OATAO is an open access repository that collects the work of Toulouse
researchers and makes it freely available over the web where possible
Any correspondence concerning this service should be sent
to the repository administrator:
tech-oatao@listes-diff.inp-toulouse.fr
This is an author’s version published in:
http://oatao.univ-toulouse.fr/25156
To cite this version:
Brunet, Magali and Malard, Benoît
and Ratel-Ramond, Nicolas and
Deshayes, Christophe and Joulié, Sébastien and Warot-Fonrose, Bénédicte and
Sciau, Philippe and Douin, Joël and De Geuser, Frédéric and Deschamps,
Alexis Precipitation in original Duralumin A-U4G versus modern 2017A
alloy. (2019) Materialia, 8. 100429. ISSN 2589-1529
Precipitation in original Duralumin A-U4G versus modern 2017 A alloy
Magali Brunet
a,
*
, Benoit Malard
b, Nicolas Ratel-Ramond
a, Christophe Deshayes
a,
Sébastien Jouli
e
, Bénédicte Warot-Fonrose
a, Philippe Sciau
a, Joël Douin
a, Frédéric De Geuser
c,
Alexis Descham ps
c• CEMES, CNRS, UniYemti de Toulouse, 29 rue Jeanne MIJJ'Vi& BP 94347 31055 Toulouse Cedex 4, Fronce • CIRIMA T, TNP Toulouse, CNRS, 4 aHé.e Emile Manso, 31030 Toulouse, France
• UniYemté Grenoble Alpes, CNRS, Grenoble TNP, SJMoP, F-38000 Grenoble, France
ARTICLE INFO ABSTRACT
Keyword.s: Duralumin Al-Co-Mg-Si alloy Predpitation SAXS STEM-HMDF
Precipitation in Duralumin, a historie quatemary alloy of the type: Al-Cu-Mg-Si, was never fully studied nor observed bycurrentelectron microscopytechniques. This article presents the full characterization and comparison of two alloys: a Duralumin (A-U4G) from the 19SOs collected on a vintage aircraft and its modern equivalent: a 2017 A alloy. The as-received and peak-aging states were analysed with DSC, SAXS and TEM advanced techniques. It is shown that old Duralumin and modern 2017A present a similar nanoprecipitation in the as-received state and behave similarly upon artificial aging. As opposed to what has been reported in the past, three types of precipitates participating in hardening were found upon aging: 8'-Al2Cu, Q'(Q)-AICuMgSi and Q-Al2Cu.
1. Introduction
Duralumin, with a composition close to 4 Cu 0.7 Mg 0.6 Mn 0.6 Si wt°/4, is the oldest age hardening aluminium alloy. Discovered in 1906 by Alfred Wilm, a German metallurgist [1], it triggered the develop ment of aeronautics at the beginning of the XXth century. At the sci entific and technical research centre in Neubabelsberg, Wilm experi mented many treatments on Al Cu Mn alloys with small amounts of magnesium (0.5 wt%). He found out that by quenching from tempera tures below its melting point (about 450'C) and by letting it age natu rally for a few days, the new alloy exhibited enhanced mechanical prop erties (strength and hardness). Thanks to its low density and strength, Duralumin soon became the prime choice for airplanes construction, well illustrated by the airplane Breguet 14 whose production reached 12,000 during World War I [2]. From then on, aircraft production, pre viously a craftsmen business, transformed into a real industry. Following on this discovery and for reaching even higher performance, metallur gists in the 1920s tuned aluminium alloys via the addition of microal loying elements. This was done empirically, as no explanation could yet be provided for the age hardening phenomenon. Merica et al [3] were the first team in 1929 to explain that the hardening during aging of du ralumin was due to fine and highly dispersed Al2Cu precipitates. But the concept of dislocation, essential to a complete understanding of the structural hardening mechanism, was introduced by Orowan in 1934 [4]. The discovery of coherent copper rich zones in the Al Cu 4% al loy was achieved independently by Guinier [5] and Preston [6] with X ray experiments in 1938. lt took even longer to be able to observe
• Corresponding author.
E-mail address: magali.brunet@cemes.fr (M. Brunet).
directly the nanoprecipitates responsible for the hardening: first obser vations were made on oxide replicas in France in 1952 by Saulnier and
Syre [7].
Original Duralumin was thus improved experimentally by increas ing the magnesium content (up to 1.5wt%), leading to a new alloy, called Duralumin FR in France in the early 1930s [8]. Duralumin and Duralumin FR became A U4G and A U4Gl respectively in 1943, to fol low a new designation defined by AFNOR1 [9]. From the 1930s to the 1950s, because of its lack of strength and ductility, A U 4G was replaced gradually in aeronautics construction by A U4Gl. ln 1954, international standardization of aluminium alloys took place [10]: the numerical des ignation with 4 numbers was adopted. A U4G was designated as 2017 A and A U4Gl as the well known 2024.
Original Duralumin has not received a major focus in the scientific literature related to the precipitation at nanoscale. ln fact, published works on the precipitation occurring in Duralumin and even on the modern 2017 A alloys are scarce. Original studies on the precipitation of industrial Duralumin were published in the 1950s. ln 1950, Lambot studied the precipitation of an industrial Duralumin by X ray abnor mal scattering [11]. ln accordance with results found by Guinier and Preston in Al Cu alloys, after quenching and room temperature aging, he detected scattering due to copper clusters or Guinier Preston Zones (GPZ). Then, upon aging at moderate temperatures, a first form of pre cipitates was evidenced, with a structure of three aluminium rich planes
1 Created in 1926, AFNOR is the French association for standardization.
Table 1
Elementalcompositionofalloys(inwt%)obtainedbyICP-OES.
Al Cu Mg Mn Si Fe Ti Zn Cr
2017A Base 4.32 ± 0.08 0.68 ± 0.01 0.611 ± 0.002 0.618 ± 0.006 0.34 ± 0.01 0.043 ± 0.001 0.20 ± 0.04 0.029 ± 0.003
A-U4G Base 4.18 ± 0.04 0.710 ± 0.005 0.67 ± 0.01 0.61 ± 0.01 0.285 ± 0.008 0.010 ± 0.001 0.082 ± 0.008 0.007 ± 0.0001
in between two copper richplanes. This phase evolved tothe well
distinguished𝜃’ Al2Cuphasewhenagingathighertemperatures.Other scatteringpatterns,superimposedtotheonesoriginatingfromAl2Cu precipitates,weredetected.TheywereattributedtotheAl4CuMg5Si4 phase.Thisphaseisexpectedatequilibriumstate,inalloysofMg/Si ratioslowerthan1.73[12].Twoyearslater,SaulnierandSyre[7]iden tifiedinasimilarindustrialDuralumin,thankstoelectronmicroscopy (usingoxidereplicas),thepresenceoftheso calledphaseX(renamed 𝜃’’later)afteragingat260°Cevolvingintothe𝜃’ Al2Cuphaseat290°C,
confirmingthefindingsofLambot.Anotherphasewasevidencedwith
thermaldilatometry.However,itwasattributedtoMg2Sineedles.In thiswork,theQ Al4CuMg5Si4phasecouldnotbeevidenced.Similarly,
YanoandKodain 1968[13], observedby transmissionelectronmi
croscopy(TEM),thepresenceofthe𝜃’ Al2CuandMg2SiphasesinDu ralumin.Morerecently,Härteletal.[14]studiedthe2017Aalloyand observedthe𝜃’phaseandanotherphase,ΩAlCu.However,theydidnot mentionthepresenceofQ AlCuMgSi,neitherMg2Si.Itistobenoted thoughthatthesiliconcontentwas,intheircase,verylow(0.08wt%).
