i
Paper-based Diagnostic Biosensors for Protein and
DNA Detection
by
Hao Fu
Department of Mechanical Engineering
McGill University
Montreal, Quebec, Canada
August 2019
A thesis submitted to McGill University in partial fulfillment of the requirements for a
doctoral degree
ii
Dedication
To my dearly-beloved parents and friends, as well as mentors
Qingjun
and
Jinling
for their love, support, and integrity-oriented family education.
To my brother
Xiaoyong
and my sister
Yanting
who accompany me through the
hard times and guide me to be a mentally-mature person.
iii
Table of Contents
Table of Contents ... iii
List of Figures ... viii
List of Tables ... xvi
Abstract ... xvii
Résumé ... xx
Acknowledgment ... xxiii
Contributions of the Author ... xxiv
Chapter 1: Introduction ... 2
1.1 Thesis objectives ... 4
1.2 Thesis organization ... 4
1.3 References ... 8
2 Chapter 2: State-of-the-art advances and challenges of incorporating functionality into microfluidic paper-based analytical devices ... 11
Abstract ... 11
2.1 Introduction ... 12
2.2 Brief state-of-the-art advances of μPAD technology ... 13
2.3 Detection and readout... 15
2.4 Programming and timing ... 17
2.5 Multi-step processing ... 19
2.6 Surface chemistry ... 21
2.7 Emerging challenges and remaining opportunities ... 23
2.8 Conclusion and outlook ... 25
2.9 References ... 26
iv
3 Chapter 3: An all-in-one, paper-based origami biosensor for rapid diagnosis of cardiac
diseases ... 36
Abstract ... 36
3.1 Introduction ... 37
3.2 Experimental methods ... 40
3.2.1 Design and fabrication of E-μPADs ... 40
3.2.2 ZnO-NW growth on WEs ... 42
3.2.3 Biofunctionalization of ZnO-NW-grown WEs ... 43
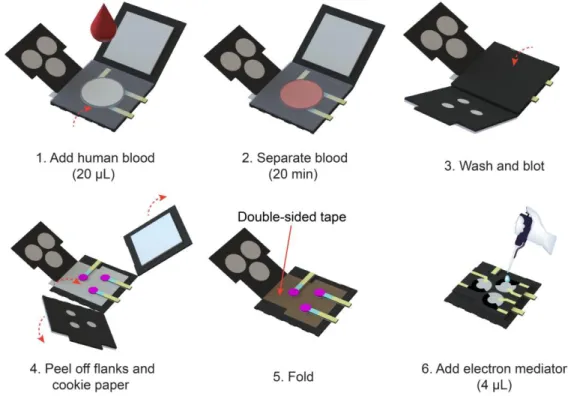
3.2.4 E-μPAD operation process ... 44
3.3 Results and discussion ... 45
3.3.1 Electrochemical characterization of the E-μPAD ... 45
3.3.2 Detection of cardiac biomarkers in ABP ... 48
3.3.3 Detection of cardiac biomarkers in real human blood ... 49
3.4 Conclusion ... 52
3.5 Acknowledgments ... 53
3.6 References ... 53
3.7 Supporting information ... 57
Link between chapter 3 and chapter 4 ... 59
4 Chapter 4: A paper-based microfluidic platform with shape-memory-polymer-actuated fluid valves for automated multi-step immunoassays... 61
Abstract ... 61
4.1 Introduction ... 62
4.2 Experimental section ... 64
4.2.1 Materials and reagents ... 64
v
4.2.3 Integration of the μPAD with a colorimetric reader ... 68
4.2.4 Fabrication and preparation of the µPAD ... 70
4.3 Results and discussion ... 71
4.3.1 Determination of the operation parameters... 71
4.3.2 Self-checking mechanism for valve malfunction ... 73
4.3.3 Automatic direct ELISA of rabbit IgG antigen in PBS ... 75
4.3.4 Autonomous sandwich P-ELISA for animal tissue samples ... 77
4.4 Conclusion ... 80
4.5 Acknowledgments ... 81
4.6 References ... 81
4.7 Supporting information ... 86
Link between chapter 4 and chapter 5 ... 93
5 Chapter 5: A point-of-care paper-based microfluidic platform for rapid diagnosis of human patients with clinically suspected laryngopharyngeal reflux ... 95
Abstract ... 95
5.1 Introduction ... 96
5.2 Experimental section ... 99
5.2.1 Platform overall design ... 99
5.2.2 Automated sandwich ELISA protocol ... 100
5.2.3 Human sample collection ... 103
5.2.4 Secretion collection and benchmark test ... 104
5.3 Results and discussion ... 105
5.3.1 Automated sandwich ELISA on the μPADs ... 105
5.3.2 Human sample analysis... 107
vi
5.5 Acknowledgments ... 110
5.6 References ... 110
5.7 Supporting information ... 115
Link between chapter 5 and chapter 6 ... 116
6 Chapter 6: Comparing surface chemistries for biomolecule immobilization on paper-based microfluidic devices ... 118
Abstract ... 118
6.1 Introduction ... 119
6.2 Experimental section ... 121
6.2.1 Materials and reagent ... 121
6.2.2 Surface chemistries and multi-well plate fabrication ... 121
6.2.3 Surface characterization of modified papers ... 123
6.2.4 Fluorometric assays for examining immobilization strength... 124
6.2.5 Colorimetric ELISAs on the modified papers ... 124
6.3 Results and discussion ... 125
6.3.1 Surface characterization of modified papers ... 125
6.3.2 Quantification of immobilization performance ... 127
6.3.3 Stability of modified cellulose paper ... 131
6.4 Conclusion ... 133
6.5 Acknowledgments ... 134
6.6 References ... 134
Link between chapter 6 and chapter 7 ... 138
7 Chapter 7: A fully-automated paper-based biosensor integrated with few-layered MoS2 nanosheets for DNA detection ... 140
vii
7.1 Introduction ... 141
7.2 Experimental section ... 143
7.2.1 Materials, reagents, and apparatus ... 143
7.2.2 Few-layered MoS2 nanosheet fabrication ... 144
7.2.3 The µPAD manufacture and fluorescent sensing mechanism ... 145
7.2.4 Automation on µPAD and the portable platform development ... 147
7.3 Results and discussion ... 149
7.3.1 Characterization of the synthesized MoS2 nanosheets ... 149
7.3.2 Optimization of detection conditions and calibration of DNA sensing ... 149
7.3.3 Automated DNA sensing on the µPAD ... 153
7.4 Conclusion ... 155
7.5 Acknowledgments ... 155
7.6 References ... 155
7.7 Supporting information ... 160
8 Chapter 8: Conclusions and future work ... 169
8.1 Summary of contributions ... 169
8.2 Future works ... 171
8.3 Publications ... 173
8.3.1 Refereed journals ... 173
8.3.2 Journal articles in preparation and submission ... 174
viii
List of Figures
Figure 2-1. Representative designs for incorporating functionality into μPADs. (a) A μPAD
integrated with blood membrane for human blood plasma separation. Reprinted from [34]. (b) A two-dimensional (2D) paper network for a manually-folding operations to perform a sandwich immunoassay; the test line is fabricated at the end of the μPAD for mixing with sequentially-transported reagents. Reprinted from [35]. (c) A magnetic timing valve manufactured in μPAD for manipulating fluid flow. Reprinted from [36]. (d) A smartphone developed for electrochemical sensing and reading on a μPAD. Reprinted from [37]. ... 14
Figure 2-2. (a) A smartphone-based colorimetric determination for pH and nitrite detection on
μPAD. Reprinted from [43]. (b) A commercial glucose meter tuned for human blood analysis on paper-based microfluidics. Reprinted from [44]. ... 16
