Development of new dairy ingredient with
prebiotic and antioxidant properties through
the electro-activation processing of sweet cheese
whey
Thèse
Ourdia Kareb
Doctorat en sciences et technologie des aliments
Philosophiae Doctor (Ph. D.)
Québec, Canada
Development of new dairy ingredient with
prebiotic and antioxidant properties through
the electro-activation processing of sweet cheese
whey
Thèse
Ourdia Kareb
Sous la direction de :
Mohammed Aider, directeur de recherche
Claude P. Champagne, codirecteur de recherche
iii
RÉSUMÉ
Le but de ce projet était de développer la technologie d'électro-activation comme une approche novatrice pour la valorisation intégrale des constituants du lactosérum. Ce sous-produit est riche en composants de valeur avec des propriétés biologiques et fonctionnelles prometteuses. La première étape de ce travail visait à déterminer les conditions optimales pour la production du lactulose en utilisant le lactosérum comme source de lactose. Un rendement de 35% a été obtenu avec une pureté élevée à des températures de 0, 10 et 25°C et un court temps de réaction (≅ 40 minutes). En outre, l'analyse protéomique a révélé l'hydrolyse des protéines de lactosérum et la formation simultanée de produits de réaction Maillard aux propriétés antioxydantes élevées. La deuxième étape visait à élucider les mécanismes antioxydants qui régissaient les hydrolysats de protéines de lactosérum à réaction de Maillard (MRP-whey) et à caractériser leurs structures. L'effet antioxydant a été attribué à de multiples propriétés et pourrait être utilisé à la fois comme antioxydants primaires et secondaires. La structure moléculaire du MRP-whey a été caractérisée comme étant des bases de Schiff. De plus, des peptides bioactifs multifonctionnels, avec des propriétés biologiques ont également été identifiés. Dans la troisième étape, l'efficacité du lactosérum électro-activé (lactosérum-EA) possédant des propriétés prébiotique et antioxydante sur la croissance des bactéries probiotiques testées a été démontrée. Le lactosérum-EA avait comparativement un meilleur effet bifidogène que le lactulose. De plus, on a constaté que le lactosérum-EA produisait un effet protecteur sur L. johnsonii La-1 durant sa croissance en présence d'oxygène. Cet effet peut être lié, en partie, à la capacité du lactosérum-EA à éliminer le peroxyde d'hydrogène et à prévenir son accumulation dans le milieu de croissance. Les spectres FTIR ont montré que le lactosérum-EA empêchait l'oxydation des lipides de la membrane cellulaire en limitant le changement dans l'ordre structurel des acides gras. Dans l'ensemble, le lactosérum-EA comme prébiotique possédant une capacité antioxydante a un grand potentiel d'application, non seulement dans l'industrie laitière, mais aussi comme ingrédient fonctionnel avec diverses bioactivités et fonctionnalités.
iv
ABSTRACT
The goal of this project was to develop the electro-activation technology as innovative approach for an integral valorization of whey constituents. This by-product is rich in valuable components with promising biological and functional properties. The first step of this work aimed to determine the optimal conditions of lactulose production using whey as a source of lactose and a yield of 35% was obtained with high purity under temperatures of 0, 10 and 25°C and short reaction time (≅ 40 minutes). Moreover, the proteomic analysis revealed the hydrolysis of whey proteins and simultaneous formation of Maillard reaction products with high antioxidant properties. The second step aimed to elucidate the antioxidant mechanisms that governed the Maillard-reacted-whey protein hydrolysates (MRPs-Maillard-reacted-whey) and to characterize their structures. The antioxidant effect was ascribed to a multiple properties and could be used as both primary and secondary antioxidants. The molecular structure of the MRPs-whey was characterized to be Schiff base compounds. Additionally, multifunctional bioactive peptides, with biological properties were also identified. In the third step, the efficacy of electro-activated whey (EA-whey) as a combined prebiotic and antioxidant component on the growth of tested probiotic bacteria was demonstrated. The EA-whey was comparatively a better bifidogenic factor than lactulose. Additionally, EA-whey was found to elicit a protective effect on L. johnsonii La-1 during its growth in the presence of oxygen. This effect may be related, in part, to the ability of EA-whey to scavenge hydrogen peroxide metabolites and prevent its accumulation in the growth medium. FTIR spectra showed that EA-whey prevented the cell membrane lipids oxidation by limiting the change in the structural order of fatty acids. Overall, EA-whey as a prebiotic supplement possessing antioxidant capacity has great potential for application not only in the dairy industry but also as a functional food ingredient with potent bioactivities and functionalities.