Asshownhere,literaturedoesnotprovideaclearandcomprehensive
reportonprecipitationoccurringinDuralumin.
This article focuses on the observation and identification of the
metastablephasesandprecipitationsequencesoccurringinaDuralu
min(A U4G)of1958collectedonanoldaircraftandagedatambient
temperatureformorethan60years.Itwillbecomparedtoamodern
equivalent,the2017Aalloy.Whenlookingattheinternationalstan
dards[10],itisinterestingtonotethat,asopposedtotheA U4G1which
compositionissignificantlydifferentfromthemodernequivalent2024,
thecompositionofA U4G/2017Ahasbarelyevolvedovertime.Asthe
collectedDuraluminismorethan60yearsold,bycomparingits be
haviourandmicrostructuretothemodernequivalent,itisalsotheoc casiontostudylong termagingonthistypeofalloy.Thisconstitutesa firstmotivationforstudyingthisalloy.Thesecondmotivationisrelated toCulturalHeritage.Duraluminconstitutesmostoftheairplanes’fuse
lageandstructurebeforeandduringWorldWarII.Preciousspecimens
conservedinMuseumshouldbepreserved.Byunderstandinganddoc
umentingtheintrinsicconstitutionofthealloyanditsbehaviourover time,itwillbeeasiertoanticipateproblemssuchaslackofmechanical resistanceand/orcorrosion.
ThemicrostructureoftheA U4Gand2017Aindifferentstates,as
receivedandafterartificialaging,willbereportedhereafterbycom biningdifferenttechniques:conventionalbrightanddarkfieldTEM,se lectedareadiffractionpatterns(SADP),scanningtransmissionelectron
microscopycoupledwithenergydispersiveX rayspectroscopy(STEM
EDS),withhighangleannulardarkfield(HAADF)imagingorwithelec
tronenergylossspectroscopy(EELS),differentialscanningcalorimetry (DSC)andsmallangleX rayscattering(SAXS)experiments.Itisshown that,comparedtowhatcouldbeexpectedfromtheliteratureonquater
naryAl Cu Mg Sialloys[15,16]witharangeof2.5 4.5wt%ofcop
per,andaratioofMg:Sicloseto1orwhatwasobservedinthe1950s
and1960sonDuralumin[7,13],acomplexandspecificprecipitation
sequenceoccursinthesealloys.
2. Materialsandcharacterizationtechniques
2.1. Materials
ThestudiedalloyisaDuralumin,identifiedasA U4Gintheshapeof plate(about1mmthick)collectedonanaircraftfrom1958,aBreguet
Sahara765:thesamplewasextractedfromaflapofthelandinggear,
a partnotsubjectedtohottemperaturesrelatedtoengine proximity
(seeFig.S1ofsupplementaryinformation).Theequivalentmodernal
loy,ENAW 2017A(labelledhereafter2017A)wasboughtfromBikar
MetalleGmbH.ItwasreceivedinT4state.Ingeneral,for2017Aand
2024alloys,thestatesT3andT4arecommonforpartsoflowthickness (<12mm).Inthesestates,thealloyexhibitsagoodcorrosionresistance, ahightoughnessandagoodfatigueresistance[17].Theplatecollected ontheplanereceivedalsoaT4treatmentuponfabrication:itwasclearly mentionedintheBreguetstandards[18].However,forthisalloy,theas receivedstatecorrespondsinfact,toaT4stateplus10yearsofservice and50yearsinoutdoorsconditions.
For each alloy, the inductively coupled plasma optical emission
spectrometry (ICP OES) measurements, performed byEvans Analyti
cal GroupSAS,is reportedin Table1.It isconfirmedthatthecom
positionsaresimilar.Currently,2017Acontainssomezincwhereasold
versionof thisalloydoesnot.TheratiosMg:Siare1.1and1.16for
2017AandA U4G,respectively,whichcorrespondtoreportedvaluesfor Duralumin[7].
Forthestudyofhardeningprecipitation,twostateswerecompared:
as received andpeak agingconditions. Tofindpeak agingcondition,
received sampleswereheat treated at 180°C during different times.
Thesampleswerethencutalongthreeperpendiculardirectionsrela
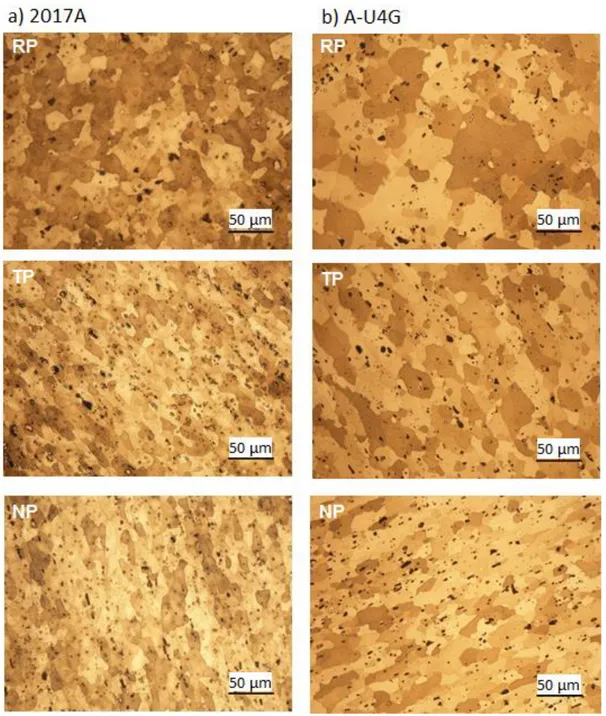
tivetotherollingdirectionandmeasurementsofmicro hardness(Vick ers)werecarriedoutinthecorrespondingplanes(RP:rollingplane;NP: normalplane;TP:transverseplane).