Figure 2-3. (a) A shunt used as a dissolvable bridge to connect two paper channels. Reprinted
from [58]. (b) A two-terminal diode in a paper-based device for time series operations. Reprinted from [59]. (c) A magnetic two-way valving design for automated fluid manipulation in a μPAD. Reprinted from [60]. ... 18
Figure 2-4. (a) A “maze-like” paper-based device for detection of human chorionic gonadotropin
(hCG) based on an ELISA mechanism. Reprinted from [65]. (b) A dissolvable-material-based timing design for programming multi-step assays in a μPAD. Reprinted from [61]. (c) A versatile valving toolkit for automating fluidic operations in paper-based microfluidics. Scale bar is 1 cm. Reprinted from [26]. ... 20
Figure 2-5. (a) A potassium-periodate-oxidized paper for polymerization-based amplification in
the immunoassay for detection of PfHRP2 (analyte). Reprinted from [27]. (b) A modification strategy of cellulose surfaces with PDITC for DNA immobilization and subsequent rapid hybridization for target DNA sensing. Reprinted from [73]. (c) Chemical biofunctionalization of cellulose paper using DNA for biomolecule immobilizations. Reprinted from [75]. ... 22
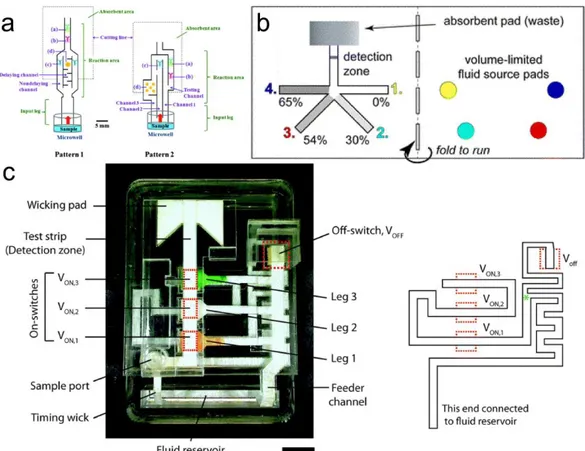
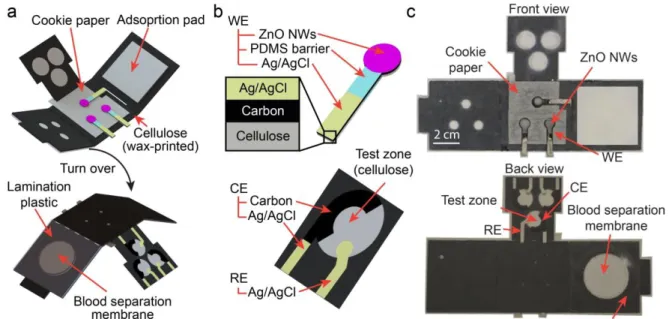
Figure 3-1. Schematic illustration of the all-in-one μPAD. (a) Schematic of the assembled
E-μPAD with all components. (b) Three-electrode (working, reference, and counter electrodes) configurations on the E-μPAD. (c) Photographs of the all-in-one E-μPAD. ... 40
ix
Figure 3-3. SEM images of (a) pristine cellulose, (b) carbon-printed WE (C), and (c)
ZnO-NW-coated WE. ... 45
Figure 3-4. Schematic illustration and experimental performance of electrochemical sensing
principle of the all-in-one E-μPAD. (a) Upper: equivalent circuit model for interpreting the Nyquist plots; lower: EIS biosensing principle based on different surface conditions on ZnO NWs before and after adding antigen. (b) Simulated Nyquist plots upon different surface conditions on ZnO NWs before and after adding antigen. (c) CV measurements under varied WE surface conditions. (d) Representative Nyquist plots in the presence of 25 µg/mL capture antibody (anti-rabbit-IgG) and 1 ng/mL antigen (rabbit-IgG)-immobilized capture antibody through EIS measurements in the frequency range of 20 kHz - 20 Hz. ... 46
Figure 3-5. (a) Representative Nyquist plots and (b) calibration curves from all-in-one EμPADs
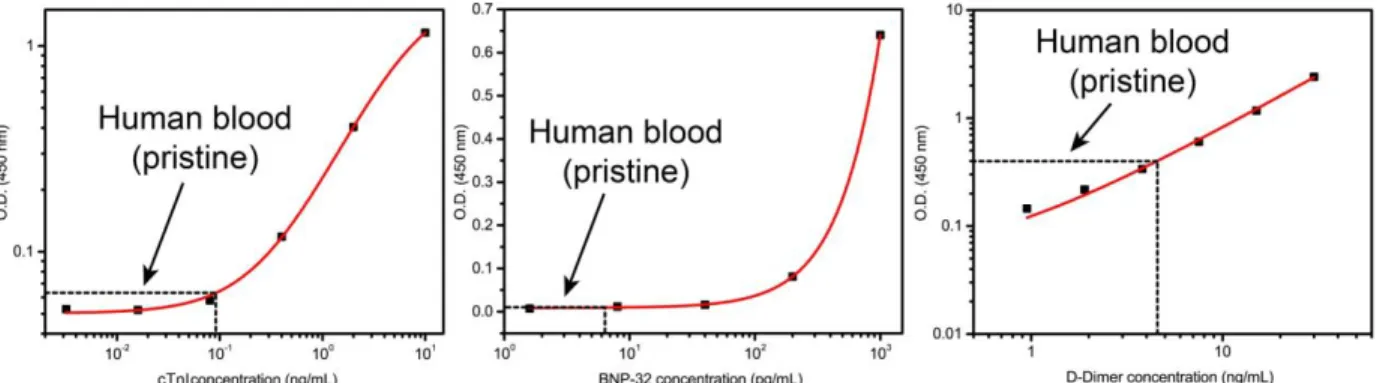
response to 10-fold spiked dilution in human artificial plasma for the detection of cTnI, BNP-32, and D-Dimer, respectively (n = 5). ... 48
Figure 3-6. (a) Schematic illustrations of human blood separation process on the all-in-one
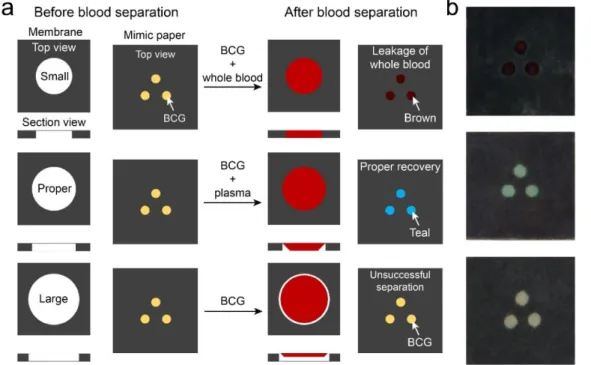
E-μPAD. Membrane: top views and section views of blood separation membranes in small, proper, and large sizes. Mimic paper: color changes observed on the mimic paper (added with 3 µL of BCG on each spot) in the E-μPAD when BCG reacted with whole blood (brown) and plasma (teal), indicating leakage of blood and proper separation of blood separations processes on the mimic paper (added with 3 µL of BCG on each spot) in the E-μPAD. The color of BCG keeps the same if the separation is unsuccessful. (b) Photographs of the mimic papers upon various separation conditions. ... 50
Figure 3-7. Calibration curves for detecting cTnI, BNP-32, and D-Dimer in human blood with the
all-in-one E-μPADs (n = 5). ... 52
Figure 3-S1. Original concentrations of three cardiac biomarkers in human blood measured by
standard ELISA kits. ... 58
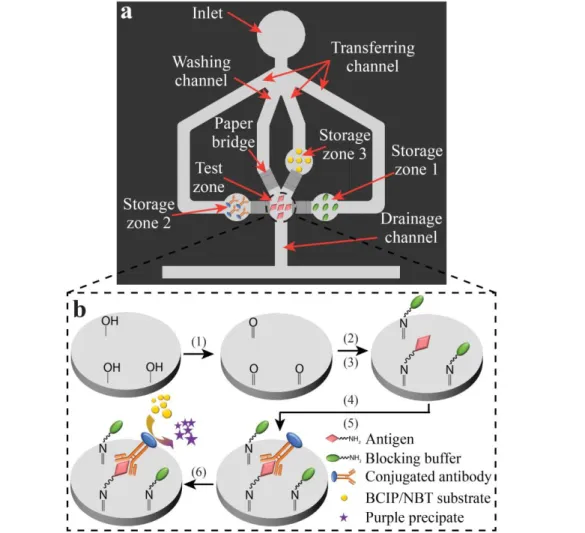
Figure 4-1. Schematic diagrams of (a) top view of a µPAD integrating four SMP-actuated valves
(the valves are attached to the back of the µPAD) and (b) the protocol of a direct ELISA that can be performed in the test zone of the µPAD. The protocol of a direct ELISA is carried out in six steps: (1) modifying the paper test zone surface with aldehyde groups using periodate potassium; (2) immobilizing antigens on the modified paper through covalent bonding; (3) blocking the test zone surface with blocking buffer; (4) labelling antigens with enzyme-conjugated antibodies; (5)
x
washing away un-bound antibodies; and (6) adding enzyme substrate for colorimetric signal production. ... 66
Figure 4-2. Principle and operation of the SMP-actuated valve. (a)(b) The initial state of a valve
is bent up. (c) The SMP is first activated to reach its temporary flat shape (“activation #1”), which bends down the paper arm and makes the paper bridge connect the reagent storage zone and the test zone for reagent transfer. (d) The SMP is then activated again to recover its permanent curved shape (“activation #2”), which disconnects the reagent storage zone and the test zone and stops the transfer process. ... 67
Figure 4-3. Integration of the μPAD with a colorimetric reader. (a) Photograph of the colorimetric
reader that accommodates a µPAD for activation of the valves and readout of the colorimetric signal. (b) Exploded view of the combined architecture of the µPAD and the operation cell of the colorimetric reader. ... 68
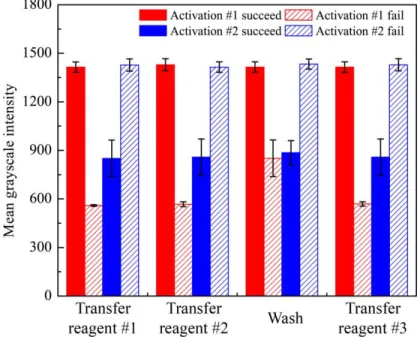
Figure 4-4. Light transmittance signals (n = 5) measured from the test zone at each step of the
direct ELISA (Figure 1b), right after the valve is in activation #1 and one minute after the valve is in activation #2. Reagents #1, #2, and #3 are the blocking buffer, the enzyme-conjugated antibody, and the enzyme substrate. When the valve activation fails to work after each step, the measured light transmittance signals of the test zone are different from the normal state based on the unusual wetting conditions, enabling the detection of the valve malfunction. ... 74
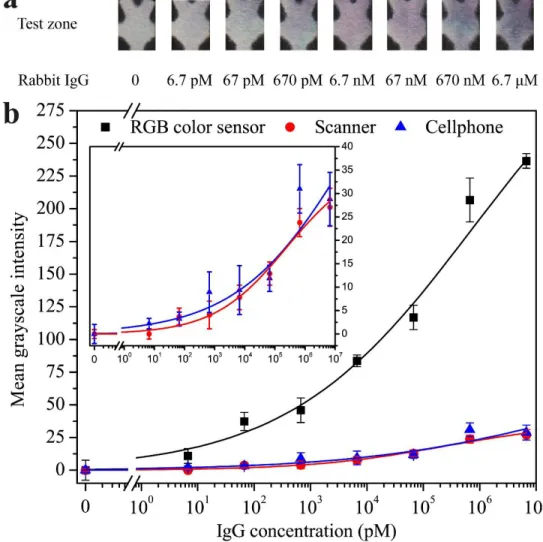
Figure 4-5. Results of direct ELISA on the μPAD for detection of rabbit IgG in PBS. (a)
Photographs of test zones at different IgG concentrations. (b) Calibration curves of the mean grayscale intensity signal versus the IgG concentration on the test zone (n = 5). Signals were measured from the same test zones by the RGB color sensor (R2 = 0.993), a desktop scanner (R2 = 0.970), and a cellphone camera (R2 = 0.894). The RGB color sensor provides higher intensity signals than the scanner and cellphone. ... 76
