 93 Figure 7 ... 94 Figure 8 ... 95 Figure 9 ... 96 Chapter 3 Fig. 1 ... 118
xi Fig. 2 ... 119 Fig. 3 ... 120 Fig. 4 ... 121 Fig. 5 ... 122 Fig. 6 ... 123 Chapter 4 Figure 1 ... 140 Figure 2 ... 141 Appendices Review article Figure 1: ... 187 Figure 2: ... 188 Book chapter Figure 1 ... 240 Figure 2 ... 241
xii LIST OF ABBREVIATIONS 17β-HSD: 17beta-hydroxysteroid dehydrogenase 3D: Three dimensions 3β-HSD: 3beta-hydroxylsteroid dehydrogenase 4-dione: androstenedione 4-MA: 17-N,N-diethylcarbamoyl-4-methyl-4-aza-5-androstan-3-one A-dione: androstanedione
ADT: androgen deprivation therapy AKR1C: aldo-ketoreductase 1 C AR: androgen receptor
ASP: acylation stimulating protein BMI: body mass index
CD31: cluster of differentiation 31
CYP11A1 (P450SCC): side-chain cleavage enzyme CYP11B1: 11β-hydroxylase
CYP11B2: aldosterone synthase CYP21B: 21-hydroxylase CYP7B1: 7α-hydroxylase
DEXA: dual-energy x-ray absorptiometry DHEA: dehydroepiandrosterone
DHEA-S: dehydroepiandrosterone sulphate DHT: dihydrotestosterone
DMEM: Dulbecco’s Modified Eagle Medium E1: estrone
E2: estradiol
EST: estrone sulfotransferase FFA: free fatty acids
FM: total body fat mass
G3PDH: glycerol-3-phosphate dehydrogenase GAPDH: glyceraldhyde 3-phosphate dehydrogenase HAMEC: human adipose microvascular endothelial cells HDL: high-density lipoprotein
HEK-293: human embryonic kidney 293 cells HSD17B: 17β-hydroxysteroid dehydrogenase HSD3B: 3β-hydroxysteroid dehydrogenase IL: interleukin
LPL: lipoprotein lipase
mRNA: messenger ribonucleic acid NAD: nicotinamide dinucleotide
OATP: transporter from the organic anion transport polypeptide family OM: omental
p450Aro (Cyp19A1): aromatase P450c17 (CYP17): 17α-hydroxylase PAI1: plasminogen activator inhibitor-1 PCOS: poly cystic ovary syndrome
xiii
PPARγ: peroxisome proliferative factor gamma RU486: mifepristone
SC: subcutaneous
SRD5A: steroid 5α-reductase ST: sulfotransferase
StAR: steroidogenic acute regulatory protein STS: steroid sulfatase
Testo.: testosterone THF: tetrahydrocortisol
TLC: thin layer chromatography TNF-α: tumor necrosis factor-alpha UDP: uridine dinucleotide phosphate WAT: white adipose tissue
WC: waist circumference α: alpha
xiv
xv
AKNOWLEDGEMENT
First of all, I would like to thank Allah who gave me the health and power to complete my PhD thesis. I would like also to thank my research director, Dr. André Tchernof. Personally, Dr. Tchernof is kind and always a gentleman. During my thesis, Dr. Tchernof helped me countless times and in all aspects of my research. I learned from him the ways of scientific research and scientific thinking. I would also like to thank my research co-director, Dr. Katherine Cianflone who enabled me to come to study at Laval University. I also cannot forget to thank the Egyptian ministry of higher education for a mission to undergo PhD training in Laval University. I would like also to express sincere gratitude to my colleges in the lab of Dr. Andre Tchernof, especially Melissa Pelletier, who helped me in everything I did in the laboratory throughout my thesis work. I would like to thank my professors and my colleges in the department of Biochemistry, Faculty of Veterinary Medicine, Zagazig University, who allowed me to come for studies at Laval University. I would like to thank all our collaborators in the Endocrinology and Nephrology axis at CHU de Québec Research Center and at the Québec Heart and Lung Institute Research Center. Their invaluable help allowed us to have access to the biological material needed for our studies. This included help with participants, surgeons, nurses, radiological technicians and gene expression platform professionals.
In closing it also a great chance to thank my mother, father, wife and sisters as well as mother, father, brother of my wife who always made their best efforts to help me psychologically through all the difficulties encountered during my thesis. I also want to thank my lovely children, Youseif and Omar.
xvi
FOREWORD
This thesis includes studies of steroid hormone converting-enzymes mainly 5α-reductases and 17β-HSD type 2, in human adipose tissue. The introduction of this thesis was based on my two publications which are included in the appendix of this thesis.
1. Tchernof, A., Mansour, M. F., Pelletier, M., Boulet, M. M., Nadeau, M., & Luu-The, V. (2015). Updated survey of the steroid-converting enzymes in human adipose tissues. J Steroid Biochem Mol Biol, 147, 56-69.
2. Fouad Mansour M, Chan CWJ, Laforest S, Veilleux A and Tchernof A. Sex differences in body fat distribution. Book chapter in Adipose Tissue Biology, second edition. In press.
The first chapter is a study of 5α-reductase isoenzymes in adipose tissue from men. This
study was published in Journal of Steroid Biochemistry and Molecular Biology in 2016. In this study, I contributed to experiments, data interpretation and writing of the manuscript. Melissa Pelletier contributed to the study design, experiments, analysis of the data and writing of the manuscript. The gene expression was performed by the CHU de Quebec Research Center (CHUL) Gene Expression Platform (Quebec, Canada).
The second chapter presents a study that characterized 17β-HSD type 2 in adipose tissue
from men. This paper was published in Molecular and Cellular Endocrinology in 2015. I contributed to experiments, data analysis, data interpretation and writing of the manuscript. Melisa Pelletier contributed to laboratory experiments, data analysis, data interpretation and writing of the manuscript. Johanne Ouellet performed the immunohistochemistry at CHU de Quebec Research Center (CHUL). The gene expression was performed by the CHU de Quebec Research Center (CHUL) Gene Expression Platform (Quebec, Canada). The confocal imaging materials were obtained through the Funds of the leaders program from Canada foundation for Innovation (CFI) to J.F.
The third chapter is a study of estradiol inactivation in adipose tissues of women to be
xvii
interpretation and writing of the manuscript. Melissa Pelletier contributed to the study design, experiments and writing of the manuscript. Johanne Ouellet performed the immunohistochemistry in CHU de Quebec Research Center (CHUL). Gene expression was measured by the CHU de Quebec Research Center (CHUL) Gene Expression Platform (Quebec, Canada).
The fourth chapter contains a study of the sex dimorphism in 17β-HSD type 2 expression
and activity in human adipose tissue. This paper will be submitted soon for publication as a short communication. I contributed to the study design, experiments, data analysis, data interpretation and writing of the manuscript. Melissa Pelletier contributed to the study design, experiments and writing of the manuscript. The gene expression was measured by the CHU de Quebec Research Center (CHUL) Gene Expression Platform (Quebec, Canada).
