© Kathleen Anne MacGregor, 2020
Individual - and population - level responses to the
environment : Environmental modification of movement
behaviour in the green sea urchin, Strongylocentrotus
droebachiensis
Thèse
Kathleen Anne MacGregor
Doctorat en biologie
Philosophiæ doctor (Ph. D.)
Individual- and population-level responses to the
environment:
Environmental modification of movement behaviour in the green
sea urchin, Strongylocentrotus droebachiensis
Thèse de doctorat
Kathleen Anne MacGregor
Sous la direction de :
ii
Résumé
La quête de nourriture est l’activité la plus importante dans la vie d’un organisme, fournissant des individus avec l’énergie nécessaire pour grandir et se reproduire. L’approvisionnement d’un individu se rattache à ses déplacements dans un paysage hétérogène. Dans les écosystèmes marins, les organismes mobiles sont exposés à des défis uniques en raison des forces hydrodynamiques importantes que l’eau exerce sur leur corps. Le type de substrat joue un rôle déterminant en créant une résistance environnementale qui accélère ou ralentit le déplacement des organismes benthiques mobiles. Les oursins (Phylum Echinodermata, Classe Echinoidea) représentent un des plus importants herbivores marins. À des abondances très élevées, les oursins peuvent brouter les bancs de macroalgues brunes (laminaires) jusqu’à les éliminer complètement pour former des zones dénudées. Dans ces zones, les oursins se retrouvent donc dans un état perpétuel de manque de nutriments ; la détection et utilisation rapide de parcelles contenant des ressources, particulièrement des morceaux de laminaires provenant de lits de macroalgues adjacents, est critique. Cependant, très peu d’études décrivant en détail le comportement de mouvement des oursins existent et aucune de celles-ci ne prend en considération à la fois l’hétérogénéité spatiale à petite échelle et la présence de nourriture. Il devient donc difficile de prédire les réponses des communautés aux changements environnementaux.
Ma thèse se concentre sur les facteurs environnementaux qui modifient le comportement de déplacement des oursins dans les zones dénudées. J’évalue comment ces facteurs environnementaux peuvent modifier la réaction de ces zones dénudées à certaines perturbations, telle la pêcherie. En premier lieu, j’ai combiné une revue de la littérature portant sur les perturbations expérimentales comprenant des réductions de densité d’oursins avec ma propre expérience pour explorer la généralité des liens de cause à effet entre l’abondance d’oursins et la recolonisation par les macroalgues. Dans la revue de la littérature, les réductions de densité d’oursins permettaient la recolonisation par les laminaires dans seulement deux tiers des cas, tandis que mes propres manipulations révélaient que le site où les manipulations étaient effectuées était le facteur le plus important. Cette forte variabilité spatiale peut possiblement être expliquée par des différences d’intensité d’interactions indirectes telle une compétition entre les grands et les petits oursins ainsi que par des différences dans les paysages sous-marins. Afin de décrire les comportements de déplacements d’individus pouvant expliquer les patrons observés lors des perturbations, j’ai ensuite manipulé la composition du substrat et la présence de morceaux de laminaire sur le terrain pour observer les mouvements d’oursins à travers ces zones. Le substrat instable (sable) ne fonctionnait pas comme barrière imperméable ; nous y trouvions cependant une densité d’oursin plus faible et la vitesse de consommation de morceaux de laminaires était réduite par rapport au substrat rocheux. Encore une fois, il y avait une différence claire entre les sites, s’expliquant possiblement par la structure de tailles des oursins. Le sable semblait empêcher les très grands oursins (diamètre de test > 50 mm) de traverser, sans affecter les moyens oursins (diamètre de test de 20-50 mm).
iii
Finalement, j’ai utilisé la chronophotographie pour démontrer comment les paysages benthiques hétérogènes et la présence de morceaux de laminaire modifient les comportements de déplacement des oursins verts. Les oursins verts peuvent détecter la présence de laminaire et changent leurs comportements de mouvement en leur présence, mais ne peuvent pas se diriger vers eux. Les paysages avec une plus grande proportion de substrat rocheux ont facilité une augmentation de déplacement en présence de laminaires, mais seulement en été (juillet et août). Cette relation intrigante entre les comportements de déplacement et la taille des oursins à certains sites, combinés avec la variabilité temporale observée, indique que le rôle du paysage dans la détermination des comportements de déplacement d’oursins est complexe et modifié par des facteurs intrinsèque et extrinsèque. Mes résultats sont les premières observations détaillées des comportements de déplacements des oursins dans un contexte spatial explicite. Ces résultats démontrent clairement qu’une connaissance mécanistique des réponses écosystémiques de zones dénudées face aux perturbations futures devrait inclure des informations détaillées sur les modifications du comportement par l’environnement.
iv
Abstract
Feeding and the search for food are one of the most important activities of all mobile animals, providing individuals with the energy and resources to grow and reproduce. Foraging is fundamentally concerned with movement through an explicit landscape. Marine environments present a set of unique opportunities and challenges for mobile foragers because water is much denser than air and exerts significant drag and lift forces on benthic organisms moving across the bottom. Substratum type, therefore, functions as a key determinant of movement in subtidal marine environments.
Urchins (Phylum Echinodermata, Class Echinoidea) are one of the most important marine grazers worldwide. When urchin populations are large, they can overgraze brown macroalgal (kelp) beds and form urchin barren grounds, characterized by a complete lack of kelp and high densities of urchins. Under such conditions, the foraging of adult urchins largely determines the state of subtidal benthic habitats by limiting the recolonization of macroalgae. Urchins in barren grounds thus exist in a resource-limited state, and the rapid detection and consumption of resource patches, particularly pieces of macroalgae from adjacent algal beds, is therefore key in determining individual growth and reproduction. However, there are very few detailed examinations of urchin movement and none which explicitly take both seascape and the presence of food into account, making predictions about community responses to environmental changes difficult.
My objective in this thesis was to evaluate the environmental factors modifying the movement behaviour of urchins in barren grounds, including the role of environment in determining the response of these communities to perturbations such as a fishery or disease outbreak which entail a reduction in urchin numbers and biomass. First, I combined a global literature review of previous experimental manipulations of urchin abundances with my own replicated urchin removal experiment in order to examine the causal link between grazing pressure exerted by urchins and macroalgal colonization and growth. In the published literature, urchin removals result in kelp colonization in only two thirds of cases worldwide. In my own manipulations in the Gulf of St. Lawrence, site was the most important determinant of successful reduction of urchin densities. Spatial variability, possibly related to the strength of indirect interactions such as competition between large and small urchins, was essential in determining the outcomes of perturbation experiments. In order to examine the movement behaviour that could explain the spatial variability in community response in detail, I then experimentally manipulated substrata composition in the field to examine the interacting effects of substrata and the presence of drift kelp on urchin movement behaviour. Unstable substrata (sand) did not function as absolute barriers to urchin movement in barren grounds, but urchin densities were lower on sand relative to adjacent rocky substrata, and sand barriers slowed cumulative consumption of drift kelp. Once again, however, there were clear and consistent site-to-site differences in movement behaviour, possibly related to the size-structure of urchin populations. Sand patches
v
appear to reduce movement of very large urchins (test diameter > 50 mm) but not of medium-sized urchins (test diameter of 20-50 mm).
