Differential Compensatory Responses in Colorado Potato
Beetle Fed Different Plant Diets or Protease Inhibitor
Variants
Thèse
Asieh Rasoolizadeh
Doctorat en Biologie vegétale
Philosophiæ Doctor (Ph.D.)
Québec, Canada
Differential Compensatory Responses in Colorado Potato
Beetle Fed Different Plant Diets or Protease Inhibitor
Variants
Thèse
Asieh Rasoolizadeh
Sous la direction de :
Dominique Michaud, directeur de recherche
iii
De nombreuses études ont été publiées sur le potentiel des inhibiteurs de protéases en phytoprotection et les stratégies développées par les insectes herbivores pour éviter l’effet antidigestif de ces protéines. Le but du projet était de documenter plus à fond les mécanismes compensatoires mis en place chez les insectes en réponse à la diète ou aux inhibiteurs de protéases, en utilisant comme système d’étude des larves du doryphore de la pomme de terre (Leptinotarsa decemlineata) se nourrissant de différentes Solanacées ou de lignées transgéniques de pomme de terre (Solanum tuberosum) exprimant différents variants d’un inhibiteur de protéases, la cystatine de tomate SlCYS8, générés par mutagénèse dirigée. Des bioessais ont été réalisés pour étudier l'impact de la diète sur la croissance, la consommation foliaire et l'abondance des protéases digestives sécrétées par l'insecte. D'autres bioessais ont été réalisés pour étudier l'impact des variants SlCYS8, administrés ex planta sur feuilles de pomme de terre ou in planta par voie transgénique. Les insectes étaient comparés à l’aide d’indicateurs macroscopiques (prise de poids, consommation foliaire) et biochimiques (protéases digestives, protéome digestif). Des analyses protéomiques étaient notamment réalisées pour estimer l'impact global de la diète sur les protéases digestives de l'insecte et identifier les protéases sensibles aux variants cystatine à l’étude. Ce projet aura contribué, dans l’ensemble, à une meilleure compréhension des mécanismes de compensation digestive chez les insectes herbivores. Il aura aussi permis de confirmer le potentiel des approches courantes du génie des protéines pour une amélioration rationnelle des inhibiteurs de protéases à des fins de phytoprotection.
iv
RÉSUMÉ ... iii
TABLE OF CONTENTS ... iv
LIST OF FIGURES ... vii
LIST OF TABLES ... viii
ABBREVIATIONS ... ix
ACKNOWLEDGMENTS ... x
FOREWORD | AVANT-PROPOS ... xi
GENERAL INTRODUCTION ... 1
CHAPITRE 1 The research problem ... 4
CHAPITRE 2 The research project ... 16
CHAPITRE 3 Population-associated heterogeneity of the digestive Cysteine protease complement in colorado potato beetle, Leptinotarsa decemlineata ... 21
3.1 Résumé ... 22
3.2 Abstract ... 23
3.3 Introduction ... 23
3.4 Methods ... 26
3.4.1 Insect feeding assay ... 26
3.4.2 Insect midgut proteins ... 26
3.4.3 Protease and protease inhibitory assays ... 27
3.4.4 Mass spectrometry ... 27
3.4.5 Protein identification ... 28
3.4.6 Spectral count analyses ... 28
3.5 Results ... 29
3.5.1 Midgut protease activities in freshly molted 4th instars are population-dependent 29 3.5.2 Midgut intestain profiles in freshly molted 4th-instar larvae are population-dependent ... 32
3.5.3 Diet-induced adjustments of protease profiles in 4th-instar larvae also are population-dependent ... 33
3.5.4 Single variants of tomato cystatin SlCYS8 with improved inhibitory potency towards Cys proteases show improved, albeit differential, potency against cathepsin L-like enzymes of both Québec and Maryland insects ... 36
3.6 Discussion ... 37
v
CHAPITRE 4 Positive selection of digestive Cys proteases in herbivorous Coleoptera ... 40
4.1 Résumé ... 41
4.2 Abstract ... 41
4.3 Introduction ... 42
4.4 Methods ... 44
4.4.1 Sequence variability inferences ... 44
4.4.2 Intestain::cystatin interactions ... 44
4.4.3 Recombinant cystatins ... 45
4.4.4 Insect feeding assay ... 46
4.4.5 Insect midgut proteins ... 46
4.4.6 Protease assays ... 47
4.4.7 Mass spectrometry ... 47
4.4.8 Protein identification ... 48
4.4.9 Spectral count analyses ... 49
4.5 Results ... 49
4.5.1 The digestive Cys proteases of herbivorous Coleoptera are positively selected .. 49
4.5.2 A reciprocal positive selection pathway for plant cystatins and Coleoptera Cys proteases? ... 50
4.5.3 Altered digestive protease patterns in Leptinotarsa decemlineata fed a positively selected cystatin ... 54
4.5.4 Differential intestain profiles in L. decemlineata fed functional variants of a rapidly evolving cystatin ... 56
4.6 Discussion ... 59
4.7 Acknowledgements ... 61
CHAPITRE 5 Single substitutions to closely related amino acids contribute to the functional diversification of an insect-inducible, positively selected plant cystatin ... 62
5.1 Résumé ... 63
5.2 Abstract ... 63
5.3 Introduction ... 64
5.4 Experimental Procedures ... 66
5.4.1 Plant cystatin sequence data ... 66
5.4.2 SlCYS8 variants ... 67
5.4.3 Leaf feeding assay ... 67
5.4.4 Insect midgut proteins ... 68
5.4.5 Protease assays ... 68
5.4.6 Mass spectrometry ... 69
5.4.7 Protein identification ... 70
5.4.8 Spectral count analyses ... 70
vi
5.5.1 Structurally related amino acids are found at positively selected sites of plant
cystatins ... 71
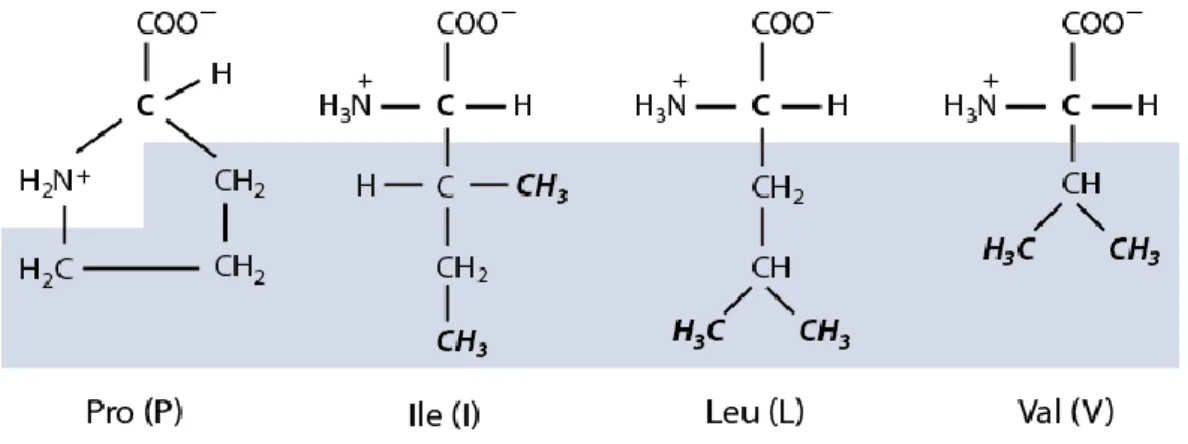
5.5.2 P2I, P2L and P2V show differential binding profiles for L. decemlineata intestains ... 73
5.5.3 P2I, P2L and P2V differentially alter growth, leaf consumption and digestive protease profiles of L. decemlineata larvae ... 75
5.5.4 The differential effects of P2I, P2L and P2V on Cys protease profiles are intestain family-specific ... 78
5.6 Discussion ... 80
5.7 Acknowledgements ... 82
CHAPITRE 6 Functional proteomics-aided selection of protease inhibitors for herbivore insect control ... 83
6.1 Résumé ... 84
6.2 Abstract ... 84
6.3 Introduction ... 85
6.4 Results and discussion ... 87
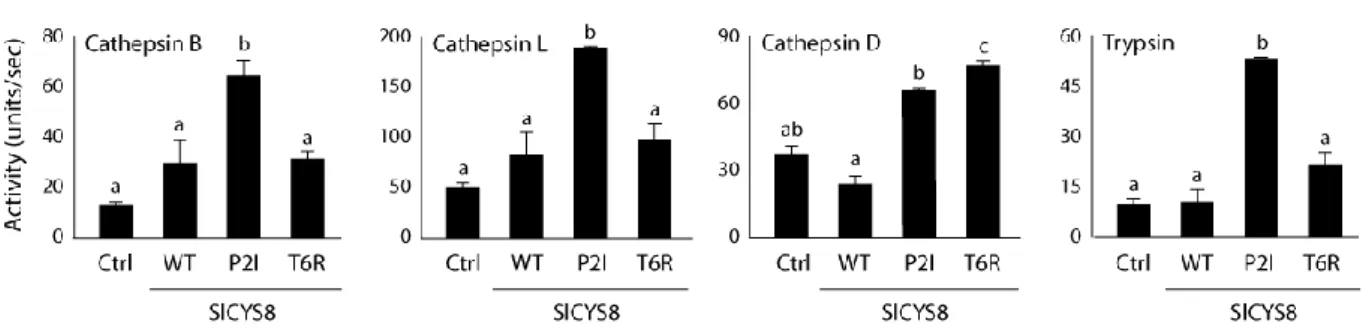
6.4.1 Tomato SlCYS8 variant P2V as a promising inhibitor for L. decemlineata control. 87 6.4.2 SlCYS8 variants P2V and T6R expressed in potato show differential effects on L. decemlineata larvae. ... 89
6.4.3 T6R- and P2V-expressing potato lines differentially alter digestive protease profiles in L. decemlineata. ... 93
6.5 Conclusion ... 96
6.6 Methods ... 97
6.6.1 Transgenic plant lines ... 97
6.6.2 Cystatins and ß-glucanases in leaves ... 98
6.6.3 Insect feeding assay ... 98
6.6.4 Insect midgut proteins ... 99
6.6.5 Protease assays ... 99
6.6.6 Mass spectrometry ... 100
6.6.7 Protease identification ... 100
6.6.8 Spectral count analyses ... 101
CHAPITRE 7 Discussion & perspectives ... 102
REFERENCES ... 110
Annex 1 – Supplementary data for Chapter 3 ... 125