1
INTRODUCTION
Consequent to modern lifestyles which have been increasingly characterized by reduced physical activity and consumption of widely available low-cost, energy-dense foods, an obesity epidemic has emerged in many industrialized societies [1]. Yet, the individual responses to this “obesigenic environment” remain highly variable, and consequently, body fatness is extremely heterogeneous [2]. Similarly, the susceptibility to develop complications as a result of excess body weight is also highly variable among overweight and obese individuals. Some appear to be relatively protected from the development of health problems in relation to their excess body fatness, even in the obese state [3, 4]. In this regard, one of the most critical determinants of disease in overweight or obese men and women is the presence of visceral obesity, which is characterized by large, centrally located fat stores within the mesentery and greater omentum [5, 6]. Moreover, visceral obesity has now clearly emerged as one of the most prevalent manifestations of the metabolic syndrome and represents an essential feature of the current obesity epidemic [7]. One of the critical determinants of elevated cardiometabolic risk or the metabolic syndrome is adipose tissue function impairments, which include adipocyte hypertrophy, low free fatty acid uptake, reduced triglyceride synthesis, impaired adipogenesis, resistance to the inhibitory effect of insulin on lipolysis, immune cell infiltration and inflammatory cytokine secretion [8].
Human body composition is sexually dimorphic as women are often characterized by higher body fat percentages compared to men, who proportionally have higher muscle and bone masses [9-12]. The distribution of body fat is also sexually dimorphic in humans [13]. Men usually have an android body fat distribution pattern, with adipose tissue accumulation in the abdominal region and women often display a body fat distribution pattern described as gynoid with more fat deposition in the gluteal and femoral regions. The abdominal cavity contains subcutaneous and visceral adipose tissues (Figure 1) (reviewed in [14]). The fat located inside the abdominal cavity, termed intra-abdominal or visceral fat, includes omental, mesenteric and retroperitoneal fat [15]. Compared to subcutaneous fat, visceral fat is more vascular, cellular, innervated and contains a large number of inflammatory and
2
Subcutaneous adipose tissue Visceral adipose tissue
Visceral adipose tissue
Subcutaneous adipose tissue
immune cells (reviewed in [14]). Moreover, visceral adipose tissue has lower preadipocyte differentiation capacity and higher percentage of large adipocytes (reviewed in [14]). Visceral adipose tissue also contains higher levels of glucocorticoids and androgen receptors than the subcutaneous depot (reviewed in [14]).
Figure 1: Axial computed tomography image obtained at the L4-L5 vertebrae level.
Regions of interest delineate visceral and subcutaneous abdominal adipose tissues in two patients with identical body fat mass but either low visceral fat accumulation (patient in panels A and C) or high visceral fat accumulation (patient in panels B and D). Figures C and D show highlighted visceral adipose tissue. Adapted from [16].
Visceral fat is significantly more abundant in men compared to women at every body size [8]. Within a given sex however, large interindividual variations are found in the amount of visceral fat: approximately 10-fold in samples of lean to moderately obese Caucasian men and women [13]. As mentioned, large accumulation of visceral adipose tissue is a critical determinant of obesity-related metabolic alterations which are known to increase the risk of type 2 diabetes and cardiovascular disease [17, 18]. Moreover, the presence of excess
3
visceral adipose tissue better predicts mortality than subcutaneous adipose tissue (reviewed in [14]).
Regarding the various subcutaneous adipose tissue depots, in vivo studies reported positive associations between formation of adipocytes with that of preadipocytes in the abdominal and femoral depots. However, preadipocytes and adipocytes have higher formation rates in the subcutaneous femoral depot compared to subcutaneous abdominal fat [19]. Furthermore, 284 genes were differently expressed between human abdominal and gluteal subcutaneous depots either specifically in males or females or in both sexes [20]. These results suggest distinct developmental signatures of these subcutaneous depots [20]. Regarding adipocytokines, the interstitial concentration of leptin was significantly higher in the subcutaneous femoral depot compared to the subcutaneous abdominal depot. However, no significant differences were detected between these depots in the interstitial concentration of adiponectin, resistin, monocyte chemoattractant protein-1, IL-6 and IL-8 [21]. Moreover, no significant regional difference in adipose tissue blood flow was detected between the subcutaneous femoral depot and the subcutaneous abdominal depot [21].
1. SEX DIMORPHISM IN BODY COMPOSITION AND FAT DISTRIBUTION
Over the course of childhood, weight gain is slightly higher in boys than girls. Boys weigh slightly more before puberty, although in both sexes, lean mass appears to be relatively similar. Total body fat mass is also comparable between boys and girls before the age of 7. After adrenarche, girls accumulate fat mass more rapidly and eventually reach slightly higher levels than that of boys [10, 22, 23]. Hence, differences in body composition can be detected. However, these differences remain relatively small in magnitude before puberty. Between ages 10 to 20 years, boys accumulate approximately twice the amount of lean mass compared to girls (33 kg and 16 kg, respectively) [12]. On the other hand, total fat mass increases proportionately more in girls [12]. As a result, adult women have significantly higher levels of fat mass and relatively lower lean mass compared to men [10, 11]. Despite minor variation prior to puberty, studies reported that pre-puberty females have a smaller waist compared to pre-puberty males [23]. In healthy subjects, average percentage of body fat mass values range 10-15% for men and 20-30% in women, although
4
values can obviously reach higher levels in various populations [10, 12, 24]. Women with aging tend to have a slightly higher propensity to gain fat mass than men [10]. This may be attributable to hormonal changes at the menopause, although the impact of these changes remains controversial and difficult to demonstrate consistently [25-27]. Available studies rather show that the impact of menopause may manifest more specifically on accumulation of abdominal fat [26-29].
The sex dimorphism in distribution of body fat becomes apparent at puberty [24]. The amount of fat that accumulates in the abdominal region can be estimated using imaging techniques such as magnetic resonance imaging or computed tomography [30-32]. These studies reported that despite having higher percent body fat mass than men, women generally have significantly lower accumulation of visceral adipose tissue. The data from the Quebec Family Study [33] and the Heritage Family Study [34] allowed us to examine this sex dimorphism in the adult Caucasian population. For example, in the Quebec Family Study, a subsample of 203 men and 219 women that were on average 40 years old, the sex dimorphism in body composition was readily apparent with 23% in men vs 32% fat in women. On the other hand, body fat-free mass was 61 kg in men vs. 46 kg in women. Despite such apparent differences, men had a 37% higher visceral adipose tissue area compared to women. However, abdominal subcutaneous adipose tissue area was 50% higher in women [33]. Very similar differences can be detected in other Caucasian populations [34, 35].