Finally, I used time-lapse photography to describe the movements of individual urchins in relation to the presence of kelp and differences in seascape substratum composition. Green sea urchins were able to detect the presence of drift kelp in barren ground habitats and alter their movement behaviour in response but did not move directly towards the kelp. Seascapes with increased proportions of rocky substrata facilitated increased urchin movement in response to the presence of drift, but only in the summer and not in spring. The intriguing relationships between movement behaviour and urchin size at specific sites combined with the observed seasonal variability indicate that the role of seascape in determining the movement behaviour of urchins is complex and modified by important intrinsic and extrinsic factors. My results are the first detailed observations of urchin movement in a spatially explicit context and clearly demonstrate that a mechanistic understanding of the responses of barren ground systems to future perturbations must include detailed information on environmental modification of behaviour.
vi
Table des matières
Résumé ... ii
Abstract ... iv
Table des matières ... vi
Liste des figures ... ix
Liste des tableaux ... xi
Remerciements ... xiv
Avant-propos ... xvi
Introduction ... 1
Chapter 1 : Experimental removal of urchins in barren grounds: reduced density is not the only determinant of macroalgal colonization ... 8
1.1 Résumé ... 8
1.2 Abstract ... 9
1.3 Introduction ... 10
1.4 Material and Methods ... 13
1.4.1 Literature Review ... 13
1.4.2 Urchin removals in the Gulf of St. Lawrence ... 14
1.5 Results ... 18
1.5.1 Literature Review ... 18
1.5.2 Urchin removals in the Gulf of St. Lawrence ... 24
1.6 Discussion ... 28
1.6.1 Literature Review ... 28
1.6.2 Replicated disturbance manipulations in the field: urchin removals ... 31
1.6.3 Conclusions ... 35
Chapter 2 : Habitat heterogeneity and foraging success: sea urchin movements and resource discovery in barren grounds ... 37
2.1 Résumé ... 37
2.2 Abstract ... 38
2.3 Introduction ... 39
2.4 Material and Methods ... 41
2.4.1 Study areas ... 41
2.4.2 Site characterization ... 42
2.4.3 Temporal pattern and spatial extent of urchin aggregation on kelp (Sets 1, 2 and 3) ... 43
vii
2.4.5 Kelp consumption ... 44
2.4.6 Statistical analyses ... 44
2.5 Results ... 45
2.5.1 Site characterization ... 45
2.5.2 Temporal pattern and spatial extent of urchin aggregation on kelp (Sets 1, 2 and 3) ... 46
2.5.3 Barriers to movement: unstable substrata and aggregation on kelp (Sets 4, 5 and 6) ... 48
2.5.4 Kelp consumption ... 50
2.6 Discussion ... 50
2.6.1 Foraging in urchin barren grounds: blind scramble or directed movement? ... 50
2.6.2 Spatially and temporally variable barriers to movement ... 53
2.6.3 Conclusions and directions for future research ... 55
Chapter 3 : Foraging behaviour in subsidized habitats: sea urchin movement across heterogeneous benthic seascapes ... 56
3.1 Résumé ... 56
3.2 Abstract ... 57
3.3 Introduction ... 58
3.4 Material and methods ... 61
3.4.1 In situ observations of individual movement: time-lapse photography ... 62
3.4.2 Extracting urchin movement tracks from image sequences ... 63
3.4.3 Characterization of substrata within image sequences ... 63
3.4.4 Statistical analysis of urchin movement data ... 64
3.5 Results ... 66
3.5.1 Characterization of substrata and sites ... 66
3.5.2 Individual movement behaviour ... 68
3.6 Discussion ... 75
3.6.1 Urchin foraging behaviour: aggregation and patch discovery ... 75
3.6.2 The effect of seascape on urchin foraging behaviour ... 77
3.6.3 Seasonality of movement behaviour ... 78
3.6.4 Urchin foraging strategy ... 79
3.6.5 Conclusions and future work ... 80
Conclusion ... 81
Heterogeneous benthic seascapes ... 81
Spatial scale of seascapes: grazing refuges for kelp ... 85
Perturbations in marine subtidal benthic habitats ... 86
Seasonality ... 88
viii
Bibliographie ... 92
Annexe A : Chapter 3 - Supplementary Material I ... 115
Processing images in imageJ to get x,y coordinates of tracks ... 115
Annexe B: Chapter 3 - Supplementary Material II ... 119
Procedure used to extract substrata map from photos using QGIS and combine with urchin tracks using R ... 119
ix
Liste des figures
Figure 1.1. Experimental manipulations in the Gulf of Saint Lawrence (Quebec, Canada). a) Maps of study area
in the Northwest Atlantic Ocean, three regions where manipulations were carried out (Pointe Mitis, the Mingan Archipelago and Punchbowl) and three replicate sites within the Mingan Archipelago. Regions and sites are colour-coded to indicate whether clearings were done at that region/site in 2011 (white), 2012 (striped) or in both years (solid gray). b) Conceptual zones of individual clearings and locations of transects. The interior zone (2011: 2-m radius; 2012: 3-m radius) is the inner grey circle, the exterior is the band surrounding that (2011: 1 m wide; 2012: 2 m wide), and the outer ring is the area monitored but never cleared (only monitored in 2012: 3 m wide). Four transects (red dotted lines) ran from the center of the clearings out to the end of the exterior zone (2011) or never-cleared area (2012). ... 16
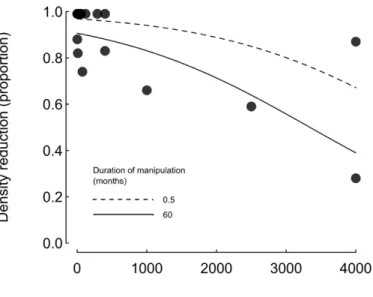
Figure 1.2. Relationship between the proportion density reduction reported in manipulative urchin clearing
experiments and the most important predictor of the analysis, Area of cleared area, in m2. Predicted values
illustrating the model results are shown for two example experimental durations, the minimum observed duration (0.5 month) and the maximum observed duration (60 months). ... 22
Figure 1.3. Proportion density reduction (from 0 to 1 with 1 being 100% reduction of original urchin density) in
experimental areas presented by geographic region and distinguished by whether kelp/fucoid recolonization was observed or not (circles = no; triangles = yes). Principal species of urchin are shown corresponding to regions. ... 22
Figure 1.4. Kelp recolonization as a function of the duration of the manipulation for two removal techniques
(killing or removing). Predicted values with 95% Confidence Intervals for removing urchins (solid line) and elevated predicted values for killing urchins in situ (dashed line). ... 24
Figure 1.5. Clearing trajectories through time. Density of large urchins in the interior zone before clearing, and
then through time. a) 2011 pulse clearings. b) 2012 pulse clearings. c) 2012 press clearings. For Cap du Corbeau (CC), Île-du-Havre (IH) and Pointe-Mitis (PM), each point is the mean of 2 replicate clearings at a site (mean ± 1 standard error). At Punchbowl (PB), only 1 clearing was done, so no measure of variation is possible; in all other cases where error bars are not visible it is because they fall within the plotting symbol. Dotted lines connect subsequent densities and, for press clearings (c), include the reclearing visits when densities were reset to zero. ... 25
Figure 1.6. Abundance of urchins in the exterior zone at the time of the first visit (10-14 d after clearing) split
into direction relative to shore. Points are labelled to indicate the direction (away: away from shore; towards: closest to shore; parallel 1 and parallel 2: transects running parallel to shore). Points are mean values ± 1 standard error. ... 26
Figure 1.7. Estimated biomass of large urchins in clearings before clearing, at the first visit after 1-2 weeks, and
at the final visit 2.5-3 months after clearing for (a) pulse clearings and (b) press clearings in 2012. Each point is the mean of 2 replicate clearings at each site (± 1 standard error). Dotted lines indicate the threshold biomass (150 g.m-2) considered necessary to maintain urchin barren grounds. ... 27
Figure 1.8. Density of large urchins in the area surrounding the manipulations (i.e., never manipulated) before
clearing and then through time: (a) 2012 pulse clearings and (b) 2012 press clearings. Each point is the mean of 2 replicate clearings at each site (± 1 standard error). Dotted lines connect subsequent measures. ... 28
Figure 2.1. Map of study site locations in the Gulf of Saint Lawrence (Quebec, Canada). Two sites are in the
Mingan Archipelago on the north shore (Petite Île au Marteau [PIM] and Île aux Goélands [IG]) and one site on the south shore of the maritime estuary (Pointe Mitis [PM]). ... 42
Figure 2.2. Size distribution and biomass distribution for the three sites. Size distributions are shown as
urchins.m-2 within 5-mm bins for test diameter for PIM (a), IG (c) and PM (e). The 5-mm bins are labelled with
the largest measure for that size class (i.e., the first size class labelled ‘5’ contains urchins ranging from 0 to 5 mm test diameter). Biomass is shown as kg.m-2 within the same 5-mm bins of test diameter for PIM (b), IG (d)
x
and PM (f). The red dashed line indicates the limit of what was considered large urchins in the present manipulations (to the right of the line are urchins > 20 mm test diameter). ... 46
Figure 2.3. Temporal pattern and spatial extent of urchin aggregation on kelp. Small points are data points from
individual experimental units. Lines are large urchin numbers through time (predicted values from GLMM [a and c] and LMM [b and d] with shaded 95% Confidence Intervals). Kelp treatments (a and b) and no kelp treatments (c and d) are shown for the inner and outer zones. The dashed line in each panel indicates the overall mean large urchin density determined using urchins removed from experimental plots. ... 47
Figure 2.4. The number of large urchins in both the inner and outer zones after 48 hours. Sets of manipulation
are presented separately. Numbers of large urchins in the inner zone (a, c and e) and outer zone (b, d and f) are shown with no kelp treatments on the left and kelp treatments on the right; bars are means ± 1 standard error and individual data points are shown (n=3 per treatment). Substratum treatments are shown as different coloured bars: control clearings are dark grey, procedural controls are medium gray and sand is light grey. The dashed line in each panel indicates the pre-manipulation mean large urchin density for that Set determined using urchins removed from experimental plots. ... 49
Figure 2.5. Proportion of kelp consumed in all manipulations (during the 2nd 24-hour period of manipulations).