Annex 2 – Supplementary data for Chapter 4 ... 134
Annex 3 – Supplementary data for Chapter 5 ... 153
vii F 1.1. ... 13 FIGURE 1.2. ... 15 FIGURE 3.1 ... 30 FIGURE 3.2. ... 31 FIGURE 3.3. ... 33 FIGURE 3.4. ... 35 FIGURE 3.5. ... 36 FIGURE 4.1 ... 50 FIGURE 4.2.. ... 53 FIGURE 4.3. ... 55 FIGURE 4.4. ... 56 FIGURE 4.5 ... 58 FIGURE 5.1.. ... 66 FIGURE 5.2. ... 73 FIGURE 5.3. ... 74 FIGURE 5.4. ... 76 FIGURE 5.5. ... 78 FIGURE 5.6.. ... 79 FIGURE 6.1. ... 88 FIGURE 6.2.. ... 90 FIGURE 6.3. ... 91 FIGURE 6.4. ... 93 FIGURE 6.5. ... 95 FIGURE 7.1. ... 108 FIGURE 7.2. ... 109 FIGURE S4.1 ... 134 FIGURE S4.2 ... 146 FIGURE S5.1 ... 153
viii TABLE 4.1. ... 51 TABLE 4.2 ... 54 TABLE 5.1 ... 72 TABLE 6.1. ... 95 TABLE S3.1……….. 125 TABLE S3.2……….. 125 TABLE S3.3……….. 126 TABLE S3.4……….. 129 TABLE S3.5……….. 129 TABLE S3.6……….. 130 TABLE S3.7……….. 133 TABLE S4.1……….. 147 TABLE S4.2……….. 148 TABLE S4.3………... 149 TABLE S4.4………... 152 TABLE S5.1……….. 157 TABLE S5.2……….. 159 TABLE S5.3……….. 162 TABLE S6.1……….. 163 TABLE S6.2……….. 164
ix
ANOVA Analysis of variance
CPB Colorado potato beetle
cDNA Complementary DNA
Int Intestain
IntA, IntB, IntC, IntD, IntE, IntF Leptinoatarsa decemlineata digestive Cys proteases of the intestain A, B, C, D, E and F functional families
Ldp30 Leptinotarsa decemlineata 30-kDa
cystatin-sensitive midgut Cys protease
CaMV 35S Cauliflower Mosaic Virus 35S promoter
PCR Polymerase chain reaction
P2I, P2L, P2V and P2Y Single functional variants of tomato cystatin SlCYS8 bearing an isoleucine (I), a leucine (L), a valine (V) or a tyrosine (Y) in place of the original proline (P) at position 2 in the N-terminal region
RCR Relative consumption rate
RGR Relative growth rate
SDS-PAGE Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis
SlCYS8 Tomato multicystatin 8th subunit
T6R Single functional variant of tomato cystatin
SlCYS8 bearing an arginine (R) in place of the original threonine (T) at position 6 in the N-terminal region
x
During my Ph.D. program, I have met several people at Laval U who have shared their knowledge and experience with me to make my work both feasible and pleasant.
I take this opportunity to thank them all from the bottom of my heart. More specifically, I would like to thank my supervisor, Prof. Dominique Michaud. He generously welcomed me in his lab, provided me with scientific training on a daily basis and gave me the opportunity to develop my competences. It has always been a great pleasure to share new results with him, and his constant cheering, interest and enthusiasm for my work allowed me to push through and get through several difficult tasks.
I would also like to express my deepest appreciation to my co-supervisor, Prof. Conrad Cloutier, for his keen interest on my research and experiments, for his kind and always relevant suggestions, and for taking his precious time to assess my manuscripts.
I would like to express my gratitude to the members of our laboratory. I thank Marie-Claire Goulet, Stéphanie Robert and Ann-Julie Rhéaume, who have helped me by providing valuable and friendly support during my experimentations.
Last, but not least, I wish to express my profound gratitude to my love, Reza, and to my parents. Reza was always besides me and helped me to overcome the obstacles and continue on my way. Although I am living far away from my parents, they are always in my heart. The distance did not keep them from providing valuable advice and generous support. I have always benefited from their gracious words and encouragements, which allowed me, during the hardest times, to keep moving forward and continuing my career path.
xi FOREWORD | AVANT-PROPOS
This thesis includes four primary research articles published over the last two years in peer-reviewed scientific journals. To meet the requirements of Laval U’s Faculté des études supérieures et postdoctorales, those chapters presented in English in the document are preceded by a summary in French. A consolidated list of references including references cited in all chapters, including original manuscripts, is presented at the end of the document. Co-authors’ contributions to my articles are summarized here below.
CHAPITRE 3
Population-associated heterogeneity of the digestive Cys protease complement in
Leptinotarsa decemlineata
Asieh Rasoolizadeh, Marie-Claire Goulet, Jean-Frédéric Guay, Conrad Cloutier & Dominique Michaud
Cet article a été rédigé sous la supervision du Prof. Michaud et la co-supervision du Prof. Cloutier. Il a été publié en 2017 dans le périodique scientifique Journal of Insect Physiology. J'ai bénéficié pendant ces travaux de l'aide précieuse de Mme Marie-Claire Goulet et de M. Jean-Frédéric Guay, qui m’ont assistée aussi bien pour la réalisation des expériences que pour l’analyse des données.
CHAPITRE 4
Positive selection of digestive Cys proteases in herbivorous Coleoptera
Juan Vorster, Asieh Rasoolizadeh, Marie-Claire Goulet, Conrad Cloutier, Frank Sainsbury & Dominique Michaud
Cet article a été rédigé sous la supervision du Prof. Michaud et la co-supervision du Prof. Cloutier. Les travaux ont été réalisés en étroite collaboration avec un ancien chercheur postdoctoral du laboratoire, le Dr. Juan Vorster, qui s’est attardé en particulier aux analyses structurales et statistiques suggérant la sélection positive des protéases étudiées. Je me suis attardée pour ma part aux démonstrations empiriques de l’étude, nécessaires pour appuyer les inférences in silico de mon collègue. L’article résultant de ces travaux a été publié en 2015 dans
xii
le périodique scientifique Insect Biochemistry and Molecular Biology, avec le Dr. Vorster et moi-même comme co-premiers auteurs.
CHAPITRE 5
Single substitutions to closely related amino acids contribute to the functional diversification of an insect-inducible, positively selected plant cystatin
Asieh Rasoolizadeh, Marie-Claire Goulet, Frank Sainsbury, Conrad Cloutier & Dominique Michaud
Les travaux et la rédaction de cet article ont été faits sous la supervision du Prof. Michaud et la co-supervision du Prof. Cloutier. L’article en découlant a été publié en 2016 dans la revue FEBS Journal. J'ai bénéficié lors de ces travaux de l'aide précieuse de notre professionnelle de recherche, Mme Marie-Claire Goulet,qui a généré entre autres les constructions génétiques pour les cystatines recombinées. Le Dr. FrankSainsbury, un ancien chercheur postdoctoral du laboratoire, m’a assistée pour le volet protéomique des travaux, en particulier pour l’analyse des données spectrométriques.