Other ethnicities also generally exhibit this pattern of sex differences, although there are marked ethnicity-related disparities in total adiposity and the propensity to store visceral fat. In African American individuals [36], a lower proportion of visceral fat is detected for any given total body fat mass value, suggesting a reduced susceptibility to visceral obesity in this population. The opposite is true for other ethnic groups such as the South East Asians and Canadian Aboriginals [35, 37, 38]. In an analysis of available literature on computed tomography studies in various ethnic subgroups [8], we demonstrated that for any given adiposity level, the ratio of visceral-to-subcutaneous adipose tissue areas (reflecting the relative amount of visceral fat) was higher in Asian and Caucasian compared
5
to African American populations. The tendency of some populations to preferentially accumulate adipose tissue in the visceral region may have an impact on their susceptibility for type 2 diabetes and cardiovascular diseases.
A most striking feature is the very large inter-individual variability in visceral adipose tissue area in both sexes. Despite a generally lower visceral adipose tissue accumulation in women, relatively important and physiologically significant accumulations of visceral fat can still be observed in this sex, even in the normal BMI range. As mentioned, persuasive evidence is now available supporting the notion that not only in men but also in women, abdominal, visceral obesity is closely associated with a cluster of metabolic abnormalities including insulin resistance, dyslipidemia as well as a chronic, low-grade inflammatory state [7, 39-41]. Hypertriglyceridemia and low HDL cholesterol are two major abnormalities of the blood lipid profile associated with visceral adiposity. Such dyslipidemic state associated with visceral obesity is an important risk factor in the pathophysiology of cardiovascular diseases (reviewed in [8]).
2. SEX DIFFERENCES IN ADIPOSE TISSUE CELLULARITY AND LIPOLYSIS
2.1 Adipose tissue cellularity
The absolute number of adipocytes and their sizes are distinctly related to obesity in adipose tissues of women and men. More adipocytes are found in the lower-body adipose tissue compartments (i.e. gluteal and femoral) of obese than lean women [42]. In non-obese men, lower-body fat responds to overfeeding by adipocyte hyperplasia [43]. However in obese men, lower-body adipocytes have been shown to be larger, but there is no report of adipocyte hyperplasia in obese men compared to lean men [42]. These findings indirectly suggest that during weight gain, lower-body adipose tissue tends to expand mainly through hyperplasia in women, but through hypertrophy and/or hyperplasia in men [42, 43]. Accordingly, lower-body subcutaneous adipocytes of women tend to be larger than those of men with the same fat mass, however no sex difference is detected in abdominal subcutaneous adipocyte size [42, 44-46]. A strong association is observed between abdominal subcutaneous adipocyte size and total body fat mass in lean to moderately obese
6
individuals of both sexes, suggesting that the contribution of adipocyte hypertrophy to expansion of adipose tissue may be similar in men and women [42]. As a consequence, part of the higher subcutaneous fat mass values detected in women compared to men may be attributable to increased adipocyte number [42]. Accordingly, a higher adipocyte number is already detected in subcutaneous adipose tissue of adolescent girls suggesting sustained adipose tissue hyperplasia in young girls compared to boys [47].
In adult women, expression of genes involved in the differentiation of preadipocytes is relatively higher in subcutaneous than in visceral adipose tissue [48]. Furthermore, only subcutaneous expression of these genes tracked with adiposity measures, suggesting that in women, expansion of the subcutaneous adipose tissue depot relies more heavily on adipocyte hyperplasia than the visceral adipose tissue depot, which may be predominantly hypertrophic [48]. This state of hypertrophy is associated with alterations in adipocyte lipolysis and in the expression of genes implicated in adipose metabolism and inflammation [49]. Taken together, these findings may suggest that depot-specific differences in the cellularity of adipose tissue reflect the propensity of premenopausal women to store more lipids in lower-body compartments through adipocyte hyperplasia, while intra-abdominal adipose tissue depots of men (and postmenopausal women) are more prone to manage incoming lipids through adipocyte hypertrophy (reviewed in [50]).
2.2 Lipolysis
Lipolysis is one of the major functions of adipose tissue by which adipose tissue supplies the body tissues with the required energy during fasting, exercise or starvation in the form of free fatty acids (reviewed in [51]). The net accumulation of lipids in a given fat depot reflects the balance between triglyceride storage that is mainly regulated through LPL activity or triglyceride synthesis and the rates of lipolysis that is primarily controlled by insulin and catecholamines. The size of the fat cell is a main determinant of lipolytic responsiveness [51, 52]. On the other hand, the release of stored fatty acids from the femoral region seems to be resistant to stimulation of adrenaline [53] suggesting that femoral adipose tissue possibly contributes to the prevention of ectopic fat deposition [53]. Moreover, increasing accumulation of visceral fat is associated with increasing the lipolytic
7
visceral FFA delivered to liver [54]. Thus, in visceral obesity, omental and mesenteric adipose tissues may play an important role in releasing not only fatty acids but also IL-6 to the liver (reviewed in [55]). IL-6 is considered as an efficient stimulator for lipolysis in humans [56].
Free fatty acid release differs not only as a function of regional body area but also according to sex [57]. In vivo studies have suggested, however, that regional differences in lipolysis seem to play a minor role in determining sex differences in the distribution of body fat, with greater contribution of regional meal FFA storage (reviewed in [58]). In absolute values, lipolytic activity is higher in women than men [59, 60]. Furthermore, healthy non obese women have higher rates of non-oxidative free fatty acid disposal than men [61]. Accordingly, women have higher FFA storage rates (palmitate) in upper- and lower-body subcutaneous fat compartments than men [62]. The kinetics of adipose tissue lipolysis also differ between obese male and female, suggesting higher FFA release among females [59, 63]. Furthermore, leg and splanchnic tissues are the main source of FFA in obese men and women compared to lean individuals (reviewed in [50]).
In women, the greater lipolysis rate is associated with lower activation of α2-adrenergic receptors [60]. Yet in obesity, catecholamine-induced lipolysis is reversibly attenuated [64]. After menopause, increased accumulation of lipids and enlargement of adipocytes in the visceral fat depot is associated with higher lipolytic rates [65]. In healthy men, acute exposure to cold increased the release of FFA from WAT lipolysis associated with metabolic activation of brown adipose tissue [66]. In another interesting finding, sleep restriction also increases nocturnal and early morning FFA which may be contributing to insulin resistance [67].
Some animal studies contributed to a better understanding of the sex dimorphism in lipolysis. In a study of male and female mice, only female mice showed increases in adipose tissue forskolin-stimulated lipolysis, as shown by significantly higher glycerol release [68]. In the same study, female mice exhibited efficient weight loss, whereas male mice exhibited higher gain in body weight than females [68]. Another study detected
8
increased FFA concentrations and adipose tissue lipolysis in female mice after training, while FFA levels in male mice were decreased [69]. Sex steroid hormones, and more generally steroid hormones, have long been recognized as important modulators of body fat distribution [8]. However, many etiological factors remain to be identified for understanding the preferential deposition of fat within the abdomen in some individuals in case of excess energy intake [8]. Of note, the mechanisms by which steroid hormones influence the various aspects of adipose tissue function have generally remained elusive, especially in humans [70]. The importance of peripheral conversion of steroids is well established (Figure 2) [71].