a) Small points are individual measures, coloured by the substratum treatment (predicted values from model for one representative set [Set 5] and Control clearings are shown as regression line). b) proportion of kelp consumed by substratum treatment, all sets combined (Control n = 24; Procedural Control n = 9; Sand n = 9). The top and the bottom of the box are the 25th and 75th percentile, the band is the median, and the ends of the whiskers are either the quartiles + or – 1.5 * the interquartile range or, if this is further from the box than the most extreme value, the end of the whisker is the most extreme value. ... 50
Figure 3.1. Map of study sites in the Mingan Archipelago on the north shore of the Gulf of Saint Lawrence
(Quebec, Canada). The three rocky sites (R-1, R-2 and R-3) are denoted by white points and the three sandy sites (S-1, S-2 and S-3) by black points. ... 62
Figure 3.2. Description of the six study sites a) proportion of buffered solid substrata, with rocky sites in white
and sandy sites in grey; b) relationship between the proportion of solid area and the nearest neighbour distance; c) area of individual solid patches and number of solid patches; and d) size-frequency distributions of urchins at the six sites with median diameter indicated with an arrow. ... 67
Figure 3.3. Densities (ind.0.25m-2) in image sequences at the beginning and end of sequences (three to four
hours total time), with and without a piece of kelp in the center of the area (all sites and deployments combined; n=18 for each point). Points are means ± 1 standard error. ... 68
Figure 3.4. Net displacement (cm.hr-1) of urchins. The significant interaction between kelp and month of
deployment is shown (n=6 for each point). Points are means ± 1 standard error. ... 71
Figure 3.5. Proportion of time urchins spent moving. Points are means ± 1 standard error (n=3 for each point; 12 points per panel). a) May b) July and c) August. In (b), two sites with Kelp have almost identical proportions of substrata (0.9) and proportions of time moving (0.1) and points are almost completely overlapping. ... 72
Figure 3.6. Net distance moved closer to the center marker or piece of kelp (cm). A positive value indicates that
the urchin ended the sequence closer to the kelp or central marker than at the beginning while a negative value indicates that the urchin ended further from the kelp or central marker than at the beginning. ... 73
Figure 3.7. Probability of reaching the piece of kelp is influenced by the initial distance from the kelp, month,
and urchin size (test diameter). Data points are shown classed by season (circles for May, squares for July and triangles for August). Lines show the predicted values for a minimal scenario (May with a low proportion of solid substrata) and a maximale scenario (August with a large proportion of solid substrata) for both large (dashed line: 50 mm test diameter) and small (solid line: 20 mm test diameter) urchins. 95% confidence intervals are shown as shaded areas for August predictions only. ... 74
xi
Liste des tableaux
Table 1.1. Description of replication of urchin removals at five sites during two years in the Gulf of Saint Lawrence
(Mingan Archipelago and Maritime Estuary) and in Newfoundland. ... 17
Table 1.2. Information extracted from 21 manipulative subtidal field experiments in which urchin densities were
reduced and proportional reduction in urchin densities and algal recolonization assessed. ... 19
Table 1.3. The effect of area, duration of manipulation and removal on the proportion reduction in density of
urchins in manipulation experiments: estimates of coefficients and 95% Confidence Intervals are presented (link scale: logit) and Analysis of Deviance (Type II log-likelihood tests) with significance of individual parameters. Significant effects are indicated in bold while marginally significant trends are underlined. ... 21
Table 1.4. Analysis of Deviance table (Type II log-likelihood tests) for binomial model testing the effect of
experimental parameters on the probability of kelp recolonization. The effect of proportion density reduction, area, duration of manipulation, removal and minimum depth of manipulation on the probability of kelp recolonization: estimates of coefficients and 95% Confidence Intervals are presented (link scale: logit) and Analysis of Deviance (Type II log-likelihood tests) with significance of individual parameters. Significant effects are indicated in bold while marginally significant trends are underlined. ... 23
Table 1.5. Analysis of Variance table (Type II Wald Chi square tests) for generalized linear mixed effects model
on density of large urchins in the interior zone of clearings carried out in 2012. Significant effects are indicated in bold while marginally significant trends are underlined. ... 26
Table 1.6. Analysis of Variance table (Type II Wald Chi square tests) for generalized linear mixed effects model
on density of large urchins in the exterior zone at the first visit in 2012, based on direction. Significant effects are indicated in bold while marginally significant trends are underlined. ... 26
Table 3.1. Description of the six response variables used to analyze individual movement behaviour of urchins.