2.2. Characterizationtechniques
Thealloyswereanalysedbydifferent techniques,which required
adapted preparations.Forhardnessmeasurements aswell asfor the
observation of the microstructure by optical microscope (OM) and
scanningelectronmicroscope(SEM),eachspecimen(inthe3planes)
wasembeddedinanepoxyresinandmechanicallypolishedonwater
lubricatedabrasivepapers(siliconcarbide):P600thenP1200andeven
tually on polishing cloths with diamond paste (from 3μmdown to
1μm).
ForTEMobservations,thepreparationwasthefollowing:tobring
thesamplethicknessdownto25μm,amechanicalpolishingwithpaper
gradeupto2400SiC,wasperformed.Thespecimenswerethenelec
trochemicallythinnedusingaTenupol 5Struersapparatusoperatingat 60Vinasolutionofmethanolandnitricacid(3:1)at−15°C.Observa tionsofthenanostructurewerecarriedoutonaJEOL2010operatingat
200kV.Brightfieldimagesweretakenwithanorientationslightlyoff
the[100]Alzoneaxis.ChemicalelementswereidentifiedwithaCM20
FEGTEM/STEMmicroscope,operatingat200kVandequippedwithan
energydispersiveX rayspectroscopy(QuantaxEDSsystemwithsilicon
driftdetectorfromBruker).ForEDSmapping,anareaof235×88pix
elswas scannedwithaprobesizeofabout5nmandahighstatistic
(20000cps).
HAADFscanningTEMmicrographsofas receivedandheat treated
sampleswereacquiredusing aJEOLcold FEGJEM ARM200Finstru
mentoperatedat200kV(energyresolution,0.3eV)andequippedwith
aprobeCscorrectorwithaspatialresolutionof0.078nm.Toidentify thephasesandchemistry,energyelectronlossspectroscopy(EELS)spec
trawereacquiredusingaGIFQuantumERimagingfilter,between850
and1850eVinorder toincludetheCu L2,3 (931 951eV),theMg K
(1305eV),theSi K(1839eV)andtheAl K(1560eV)edges.EELSwas
performed onlinescansacrossprecipitatesaswellason somefixed
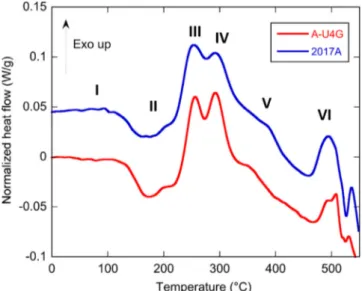
Fig. 1. DSCcurvesofA-U4Gand2017A,as-receivedstate.Theheatingrateis 20°C/min.2017Acurveisshiftedupwardforabettervisualisation.
DSCexperiments were alsocarried out.For this,sampleswith a
thicknessof1mmandaweightofabout20mgwereprepared.They
weresubjectedtorampheatingbetween−10°Cand550°Cat20°C/min inaMettler ToledoDSC3+.Resultswerecorrectedforbaselineandnor malizedforthesampleweight.
SAXSmeasurementswereperformedonanXeussequipmentpro
posedbyXenocs.TheX raysourceisequippedwithaCu anodeand
theSAXSsetupisequippedwithanti scatteringslitsand6MPilatusde
tector.Thebeamsizeis1mm2.ForSAXSexperiments,sampleswere
thinneddownto100μm.Inordertoinvestigatethelargeq rangeavail able,3sample to detectordistanceswereconsidered(40cm,1.2mand 2.5m).Inaddition,afixeddetectorcollectedtheintensityscatteredat wideangles,andallowedtoobtainthefirstdiffractionpeaksofthema
terial.Transmissionandbackgroundcorrectionsweremadeusingstan
dardprotocols.Thequantitativeanalysiswascarriedoutaccordingto theapproachdescribedinapreviousarticle[19].
3. Results
3.1. Asreceivedstate
DSCresultsforA U4Gand2017Ainas receivedstatearepresented
inFig.1.Sixdifferentregionscanbeidentified.Peaksaredirectlyre latedtoprecipitatesnucleationandgrowthinthealloy.Thefirstregion (I),fromroomtemperatureupto150°Cisflat.Itisgenerallyassumed
thatinthistemperaturerange,Guinier Prestonzonesshouldappear.
Here,intheas receivedalloys,GPZaresupposedlyalreadyformed.A
secondregion(II)containsanendothermicpeakbetween150°Cand
180°Candasmallexothermicpeakaround200°C,ascribedtothedis solutionofGPZandtheformationof𝜃’’phase,respectively.Thethird andfourthregions(IIIandIV)exhibittwolargeexothermicpeaks:one
between 220°C and280°C (III)and onebetween 280°C and320°C
(IV).InliteratureonAl Cu Mg Sialloys,thesepeakswereattributed todifferentphases.ItwasfirstobservedbySaulnierandSyre[7]that
anexothermic peakat290°Ccoincidedwith thepresenceof the𝜃’
Al2Cuphasewhereasthepeakat350°Cwasmarkedbytheapparition
ofaneedleshapeprecipitation,supposedlyMg2Si.Morerecentworks [20 22]reportapeakaround250°Casthegrowthofthequaternary(Q’ or𝜆’) AlCuMgSiphaseandapeakaround280°Castheapparitionofthe 𝜃’ Al2Cuphase.Thefifthregion(V)isasmallexothermicpeakbetween 350°Cand400°C,associatedbysomeauthorstotheincoherent𝜃 Al2Cu phaseprecipitation[22].However,insteadofaprecipitationpeak,this
shouldercouldcorrespondtothestartofthedissolutionofprecipitates presentinthealloy,whichoccursherebetween450°Cand520°C(re gion VI)andthenproceedstofullsolidsolutionatthesolutionizing temperature.
ThemainobservationfromFig.1isthesimilarityinbetween the
A U4Gand2017Abehaviour.Inthemeasurements,theobservedpeaks
areclosetowhatisreportedfortheDuraluminin1952bySaulnierand Syre[7]andforthe2017AalloybyHärteletal.[14].Similarprecip
itationshouldthusbeexpected.PeakVishoweverslightlyshiftedto
highertemperatures(around400°C)for2017AcomparedwithA U4G
(around350°C).Thiscouldbethesignofanearlierstartforprecipitate dissolutionintheA U4Galloy.
Representativebright fieldTEMimagesinconventionalmodeforthe
as receivedsamplesorientedin[001]AlzoneaxisareshowninFig.2(a)
and(b).Intheas receivedsamples,onlydispersoids(AlMnSi)canbe
observedinbrightfieldmode:theyarelargerinsize(125nm±51nmin
average)in2017AthanintheA U4G(57nm±25nminaverage),both
presentingalargestandarddeviation.Diffractionpatterns(notshownin Fig.2)donotpresentanyvisiblespotthatcanbeattributedtocoherent precipitation.