CHAPITRE 6
Functional proteomics-aided selection of protease inhibitors for herbivore insect control Asieh Rasoolizadeh, Aurélie Munger, Marie-Claire Goulet, Frank Sainsbury, Conrad Cloutier & Dominique Michaud
Les résultats de ces travaux ont été publiés en 2016 dans le périodique Scientific Reports. L'ensemble des travaux et la rédaction de cet article ont été réalisés sous la supervision du Prof. Michaud et la co-supervision du Prof. Cloutier. J'ai bénéficié pour ces travaux de l'aide précieuse de notre professionnelle de recherche, Mme Marie-Claire Goulet, et de Mme Aurélie Munger, alors étudiante à la maîtrise au laboratoire, avec qui j’ai produit les lignées de pommes de terre transgéniques utilisées pour les bio-essais.
1
GENERAL INTRODUCTION
Numerous studies have reported the potential of plant protease inhibitors such as Kunitz inhibitors and cystatins to develop insect-resistant transgenic crops (Schlüter et al., 2010; Macedo et al., 2015). These proteins direct an interruption of dietary protein digestion in the target midgut leading to amino acid shortage, detrimental growth delays and eventual death of the herbivore (Michaud, 2000).
Despite promising developments, the successful use of protease inhibitors in plant protection still remains confined to specific examples. Insect herbivores have developed efficient strategies to escape the inhibitory effects of plant protease inhibitors, ranging from the overexpression of ‘insensitive’ proteases to partial or complete degradation of the inhibitors in the midgut lumen (Zhu-Salzman & Zeng, 2015). An interesting example is the coleopteran pest of potato Colorado potato beetle (CPB), which has shown an astonishing ability to adapt to plant dietary cystatins. The over-expression of cystatin-targeted cysteine (Cys) proteases, the de novo expression of Cys proteases recalcitrant to cystatin inhibition, the degradation of defensive protease inhibitors using non-target proteases and the over-expression of proteases from alternative functional classes are well described strategies used by CPB larvae to elude the negative effects of dietary cystatins (Schlüter et al., 2010). In practice, the development of recombinant cystatins and other protease inhibitors with strong effects against this insect remains potentially valuable but is still challenging.
Our research group has shown some ten years ago that plant cystatins, like other defense-related proteins involved in host–pest interactions, follow an adaptive evolutionary scheme involving the preferential fixation, or positive selection, of amino acid residues located at strategic positions on the protein (Kiggundu et al., 2006). Based on this, we assessed the potential of site-directed mutagenesis at hypervariable –and functionally relevant– amino acid sites in recent years to improve the inhibitory functions and eventual practical effectiveness of plant cystatins against target pest digestive proteases. For instance, we used the 8th subunit of tomato multicystatin, SlCYS8 (Girard et al., 2007), as a model to produce single cystatin variants with strong inhibitory efficiencies against various Cys protease models, including the
2
digestive Cys proteases of CPB larvae (Kiggundu et al., 2006; Goulet et al., 2008). Overall, these studies have confirmed the potential of site-directed mutagenesis at positively selected amino acid sites to engineer recombinant cystatins with improved value for insect pest control. Despite these developments, a more thorough assessment of the CPB digestive protease complement remains essential to improve our basic knowledge on the actual effects of improved SlCYS8 variants, notably to detect differential compensatory responses eventually taking place in the insect fed cystatins with alternative inhibitory spectra. Such studies would be useful in practice to measure and improve the potential of engineered cystatins for herbivore pest control, and to get some more clues about how to improve these proteins for use in an agricultural context. Considering the close relationships established over time between plants and their insect enemies, we hypothesized that herbivorous insects’ digestive Cys proteases are involved in an evolutionary “arms race” with the rapidly evolving cystatins of their plant hosts. To challenge this idea, we here looked for the existence of a reciprocal adaptive evolution process on the insect side using digestive Cys proteases of herbivorous Coleoptera, including the CPB, as a model. We also assessed the impact of plant diet on protease forms and sensitivity to inhibition among CPB populations. Early experiments in our laboratory indicated that CPB larvae may quickly adapt their midgut proteolytic system to different host plants (Overney et al., 1997) but a more thorough analysis of these compensatory responses at the proteome level remained essential to get a more precise idea of the phenomenon. Possible variation among CPB populations of different origins also remained to be assessed to measure how robust and representative predictive models are when studying compensatory responses to protease inhibitors in a given target insect population. Numerous data can be found in the literature suggesting variable adaptations to host plants, environmental conditions or agricultural practices among CPB populations of different geographical origins (e.g. Hare, 1986; Hsiao, 1985; Jiang et al., 2010; Lyytinen et al., 2012; Lehmann et al., 2014). On the other hand, very little information was available, when initiating this project, about an eventual variation of digestive protease complements in different CPB populations.
To address these questions and to gain further knowledge about basic compensatory responses to protease inhibitors in the CPB, we performed a number of diet bioassays and detailed digestive protease analyses at the proteome scale, assessing in particular the impacts of plant leaf diet, geographical origin and cystatin variant inhibitory spectrum against this insect’s
3
digestive protease complement taken as a whole. The core of this thesis includes an introductory (literature review) chapter to introduce the project’s research problem, a short complementary chapter to present research hypotheses and objectives, four scientific manuscripts recently published in peer-reviewed journals, and a concluding chapter summarizing our main findings and conclusions. A literature review is first presented in Chapter 1 that summarizes current knowledge and recent advances on protease::inhibitor interactions in plant–insect systems, with special emphasis on the potato–CPB interaction. Research hypotheses and objectives are then presented in Chapter 2, followed by primary research articles containing most of the original data. Chapter 3 (Article 1) assesses the impact of plant diet on CPB digestive proteases, and the influence of geographical origin (or insect population) while drawing conclusions about digestive compensatory responses in CPB larvae. Chapter 4 (Article 2) confirms the occurrence of positively selected (hypervariable) amino acid sites in coleopteran herbivore digestive Cys proteases, supporting the idea of a co-evolutionary arms race at the genome level between herbivore insect Cys proteases and plant cystatins. In complement to Chapter 4, Chapter 5 (Article 3) confirms a link between amino acid hypervariability among CPB midgut proteases and susceptibility of these enzymes to plant cystatins. This chapter also shows that structural variations as small as the spatial orientation of the R group on a positively selected amino acid or the distance separating this functional group and the alpha carbon can induce differential compensatory responses in CPB larvae. Despite such findings again highlighting the striking adaptive capacity of the CPB to protease inhibitors, Chapter 6 (Article 4) confirms the potential of a promising SlCYS8 variant, P2V, to provide potato plants with partial resistance to this insect, and thus points to a possible limit of its compensatory ability to dietary cystatins. A General discussion and perspectives chapter (Chapter 7) concludes the thesis, which summarizes the project’s main findings and proposes new ideas to successfully harness the potential of Cys protease inhibitors for CPB control. Original data are presented in this last chapter that suggest the usefulness of a new class of Cys protease inhibitors, the mushroom macrocypins (Smid et al., 2013), as a complement to plant cystatins for an efficient, broad-spectrum inhibition of CPB digestive Cys proteases.
4
CHAPITRE 1 THE RESEARCH PROBLEM
Herbivorous insects and their host plants account for an important fraction of Earth's biodiversity (Stotz et al., 1999). These organisms have established continuous and durable interactions through millions of years and are often considered as a model case of between-species coevolution at the biology timescale.
1.1
Plant–insect interactions at the molecular scale
THE PLANT’S DEFENSE RESPONSE TO INSECT HERBIVORY While, by definition, herbivorous insects continuously threaten them, plants have developed the ability to detect insect feeding by the recognition of ‘defense elicitor’ molecules in the oral secretions or saliva of the herbivores (Chung et al., 2013; Furstenberg-Hagg et al., 2013; Frost et al., 2008; Pare et al., 1999). Plants deploy a variety of defense mechanisms to overcome herbivory, notably resulting in the expression of numerous toxic, antidigestive or antifeedant compounds in both wounded and distant tissues (Chen, 2008). Organic chemicals such as alkaloids, terpenes and cyanogenic glucosides are well-known examples of plant defense compounds (Furstenberg-Hagg et al., 2013; Franco et al., 2002). These phytochemical repellants make plant tissues unpleasant or even toxic to insect herbivores or pathogens, and also attract, under their volatile forms, natural enemies of the herbivores such as insect parasitoids and predators (Saha et al., 2013).
Defense-related polypeptides and proteins like systemin, arcelins, vicilins, chitinases, -1,3-glucanases, lectins, protease inhibitors and α-amylase inhibitors are another key component of the plant’s defensive arsenal against insect herbivores (Farrokhi et al., 2008; Dang and Van Damme, 2015). Enzyme inhibitors, in particular, are deposited in storage tissues or induced in leaves upon herbivory to block dietary macromolecule breakdown in the insect midgut lumen (Furstenberg-Hagg et al., 2013; Franco et al., 2002). For instance, α-amylase inhibitors accumulated in storage tissues during seed or vegetative storage organ development
5
significantly delay starch digestion, and hence carbohydrate and energy uptake, in the midgut of seed-feeding insects (Svensson et al., 2004). Similarly, protease inhibitors are accumulated in developing storage organs or induced in leaves upon herbivory to limit herbivores’ ability to digest dietary proteins and absorb amino acids essential for protein biosynthesis (Felton et al., 2005; Bhattacharjee et al., 2012).