... 66
Table 3.2. Results of analysis of individual movement behaviour for track-level response variables. Analysis of
Variance tables (Type II Wald Chi square tests appropriate for unequal sample sizes), beta-coefficients and 95% Confidence Intervals, backtransformed where necessary to facilitate interpretation. Coefficients for Total Displacement and Net Displacement correspond to the percent change in response variable per unit increase in the predictor (for continuous predictors) or as compared to the reference level (for factors), such that a coefficient of 0.22 indicates a 22% increase in the response variable and a coefficient of -0.15 indicates a 15% decrease in the response variable. In this case, confidence intervals that do not overlap with 0 indicate a significant effect. Coefficients for Net Distance Moved Closer to Kelp are on the scale of the response variable such that coefficients correspond to unit increase in the response variable associated with a unit increase in the predictor variable or as compared to the reference level. Coefficients for Proportion of Time Spent Moving and for Probability of Reaching Kelp correspond to the change in odds associated with a unit increase in the predictors or as compared to the reference level such that a coefficient of 1.78 represents a 78% increase in odds and a coefficient of 0.25 represents a 25% decrease. In this case, confidence intervals that do not overlap 1 indicate a significant positive or negative effect. Significant effects are indicated in bold while marginally significant trends (p-value between 0.1 and 0.05) are underlined. ... 69
Table 3.3. Results of analysis of individual movement behaviour for the observation-level response variable of
the length of individual moves (movement speed). Analysis of Variance tables (Type II Wald Chi square tests) for generalized linear mixed effects model. Significant effects are indicated in bold while marginally significant trends are underlined. ... 75
xii
< For my mother, who convinced me I could
do anything and whose strength, passion and
willingness to fight for what is important
continue to inspire me >
xiii
< "[F]rom the least to the greatest in the
zoological progression, the stomach sways
the world; the data supplied by food are the
chief among all the documents of life." >
Fabre 1913
xiv
Remerciements
This thesis was definitely a journey and I would first like to thank my guide and advisor, Ladd Johnson. Thank you for sharing your passion so freely, for many inspiring conversations and fun times, for leading by example and always assuming I was capable of anything. Your trust and confidence in my abilities gave me the confidence to discover what I found interesting in the observations I made, to figure out ways to experimentally answer tough questions, and to place my results into an ecological ‘story’ that (sometimes!) makes sense. Your insistence on not only great science but also good company and a healthy work-life balance convinced me that I could indeed do what I loved and still maintain the rest of my life. You are not only an inspiring and passionate scientist but also an amazing advisor and mentor; your kindness and love of teaching made my time in the lab an absolute pleasure. Thank you.
I would also like to express my gratitude to my committee (Nadia Aubin-Horth and Daniel Fortin) who contributed enormously to the development of this project by asking great questions, forcing me to think about parallels to my work in other systems and offering encouragement and support. It was particularly important to me to have the guidance and insightful critique of a woman, to have a role model of a female scientist (still a minority in Laval University’s biology department) and to see myself reflected in the expertise represented on my committee: thank you Nadia.
Without the collaborative and concerted effort of many people, the diving fieldwork necessary for this project would have been impossible. Thank you to all the cheerful cold-water divers who worked so hard with me: Carla Narvaez, Thew Suskiewicz, Kristen LeGault, Leo Miranda, Benoit Dumas, Anne Provencher St-Pierre, David Grimard, Amélie Robichaud, Julien Arsenault-Hetu, Anne-Sara Sean, François-Étienne Sylvain, Guillaume Borgia, Audrey Desaulniers, Laurie Desbiens, Guadalupe Fernandez Nieto, and Filippo Ferrario. I was fortunate to have the amazing help and company of all you wonderful people!
I would particularly like to thank Carla, Filippo and Thew for invaluable support, discussions, and help not only in the field, but throughout the entire project. I learned so much working with you and I am grateful not only for your input and effort in this project, but also for your company, good will, and friendship; I am exceptionally lucky to have had such a great team. As challenging as the fieldwork was, this project was also helped innumerable times by the entire Benthos lab (current, past and honorary) who gave feedback and comments, asked great questions, and provided help and support (both academic and moral) when necessary: Anissa Merzouk, Sam Collin, Nico Le Corre, Annick Drouin, Jordan Ouellette-Plante, Meike Kern, Filippo Ferrario, Heather Hawk, Thew Suskiewicz, Carla Narvaez, Leo Miranda, Guadalupe Fernandez Nieto, Anne Fontaine, Kevin Ma, Nathan Haag, Manon Picard, Marie-Hélène Armaly St-Gelais, Charlotte Carrier-Belleau, Pauly Brüning and Ignacio Garrido.
xv
And of course, special thanks to my parents Janice Hayward and Garry MacGregor. Thank you for believing curiosity was important, for keeping me supplied with a stack of books, and, most importantly, for all the love and support through this long process. And thank you to my sister, Heather MacGregor, who inspires me with her passion, insightful questions, and pure stubborn dedication. I am so lucky to have such a wonderful sister (and friend)! Long skype conversations with all three of you were essential to keeping me happy and motivated; I love you all.
And last but certainly not least, I need to thank Claudine Durand for her patience, love and support throughout the (many) years that this project took; I couldn’t have done it without you.
Financial support for this project came from an NSERC Strategic and Discovery grants program awarded to Ladd Johnson, and graduate student support from the FQRNT, NSERC, Québec Océan and the Biology department at Université Laval to Kathleen MacGregor. Invaluable (and much appreciated) logistical and field support were provided by the Fisheries and Oceans Canada’s Maurice-Lamontagne Institute.
xvi
Avant-propos
Cette thèse comprend une introduction générale, trois chapitres sous forme d’article et une discussion générale. Les trois chapitres, qui présentent les résultats de mes travaux de recherche, ont été rédigés en anglais sous forme d’articles scientifiques et seront soumis pour publication suite à la soutenance.
Chapitre 1: MacGregor KA and Johnson, LE. Experimental removal of urchins in barren grounds: reduced density is not the only determinant of macroalgal colonization.
Chapitre 2: MacGregor KA and Johnson, LE. Habitat heterogeneity and foraging success: sea urchin movements and resource discovery in barren grounds.
Chapitre 3: MacGregor KA and Johnson, LE. Foraging behaviour in subsidized habitats: sea urchin movements across heterogeneous benthic seascapes
J’ai conçu et réalisé toutes les expériences et observations sur le terrain, extrait toutes les données des images récoltées sur le terrain, effectué toutes les analyses statistiques, et procédé à la rédaction des articles pour soumission et publication avec l’aide de mon directeur de recherche, Ladd Johnson. Les résultats de cette thèse ont été présentés lors des congrès nationaux et internationaux suivants :
Communications orales aux congrès
MacGregor, K.A. and L.E. Johnson. Kelp bed restoration in the Gulf of Saint Lawrence: No easy task! World Conference on Marine Biodiversity, May 13-16, 2018, Montreal, Québec, Canada.
MacGregor, K.A. and L.E. Johnson. What happens when the herbivores always win? Kelp beds and urchin barens in the Gulf of Saint Lawrence (Québec). Pacific Ecology and Evolution Conference, February 23-25, 2018, Bamfield, British Columbia, Canada.
MacGregor, K.A., T.S. Suskiewicz, C.A. Narvaez and L.E. Johnson. Primary productivity and mobile invertebrates in shallow rocky arctic environments: Understudied interactions. Arctic Change, December 11-15, 2017, Québec, Québec, Canada.
MacGregor, K.A. and L.E. Johnson. Finding food in the Saint Lawrence: Green sea urchin movement behaviour in densely populated urchin barrens. Benthic Ecology Workshop, October 24-25, 2016, Saint Andrews, New Brunswick, Canada.
MacGregor, K.A. and L.E. Johnson. Sitting tight! Green sea urchins move less when tidal flow increases but can detect drift kelp. Benthic Ecology Meetings, March 16-19, 2016, Portland, Maine, USA.
MacGregor, K.A. and L.E. Johnson. Facing up to a difficult environment: Green sea urchins (Strongylocentrotus
droebachiensis) alter their movement behaviour in response to water flow and the presence of food. Société
xvii
MacGregor, K.A. and L.E. Johnson. The search for food: Do green sea urchins (Strongylocentrotus
droebachiensis) locate 'windfall' food in a random or directed manner? International Echinoderm Conference,
May 24-29, 2015, Playa del Carmen, Mexico.
MacGregor, K.A. and L.E. Johnson. Fill up and move along! Green sea urchin (Strongylocentrotus
droebachiensis) densities in space and time. ACCESS (Atlantic Canada Coastal and Estuarine Science Society),
May 10-12, St. Andrews, New Brunswick, Canada.
MacGregor, K.A. and L.E. Johnson. Crowded together and drifting apart: Temporal and spatial extent of green sea urchin (Strongylocentrotus droebachiensis) aggregations. Benthic Ecology Meetings, March 5-7, 2015, Quebec City, Québec, Canada.