AtomicresolvedSTEM HAADFexperimentswereperformedonthe
as receivedalloys,seeFig.3fortheimagesofA U4Gin[001]Alzone axis.Precipitationinthematrixwashardlydetectable.InHAADF mode, seeFig.3(b)and(d),theintensityisproportionaltoZ1.5–1.8,Zbeing
theatomicnumber[23].Brightdots/areascorrespondtotheheavier
elementandarethusrepresentativeofcopperatoms.TheEDSanalysis
confirmedthepresenceofcopperinhigherconcentrationinthesezones
comparedwiththematrix.Thus,Cu richzones,notverywelldefined
andhavingasizeofafewnanometres,weredetectedhereandtherein thematrix(seeFig.3(a)and(b)).Someclusterswerealsolocatedatthe matrix/dispersoidinterfaces(seeFig.3(c)and(d)).
SAXSmeasurements,performedonA U4Gand2017Aalloysinthe
as receivedstate,areshowninFig.4.TheSAXSdataobservedonboth
specimensshowaverysimilarbehaviourthatcanbedescribedbytwo
maincontributions.Inthelowqrange(0.01<q<0.1Å−1),aclear q−4contributionisobserved,whichcanbeattributedtolargeprecip itatessuchasthedispersoids.Intherange0.1<q<1Å−1,aplateau isobserved,followedbyanintensitydecreaseasqincreases.Wehave modelledthiscontributionbyadistributionofsphericalprecipitatesof uniformelectrondensity(i.e.,composition)withapolydispersitygiven bytheSchultzdistribution(see[24]forthedetailedexpressionofthis
contribution). Aleast square fitof thedatagives meandiametersof
about8Å,whichisconsistentwiththeexistenceofGPZorsmallclus tersinthealloymatrix.Theexactnatureoftheclustersisatthisstage
notdetermined.
3.2. Artificialagingtreatmentat180°Candidentificationofprecipitates Fig.5presentstheVickershardnessasafunctionofagingtimeforthe
twostudiedalloys:A U4Gand2017A.OnlyRPhardnesswasplottedas
measurementsonotherplanesgavesimilarresults.TheA U4Gisharder
thanthemodernalloy2017Abyabout10 HVthroughouttheaging
curveexceptforlongover agingtimeswheretheA U4Ghardnessdrops
faster.Inbothcases,theconditionscorrespondingtothemaximumpeak hardnesswerereachedafter8hat180°C.Thesepeakageingconditions wereselectedfortheinvestigationofthemicrostructure.
Fig.6showstheSAXSmeasurementsafteragingfor8hat180°C.
Onceagain,bothalloysbehavesimilarly.Fromthesemeasurements,it
isseenthatthesignal,intherangeof0.1<q<1Å−1,disappearedin favourofasignalintherange0.04<q<0.2Å−1,whichtranslatesinto thedisappearanceofsmallclustersandtheformationoflargerparticles. Thiscontributiontothescatteredintensitycanbereproducedbyusing
aflatcylindermodel.Dimensionsofprecipitatesare:4nmthick and
70nmlongforA U4Gand3nmthickand40nmlongfor2017A.
Themicrostructurescorresponding tothispeak agedcondition in
Fig. 2. TEM bright field images of as-received al-loys(a)2017A;(b)A-U4Gshowingmainly AlMnSi-dispersoidsinthematrix.
Fig. 3. STEMimagesofas-receivedA-U4Galloyin[001]Al
zoneaxis;(a)and(c)Annularbrightfield;(b)and(d)high angleannulardarkfield.Images(a)and(b)ononesideand(c) and(d)ontheothersidecorrespondtotheexactsamearea. Aclusterisindicatedwiththewhitearrowattheinterface dispersoid/matrix.
Fig. 7: bright field images and corresponding selected area electron
diffractionpatternsarepresented.Fig.8showstheSTEM EDSmapping
fortheA U4Galloy.
After8hat180°C,averydenseprecipitationisobservedinsidethe matrix(Fig.7)andatgrainboundaries(Fig.8).Inbothalloys(Fig.7(a)
and(c)), precipitateslyingin {100}Al planes areobserved:some as
plateletsandothersasrods,withprecipitationondislocations.Asthe
observedprecipitationissimilarinbothA U4Gand2017Aalloys,ad
vancedanalysesresultsarethenonlyshownforA U4Galloy.
InFig.8,precipitatesatgrainboundaries wereidentifiedbyEDS:
theyare(Mg,Si) precipitatesandcopper precipitates.OntheseEDS
maps,dispersoidsofAlMnSinaturearepresent.Aprecipitationinthe matrixisalsovisiblebuthardlyidentifiable.
Asamatter of fact,different precipitatespopulationsco exist in
sidethegrainsandtheiridentificationwasmadepossiblebycombin
ingdifferentelectronmicroscopytechniques.FromSADPtakenalong
[001]Alzoneaxis,showninFig.7(b)and(d),thepresenceof𝜃’ Al2Cu phasewasevidencedthankstothestrikesalong<001>Aldirectionsand faintspots(110)𝜃’at 12 of(220)Al[25].Theycorrespondtothreevari
antsofplate shapesemi coherentprecipitatesgrowinginthe{100}Al planes:oneface onandtwoedge on.The𝜃’ Al2Cuprecipitatenature
wasconfirmedthankstoSTEM HAADFasshowninFig.9(b).Similarly
toHAADFimagestakenonas receivedstates,brightdotscorrespondto
copper richatomiccolumns.Seenedge on,thecrystalstructureofthis
plateletisbody centredtetragonalaspreviouslyshownbyBourgeois
etal.[26]andShenetal.[27].Thepresenceofpre𝜃’−1(Al2Cu)pre cipitatescoherentonthe{100}Alplanes,wasalsorevealedwithSTEM HAADF,asshowninFig.9(c)):thesetypesofprecipitateswererecently identifiedbyLiuetal.[28].Theyconsistofthreeparallelcopperplanes,
eachseparatedbytwoaluminiumplanes.OnSADP,diffractionpatterns
ofthepre𝜃’−1coincidewithdiffractionpatternsof𝜃’ Al2Cu.Bothtypes arethus,difficulttodifferentiate.Atpeak agedconditions,thisphase, whichcorrespondstothefirststageof𝜃’ Al2Cuformation,isnotpre dominant:mostofthepopulationhasevolvedtothe𝜃’ Al2Cuphase.
OtherprecipitatesintheshapeofrodswereobservedintheA U4G
alloyafter8hat180°C,asshowninthebrightfieldimagesofFig.7(a) andintheHAADFimageofFig.9(a).Therods,seenend on,areoriented along<001>Aldirections.Noclearidentificationbeingpossiblewith
Fig. 4. SAXScurvesfor2017AandA-U4Gintheas-receivedstate.Themodel wassuperimposedtotheexperimentalcurves.
Fig. 5. Hardnessmeasurementonrollingplaneforold(A-U4G)andmodern (2017A)alloysheattreatedat180°C.