Figure 4-6. Sandwich P-ELISA for TNF-α in protein extractions from surgically injured rat vocal
fold tissue tested by our platform and the standard ELISA. (a) Schematic of a sandwich ELISA on the µPAD. HRP substrates were catalyzed by HRP into blue products. The catalyzing reaction was stopped with sulfuric acid to produce a yellow color. (b) Calibration curve of the mean grayscale intensity signal measured from our platform versus the TNF-α concentration (n = 5). (c) The comparison of testing data from our platform and the standard ELISA on extraction samples of rat
xi
vocal fold tissues two days and four weeks after surgery. (d) Bland-Altman analysis on the diagnostic methods based on our platform and the standard ELISA. ... 79
Figure 4-S1. Interface of the control pad on a smartphone for the Bluetooth module to the platform.
A user can control the platform by the Bluetooth module to start an assay and receive the test result after the assay is finished. R, G, B, and GS presents the red, green, blue value, and mean grayscale intensity, respectively. ... 86
Figure 4-S2. Surface chemistry modification of the paper test zone for covalent binding of proteins.
(a) Potassium periodate (pH = 5, 0.031 M KIO4) is utilized to oxidize the C2-C3 vicinal hydroxyl
groups into dialdehyde. (b) Amino groups of the residues (i.e. lysine, asparagine, arginine, or alutamine) in proteins can be covalently bound to the aldehyde groups of modified cellulose surface on the test zone via Schiff base linkage. ... 87
Figure 4-S3. Characterization of biofunctionalized test zone using Fourier-transform infrared
spectroscopy (FTIR). (a) FTIR spectra of unmodified cellulose paper. (b) FTIR spectra of KIO4
modified cellulose paper. The absorption peak at 1726cm-1 showed evidence of aldehyde groups due to the stretching vibration of the C=O double bond. ... 88
Figure 4-S4. Optimization of the reagent transfer time and the washing time. (a) Normalized
fluorescence intensity of FITC-conjugated rabbit IgG antibody transferred to the test zone of a µPAD after certain periods of reagent transfer time (n = 5). (b) Normalized fluorescence intensity of residual FITC-conjugated rabbit IgG antibody in the test zone after certain periods of washing time by PBS. All the results are shown in (a) and (b) were normalized to the maximum fluorescence intensity during the experiment including transferring and washing process... 89
Figure 4-S5. The signal-to-noise ratios (STNRs) of different quantification methods including
RGB color sensor (black), scanner (red), and camera (blue) based on direct ELISAs for rabbit IgG in 10-fold dilutions (6.7 pM – 6.7 μM) on the platform (n = 5). The dashed line indicates the average value of each quantification method. ... 90
Figure 4-S6. The mean grayscale intensities of the sandwich ELISA results for TNF-α in PBS by
using two slightly different protocols: (i) adding secondary antibody and HRP streptavidin sequentially to the test zone, and (ii) pre-mixing of secondary antibody and HRP streptavidin and then added the mixture to the test zone (our final protocol adopted). No significant difference (student t-test, p = 0.132; n = 7) was observed between the results based on the two protocols.. 91
xii
Figure 5-1. (a) Photograph of loading a µPAD into the SIAO portable platform and functional
modules of the platform. (b) Diagram of a µPAD for multi-step assays. (c) An SMP sheet is attached to the pre-cut hinge of a paper arm and a hydrophilic tissue-paper bridge is attached to the paper arm tip. Underneath the SMP, a patterned copper resistor can activate the SMP by heating. An SMP sheet is capable to lift a paper arm to connect or disconnect a paper channel with a paper bridge by localized heating of a copper heater. ... 99
Figure 5-2. (a) Top view of the μPAD pre-loaded with reagents for performing a sandwich ELISA.
(b) Schematic of the sandwich ELISA for detecting analytes. (c) Enlarged view of the region shaped with dashed lines for showing automated steps on the platform to perform the sandwich ELISA on the μPAD. ... 102
Figure 5-3. Human laryngeal secretion collection process. (a) A dual-channel
video-laryngostroboscopic endoscope was inserted near to a human vocal fold tissue. (b) A tiny extraction pipe was loaded into a channel of the laryngoscope. (c) A vacuum pump was connected to the other end of the pipe. (d) The pump was turned on and secretions were transferred to a suction source... 104
Figure 5-4. (a) Bland-Altman analysis for detecting pepsin in the same human patient samples
between SIAO platform and conventional ELISA kit. (b) Detection performance for human TNF-α (200 pg/mL) and (c) IL-1β (600 pg/mL) on the SIAO platform. ... 105
Figure 5-5. (a) Mean and standard errors of VHI and RSI scores in asymptomatic controls (n = 9)
and clinically suspected LPR group (n = 9), *p < 0.05. (b) Mean and standard errors of protein levels for LPR biomarkers in asymptomatic controls (n = 9) and clinically suspected LPR (n = 9) group. ng/ml for pepsin; pg/ml for IL-1β and TNF- α (*p < 0.05). ... 107
Figure 5-S1. Calibration curves of human pepsin, IL-1β, and TNF-α detected on the μPADs in the
portable platform. ... 115
Figure 6-1. Schematic of five surface chemistries for chemically modifying cellulose paper and
immobilizing biomolecules. Potassium periodate (KIO4), carbonyldiimidazole (CDI), divinyl
sulfone (DVS), phenylene diisothiocyanate (PDITC), and chitosan/glutaraldehyde (GA) were utilized to modify cellulose paper and generate functional binding sites to covalently bind with amino groups on biomolecules (e.g. antigen/antibody and aminoed nucleic acid). ... 122
Figure 6-2. SEM micrographs at 500× magnification of cellulose paper with scale bar = 25 μm:
xiii
paper; (d) DVS modified cellulose paper; (e) PDITC modified cellulose paper; (f) chitosan/GA modified cellulose paper. ... 125
Figure 6-3. Infrared spectra of unmodified cellulose paper and modified cellulose paper with KIO4.