THE INSECT’S COMPENSATORY RESPONSE TO PLANT DEFENSE BARRIERS Investigation of plant–insect interactions not only led to the elucidation of host plant defenses, but also to a better understanding of how insects respond to plant chemical or antidigestive challenges. The insect digestive tractus is not a passive target of plant defense action; it can adapt to nutritional deficiency and cope with plant toxins or antifeedant metabolites (Zhu-Salzman & Zeng, 2015). Natural selection has given herbivorous insects the ability to elude plant defenses by different means, including adaptation of their mouthparts or digestive system structure in such a way as to sequester phytotoxic compounds in harmless locations (Wise & Abrahamson, 2005). Insects also have evolved an array of detoxifying enzymes to mitigate the harmful effects of plant-derived toxicants by oxidation, reduction, hydrolysis or conjugation to specific molecules (Scott & Wen, 2001; Yu, 2005). One early example is the parsnip webworm Depressaria pastinacella, shown to use furanocoumarin-metabolizing enzymes to deactivate furanocoumarin derivatives of the wild parsnip Pastinaca sativa (Berenbaum & Zangerl, 1998). Another example is the black swallowtail Papilio polyxene, that uses gene duplication-derived variants of cytochrome P450 monooxygenases to inactivate a range of toxins in different plant tissues (Wen et al., 2006).
Protease inhibitors are another class of defense compounds against which herbivorous insects had to deal with over time (Ryan, 1990). Herbivorous arthropod genomes include large families of digestive protease genes (Srinivasan et al., 2006; Tribolium Genome Sequencing Consortium, 2008; Grbic et al., 2011) that allow for the expression of protease functional isoforms under a range of environmental or physiological conditions (e.g. Jongsma et al., 1995; Bown et al., 1996; Zhu-Salzman et al., 2003; Gruden et al., 2004; Sainsbury et al., 2012). From a physiological standpoint, such complex digestive protease complements allow the herbivores to elude the detrimental effects of defense-related protease inhibitors in different ways, including by the secretion of midgut proteases from several functional families, by the
6
overexpression of inhibitor-sensitive proteases to outnumber the ingested inhibitors, by the up-regulation of protease isoforms weakly sensitive to inhibition, or by degradation of the plant inhibitors using non-target proteases (Broadway, 2000; Zhu-Salzman & Zeng, 2015). From a practical standpoint, the development of complex digestive protease complements has given insects a range of options to successfully overcome the effects of protease inhibitors and has significantly hindered the large-scale implementation of these proteins in plant protection strategies (Schlüter et al., 2010; Zhu-Salzman & Zeng, 2015).
A well-documented case is the coleopteran herbivore pest Colorado potato beetle (CPB) Leptinotarsa decemlineata Say (Maharijaya and Vosman, 2015). This insect uses a complex set of proteases for leaf protein digestion, notably including cysteine (Cys) proteases which account, overall, for more than 90% of total proteolytic activities in crude midgut extracts (Thie & Houseman, 1990; Michaud et al., 1993, 1995; Novillo et al., 1997; Gruden et al., 2003, 2004; Sainsbury et al., 2012; Srp et al., 2016). A range of effects have been described for transgenic potato lines expressing Cys protease inhibitors on the CPB, from toxicity causing larval mortality (Lecardonnel et al., 1999; Outchkourov et al., 2003) or growth delays (Cingel et al., 2014; 2015; Outchkourov et al., 2003) to development acceleration and over-compensatory feeding concomitant with increased protease levels in the midgut lumen (Cingel et al., 2015; Cloutier et al., 1999; 2000). Proposed explanations for such diverse effects from one study to another involved the amount of recombinant inhibitor in host plant tissues, variable stability of the inhibitor in planta, specific efficiency of the inhibitor towards insect proteases, and experimental biases impacting insect fitness. Our general goal in the present project was to gain more insight about digestive protease-related compensatory processes in CPB larvae consuming plant protease inhibitors, useful then to harness the full potential of these antidigestive proteins as valuable compounds in plant protection.
1.2
The CPB: A short literature review
For more than a century now, farmers have suffered huge damage by the CPB. This insect feeds on leaves of Solanaceae plants, including tomato, pepper, horse nettle, eggplant and, most importantly, cultivated potato Solanum tuberosum (Capinera, 2001). In potato fields, large populations of potato beetles can defoliate entire plants and lead to significant economic losses (Alyokhin, 2009; Hare, 1990).
7 1.2.1 Geographical distribution and life cycle of the CPB
Almost two centuries ago, American entomologist Thomas Say identified the CPB in Missouri and Arkansas while collecting insects on the buffalo bur Solanum rostratum (Dunal) (Say, 1824). Mexico is the primary origin of this insect, which then migrated to North America in the early 1800s (Alyokhin, 2009; Hare, 1990). Once reaching cultivated potato areas, its geographical distribution expanded fastly and it soon happened to be the most damaging pest of potato in North America (Hare, 1990). The first reports identifying CPB as a pest to potato crops came from Nebraska in 1859 (Capinera, 2001). Reports on its dissemination in China and Iran in recent decades confirmed its status as a global pest of potato and other Solanaceae crops worldwide (Alyokhin et al., 2008; Sharif et al., 2007).
The CPB has a complex life cycle that is well suited to agricultural environments. In Northeast U.S. and Canada, beetles remain below the soil surface for several months during the winter season. Adults emerge in the spring season to feed, mate, and deposit eggs on the underside of leaves of early-planted potatoes. Emerging larvae start feeding on potato leaves until molting, and so on until reaching their fourth larval stage. Larval development lasts ten to twenty days depending on daylength, ambient temperature and host plant quality. Mature larvae then fall down the leaves and go for five to ten days into the soil for pupation, to transform into adults (Capinera, 2001; Hare, 1990). This first generation of adults emerges during the growing season to mate and lay eggs, producing a second generation of larvae with unquenchable appetites. After several weeks, second generation-adults migrate below the soil surface to overwinter, thus completing a new cycle prior to the next season (Alyokhin, 2009; Capinera, 2001). This complex life cycle of the insect is well suited to the variable agricultural and climatic conditions of potato culture areas, and contributes to make it a pest difficult to control (Cingel et al., 2016; Sablon et al. 2013). Several approaches have been proposed over the years to control CPB populations in potato fields, used alone or in combination in an integrated pest management program.
1.2.2 Current approaches for CPB control
Approaches to mitigate CPB infestations in potato fields involve alternative cultural practices, chemical pesticides, biocontrol agents and/or potato varieties resistant to the insect.
8
CULTURAL PRACTICES Cultural practices such as crop rotations and alternating planting times were shown to reduce seasonal defoliation rates by CPB larvae and adults in the field (Alyokhin, 2009). For instance, the density of beetle egg masses was decreased to less than 10% in rotated potato fields compared to non-rotated control fields (Lashomb & Ng 1984), while early season adult densities were decreased by 96% with certain crop rotation practices (Wright, 1984). The manipulation of planting time was also proposed, with the objective of restraining the size of second-generation larval populations and the number of adults then migrating below the soil surface for winter (Alyokhin, 2009). The use of trap crops and mulching was also proposed, to keep adult beetles away from the main crop and to increase time between soil emergence and leaf damage in the field (Alyokhin, 2009). While interesting in theory, the adoption of control strategies based on alternative cultural practices is often ruled out for economic considerations. Agronomic constraints compromising the practical feasibility of these approaches also limit their implementation, such as the need to establish crop rotations more than 300-900 m away from cultivated potato fields to counteract the mobility of CPB adults from one field to another (Alyokhin, 2009).
BIOCONTROL AGENTS Several biological control (or “biocontrol”) agents have been considered to mitigate CPB infestations in the field, including arthropod predators, parasitoids and microbial pathogens. Examples are the specialist predators two-spotted stinkbug Perillus bioculatus (Hemiptera: Pentatomidae), spined soldier bug Podisus maculiventris (Hemiptera: Pentatomidae) and ground beetle Lebia grandis (Coleoptera: Carabidae), which also behaves as an obligate parasitoid of CPB pupae at the larval stage (Alyokhin, 2009). Other biocontrol agents are the solitary egg parasitoid Edovum puttleri (Hymenoptera: Eulophidae), the tachinid larval parasitoids Myiopharus aberrans (Diptera: Tachinidae) and Myiopharus doryphoeae (Diptera: Tachinidae), the entomopathogenic fungus Beauvaria bassiana and the soil bacterium Bacillus thuringiensis, var. tenebrionis (Wraight & Ramos, 2002; Alyokhin, 2009; Weber, 2013). A number of cases were reported where biocontrol enemies could reach satisfying population densities and exert pressure on the target pest sufficient to reduce CPB numbers below economically damaging levels in the field. However, high fecundity rates of CPB females and the diversity of environmental and cultural parameters influencing their action often compromise the efficacy of these organisms in practice (Cloutier & Jean, 1998; Cloutier & Bauduin; 1995; Cloutier & Boiteau; 2002; Alyokhin, 2009).