SADP,thisphasewasidentifiedthankstoSTEM EELS.Rodsappeared
tobetheQ AlCuMgSiphaseoroneofitsprecursors(Q’).Thecomposi tionfoundforthisphasebySTEM EELSwasinaverage73±10Al 10±4 Cu 12±6Mg 5±5Siat%.Aluminiumcontentforthisphaseisoveresti
matedaspartofthematrixisalsoprobed.Moreover,asobservedon
theSTEM HAADFimage(Fig.10(b))andconfirmedbySTEM EELS,the
precipitateshaveacore shellstructurewithacorerichinsiliconand
magnesiumandacopper richshell.Thiscore shellstructureobserved
herecoincideswithresultsreportedbyMatsudaetal.in2007[29]and Biswasetal.in2014[21].
InFig.9(b),oneofthequaternaryprecipitateisseenedge on,co precipitatedwitha𝜃’ Al2Cuprecipitate.Inthisorientation,bySTEM
HAADF,theQ’(Q) AlCuMgsiphase canbe recognizedbytheCu rich
columns (highintensity due tohighatomic number) surrounded by
lowerintensitycolumns(Si,AlandMg)similartothecross sectionstruc tureviewedalong<100>AlzoneaxisshowninDingetal.[30].
Eventually,athirdphase is present,detectedthankstothespots
at1/3and2/3ofthe[022]AlorientationinSADP(Fig.7(b)and(d)). STEM HAADFintwoorientations[110]Aland[112]Al(seeFig.11)was necessarytoidentifythisphaseastheΩAl2Cu.Thisphaseisknownto
haveanorthorhombicstructure,spacegroupFmmmwitha=0.496nm;
b=0.859nmandc=0.848nmandtoprecipitateinthe{111}Alplanes
[31].MeasurementsofCu CudistancesonSTEM HAADFimagesalong
Fig. 6. SAXScurvesfor2017AandA-U4Gatpeak-agingstate:after8hat 180°C.Themodelwassuperimposedtotheexperimentalcurves.
[001]Ω,[100]Ωand[010]Ωcorrelatewiththeexpectedlatticeparame ters.Theseprecipitatesgrowpreferentiallyondispersoids(AlMnSi).
Alltheobservationsprovidedaclearevidenceonthephasespresent
uponartificialaging: atgrain boundaries,copperandMg Si precipi
tateswereformedwhileinthematrix,𝜃’ Al2Cu,Q’(Q) AlCuMgSiand
ΩAl2Cuwereclearlyidentified.
4. Discussion 4.1. Hardnessevolution
ThehigherhardnessofA U4Gcomparedto2017Aintheas received
state(about10HVdifference)couldbeexplainedbydifferentfactors.
Whenconsidering precipitationat nanoscale,SAXS experiments pro
videdevidenceofthepresenceofclustersinas receivedstatewhether
inA U4Gorin2017A.DSCconfirmedthepresenceofthispopulation
intheas receivedstate,asadissolutioneventoccurredaround180°C. These clusters,withadiameterin therangeof8Å,aretheprincipal
causeofhardnessinDuralumin.However,itwasshownthatthenano
precipitationisidenticalinbothalloys,whichrulesoutthisfactoras theprimarycauseinthehardnessdifference.Secondly,dispersoidscon tributepartlytohardnessbycontrollingthegrain size.Itwasshown
thattheyaresmallerandmorenumerousinA U4Gthanin2017A.This
couldbeanexplanationforthedifferenceinhardness.However,since
thegrainsizeiscloseinbothalloys(seeFig.S2inSupplementaryin
formation), thecontributionofdispersoidsshould notbe significant.
Thethirdexplanationcomesfromtheplates’conditions.Theoldplate
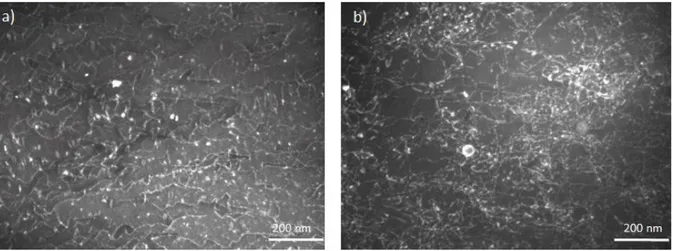
wasbentwhencollected.Thisactiongeneratedstrainhardening,with
ahigherdensityofdislocations(seeFig.S3inSupplementaryinforma tion).Thepresenceofthesedislocationsisbelievedtobethemainfactor inthehigherhardnessoftheA U4Galloyintheas receivedstate.
Forover ageingconditions,thehardnessoftheA U4Galloydrops
fasteruntilreachingthehardnessofthemodernalloy.Itcanbehypoth esized thatafterartificialageing,thehistoricalalloylosespartofits additionaldislocationdensitythroughfasterrecovery.Thefinalpartof thehardnesscurvecouldalsobeexplainedbethefactthatalloysage fasterwhenadditionaldislocationsareintroduced,becausethedisloca
tionnetworktransportssoluteatomsthroughpipediffusion[32]and
thusacceleratesgrowthandcoarseningof𝜃’precipitates,knowntonu cleateondislocations[33].Thisgrowthandcoarseningwillinducea decreaseinhardness.
Fig. 7. TEM bright-field images andcorresponding SADPin[001]Al zoneaxisaftertreatmentat180°C–
8hfor2017A:(a)and(b) andforA-U4G:(c) and (d).(110)𝜃’spotsandstrikesalong[001]Aldirections
forthe𝜃’phaseareindicatedwitharrows.Spotsat 1/3and2/3of(022)AlcorrespondingtoΩphaseare
surrounded.
Fig. 8. STEM-EDSmappingaftertreatmentat180°C–8hinA-U4Gshowingprecipitationatgrainboundaries:MgSiandCuaswellasinthematrix.
4.2. Nano structureintheas receivedstate
About thenano clusters presentin theas received states, several
pointscanbemade.Thecopper richclustersobservedbyatomically
resolvedTEMimagesinHAADFmodewerenotsufficientlynumerous
tocorrelatewiththemeasuredSAXSsignal.Electronmicroscopyisthus
notfullysucceedinginobserving andanalysingnanoclusters.Onan
otherhand,atthisstage,ourexperimentscannotdifferentiatebetween GPZ(flatclustersgrowingintheplanesparalleltothe<100>Alaxes)
anddisorderedclusters.GPZareusuallyfoundinthemodelAl 4%Cu
alloyagedatroom temperature,asitwasfirstlyprovedbyGuinierand
PrestonbyXRDin1938.Lambot[11],whostudiedbothAl 4%Cuand
DuraluminbyX rayscattering,provedthatthescatteringsignalwasper fectlyidenticalinbothalloys,concludingthusthatGPzonesarepresent inDuraluminagedatroomtemperature.Itistobenotedthattheirstud ieswerecarriedoutonsinglecrystals.Inthepresentcase,SAXSsignal
isanaverageofthemeasurementsmadeonseveralgrains:theinfor
Fig. 9. (a)STEMBright-fieldimageofA-U4G alloy in [001]Al zone axisshowing𝜃’-Al2Cu
(blackarrows)andQ-AlCuMgSirods(white ar-rows),(b)HAADFimagesof𝜃’-Al2Cuplatelets
seenedge-onwithco-precipitation of Q’(Q)-AlCuMgSiand(c)pre-𝜃’−1Al2Cudisksseen
edge-on.