Figure 2: pathways of andrgen and estrogen secretion and transformation in men (A) and premenopausal women (B) [71].
While, for many years the physiologists have been aware of the local uptake and conversion of steroid hormones in adipose tissue [2], not all studies have taken into consideration the presence of numerous steroid-converting enzymes that may alter the ultimate fate and action of a given steroid hormone entering adipose tissue. These enzymes
9
may be involved in the pathogenesis of chronic conditions such as abdominal obesity, diabetes or hormone-dependent cancers (reviewed in [72]). The potential role of steroid-converting enzymes expressed in adipose tissue is the main topic of this doctoral thesis. The next section will describe literature relating sex hormones and their adipose tissue conversions to abdominal obesity in humans.
3. PATHWAYS OF ANDROGEN DYNAMICS
Androgens not only have a pivotal actions in various body tissues (Figure 3) [73] but they also have important effects on adipose tissue functions and metabolism (Figure 4, Table 1) [72]. They likely are involved in the modulation of body fat distribution patterns in men and women [70, 74, 75]. Various studies showed that low plasma testosterone concentrations are often associated with abdominal obesity and elevated accumulation of visceral fat [76-79]. Many studies reported the relationship between endogenous androgens and body fat accumulation (Table 2).
3.1 HORMONAL THERAPY
Supplementation of testosterone with physiological doses in men with initially low endogenous levels generally leads to a decrease in accumulation of visceral fat (reviewed in [80, 81]). These effects appear to be dose-dependent [81] and furthermore, lead to concomitant improvements of glucose and insulin homeostasis [82-84], but they have little effects on the lipid profile [85]. Testosterone replacement in young hypogonadal men (age<50 years) leads to a number of beneficial effects not only on reproductive function but also by increasing skeletal muscle mass [86] and by decreasing total body fat in both hypogonadal and aging men [87]. Saad et al. showed that testosterone therapy leads to weight loss and reduction in waist circumference in all three classes of obesity. Furthermore, elderly men (over 65 years) with late-onset hypogonadism seem to benefit from testosterone treatment as much as younger men [88, 89]. One possible effect of testosterone on body fat is inhibition of preadipocyte differentiation which may explain the reduction of fat mass in men treated with testosterone [90] and reviewed in [91].
10
Figure 3: Androgen actions in various tissues of males [73].
Haider et al., have reported that long term testosterone therapy up to 6 years leads to significant weight loss and improvement of type 2 diabetes and cardiometabolic risk factors in patients suffering from obesity, diabetes and testosterone deficiency [92]. In an observational study, testosterone administration improved body weight and metabolic risk factors in men with hypogonadism. However, discontinuation of testosterone administration reversed these effects, which returned again when testosterone therapy was resumed [93]. In men with testosterone deficiency, the effects of long-term testosterone therapy on weight loss, waist circumference and BMI seems to be attributed to improvement of adipose tissue function, energy utilization, mitochondrial function, increased motivation and vigor which in turn improve the cardiometabolic function and allow increases in physical activity (reviewed in [84]). On the other hand, androgen deprivation therapy (ADT), the first line of treatment and management of advanced prostate cancer in men represses the impact of androgen/androgen receptor signals. This type of
11 Glucocorticoids • ↑ in vitro LPL activity in human primary adipocytes; • ↑ in vivo basal lipolysis in men & women; • ↑ in vitro basal lipolysis in rat primary adipocytes; • ↑ in vitro catecholamine‐stimulated lipolysis in human fat samples & rat primary adipocytes; • ↓ preadipocyte proliferation in rodent primary adipocytes; • ↑ preadipocyte differentiation. Progesterone
• Antiglucocorticoid effect: Blocks the action of dexamethasone on LPL and lipolysis in rodent adipose tissue;
• Role in fatty acid synthesis control; • ↑ leptin & resistin mRNA expression & ↓
adiponectin in female rats. Absence of these effects in male rats;
• Many progesterone metabolites generated.
Estradiol
• ↓ premenopausal & ↑ postmenopausal women in vivo LPL activity; • ↑/↓ in vitro LPL activity in human primary adipocytes; • ↓ in vivo basal lipolysis in postmenopausal women; • ↑ in vitro basal lipolysis in human primary adipocytes; • ↑ preadipocyte proliferation rate. Androgen
• ↓ OM in vivo LPL activity & TG accumulation and lesser effect on SC; • ↓ OM & SC in vitro LPL activity; • ↑SC in vivo catecholamine stimulated lipolysis; • ↑ in vitro catecholamine stimulated lipolysis in rat & human primary adipocytes; • ↓ primary preadipocyte differentiation.
treatment may be accompanied by obesity, insulin resistance, dyslipidemia and cardiometabolic alterations [94].
Figure 4: Summary of steroid hormone effects on adipose tissue function and metabolism [72].
Hence, within the physiological range, higher testosterone concentrations are associated with a favorable metabolic profile, either when considering endogenous levels or following physiological replacement in men with low baseline testosterone concentrations [33, 70, 95-97]. In women, testosterone therapy is effective in the treatment of female sexual
12
dysfunction [98]. In women with low testosterone levels who were subjected to testosterone replacement therapy in various doses for 24 weeks, no adverse effects in cardiometabolic risk factors were detected [86]. Moreover, testosterone therapy up to 12 months has no adverse impact on female voice [99].
Long-term studies are required to establish testosterone therapy safety and its possible benefits in women [98]. In vitro experiments indicate that androgen treatment of abdominal adipocytes or adipose tissue explants does not lead to increased adipogenesis or higher uptake of lipids as assessed by LPL activity [80]. Indeed, androgens had the opposite effect as they inhibited these indirect measures of lipid storage, even at high doses [80]. On the other hand, an increasing body of evidence seems to suggest that prenatal androgenization of the fetus may be a significant etiologic factor for PCOS and related metabolic alterations (reviewed in [100]). In fact, hyperandrogenemia in female leads to many adverse effects in various body tissues (Figure 5) [73].
13
It has been known for a long time that androgens are detectable in adipose tissue [101-105]. DHEA, androstenedione (4-dione) and testosterone are the most abundant [103, 106]. Our research group also previously detected the most potent androgen, DHT, using more sensitive techniques [106]. Although most of androgenic steroid levels in adipose tissue are strongly correlated with levels in the circulation, the amount of steroids is generally higher in adipose tissue than blood [102-104, 106]. Such plasma-to-adipose tissue gradient in the level of steroids indirectly supports the notion that adipose tissue is a site for androgen uptake, metabolism and action [102].