MacGregor, K.A., T. Suskiewicz and L.E. Johnson. The biggest, the best and the most impressive! Kelp-urchin dynamics in the Arctic and the discovery of a kelp 'hotspot' in Northern Labrador. Arctic Change 2014, December 9-12, 2014, Ottawa, Ontario, Canada.
MacGregor, K.A. and L.E. Johnson. Temporal and spatial extent of aggregation in a herbivore population: Drift subsidies and the green sea urchin (Strongylocentrotus droebachiensis) in barren zones of the Saint Lawrence. Annual meeting of Québec Océan, November 18-19, 2014, Rivière-du-Loup, Québec.
MacGregor, K.A. and L.E. Johnson. Crazy for Kelp? Movement behaviour of the green sea urchin (Strongylocentrotus droebachiensis) in the presence of a preferred food. Genomes to/aux Biomes, May 25-29, 2014, Montreal, Québec, Canada.
MacGregor, K.A., L.E. Johnson and J-S. Lauzon-Guay. Feels like walking, walking on broken glass? Context-dependent behaviour of the green sea urchin (Strongylocentrotus droebachiensis). Benthic Ecology Meetings, March 20-24, 2013, Savannah, Georgia, USA.
Sessions d'affiches
MacGregor, K.A. and L.E. Johnson. Facing up to a difficult environment: Green sea urchins (Strongylocentrotus
droebachiensis) alter their movement behaviour in response to water flow and the presence of food. Annual
General Meeting, Québec Océan, November 10-11, 2015, Québec, Canada.
MacGregor, K.A. and L.E. Johnson. If it feels right, go for it! Watching the green sea urchin (Strongylocentrotus
droebachiensis) navigate patchy landscapes. Annual meeting of the Société québécoise pour l'étude biologique
du Comportement, November 8-10, 2013, Montréal, Québec, Canada.
MacGregor, K.A. and L.E. Johnson. If it feels right, go for it! Watching the green sea urchin (Strongylocentrotus
droebachiensis) navigate patchy landscapes. Annual meeting of Québec Océan, November 13-15, 2013,
Rivière-du-Loup, Québec.
MacGregor, K.A., L.E. Johnson and J-S. Lauzon-Guay. Does the green sea urchin notice its surroundings? Environmental modification of movement in Strongylocentrotus droebachiensis. Annual meeting of Québec Ocean, November 5-7, 2012, Québec, Québec, Canada.
MacGregor, K.A., L.E. Johnson and J-S. Lauzon-Guay. Movement in the green sea urchin (Strongylocentrotus droebachiensis): Context dependency in recovery responses to perturbations. Benthic Ecology Meetings, March 22-26, 2012, Norfolk, Virginia, USA.
1
Introduction
Feeding and the search for food are one of the most important activities of mobile animals, providing individuals with the energy and resources to grow and reproduce (Bell, 1990; Stephens and Krebs, 1986). Obtaining those resources throughout an organism’s life will determine not only survival but also reproductive success and influence an organism’s fitness. Satisfying this need for energy is known as foraging; foraging is not only the act of eating, however, but also the search for food, the detection and recognition of resources and the ability to capture and ingest the food item. Ecology has been concerned with foraging since the earliest naturalists’ observations because of the essential link between eating and survival. An organism must find enough to eat, always, or risk starving to death. Foraging is a critically important activity and, although many animals spend a surprisingly large amount of time doing nothing (Herbers, 1981), many of the behaviours observed by naturalists and ecologists are therefore foraging behaviours. Indeed, understanding why many animals do not spend more time foraging has preoccupied many researchers (e.g., Bénichou et al., 2018; Herbers, 1981).
Much attention has been given to describing a species’ requirements in terms of its niche: an ensemble of requirements and specifications describing the environment and resources used by a species (Chase and Leibold, 2003; Levine and HilleRisLambers, 2009). The distribution and abundance of species are understood, in this framework, to be a function of the distribution and availability of the resources necessary for their survival and reproduction. Foraging theory seeks to explain why animals use particular resources and how the strategies of using those resources provide a successful or optimal mode of resource acquisition for an organism. Indeed, both Charles Darwin and Alfred Russel Wallace, who concurrently developed theories of evolution through natural selection, were initially inspired by Malthus’s “An Essay on the Principle of Population” (1798) detailing the importance of food in limiting human populations. Darwin’s famous observations of beak specialization in finch species of the Galapagos Islands were in fact observations of foraging behaviour; different beak shapes are specialized to allow species to obtain and ‘capture’ or handle different seed types (Weiner, 1994).
Organisms must compromise between the effort and energy expenditure necessary to locate, detect, and capture resources (costs) and the energy gained (benefits) from consuming that resource. In the 1960s, a theory of optimal foraging in a patchy environment wherein the balance between costs and benefits of foraging is explicitly modified by the surrounding environment and distribution of resources was developed (Charnov, 1976; MacArthur and Levins, 1964; MacArthur and Pianka, 1966). Essentially, foraging theory has since developed into four main areas of study: 1) what should an organism eat (optimal diet); 2) where should an organism eat (location and choice of patches); 3) how much time should an organism spend in a patch or with a single item before moving on to begin searching again (allocation of time to each patch; when to leave); and
2
4) how should an organism move (direction, pattern, speed) in order to maximize its chances of detecting and capturing prey. Although early models of predator-prey dynamics and much of the early language of foraging theory (e.g., ‘capture’) evokes the dynamics of carnivores, the theory and terminology can also be applied to many herbivore populations and the resources (plants) they need to survive (Lubchenco, 1979).
Foraging of herbivores on plant resources has often been examined using insects as model species for two reasons. Firstly, insects and the plants they graze on are of a manageable size to allow observation and manipulation of multiple individuals and even entire populations through multiple generations. Secondly, insects graze on plants that are often of economic or agricultural importance to people (e.g., Bourhis et al., 2015). In-depth examinations of insect-plant model systems have therefore been common in the ecological literature dealing with herbivore foraging impacts (e.g., Underwood and Rausher, 2000). Of principal interest to agriculture, particularly since there is evidence that climate warming may be driving increasing insect damage (Meineke et al., 2018), are the effects of grazers on plant populations (e.g., crop damage). However, the effects of plant populations on herbivore distribution, abundance and behaviour are also of fundamental interest to ecologists in order to describe why we find particular species in certain places at certain times (e.g., migrations to follow cycles of plant productivity seasonally; Fryxell and Sinclair, 1988; Leimgruber et al., 2001). There are also effects of grazing on the surrounding community and associated species; for example, grazing can limit the growth of competitively dominant species, allowing weaker competitive species to persist or thrive in the environment (e.g., Morrison et al., 2018). In fact, the effects of grazing on the biodiversity, structure and functioning of ecosystems can be enormous. Sometimes this effect becomes evident only when the grazing pressure changes; for example, the reintroduction of wolves in Yellowstone Park had dramatic effects on the types of vegetation that flourished in the park by increasing the risk of predation for elk in certain areas and therefore decreasing grazing pressure (Ripple and Beschta, 2003; Smith et al., 2003). Grazing can strongly affect communities and ecosystems, and alterations in grazing pressure have far-reaching consequences for the habitat and species composition in an area.