The characteristic absorption peak of the aldehyde group at 1736 cm-1 was observed on the infrared spectra. ... 126
Figure 6-4. FITC anti-rabbit IgG (50 μg/mL) was used to investigate the immobilization
performance of unmodified cellulose paper and five surface modified cellulose paper (N = 7). Randomly selected photographs of paper spots on the multi-well paper plates are shown below the corresponding bars. Error bars represent standard deviations. ... 129
Figure 6-5. Colorimetric paper-based ELISA results on the unmodified cellulose paper and five
surface modified papers: (a) Schematic of colorimetric ELISA for detecting rabbit IgG. (b) Mean grayscale intensity values of control zone signals (background noise) and valid assay signals (test zone signal minus background noise) (n = 7). Randomly selected photographs of paper spots on the multi-well paper plates are shown below the corresponding bars. Error bars represent standard deviations. ... 130
Figure 6-6. Colorimetric paper-based ELISA results on the modified papers after 30 days of
storage (n = 7). Error bars represent standard deviations. ... 132
Figure 7-1. Schematic illustration of the µPAD and fluorescent sensing mechanism. (a) Top view
of a µPAD design integrating three SMP-actuated valves (the valves are attached to the back of the µPAD). The region indicated with dashed lines is a pre-cut paper cantilever beam. (b) Side view and photograph of a µPAD. (c) Fluorescent sensing mechanism for target ssDNA. ... 145
Figure 7-2. (a) Architecture of the autonomously-portable platform. Photographs of the platform
from top view when it is (b) closed and (c) open. ... 148
Figure 7-3. Fluorescence emission spectra of (a) SP1 (10 nM) in 2, 1, 0.5, 0.2, and 0.1 mg/mL
MoS2 nanosheet solution in presence of ST1 (10 nM) and (b) LP1 (10 nM) in 2, 1, and 0.5 mg/mL
MoS2 nanosheet solution in presence of LT1 (10 nM). Kinetic study for the fluorescence changes
of (c) SP1 (10 nM) and duplex of the SP1 and ST1 (10 nM), and (d) LP1 (10 nM) and duplex of the LP1 and LT1 (10 nM) in the presence of 1 mg/mL MoS2. Excitation and emission wavelengths
are 475 and 520 nm, respectively. ... 150
Figure 7-4. (a) Representative fluorescence spectra of SP1 (10 nM) in 1 mg/mL of MoS2 nanosheet
xiv
(b) Calibration curve for the short ssDNA detection. Inset: the calibration curve at the concentration range from 0 to 10 nM (n = 5). (c) & (d) Representative fluorescence spectra and calibration curve for the long ss DNA detection (n = 5). Excitation and emission wavelengths are 475 and 520 nm, respectively. ... 152
Figure 7-5. Calibration curves for the detection of (a) ST1 and (b) LT1 on the platform. The
mixture of P1 (10 nM) and 1 mg/mL MoS2 nanosheet solution was added onto the test zone of
μPAD (n = 5). ... 154
Figure 7-S1. Fabrication process of few-layered MoS2 nanosheets. ... 161
Figure 7-S2. Automated transferring processes on the µPADs for detection of (a) short ssDNA
detection; step 1: direct transferring of analytes from storage zone to test zone for quantification (Sequence of SMP valving: 1 close > 1 open > 2 close > 3 close > 3 open > 2 open). (b) long ssDNA detection; step 1: transferring of analytes from storage zone to amplification zone (Sequence of SMP valving: 1 close > 1 open); step 2: isothermal DNA amplification; step 3: transferring of amplified analytes to test zone for quantification (Sequence of SMP valving: 2 close > 3 close > 3 open > 2 open). ... 162
Figure 7-S3. The comparison experiment of platform architecture and quantification type. .... 163 Figure 7-S4. Temperature control performance for the isothermal amplification module in the
platform. The sampling frequency in the closed-looped system was set to be 1 s. ... 163
Figure 7-S5. (a) AFM images of synthesized MoS2 nanosheets (1 mg/mL MoS2 solution coated
on a piece of 20 mm × 20 mm silicon waver). (b) UV-Vis absorption spectra of the 1 mg/mL MoS2
nanosheet solution. (c) XPS spectra of Mo 3d and S 2p for the MoS2 nanosheets. ... 164
Figure 7-S6. Fluorescence emission spectra of SP1 (10 nM) in MoS2 nanosheet solution (1 mg/mL)
in presence of ST1 (10 nM) after 10 min under excitation wavelength of 470, 475, and 480 nm, respectively. Emission wavelength was set at 520 nm. ... 165
Figure 7-S7. (a) UV-Vis absorbance spectra of target DNA before and after LAMP process. In
solution: the WarmStart LAMP Kit DNA (NEB #E1700S) was used to amplify DNA in solution instructed by the manual. On µPAD: the amplification zone for amplifying DNA on µPAD was performed an LMAP and then removed from the µPAD and resolved in the Tris-HCl buffer to vibrate for 10 minutes before the spectra collection. Original concentration of template was 0.05 µg/mL. (b) The sample was detected after a LAMP process on the µPAD with a comparable response with a 104 fold concentrated spiked sample. ... 166
xv
Figure 7-S8. Fluorescence emission spectra responses of 1 mg/mL of MoS2 nanosheet solution in
the presence of (a) SP1/ST1 (10 nM/10 nM) duplex, SP1 (10 nM) and SC1 (10 nM) and SP1 (10 nM), and (b) LP1/LT1 (10 nM/10 nM) duplex, LP1 (10 nM) and LC1 (10 nM) and LP1 (10 nM), respectively. Excitation and emission wavelengths are 475 and 520 nm, respectively ... 167
xvi
List of Tables
Table 3-1. Separation performance of the various sizes of blood membrane for a separation of 20
µL of human blood on the EμPADs (n = 21). ... 51
Table 4-S1. Comparison of valving performance of three SMPs when SMP-actuated valves (n =
15) were on the platform. Time of activation #1 was the heating time of an SMP to its temporary flat shape. Time of activation #2 was the heating time of the SMP returning to its permanent curved shape. A successful valve activation was counted only with success in both activation #1 and activation #2. ... 92
Table 5-1. Comparisons of three diagnostic tools for LPR detection. *Estimated, **Based on the
Instructions for use from Peptest official website. ... 106
Table 6-1. Characterized relative compositions of unmodified and modified cellulose papers by
XPS. ... 127
Table 6-2. Comparison of the five performed modification methods. *A glucose unit in the
xvii
Abstract
Since its original development in 2007, the microfluidic paper-based analytical device (μPAD) technology has started to gain worldwide acceptance. The μPADs feature low cost, favorable biocompatibility, ease of operation, and superior portability. These advantages indicate the great potential of μPADs as miniaturized biosensors with different detection mechanisms, including electrochemistry, colorimetry, fluorometry, and chemiluminescence. Various assays such as immunoassays and hybridization-based nucleic acid detection have been demonstrated on μPADs. The measurements are easy to interpret in semi- or quantitative results with certain portable instruments. The μPADs reveal promising possibilities as point-of-care (POC) tools for personal healthcare monitoring and early-state disease diagnosis, especially in resource-limited settings.
In the past decade, the development of μPADs has been focused on performing more powerful analyte detections with ultrasensitive results by introducing innovative sensing mechanisms and smart device designs. In my doctoral research, I first developed an all-in-one electrochemical μPAD (E-μPAD) for multiplexed testing of cardiac biomarkers in real human blood. Then, I designed a novel paper-based microfluidic valve actuated by thermally-responsive shape memory polymers (SMPs), which led to the development of a fully-automated diagnostic μPAD. The platform was engineered into a handheld format, and is customizable to carry out any multi-step assays on a μPAD in an automated manner for POC diagnosis.
In-situ growth of zinc oxide nanowires (ZnO NWs) on the all-in-one E-μPAD was employed
to increase the surface-area-to-volume ratio and electron transfer property of the E-μPAD. Compared to conventional carbon working electrodes (WEs) on μPADs, electrochemical signals were enhanced of 550% by introducing ZnO NWs on the WE in cyclic voltammetry measurements. By using electrochemical impedance spectroscopy (EIS) sensing mechanism, I first completed the calibration of three cardiac biomarkers, including troponin I, brain-natriuretic-peptide (BNP)-32, and D-dimer, in spiked artificial human plasma on the all-in-one E-μPAD. The limits of detection (LODs) were confirmed to be 190 fM, 40 fM, and 730 fM, respectively. Then, I demonstrated the detection of the three cardiac biomarkers in spiked real human blood. The all-in-one μPAD is highly integrated into a compact size and easy to be operated in an origami fashion. It offers
xviii
ultrasensitive and rapid diagnosis of bloodborne protein markers, promising its significant application to POC testing.