Our research group previously examined differences in the steroid content of subcutaneous and omental adipose tissue in men [106]. Similar testosterone levels were observed in both adipose tissue depots. However, DHEA, 4-dione and DHT levels were higher in omental compared to subcutaneous adipose tissue. We postulate that regional differences in the enzymes that modulate steroid hormone activities may partly explain depot differences in the availability of active androgens as discussed below. In obese men, levels of testosterone and DHT in omental fat tissue were negatively associated with waist circumference [106]. Moreover, tissue 4-dione, testosterone and DHT levels were all positively correlated with adipocyte lipolytic responsiveness to catecholamine stimuli. These correlations were stronger in omental than in subcutaneous adipose tissue and are consistent with the stimulatory effect of androgens on lipolysis [106]. These results support the notion of a depot-specific regulation of androgen action in adipose tissue (reviewed in [72]).
A review of the relationship between endogenous DHEA and abdominal obesity [107] indicated that most studies assessing the free form of this steroid in men found a significant negative association between DHEA levels and accumulation of abdominal fat [108-110]. Studies which examined the correlation between the measurements of visceral adipose tissue area by computed tomography and plasma DHEA also detected a negative correlation, suggesting that low DHEA levels are associated with greater accumulation of abdominal fat [109, 110].
14
Table 1: Summary of androgen actions on fat accumulation, metabolic profile and adipose tissue metabolism
Exogenous androgen administration
Outcome Model Hormone exposure Effect References
Total and subcutaneous adiposity
Men Testosterone tx [111, 112]
Postmenopausal women DHT tx [113]
Female transsexuals Testosterone tx [114-116]
Abdominal/Visceral fat accumulation
Men Testosterone tx [81, 117-119]
Postmenopausal women DHT tx [113]
Female transsexuals Testosterone tx [114, 116, 120]
Body weight, BMI and waist
circumference Men Testosterone tx [88, 89, 92, 93, 121, 122]
Insulin resistance Men Testosterone tx / [83, 118, 123, 124]
Female transsexuals Testosterone tx NS [116, 125]
Atherogenic lipid profile Men Testosterone tx /NS [85, 112, 123]
Female transsexuals Testosterone tx [116, 125]
: increased, : decreased or NS: no significant change in outcome reported following hormone exposure, tx: pharmacological treatment, DHT: dihydrotestosterone, BMI: body mass index.
15
Table 2: Associations between endogenous androgen levels, fat accumulation, insulin resistance or atherogenic lipid profile
Endogenous androgen levels
Outcome Model Hormone exposure Association References
Total and subcutaneous adiposity
Men Testosterone and DHT – [76, 97, 126, 127]
Women Testosterone – [128-130]
Visceral fat accumulation
Men Androstenedione, testosterone, DHEA
and DHT – [76, 77, 97, 109, 110, 126, 127, 131, 132] Women Testosterone +/–/NS [77, 128-130, 133-136] Insulin resistance Men Testosterone – [76, 77, 97] Women Testosterone – [129]
Atherogenic lipid profile Men Testosterone – [137, 138]
16
The association between plasma levels of sulphated DHEA (DHEA-S) and distribution of body fat is less consistent. Some studies reported a negative association between plasma DHEA-S and accumulation of central fat [108, 109], and other studies reported the opposite [110, 139]. The association between visceral adipose tissue areas were measured by computed tomography and DHEA-S was negative in one study [109] and positively associated in another [110]. Studies of DHEA replacement continue to be notoriously discordant with respect to their impact on distribution of body fat and parameters of the metabolic profile in humans [107, 140, 141]. Some studies convincingly demonstrated that this hormone precursor had relatively little effects which could not be sustained in long-term therapies when given orally [140, 142-144]. Several contradictions were observed in previous studies on DHEA and visceral obesity could actually result from interindividual differences in the ability of peripheral sites such as adipose tissues to transform DHEA into more potent steroid hormones (reviewed in [72]).
We previously reviewed the impact of androgens on adipose tissue function [75]. Conflicting results were often reported. The summarized androgenic effects on selected aspects of adipose tissue function is summarized in Figure 4. At least 3 studies reported that androgens had no effect on preadipocyte proliferation in cultures from rodent and human adipose tissues [145-147]. On the other hand, a clear inhibitory effect of testosterone and DHT has been detected on adipogenesis in several models [90, 148-151], including human primary preadipocytes from our patients [80]. We [80] and others [90, 151] detected that these effects were partially reversed by anti-androgens flutamide or bicalutamide. One study reported the inhibition of adipogenesis by DHEA specifically in omental fat [152] which could be mediated by androgenic metabolites of DHEA [153].
The studies of the androgenic impact on lipolysis are not unanimous. Testosterone treatment enhanced norepinephrine-stimulated lipolysis in abdominal subcutaneous adipose tissue of normal men [154]. Studies on human and rodent adipocytes confirmed this finding using testosterone [155] and DHEA [156]. However, another [157] detected an inhibitory effect of testosterone on catecholamine-induced lipolysis in differentiated subcutaneous preadipocytes. Modulation of β-adrenoreceptors and hormone-sensitive lipase as well as adenylate cyclase activity had been proposed as mediators of androgenic action on lipolysis [157-161]. Flutamide
17
blunted the androgenic effects on lipolysis, indicating that these effects are likely to be mediated through the androgen receptor [145]. Most studies also reported that androgens decrease lipid uptake and synthesis in adipose tissue. Testosterone supplementation in men reduced lipoprotein lipase activity and triglyceride uptake in abdominal adipose tissue compartments [154, 162]. Discrepant effects were reported in isolated mature adipocytes [145] or in adipose tissue from monkeys that were castrated and replaced with testosterone [163].
Finally, androgens may also influence adipokine/cytokine concentrations. Testosterone administration in men decreased plasma adiponectin and leptin levels [164-166]. Leptin and adiponectin secretion were also reduced by DHT in subcutaneous explant cultures and in differentiated 3T3-L1 cells [167, 168]. Other [169] corroborated the inhibitory effect of androgens on leptin, but reported that adiponectin was not modulated by androgens. Moreover, DHEA-S also inhibited adiponectin expression in omental adipocytes [170]. Finally, testosterone replacement in hypogonadal men also decreased TNF-α, IL-1β and increased IL-10 plasma levels [171].