9
CHEMICAL PESTICIDES At this stage, chemical pesticides still represent the most convenient way of controlling CPB populations in commercial potato fields. Indeed, the economic importance of the CPB has played a significant role in the development and adoption of synthetic pesticides worldwide, with hundreds of chemicals tested over the years against this pest (Alyokhin et al., 2008). Potato beetles have been an early target for DDT in 1939, and more than 30 insecticides are currently used in the U.S. against this particular insect (Alyokhin et al., 2008). On the other hand, and despite their efficiency and ease of use, the effectiveness of these compounds cannot be guaranteed in the long term (Alyokhin et al., 2008). High selection pressures exerted on this pest by the large-scale use of synthetic pesticides has led, over the years, to the establishment of insecticide-resistant populations (Alyokhin et al., 2008). A first evidence of this was reported for DDT in the early 1950’s (Quinton, 1955), followed by the rapid appearance of populations resistant to other pesticides (Alyokhin, 2009).
From a mechanistic viewpoint, CPB resistance to chemical insecticides may be explained by increased target site insensitivity, decreased insecticide penetration, behavioral adaptation to avoid physical contact with the pesticides, and, most importantly, expression of detoxifying enzymes such as carboxylesterases, monooxygenases and/or esterases to produce harmless derivatives of the toxic compounds (Alyokhin & Ferro, 1999; Hoy & Head, 1995; Alyokhin et al., 2012). From an evolutionary viewpoint, different factors may explain the natural predisposition of the CPB to develop pesticide resistance (Alyokhin et al., 2008). Its coevolution history with Solanaceae species likely is one factor, given the high levels of glycoalkaloids in the foliage of these plants and hence the need, for the coevolving herbivore, of developing strategies to detoxify, or at least tolerate, these constitutive poisoneous compounds. Another likely factor is the high growth, developmental and reproductive rates of the insect, that maximize the incidence of random mutations and generation of pesticide-resistant mutants in field populations. Overall, high selection pressures exerted on CPB populations under changing environmental conditions, together with a natural ability of the insect to cope with toxic substances, likely contributed to the large number of insecticide-resistant populations described over the years by many authors (Ferro et al., 1993; Zhao et al., 2000; Alyokhin et al., 2006, 2008; Alyokhin & Dively, 2007; Alyokhin, 2009).
HOST PLANT RESISTANCE While for practical reasons chemical pesticides remain –and will likely remain– a predominant approach for CPB control in potato fields, evolutive resistance
10
and environmental concerns raised by the large-scale use of these compounds in agriculture always remind the relevance of developing effective and durable alternatives for potato protection (Alyokhin, 2009). The eventual adoption of environment-friendly cultural practices and biocontrol agents has been addressed above; host plant genetic resistance also is an interesting avenue (Gatehouse, 2008; Pelletier et al., 2013). Breeding attempts to develop potato varieties resistant to the CPB have mostly relied until now on germplasm integration from closely-related, CPB-resistant Solanum species by crosspollination or protoplast electrofusion. A useful trait to implement insect resistance in potato is the pattern of trichomes on the leaf surface, as exemplified with Solanum ployadenium, Solanum berthaultii and Solanum neocaardenasii, three relatives of potato that show resistance to CPB and aphids (Pelletier et al., 2013). Another useful trait for improvement is the glycoalkaloid content in leaves. Leptin, in particular, works as an antibiotic against the CPB to compromise development, feeding and survival of both larvae and adults. Potato hybrids produced by the crossing of a commercial potato cultivar with Solanum chacoense, a leptin-rich potato relative, showed high resistance to the CPB (Pelletier et al., 2013). As well, the commercial potato cultivar Dakota Diamond, with high levels of foliar leptin, was less sensitive to herbivory than other cultivars showing lower leptin levels (Thompson et al., 2008).
Another promising, yet controversial, option for potato improvement against the CPB is the development of insect-resistant transgenic, or genetically-modified (GM), lines by genetic transformation (Alyokhin et al., 2008). The successful expression of a B. thuringiensis Cry3a toxin in potato some 25 years ago (Perlak et al., 1993) has led to the commercial development of potato lines highly resistant to the CPB in the late 1990’s. Even though, for different reasons including consumer concerns and unresolved environmental issues, these plants were discontinued after only five years on the market (Cloutier et al., 2008), the potential of genetic transformation to implement insect resistance into major crops still remains attractive, including for potato (Barrell et al., 2013). Cry toxin-expressing varieties of corn, cotton and soybean for insect control are now grown each year over millions of hectares worldwide (James, 2015), and agricultural areas sown with GM rice varieties engineered to express a Cry toxin, alone or in combination with a plant protease inhibitor, have increased steadily in recent years (Chen et al., 2011; Li et al., 2016).
11
1.3
Protease inhibitors for insect control
Numerous studies have assessed the potential of protease inhibitors as a complement to Cry toxins in crop protection, after the publication of a seminal paper by Hilder et al. (1987) some 30 years ago reporting the detrimental effects of a cowpea trypsin inhibitor-expressing tobacco line against the lepidopteran pest tobacco budworm, Heliothis virescens (Haq et al., 2004; Schlüter et al., 2010; Macedo et al., 2015). This approach for pest control consists of interrupting dietary protein digestion in target insects via the ectopic expression of a protease inhibitor in host plant tissues (Michaud, 2000). Inhibition of protein digestive functions in the midgut lumen upon leaf ingestion causes dietary protein wastage and nutritional deprivation, which in turn interfere with growth, development and reproduction of the herbivore (Xu et al., 1996; Gatehouse et al., 1997; Chen et al., 2009).
1.3.1 Target proteases in the insect midgut
Proteases are predominant hydrolytic enzymes in herbivorous insects, mostly involved in dietary protein digestion (Macedo & Freire, 2011). These enzymes are categorized into four major classes based on the identity of functionally relevant amino acid residues at their active catalytic site:
. The serine (Ser) proteases, such as trypsin and chymotrypsin, that rely on a catalytic triad composed of an aspartate (Asp), a Ser and a histidine (His);
. The cysteine (Cys) proteases, such as cathepsin L, cathepsin B and papain, that rely on a catalytic triad composed of a Cys, a His and an Asp;
. The aspartate (Asp) –or acidic– proteases, such as pepsin and cathepsin D, that rely on two Asp residues; and
. The metalloproteases, that depend on a metallic cation, usually zinc, to exhibit activity. Herbivorous insects use complex complements of proteases for protein digestion, often including Ser or Cys proteases as the predominant forms (Haq et al 2004). Lepidoptera, with a midgut lumen in the alkaline pH range, mainly use trypsin- and chymotrypsin-like Ser proteases for protein digestion (Srinivasan et al., 2006). Herbivorous Coleoptera, with a midgut lumen in
12
the mildly acidic pH range, often rely on Cys and Asp proteases (Murdock et al., 1987; Macedo & Freire., 2011).
Similar to proteases, protease inhibitors are categorized into four functional classes, based on their preferred protease targets, i.e. the Ser protease inhibitors, active against Ser proteases; the Cys protease inhibitors, active against Cys proteases; the Asp protease inhibitors, active against Asp proteases; and the metalloprotease inhibitors, active against metal-dependent peptidases. Most protease inhibitors, regardless of their functional class, bind target proteases at the catalytic site to form a ‘physiologically stable’ bipartite complex blocking access to protein substrates (Michaud, 2000). The inhibitor thus acts as a pseudo-substrate to prevent hydrolysis of peptide bonds by the target enzyme.