Although the all-in-one μPAD shows the feasibility in clinical uses, its operation requires a certain level of professional skills and manual involvements (e.g., use of a pipette and a potentiostat), which may restrict the scope of its on-site applications, especially for use by non-professional operators. In this regard, I spent efforts on developing an automated μPAD platform to realize sample-in-answer-out (SIAO) testing. In the platform, I utilized thermally-responsive SMPs as localized actuators to lift pre-cut paper valves to connect and disconnect paper channels on a single-layer μPAD. By turning on and off copper heaters to activate the SMPs, reagent transferring and washing processes for multi-step assays were completed on the μPAD in a fully-automated way. The minimum activation times to open and close the paper valves were measured to be 22.7 ± 3.7 s and 24.4 ± 5.3 s, respectively.
I carried out direct ELISA calibration of rabbit IgG in phosphate buffered saline (PBS) with the SIAO platform and achieved a low LOD of 27 pM (comparable to standard paper-based ELISAs under manual operations). I also detected rat tumor-necrosis-factor (TNF)-α in protein extractions from rat laryngeal tissues (i.e., vocal folds) by sandwich ELISA with the platform and the LOD was determined to be 22 pM. Furthermore, I tested pepsin in human laryngeal surface secretions on the platform with a LOD of 5.8 pM. The measurements for rat and human samples were both benchmarked by standard ELISAs. By Bland-Altman analysis, our platform revealed 94.4% agreement (within 95% confidence interval around the mean) to the standard ELISAs. The platform has also been extended to DNA detection. By coating few-layered molybdenum disulfide (MoS2) nanosheets (under five layers) onto a μPAD as quenching substrate, the fluorophore-tagged
DNA probes (pre-attached to MoS2 nanosheets) were used to detect the presence of DNA target
(complementary to the DNA probe in nucleotide sequences) by quantifying the fluorescence signal change. The DNA hybridization was fulfilled automatically on the platform and achieved a LOD of 610 pM.
In the development of the SIAO platform, I also carried out an experimental study of comparing five popular surface biofunctionalization methods to enhance adsorption of biomolecules (e.g., proteins and nucleic acid probes) on μPADs. Cellulose paper surface can be
xix
modified with active sites for covalent immobilizations to reduce or eliminate non-specific bindings and improve signal outputs. I experimentally investigated modification performance of the methods by carrying out standard paper-based direct ELISAs. The method with potassium periodate (KIO4) was observed with 59% decreased background noise and 53% increased
colorimetric signal outputs, and then chose to chemically modify μPADs in the automated platform works.
My doctoral research made original contributions to the field not only on new μPAD designs for electrochemical sensing and fully-automated device operation, but also on the demonstration of practical uses of the developed devices for testing rat and human samples. This work provides promising and reliable POC tools for use by unskilled professionals and end users to monitor patient health status and conduct early-state disease diagnosis.
xx
Résumé
Depuis son développement initial dans la 2007, la technologie du dispositif analytique à base de papier microfluidique (μPAD) a commencé à être acceptée dans le monde entier. Les μPADs sont les caractéristiques d'un faible coût de fabrication, une bonne biocompatibilité, une bonne portabilité, etc. Ces avantages indiquent que les μPADs a un grand potentiel en tant que biocapteur miniature et sensible avec différents mécanismes de détection, (y compris l'électrochimie, la colorimétrie, la fluorométrie et la chimiluminescence). Divers tests, tels que l'immunodosage et l'hybridation d'ADN, ont été montrés sur μPADs. Les mesures sont faciles à interpréter dans la moitié de résultats ou les résultats quantitatifs avec certains instruments portatifs. Les μPADs révèlent de bonnes possibilités en tant qu'outils de point de soins (POC) pour les utilisateurs finaux sur la surveillance des soins de santé personnels et le diagnostic des maladies à l'état précoce, en particulier dans les environnements à ressources limitées.
Au cours de la dernière décennie, les μPADs se sont concentrés sur l'exécution de tests plus complexes avec des résultats ultra-sensibles en introduisant des mécanismes de détection innovants et des conceptions de dispositifs intelligents. Dans ma recherche de doctorat, j'ai d'abord développé un tout-en-un μPAD pour tester les biomarqueurs cardiaques dans le sang humain réel. Ensuite, j'ai conçu un nouveau type de valve microfluidique à base de papier actilée par la réponse thermique de la forme polymère de mémoire (SMPs), pour réaliser le diagnostic automatique de μPADs. La plate-forme est conçue comme un format de poche qui peut être personnalisé et peut être automatisé toute détection multi-étape sur μPAD pour réaliser les diagnostics POC.
La croissance in situ des nanofils d'oxyde de zinc (ZnO NWs) sur le μPAD tout-en-un est employée pour améliorer le rapport entre la surface micro-superficielle et le volume et la performance du transfert d'électrons de μPAD. Comparé aux électrodes conventionnelles de travail du carbone (WEs) sur μpads, les signaux électrochimiques ont été amplifiés par 5,5 fois en introduisant des ZnO NWs sur WE de voltampmétrie cyclique. En utilisant le mécanisme de détection de la spectroscopie d'impédance électrochimique (EIS), j'ai d'abord effectué l trois biomarqueurs cardiaques dans le plasma sanguin artificiel dopé sur le tout-en-un μPADs, y compris la troponine I, les peptides cérébraux naturels (BNP)-32 et d-deux agrégats. La limite des détections (LODs) a été confirmée à 190 fM, 40fM et 730 fM, respectivement. Puis j'ai démontré
xxi
les détections des trois biomarqueurs cardiaques dans le sang humain réel dopé. Le tout-en-un μPAD est fortement intégré dans une taille compacte et facile à être utilisé dans une mode origami. Il offre un diagnostic ultrasensible et rapide des marqueurs protéiques transmissibles par le sang, montrant son application significative aux tests de POC. Bien que le tout-en-un μPAD étende sa faisabilité dans les cliniques, ses opérations exigent un certain niveau de compétence professionnelles les implications manuelles (par exemple l’utilisation d’une pipette et d’un potentiostat), ce qui peut restreindre la possibilité de sea applications sur le site, en particulier pour les utilisateurs finaux non professionnels. J'ai ensuite passé des efforts pour développer une plate-forme automatisée sur les μPADs pour implémenter les tests de rappel de l'échantillon (SIAO). Dans la plate-forme, j'ai utilisé des SMPs thermosensibles comme actionneurs localisés pour soulever des vannes de papier pré-coupées pour connecter et déconnecter les canaux de papier Isconnect sur le μPAD. En contrôlant l'ouverture et la fermeture des radiateurs en cuivre pour activer les SMPs, les processus de transfert et de lavage des réactifs pour les essais en plusieurs étapes ont été complétés sur le μPAD de façon entièrement automatisée. Les durées minimales d'activation pour ouvrir et fermer les vannes de papier ont été mesurées à 22,7 ± 3,7 s et 24,4 ± 5,3 s respectivement.
J'ai effectué l'étalonnage ELISA direct de l'IgG de lapin dans le tampon de phosphate de saumure (PBS) avec la plate-forme SIAO et atteindu une faible LOD du pM (comparé aux ELISAs standard à base de papier dans le cadre d'opérations manuelles). J'ai aussi détecté la tumeur de rat-nécrose-facteur (TNF)-α des extractions de protéine du tissu laryngé de rat (c’est-à-dire, plis vocaux) par Sandwich ELISA avec la plate-forme et le LOD qui est déterminé à 22 pM. En outre, j'ai testé la pepsine dans les sécrétions de surface laryngée sur la plate-forme avec un LOD de 5,8 pM. Les mesures pour les rats et les échantillons ont été évaluées à la fois par des ELISAs standard. Par l'analyse de Bland-Altman, nous avons révélé 94,4% d'accord (dans un intervalle de confiance de 95% autour de la moyenne) à la St. Andard ELISAs. La plate-forme a aussi été étendue à la détection d'ADN. En enduisant du nanomètre de disulfure de molybdène monocouche (MoS2) (sous cinq couches) sur le μPAD comme substrat de trempe, les sondes d'ADN marquées par le fluorophore (pré-attachées aux nanomètres de MoS2) ont été utilisées pour détecter la présence de la cible d'ADN (complémentaire à la sonde d'ADN dans les séquences de nucléotides) en
xxii
quantifiant le changement de signal de fluorescence. L'hybridation de l'ADN a été réalisée automatiquement sur la plate-forme et a atteint un LOD de 610 pM.
Dans le développement de la plate-forme SIAO, j'ai aussi réalisé une étude expérimentale de la comparaison de cinq méthodes populaires de biofonctionnalisation de surface pour améliorer l'adsorption des biomolécules (par exemple, les protéines et les sondes d'acide nucléique) sur les μPADs. La surface du papier cellulosique peut être modifiée avec des sites actifs pour les immobilisations covalentes afin de réduire ou d'éliminer les liaisons non spécifiques et d'améliorer les sorties du signal. J'ai étudié expérimentalement les performances de modification des méthodes en effectuant des ELISAs directes à base de papier standard. La méthode avec le periodate de potassium (KIO4) a été observée avec 59% de bruit de fond diminué et 53% d'augmentation des
sorties de signal colorimétrique, puis a choisi de modifier chimiquement les μPADs dans les travaux de la plate-forme automatisée.