v
TABLE OF CONTENTS
RÉSUMÉ ... iii
ABSTRACT ... iv
TABLE OF CONTENTS ... v
LIST OF TABLES ... xiii
LIST OF FIGURES ... xiv
LIST OF ABBREVIATIONS ... xviii
DEDICATIONS ... xix
ACKNOWLEDGMENTS ... xx
FOREWORD ... xxii
INTRODUCTION ... 1
1. CHAPTER 1: Literature review ... 4
1.1 Functional foods ... 4
1.1.1 Probiotics ... 6
1.1.1.1 Probiotics concept ... 6
1.1.1.2 Commercially used probiotic foods ... 6
1.1.1.3 Selection of potential probiotics ... 8
1.1.1.4 Mechanisms of action of probiotics ... 10
1.1.1.5 Beneficial health effects of probiotics ... 12
1.1.1.6 Factors affecting the survival of probiotics in dairy products .. ... 14
1.1.2 Prebiotics ... 16
1.1.2.1 Prebiotics concept ... 16
1.1.2.2 Criteria of prebiotics ... 17
vi
1.1.2.4 Beneficial health effects of prebiotics ... 20
1.1.2.5 Prebiotics as functional food ingredients ... 21
1.1.2.6 Synbiotic concept ... 23
1.1.2.7 Synbiotic in dairy products ... 24
1.2 Whey ... 26
1.2.1 Whey in environmental consideration ... 26
1.2.2 Whey composition ... 27
1.2.2.1 Lactose whey ... 28
1.2.2.2 Lactose-derived lactulose ... 28
1.2.2.2.1 General properties of lactulose ... 28
1.2.2.2.2 Chemistry of lactulose synthesis ... 29
1.2.3 Methods of lactulose production ... 31
1.2.3.1 Chemical synthesis ... 31 1.2.3.2 Enzymatic synthesis ... 31 1.2.3.3 Electro-activation synthesis ... 32 1.2.4 Lactulose applications ... 33 1.2.4.1 Pharmaceutical applications ... 33 1.2.4.2 Food applications ... 34 1.2.5 Whey proteins ... 35
1.2.5.1 Nutritional value of whey proteins ... 36
1.2.5.2 Whey proteins in food as a functional ingredient ... 37
1.2.5.3 Physiological importance of whey proteins ... 39
1.2.5.4 Bioactive peptides generated from whey ... 40
1.2.5.4.1 Processing of bioactive peptides in whey ... 41
vii
1.2.5.4.2.1 Antihypertensive peptides ... 43
1.2.5.4.2.2 Antioxidative peptides ... 44
1.2.5.4.2.3 Antimicrobial peptides ... 45
1.2.5.4.2.4 Immunomodulatory peptides ... 45
1.2.5.4.2.5 Other bioactive peptides ... 46
1.2.5.5 Maillard reaction products from whey ... 47
1.2.5.5.1 Maillard reaction stages ... 48
1.2.5.5.2 Maillard reaction processing ... 50
1.2.5.6 Some proprieties of Maillard reaction products ... 51
1.2.5.7 Antioxidant properties of MRPs-whey ... 51
1.3 Electro-activation ... 53
1.3.1 The concept of electro-activation and devolvement ... 53
1.3.2 Principles of electro-activated aqueous solutions ... 53
1.3.3 Application of electro-activation solutions ... 56
1.3.4 Electro-activation technology for whey valorization ... 57
2. CHAPTER 2: Problematic, Hypothesis and Objectives ... 59
2.1 Problematic ... 59
2.2 Hypothesis ... 59
2.3 Main objective ... 60
2.4 Specific objectives ... 60
3. CHAPTER 3: Contribution to the production of lactulose-rich whey by in situ electro-isomerization of lactose and effect on whey proteins after electro-activation as confirmed by MALDI-TOF MS spectrometry and SDS-PAGE gel electrophoresis . ... 61
3.1 RÉSUMÉ ... 62
viii
3.3 INTRODUCTION ... 64
3.4 MATERIALS AND METHODS ... 67
3.4.1 Chemicals ... 67
3.4.2 Electro-activation reactor and configuration ... 67
3.4.3 Electro-activation reaction ... 69
3.4.4 HPLC analysis of the reaction products ... 69
3.4.5 Total protein content ... 70
3.4.6 Gel Electrophoresis (SDS-PAGE) ... 70
3.4.7 MALDI-TOF-MS assessment ... 71
3.4.8 Determination of free amino acids by UPLC-UV florescence detection ... 71
3.4.9 Oxygene radical capacity (ORAC) of electro-activated whey .... 72
3.4.10 Statistical Analysis ... 72
3.5 RESULTS AND DISCUSSION ... 73
3.5.1 Process of whey solution EA: Evolution of whey solution alkalinity ... 73
3.5.2 Assessment of lactulose formation during whey electro-activation ... 76
