IN VITRO AND EX VITRO POTATO PLANTLETS
(SOLANUM TUBEROSUM) METABOLIC RESPONSE
TO EXOGENOUSLY SUPPLIED SUCROSE: A
METABOLOMIC APPROACH
Thèse présentée
à la Faculté des études supérieures de l'Université Laval dans le cadre du programme de doctorat en biologie végétale
pour l'obtention du grade de philosophiae doctor (PH.D.)
DEPARTEMENT DE PHYTOLOGIE
FACULTÉ DES SCIENCES DE L'AGRICULTURE ET DE L'ALIMENTATION UNIVERSITÉ LAVAL
QUÉBEC
2011
Résumé court
L'application commerciale de la culture in vitro est limitée à la propagation massive de certaines espèces végétales en raison des pertes excessives rencontrée lors de la phase de l'acclimatation. On pense que le saccharose ajouté au milieu de culture serait responsable de ce problème. L'objectif de cette thèse était d'approfondir nos connaissances sur les effets du saccharose exogène et des conditions in vitro sur la croissance, la physiologie et la biochimie des plantules de pomme de terre (Solanum tuberosum L.) in vitro et ex vitro. Comme prémisse à la recherche nous nous sommes intéressés à déterminer comment le sucre est absorbé et transporté dans les plantules. Les plantules autotrophes et mixotrophes absorbent et métabolisent le sucre de manière très distincte. Les premières absorbent et transloquent de faibles quantités de saccharose et relâchent même de fortes quantités des sucres dans le milieu. Les plantules mixotrophes accumulent des sucres radioactifs dans la tige et dans les feuilles. Grâce à une approche métabolomique nous avons étudié de manière approfondie la réponse métabolique des plantules in vitro et ex vitro de sucres exogènes. Nos données de métabolomique expliquent certains des problèmes trouvés dans les conditions de culture in vitro et leur impact sur l'acclimatation ex vitro. Par exemple, l'analyse métabolomique révèle que les conditions de culture in vitro ont provoqué des changements très importants des métabolismes de l'azote et du carbone; des niveaux plus élevés d'acides aminés, de metabolites de stress, de polyamines, d'intermédiaires de la photorespiration, de sucres alcools osmotiquement actifs, et des niveaux inférieurs des intermédiaires du cycle de Krebs et de la glycolyse, caractérisent le phénotype métabolique distinct des plantules in vitro mixotrophes. Nous suggérons que ces changements dans les niveaux d'accumulation des metabolites représentent une réponse adaptative de la plantule in vitro à la quantité excessive d'azote dans le milieu de culture MS et aux conditions de stress osmotique causés par la présence de sucre exogène.
Résumé long
La micropropagation est largement utilisée pour la propagation rapide de certaines espèces végétales. Malgré son potentiel, cette technique présente plusieurs problèmes qui réduisent son efficacité et sa rentabilité. Une des limitations la plus importante est le faible pourcentage de survie à l'environnement naturel après le transfert des conditions in vitro à ex vitro. L'environnement in vitro est complètement différent de celui ex vitro. Les conditions de culture in vitro sont caractérisées par une forte humidité relative, une mauvaise ventilation, une faible luminosité, une faible concentration de CO2 au cours de la photopériode et une concentration élevée en sucre, en azote et en phytohormones dans le milieu. Ces conditions nutritionnelles et physiques sont responsables de l'anormalité de la morphologie, de l'anatomie, de la physiologie et de la biochimie des plantules cultivées in vitro. Dans de nombreux cas, ces anormalités rendent l'acclimatation aux conditions ex vitro plus difficile. On présume que le saccharose dans le milieu de culture contribue au problème de l'acclimatation par ses effets négatifs sur la photosynthèse et le métabolisme carboné et azoté au cours de la phase in vitro. Le but de ce travail est d'approfondir les connaissances sur les effets du saccharose exogène et des conditions in vitro sur la croissance, la physiologie et la biochimie (métabolomique) des plantules in vitro et ex vitro. Premièrement, nos résultats indiquent que les plantules adaptées aux conditions mixotrophes (culture sur milieu MS avec 3% de saccharose pour 10 jours) ont hydrolyse et métabolisé efficacement le saccharose du milieu et que les hexoses (fructose) se sont accumulés en particulier dans leurs feuilles. Cependant, les plantules adaptées aux conditions autotrophes (culture sur milieu MS sans saccharose pendant 10 jours) ont été moins efficaces dans le métabolisme du saccharose, et ont plutôt relâché plus de sucre dans le milieu de culture (2 fois plus que les plantules mixotrophes). Par ailleurs, nous avons observé une conversion du fructose radioactif en glucose dans les plantules autotrophes et mixotrophes, ce qui suggère que le sucre entre dans le métabolisme cellulaire et qu'il n'est pas transloqué passivement dans le xylème.
Deuxièmement, à l'aide de la technique de métabolomique nous avons caractérisé de manière globale, et pour une première fois, l'impact du saccharose du milieu de culture sur le profil des metabolites des plantules pendant le stade d'enracinement in vitro. Des
ni
plantules ont été mises en culture pendant 5 semaines in vitro en présence (3%) ou en absence de saccharose exogène en conditions non limitantes de CO2. Sous ces conditions de culture, les plantules mixotrophes avaient une faible activité photosynthétique et étaient peu développées. La plupart des paramètres de croissance tels que la hauteur de plantule, le poids des feuilles, le nombre de feuilles et la surface foliaire par plante ont été significativement plus faibles en présence de saccharose dans le milieu de culture. À l'inverse de ce qui est généralement reconnu, les plantules mixotrophes in vitro sont soumises à un stress important, ce qui explique l'accumulation de plusieurs metabolites comme la proline, l'hydroxyproline, l'asparagine, le GABA, le fructose, le sucrose, le glucose et le myo-inositol. Nos résultats suggèrent aussi que les plantes in vitro doivent lutter contre une teneur très élevée en azote dans le milieu de culture par une accumulation de quantités très importantes d'acides aminés comme la glutamine, l'acide aspartique et le glutamate.
Dans la troisième expérience, nous avons évalué l'effet du saccharose ajouté au milieu de culture sur les profils métaboliques de plantules in vitro. Un suivi du métabolisme des plantules mixotrophes et autotrophes a été réalisé pour comprendre comment elles s'adaptaient métaboliquement lors de l'acclimatation. Pour ce faire, des plantules ont été cultivées pendant 4 semaines in vitro dans les conditions usuelles, c'est-à-dire sans échange gazeux et en présence (3%) ou en absence de saccharose exogène. Sous ces conditions et de manière assez surprenante, les plantules mixotrophes ont montré une plus forte photosynthèse que les plantules autotrophes. L'analyse métabolomique par GC-MS a permis d'établir pour la première fois une signature métabolique pour une plante in vitro et ex vitro. L'analyse des données par L'Analyse en Composantes Principales (PCA) a clairement permis de séparer quatre catégories de plantes selon leur profil d'accumulation de metabolites, correspondant aux plantes mixotrophes in vitro, autotrophes in vitro, mixotrophes et autotrophes ex vitro après 8 jours d'acclimatation et mixotrophes et autotrophes ex vitro après 16 jours d'acclimatation. En général, les plantules mixotrophes in vitro ont accumulé de nombreux metabolites liés au stress (la proline et le GABA) et plusieurs solutés osmotiquement compatibles (acides aminés, sucres et sucres alcools). De plus, de nombreux composés azotés (l'urée, uréides, polyamines et catecholamines) se sont accumulés sous les conditions de culture in vitro chez les plantules autotrophes et
mixotrophes. En outre, les plantules in vitro autotrophes et mixotrophes se caractérisaient par des niveaux très bas de glycolyse et du cycle des TCA par rapport aux plantes ex vitro. Enfin, elles se caractérisaient aussi par une forte accumulation d'intermédiaires de la photorespiration et du cycle de l'urée. En somme, ces résultats suggèrent que les plantules mixotrophes modifient leur métabolisme global pour s'adapter aux stress multiples trouvés in vitro. En d'autres mots, elles modulent leur métabolisme en fonction du niveau de toxicité de l'azote (le milieu MS contient une forte teneur en N), de la forte pression osmotique engendrée par la concentration élevée en sucres dans le milieu, de la mauvaise ventilation et de la teneur de l'atmosphère in vitro en CO2.
En résumé, la présence de sucres dans le milieu de culture cause un stress important aux plantules qui réagissent en accumulant plusieurs metabolites de stress. Le sucre exogène perturbe aussi les métabolismes carboné et azoté en réorientant la plantule dans la synthèse de très grandes quantités d'acides aminés et en réduisant les intermédiaires de glycolyse et du cycle des TCA. Il contribue au déséquilibre du métabolisme et à la physiologie atypique de la plantule, ce qui rend problématique l'acclimatation aux conditions ex vitro.