Overall, active androgens testosterone and possibly DHT seem to favor fat mass reductions that manifest through inhibition of adipogenesis and lipogenesis and possible stimulation of lipolysis
(Table 3). Adipokine and inflammation are likely modulated, but the impact on adiponectin is
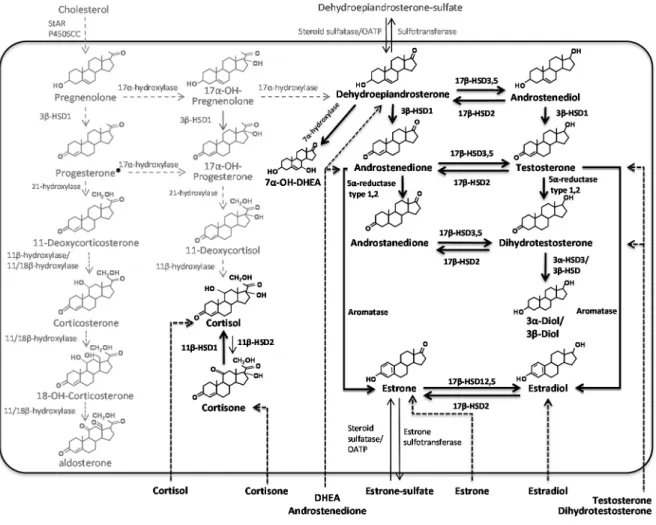
controversial. Effects have been reported to vary according to the fat depot examined and as a function of the nature and dose of the tested androgen. Considering the clear impact of androgens on adipose tissue distribution patterns, local synthesis or inactivation of active androgens could logically have depot-specific effects on androgen availability and possibly the accumulation of adipose tissue (reviewed in [72]). In Figure 6, we have provided an updated representation of the steroid-converting enzymes that are detected or may be present in adipose tissue. The large body of evidence supporting the existence of such an elaborate network of steroid-converting enzymes in adipose tissue can no longer be ignored when considering the biological impact of steroid hormones on adipose tissue. We described the role of some enzymes which may be involved in the modulation of steroid hormone effects in adipose tissue. The next section describes steroid-converting enzymes which have been described in adipose tissue.
18
Figure 6: Pathways of steroid hormone metabolism in adipose tissue. Grey arrows and
steroids indicate putative pathways requiring confirmation. Black arrows and steroids indicate confirmed pathways. *: Progesterone can be also transformed by AKR1C1 into 20α-hydroxyprogesterone [72].
3.2 MAJOR STEROID-CONVERTING ENZYMES INVOLVED IN THE SYNTHESIS OR INACTIVATION OF ANDROGENS
3.2.1 5α-reductases
5α-reductases are microsomal enzymes responsible for the formation of 5α-dihydrotestosterone (DHT), either indirectly through conversion of androstenedione (4-dione) to androstanedione (A-dione) and subsequent 17-oxoreduction, or through direct conversion of testosterone (testo) to
19
DHT [172]. For the indirect pathway, 17-HSDs are responsible for the subsequent 17-oxoreduction of A-dione to DHT. Literature in general assumes that the main reaction is that of testosterone to DHT [173]. However, in various tissues, DHT formation likely results mostly from 4-dione transformation [172, 174, 175]. The latter hypothesis is also in agreement with results of Perel et al. [176], demonstrating formation of 5α-reduced androgen metabolites.
In fact, Perel et al. [176] demonstrated the formation of 5α-reduced androgen metabolites such as androsterone, androstanedione and DHT in stromal cells from breast adipose tissue incubated with 4-dione. The production of 5α-reduced metabolites exceeded estrone production by 100 fold. The same research group reported no statistical difference in the activity of 5α-reductase between flank and abdominal adipose tissue cell cultures [177]. Moreover, work by our group in the sebaceous gland had shown that the formation of DHT also likely resulted from 4-dione transformation [174]. The enzymology data indirectly support that this possibly also applies to adipocytes [172, 175]. In primary preadipocytes, we reported that induction of differentiation significantly decreased DHT formation from testosterone [178].
Literature assumed that one out of two isoenzymes of 5α-reductase mediates local DHT production, but a third isoform of 5α-reductase has been identified [179]. This third 5α-reductase isoenzyme designated as type 3 was detected in prostate tissue and was reported to be poorly inhibited by dutasteride at high androgen concentrations in vitro [180]. It was reported to be expressed in several tissues or organs [179]. Moreover, this isoenzyme plays a role in the glycosylation of N-linked protein, and mutations in this gene cause a rare Mendelian disease [181]. The three isoenzymes of 5α-reductase are the products of three different genes: SRD5A1, SRD5A2, SRD5A3 [182]. The type 1 and type 2 isoenzymes of SRD5A have a low homology; they have different chromosomal localizations and kinetic parameters; they also differ in their patterns of distribution in androgen target tissues [183]. Upreti et al. [184], reported that SRD5A1, but not SRD5A2, was expressed in human subcutaneous adipose tissue. These results are consistent with those of Blouin et al. [178], in which we reported that SRD5A1 was not influenced by differentiation in both abdominal adipose tissue compartments. On the other hand, Wake et al. [185], reported that messenger RNA level of SRD5A1 in human SC adipose tissue did not predict the amount of body fat or its distribution.
20
The type 3 enzyme seems to be expressed in many tissues, although adipose tissue was not tested [179]. However, the messenger RNA expression of 5α-reductase type 3 was the highest of all steroid reductase isoform transcripts in many body tissues (Figure 7) [186]. This isoenzyme is highly expressed in skin, brain and mammary gland suggesting its importance in the synthesis of androgens in these tissues [186]. The role 5α-reductase isoenzymes in adipose tissue androgen homeostasis remains to be determined.
In addition to androgens, 5α-reductases may contribute to the metabolism of other steroid hormones including progesterone metabolites [187], glucocorticoids [188] and mineralocorticoids (reviewed in [189]). Formation of 5α-reduced metabolites from other steroids may also be relevant in adipose tissue. Our group reported that pregnane-3α/β-ol-20-one, 5α-pregnane-3,20-dione and 5α-pregnane-20α-ol-3-one were major metabolites of progesterone in cultures of OM and SC preadipocytes [190]. Regarding cortisol, Tomlinson et al. [188] reported decreased reductase activity after weight loss, which was based on the ratio of circulating 5α-THF over 5α-THF. Moreover, Tsilchorozidou et al.[191]reported that 5α-reductase activity toward cortisol was positively associated with BMI in a cohort of polycystic ovary syndrome (PCOS) women. Studies demonstrated the association of 5α-reductase isoenzymes in various diseases. In women, SRD5A1 haplotypes were associated with PCOS and hirsutism and SRD5A2 haplotypes were associated only with PCOS [192]. Supporting the importance of these isoenzymes in the pathogenesis of PCOS, but only 5α-reductase type 1 isoenzyme is important in hirsutism [192]. The results of PCOS, supported by increases in 5α-reductase activity in PCOS follicles was 4-folds higher than that of control follicles [193].