Fig. 10. (a)STEMBright-fieldimageofA-U4G alloyin[001]Alzoneaxis(b)STEM-HAADF
im-ageofQ-AlCuMgSirodsseentop-on.
Furtherinvestigationisrequiredtoconfirmthestructuralnatureofthese clusters.
4.3. Uponartificialaging identificationofprecipitation
Uponartificialaging(at180°C for8h),itwasdemonstratedthat
threepopulationsofprecipitatescoexistinthematrix:𝜃’ Al2Cu,Q’(Q)
AlCuMgSiandΩAl2Cu.LinkingbacktheTEMobservationswithDSC
resultsandthankstoresultsreportedintheliteratureforAl Cu Mg
Sialloys,itcanbe hypothesizedthatregionIII(between220°Cand
280°C)isrelatedtothegrowthofQ’(Q) AlCuMgSiphaseandregionIV (between280°Cand320°C)tothegrowthofthe𝜃’ Al2Cuphase.Asfor theΩAl2Cuphase,havingthesamestoichiometryasthe𝜃’ Al2Cuphase andifitsgrowthiscontrolledbydiffusion,therecouldbesuperimposi tionofpeaksintheDSCcurve.
The𝜃’ Al2Cuphase,foundinlargequantity,isnotsurprising.Itcon
stitutesthemainhardeningphasein the2xxxfamilyalloysandwas
reportedinallstudiesonDuraluminor2017Aalloy[7,11,13].
The presence of the Q AlCuMgSi phase or one of his precursors
(Q’)wasevidenced,inbotholdandmodernalloys.Althoughnotsys
tematicallyrevealedinprevious studiesof Duralumin,thesefindings
confirmtheequilibriumdiagramsdatareportedbyMondolfoin1943
[12]whichpredictsthatwithMg:Siratio<1.73,hardeningconstituents shouldleadtotheAl2CuandAl4CuMg5Si4equilibriumphases.Thesil
icon contentisindeedhigherthanthatrequiredfortheformationof
Mg2Si(Mg:Si=1.08):theexcessof silicon willcombinewithcopper
andmagnesiumtoformthequaternaryphase.Theglobalstoichiomet
riccompositionfortheQphasehasbeen,however,acontroversialmat ter.Mondolfopredictstwopossiblequaternaryphases:Al4CuMg5Si4or Al4CuMg4Si4.Inmorerecentliterature,mainlyforalloysofthe6xxx family(Al Mg Siwithadditionsofcopper)[15],butalsofor2014al loys[20],thestoichiometryreportedinbulkalloyswereAl5Cu2Mg8Si6, Al4Cu2Mg8Si7, Al3Cu2Mg9Si7 etc.However, the nanometre scale of theseprecipitatesmakesthequantitativeanalyseschallenging.Biswas
et al.[21]usedatomprobe analysisandprovedthat theQprecipi
tatesevolved,uponaging,fromacopper richcomposition(44Al 22Cu
16Mg 16,5Siat%)toamagnesiumandsilicon richcomposition(28Al
9Cu 37Mg 26Siat%.)after4hat260°C.Inaveryrecentpaper,Ding
etal.[30]wereabletoprovide,bycombiningatomprobetomography
andatomiccolumnintensityquantificationinSTEM HAADFimages,the
reasonwhyvaryingcompositionwerereportedinpastwork:itwasin
deedprovedtobeduetotheoccupancychangeoftheatomiccolumns.
ThestoichiometryoftheQphaseanditsprecursor,theQ’phasewere
Fig. 11. STEMBright-fieldimagesandHAADF imagesofA-U4Galloyin(a)and(b):[110]Al
zoneaxis;(c)and(d):[1̄12]Alzoneaxis
show-ingΩ-Al2Cuplatelets(whitearrows)growing
ondispersoids.
matrixeffectscannotbeexcludedwithEELS,theelementalcomposi
tionfoundherebyEELSinDuralumin(73±10Al 10±4Cu 12±6Mg
5±5Siat%)isinallcasesmuchleanerinmagnesiumandsiliconthan whatisusuallyreported.Thisresultsshouldbecorroboratedwithother
techniquestoconfirmthetendency.Theheterogeneousnucleationof
𝜃’ Al2CuphaseandtheQ’(Q) AlCuMgSiphase,observed inFig.9(b), wasalsoreportedbyotherauthors[21,34].Itcouldbecausedbythe strainfieldofQ phaseprecipitatesonwhichthe𝜃’ Al2Cunucleate.
Anotherphase,ΩAl2Cu,ishereobserved.TheΩphaseisusually
foundinhighdensityinAl Cu Mg Agquaternaryalloyswithahigh
Cu:Mgratio[25,31].Itpresents oneofthehigheststrengtheningpo
tentialin aluminiumalloys. Inparticular, Al Cu Mg Agalloys have
beendevelopedforelevated temperatureaerospaceindustryapplica
tions[35]duetotheenhancedthermalstabilityoftheΩphase.Some
authorsprovedthatsilverwasnotrequiredfortheformationofΩpre cipitatesalthoughitspresenceincreasestheirdensity[36].TheΩphase
wasindeedobservedin2024and2124alloysbyWangetal.[37]and
wasactuallyfoundin2017Aalloyaswell[14].In2024and2014al
loys,theΩphasewasfoundtoformpreferentiallyonmanganese based
dispersoids.Thisheterogeneousgrowthisclearlyevidencedinourre
portedresults.TounderstandwhysuchaphaseappearedinDuralumin,
itisinterestingtodiscussitsnucleationprocess.InAl Cu Mg Agalloys,
theprocesshasbeenwidelyinvestigated:uponaging,magnesiumand
silverco cluster,thencopperatomsaggregatetothe{111}Al planes. Duringcoarsening,whencopperconcentrationbecomesclosetoAl2Cu, silverandmagnesiumstarttomigratefromtheinteriortotheinterface oftheprecipitate.Theinterfacialstructurewasprovedtobeadouble
layercomposedofAginhexagonalstructureandMgandCubelowthe
centreofthehexagon[38].Magnesiumisconsiderednecessaryforthe
nucleationoftheΩphase.Asforsilver,itsroleistoacceleratetheaggre gationofmagnesiumatomsbyretainingvacanciesandtoassistinthis waythemagnesiumdiffusion[39].Siliconisalsoanelementthatinflu encesthepresenceofΩphase.FromPolmearetal.[35],silicon(and iron)shouldremainlow(lessthan0.10wt%)tominimisetheformation
oflowmeltingpointeutecticsatgrainboundariesandtomaximiseΩ
precipitation.Infact,highlevelsofsiliconinterferewithprecipitationof Ωbecausesiliconinteractswithmagnesiumandthusreducestheforma tionMg Agco clusters,whichgenerallyfacilitatenucleationofΩ.This wasalsoobservedintheworkbyÜnluetal.[40].However,theauthors showedthatitisrathertheMg:Siratiothathadtobeconsidered:some
Ωprecipitationwasfoundinalloyswithsiliconlevelsashighas0.5
and0.65wt%withcorrespondingMg:Siratiosof3.0and2.23.Inthe
presentalloys,theobservationthatΩformsprincipallyondispersoids
intheDuraluminand2017Acanbeexplainedbythepresenceofcop
perclustersatthematrix/dispersoidinterfaceintheas receivedstate.