Short abstract
The commercial application of tissue culture is restricted to mass propagation of certain plant species due to an important loss during hardening stage. Sucrose in the culture medium is purported to be one ofthe principal causes of this problem. The objective of this thesis was to understand the effects of exogenous sucrose and in vitro conditions on the growth, physiology and biochemistry of tissue cultured potato plantlets (Solanum tuberosum L.) both in and ex vitro. As a premise to the research, we determined how sugar was absorbed and translocated in plantlets. Photoautotrophic and mixotrophic plantlets absorb and metabolize sugar in very distinct manners. The former do not absorb and translocate large quantities of sucrose and even release sugars in the medium. Mixotrophic plantlets translocate radioactive sugars in the stem and leaves. Through a metabolomic approach we comprehensively studied the metabolic response of in vitro and ex vitro plantlets to exogenous sugars. Our metabolic profiling data contribute to better explain many problems found in vitro and their impact on ex vitro acclimatization stage. For instance, metabolomic analysis revealed that tissue culture conditions caused a very important change in nitrogen and carbon metabolisms depicted by higher levels of amino acids, stress metabolites, polyamines, photorespiratory intermediates, osmoactive sugar alcohols, and lower levels of TCA cycle and glycolysis intermediates, all of which typifies the metabolic phénotype of a mixotrophic tissue cultured plants. We suggest that these changes in metabolites levels represent an adaptive response of the in vitro plantlets to the excessive amount of nitrogen found in MS culture medium and to osmotic stress conditions caused by exogenous sugars.
Long abstract
Micropropagation is largely utilized for the rapid propagation of certain plant species. However, despite its potential, this technique is plagued with many problems which reduce the efficiency of the technique and increase its costs. One of the most important limitations is the low percentage of survival to the natural environment after transfer from in vitro to ex vitro conditions. The in vitro environment differs greatly from the ex vitro conditions. Tissue culture conditions are characterized by high relative humidity, poor ventilation in the vessels, low light level, low CO2 concentration in the culture vessel during photoperiod, presence of high sugar, nitrogen and phytohormones in the medium. These nutritional and physical conditions are responsible for the abnormal morphology, anatomy, physiology and biochemistry of in vitro grown plantlets. In many cases, these abnormalities render the acclimatization of plantlets to ex vitro conditions more difficult. Sucrose in the culture medium is purported to contribute to acclimatization problems by its negative effects on photosynthesis and carbon and nitrogen metabolism during in vitro stages. The aim of this work is thus to understand in greater depth the effects of exogenous sucrose and in vitro conditions on the growth, physiology and biochemistry (metabolic profiling) of tissue cultured plantlets and acclimatized potato plants.
First, our results indicate that plantlets adapted to mixotrophic conditions (cultured on MS medium with 3% sucrose for 10 days) were more efficient in medium sucrose hydrolysis and hexoses (fructose) accumulation especially in their leaves. However, plantlets adapted to autotrophic conditions (cultured on MS medium without sucrose for 10 days) were less efficient in sucrose accumulation; instead they released two times more sugar in the culture medium compared to mixotrophic plantlets. In addition, we observed the conversion of fructose to glucose in both autotrophic and mixotrophic plantlets.
Second, using a metabolomics approach we comprehensively characterized and for the first time the impact of sucrose in the culture medium on the metabolites of the plantlets during the in vitro rooting stage. Plantlets were grown for 5 weeks in vitro in the presence (3%) or absence of exogenous sucrose and non-limiting CO2. Under mixotrophic conditions, plantlets had a low photosynthetic activity and were poorly developed (most growth parameters such as, shoot height, leaf weight, leaf number and leaf area/plant were
vu significantly lower in the presence of sucrose in the culture medium). Unlike what is generally believed, the mixotrophic plantlets in vitro are subjected to significant stress as evidenced by the accumulation of several metabolites such as proline, hydroxyproline, asparagine, GABA, some sugars (fructose, sucrose, glucose) and myo-inositol. Our results also suggest that in vitro plantlets are struggling against a very high content of nitrogen in the culture medium. They do so by accumulating large quantities of amino acids such as glutamine, aspartic acid and glutamate.
In a third experiment, we evaluated the effect of sucrose added to the culture medium on the metabolic profile of in vitro plantlets and measured the metabolism of autotrophic and mixotrophic plantlets to understand how they metabolically adjusted to the acclimatization conditions. To do this, plantlets were grown for 4 weeks in vitro under conventional conditions, without gas exchange, and in the presence (3%) or absence of exogenous sucrose. Under these conditions and rather surprisingly, the mixotrophic plantlets showed greater photosynthesis than autotrophic plantlets. The metabolomic analysis by GC-MS has established for the first time a metabolic signature for in vitro and ex vitro plantlets. The data analysis by Principal Component Analysis (PCA) has clearly separated four categories of plants according to their patterns of accumulation of metabolites, corresponding to in vitro mixotrophic plantlets, in vitro autotrophic plantlets, ex vitro autotrophic and mixotrophic after 8 days of acclimatization and ex vitro autotrophic and mixotrophic after
16 days of acclimatization. In general, the in vitro mixotrophic plantlets have accumulated many metabolites related to stress, for example, proline and GABA, and have also accumulated more compatible solutes, such as, amino acids, sugars and sugar alcohols. In addition, several nitrogen compounds such as urea, ureides, polyamines and catecholamines, accumulated under in vitro culture conditions (in both autotrophic and mixotrophic plantlets). Moreover, in vitro plantlets were characterized by very low levels of glycolysis and the TCA cycle compared with ex vitro plants. They were also characterized by a high accumulation of intermediates of photorespiration and the urea cycle. All in all, these results suggest that mixotrophic plantlets change their overall metabolism to adapt to multiple stressors found in vitro, i.e. it adjusts its metabolism depending on the level of toxicity of nitrogen (the MS medium contains a high content of N), the osmotic pressure
generated by the high concentration of sugar in the medium, poor ventilation in the culture vessels.
In brief, the presence of sugars in the culture medium causes an important stress for the plantlets that react by accumulating several stress metabolites. Exogenous sugars disrupt the carbon and nitrogen metabolisms by redirecting metabolic pathways of plantlets in the synthesis of very large amounts of amino acids and reducing the intermediates of glycolysis and the TCA cycle. It contributes to the metabolic and physiological imbalances of the plantlets, which makes them difficult to acclimatize to ex vitro conditions.
Avant-propos
Cette thèse comprend cinq chapitres, une introduction et une conclusion générale (Chapitres 1 et 5) et trois chapitres (2, 3, et 4) sont présentés sous forme d'articles scientifiques, devant être publiés dans des revues scientifiques sous peu. Les contributions respectives pour chacun des articles sont détaillées, ci-après:
Chapitre 2
Sugar uptake and metabolism in tissue cultured potato plantlets cultured in liquid medium
Cet article a été publié dans la revue Acta Horticulturae, 748 (2007), 265-273. La rédaction de l'article a été sous la supervision de mon directeur de recherche, le Dr. Yves Desjardins. J'ai réalisé l'ensemble des travaux de cet article.
Chapitre 3
Metabolic profiling of photoautotrophic and photomixotrophic potato plantlets (Solanum tuberosum L.) provides a new insights into the acclimatization problems Cet article est soumis a la revue Plant Cell, Tissue and Organ Culture. J'ai réalisé les travaux de « metabolic profiling » qui sont présentés dans cet article dans le laboratoire d'analyses biochimiques au département des sciences et technologies des aliments, sous la supervision de mon co-directeur, le Dr. Paul Angers et avec l'aide précieuse de M. Claude Gosselin. J'ai effectué la rédaction de cet article sous la supervision de mon directeur de recherche, le Dr. Yves Desjardins.
Chapitre 4
Comprehensive analysis of /'// vitro to ex vitro transition of tissue cultured potato plantlets grown with or without sucrose using metabolic profiling technique
Cet article est soumis à la revue Physiologia Plantarum. J'ai réalisé les analyses de «metabolic profiling» qui sont présentés dans cet article dans le laboratoire d'analyses
de mon co-directeur, le Dr. Angers, avec l'aide précieuse de M. Ronan Corcuff. J'ai effectué la rédaction de cet article sous la supervision de mon directeur de recherche, le Dr. Yves Desjardins.
Xl
Remerciements
Tout d'abord, je tiens à remercier très sincèrement mon directeur de thèse, Dr. Yves Desjardins, pour son soutien continu durant mes études doctorales. M. Desjardins a toujours été là pour m'écouter et pour me conseiller. Il m'a appris à exprimer mes idées et réaliser mes objectifs. Aussi, je le remercie pour son soutien et ses encouragements lors de la rédaction des manuscrits. Sans ses encouragements et constante orientation, je n'aurais pu terminer cette thèse.
Je remercie également mon co-directeur, Dr. Paul Angers, responsable du laboratoire d'analyses biochimique au département des sciences et technologie des aliments, pour m'avoir permis de réaliser mes analyses de « metabolic profiling » et pour son aide notamment en ce qui concerne le GC-MS.
Je désire remercier le gouvernement égyptien pour son appui financier durant mes études au programme de doctorat.
Je veux aussi remercier MM. Claude Gosselin et Ronan Corcuff pour leur aide lors de mon apprentissage du GC-MS.