5α-reductase inhibition
Finasteride and dutasteride act as inhibitors for 5α-reductases (reviewed in [189]). Finasteride is used for treatment of male androgenic alopecia [194] and symptoms associated with benign prostate hyperplasia [195]. Dutasteride also reduces the symptoms of benign prostate hyperplasia [196]. On the other hand, the inhibition of 5α-reductase could contribute to the pathogenesis of
21
Figure 7: Messenger RNA expression of 5α-reductase isoenzymes in various body tissues
22
obesity, insulin resistance, vascular diseases and metabolic syndrome (reviewed in [189]). One possible mechanism is that the inhibition of 5α-reductase activity by finasteride and dutasteride would reduce the clearance of glucocorticoids and mineralocorticoids, supporting the development of insulin resistance, diabetes and vascular diseases (reviewed in [189]). Moreover, 5α-reductase inhibitors can produce adverse effects in some individuals including erectile dysfunction, loss of libido, ejaculatory dysfunction and depression [197]. Therefore, caution should be taken when prescribing of 5α-reductase inhibitors [197].
3.2.2 17β-hydroxysteroid dehydrogenases (17-HSDs)
17β-HSDs play a critical role in the biological activity of androgens and estrogens by catalyzing the reduction of 17-ketosteroids or the oxidation of 17β-hydroxysteroids using NAD(P)H or NAD(P)+ as cofactor [198]. The enzyme activities associated with the various 17β-HSD isoenzymes are widespread in human tissues, not only in classic steroidogenic tissues such as the testis, ovary, and placenta, but also in a large series of peripheral tissues [199]. In the nineties, several new types of 17β-HSDs were reported, indicating a fine, tissue-specific regulation. To date, 14 17β-HSD isoenzymes were detected in mammalians. Even if they participate in sex steroid formation, certain types are expressed exclusively in some peripheral tissues [198]. More importantly, many 17β-HSDs have a directional activity in intact cells (reductive or oxidative), a selective substrate affinity, and a particular tissue distribution. These characteristics are significant determinants of the activity of the 17β-HSD family. The next sections will provide descriptions of 17β-HSDs that are relevant to androgen metabolism in adipose tissue.
3.2.2.1 17-HSD type 3
17β-HSD type 3 is involved in the conversion of 4-dione to testosterone [198]. It is expressed in human subcutaneous and visceral adipose tissue compartments [178, 200, 201]. However, no difference in the expression levels of this enzyme was observed between these adipose tissue depots [178]. Induction of preadipocyte differentiation tends to increase expression of this enzyme [178, 202]. At this time, the specific contribution of 17β-HSD type 3 to the availability of androgens in adipose tissue remains unclear. The ratio of 17-HSD3-to-aromatase mRNA in intra-abdominal adipose tissue was positively associated with BMI in a study [203], which led
23
Table 3: Impact of androgens on adipose tissue LPL activity, catecholamine-stimulated lipolysis and adipogenesis
Adipose tissue metabolism
Outcome Tissue or model Hormone exposure Effect References
In vivo LPL activity and triglyceride accumulation Visceral adipose tissue Testosterone [204]
SC adipose tissue Testosterone NS [154, 204]
In vitro LPL activity SC and OM human adipose tissue Testosterone and DHT [80]
In vivo catecholamine-stimulated lipolysis
Abdominal SC adipose
tissue Testosterone [154]
Femoral SC adipose
tissue Testosterone NS [154]
In vitro catecholamine-stimulated lipolysis Rat and abdominal OM and/or SC adipose tissue Testosterone, DHT and DHEA-S / [145, 156, 157, 205] Preadipocyte proliferation 3T3-L1 and human primary preadipocytes Testosterone, DHT and DHEA NS/ [146, 147, 206]
Preadipocyte differentiation
3T3-L1 and rat primary
preadipocytes Testosterone and DHT [90, 146, 207]
SC and OM human
primary preadipocytes Testosterone and DHT [80, 151]
: increased, : decreased or NS: no significant change in outcome reported following hormone exposure, DHT: dihydrotestosterone, LPL: lipoprotein lipase, SC: subcutaneous, OM: omental.
24
the authors to suggest increased androgenicity in visceral adipose tissue. This hypothesis remains to be functionally examined in light of the presence of 17-HSD type 5, which is expressed at much higher level than 17-HSD type 3 (reviewed in [72]).
3.2.2.2 17-HSD type 5 (AKR1C3)
17-HSD type 5 is involved in the formation of testosterone from 4-dione. The expression level of the enzyme was found to be associated with visceral and overall adiposity indices as well as with the waist-hip ratio [185]. The expression of this enzyme is strongly induced by adipocyte differentiation. For example, testosterone formation is increased by 5 folds in differentiated adipocytes from the subcutaneous and omental adipose tissues, and AKR1C3 expression levels follow a similar pattern [178, 208, 209]. The expression levels of AKR1C3 are higher in the subcutaneous depot [178, 209]. Moreover, the size of adipocytes could also influence AKR1C3 expression since some observed that it is expressed at higher levels in larger than in smaller adipocytes from the same subject [210]. It remains to be confirmed whether increasing expression and activity levels of this enzyme with obesity contribute to make adipose tissue more androgenic. The role of 17-HSD type 5 in the synthesis of prostaglandins, which are known modulators of PPARγ [211], or its impact on estrogens could also mediate its relationship to obesity.
3.2.2.3 17β-hydroxysteroid dehydrogenase type 2 (17β-HSD type 2)
17β-HSD type 2 is 1.4- kilobase cDNA encoding 387 amino acids with a MW of 42782 [212]. The enzyme catalyzes the conversion of active 17β-hydroxysteroids into less active 17-ketosteroids, which for example decreases tissue levels of active androgens and estrogens using NAD+ as a cofactor [213]. The gene of this enzyme is localized on chromosome 16, 16q24 [214]. Regarding the protein product, 17β-HSD type 2 is a trans-membrane protein which has an unknown 3D-structure [213]. This protein contains a carboxyl-terminal endoplasmic reticulum retention motif which suggested the association of 17β-HSD type 2 to the membrane of the endoplasmic reticulum [212]. Specifically, it catalyzes the conversion of testosterone to androstenedione (4-dione), of estradiol (E2) to estrone (E1) and of 20α-dihydroprogesterone to progesterone [212]. 17β-HSD type 2 also exhibits 3β-HSD activity in homogenates and intact
25
cells of transfected HEK 293 cells [215]. The messenger RNA of 17β-HSD type 2 was detected in endometrial tissue [212, 214], placenta, human fetal liver, gastrointestinal tract and urinary tract at 20 weeks of gestation. Furthermore, 17β-HSD type 2 immunoreactive protein was detected in surface epithelial cells of the stomach, small intestine, hepatocytes, colon and renal medulla [212, 216]. Other studies indicated that, 17β-HSD type 2 mRNA and activity were high in the cultures of endothelial cells from umbilical artery (HUAEC) and vein (HUVEC). In arteries, the higher activity level of 17β-HSD type 2 was comparable to that of the placenta, which suggests the presence of an expression gradient [217]. Furthermore, 17β-HSD type 2 may be involved in maintaining progesterone level in pregnancy by inactivating androgens and estrogens in the placenta [212]. The enzyme also likely serves as a barrier that reduces E2 secretion rates toward the foetal blood circulation [218]. Moreover, the high expression of 17β-HSD type 2 in the placenta suggesting its role in pregnancy [212]. In adipose tissue, the messenger RNA of 17β-HSD2 is expressed at higher levels in omental adipose tissue than in the subcutaneous depot [178]. Its relevance for adipose tissue physiology and obesity is still unknown (reviewed in [72]).