Two of the questions involved in herbivore foraging, the detection and choice of patches (where to eat) and the process of locating and exploiting resource patches (how to move) both involve moving through an explicit landscape. The landscape, a mosaic of patches of habitat with characteristic features such as a dominant vegetation type, is a fundamental element of foraging. Not all landscapes or patches in a landscape will provide the same opportunities for movement or detection of both resources and threats. One way of classifying landscape patches is by characterizing the resistance they provide for movement through them (Gherghel and Papeş, 2015; Schooley and Wiens, 2004). The increase in detailed individual movement data (e.g., technological advances in tracking methods) combined with detailed mapping (e.g., based on satellite imagery) has increasingly allowed in-depth examinations of movement through patchy landscapes (e.g., Béguer-Pon et al.,
3
2016; Harvey and Fortin, 2013; Haynes and Cronin, 2006; Leblond et al., 2011; Singh et al., 2016; Vanbianchi et al., 2018). For example, research has shown that wolves use man-made patches such as trails and roads to facilitate their movement through a landscape, which has altered their interactions with prey such as caribou (James and Stuart-Smith, 2000).
It is essential to consider the influence of the landscape through which an organism is moving when looking at individual movement behaviours. Landscape patchiness has been demonstrated to fundamentally alter the types of movement that an organism displays (Crist et al., 1992; Eycott et al., 2012; Hovel and Wahle, 2010; Johnson et al., 1992b; Lima and Zollner, 1996). Some landscape patches create high resistance to movement and can be thought of as barriers. Impermeable barriers completely block movement while partial or permeable barriers can slow or reduce movement to varying degrees (e.g., Gherghel and Papeş, 2015; Pépino et al., 2012). Resistance to movement can be created as either physical or as behavioural impediments to moving. For example, physical impediments to movement such as thick undergrowth or deep snow may slow movement rates (Morales and Ellner, 2002) or areas of elevated predation risk may discourage movement through certain patches (e.g., Madin et al., 2011).
Marine environments present a set of unique opportunities and challenges for mobile organisms because water is 780 times denser than air. Although pelagic organisms such as fish that swim through the water column have morphological and behavioural adaptations that rely on the density and incompressibility of water both to remain buoyant and to allow diverse strategies of propulsion (Fish et al., 2014; Lighthill, 1969; Sfakiotakis et al., 1999), many marine organisms ‘walk’ along the bottom of the ocean. Unlike terrestrial organisms, for which gravity is often the largest external force to overcome in order to move, benthic marine organisms face a very particular set of challenges as the dense water medium through which they move flows over them and creates significant drag and lift forces (Denny, 1987; Hui, 1992; Martinez, 2001, 1996). Benthic organisms must constantly ensure that they remain firmly attached to the bottom (i.e., counter the drag and lift force exerted on their body by moving water; Martinez, 1996), expending energy not only to generate forward movement but also to remain firmly in place.
Benthic seascapes, like landscapes, are mosaics of patches with different characteristics where patches can be classified by determining a permeability or resistance to movement. The most critical distinction in terms of seascape patch identity in benthic subtidal habitats is the type of primary substrata, and the most basic division of substrata types can be made between solid rocky areas and sandy or muddy areas. Because many mobile marine benthic invertebrates walk along the bottom, the type of substratum is critical for determining the environmental resistance to movement through that patch. In general, hard substratum provides a solid surface for movement and attachment of many species, particularly mobile benthic invertebrates that rely on
4
pushing and pulling against solid elements to generate movement. For these species, hard substratum creates an environment that facilitates movement whereas sand and mud complicate movement. Substratum type, therefore, functions as a key determinant of environmental resistance to movement in subtidal marine environments. However, there are very few detailed examinations of invertebrate movement in marine habitats which take seascape (i.e., explicit patches of different substratum types) into account (e.g., Laur et al., 1986).
Urchins (Phylum Echinodermata, Class Echinoidea) are one of the most important marine herbivores worldwide. Like many benthic invertebrate species, urchins have a pelagic larval stage lasting weeks to months that accounts for large-scale dispersal and population connectivity (Lawrence, 2012). However, once young urchins settle and recruit at a specific site, movement of adults occurs along the bottom. Urchins move by using tube feet, powered using a system based principally on water pressure and suction, although tube feet may also have chemically adhesive properties (Lawrence, 2012; Santos et al., 2005). Urchins have a protective hard shell (called a test) covered by spines and are voracious herbivores that feed primarily on macroalgae although some species are opportunistic omnivores and will consume animal as well as algal material if available (Lawrence, 2012). Early observations, for example, note that although “the sea-urchin is thus a vegetarian, yet near the fishing stations it may often be seen to feed greedily on the garbage of the fisheries” (Dawson, 1867). Urchins in temperate zones worldwide graze preferentially on large brown macroalgal species, particularly those of the order Laminariales (kelp), and the interactions between urchins and kelp are a key marine herbivore-plant interaction.
Kelp grows in dense beds or forests in many temperate regions, creating a highly productive habitat that, because of the complex three-dimensional structure created by high densities of productive kelp fronds, creates a unique habitat supporting both a high diversity and density of associated species (Bertocci et al., 2015; Miller et al., 2017; Steneck et al., 2002; Ware et al., 2019; Wilce and Dunton, 2014). Kelp have been classified as ecosystem engineers because of their fundamental and structuring influence on subtidal coastal environments (Teagle and Smale, 2018). Kelp bed habitats not only have a high ecological value as diverse and productive areas; they also function as nursery areas and habitat for many commercially important species and contribute to the economic value of coastal fisheries by maintaining healthy and sustainable populations. When urchin populations are large, they can overgraze kelp beds, forming dense feeding fronts along the seaward edges of kelp beds. In many cases, urchin grazing is so destructive and the numbers of urchins so high, that kelp is completely eliminated and urchin barren grounds are formed (Lawrence, 1975). Urchin barrens are characterized by a complete lack of kelp, high densities of urchins and a rocky substratum dominated by crustose coralline algae. Urchin barren grounds have lower primary productivity than kelp beds. However, they are not ‘barren’ in the sense of a complete absence of life and productivity. The quantities and possible importance to urchin populations of additional sources of nutrients such as bacterial film, microalgae, whelk egg cases (Dumont
5
et al., 2008), spawning forage fish such as capelin (Crook and Davoren, 2016) and the accumulation of faeces (notably from high densities of urchins: see Sauchyn et al., 2011) are largely unexplored, although urchins in barren grounds have been described as generally more omnivorous than those in kelp beds (Himmelman, 1984).
Kelp beds and barren grounds are further linked through the export of kelp plants and fragments. Pulsed spatial subsidization of ecosystems is increasingly recognized as an important source of nutrients in many systems. For example, extensive and ongoing work in the Pacific northwest continues to demonstrate the importance of salmon runs for coastal terrestrial ecosystems. The resource pulse contained in the huge abundances of carcasses which flood watersheds during salmon runs represents an important source of nutrients for the entire ecosystem (Christie and Reimchen, 2008; Hocking and Reimchen, 2009). Because of the extremely high primary productivity of kelp, enormous amounts of carbon are produced annually (Mann, 1973), and much of this production is exported to adjacent ecosystems as the kelp blades continually fragment and degrade at the distal ends or as entire plants are detached from the bottom by waves and storms (Filbee-Dexter et al., 2018; Krumhansl and Scheibling, 2011). The export of carbon from kelp beds has even been identified as a potential pathway for carbon sequestration in the deep ocean (Krause-Jensen et al., 2018; Krause-Jensen and Duarte, 2016; Lyons et al., 2016). This exported carbon is an important source of nutrients for barren grounds which have very low levels of in situ primary productivity (Filbee-Dexter and Scheibling, 2014a; Vanderklift and Wernberg, 2008). Because in situ primary productivity is so low, subsidization through windfall resource patches (e.g., drift kelp) may be a defining feature of barren grounds and could provide a major source of nutrients for barren ground urchins (e.g., Vanderklift and Wernberg, 2008).