J'aimerais remercier tous mes amis et collègues, en particulier Charaf Amhoach, Boubacar Sima, Pascal Dubé, Régis Baziramakenga, Ayman El-Sayed, M. Ehab Kheadr, Moustafa Khalf, Mourad Assidi, Omar Seddiki, Chams El-Deen pour leur support continu, leurs conseils et leurs encouragements pendant mes études.
Je tiens à remercier Drs. Gilbert Éthier and Steve Pépin pour leur aide et les nombreuses discussions fructueuses sur la photosynthèse.
Je pense également à tout le personnel de l'INAF et du CRH qui m'ont apporté leur aide ou qui ont contribué à créer une ambiance agréable durant mes étues.
Je remercie ma famille, mon frère et mes sœurs et mes neveux et nièces et m'excuse du fait que je n'ai pas eu beaucoup de temps à leur consacrer. J'aimerais leur dire que je les porte dans mon cœur où que je sois.
J'adresse évidemment mes sincères remerciements à mon épouse, Mona Hussein, ma fille Sara, mon garçon Ali, qui ont partagé ma vie au Québec et m'ont soutenu de tant façons que cette thèse n'aurait pas été possible sans leur support. Je remercie Mona de m'avoir enduré durant les périodes difficiles. Je la remercie pour ses encouragements et son appui précieux. Je ne terminerai pas sans adresser un immense merci à mon beau-père, ma belle-mère, ma belle-sœur et mes beaux-frères pour leurs encouragements pendant mes études.
Je n'oublie pas mes parents qui sont décédés au cours de mes études. Cette thèse est le fruit de leurs efforts et de l'éducation qu'ils m'ont donnée. Je leur demande la miséricorde de mon seigneur pour tout ce qu'ils ont fait et pour le soutien qu'ils m'ont apporté durant toute ma vie.
A mes parents, ma femme et mes enfants qui ont fait beaucoup pour moi et m'ont permis
Résumé court i Résumé long ii Short abstract v Long abstract vi Avant-propos ix Remerciements xi Chapter 1: Introduction 1
1.1 Plant in vitro culture 2 1.1.1 Micropropagation stages 3
1.2 Plant in vitro culture: acclimatization problems 5 1.2.1 The effects of in vitro conditions on growth and development of plants 5
1.2.2 The effect of exogenous sugars at the in vitro plantlets and their
subsequent effect on acclimatization stage 8 1.3 Exogenous sugar uptake and metabolism 10
1.4 Metabolomics 13 1.4.1 Metabolomics to obtain biochemical insight into the effect of
metabolic changes in the plant physiological status 16
1.4.2 Multivariate data analysis 18 1.4.3 Current applications of metabolomics coupled with multivariate
statistical analysis 19 1.5 Hypotheses 21 1.6 Objectives 22 Chapter 2: Sugar uptake and metabolism in tissue cultured potato plantlets
cultured in liquid medium 23
2.1 Résumé 24 2.2 Abstract 25 2.3 Introduction 26 2.4 Materials and methods 27
2.4.1 Plant material and growth conditions 27 2.4.2 [ H]-(fructosyl)-sucrose uptake by plantlets 28 2.4.3 Analysis of labeled sucrose, glucose and fructose 28
2.5 Results 29 2.5.1 Analysis of sugar content in the culture medium after different chase
periods 29 2.5.2 Distribution and metabolism of sucrose radioactivity in different parts of
plantlets 29 2.6 Discussion 33
2.6.1 Medium sugars release by in vitro plantlet roots 33 2.6.2 Transport and metabolism of sucrose radioactivity in the different
plantlets parts 35 2.7 Conclusions 36 2.8 Acknowledgements 36
XV
Chapter 3: Metabolic profiling of photoautotrophic and photomixotrophic potato plantlets (Solanum tuberosum L.) provides a new insights into the
acclimatization problems 37
3.1 Résumé 38 3.2 Abstract 39 3.3 Introduction 40 3.4 Materials and methods 43
3.4.1 Plant material and growth conditions 43 3.4.2 Photosynthesis and growth parameters measurements 43
3.4.3 Potato leaf metabolite sample preparation for gas chromatography-mass
spectrometry 44 3.4.4 GC-MS Analysis 44
3.4.5 Statistical analysis of Data 45
3.5 Results 45 3.5.1 Comparison of growth parameters and photosynthesis under autotrophic
and mixotrophic conditions 45 3.5.2 Comparison of metabolites abundance in the photoautotrophic and
photomixotrophic plantlets 47 3.5.3 Principal component analysis and hierarchical cluster analysis of
metabolite levels in photoautotrophic and photomixotrophic potato leaves 53
3.6 Discussion 57 3.6.1 Growth parameters and photosynthesis 57
3.6.2 In vitro potato plantlets were stressed by the presence of sucrose in the
culture medium 58 3.6.2 Accumulation of a wide-spectrum of amino acids in mixotrophic
plantlets 59 3.6.3 Photomixotrophic conditions increased organic acids biosynthesis 60
3.6.4 Photomixotrophic leaves accumulated large concentration of sugars and
sugar alcohols 61 3.6.5 Photomixotrophic leaves accumulated large quantity of catecholamines 61
3.7 Conclusion 62 3.8 Acknowledgements 63
Chapter 4: Comprehensive analysis of m vitro to ex vitro transition of tissue cultured potato plantlets grown with or without sucrose using metabolic
profiling technique 64 4.1 Résumé 65 4.2 Abstract 67 4.3 Introduction 69 4.4 Materials and methods 71
4.4.1 Plant material and in vitro growth conditions 71
4.4.2 Acclimatization 71 4.4.3 Photosynthesis and growth parameters measurements 72
4.4.4 Potato leaf metabolite sample preparation for gas chromatography-mass
spectrometry 72 4.4.5 GC-MS Analysis 73
4.4.6 Statistical analysis of Data 74
4.5 Results 74 4.5.1 Growth parameters and photosynthesis 74
4.5.2 Effect of photoautotrophic and photomixotrophic conditions on the
levels of metabolites in potato leaves during in vitro and ex vitro stages 77 4.5.3 Response of amino acids to sugar medium concentration and culture
conditions 81 4.5.4 Response of organic acids to medium sugar concentration and culture
conditions 83 4.5.5 Response of sugars and sugar alcohols to medium's sugar concentration
and culture conditions 84 4.5.6 Response of urea, ureides and other miscellaneous compounds to
medium's sugar concentration and culture conditions 90 4.5.7 Application of principal component analysis to metabolite data set 92
4.6 Discussion 94 4.6.1 The impact of sucrose on growth and photosynthesis during in vitro and
acclimatization stages 94 4.6.2 Metabolite abundance change during tissue culture and acclimatization
stages in photoautotrophic and photomixotrophic plants 96 4.6.3 Amino acids, TCA cycle and glycolysis intermediates levels is altered in
in vitro leaves 97 4.6.4 Induction of antioxidant defence system under in vitro and ex vitro
conditions 100 4.6.5 Sugars and polyols accumulation in tissue cultured PMT plants 101
4.6.6 Up-regulation of photorespiration and anaerobic respiration in in vitro
plantlets 102 4.6.7 Histidine, oxalate, tartrate and other organic acid accumulation in tissue
cultured plants 103 4.6.8 Sequestration of N in urea, allantoin and polyamines in tissue cultured
plantlets 105 4.7 Summary 108 4.8 Acknowledgements 111
Chapter 5: Conclusion 112 5.1 Sugar uptake and metabolism by intact potato plantlet 113
Liste des tableaux
Table 3.1: Rooting stage growth parameters of potato plantlets under
photoautotrophic and photomixotrophic conditions. For treatment codes, PAT Photoautotrophic plantlets (without sucrose in the medium); PMT