Moreover,itdoesnotformasaphasedispersedinthematrixbecause
oftheabsenceortheverylowdensityofmagnesiumclustersinthema trix.Themagnesiumclustersareinthiscaseimpededbysilicon,which ratherfacilitatestheformationoftheQ AlCuMgSiphase.
Conclusion
AnoldDuralumin(A U4G)anditsmodernequivalent(2017A)were
studiedwithdifferentcharacterizationtechniquestoclarifytheprecip itationprocesses.Themainfindingsare:
TheprecipitationsequenceinDuraluminandmodern2017Aupon
artificialaging issimilar. Itconsistsin 𝜃’ Al2Cu,Q’(Q) AlCuMgSi andΩAl2Cuinthematrix.Atgrainboundaries,variousprecipitates
arefound:copper basedand(Mg,Si)precipitateswithunknownsto ichiometry.
TheQ AlCuMgSiphaseoroneofitsprecursors(Q’)isobservedin
thematrixbutthe𝛽 Mg2Siphase,previouslyreportedinDuralumin, isnotpresent.Thisphasefacilitatesthenucleationofthe𝜃’ Al2Cu phasethroughstrainfield.
TheΩphaseisobservedanditgrowspreferentiallyondispersoids.
Itpresumablynucleatesdue tothepresenceof copperclustersat
thematrix/dispersoidinterface.Thisphaseisaminorphasedueto theabsenceorrareoccurrenceofmagnesiumclustersinthematrix.
ThenucleationmechanismsfortheΩphaseondispersoidsinthese
Al Cu Mg Sialloysremainstobeevidenced.
Intheas receivedstate(naturallyaged),thepresenceofclustersis evidenced,withcopperasthemainelementbutthestructuralnature oftheseclustersisyettobedetermined.
Despitesomedifferencesinthemicrostructure,suchasthedisper
soids’sizeanddensity,Duraluminexhibitsasimilarnanoprecipita tionasitsmodernequivalentinthenaturallyagedconditions,andit behavesalsosimilarlyuponartificialaging.Theseobservationslead
totheconclusionthattheconditionsexperiencedbythecollected
plate(10yearsofserviceplus50yearsatambienttemperature)had
nosignificantimpactonthenanostructure.
Overall,theseresultsconstituteabaseforassessingtheverylong termagingofaluminiumalloysinaeronautics.
DeclarationofCompetingInterest
None
Acknowledgments
TheauthorswhichtothankPierreRoblinfromFRFERMAT,Uni
versité deToulouse,CNRS,Toulouse,France,forSAXSmeasurements,
TeresaHungria Hernandez,fromRaimondCastaingMicroanalysisCen
tre,ToulouseforSTEM(HAADF,EDSandEELS)experimentsandthe
volunteersofLesAilesAnciennesToulouseforprovidinguswiththeplate collectedontheBreguetSahara765aircraft.
ThisstudyhasbeenpartiallysupportedthroughthegrantNanoXn°
ANR 17 EURE 0009intheframeworkofthe“ProgrammedesInvestisse
mentsd’Avenir”.
Supplementarymaterial
Supplementarymaterialassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.mtla.2019.100429.
References
[1] O. Hardouin Duparc , Alfred Wilm et les débuts de Duralumin, Cah. d’hist. de l’alum. Inst. Pour l’Hist. de l’Alum. Paris (2005) 63–77 .
[2] M. Victor , Comment a été construit le premier avion en Duralumin, Rev. Alum. (1951) 339–340 .
[3] P.D. Merica , R.G. Waltenberg , H. Scott , Heat treatment of Duralumin, Sci. Pap. Bur. Stand. 15 (1919) 271–316 Scientific Paper 347 (S347) .
[4] E. Orowan , Zur kristallplastizität. III, Z. Phys. 89 (9) (1934) 634–659 .
[5] A. Guinier , Structure of age-hardened aluminium-copper alloys, Nature 142 (1938) 569 .
[6] G.D. Preston , LXXIV. The diffraction of X-rays by an age-hardening alloy of alu- minium and copper. The structure of an intermediate phase. The London, Edinburgh, and Dublin, Philos. Mag. J. Sci. 26 (178) (1938) 855–871 .
[7] A. Saulnier , R. Syre , Maturation, revenu et réversion des alliages Al–Cu 4%, Al–Cu–Mg et Al–Cu–Mg–Si de haute pureté, Rev. Met. Paris 49 (1) (1952) 1–21 .
[8] Duralumin, notice générale, Société du Duralumin, Paris, 1938.
[9]H. Lebouteux , La symbolisation de l’aluminium et de ses alliages en France et dans quelques pays étrangers, Encycl. Trav. Alum. Extrait Rev. Alum. Paris (1956) 1–3 .
[10] The-Aluminum-Association, International alloy designations and chemical com- position limits for wrought aluminum and wrought aluminum alloys, 2015
http://www.aluminum.org/resources/industry-standards/aluminum-alloys-101 .
[11]H. Lambot , Etude cristallographique de la précipitation structurale dans les duralu- mins, Rev. Met. Paris 47 (10) (1950) 709–726 .
[12]L.F. Mondolfo , Metallography of Aluminum Alloys, John Wiley & Sons, Inc., New York, USA, 1943 .
[13]S. Yano , S. Koda , Electron microscope observation of duralumin, J. Jpn. Inst. Light Metals 18 (7) (1968) 371–376 .
[14] M. Härtel , S. Wagner , P. Frint , M.F.-X. Wagner , Effects of particle reinforcement and ecap on the precipitation kinetics of an Al–Cu alloy, IOP Conf. Ser.: Mater. Sci. Eng. 63 (1) (2014) 012080 .
[15]D.G. Eskin , Decomposition of supersaturated solid solutions in Al–Cu–Mg–Si alloys, J. Mater. Sci. 38 (2) (2003) 279–290 .