Ma recherche de doctorat a fait des contributions originales sur le terrain à de nouvelles conceptions de μPAD pour la détection électrochimique et le fonctionnement entièrement automatisé de l'appareil, mais aussi sur la démonstration des utilisations pratiques des dispositifs développés pour tester des échantillons de rats et humains. Ce travail fournit aux utilisateurs finaux des outils de PDP prometteurs et fiables pour surveiller leur état de santé et diagnostiquer les maladies de l'état précoce.
xxiii
Acknowledgment
First and foremost, I would like to sincerely thank my supervisor, Prof. Xinyu Liu, for providing me the opportunity to complete my Ph.D. degree. What I have learned from his diligent and enthusiastic attitude for research has become precious fortune to me and will surely instruct me for my future career and work. I want to express my sincere appreciation to Prof. Damiano Pasini and Prof. Maryam Tabrizian for not only serving as my Ph.D. supervisory committee but their guidance and suggestions. I also really want to thank my co-supervisor Prof. Yaoyao Fiona Zhao for her patient help and support on my doctoral research progress.
This research could not be accomplished without my supportive collaborators. Especially, I am extremely grateful for Prof. Nicole Y. K. Li-Jessen as my collaborator. She shares with me her valuable insights and expertise in my research. Besides, her generosity and kindness always help me out in my academic or normal life. I want to thank Dr. Lihong Shang and Mr. George Kretschmann for their assistance on microscopical and chemical characterizations.
I appreciate the assistance from all my colleagues in Liu’s lab and other friends who are always helpful: Dr. Pengfei Song, Sina Kheiri, Peng Pan, Dr. Peng Ran, Dr. Weize Zhang, Zhen Qin, Dr. Qigao Fan, Yu-Hsuan Wang, Tyler Clancy, Dr. Longyan Chen, Dr. Xiao Li, Xianke Dong, Juntian Qu, Qiyang Wu, Binbin Ying, Yueyue Pan, Dr. Huijie Wang, and Zhen Yin.
I would like to express my great appreciation for the financial support from the Natural Sciences and Engineering Research Council of Canada, and the Faculty of Engineering at McGill University.
Finally, I owe my deepest gratitude to my parents Qingjun Fu and Jinling Niu for their endless love. Their financial and more importantly emotional support allows me to pursue whatever I want and stretch the depth and dimension of my life. Also, I feel extremely lucky that I have my brother Xiaoyong Liu and sister Yanting Jin who stand by me all the time through the hard times. I would never grow up to be a mature person like now without their guidance and support. With them, I will definitely know there is always a reserved door open for me during my journey to face fear.
xxiv
Contributions of the Author
This is a manuscript-based thesis consisting of six journal articles. The title of the articles, name of the authors, and their contributions are listed below:
1) State-of-the-art advances and challenges of incorporating functionality into microfluidic paper-based analytical devices
Hao Fu1,2, Pengfei Song1,2, and Xinyu Liu1,2
1Department of Mechanical Engineering, McGill University, Montreal, Quebec H3A 0C3, Canada 2Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, Ontario
M5S 3G8, Canada
to be submitted.
Author contributions:
H. Fu: Organized the literature, planned the review architecture, and wrote the manuscript. P. Song: Co-organized the literature and helped to edit the text.
X. Liu: Supervised and planned the review architecture, and wrote the manuscript.
2) An all-in-one, paper-based origami biosensor for rapid diagnosis of cardiac diseases
Hao Fu1,2, Xiao Li1, and Xinyu Liu1,2
1Department of Mechanical Engineering, McGill University, Montreal, Quebec H3A 0C3, Canada 2Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, Ontario
M5S 3G8, Canada
submitted to Biosensors and Bioelectronics.
Author contributions:
H. Fu: Designed and performed the experiments, analyzed the data, prepared the presentation
items and wrote the manuscript.
xxv
X. Liu: Proposed the idea, designed the experiments, analyzed the data, and wrote the manuscript. 3) A paper-based microfluidic platform with shape-memory-polymer-actuated fluid valves for automated multi-step immunoassays
Hao Fu1,2, Pengfei Song1,2, Qiyang Wu1,2, Chen Zhao1, Peng Pan1,2, Xiao Li2, Nicole Y. K. Li-Jessen3, and Xinyu Liu1,2
1Department of Industrial and Mechanical Engineering, University of Toronto, Toronto, ON M5S
3G8, Canada
2Department of Mechanical Engineering, McGill University, Montreal, QC H3A 0C3, Canada 3School of Communication Sciences and Disorders, McGill University, Montreal, QC H3A 1G1,
Canada
accepted by Microsystems and nanoengineering.
Author contributions:
H. Fu: Designed and performed the experiments, analyzed the data, prepared the presentation
items, and wrote the manuscript.
P. Song: Carried out the protocol optimization experiments and collected the data. Q. Wu: Wrote the codes for the platform automation.
C. Zhao: Wrote the codes for the Bluetooth communication. P. Pan: Fabricated the colorimetric reader system.
X. Li: Prepared the paper devices.
N. Li-Jessen: Provided the animal specimens and data.
X. Liu: Proposed the idea, designed the experiments, analyzed the data, and wrote the manuscript.
4) A point-of-care paper-based microfluidic platform for rapid diagnosis of human patients with clinically suspected laryngopharyngeal reflux
Hao Fu1,2, Pengfei Song1,2, Peng Pan1,2, Neil Lui1, Karen Kost3, Nicole Y. K. Li-Jessen4, and Xinyu Liu1,2
xxvi
2Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, Ontario
M5S 3G8, Canada
3Department of Otolaryngology, McGill University, Montreal, QC H3A 1A1, Canada
4School of Communication Sciences and Disorders, McGill University, Montreal, QC H3A 1G1,
Canada
submitted to Clinical Chemistry.
Author contributions:
H. Fu: Designed and performed the experiments, analyzed the data, prepared the presentation
items, and wrote the manuscript.
P. Song: Performed the experiments.
P. Pan: Assisted in human sample collection and data analysis. N. Lui: Prepared the paper devices.
K. Kost: Collected the human patient samples.
N. Li-Jessen: Evaluated the patients upon reflux symptom index and wrote the manuscript.
X. Liu: Proposed the idea, designed the experiments, analyzed the data, and wrote the manuscript.
5) Comparing surface chemistries for biomolecule immobilization on paper-based microfluidic devices
Hao Fu1,2 and Xinyu Liu1,2
1Department of Mechanical Engineering, McGill University, 817 Sherbrooke Street West,
Montreal, Quebec H3A 0C3, Canada
2Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, Ontario
M5S 3G8, Canada
to be submitted.
Author contributions:
H. Fu: Designed and performed the experiments, analyzed the data, prepared the presentation
items, and wrote the manuscript.
xxvii
6) A fully-automated paper-based biosensor integrated with few-layered MoS2 nanosheets
for DNA detection
Hao Fu1,2, Peng Pan1,2, Neil Lui1, and Xinyu Liu1,2
1Department of Mechanical Engineering, McGill University, 817 Sherbrooke Street West,
Montreal, Quebec H3A 0C3, Canada
2Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, Ontario
M5S 3G8, Canada
to be submitted.
Author contributions:
H. Fu: Designed and performed the experiments, analyzed the data, prepared the presentation
items, and wrote the manuscript.
P. Pan: Performed the experiments. N. Lui: Prepared the paper devices.
1
Chapter 1
2
Chapter 1: Introduction
Microfluidic paper-based analytical devices (μPADs) have emerged as a new platform technology, targeting to improve accessibilities to medical diagnostics [1]. Regular filter paper and chromatography paper are major raw materials as substrates for μPADs [2]. Their abundance, mass producibility, and flexibility enable widespread use of μPADs [3]. Benefitting from the porous structures in paper-based substrates, their functional capabilities exempt external force-driven pumps during assays, miniaturizing the experimental setups of μPADs. Thus, the μPAD has become one of the most promising diagnostic tools to achieve low-cost and easy-to-interpret tests in a point-of-care (POC) way. It contributes to improvements in simplifying personal healthcare monitoring and disease diagnosis processes, especially in developing countries or at resource-limited sites.
In the most recent decade, the research on μPADs has exhibited profound achievements from device fabrication to quantitative analysis, with the ultimate aim of practical applications [4]. Particularly with currently-summative advancements, μPADs have been demonstrated as reliable POC platforms across a range of areas, including personal healthcare monitoring [5-8], and disease diagnoses [9-11]. Taking advantaging of the abundance of paper materials and convenience of wax-printing or inkjet-dispensing techniques for channel pattering, key techniques for mass production of μPADs have already been met [3].