3.5.3 HPLC analysis of lactulose and other reaction by-products ... 78
3.5.4 Effect of temperature ... 81
3.5.5 Effect of the electric current intensity ... 83
3.5.6 Effect of the volume reaction ... 85
3.5.7 Effect of the feed whey concentration ... 87
3.5.8 Proteomic analysis of the EA-whey ... 89
3.5.8.1 SDS-PAGE profiles of EA-whey proteins ... 89
ix
3.5.9 Total antioxidant capacity of EA-whey proteins ... 92
3.6 CONCLUSION ... 96
4. CHAPTER 4: Impact of electro-activation on the antioxidant properties of defatted whey ... 97
4.1 RÉSUMÉ ... 98
4.2 ABSTRACT ... 99
4.3 INTRODUCTION ... 100
4.4 MATERIALS AND METHODS ... 102
4.4.1 Chemicals, reagents, membranes and electrodes ... 102
4.4.2 Electro-activation of whey induced Maillard reaction products 104 4.4.3 Determination of antioxidant activity ... 104
4.4.3.1 Determination of reducing power of EA-whey ... 104
4.4.3.2 Determination of 2,2-diphenyl-1-picrylhydrazyl radical-scavenging activity of EA-whey ... 105
4.4.3.3 Determination of 2,2-diphenyl-1-picrylhydrazyl radical-scavenging activity of EA-whey ... 105
4.4.3.4 Determination of hydroxyl radical scavenging activity of EA-whey ... 106
4.4.3.5 Determination of chelating activity on Fe2+ of EA-whey ... 106
4.4.4 Measurement of browning intensity and fluorescence of electro-activated whey ... 107
4.4.5 Statistical analysis ... 107
4.5 RESULTS AND DISCUSSION ... 108
4.5.1 Oxygen radical absorbance capacity ... 108
4.5.2 Reducing power ... 110
x
4.5.4 2,2-Diphenyl-1-picrylhydrazyl scavenging activity ... 114
4.5.5 Hydroxyl radical scavenging activity ... 116
4.5.6 Effect of pH on brown colour development of electro-activated whey ... 119
4.6 CONCLUSION ... 122
5. CHAPTER 5: Electro-activation of sweet defatted whey: Impact on the induced Maillard reaction products and bioactive peptides ... 123
5.1 RÉSUMÉ ... 124
5.2 ABSTRACT ... 125
5.3 INTRODUCTION ... 126
5.4 MATERIALS AND METHODS ... 128
5.4.1 Chemicals and reagents ... 128
5.4.2 Maillard reaction products (MRPs) generation from EA-whey 128 5.4.3 Determination of free and sugar-bound amino acids ... 129
5.4.4 Determination of reducing sugars in MRPs-whey ... 130
5.4.5 Structure characterization of MRPs-whey induced by electro-activation ... 130
5.4.5.1 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) ... 130 5.4.5.2 FT-IR measurements ... 130 5.4.5.3 LC/MS-MS analysis ... 131 5.4.5.3.1 Samples preparation ... 131 5.4.5.3.2 Mass spectrometry ... 131 5.4.5.3.3 Database searching ... 132
5.4.5.3.4 Criteria for protein identification ... 132
xi
5.6 RESULTS AND DISCUSSION ... 133
5.6.1 Changes in free amino acids and reducing sugars ... 133
5.6.2 FTIR spectroscopy analysis ... 138
5.6.3 Identification of bioactive peptides from electro-activated whey ... ... 140
5.7 CONCLUSION ... 143
6. CHAPTER 6: Effect of electro-activated sweet whey on growth of probiotic bacteria of Bifidobacterium, Lactobacillus and Streptococcus genera under model growth conditions ... 144
6.1 RÉSUMÉ ... 145
6.2 ABSTARCT ... 146
6.3 INTRODUCTION ... 147
6.4 MATERIALS AND METHODS ... 150
6.4.1 Chemicals and reagents ... 150
6.4.2 Preparation of electro-activated whey ... 150
6.4.3 Microorganisms and culture conditions ... 152
6.4.4 Experimental design ... 153
6.4.5 Evaluation of growth performance ... 153
6.4.5.1 Effect of MRS supplementation on the growth of probiotic bacteria under anaerobic conditions ... 153
6.4.5.2 Effect of MRS supplementation on the growth of La under aerobic conditions ... 154
6.4.5.3 Fourier transform infrared spectroscopy analysis ... 154
6.5 Statistical analyses ... 155
6.6 RESULTS AND DISCUSSION ... 156
xii
6.6.1.1 Effect of MRS supplementation on the growth of probiotic