1.3.2 Plant cystatins for CPB control
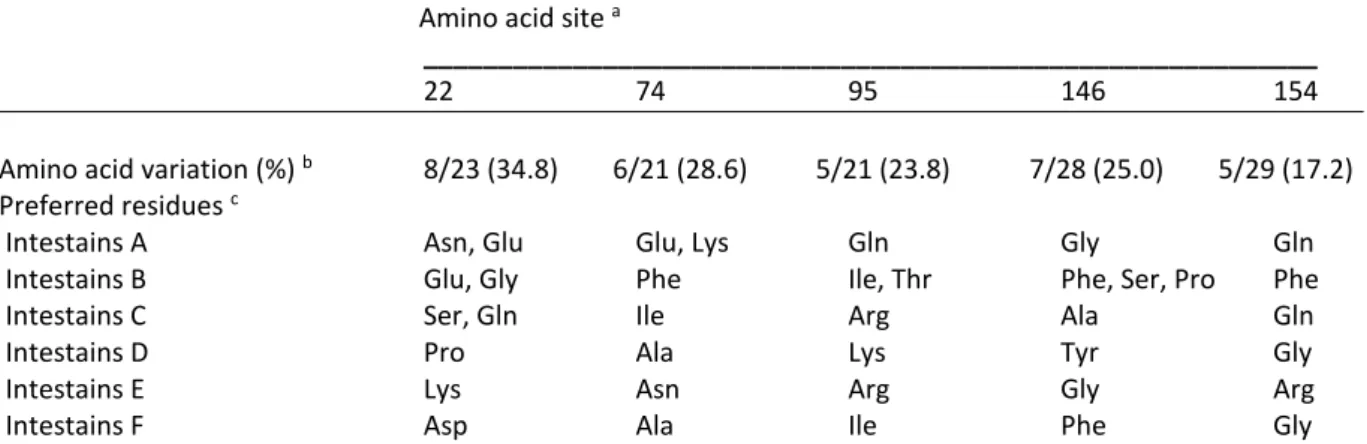
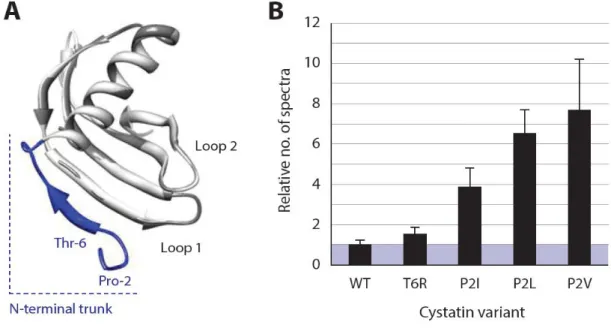
The fact that phytopaghous Coleoptera, including the CPB (Thie & Houseman, 1990; Michaud et al., 1995; Novillo et al., 1997; Gruden et al., 2003; Srp et al., 2017), mainly use Cys proteases for plant protein digestion has prompted several groups to assess the potential of plant Cys protease inhibitors in plant protection. As inferred from structural data for the model inhibitor oryzacystatin I (Protein Data Bank Accession No. 1EQK), these proteins present three conserved regions forming a tripartite wedge that penetrates the active site cleft of the target enzyme to prevent peptide bond hydrolysis (Nagata et al., 2000). The inhibitor physically interacts with the active site of the target enzyme through two hairpin loops that each include a conserved amino acid motif essential for inhibitory activity (Figure 1.1). Like for mammalian cystatins, the N-terminal region–or ‘N-terminal trunk’–of plant cystatins includes a conserved glycine (Gly) residue involved in the inhibitory process. This region of the protein does not physically interact with the active site but stabilizes the binding process and strongly influences the strength and specificity of the resulting enzyme::inhibitor complex (Benchabane et al., 2010).
13
Figure 1.1 (ADAPTED FROM BENCHABANE ET AL.,2010) Structural models for the plant cystatin OC-I. Front (left) and side (right) views are presented, showing the main structural elements of the protein, namely a five-stranded ß-sheet core (in blue), a -helix with the conserved amino acid motif LARFAVDEHN (in red), an N-terminal region (or N-terminal trunk) with the conserved Gly residue, and two hairpin loops harbouring the conserved Gln–Xaa–Val–Xaa–Gly and Pro-Trp motifs entering the active site cleft of target
enzymes. The models were built using the modelling software Discovery Studio 2.5 (Accelrys, San Diego CA), based on the structural spatial coordinates of oryzacystatin I (Protein Data Bank Access. No. 1EQK;
http://www.wwpdb.org) (Nagata et al., 2010).
Successful cases of resistance to herbivorous pests have been reported for a number of plants expressing recombinant cystatins (e.g. Chan et al., 2010; Senthilkumar et al., 2010; Carrillo et al., 2011a, 2011b; Roderick et al., 2012; Santamaria et al., 2012; Vieira et al., 2015), but the pesticidal effects of these proteins proved unsufficient in many other instances despite observable protease inhibitory effects in vivo (Benchabane et al., 2010; Schlüter et al., 2010; Macedo et al.., 2015). As discussed above (Section 1.1), insects have evolved different strategies to cope with plant protease inhibitors (Zhu-Salzman & Zeng, 2015), notably involving complex digestive systems with proteases from diverse functional classes (Hernandez et al., 2003; Prabakhar et al., 2007; Vinokurov et al., 2009; Kiggundu et al., 2010), target protease overexpression to outnumber the inhibitory proteins (Cloutier et al., 2000; Ahn et al., 2004) and the production of protease isoforms weakly sensitive to inhibition (Jongsma et al., 1995; Bown et al., 1997; Girard et al., 1998a; Cloutier et al., 2000; Mazumdar-Leighton & Broadway, 2001; Zhu-Salzman et al., 2003; Brunelle et al., 2004; Gruden et al., 2004; Liu et al., 2004; Koo et al., 2008; Ahn et al., 2010). Other strategies are the up-regulation of proteases from alternative functional classes (Zhu-Salzman et al., 2003; Rivard et al. 2004; Oppert et al. 2005; Vila et al., 2005), the degradation of protease inhibitors by nontarget midgut proteases (Michaud, 1997; Girard et al., 1998b; Giri et al., 1998; Gruden et al., 2003; Zhu-Salzman et al.,
14
2003; Ahn et al., 2009; Yang et al., 2009) and a reallocation of metabolic resources towards inhibitor-induced compensatory responses (Liu et al., 2004; Chi et al., 2009). It is now well established that protease–inhibitor interactions in plant–insect systems are the result of a long coevolutive process that triggers the continuous diversification of insect protease and plant protease inhibitor functions (Lopes et al., 2004; Christeller, 2005; Kiggundu et al., 2006). It is also established that the ectopic expression of protease inhibitors in transgenic plants not only alters the activity of target proteases in the midgut lumen of naive herbivores, but also induces a significant remodelling of both the midgut transcriptome and digestive protease complement (Brunelle et al., 2004; Liu et al., 2004; Rivard et al., 2004; Chi et al., 2009).
Different approaches have been proposed to broaden the range of susceptible proteases and overcome compensatory effects in the insect midgut following inhibitor intake (Sainsbury et al., 2012), based on (1) the use of inhibitor combinations effective against different sets of proteases in the target pest or (2) the engineering of inhibitor variants with extended inhibitory spectra. Fusion proteins integrating Ser and Cys protease inhibitor sequences were shown for instance to exhibit improved potential against target herbivores compared to the inhibitors expressed alone (Urwin et al., 1998; Inanaga et al., 2001; Outchkourov et al., 2004; Brunelle et al., 2005; Benchabane et al., 2008), similar to transgene stacking approaches for the simultaneous expression of two or more inhibitors in host plant tissues (Abdeen et al., 2005; Senthilkumar et al., 2010; Dunse et al., 2010; Chan et al., 2010; Santamaria et al., 2012). Protein engineering schemes involving random or site-directed mutations in functionally relevant regions of the native protein have also been proposed to improve the potency of plant protease inhibitors (Jongsma et al., 2015; Urwin et al., 1995; Koiwa et al., 2001; Ceci et al., 2003; Melo et al., 2003; Kiggundu et al., 2006; Goulet et al., 2008). Site-directed mutagenesis at positively selected (hypervariable) amino acid sites was used for instance to generate functional diversity among cystatin variants, helpful to devise candidate inhibitors with broader or complementary inhibitory profiles towards Cys proteases (Kiggundu et al., 2006). Single variants of tomato cystatin SlCYS8 (Girard et al., 2007) were developed using this approach, that show a range of inhibitory specificities against CPB digestive proteases (Goulet al., 2008) (Figure 1.2) and represent, together, a valuable source of candidate cystatins for potato improvement.
15 1.3.3 Unsolved questions for the present project
The challenge at present is to translate those theoretical developments and empirical advances generated in vitro into applications of practical significance. Major progress has been made over the years to produce plant cystatins highly active against herbivorous insect proteases (Schlüter et al., 2010; Sainsbury et al., 2012) but several questions remain about the actual potential of these improved inhibitors from a plant protection perspective. For instance:
1. Is the inhibitory potency of a plant cystatin like tomato SlCYS8 against CPB digestive proteases similar for insects of different origins?
2. Are digestive Cys proteases in the CPB following a rapid evolution pathway as reported previously for their plant cystatin interacting counterparts?
3. Are potent functional variants of SlCYS8 produced by site-directed mutagenesis, such as P2F, P2V or T6R (Figure 1.2), still inducing compensatory responses in the target insect? 4. Are these promising variants identified by in vitro means useful to generate potato lines
resistant to the CPB?
This project aimed at providing answers to these four questions, with the ultimate goal of confirming the potential of plant cystatins for potato improvement against the CPB.