The ongoing efforts on μPADs are dedicated to seeking to investigate potential incorporating functionality, including i) signal detection and readout, ii) programming and timing, iii) multi-step processing, and iv) surface chemistry from laboratory-scale research to commercialization [3, 12]. Integrating functionality of detection and readout into μPADs is mainly involved for improvements on test sensitivity and sensing mechanism innovations [12]. Although many preliminary analytical technologies of μPADs have been demonstrated, more accurate and sensitive detection capabilities can enable effective and meaningful healthcare monitoring and disease diagnoses (i.e., confirming the presence of targets under the clinically-acknowledged limit of detections, LODs) [3]. Nevertheless, professional involvements and trained skills of pipetting and instrument-related interoperations during performing assays are still required to obtain results [13-15]. These limit accessibilities of μPADs to end-users as commercial products. Further attempts are then conducted to realize automation (i.e., programming and timing) on μPADs [16, 17]. Automated control will
3
significantly extend feasibilities of a μPAD from laboratory-based demonstrations to practical applications such as on-site testing. An automated platform for μPADs is a prerequisite to fulfill multi-step processing. Realizing multi-step assays on μPADs is essential to increasing its functionality on complex measurements (e.g., immunoassays and DNA analysis) [12]. Besides, various surface chemistries of paper provide effective techniques for reducing background noise and enhancing signal outputs. To incorporate above-mentioned functionalities into μPADs, new developments are needed to convert μPADs into reliable alternatives to traditionally-standard analytical tools.
In my research, I explored new designs and strategies to expand currently incorporated functionalities of μPADs for a diverse set of practical applications, especially for clinical uses. I first developed an all-in-one electrochemical μPAD (E-μPAD) to improve the device functionality on signal detection and readout and applied it to quantifying cardiac biomarkers in real human blood. Zinc oxide nanowires (ZnO NWs) were introduced to the working electrode (WE) of the E-μPAD for enhancing the surface-to-volume ratio and electron-transferring properties of the device WE, which eventually amplified electrochemical signal outputs on μPADs. The ZnO NWs were grown on carbon-printed paper WEs by an in-situ hydrothermal synthesis process. By implementing a label-free electrochemical sensing mechanism (namely electrochemical impedance spectroscopy or EIS), the ZnO-NW-decorated μPAD was employed in protein biosensing with equivalent analytical performance to commercial enzyme-linked immunosorbent assay (ELISA) kits. The all-in-one E-μPAD enabled portable, utility, and user-friendly detection in a highly integrated device format. This device can be operated through paper origami without complex manipulations. To incorporate programming and timing functionalities and implement multi-step immunoassays on μPADs, I established an automated platform to turn on and off thermally-responsive shape-memory-polymer (SMP) actuators to turn on and off paper fluidic valves and thus connect/disconnect paper channels on a μPAD. The developed platform is capable of realizing sample-in-answer-out (SIAO) diagnostic assays in a fully-automated fashion, and of conducting multi-step assays such as ELISAs. I demonstrated its practical feasibility of on-site testing for rat and human samples by running automated ELISAs. The platform can be further extended to multi-step DNA sensing and enable another important category of diagnostic assay. I also carried out a systematic investigation of comparing five single-step surface chemistry routes
4
for covalent immobilization of proteins (antigens and antibodies) on a μPAD made from cellulose paper. The chemically-modified μPAD provided more active binding sites on its paper surface for covalent binding of proteins. The modification methods were compared and the potassium periodate (KIO4)-based biofunctionalization route was found to be the most efficient, and thus was
utilized for biofunctionalization of the automated ELISA platform.
1.1 Thesis objectives
The overall research goal of this thesis is to integrate novel functionalities (e.g., high-performance biosensing mechanism, all-in-one design for device operation, and automatic fluid manipulation) into μPADs for a variety of practical applications. The invention of new μPAD functionalities in my doctoral work is required to facilitate current sensing mechanisms by advancing detection sensitivity and achieving device portability. Besides, the developed μPADs can be operated by any individual to accomplish automated multi-step assays with an SIAO manner. The detailed targets of this thesis include:
To develop an origami all-in-one E-μPAD with a ZnO-NW-coated WE for highly-sensitive electrochemical sensing of cardiac biomarkers in human blood.
To establish an autonomous SIAO platform that manipulates localized SMP actuators as paper-based valves for running automated multi-step assays on μPADs.
To demonstrate the feasibility of the autonomous SIAO platform in clinical use by testing rat and human vocal-fold secretion samples with it.
To systematically investigate and compare five biofunctionalization methods on μPADs for improvement of protein immobilization.
To extend the autonomous SIAO platform to automated DNA analysis by coating few-layer MoS2 nanosheets on the μPAD as superior fluorescence quenching substrate.
1.2 Thesis organization
This thesis is manuscript-based and consists of eight chapters. The current chapter as chapter 1 is an introduction that briefly summarizes the basic concepts and present status of μPADs, followed by the objectives of this thesis.
5
Chapter 2 presents a comprehensive review in the field of μPADs. Starting from depicting the recent developments of μPAD technology, I seek to highlight its most impactful and promising applications for real uses, especially in personal healthcare monitoring and disease diagnosis. To incorporate functionality into μPADs is the main flow of this review to be discussed. Both advantages and limits of μPADs for practical advancements are analyzed. In the end, the future outlook, emerging challenges, and remaining opportunities of μPADs are proposed. It should be mentioned that four primary directions of incorporating functionality of μPADs: i) detection and readout, ii) programming and timing, iii) multi-step processing, and iv) surface chemistry, are to be further researched and developed. These four topics set the detailed goals for this thesis. Chapter 3 is involved with a highly-integrated all-in-one E-μPAD for electrochemical sensing in human blood, broadening the functionality of μPADs on detection and readout. Chapters 4, 5, and 6 focus on the development of an autonomous platform by controlling SMP actuators to realize automated multi-steps assays on μPADs (i.e., functionalities of programming and timing, and multi-step processing). Chapter 7 presents a fundamental study on surface chemistries for μPADs by comparing five facile modification methods and investigating their biofunctionalization performance for biomolecules.
In Chapter 3, I introduce an all-in-one E-μPAD that is highly integrated with all essential components for testing human blood. The developed all-in-one E-μPAD features ease of operation, portability, and ultrasensitive protein detection, making early-state diagnosis of cardiac diseases possible. I fabricated in-situ ZnO NWs on working electrodes in the E-μPAD by a hydrothermal method in order to amplify electrochemical signals. The ZnO NWs allowed superior electron-transferring capability of the WE and importantly increased its surface-to-volume ratio. Compared to conventional carbon-printed working electrodes on μPADs, the ZnO-NW-coated WE revealed a 550% improvement of its electrochemical sensing performance in cyclic voltammetry measurements. Implementing a label-free detection mechanism based on electrochemical impedance spectroscopy (EIS), I performed calibration experiments for three cardiac biomarkers, including troponin I, brain-natriuretic-peptide (BNP)-32, and D-dimer, in spiked artificial human blood plasma. The limits of detection (LODs) were determined to be 190 fM, 40 fM, and 730 fM, respectively. Finally, I demonstrated the feasibilities of the all-in-one E-μPADs for detecting the cardiac biomarkers in human blood.
6
Chapter 4 introduces an autonomous platform for achieving automated multi-step assays on μPADs. Thermally-responsive SMPs were employed as actuators to control paper cantilever beams to connect and disconnect channels on μPADs. The SMPs provide functionality of programming and timing on μPADs to achieve an accurate control on paper channel connection (22.7 ± 3.7 s) and disconnection (24.4 ± 5.3 s). When running an assay on the platform, any end-user just needs to add sample to a test zone and reagent-transferring buffer to an inlet of a μPAD, close the door, wait for a period, and the tested results will be displayed on the screen of the platform at the end of the assay. In order to eliminate the invalid tests caused by malfunction operations of any individual SMP, I compared various SMP products and chose the one with the highest success rate (> 97%) for later use. Moreover, I integrated a self-checking mechanism for valve malfunction by detecting the wettability of μPADs to identify any mal-operation of SMP actuators. The mechanism can remind a user to replace another μPAD if a malfunction occurs and render the platform reliable in real assays. The platform can realize a true SIAO diagnostic detection on μPADs. I calibrated the detection of rabbit IgG in PBS on the platform. The assay was optimized as direct ELISAs on μPADs and showed a low limit of detection (LOD) of 27 pM (comparable to that of standard and paper-based ELISAs). I also conducted sandwich ELISAs on this platform to calibrate the detection of rat tumor necrosis factor (TNF)-α with a low LOD of 22 pM. To demonstrate the clinical application of the multi-step assay platform, I quantified TNF-α in rat samples (specifically surgically injured laryngeal tissues of rats). The tested results of TNF-α were shown similar between the standard and μPAD ELISAs.