bacteria under anaerobic conditions ... 156
6.6.1.2 Effect of MRS supplementation by EA-whey on the growth of L. johnsonii La-1 under aerobic conditions ... 161
6.6.1.3 FTIR analysis ... 163
6.6.2 DISCUSSION ... 165
6.6.2.1 Justification of the used strategy ... 165
6.6.2.2 Response of the bifidobacteria to EA-whey ... 166
6.6.2.3 Response of the lactobacillus to EA-whey ... 167
6.6.2.4 Response of the specific yogurt cultures to EA-whey ... 168
6.6.2.5 FTIR analysis of L. johnsonii membrane lipids ... 170
6.7 CONCLUSION ... 173
CHAPTER 7: General conclusion and perspectives ... 174
6.1 GENERAL CONCLUSION ... 174
7.2 PESPECTIVES ... 176
xiii
LIST OF TABLES
Table 1.1: Main approaches used for the production of prebiotic carbohydrates
(Playne & Crittenden, 2009). ... 19
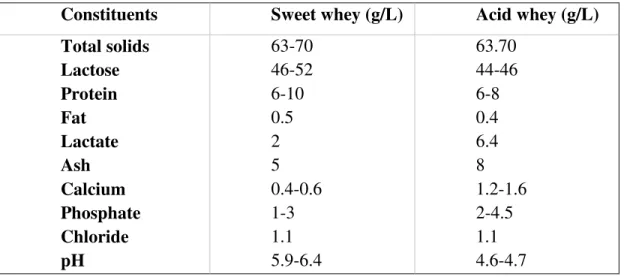
Table 1.2: Food applications of prebiotics (Wang, 2009). ... 22 Table 1.3: Proximate composition and pH of sweet and acid whey (Yadav et
al., 2015). ... 27
Table 1.4: Protein composition and basic characteristics of the whey proteins
(Yadav et al., 2015). ... 36
Table 1.5: Examples of application of whey protein ingredients in certain foods
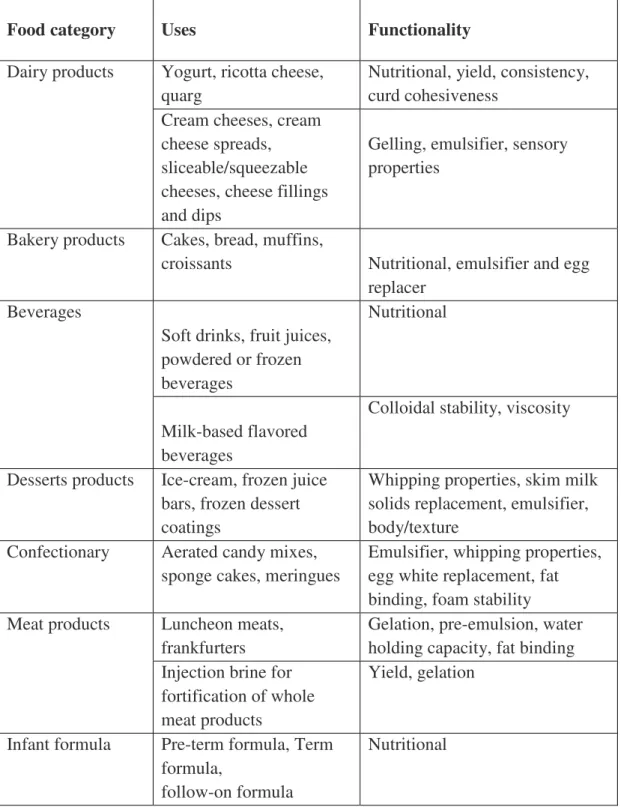
and their functional properties adapted from (Bansal & Bhandari, 2016). ... 38
Table 3.1: Oxygen radical absorbance capacity of whey 7% (w/v) as a function
EA time at 10°C and 400 mA. ... 94
Table 5.1: Comparison of amino acids composition of native whey and
EA-Whey at different concentrations. ... 134
Table 5.2: List of the identified amino acid sequences in the electro-activated
whey (EA-Whey) and their potential biological activity. ... 141
Table 6.1: Main proximate composition and antioxidant properties of whey and
EA-whey used in this study. ... 152
Table 6.2: ODmax of the bacterial strains in supplemented MRS medium as a
function of the concentration and type of carbon source. ... 157
Table 6.3: µmax (h-1) of the bacterial strains in supplemented MRS medium as
a function of the concentration and type of carbon source. ... 159
Table 6.4: ODmax and µmax (h-1) of L. johnsonii La-1 was cultured in
MRS-supplemented medium under anaerobic conditions. ... 162
Table 6.5: ODmax and µmax (h-1) of L. johnsonii La-1 was cultured in
xiv
LIST OF FIGURES
Figure 1.1: Some most used probiotic species (Holzapfel et al., 2001; Kumar et al., 2016). ... 7
Figure 1.2: Desirable criteria for the selection of probiotics in commercial applications adopted from (Mortazavian et al., 2012; Tripathi & Giri, 2014). ... 8
Figure 1.3: Schematic diagram illustrating potential or known mechanisms whereby probiotic bacteria might affect the microbiota. These mechanisms include (1) competition for dietary ingredients as growth substrates, (2) bioconversion of, for example, sugars into fermentation products with inhibitory properties, (3) production of growth substrates, for example, EPS or vitamins, for other bacteria, (4) direct antagonism by bacteriocins, (5) competitive exclusion for binding sites, (6) improved barrier function, (7) reduction of inflammation, thus altering intestinal properties for colonization and persistence within, and (8) stimulation of innate immune response. IEC: epithelial cells, DC: dendritic cells, T:T-cells (O'Toole & Cooney, 2008). ... 12
Figure 1.4: Health benefits from probiotic consumption (Parvez et al., 2006). ... 14 Figure 1.5: Schematic representation of the chemistry mechanism of lactulose isomerization trough LA transformation, adapted from Aissa & Aïder (2013a). ... 30
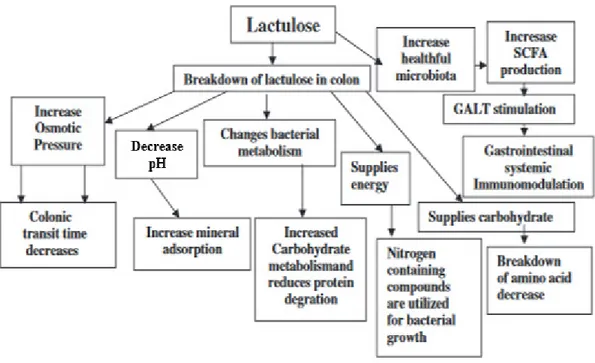
Figure 1.6: Mechanism of action of lactulose and significance of the bacterial metabolism of lactulose (Panesar & Kumari, 2011). ... 34