photomixotrophic plantlets (with 3% sucrose in the medium) 46 Table 3.2: List of 51 metabolites identified from a methanol potato leaf tissue
extraction 48 Table 4.1: Effect of sucrose on the photosynthetic parameters ofthe potato
plantlets under in vitro (before acclimatization, day 0) and ex vitro (after 8 and 16 days of acclimatization) culture conditions. PAT, photoautotrophic
and PMT, photomixotrophic plants 77 Table 5.1: Some important metabolites variations in potato leaves (Solanum
tuberosum L., cv Norland) under photoautotrophic and photomixotrophic
Figure 1.1: General description ofthe in vitro environment in conventional
micropropagation 6 Figure 1.2: Changes in plantlet genes, metabolism and physiology caused by
oxidative stress which can lead to aberrant morphology, recalcitrance,
hyperhydricity and somaclonal variation 7 Figure 1.3: Model describing sucrose uptake by tissue culture material 12
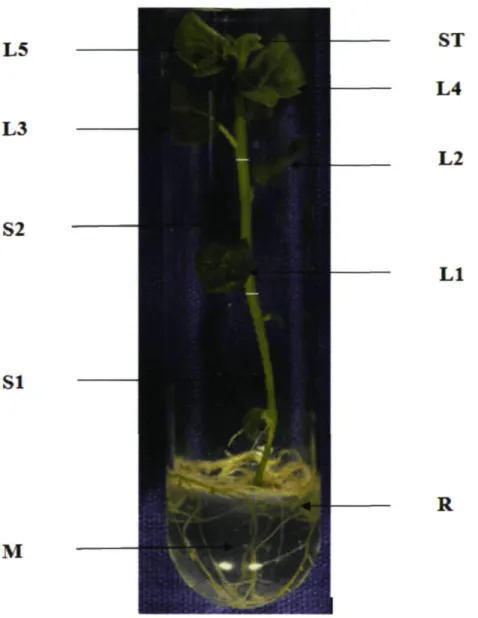
Figure 2.1: Picture of a typical in vitro potato plantlet to depict were sampling of radioactivity was carried during experiment .The culture medium (M) and different plant parts like roots (R), stem (base SI, middle S2), shoot tip (ST)
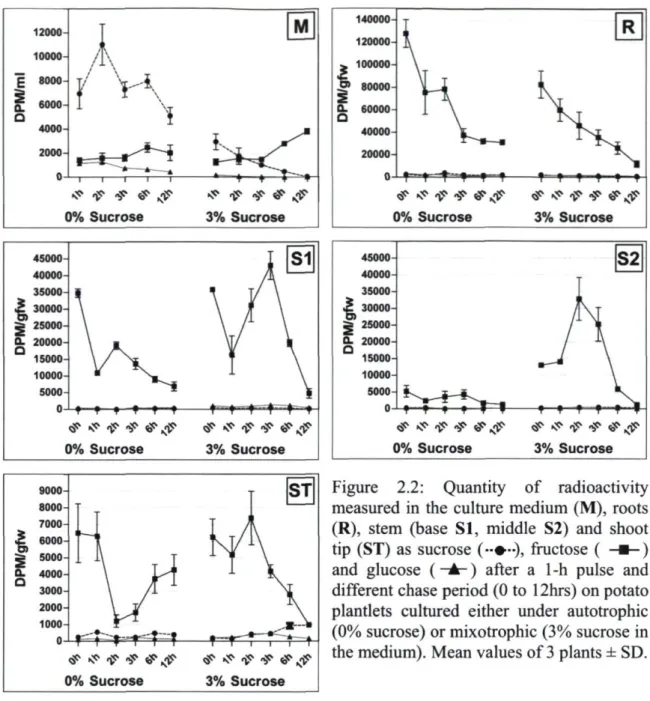
and five leaves (Ll, L2, L3, L4 and L5) 31 Figure 2.2: Quantity of radioactivity measured in the culture medium (M), Roots (R),
stem (base SI, middle S2) and shoot tip (ST) as sucrose, fructose and glucose after a lhr pulse and different chase period (0 to 12hrs) on potato plantlets cultured either under autotrophic (0% sucrose) or mixotrophic (3% sucrose in
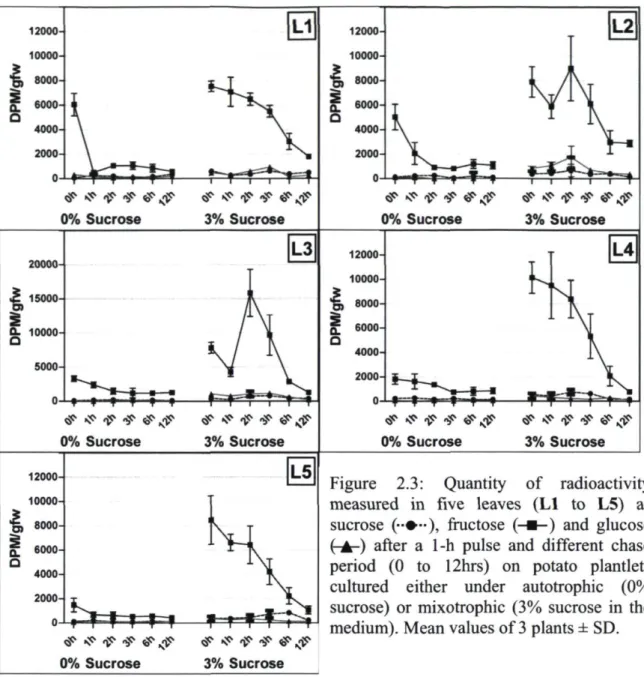
the medium) 32 Figure 2.3: Quantity of radioactivity measured in five leaves (Ll to L5) as sucrose,
fructose and glucose after a lhr pulse and different chase period (0 to 12hrs) on potato plantlets cultured either under autotrophic (0% sucrose) or mixotrophic
(3% sucrose in the medium) 33 Figure 3.1: Photoautrophic plantlets (growing on sugar-free MS medium) and
photomixotrophic plantlets (growing on MS medium with 3% sucrose) of potato
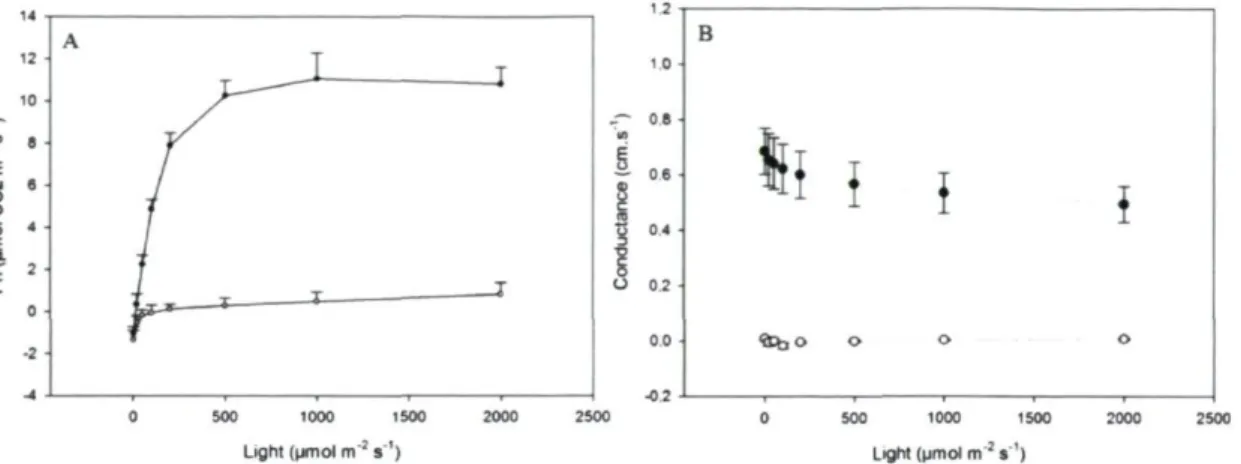
(Solanum tuberosum L., cv Norland) at the end of rooting stage 46 Figure 3.2: (A) The light response curve of net photosynthesis (Pn) and (B) the
stomatal conductance for leaves of autotrophic (closed circles) and mixotrophic (open circles) potato plantlets (Solanum tuberosum L., cv Norland) with
increasing light intensity 47 Figure 3.3: Comparison of GC/MS chromatogram of potato (Solanum tuberosum L.,
cv Norland) leaves extract produced under photoautotrophic and
photomixotropic conditions. By visual inspection of GC/MS chromatograms we can observe dramatic differences between photoautotrophic and
photomixotrophic potato leaves which indicate the strong effects of exogenous
sucrose on over all leaf metabolism 49 Figure 3.4: Changes in metabolite levels of potato (Solanum tuberosum L., cv
Norland) grown under photomixotrophic or photoautotrophic conditions in vitro. (A) show all polar metabolites detected in the leaf, (B) amino acids, (C)
organic acids, (D) sugars and sugar alcohols, (E) aromatic amines and urea 49 Figure 3.5: The principal component analysis (PCA) scores plot demonstrates a
distinct separation between photoautotrophic (A) and photomixotrophic (M) potato plantlets. PCA factor 1 and factor2 represent more than 70% ofthe total
variance 54 Figure 3.6: PCA loadings plot representation ofthe contribution of individual
XIX
photomixotrophic potato plantlets samples. Metabolites marked M > A were most abundant in mixotrophic tissues and vice versa for A > M. However, those
marked A = M were at the same levels under the two conditions 55 Figure 3.7: HCA dendrogram grouping of metabolites based on significant
differences in relative abundance in autotrophic (A) and mixotrophic (M) potato
leaf tissues 56 Figure 4.1 : Photoautrophic plantlets (growing on sugar-free MS medium) and
photomixotrophic plantlets (growing on MS medium with 3% sucrose) of potato (Solanum tuberosum L., cv Norland). (A) after 8 days of ex vitro acclimatization
and (B) after 16 days of ex vitro acclimatization 75 Figure 4.2: Growth parameters of autotrophic (circles) and mixotrophic (squares)
potato plantlets (Solanum tuberosum L., cv Norland) at the end of rooting stage (in vitro), after 8 days of ex vitro acclimatization and and after 16 days of ex
vitro acclimatization 76 Figure 4.3: Changes in all identified metabolites recovered in methanolic extracts
from leaves of potato (Solanum tuberosum L., cv Norland) grown under
photomixototrophic (A) and photoautotrophic (B) conditions during in vitro (1) and ex vitro (2 and 3) acclimatization stages. Metabolites are shown in order of
decreasing normalized peak area in PMT leaves tissue (in vitro) 78 Figure 4.4: Changes in the quantity of all identified metabolites in potato leaves
(Solanum tuberosum L. cv Norland) through in vitro and ex vitro stages. The percentage of each individual group relates to the total amount of identified
metabolites 81 Figure 4.5: Changes in amino acids (A) and organic acids (B) in potato leaves
(Solanum tuberosum L., cv Norland) during the in vitro and the ex vitro acclimatization stages. The percentage of each individual metabolite related to
its group is showed on the histograms 82 Figure 4.6: Changes in sugars (A) and sugar alcohols (B) in potato leaves (Solanum
tuberosum L., cv Norland) during the in vitro and the ex vitro acclimatization stages. The percentage of each individual metabolite related to its group is
showed on the histograms 85 Figure 4.7: Changes in metabolites extracted from the leaves of potato (Solanum
tuberosum L., cv Norland) grown under photoautotrophic and photomixotrophic
conditions during in vitro and ex vitro acclimatization stages 86 Figure 4.8: The principal component analysis (PCA) demonstrates a distinct
separation between in vitro PAT and PMT potato plantlets. Principal component
1 and 2 represent more than 70% ofthe total variance 93 Figure 4.9: PCA loading plot representing the contribution of individual metabolites
to principal component clustering of PAT and PMT potato plantlets samples 94 Figure 4.10: Schematic showing significant variations ofthe most important
metabolites abundance mapped onto the metabolic network. Colored boxes indicate changes in particular metabolite in the: 1- PMT vs. PAT plantlets under in vitro conditions, 2- PMT in vitro plantlets vs. PMT ex vitro plants (after 16
Figure 5.1: A model represents the effects of culture stage (in vitro PMT, A and ex vitro PMT, B) on some essential pathways in potato leaves (Solanum tuberosum L., cv Norland). The width ofthe arrows represents the importance ofthe