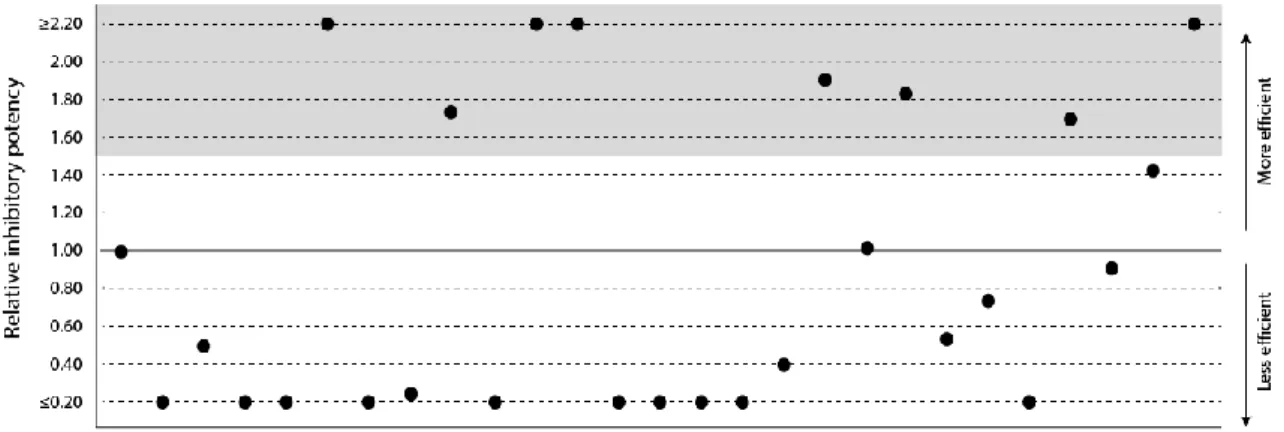
Figure 1.2 Relative inhibitory potency of tomato cystatin SlCYS8 and single variants mutated at
positively selected sites 2, 6 or 31 (see Kiggundu et al., 2006). Protease inhibitory activities were determined by fluorimetry against CPB midgut Cys proteases, using the synthetic peptide Z-Phe– Arg-methylcoumarin as a model substrate. Data are presented relative to the activity of native SlCYS8 (IC50 20 nM; relative potency of 1.0). Datapoints were inferred from Goulet et al., 2008.
16
CHAPITRE 2 THE RESEARCH PROJECT
Four working hypotheses were tested during the project, related to the four questions raised in Chapter 1 (Section 1.3.3). A dozen of research objectives involving genomic database searching, protein in silico modelling, functional proteomics, enzymology and insect feeding bioassays were pursued to address these hypotheses, using potato, single variants of SlCYS8 (Figure 1.2) and CPB 4th instars as a tripartite plant–inhibitor–insect model.
2.1
Working hypothesis No. 1
(QUESTION 1;CHAPTER 3)… ON DIGESTIVE PROTEASE COMPLEMENTS IN CPB LARVAE OF DIFFERENT ORIGINS
Many studies have documented local adaptation to plant diet patterns (Hsiao et al., 1985; Hare & Kennedy, 1986; Horton et al., 1988), environmental cues (Lehmann et al., 2012, 2014; Lyytinen et al., 2012) or agricultural practices (Jiang et al., 2010) among CPB populations of North America or Europe. An early study by Girard et al. (1998) also reported the differential susceptibility of two populations of cabbage seed weevil Ceutorhynchus assimilis to transgenic oilseed rape expressing a rice cystatin. We expected local adaptation also to occur among CPB populations in terms of midgut protease complements and susceptibility to protease inhibition, and hence formulated the following hypothesis:
“CPB populations of different geographical origins present distinct digestive protease complements and compensatory responses to the plant diet, in terms of susceptibility to cystatin inhibition and protease isoforms in the midgut lumen.”
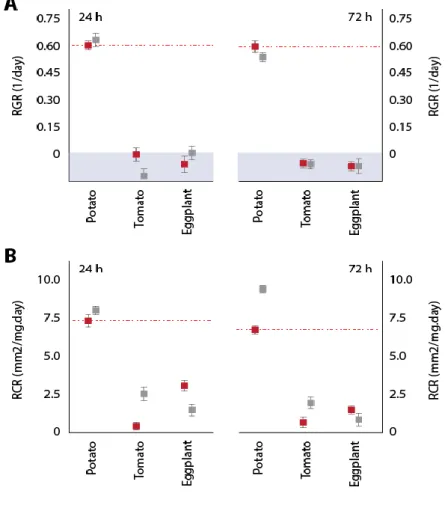
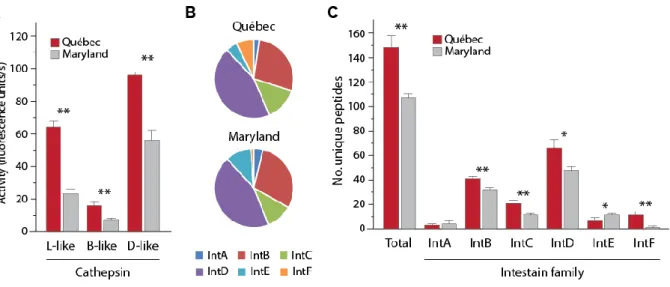
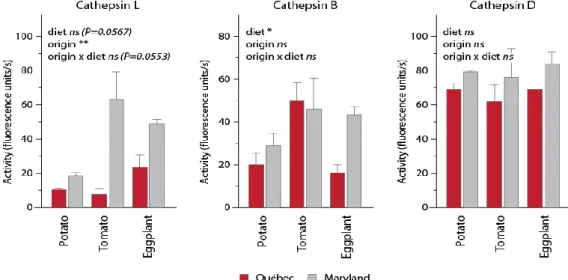
The following research objectives were pursued to test this hypothesis, using in vitro assays for protease activity and inhibition measurements, cystatin activity-based functional proteomics for Cys protease isoform (‘intestain’) characterization, and feeding bioassays with potato, tomato and eggplant for larval growth and leaf consumption monitoring:
17
. Objective 1.1 To compare growth and leaf consumption rates of potato, tomato and eggplant
fed CPB 4th instars derived from insect populations collected in two geographically distant potato fields.
. Objective 1.2 To compare midgut protease activities and intestain profiles in potato, tomato and eggplant fed CPB 4th instars of the two insect populations.
. Objective 1.3 To compare the inhibitory potency of SlCYS8 and SlCYS8 functional
variants against midgut proteases of CPB 4th instars from the two insect
populations.
2.2
Working hypothesis No. 2
(QUESTION 2;CHAPTER 4)… ON THE POSITIVE SELECTION OF COLEOPTERA (INCLUDING CPB)CYS DIGESTIVE PROTEASES
Ten years ago, our research group has identified Darwinian positive selection as contributing to the rapid functional diversification of plant cystatins in response to selective pressures exerted by the digestive proteases of their herbivorous enemies (Kiggundu et al., 2006). In line with the widely accepted idea of a coevolutionary pathway for plant protease inhibitors and herbivorous insect digestive proteases (Lopes et al., 2004; Jongsma & Beekwilder, 1991), we expected a reciprocal process to occur on the insect side and formulated the following working hypothesis:
“Herbivorous Coleoptera, including CPB, digestive Cys proteases present hypervariable, positively selected amino acid sites in regions of their primary sequence that interact with dietary protein substrates and plant pseudosubstrate inhibitors.”
The following research objectives were pursued to test this hypothesis, using statistical models for the detection of amino acid hypervariability among protein sequence datasets, in silico protein structure modelling for the visualization of intestain::cystatin interactions, in vitro assays for protease activity measurements, cystatin activity-based functional proteomics for intestain characterization and potato feeding bioassays for larval growth and leaf consumption monitoring:
18 . Objective 2.1 To look for the onset of positively selected amino acid sites among
herbivorous Coleoptera digestive Cys proteases.
. Objective 2.2 To determine the position of positively selected amino acid sites on CPB intestains (if any), in relation with the position of positively selected amino acid sites on their plant cystatin interacting counterparts.
. Objective 2.3 To compare growth and leaf consumption rates of CPB 4th instars fed SlCYS8
or SlCYS8 single variants mutated at positively selected amino acid sites. . Objective 2.4 To compare midgut protease activities and intestain profiles in CPB 4th instars
fed SlCYS8 or SlCYS8 single variants mutated at positively selected amino acid sites.