Figure 1.7: Alternative modes of bioactive peptide generation (Madureira et al., 2010). ... 41
Figure 1.8: Scheme of the Maillard reaction adapted from (Hodge, 1953). ... 49 Figure 1.9: Schematic illustration of a basic water electrolysis system (Zeng & Zhang, 2010). ... 55
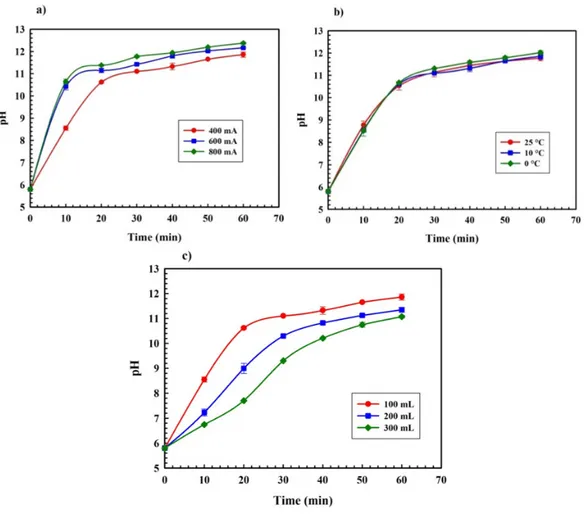
Figure 3.1: Schematic representation of the experimental set-up for lactose-whey isomerization. ... 68 Figure 3.2: Profile of pH as a function of EA time in whey (Cwhey = 7% w/v), at
different experimental conditions (a) current intensities (400, 600, and 800 mA) at 10°C and 100 mL, (b) working temperatures (0, 10, and 25°C) at 400 mA and 100 mL, and (c) volume conditions (100, 200, and 300 mL) at 10°C and 400 mA. ... 73
xv
Figure 3.3: Profile of lactulose yield as a function of EA time using different feed solutions. The experimental conditions were; whey (Cwhey = 7% w/v), lactose
(CLactose = 5% w/v) at 10°C, 400 mA and 100 mL conditions. Data represent the mean
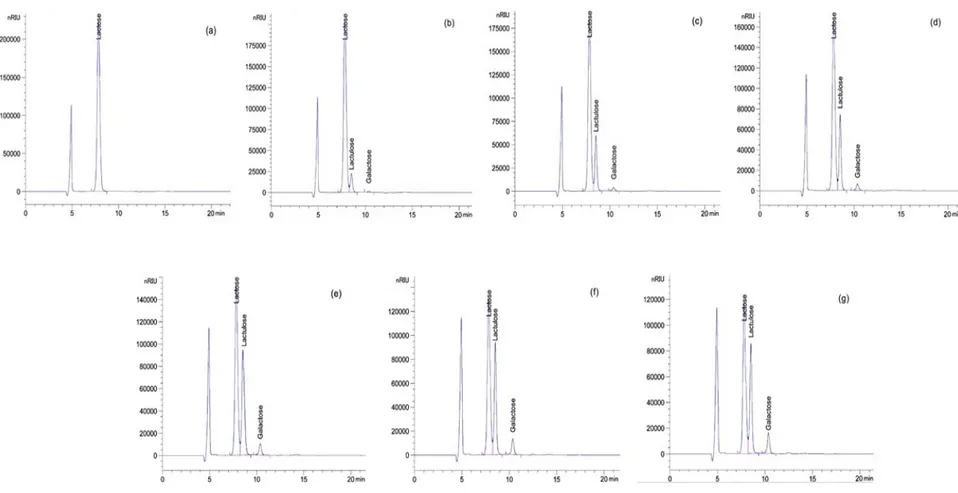
± standard deviation of three experiments. ... 77 Figure 3.4: Chromatogram profiles of whey sugars after EA at 7% (w/v) concentration, temperature T = 10°C, current intensity, I = 400 mA and V=100 mL: (a) t = 0 min, (b) t = 10 min, (c) t = 20 min, (d) t = 30 min, (e) t = 40 min, (f) t = 50 min, (g) t = 60 min. ... 79
Figure 3.5: Mechanism of the electro-isomerization of lactose to lactulose in whey and the possible galactose formation pathways under low temperature using EA process. ... 80
Figure 3.6: Profiles of lactulose and galactose produced as a function of EA time at different processing temperatures (0, 10, 25°C), whey concentration 7% (w/v), temperature T = 10°C, current intensity I = 400 mA and V = 100 mL. ... 82
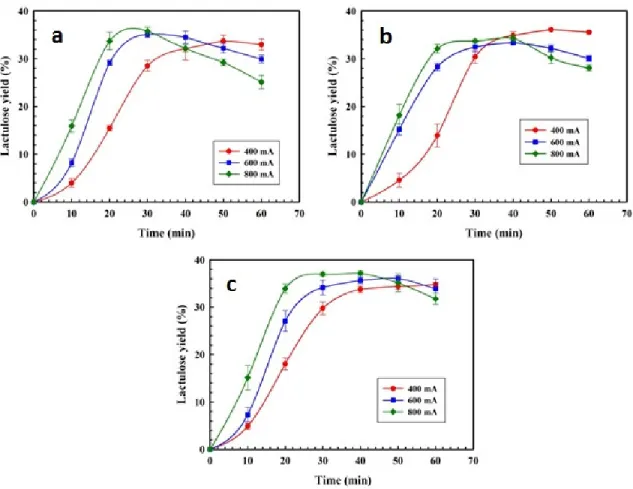
Figure 3.7: Profiles of lactulose yield as a function of EA time at different current intensities (400, 600, and 800 mA). The concentration of feed whey and volume were fixed at Cwhey = 7% (w/v) and V = 100 mL, respectively. The working
temperatures were varied, (a) at 25°C, (b) at 10°C and (c) at 0°C... 84 Figure 3.8: Profile of lactulose yield produced as a function of EA time at different volume conditions (100, 200 and 300 mL). The concentration of feed whey and temperature were fixed at Cwhey = 7% (w/v) and T = 10°C, respectively. The current
intensities were varied, (a) at 400 mA, (b) at 600 mA and (c) at 800 mA. ... 86 Figure 3.9: Profiles of lactulose yield produced as a function of EA time at different whey concentration (7, 14 and 28% w/v). The temperature and volume conditions were fixed at T = 10°C and V = 100 mL, respectively. The current intensities were varied, (a) at 400 mA, (b) at 600 mA and (c) at 800 mA. ... 88
Figure 3.10: Electrophoretic pattern of EA whey proteins as a function of reaction time under non-reducing conditions (a) and reducing conditions (b). The temperature and current intensity were fixed at T = 10°C and I = 400 mA, respectively. MW, molecular weight of protein standard; BSA, bovine serum albumin; β-LG, α-LA and IGs. ... 90
xvi
Figure 3.11: MALDI mass spectrum acquired in the linear mode: (a) untreated whey and (b) after 40 min of the EA. MALDI mass spectra acquired in the reflectron mode: (c) untreated sample and (d) after 40 min of EA. ... 93
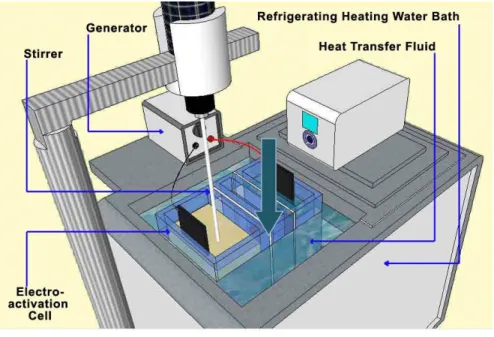
Figure 4.1: Schematic representation of the reactor used for whey EA: AEM and CEM indicate the anion and cation exchange membrane, respectively. ... 103
Figure 4.2: Effect of EA time and current intensities on the ORAC activity of whey at different concentrations: (a) at 7%, (b) at 14% and (c) at 21% (w/v). Error bars show standard deviation (n = 3). ... 109