Plant in vitro culture is the science and art of growing plant cells, tissues or organs on artificial media by isolating them from a stock plant (George et al, 2007). It forms the backbone of plant biotechnology, i.e. micropropagation, production of disease-free plants, haploid production, triploid production, in vitro pollination and fertilization, somatic hybridization and cybridization, somaclonal and gametoclonal variant selection, germplasm conservation, secondary metabolite production, and genetic transformation. Plant cell and tissue culture has already contributed significantly to crop improvement and keeps great potential for the future (Kumar and Kumar, 1996). Research efforts in this area have always been very important worldwide. For instance, in vitro culture techniques are now being widely applied for improvement of field crop, forest, and horticulture and plantation crops and for increased agricultural and forestry production. Tissue culture technologies are also being exploited for large-scale production or micropropagation of elite planting material with desirable characteristics. This technology is currently being commercialized globally and significantly contributes towards the improved production of high quality planting material.
According to Murashige and Skoog (1974), plant cell and tissue culture is defined as the ability to regenerate and propagate plants from single cells, tissues and organs under sterilized and controlled environmental conditions. The origin of this technique dates back in the early 1900's when Gottlieb Haberlandt developed the concept of in vitro cell culture. Haberlandt was able to keep isolated leaf mesophyll alive and expanding (Cocking, 1986). This author was the first to propose the concept of totipotency, or the ability to regenerate a whole organism from a single cell (Cocking, 1986). In his cultures, cells that produced starch and enlarged in size survived for many weeks while none of them divided. He expected the needs for cell division under experimental conditions a requirement that have been proved through time. Following Haberlandt, many researchers continued working on plant in vitro cultures. In 1939, Gautheret cultivated cambial tissues of carrot root, Nobecourt worked on carrot, and White worked on tobacco, maintained cultures for prolonged periods of time and these were considered to be the first true plant tissue cultures (Chawla, 2002).
To establish an axenic culture, plant cells, tissues or organs are cultured on a defined medium under strict aseptic conditions and incubated under artificial environment to induce growth and development. The developmental fate of these tissues can be oriented towards desired ends by inclusion of sugar, growth regulators and other chemicals to the medium. Although growth of isolated plant tissues in vitro was achieved, little was known about the optimal nutrient medium for growing plant tissues. The nutritional composition varied depending on the type of cells, tissues, organs, protoplasts and the plant species used. There is also a difference in nutritional requirement among genotypes or cultivars of the same species. Thus, specific media had to be developed for each species, containing distinct mineral salts, carbon source, vitamins, plant growth regulators and other organic supplements. Among the culture media developed, the MS medium (Murashige and Skoog,
1962) is the most widely used in different plant tissue culture systems. Indeed, Murashige and Skoog worked with tobacco callus culture and developed an efficient medium based on the chemical analysis of incinerated tissues of this species. The MS medium is characterized by a nitrogen concentration 5 times higher than previously reported by Miller's (1956), 15 times higher than Hildebrandt's (1946) and 19 times higher than White's (1943) (George and Sherrington, 1993/1996). Similar increases were recommended for potassium and phosphorus. MS medium is used for culturing numerous species and is frequently used as the initial medium for protocol development of new species. This medium has been shown to have a high total ionic concentration (93.3 mM), so that high precipitates can form (George and Sherrington, 1993/1996).
1.1.1 Micropropagation stages
Murashige (1974) defined three stages (I to III) for Micropropagation; Debergh and Maene (1981) added stage '0'. Currently, we follow five stages procedure (0 to IV).
Stage 0: Mother plant selection and preparation
Stock plants must be typical of the variety or species, and free from any symptoms of disease. At this stage stock plants are grown under more hygienic conditions to reduce the risk of contamination.
Vegetative parts or reproductive parts are used for the propagation and shoot tip and auxiliary buds are often used for this. Success at this stage firstly requires that expiants be transferred to the culture environment free from microbial contaminants. In this stage, expiants are surface sterilized by treating them with disinfectant solution such as ethyl alcohol, bromine water, mercuric chloride etc. Then the expiants are established on an appropriate medium. There is not any universal culture medium; however modifications of Murashige and Skoog basal medium (Murashige and Skoog, 1962) are most frequently used.
Stage II: Multiplication of shoots
The object of this stage is to produce a large number of propagules from the established expiants. Through repeated cycles of this process, a single expiant may be multiplied from one to hundreds or thousands of plantlets. Depending on the type of tissue grown, multiplication can involve different methods and media. If the plant material grown is callus tissue, it can be cut into smaller pieces and recultured on the same type of culture medium to grow more callus tissue. If the tissue is grown as small plants called plantlets, hormones are often added that cause the plantlets to produce many small offshoots that can be removed and recultured.
Stage III: Rooting of regenerated shoots
Shoots or plantlets derived from Stage II are small, and not yet able of self-supporting growth in soil. At this stage, plantlets should be capable of carrying out photosynthesis, and survive without an artificial supply of carbohydrate. During the rooting stage, shoots are transferred on a rooting medium usually containing auxin. Elongation of shoots prior to rooting, rooting of shoots, and prehardening cultures are carried out to increase the survival rate.
Stage IV: Hardening or transfer to the natural environment
In the final stage of plant micropropagation, the plantlets are removed from the culture media and transferred to soil under greenhouse environment (ex vitro). The plantlets are placed under a high humidity environment to harden them off and for continued growth by conventional methods.
Although production or improvement of plants using tissue culture, especially for cloning and genetic engineering, seem very attractive, the acclimatization stage for some plants species is very inefficient and thus increases the production costs. At this stage, transplantation to the soil and weaning of plants is considered to be a major bottleneck in the micropropagation of many plants and the benefit of any micropropagation system can only be completely achieved once the successful transfer of plantlets from in vitro to ex vitro conditions has been obtained.