2.3
Working hypothesis No. 3
(QUESTION 3;CHAPTER 5)… ON COMPENSATORY RESPONSES TO SLCYS8 FUNCTIONAL VARIANTS IN CPB LARVAE
We previously reported a functional link between positively selected amino acids in plant cystatins and the inhibitory range of these proteins against insect digestive proteases (Kiggundu et al., 2006; Goulet et al., 2008). Given the key role of protein structure on biological function (Redfern et al., 2008) and assuming a rapid evolutionary pathway for CPB digestive proteases (HYPOTHESIS 2), we expected differential compensatory responses to occur in CPB challenged with closely related cystatin variants, and formulated the following working hypothesis:
“Single substitutions to closely related amino acids at positively selected sites of plant cystatins induce differential compensatory responses in CPB leading to specific adjustments of the digestive protease complement.”
The following research objectives were pursued to test this hypothesis, using public genomic databases for cystatin sequence searching, in vitro assays for protease activity measurements, cystatin activity-based functional proteomics for intestain characterization and potato feeding bioassays for larval growth and leaf consumption monitoring:
19 . Objective 3.1 To look for differential amino acid patterns at positively selected sites in the
N-terminal trunk of plant cystatins.
. Objective 3.2 To compare growth and leaf consumption rates of CPB 4th instars fed SlCYS8
or single variants with distinct, but closely related amino acid residues in the N-terminal area.
. Objective 3.3 To compare midgut protease activities and intestain profiles in CPB 4th instars
fed SlCYS8 or single variants with distinct, but closely related amino acid residues in the N-terminal area.
2.4
Working hypothesis No. 4
(QUESTION 4;CHAPTER 6)… ON THE INSECT RESISTANCE STATUS OF SLCYS8 VARIANT-EXPRESSING POTATO LINES
Despite unsuccessful attempts to use protease inhibitors in plant pest control, a number of promising cases have been reported in recent years with recombinant cystatins (e.g. Chan et al., 2010; Senthilkumar et al., 2010; Alvarez-Alfageme et al., 2011; Carrillo et al., 2011a, 2011b; Roderick et al., 2012; Santamaria et al., 2012; Vieira et al. , 2015) that still remind the crucial role of Cys digestive proteases in plants’ herbivorous enemies and the eventual relevance of these enzymes as effective targets for pest control (Chen et al., 2011; Macedo et al., 2015). As discussed previously (Section 1.1), mitigated effects were observed with recombinant cystatins against CPB larvae and adults, which again underlined the ability of this insect to cope with dietary protease inhibitory challenges. On the other hand, larval growth delays observed in some instances with cystatin-expressing potato lines (Outchkourov et al., 2003; Cingel et al., 2014, 2015), or the adverse effects of a cystatin–Asp protease inhibitor fusion on 4th instars (Brunelle et al., 2005), suggest a compensatory limit to broad-spectrum protease inhibition in the insect, in line with the following working hypothesis:
“Transgenic potato lines engineered to express improved variants of SlCYS8 are resistant to CPB larvae.”
The following research objectives were pursued to test this hypothesis, using cystatin activity-based functional proteomics for recombinant cystatin selection and intestain characterization, Agrobacterium tumefaciens-mediated transformation for transgenic plant line
20
development, in vitro assays for protease activity measurements and potato feeding bioassays for larval growth and leaf consumption monitoring:
. Objective 4.1 To select ‘weak’ and ‘strong’ inhibitors of CPB intestains for potato genetic
transformation using a cystatin activity-based functional proteomics scheme. . Objective 4.2 To produce transgenic potato lines expressing at different levels the weak and
strong cystatin variants selected in 4.1.
. Objective 4.3 To compare growth and leaf consumption rates of CPB 4th instars fed transgenic lines produced in 4.2.
. Objective 4.4 To compare midgut protease activities and intestain profiles in CPB 4th instars fed transgenic lines produced in 4.2.
21
CHAPITRE 3 POPULATION-ASSOCIATED
HETEROGENEITY OF THE DIGESTIVE CYSTEINE
PROTEASE COMPLEMENT IN COLORADO
POTATO BEETLE, LEPTINOTARSA DECEMLINEATA
Les résultats de ce chapitre ont été publiés récemment dans le périodique scientifique Journal of Insect Physiology.1 Les co-auteurs de l’article étaient Asieh Rasoolizadeh, Marie-Claire
Goulet, Jean-Frédéric Guay, Conrad Cloutier et Dominique Michaud.
1 Rasoolizadeh A, Goulet MC, Guay JF, Cloutier C & D Michaud (2017) Population-associated heterogeneity of the
digestive Cys protease complement in Colorado potato beetle, Leptinotarsa decemlineata. Journal of Insect
Physiology doi:10.1016/j.jinsphys.2017.03.001. Des données supplémentaires rattachées à cet article sont
22
3.1 Résumé
Les insectes herbivores utilisent des systèmes protéasiques complexes pour la digestion des protéines végétales, utiles pour ajuster leurs fonctions digestives à différentes diètes et pour éluder les effets antidigestifs des inhibiteurs de protéases retrouvés dans les tissus végétaux. Nous avons ici tenté de déterminer si les protéases digestives et l’ajustement du système protéolytique dans l’intestin en fonction de la diète ingérée variaient d’une population à l’autre chez les insectes herbivores. Deux élevages de laboratoire du coléoptère nuisible Leptinotarsa decemlineata ont été utilisés comme modèles, dérivés d’insectes prélevés dans des champs de pomme de terre distants de ~1,200 km en Amérique du Nord. Des larves de stade 4 élevées sur pomme de terre ont été maintenues sur cette plante pendant trois jours, ou transférées sur des plants de tomate ou d’aubergine pour la même période, afin de comparer l’activité de leurs cathepsines digestives et leur contenu en intestaines en fonction du régime alimentaire. L’activité des cathepsines D, des cathepsines L et des cathepsines B, de même que le contenu en intestaines totales dans l’intestin, variaient d’un facteur deux d’une population à l'autre dans les quelques heures suivant l’émergence des larves. En comparaison, l’activité des cathepsines D, l’activité des cathepsines B, le contenu en intestaines totales et l’abondance des principales familles d’intestaines étaient semblables après trois jours pour les deux groupes d’insectes peu importe la diète, contrairement à l'activité des cathepsines L et l’abondance des intestaines mineures qui divergeaient d’une population à l’autre. Ces variations du système protéasique étaient associées à l’efficacité différentielle d’un inhibiteur de protéases de type cystéine, la cystatine de tomate SlCYS8, contre les cathepsines L des deux groupes d'insectes. Malgré des différences sur le plan quantitatif, des variants SlCYS8 développés pour inhiber fortement les cathepsines L d’un groupe d’insectes montraient aussi une activité améliorée contre celles de l’autre groupe. Ces données suggèrent qu’il est possible de développer des cystatines modifiées qui soient plus actives contre les protéases d’insectes d’origines différentes. Elles soulignent en revanche la pertinence de prendre en compte la variabilité naturelle des systèmes protéasiques d’une population à l’autre, éventuellement déterminante pour le succès à grande échelle des stratégies de lutte aux insectes basées sur les cystatines recombinantes.
23
3.2 Abstract
Herbivorous insects use complex protease complements to process plant proteins, useful to adjust their digestive functions to the plant diet and to elude the antidigestive effects of dietary protease inhibitors. We here assessed whether basic profiles and diet-related adjustments of the midgut protease complement may vary among populations of the insect herbivore Colorado potato beetle (Leptinotarsa decemlineata). Two laboratory colonies of this insect were used as models, derived from insect samples collected in potato fields ~1200 km distant from each other in North America. Synchronized 4th-instar larvae reared on potato were kept on this plant, or switched to tomato or eggplant, to compare their midgut cathepsin activities and content of intestain Cys proteases under different diet regimes. Cathepsin D activity, cathepsin L activity, cathepsin B activity and total intestain content shortly after larval molting on potato leaves were about two times lower in one population compared to the other. By comparison, cathepsin D activity, cathepsin B activity, total intestain content and relative aboundance of the most prominent intestain families were similar in the two populations after three days regardless of the plant diet, unlike cathepsin L activity and less prominent intestain families showing population-associated variability. Variation in Cys protease profiles translated into the differential efficiency of a Cys protease inhibitor, tomato cystatin SlCYS8, to inhibit cathepsin L activity in midgut extracts of the two insect groups. Despite quantitative differences, SlCYS8 single variants engineered to strongly inhibit Cys proteases in midgut extracts showed improved potency against cathepsin L activity of either population. These data suggest the feasibility of designing cystatins to control L. decemlineata that are effective against different populations of this insect. They underline, on the other hand, the practical relevance of considering natural variability of the protease complement among L. decemlineata target populations, eventually determinant in the success or failure of cystatin-based control strategies on a large-scale basis.
3.3 Introduction
A large body of literature has discussed the potential of plant protease inhibitors in crop protection, after the publication of a seminal paper 30 years ago reporting the engineering of trypsin inhibitor-expressing tobacco plants resistant to the lepidopteran pest Heliothis virescens (Hilder et al., 1987). Several studies have since described the usefulness of Ser and Cys protease inhibitors to develop insect-resistant plant lines (Haq et al., 2004; Macedo et al., 2015; Schlüter