Figure 4.3: Effect of EA time and current intensities on the reducing power activity of whey at different concentrations: (a) at 7%, (b) at 14% and (c) at 21% (w/v). Error bars show standard deviation (n = 3). ... 111
Figure 4.4: Effect of EA time and current intensities on the iron chelating ability of whey at different concentrations: (a) at 7%, (b) at 14% and (c) at 21% (w/v). Error bars show standard deviation (n = 3). ... 113
Figure 4.5: Effect of EA time and current intensities on the DDPH radical scavenging activity of whey at different concentrations; (a) at 7%, (b) at 14% and (c) at 21% (w/v). Error bars show standard deviation (n = 3). ... 115
Figure 4.6: Effect of EA time and current intensities on the hydroxyl radical scavenging activity of whey at different concentrations: (a) at 7%, (b) at 14% and (c) at 21% (w/v). Error bars show standard deviation (n = 3). ... 117
Figure 4.7: Changes in pH (a), absorbance at 294 nm (b), browning intensity at 420 nm (c), and fluorescence intensity (d) of whey at different concentrations (diamonds, 7%; squares, 14%; triangles, 21%) during electro-activation at 60 mA for up to 45 min; error bars show standard deviation (n = 3). ... 120
Figure 5.1: Schematic representation of the experimental set-up for the EA of whey. ... 129
Figure 5.2: Sugar profiles of EA-whey at different concentrations up to 45 min at 600 mA current intensity: (a) untreated sample, (b) 7% (w/v), (c) 14% (w/v) and (d) 21% (w/v). ... 135
Figure 5.3: Electrophoretic pattern of EA-whey proteins under non-reducing conditions (a) and reducing conditions (b)... 137
xvii
Figure.5.4: Infrared spectra of EA-whey at different concentrations after 45 min of EA treatment. ... 139
Figure 6.1: Schematic representation of the electro-activation reactor used for production of EA-whey. ... 151
Figure 6.2: Normalized FTIR spectra (3000-2800 cm-1) of L. johnsonii La-1
cells grown under (a) anaerobic conditions and (b) aerobic conditions in control MRS and BMRS culture medium supplemented with EA-whey at different concentrations. ... 164
xviii
LIST OF ABBREVIATIONS
ACE: Angiotensin-Converting Enzyme AEM: Anion Exchange Membrane ANOVA: Analysis Of the Variance ARP: Amadori rearrangement product BOD: Biological Oxygen Demand BSA: Bovine Serum Albumin CEM: Cation Exchange Membrane CFU: Colony Forming Units COD: Chemical Oxygen Demand DDPH: 2,2-Diphenyl-1-Picrylhydrazyl EA: Electro-Activation
EAS: Electro-Activated Solutions FAO: Food and Agriculture Organization FOS: Fructooligofructose
FTIR: Fourier Transform Infrared GOS: Galactooligosaccharide
GRAS: Generally Recognized As Safe
HPLC: High Performance Liquid Chromatography HRSA: Hydroxyl Radical Scavenging Activity Igs: Immunoglobulins
LC-MS/MS: Liquid Chromatography tandem Mass Spectrometry LF: Lactoferrin
LP: Lactoperoxidase
MALDI-TOF: Matrix-Assisted Laser Desorption/Ionization-Time Of Flight MRPs: Maillard Reaction Products
MW: Molecular Weight
ORAC: Oxygen Radical Absorbance Capacity PP: Proteose-peptone
ROS: Reactive Oxygen Species SCFAs: Short-Chain Fatty Acids
SDS-PAGE: Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis UPLC-UV: Ultra Performance Liquid Chromatography Ultraviolet/Visible WHO: World Health Organization
α-La: α-Lactalbumin β-Lg: β-Lactoglobulin
xix
DEDICATIONS
To the memory of my loved dad, My haven of peace And my twin You stay in me.
xx
ACKNOWLEDGMENTS
This work represents an achievement of 4 years of research that could not even have been carried out without the support and understanding of many important people that I shall therefore acknowledge.