1.2 Plant /'// vitro culture: acclimatization problems
1.2.1 The effects of in vitro conditions on growth and development of
plants
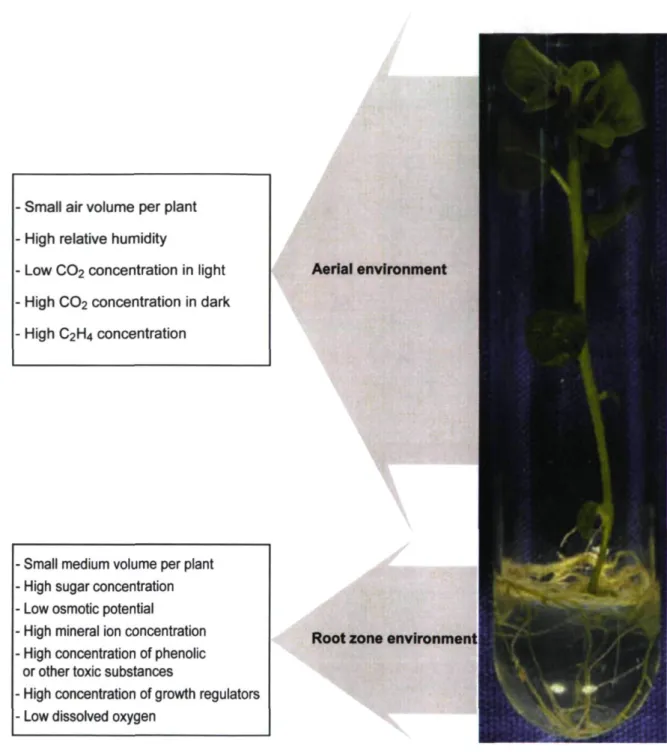
The in vitro environment differs immensely from the ex vitro or greenhouse environment (Fig. 1.1). During in vitro culture, plantlets grow under a unique microenvironment characterized by high air humidity and low irradiance. Culture vessels are kept closed to maintain aseptic condition, which consequently restricts gas exchanges (CO2, O2 and other gases) and decreases air turbulence between the inside and outside of culture vessels. Plantlets are usually supplied with large doses of growth regulators to stimulate the expiants growth and development. Moreover, the culture medium is often supplemented with ample sugar as carbon and energy sources. The presence of sugar in the culture medium considerably reduces the water potential of the medium and increases the risk of bacterial and fungal contamination. These conditions cause plantlets to develop an abnormal morphology, anatomy and physiology (Desjardins, 1995; Kozai et al, 2005; Pospisilova et al, 1992). These aberrations lead to considerable numbers of micropropagated plants death upon transfer from in vitro to ex vitro conditions.
High relative humidity
Low C02 concentration in light
High CO2 concentration in dark High C2H4 concentration
Small medium volume per plant High sugar concentration Low osmotic potential
High mineral ion concentration High concentration of phenolic or other toxic substances
High concentration of growth regulators Low dissolved oxygen
Aerial environment
Root zone environment
Figure 1.1: General description of the in vitro environment in conventional micropropagation.
Recently, it has been proposed that tissue culture conditions could be stressful for a number of plants which exhibit physiological and morphological abnormalities. Gaspar (1995;
(loss of requirement of exogenous growth regulators for maintained growth in vitro) and vitrification (hyperhydric malformations) of in vitro cultured cells and shoots, were involved in neoplastic progressions and thus leading to uncontrolled, anarchic cells in calli or shoot. In addition, it is reported that some specific physiological, morphological and genetic aberration (such as recalcitrance and somaclonal variation) that occur in tissue culture are most likely the result of oxidative stress caused by the excision of expiants, in vitro culture media and environmental factors (Fig. 1.2) (Cassells and Curry, 2001; Desjardins et a l , 2007; Gaspar et al, 2002; van Staden et al, 2006).
Explant preparation
1
Metabolism Deviation Recalcitrance Hyperhydricity Aberrant Morphology Somaclonal VariationFigure 1.2: Changes in plantlet genes, metabolism and physiology caused by oxidative stress which can lead to aberrant morphology, recalcitrance, hyperhydricity and somaclonal variation [adapted from Cassells and Curry (2001)].
1.2.2 The effect of exogenous sugars on in vitro plantlets and their
subsequent effect at the acclimatization stage
Sugars such as sucrose, glucose and fructose play a central role in plant metabolism. These sugars are important for intermediary and respiratory metabolism and are the substrate for synthesis of complex carbohydrates such as starch and cellulose. In addition, sugars provide the building blocks for amino acids and fatty acids biosynthesis and essentially all other constituents present in plants. Sugars are not only important as an energy source and structural cells constituents, they are also central regulatory molecules controlling physiology, metabolism, cell cycle, development, and gene expression in prokaryotes and eukaryotes. In higher plants, sugar affects growth and development throughout the life cycle from germination to flowering and senescence (Sheen, 1994; Thomas and Rodriguez,
1994). In addition, sugars function as physiological signals, repressing or activating plant genes related to many essential processes such as photosynthesis, glyoxylate metabolism, respiration, starch and sucrose synthesis and catabolism, nitrogen metabolism, pathogen defence, wounding response, cell cycle regulation, pigmentation, and senescence (Sheen,
1994).
In conventional micropropagation, plantlets grow under low PPF and thus require an additional source of carbon for their growth and development to compensate for low photosynthesis. Sucrose is cheap, shows already a high purity and ensures, as being a disaccharide, a less negative water potential in the media compared to hexoses when used in the same quantities. It is thus the most common sugar used in the tissue culture media, although others, such as glucose, maltose, raffinose, mannose and lactose have been used in some instances (George et a l , 2007). Plants produce sucrose by photosynthesis and can transport this molecule through vascular elements (Taiz and Zeiger, 2006). It is often hydrolyzed during autoclaving ofthe culture medium. Druart and Wulf (1993) found a 10% hydrolysis of sucrose in solution yielding monosaccharides glucose and fructose after autoclaving at 120°C for 20 minutes. This percentage increased to 95% hydrolysis to fructose and glucose when 1 % activated charcoal was added to the solution prior to autoclaving. This hydrolysis in an experimental system mimics the early steps of glycolysis, i.e. the internal hydrolysis of sucrose in the plant cells by which sucrose is broken down into glucose and fructose by the enzymes invertase or sucrose synthase (Taiz
and Zeiger, 2006). Sucrose is usually added to media in concentrations between 2-4% w/v and thus is a major medium component contributor to the osmotic potential, depending on the salt base used (George and Sherrington, 1993/1996). For example a 30 g/1 sucrose concentration contributes to about 50% of the osmotic potential of the Murashige and Skoog (MS) medium (1962) in absence of sucrose hydrolysis (George et al, 2007). When sucrose is hydrolyzed, the osmotic potential becomes more negative, but is balanced on the large run by the uptake of monosaccharides by the plant material.
The role of sugar on the overall plant carbon metabolism is still somewhat conflicting depending on the plant type and tissue culture conditions. Addition of sugar to the culture medium was reported to negatively affect growth, photosynthesis and expression of photosynthetic genes (Ehness et al, 1997; Hdider and Desjardins, 1994; Kozai et al,
1995). Hdider and Desjardins (1994) observed that high sugar concentration in culture medium was causing a feedback inhibition of photosynthesis by reducing the quantity and activation of Rubisco in cultured strawberry and potato plantlets. This response is typical of a classical feedback inhibition of photosynthesis by photosynthetic end-products, as in vitro plantlets accumulate photosynthates in their leaves and are incapable of moving them away, owing to the poor activity of sink organs. Moreover, Zobayed et al. (1999) found that removing sucrose from the medium when culturing potato promoted the normal functioning of stomata, in contrast to the abnormally formed and nonfunctional stomata of plants grown with sucrose. Also they found a lower stomatal density on in vitro formed leaves cultured on photomixotrophic medium compared to those cultured on photoautotrophic medium. Recently, Gaspar et al. (2002) reported that sucrose added to the medium could hinder chlorophyll synthesis, photosynthesis, the Calvin cycle and, as a result, decrease endogenous sugar production leading to altered carbon metabolism in in vitro cultured plants. In addition, the same researchers suggested that sugar present in the culture medium were among the most stressful factor for tissue culture plants, due to the osmotic stress. On the other hand, other authors have reported positive effects of added sugars on in vitro plantlets, such as activation of growth and photosynthesis of tobacco (Tichâ et a l , 1998), sugar beet (Kovtun and Daie, 1995), potato (Cournac et al., 1991) and sea oats (Valero-Aracama et al, 2007). In addition, supplementation of sugar to the culture media helps in
the maintenance of osmotic potential of cells and conservation of water (Hazarika, 2003). Adding sugar to the culture medium increases sucrose and starch reserves in micropropagated plants and may favour ex vitro acclimatization and accelerate physiological adjustments (Pospisilova et a l , 1999). Working with Alocasia amazonica plantlets, Jo et al. (2009) found that, increasing sucrose concentration in the culture medium increased leaf starch and sugars content, water potential and the number of stomata, while reducing the size of stomata during in vitro growth stage. Through the ex vitro acclimatization, growth parameters like the length of shoot and root, the number of leaves and roots were larger when plants were grown with 3% sucrose during in vitro stage. As mentioned above, the effects of sugars on in vitro plantlets are various and complex and it is of paramount importance to understand in a comprehensive manner their effects on biological systems, to solve the acclimatization problem.
1.3 Exogenous sugar uptake and metabolism
As previously mentioned, sucrose is the most frequently used carbon source in in vitro culture, a choice most probably attributable to its efficient uptake across the plasma membrane (Borkowska and Szczerba, 1991), which involves sucrose transporters (Slone and Buckhout, 1991). Sugar uptake into cells and subsequent vacuole accumulation across the tonoplast, have been widely studied in cell suspension cultures, particularly from specialized sucrose accumulating organs, such as sugar cane stem, sugar beet root and sycamore stem, which are considered as model systems for sugar uptake. Sucrose is not directly used in the metabolism of plant tissues, and before it can be introduced in metabolic processes, it has to be inverted into its component monosaccharides by the enzyme invertase. Invertase thus plays an essential role in sucrose uptake and utilization (Borkowska and Szczerba, 1991). For several species,sucrose in the cult ure medium is quickly hydrolyzed to glucose and fructose by apoplastic invertases (cell-wall-bound acid invertases) before entering into the cells (Shimon-Kerner et al, 2000). In addition, plants contain hexose-specific transporters in the plasma membrane, and it was thus proposed that hexoses provide an easy accessible pool of carbohydrates, available for uptake and cell metabolism. Indeed, glucose and fructose uptake have been observed in many cultured cells, such as carrot cells (Kanabus et al, 1986), Madagascar periwinkle cells
11 (Catharanthus roseus) (Sagishima et a l , 1989), bean cells (Botha and O'Kennedy, 1998) and Rudgea jasminoides cells (Kretzschmar et al, 2007).