Urchins graze preferentially on large brown algae when available, and kelp has repeatedly been demonstrated to support the highest reproductive output (Foster et al., 2015; Lemire and Himmelman, 1996; Vadas Sr. et al., 2000) although there are also benefits of a mixed diet including animal protein (Meidel and Scheibling, 1999). A more generalist diet in barren grounds probably allows urchins to persist in an environment where predictable resources are rare; foraging strategies allowing rapid and efficient detection and utilisation of these resource patches will be key for individuals. The spatially unpredictable windfalls that comprise the majority of resource inputs in barren grounds should favour the development of a foraging strategy that increases the chances of finding and profiting from periodic and unpredictable resources. However, almost no work has been done describing how urchins in barren grounds actually forage in the heterogeneous seascape through which they must walk (but see Dumont et al., 2004; Harding and Scheibling, 2015; Hernández and Russell, 2010).
Kelp beds and barren grounds can be considered as alternative stable states of rocky subtidal areas in temperate regions. Shifts between these states have been observed in many regions, and drivers and influences pushing these systems from one state to the other have been proposed (Ling et al., 2015). In the northeast
6
Pacific, a trophic cascade by which sea otters maintain reduced urchin densities through active foraging allows kelp to flourish (Estes and Palmisano, 1974). In other regions, disease (Nova Scotia) and fishing pressure (Maine) have been identified as controlling or reducing urchin populations (e.g., Scheibling and Hennigar, 1997; Steneck et al., 2013). In many regions of the world urchin barren grounds are expanding due to increasing stress on kelp beds as a result of pressures associated with climate change and new threats to kelp beds such as marine heat waves, the increase in algal turfs and detrimental invasive species such as the encrusting bryozoan
Membranipora membranacea (Filbee-Dexter and Wernberg, 2018; Krumhansl et al., 2011; Oliver et al., 2018;
Simonson et al., 2015; Vergés et al., 2014).
The biomass of urchins necessary to overgraze a healthy kelp bed is much higher than that required to maintain an urchin barren ground once kelp has been eliminated (a discontinuous phase shift; Filbee-Dexter and Scheibling, 2014b; Ling et al., 2015; Petraitis and Hoffman, 2010). For example, the biomass of green sea urchins (Strongylocentrotus droebachiensis) required to eliminate a kelp bed is high (2 to 5 kg.m-2) but the
biomass necessary to prevent recolonization by kelp is quite low (150 g.m-2) (Breen and Mann, 1976; Gagnon
et al., 2004). Many of the theories of what drives shifts between barren grounds and kelp beds are, however, based on observational studies that inevitably combine the effects of many unmeasured processes along with reductions in urchin density and grazing pressure.
Much of the work on urchin grazing in the past has focused on kelp beds and on destructive grazing in feeding fronts (e.g., Lauzon-Guay and Scheibling, 2007). In feeding fronts, dense aggregations of urchins graze aggressively on living plants in the kelp bed in contrast to barren grounds where urchins are largely distributed evenly across the substratum (i.e., do not form dense aggregations that persist in time) and forage using a dispersed browsing strategy (Scheibling and Hatcher, 2013). In many regions, such barren grounds are both spatially extensive and temporally stable. Given the importance of the urchin-kelp interaction in rocky subtidal systems and the high value (ecological and economic) of kelp beds, understanding the foraging behaviour of urchins in barren grounds is critical to understanding the urchin-kelp dynamic.
My objectives in this thesis are twofold: I first want to examine the factors contributing to shifts from urchin barren grounds to kelp beds and then explore the environmental modification of individual movement behaviour of urchins in barren grounds. Because observations of natural shifts from barren grounds to kelp beds necessarily combine the effects of many processes, I focussed on experimental perturbations of barren ground systems. Chapter 1, therefore, used both a literature review of past manipulative perturbations of urchin barren grounds and replicated experimental perturbations to examine the importance of spatial variability in determining the population-level response of urchins to perturbations. The objective of this chapter was to examine the population-level response of urchins to experimental perturbations in barren grounds, by focussing on the
7
movement of large individuals. The goal was to link reduced densities of urchins with reduced grazing pressure and therefore with an increased probability of kelp recolonization. The objective of Chapters 2 and 3 was to use smaller scale manipulations and observations to tease apart the influence of the presence of a key resource (drift kelp) and differences in seascape composition on individual movement behaviour. Chapter 2, therefore, used manipulated perturbations to infer urchin movement behaviour across a substrata barrier in the presence and absence of drift kelp by monitoring densities that immigrated into cleared areas through time. Chapter 3, by contrast, used direct observations of individuals (using time-lapse photography) combined with detailed maps of seafloor composition to describe how the movement behaviour of individuals changed in the presence of drift kelp and whether the change in behaviour was altered by the surrounding seafloor composition. Taken together, the three chapters of this thesis provide exciting and important information about the behaviour of the green sea urchin, a key marine invertebrate consumer, and will contribute to a more detailed understanding of urchin-kelp dynamics.
8
Chapter 1 : Experimental removal of urchins in
barren grounds: reduced density is not the only
determinant of macroalgal colonization
1.1 Résumé
Une des interactions fondamentales dans les communautés biologiques est celle entre un prédateur et sa proie. En conséquence, des perturbations expérimentales où des prédateurs sont enlevés du système sont essentielles pour comprendre les dynamiques des communautés. Les oursins de mer sont des herbivores voraces qui se nourrissent, par préférence, de macroalgues brunes (laminaires) et peuvent brouter à une telle intensité que des zones dénudées sont créées. Les dynamiques entre les bancs de laminaires et les zones dénudées sont d’un haut intérêt ; les changements de banc de laminaires vers des zones dénudées et vice-versa ont été observés dans plusieurs régions du monde. Des expériences qui tentent de décrire les liens de cause à effet entre l’abondance d’oursins et la recolonisation par des macroalgues ont été faites. Par contre, ce type de manipulations n’est pas très nombreux, est effectué avec des méthodologies très variables, et avec souvent très peu de réplications spatiales. Pour évaluer les liens de cause à effet entre l’abondance d’oursins et la recolonisation par les macroalgues dans les aires perturbées, nous avons combiné une revue de littérature avec notre propre expérience répliquée. Nos manipulations ont été effectuées dans les zones dénudées très stables du golfe du Saint-Laurent (nord-ouest de l’Atlantique), ou l’oursin vert (Strongylocentrotus
droebachiensis) est l’herbivore le plus important dans la zone infralittorale. La revue de littérature a démontré
que, dans deux tiers des cas, une diminution de l’abondance des oursins provoquait une recolonisation par des macroalgues. Des manipulations qui duraient plus longtemps ou qui utilisaient une méthode qui résultait en l’ajout de carcasses d’oursins morts (c.-à-d., écrasement d’oursins) ont vu une plus grande probabilité de recolonisation par des laminaires. Dans le Saint-Laurent, le site était le facteur qui avait le plus d’influence sur la probabilité de réussir à maintenir une densité d’oursins réduits pendant l’expérience. Cependant, aucune macroalgue n’a été observée pendant la durée de l’expérience, même aux sites avec des densités d’oursins qui restaient en dessous d’un seuil minimal établi auparavant, permettant la survie de macroalgues (150 g.m -2).
Les résultats de la revue de littérature, et nos expériences sur le terrain, ont clairement indiqué que les interactions, comme la compétition entre les grands les petits oursins, sont fort probablement essentielles pour déterminer les effets de changements dans l’abondance d’oursins. Ces résultats démontrent qu’il est impératif de considérer les interactions indirectes dans les zones infralittorales côtières pour être capable de prédire la réponse de ces communautés face aux perturbations. Une compréhension mécanistique du fonctionnement des communautés est encore plus essentielle étant donné que de telles perturbations vont devenir de plus en plus fréquentes dans un monde qui ressent les effets des changements climatiques.