I would like to extend the utmost gratitude to my research director Dr. Mohammed Aider for welcoming me in his research team and trusting me with this project. His availability, guidance, support, and advice were always greatly appreciated. I would also like to express my gratitude to my co-directors Dr. Claude P., Champagne and Dre. Julie Jean for their support and helpful advice. I am very grateful to Dr. Ahmed Gooma for his valuable comments and constructive criticisms in my articles.
My gratitude also extends to Amara Ait-Aissa, Pascal dubé, Veronique Richard, Diane Gagnon and, Marie Michele, Marine Béguin, Pascal Lavoie and Mélanie martineau for their help in the lab and for their incredible patience. I would like to thank Diane Lajoie and Christin Dumas for their availability in the preparation and signature of administrative documents.
To the many friends I made along the way your presence made this experience truly wonderful. I would especially like to thank Pamela, Mayank, Emna, Agathe, Mathilde, Abdel, Tahar, Lamia, Samia, Menel, Karima and Sadia. I also must acknowledge the great support I had from my colleagues in the Department of Food Sciences especially, Alseny, Abdoulaye and Omar. My deep appreciation goes to Ammar for fraternity; I could not accomplish this work without your support. I would like as well to acknowledge Mahdi, Thank you very much for great friendship and kind support.
I would also like to show my appreciation for Agathe, Khadija and Yasmée for the amazing atmosphere and love during the three years working together.
xxi
It is hard to accomplish anything in life without the care and support only your loved ones can give you. I am very fortunate to have a wonderful family that give me their immense love and support. I would especially like to extend my deep love and gratitude to my mom, who has been the great strength and motivation during the entire periods away from home. Thank you so much letting me go where I wanted to be. I would also like to show my appreciation for my wonderful brothers; Sofiane, Salim and Samir for their strong support and continuous encouragement, I express here my unconditional love “bad boys”. Special acknowledgements for my cousins, Ouisa and Hakim for their support, immense help, inspiration and insatiable encouragement they gave me. My gratitude also goes to the other members of my family: to my amazing grandparents, uncles, aunts and their families.
I would also like to show my appreciation for my family in law, especially Kahina and Djimy for their support.
Last, but not least, I am extremely thankful to my life partner, Omrak for constant support and love. I guess, there is no words to express my feelings to you. Thanks for bearing the hard times and have incredible comprehensiveness and patience during all these years spent far from you.
xxii
FOREWORD
The present thesis is submitted to the Faculty of Graduate and Postdoctoral Studies of Laval University (Faculté des études supérieures et postdoctorales de l'Université Laval) to meet the requirements for obtaining the Philosophiae Doctor es
Sciences (Ph. D) degree in Food Science and Technology at the Faculty of Agriculture
and Food Sciences (Faculté des sciences de l’agriculture et de l’alimentation).
This doctoral thesis is composed of seven chapters and the results are presented in the form of scientific articles submitted or published in international journals with refereed committee. The first chapter represents the literature review, which aims to provide the essential elements for a good understanding of the problematic of this doctorate.
The second chapter presents the problematic, hypothesis and objectives of the present work. Most experimental works and results obtained have been published or submitted for publication in appropriate scientific journals.
The third chapter presents the article entitled "Contribution to the production of lactulose-rich whey by in situ electro-isomerization of lactose and effect on whey proteins after EA as confirmed by MALDI-TOF spectrometry and SDS-PAGE electrophoresis" published in "Journal of Dairy Science". Authors: Ourdia Kareb, Claude P. Champagne and Mohammed Aider.
The fourth chapter presents the second article entitled "Impact of electro-activation on antioxidant properties of defatted whey" published in the "International Dairy Journal". Authors: Ourdia Kareb, Ahmed I. Gomaa, Claude P. Champagne, Julie Jean and Mohammed Aider.
The fifth chapter presents the third article entitled "Electro-activation of sweet defatted whey: Impact on the induced Maillard reaction products and bioactive
xxiii
peptides" published in Food Chemistry. Authors: Ourdia Kareb, Ahmed I. Gomaa, Claude P. Champagne, Julie Jean and Mohammed Aider.
The sixth chapter presents the fourth article entitled "Effect of electro-activated sweet whey on growth of probiotic bacteria of Bifidobacterium, Lactobacillus and
Streptococcus genera under model growth conditions" submitted to publication to
"International Dairy Journal". Authors: Ourdia Kareb, Ahmed I. Gomaa, Claude P. Champagne, Julie Jean and Mohammed Aider.