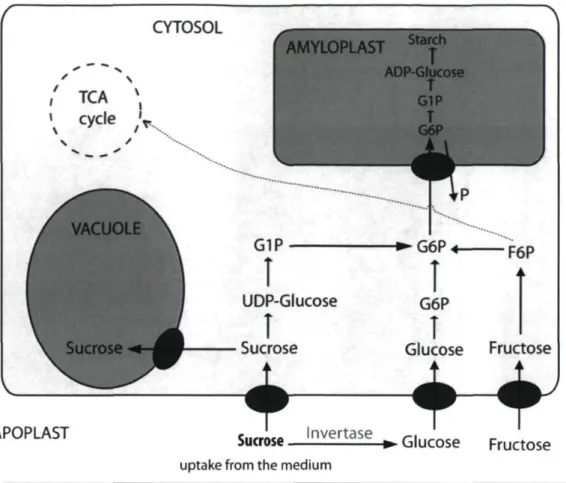
The uptake mechanism of externally supplied sugar into plant tissues would be partly passive and partly active (George et al, 2007). Active uptake is an energy-dependent process which depends on energy metabolism and ATP cellular content and requires a proton symport mechanism driven by a plasmalemma bound ATP-ase. The ATP-ase extrudes protons by hydrolysing ATP, creating a proton- and charge-gradient over the cell membrane. The resulting spontaneous influx of protons is linked to the sugar uptake by a membrane translocator protein (Slone and Buckhout, 1991). Such an uptake mechanism has been detected for both hexoses and sucrose (Felker et al, 1990; Lozada and Cardemil, 1990). In carrot cell suspensions, glucose was taken up preferentially to fructose through the first week ofthe culture, and fructose uptake followed during days 7-9 (George et al, 2007). Similar observations were reported by Kretzschmar et al. (2007) with cell suspension cultures of Rudgea jasminoides. After uptake, glucose and fructose are phosphorylated in the cytosol by an hexokinase (Kanabus et al, 1986) and converted into each other (Fig. 1.3). These phosphorylated sugars are then channelled into the primary metabolism via glycolysis, tricarboxylic acid cycle (TCA cycle) and pentose phosphate cycle, or directed towards glucuronic acid dependent pathways. The latter are synthetic pathways for cell wall components, passing over glucuronic and galacturonic acid and leading to pentoses and other cell wall carbohydrates. The surplus of imported carbon is stored as sucrose in the vacuole or deposited into starch in the plastids, dependent on the type of tissue and plant species studied.
CYTOSOL ' ' TCA S\
\
cy
cle,V
APOPLAST AMYLOPLAST Starch G1Pî
UDP-Glucoseî
Sucrose♦
T
ADP-Glucose T G1P T G6P % -►G6P + F6P G6PI
Glucose Fructose4~f
Sucrose l n v e r t a s e » Glucose Fructose
uptake from the medium
Figure 1.3: Model describing sucrose uptake by tissue culture material.
There are remarkably few data available on the uptake and metabolism of sugars in intact tissue culture plantlets. De Riek et al. (1991) showed that in Rosa multiflora multiplication cultures, up to 75% of the biomass increase originated from sucrose incorporation. However, more than 70% of the total sucrose uptake was utilized for respiration. Similar observations were made by Borkowska and Kubik ( 1990) during the in vitro rooting stage (2% sucrose) of Prunus cerasus. In this study, 23% of medium-labelled sucrose was absorbed by the plantlets, but only 5% was recovered in the plant tissues. Consequently, most ofthe labeled carbon had been respired by the plantlets. Hdider and Desjardins (1994) also showed that increasing medium sucrose leads to increase respiration. There is a paucity of data to explain which sugars are transported from the medium to the leaves. This information is essential to understand the role of sugars on leaf metabolism in vitro. The presence of sugar in the culture medium can alter leaf metabolism by disturbing normal
13 plant source-sink relationships, and also decrease leaf photosynthesis because sugar is translocated from roots to leaves.
1.4 Metabolomics
New tools to comprehensively study plant metabolism and functional genomics have been developped in recent years, including high throughput methods for transcriptomic, proteomic and metabolomic analyses. These tools have significantly enhanced the discriminating power of physiological analyses. Metabolomics is currently a rapidly growing field, and many researchers have directed their attention in developing the right research tools to explore it. Plant metabolomics is the study of the whole spectrum of metabolites in a plant system, such as a whole plant, organ, tissue or cell, at any specific growth stage or under biotic and abiotic influences such as pathogens, insects, weather and nutritional extremes (surplus or shortage). Metabolomics involves the rapid, high throughput characterization ofthe small molecule metabolites found in an organism, which are the end products of cellular processes. Thus, while gene expression data and proteomic analyses do not tell the whole story of what might be happening in a cell, metabolic profiling can give an instantaneous snapshot of the physiology of that cell. One of the challenges of systems biology and functional genomics is to integrate proteomic, transcriptomic, and metabolomic information to give a more complete picture of living organisms.
The metabolic composition of a plant system may vary widely depending on the sampling time, developmental stage, and other environmental conditions (Keil et al, 2005). The number of metabolites present in the plant kingdom is estimated to exceed 200,000 (Goodacre et al, 2004). An individual plant contains several thousands of possible metabolites, with many compounds being specific to each group of organisms (Wishart et al, 2007). Chemical composition of just about 10% ofthe plants have been reported until now (Oresic et al, 2006), and several more compounds are yet to be identified. The actual size of metabolomes may be much larger than predicted by standard metabolic pathways. For example, the number of metabolites that were expected in the bacterium Bacillus subtilis was estimated to account for a maximum of 800 compounds, but when metabolic
pathways were reconstructed from the genome sequences and enzyme coding genes, much more metabolites were identified. For example, a combination of three capillary electrophoresis methods coupled to mass spectrometry identified and quantified up to 1500 metabolites, an amount twofold higher than the previous conservative estimates (Soga et a l , 2003; Soga et al, 2002). Metabolites are chemically diverse small molecules which constitute the precursors, intermediates and end-products of metabolism in an organism. According to their functions, they can be classified into primary metabolites, which are directly involved in growth, development and reproduction of an organism; and secondary metabolites, which have special ecological roles in response to environmental stimuli. The metabolome, which includes all the metabolites of a biological system, consists of a large and interactive dynamic network of metabolites where the product/s of one reaction can become the reactant/s of another (Dunn and Ellis, 2005; Wikipedia, 2010). The metabolome is therefore subject to continuous changes depending on developmental, genetic, and environmental conditions and the type of biological system sampled (cell, tissue, organ, or entire organism) (Fiehn, 2006).
According to the scope of study and desired accuracy, metabolome analysis can be divided into different categories. For example, targeted metabolic studies or screening analyses can investigate changes in a single metabolite or small specific groups of metabolites upon changing conditions. Analytical techniques focusing on one or a few metabolites may result in better sensitivity, selectivity, and throughput compared with profiling techniques. Commonly used techniques for this type of analysis include spectrophotometric analysis, enzyme assays, mass spectrometry with ESI or MALDI ionization, nuclear magnetic resonance, thin layer chromatography, high performance liquid chromatography (HPLC), and gas chromatography (GC). With good selectivity achieved in sample preparation and detection techniques, absolute quantification of the target metabolites can be obtained with high accuracy and precision.
Multi-target metabolite profiling investigates changes in several groups of metabolites like sugars, organic acids, fatty acids, amino acids and secondary metabolites of a selected fraction such as polar compounds. The first application of this type of analysis was in human and animal body fluids (Pauling et al, 1971) and microbial systems (Oliver et al,
15 1998). Recent applications to plant systems has gained significant interest (Jeong et al, 2004; Roessner et al, 2000). The use of metabolomic approaches has increased steadily in plant research over the past few years because it complements other molecular biological approaches (Bohnert et al, 2006). The data of metabolic profiles include both quantitative and qualitative information about hundreds of metabolites, which becomes very useful in comparing different metabolic states under genetic or environmental perturbations or in estimating the functional status of an organism, tissue, or cell. Therefore, metabolomics can be powerful in studying the mode of action of drugs and herbicides (Kopka et al, 2005), in study toxicity, nutrition, pathology, disorders and biodiversity, or in allowing to link genotype to phénotype for the metabolic engineering of plants (Capell and Christou, 2004; Hanson and Shanks, 2002; Oksman-Caldentey and Saito, 2005). Chromatographic and analytical hybrids systems, such as GC-MS, LC-MS and LC-NMR, are commonly used in multi-target profiling.