Boulton et al [219] studied steroid hormones interconversion in human adipose tissue in vivo through measurement of arteriovenous concentration differences. They detected that testosterone was removed from plasma during passage through adipose tissue and that removal rates correlated with the arterial testosterone concentration. We can speculate that the conversion of active testosterone into inactive 4-dione via 17β-HSD type 2 in the vasculature of human abdominal adipose tissue may possibly affect testosterone availability and its impact on adipose tissue function and metabolism [220]. This may include influence on distribution of body fat, inhibition of preadipocyte differentiation [80, 207], and lipolysis stimulation [70].
Regarding 17β-HSD type 2 inhibition, recent studies developed various inhibitors of 17β-HSD type 2 for treatment of osteoporosis which is beneficial in the cases of lower circulating androgens and estrogens like in elderly men and postmenopausal women [213, 221, 222]. In our studies in men and women, we used specific 17β-HSD type 2 inhibitor (EM-919) which is a weak estrogen spiro-δ-lactone with a C18-steroid nucleus. It has been proposed as a valid inhibitor for the oxidase activity of 17β-HSD type 2 with 62-66% inhibition rates [223].
26 3.2.2.4 3α-HSD type 3 (AKR1C2)
To date, we published original studies suggesting increased circulating levels of the DHT metabolite 3-diol-glucuronide in abdominal obese men [132, 224, 225]. Later, a Swedish research group confirmed these initial results in a large cohort study [226]. Our work has also shown that DHT conversion to inactive androgen metabolite 3-diol was detected in adipose tissue of both men and women [209, 227, 228]. The activity was higher in the subcutaneous compared to the omental depot, and, most importantly, androgen inactivation rates in omental fat were positively associated with indices of adiposity including BMI, fat cell size and visceral adipose tissue area measured by computed tomography [209, 227, 228]. The enzyme responsible for most of the DHT conversion to 3-diol in humans is 3-HSD type 3 (AKR1C2) (reviewed in [72]).
The initial finding of higher expression and activity of AKR1C2/3α-HSD type 3 in subcutaneous vs. omental adipose tissue of both men and women [209, 227, 228] suggested that cell composition of the tissue might affect the enzyme activity. Accordingly, we detected that mature adipocytes had higher rates of androgen inactivation compared to preadipocytes [228]. Further experiments indicated that induction of fat cell differentiation increased DHT inactivation rates and messenger RNA expression of AKR1C2 [178].
3.3 ANDROGEN-GLUCOCORTICOID INTERACTION
Factors that could modulate DHT inactivation rates in preadipocytes were previously tested in our lab. We were intrigued by a robust, dose-dependent stimulation of androgen inactivation by dexamethasone alone [178]. This stimulation was apparent after only 24 hours and was completely reversed by the glucocorticoid receptor antagonist RU486. Furthermore, it did not require additional lipogenic factors (insulin or PPAR- agonist). These results suggest that this stimulation is an early event in the fat cell differentiation process.
Active glucocorticoids are synthesized locally by 11-HSD type 1 in proportion to mature adipocyte size and number and involved in the stimulation of adipogenesis [229, 230]. On the other hand, as mentioned, androgens inhibit adipogenesis and are inactivated locally by enzymes
27
that are responsive to glucocorticoids. We suggested that the stimulation of AKR1C2 expression and DHT inactivation by glucocorticoids in preadipocytes may remove some of the inhibitory effect of androgens on adipogenesis and allow the progress of adipogenesis. Other interactions had been noted in adipose tissue between both androgen and glucocorticoid signalling pathways [231, 232]. Interaction of these hormonal signals at the local level may act as an important modulator of body fat distribution patterns.
4. PATHWAYS OF ESTROGEN DYNAMICS
The central effects of estradiol (E2) have been reported on energy intake in rodents [233, 234],
but other studies also have indicated a direct impact of estrogen on adipose tissue metabolism [235]. Estrogenic action in adipose tissue is supported by the presence of estrogen receptor isoforms α and β [236, 237]. Sex- and depot-related differences in the levels of estrogen receptor have been described [178, 237-239]. The knockout of estrogen receptor α in mice is associated with increased adiposity [240]. In women, genetic variants of the genes coding for estrogen receptors α and β are associated with slightly increased body fat mass and accumulation of visceral fat [241-243]. Moreover, menopause has been related to increased central adiposity and accumulation of visceral fat, a phenotype that is attenuated by hormone replacement therapy [26-29, 244, 245]. In addition, estrogenic status influences the circulating levels of Acylation Stimulating Protein (ASP) as well as gene expression level of its receptor in adipose tissues [246].
Regarding the effects of E2 on adipose tissue, exogenous administration of the hormone to
premenopausal women decreased LPL activity in the gluteal fat [247]. However, the opposite effects were found in postmenopausal women [248]. Hormone replacement significantly reduced adipose tissue FFA release by 10 to 20% in postmenopausal women [249]. Some studies reported little effects of ovarian hormonal status on basal and catecholamine-stimulated lipolysis in subcutaneous adipose tissue [65, 248]. Furthermore, in vitro studies described higher LPL and basal lipolysis in visceral adipose tissue samples of ovarian hormone-deficient women [65]. These results suggest that E2 may reduce accumulation of abdominal fat through both central and

![Figure 2: pathways of andrgen and estrogen secretion and transformation in men (A) and premenopausal women (B) [71]](https://thumb-eu.123doks.com/thumbv2/123doknet/5572936.133654/25.918.132.793.401.818/figure-pathways-andrgen-estrogen-secretion-transformation-premenopausal-women.webp)
![Figure 3: Androgen actions in various tissues of males [73].](https://thumb-eu.123doks.com/thumbv2/123doknet/5572936.133654/27.918.133.785.138.539/figure-androgen-actions-in-various-tissues-of-males.webp)
![Figure 4: Summary of steroid hormone effects on adipose tissue function and metabolism [72]](https://thumb-eu.123doks.com/thumbv2/123doknet/5572936.133654/28.918.126.792.197.829/figure-summary-steroid-hormone-effects-adipose-function-metabolism.webp)
![Figure 5: Effects of hyperandrogenemia in females [73].](https://thumb-eu.123doks.com/thumbv2/123doknet/5572936.133654/29.918.126.799.567.995/figure-effects-hyperandrogenemia-females.webp)

![Figure 7: Messenger RNA expression of 5α-reductase isoenzymes in various body tissues [186]](https://thumb-eu.123doks.com/thumbv2/123doknet/5572936.133654/38.918.149.775.125.959/figure-messenger-rna-expression-reductase-isoenzymes-various-tissues.webp)