[16]J.-F. Nie , 20 – Physical metallurgy of light alloys, in: D.E. Laughlin, K. Hono (Eds.), Physical Metallurgy, 5th Edition, Elsevier, Oxford, 2014, pp. 2009–2156 .
[17]R. Develay , Propriétés de l’aluminium et des alliages d’aluminium corroyés, Tech. de l’Ing. M440 – V2 (1992) .
[18] Breguet, Standard forms, Vélizy factory, Archives Les Ailes Anciennes Toulouse, 1946-1954.
[19]A. Deschamps , F. de Geuser , Quantitative characterization of precipitate microstruc- tures in metallic alloys using small-angle scattering, Metall. Mater. Trans. A 44 (1) (2013) 77–86 .
[20]I. Dutta , C.P. Harper , G. Dutta , Role of Al 2O 3particulate reinforcements on precipita- tion in 2014 Al-matrix composites, Metall. Mater. Trans. A 25 (8) (1994) 1591–1602 .
[21]A. Biswas , D.J. Siegel , D.N. Seidman , Compositional evolution of Q-phase precipi- tates in an aluminum alloy, Acta Mater. 75 (Suppl. C) (2014) S322–S336 .
[22]P. Bassani , E. Gariboldi , D. Ripamonti , Thermal analysis of Al–Cu–Mg–Si alloy with Ag/Zr additions, J. Therm. Anal. Calorim. 91 (1) (2008) 29–35 .
[23]S.J. Pennycook , Z-contrast stem for materials science, Ultramicroscopy 30 (1) (1989) 58–69 .
[24]M. Kotlarchyk , S.H. Chen , Analysis of small angle neutron scattering spectra from polydisperse interacting colloids, J. Chem. Phys. 79 (5) (1983) 2461–2469 .
[25]S.C. Wang , M.J. Starink , Precipitates and intermetallic phases in precipitation hard- ening Al–Cu–Mg–(Li) based alloys, Int. Mater. Rev. 50 (4) (2005) 193–215 .
[26] L. Bourgeois , C. Dwyer , M. Weyland , J.-F. Nie , B.C. Muddle , Structure and energetics of the coherent interface between the 𝜃′ precipitate phase and aluminium in Al–Cu, Acta Mater. 59 (18) (2011) 7043–7050 .
[27]Z. Shen , Q. Ding , C. Liu , J. Wang , H. Tian , J. Li , Z. Zhang , Atomic-scale mechanism of the 𝜃″ →𝜃′ phase transformation in Al–Cu alloys, J. Mater. Sci. Technol. 33 (10) (2017) 1159–1164 .
[28]C. Liu , Z. Ma , P. Ma , L. Zhan , M. Huang , Multiple precipitation reactions and for- mation of 𝜃’-phase in a pre-deformed Al–Cu alloy, Mater. Sci. Eng.: A 733 (2018) 28–38 .
[29]K. Matsuda , D. Teguri , T. Sato , Y. Uetani , S. Ikeno , Cu segregation around metastable phase in Al–Mg–Si alloy with Cu, Mater. Trans. 48 (5) (2007) 967–974 .
[30]L. Ding , A. Orekhov , Y. Weng , Z. Jia , H. Idrissi , D. Schryvers , S. Muraishi , L. Hao , Q. Liu , Study of the Q ′ (Q)-phase precipitation in Al–Mg–Si–Cu alloys by quantifica- tion of atomic-resolution transmission electron microscopy images and atom probe tomography, J. Mater. Sci. 54 (10) (2019) 7943–7952 .
[31]K.M. Knowles , W.M. Stobbs , The structure of {111} age-hardening precipitates in Al–Cu–Mg–Ag alloys, Acta Crystallogr. Sect. B 44 (3) (1988) 207–227 .
[32]M. Legros , G. Dehm , E. Arzt , T.J. Balk , Observation of giant diffusivity along dislo- cation cores, Science 319 (5870) (2008) 1646 .
[33] C. Liu , S.K. Malladi , Q. Xu , J. Chen , F.D. Tichelaar , X. Zhuge , H.W. Zandbergen , In-situ stem imaging of growth and phase change of individual CuAlX precipitates in Al alloy, Sci Rep 7 (1) (2017) 2184 .
[34]S. Wenner , C.D. Marioara , S.J. Andersen , M. Ervik , R. Holmestad , A hybrid alu- minium alloy and its zoo of interacting nano-precipitates, Mater. Charact. 106 (2015) 226–231 .
[35]I.J. Polmear , G. Pons , Y. Barbaux , H. Octor , C. Sanchez , A.J. Morton , W.E. Borbidge , S. Rogers , After concorde: evaluation of creep resistant Al–Cu–Mg–Ag alloys, Mater. Sci. Technol. 15 (8) (1999) 861–868 .
[36]A. Garg , Y.C. Chang , J.M. Howe , Precipitation of the Ω phase in an Al–4.0Cu–0.5Mg alloy, Scr. Metall. Mater. 24 (4) (1990) 677–680 .
[37]L.M. Wang , H.M. Flower , T.C. Lindley , Precipitation of the Ω Phase in 2024 and 2124 Aluminum Alloys, 1999 .
[38]S.J. Kang , J.-M. Zuo , H.N. Han , M. Kim , Ab initio study of growth mechanism of omega precipitates in Al–Cu–Mg–Ag alloy and similar systems, J. Alloys Compd. 737 (2018) 207–212 .
[39]G.B. Winkelman , K. Raviprasad , B.C. Muddle , Stimulation of the Ω phase in an Al–1.1 at% Cu–0.5 at% Mg alloy by a duplex ageing treatment involving initial nat- ural ageing, Philos. Mag. Lett. 85 (4) (2005) 193–201 .
[40]N. Ünlü, B.M. Gable , G.J. Shiflet , E.A. Starke , The effect of cold work on the pre- cipitation of Ω and 𝜃′ in a ternary Al–Cu–Mg alloy, Metall. Mater. Trans. A 34 (12) (2003) 2757–2769 .


![Fig. 7. TEM bright-field images and corresponding SADP in [001] Al zone axis after treatment at 180 °C–](https://thumb-eu.123doks.com/thumbv2/123doknet/2965823.82010/7.892.57.769.88.911/fig-tem-bright-field-images-corresponding-sadp-treatment.webp)
![Fig. 9. (a) STEM Bright-field image of A-U4G alloy in [001] Al zone axis showing](https://thumb-eu.123doks.com/thumbv2/123doknet/2965823.82010/8.892.57.602.81.731/fig-stem-bright-field-image-alloy-zone-showing.webp)
![Fig. 11. STEM Bright-field images and HAADF images of A-U4G alloy in (a) and (b): [110] Al](https://thumb-eu.123doks.com/thumbv2/123doknet/2965823.82010/9.892.58.600.84.623/fig-stem-bright-field-images-haadf-images-alloy.webp)