There is another type of metabolite analysis that is called non-target metabolic profiling. It is a snap-shot approach to measure the wide range of metabolites present in a biological sample, using a variety of separation and analytical methods. A combination of several complementary techniques are needed for the assessment of all metabolites because a single technique is unable of accomplishing the comprehensiveness, selectivity and sensitivity required to describe the complexity of a metabolome (Morgenthal et al, 2005). GC, HPLC and CE are separation techniques frequently coupled with analytical techniques like NMR, MS, Fourier transform ion cyclotron resonance (FT-ICR) and Fourier transform infrared spectroscopy (FT-IR) in metabolite profiling techniques to maximize rapid scanning, separation, detection and identification of hundreds to thousands of metabolites in a biological sample extract (Hagel and Facchini, 2008).
Metabolic fingerprinting is a rapid and global analysis approach with high throughput for the comprehensive characterization of a sample (Fiehn, 2002). It can be used to study major variations among certain groups of metabolites, for example to distinguish among genotypic variations or to differentiate among plants exposed to different environmental conditions or genetic alterations (Dixon et al, 2006; Kopka et a l , 2004). Such studies focus on certain metabolites presented in each plant group (biomarkers) or focus on the
differences in concentration of certain metabolites upon genetic or environmental variations (Li et a l , 2006; Roessner et al, 2001 a). Metabolic fingerprinting has been used as a diagnostic diseases tool in medicine (Ellis et a l , 2007). Absolute quantitation is not required in fingerprinting approaches; instead, relative quantifications are commonly used. Metabolic profiling is the quantitative measurement of a group of related metabolites to determine the function of an entire pathway or intersecting pathways (Fiehn, 2002). It is a rapidly improving technique, used to discover comprehensive metabolic compositions of biological systems. It provides a gateway to connect and interpret the genomic and proteomic information and greatly facilitates the discovery of altered complete metabolic pathways (Fiehn, 2002).
To date, metabolome analysis has mainly been performed using two analysis platforms, MS and NMR. While NMR offers non-invasive means to comparatively characterize metabolite accumulation patterns, it exhibits limited sensitivity and resolution, and is therefore capable of detecting only the most abundant metabolites in complex samples. To acquire a comparable level of sensitivity and resolution with mass spectrometry, the cost of equipment and maintenance is comparatively several folds larger. MS analysis can be performed either directly or in combination with GC or LC separation steps. Direct-injection MS experiments have the advantage of being fast, but, like NMR, they suffer from limited resolving power. By contrast, GC- and LC-MS experiments are considerably slower, but permit more than 1000 metabolites to be analyzed for a single run (Weckwerth, 2003).
1.4.1 Metabolomics to obtain biochemical insight into the effect of
metabolic changes in the plant physiological status
Plant physiology is considered to be an important area where metabolomics will prove a precious research tool. Metabolomics may indeed be the best and most direct measure applicable to plant physiology and it is already clear that it gives us a clear and comprehensive picture of what is going on at the cell level. Metabolomics is especially useful to understand the connections and relationships between metabolites and metabolic
17
pathways. It provides greater insight into plants function and into the manner by which plants exploit the metabolic flexibility to the changing environment.
Metabolic profiling using GC-MS has become very popular lately, with the improvement in equipment sensitivity and decreased price. GC-MS has been used for a long time to measure individual metabolites in biological samples. It was also used to simultaneously measure metabolites belonging to different functional groups in potato tubers (Roessner et al, 2000) and in apricots (Katona et al, 1999). Recent analytical equipment improvement allowed quantification of a much larger and diverse group of metabolites than was done before, generating a massive metabolic data set and providing more information about the metabolic state ofthe plant in a high throughput manner.
Research work by Roessner et al. (2000) on potato physiology and tuber development were the first detailed images of metabolic profiles from single extracts for comparative biochemical analyses of soil- and in vitro-grov/n tubers. Seventy-seven compounds of various biochemical groups were detected and quantified. The GC-MS method was showed in this case to allow the simultaneous analysis of a large number of metabolite groups and the detection of clear differences between the tubers metabolism. Subsequently, combining this approach with multivariate analysis proved powerful to metabolically phénotype potato tubers which had been modified genetically or grown in different environments (Roessner et al, 2001a; Roessner et al, 2001b). For instance, Roessner et al. (2003) have employed metabolic profiling to discriminate two tomato species (transgenic and wild-type) by the metabolites composition of leaf and developing fruit tissues. Metabolomics can significantly contribute to the study of stress biology in plants and other organisms by identifying different compounds, such as by-products of stress metabolism, stress signal transduction molecules or molecules that are part ofthe acclimation response of plants. For example, Sanchez et al. (2008) demonstrated that metabolite profiling could be used to discover conserved and altered metabolic responses to salt stress in three different plant species (Arabidopsis thaliana, Lotus japonicus and Oryza sativa). Also, metabolomics has been utilized for the comprehensive comparison of total metabolites to detect the level of similarity between field-grown genetically modified and conventional potato tubers (Catchpole et al, 2005). Metabolomics also represents an important additional tool
presently used in genomics-assisted selection for crop improvement (Fernie and Schauer, 2009).
1.4.2 Multivariate data analysis
As GC-MS produces huge datasets characteristic of the metabolic status of the plant, data analysis methods used were until recently restricted to differences in individual comparison of samples between altered groups. This limited the use of metabolic profiling to obtain a biochemical insight of the variations in plant physiology. The use of multivariate data analysis techniques to analyze metabolic data (Fiehn et al, 2000) allowed to map the overall response of a plant to a genetic or environmental change and at the same time identify metabolites demonstrating differential accumulation in two systems in a non-biased way. Hence, the aims of metabolic data analysis using multivariate statistics are to categorize the overall change in metabolic state, by clustering various metabolic profiles, and to identify the metabolites showing a significant variation between the two sets of metabolic profiles. Currently, principal component analysis (PCA) and hierarchical clustering (HCL) are widely used for this purpose (Fiehn et al, 2000; von Roepenack-Lahaye et al, 2004). Without these statistical tools it would remain difficult to discriminate reliably between samples on the scale required to enable us to extract biologically meaningful information from multivariate datasets. Multivariate statistical techniques allow for the reproducible, non-biased analysis of large sets of metabolic data. Furthermore, they proved a visual comparison of overall effects of variations from large number of variables. In order to achieve this, most multivariate statistical methods use an estimation of distance, which represents the difference between two different metabolic states, based on the calculated values of each metabolite. There are several ways to measure the distance between datasets, and thus appropriate distance estimates should be chosen based on the problem at hand. Euclidean Distance and Pearson Correlation Distance are used frequently for these kinds of datasets.
PCA (Fiehn et al, 2000) and HCL (Roessner et a l , 2001a) have been used frequently in metabolic studies. Other methods, like K-means analysis (and associated FOM analysis)
19 and Significant Analysis of Microarray, which have been utilized in gene expression analysis, could also be used for metabolic profiling analysis.
1.4.2.1 Principal Component Analysis (PCA)
The principal component analysis is a technique which projects a bulky set of data onto a smaller set of variables (called principal components), which are a linear combination of the initial variables. The principal components are chosen in such a way that the first component accounts for the largest variation in the samples. For data sets with high degree or correlation between variables, the first three components together can account for more than 50% of the total variability of the system. Under such conditions, plotting metabolic profiles over the first three principal component factors allow a large fraction of the differences between plant samples to be visually recognized. Plant samples, which have almost the same metabolic status, cluster close up to each other. Therefore, if an environmental perturbation or genetic change significantly alters the metabolic state of a plant, plant samples form a separate cluster, or group.
1.4.2.2 Hierarchical Cluster Analysis (HCA)
Cluster analysis is a collection of statistical methods, which identifies groups of samples that behave similarly or show similar characteristics. The simplest method to conduct this analysis is to partition the samples using measurements that capture similarity or distance between samples. In other words, cluster analysis is an exploratory data analysis tool which aims at sorting different objects into groups in a way that the degree of association between two objects is maximal if they belong to the same group, and minimal otherwise. Given the above, cluster analysis can be used to discover structures in data without providing an explanation/interpretation. In other words, cluster analysis simply discovers structures in data without explaining why they exist.
1.4.3 Current applications of metabolomics coupled with multivariate
statistical analysis
Clustering techniques have been applied to a wide variety of research problems. For example, in the field of medicine, clustering cures or symptoms of diseases can lead to very
![Figure 1.2: Changes in plantlet genes, metabolism and physiology caused by oxidative stress which can lead to aberrant morphology, recalcitrance, hyperhydricity and somaclonal variation [adapted from Cassells and Curry (2001)]](https://thumb-eu.123doks.com/thumbv2/123doknet/5539330.132473/28.898.160.804.376.853/metabolism-physiology-oxidative-morphology-recalcitrance-hyperhydricity-somaclonal-cassells.webp)