9
1.2 Abstract
One of the most fundamental interactions in biological communities is between consumers and their resources. Consequently, experimental perturbations examining the effects of removing consumers are important in order to develop a mechanistic understanding of community dynamics. Sea urchins are voracious herbivores that graze preferentially on laminarian brown macroalgae (kelp). Therefore, the dynamics between productive kelp beds and the urchin barren grounds that result from destructive grazing have been a subject of intense interest. Shifts from kelp beds to urchin barren grounds and from barren grounds to kelp beds have been observed in many regions around the world and experimental perturbations examining the causal links between reductions in urchin abundance and recolonization by macroalgae have been carried out. However, manipulations have been infrequent, have used variable methodologies, and have generally involved very little spatial replication. In order to evaluate links between urchin density and macroalgal colonization in perturbed areas, we combined a global literature review of previous experimental manipulations with our own replicated urchin removal experiment. Our perturbation experiment was carried out in the extensive and temporally stable barren grounds of the Gulf of St. Lawrence (northwest Atlantic Ocean), where the green sea urchin (Strongylocentrotus droebachiensis) is the dominant subtidal herbivore. In the published literature, urchin removals result in kelp colonization in only two thirds of cases worldwide. Manipulations that were maintained for longer and those that employed a clearing method involving crushing urchins (and leaving the carcasses in
situ) increased the probability of kelp colonization. In the St. Lawrence, site was the most important determinant
of successful reduction of urchin densities, but no macroalgal growth was observed, even where large urchin densities were maintained below a threshold biomass previously determined to permit macroalgal regrowth (150 grams.m-2). The results of both the literature review and field manipulations indicate that indirect interactions
such as competition between large and small urchins are likely essential in determining the outcome of changes in large urchin abundance. These results clearly show that understanding indirect interactions in subtidal benthic communities is absolutely essential in order to be able to predict community responses to perturbations. A mechanistic understanding of community dynamics and responses to perturbations are particularly critical given the increasing stress placed on ecosystems by global climate change.
10
1.3 Introduction
The fundamental goal of ecology as a discipline is to provide explanations for the abundance and distribution of species. Community ecology focuses on understanding the interactions between species that coexist in the same habitat or system (Kerfoot and Sih, 1987; May, 1973). One approach is to use detailed natural history observations to describe the resources and habitat use of each species (Elton, 1927; Hutchinson, 1958; MacArthur, 1958). Another is to use experimental manipulations of species abundance to provide a mechanistic understanding of interactions between species (Connell, 1961; Diamond and Case, 1986; Hairston, 1990; Paine, 1969; Schmitz, 1998; Yodzis, 1988). In the context of classic manipulative experiments in ecology, a perturbation is defined as "selective alteration of the density of one or more members of the community" (Bender et al., 1984) and further classed as being one of two types: a PULSE manipulation involves a one-time alteration of the density of the target species followed by observations of the system as it returns to its previous state whereas a PRESS manipulation maintains the alteration while observing any shifts in the target community over time. Pulse manipulations, then, allow conclusions to be drawn only about direct interactions, whereas press manipulations provide an integrated community response that includes direct and indirect interactions between all the species present (Bender et al., 1984).
One of the most fundamental interactions in biological communities is that between consumers and resources, which can include predation, herbivory and parasitism (Lubchenco, 1979). Because of the importance of plants in ecosystems worldwide, the relationships between herbivores and the plants they consume have been some of the most-studied examples of consumption, with manipulative experiments (removals and/or exclusions) used in many systems (Diamond and Case, 1986; Hairston, 1990; Manier and Thompson Hobbs, 2007; McEachern et al., 2009; Menge, 1995; Post, 2013). Although the most obvious prediction of the resource response to a reduction in herbivore numbers is an increase in abundance or biomass, this accounts only for the direct interaction between the focal pair of species; communities are complex, however, and herbivore removals do not always have predictable effects. Significant effects of shifts in climate and other environmental factors (Ryerson et al., 2001), the functional traits of the plant species under question (Koerner et al., 2014) and herbivore diet breadth (Singer et al., 2014) have all been identified as having more influence in certain cases on community response than reductions in herbivory. In addition, indirect interactions make connections between species in communities much more complex, with effects sometimes passing through one or several mediating species before manifesting as an effect on a focal species (Nesbit et al., 2016; Schmitz et al., 1997; Wootton, 1994; Yodzis, 1988).
Coastal marine ecosystems are some of the most impacted habitats on earth, subject to a wide range of perturbations (Barbier, 2017; Halpern et al., 2008; Harley et al., 2006). The effects of climate change, including range shifts, rising temperature, increased frequency of extreme events such as heat waves, disease events or
11
storms and ocean acidification are impacting coastal ecosystems around the world (Campbell et al., 2014; Frölicher and Laufkötter, 2018; Harley et al., 2012; Oliver et al., 2018; Queirós et al., 2015; Sanderson et al., 2015; Scheibling and Lauzon-Guay, 2010; Smale and Vance, 2016; Vergés et al., 2014). Additionally, coastal areas are of enormous economic importance to a large proportion of the world’s population, providing many important ecosystem services (Barbier, 2017).
The interaction between sea urchins and their preferred food, large brown algae (principally in the orders Laminariales [hereafter referred to as 'kelp'] and Fucales), is one of the most globally important marine herbivore-plant interactions (Ling et al., 2015). Kelp are key habitat-forming primary producers in temperate rocky subtidal habitats, forming dense forests or beds and supporting diverse and abundant communities of associated species (Konar and Estes, 2003; Ling, 2008). Additionally, the productivity of kelp beds may provide important subsidies to other systems through the export of drift kelp (Filbee-Dexter et al., 2018; Kelly et al., 2012; Krumhansl and Scheibling, 2012; Reimer et al., 2018; Vanderklift and Wernberg, 2008), perhaps even contributing to carbon storage and sequestration when drift kelp is exported to the deep ocean and buried (Krause-Jensen et al., 2018). Urchins graze kelp beds, forming dense feeding fronts along their edges and, in many cases, destructively grazing these habitats to the point of elimination. The resultant areas, termed ‘urchin barren grounds’ are dominated by coralline algae and characterized by high densities of urchins and a lack of fleshy macroalgae (Lawrence, 1975). Urchin barren grounds have lower primary productivity, have lower overall value as habitat for associated species such as juvenile fish and support different assemblages of species than kelp beds (Begin et al., 2004; Kelly et al., 2011). Kelp beds and urchin barren grounds are even considered to be alternate stable states of rocky subtidal reefs (Filbee-Dexter and Scheibling, 2014b).
Shifts from kelp beds to barren grounds and from barren grounds to kelp beds have been documented in many regions of the world (Ling et al., 2015), and observational studies that take advantage of a catastrophic disturbance (e.g., disease outbreaks or drastic overfishing in which urchin densities are reduced close to zero) report algal regrowth and the reestablishment or expansion of kelp beds (Estes et al., 1978; Johnson et al., 2019; Kuwahara et al., 2010; Lauzon-Guay et al., 2009; Ling and Johnson, 2009; Moore and Miller, 1983; Pearse and Hines, 1979; Scheibling, 1986; Steneck et al., 2013). Reduced biomass of urchins in these cases is assumed to result in reduced grazing pressure which in turn leads to colonization by kelp and other macroalgae. Observational studies that take advantage of such 'natural experiments' (sensu Diamond, 1986) need, however, to be complemented by manipulative field studies to clearly elucidate the links between cause and effect. Moreover, the parameters and methods of experimental manipulations, typically targeting reductions in urchin densities and often with the goal of removing all urchins, are essential to consider. However, not all individuals in a population will exert the same grazing pressure as consumption generally scales with body size, so the largest individuals will have the largest impact (Suskiewicz and Johnson, 2017). In addition, some urchin species