In Chapter 5, I applied the autonomous platform into real use at clinical sites, for diagnosing human laryngopharyngeal reflux (LPR) by detecting three biomarkers including pepsin, interleukin (IL)-1β, and TNF-α. Nine adult patients with suspected LPR and nine adults as asymptomatic controls were recruited from the otolaryngology voice clinic at the Royal Victoria Hospital (Montreal, QC, Canada). Patient laryngeal surface secretions were collected with a tiny working channel of an endoscope that was connected to a suction source for extraction, while the patients were receiving video-laryngostroboscopic examinations. Approximately 200 L of secretions were transferred from the suction source into a centrifuge tube and then used to be quantified on μPADs with our platform and on standard ELISA kits with a plate reader. Each assay on a μPAD required 45 minutes and 3 L of time and sample, comparing to those 130 minutes and
7
75 µL using standard ELISA kits. I calibrated the tests of human pepsin, IL-1β, and TNF-α on the platform with LODs of 5.8 pM, 35 pM, and 3 pM, respectively. To investigate the clinical feasibility of our platform, the detections of pepsin in 18 human laryngeal surface secretion samples were then benchmarked by standard ELISA kits on a plate reader. Through Bland-Altman analysis, 17 out of 18 data points located in the range of 95% confidence interval (𝑑̅ ± 1.96𝑆𝑑), which reveals that our platform achieved a good agreement of diagnostic results with the standard ELISAs. I also confirmed that pepsin is a consistent and promising biomarker in diagnosing LPR of patients through this work.
Chapter 6 presents a fundamental comparison study on surface chemistries that enhance protein bindings on μPADs. The chemically-modified cellulose surface could provide active binding sites for chemical adsorption (namely covalent bonds) of proteins. The selection criteria for the candidate surface chemistries were facile, simple, and efficient, which can be used for mass production. I chose five single-step modification methods and first characterized the modified μPADs to confirm if the modification process was valid. Then I compared their biofunctionalization efficiencies by performing paper-based direct ELISA. The potassium periodate (KIO4)-modified μPADs revealed the best modification feature among the five with 59%
decreased background noise and 53% increased colorimetric signal outputs compared to unmodified μPADs. I also investigated the stabilities of five modified μPADs after storage over 30 days. All modified μPADs underwent a slight loss on biofunctionalization. The KIO4-modified
process was used to carry out surface treatment of μPADs during the ELISAs on the SIAO platform. In Chapter 7, I extended the SIAO platform to demonstrating DNA testing. I proposed, for the first time, a fluorescence-quenching-based detection mechanism for DNA sensing on μPADs. The mechanism is mainly based on fluorescent signal changes when fluorophore-tagged hairpin-looped DNA probes are folding or unfolding in the presence or absence of DNA targets. I fabricated few-layer (under five layers) MoS2 nanosheets as an effective fluorescence quenching
platforms that were pre-mixed with the DNA probes. I demonstrated the fluorescence sensing mechanism in solution with LODs of 610 pM and 230 pM for short single strand DNA (ssDNA, as potential aptamers) and long ssDNA (around 200 base pairs) testing, respectively. After coating the mixture (MoS2 nanosheets attached with DNA probes) on the μPADs, automated detection of
8
platform, respectively. Benefitting from the localized heating capability of copper heaters for manipulating SMP-based actuators, the copper heater can be also designed and integrated into the SIAO platform as an isolated heater for loop-mediated isothermal amplification (LAMP) of long target DNA. The heating temperature during the LAMP process can be controlled accurately at 65 °C ± 1 °C and enabled a stable LAMP process on the platform. This added the DNA amplification function onto the SIAO platform, making it an integrated platform for DNA application and detection.
Finally, Chapter 8 summarizes the accomplished advancements and the primary contributions of this research. The outlook and future work are also outlined in the end.
1.3 References
1. Gong, M.M. and D. Sinton, Turning the page: advancing paper-based microfluidics for
broad diagnostic application. Chemical Reviews, 2017. 117(12): p. 8447-8480.
2. Hu, J., et al., Advances in paper-based point-of-care diagnostics. Biosensors and Bioelectronics, 2014. 54: p. 585-597.
3. Yamada, K., et al., Toward practical application of paper-based microfluidics for medical
diagnostics: state-of-the-art and challenges. Lab on a Chip, 2017. 17(7): p. 1206-1249.
4. Liana, D.D., et al., Recent advances in paper-based sensors. Sensors, 2012. 12(9): p. 11505-11526.
5. Hossain, S.Z., et al., Reagentless bidirectional lateral flow bioactive paper sensors for
detection of pesticides in beverage and food samples. Analytical Chemistry, 2009. 81(21):
p. 9055-9064.
6. Nie, Z., et al., Integration of paper-based microfluidic devices with commercial
electrochemical readers. Lab on a Chip, 2010. 10(22): p. 3163-3169.
7. Nie, Z., et al., Electrochemical sensing in paper-based microfluidic devices. Lab on a Chip, 2010. 10(4): p. 477-483.
8. Apilux, A., et al., Lab-on-paper with dual electrochemical/colorimetric detection for
simultaneous determination of gold and iron. Analytical Chemistry, 2010. 82(5): p.
1727-1732.
9. Fu, E., et al., Two-dimensional paper network format that enables simple multistep assays
9 Chemistry, 2012. 84(10): p. 4574-4579.
10. Li, C.-z., et al., Paper based point-of-care testing disc for multiplex whole cell bacteria
analysis. Biosensors and Bioelectronics, 2011. 26(11): p. 4342-4348.
11. Francis, J.S., et al., Severe community-onset pneumonia in healthy adults caused by
methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clinical Infectious Diseases, 2005. 40(1): p. 100-107.
12. Cate, D.M., et al., Recent developments in paper-based microfluidic devices. Analytical Chemistry, 2014. 87(1): p. 19-41.
13. Liu, H. and R.M. Crooks, Three-dimensional paper microfluidic devices assembled using
the principles of origami. Journal of the American Chemical Society, 2011. 133(44): p.
17564-17566.
14. Connelly, J.T., J.P. Rolland, and G.M. Whitesides, “Paper machine” for molecular
diagnostics. Analytical Chemistry, 2015. 87(15): p. 7595-7601.
15. Liu, H., X. Li, and R.M. Crooks, Paper-based SlipPAD for high-throughput chemical
sensing. Analytical Chemistry, 2013. 85(9): p. 4263-4267.
16. Li, X., P. Zwanenburg, and X. Liu, Magnetic timing valves for fluid control in paper-based
microfluidics. Lab on a Chip, 2013. 13(13): p. 2609-2614.
17. Toley, B.J., et al., A versatile valving toolkit for automating fluidic operations in paper
10
Chapter 2
State-of-the-art advances and
challenges of incorporating
functionality into microfluidic
paper-based analytical devices
11
2
Chapter 2: State-of-the-art advances and challenges of incorporating
functionality into microfluidic paper-based analytical devices
Hao Fu1, 2, Pengfei Song1, 2, and Xinyu Liu2, *
1Department of Mechanical Engineering, McGill University, 817 Sherbrooke Street West,
Montreal, Quebec H3A 0C3, Canada
2Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, Ontario
M5S 3G8, Canada
*Corresponding author: xyliu@mie.utoronto.ca
To be submitted.
Abstract
Microfluidics paper-based analytical devices (μPADs) have obtained worldwide acceptance in the past decade and is becoming one of the most promising point-of-care (POC) test platforms. In order to improve the accessibility of μPAD as a new generation of alternative technology to standard and conventional laboratory testing, smart and robust functionality has been incorporated to μPAD. During this process, many challenges and opportunities have emerged in the field, targeting to resolve the existing issues all over the world, especially in developing countries. In this review, we briefly introduce the recent developments and then examine the advancements in μPAD technology for its practical uses. The state-of-the-art achievements of μPAD are first presented, which is followed by the strategies of incorporating functionality onto μPADs. Impactful advancements such as ultrasensitive detection and accurate fluid manipulation on μPAD have been accomplished and enabled analytical capabilities that are involved with multi-step assays (e.g., immunoassay and nucleic acid detection) on a paper-based platform. By analyzing the emerging challenges and remaining opportunities toward practical applications in real-world diagnosis including healthcare monitoring and disease diagnosis, we conclude this review with the future outlook for μPAD technology to meet the diverse requirements for POC uses.
Keywords: microfluidic paper-based analytical devices (μPAD); point-of-care testing; μPAD functionality; healthcare monitoring.
![Figure 2-3. (a) A shunt used as a dissolvable bridge to connect two paper channels. Reprinted from [58]](https://thumb-eu.123doks.com/thumbv2/123doknet/7426526.219454/45.918.110.806.652.939/figure-shunt-dissolvable-bridge-connect-paper-channels-reprinted.webp)