In all articles presented above, Ourdia Kareb is the first author who was in charge of the conception, experimental design and execution of experimental works, result analysis and article writing. Dr Mohammed Aider (thesis director) was involved in scientific supervision, experimental design, correction, revision and submission of manuscripts. Dr Claude champagne and Julie Jean (thesis co-directors) were involved in experimental design, correction and revision of manuscripts; Dr Ahmed Gomaa was involved in correction and revision of manuscripts.
1
INTRODUCTION
‘‘Let food be thy medicine and medicine thy food’’. In North America, more than 93% of the population adopt the idea of eating as a mean of prevention against diseases (Champagne, 2009). This social tendency attracts a keen interest within the food industry, which puts functional foods on the market with a benefit effect beyond simple nutritional function (Betoret et al., 2011; Bigliardi & Galati, 2013; Costa & Jongen, 2006). Many of these so called “healthy foods” contain functional compounds like probiotics, prebiotics, bioactive peptides and antioxidant compounds, as well as other nutrients such as vitamins and specific minerals (Grajek et al., 2005; Korhonen, 2009; Li-Chan, 2015; Lobo et al., 2010; Stanton et al., 2005; Watson & Preedy, 2015). The probiotic containing food products have been estimated to account for 60-70% of the total functional foods market (Tripathi & Giri, 2014). Probiotics are found mostly in dairy products, whith yogurts and fermented milks representing the major products sold worldwide (Granato et al., 2010a). In Canada, the regularly dose of a probiotic is estimated to approximately 109 viable cells per day to reach the targeted
health effect (Champagne et al., 2011). However, many questions remain about the effectiveness, especially their survival from design until their site of action. These microorganisms are subjected to many stresses during the manufacturing and storage of the product until the consumption due the changes in pH, total acidity, temperature, oxygen variations and depletion of nutrients leading to low survival rates (Donkor et al., 2006; Talwalkar & Kailasapathy, 2004; Tripathi & Giri, 2014). Moreover, it has been shown that probiotics exhibit slow growth in fermented dairy products due their low proteolytic activity (Marafon et al., 2011; Shihata & Shah, 2000).
One of the most important scientific challenges for the dairy industry is to promote probiotics response capacity by developing effective compounds than can enhance their growth and survival under different conditions as well as during the transit within the gastrointestinal tract, which is known to be a harsh medium for probiotics. At the other hand, prebiotics, described as non-digestible carbohydrates, are able to selectively stimulate the growth of beneficial gut microbiota, and are by far the most used ingredients which help in the growth of probiotic bacteria (Romano et al., 2015). In this regards, lactulose as a prebiotic derived from lactose is successfully used
2
in the food industry due to the nutritional and technological properties it provides in dairy products (Seki & Saito, 2012). In addition, it offers additional health benefits as a smooth laxative and is used by the pharmaceutical industry in the treatment of hepatic encephalopathy. The total annual global production of lactulose has been estimated around 50000 tons in 2009 (Playne & Crittenden, 2009). Lactulose can be produced by isomerization of lactose through chemical or enzymatic approaches. Recent works introduce electro-activation (EA) as an interesting approach of lactulose production. In contrast to both chemical and enzymatic methods, electro-activation is considered to be an economical process, is safe and is reagentless technology for the synthesis of lactulose from lactose by self-generating of high alkalinity in the reaction medium (Aider & Gimenez-Vidal, 2012; Aissa & Aïder, 2013a, 2013b, 2014a).
Other challenges for the dairy industry is to added value to the huge quantities of whey produced from cheese and casein manufacturing. About 180 million tonnes of whey were produced in 2012, and around 30-50% of the total production are still not utilized (Sitanggang et al., 2016). Despite its potential as pollutant, whey is regarded as a valuable source of numerous nutritional, functional and bioactive compounds. Whey presents an elevated content of lactose and proteins, which can be used to produce versatile benefit ingredients. The production of lactulose from whey by using electro-activation should be a commendable challenge; since it presents the both economical and environmental interests. In addition, it appears that no other study has considered the possible synergistic effect between lactose and whey protein submitted to EA. Meanwhile, the behaviour of whey proteins have not been tested in EA during lactulose synthesis. Thus, their structural and functional properties, as affected by EA, are not well known so far. It is possible that the EA of whey as non-thermal technology could allow the formation of other functional compounds of interest, mainly Maillard reaction products (MRPs) described to have excellent antioxidant properties. A better understanding of the relationship between the structure and characteristics of these conjugates will allow their use to improve functional properties of food products. Additionally, the hydrolysis of whey proteins can occur and can result in the generation of bioactive peptides. The latter have been described to perform physiological effects
3
The aim of this Ph. D project was to study the process of sweet whey electro-activation for the production, in situ, of lactulose, as well as to determine the impact of this process on whey proteins structure and functionality. Moreover, the antioxidant activity of electro-activated whey was studied in order to understand the involved compounds. Finally, the synergetic effect of electro-activated whey due its prebiotic and antioxidant properties was tested for the growth of probiotic bacteria under model growth conditions.
4
1.
CHAPTER 1: Literature review
1.1 Functional foods
It is thought that factors such as stressful lifestyle and changing dietary patterns cause the raise in the incidence of chronic diseases, and by the way, their prevention and treatment are very costly (Hu et al., 2000; Willett, 2002). Recent studies have clearly linked the balanced diet to improved health and to prevent or to reduce the risk of some diseases (Amine et al., 2002; Boushey et al., 2001; Darnton-Hill et al., 2004; Erdmann et al., 2008). These findings lead to a huge interest in the population to consume foods that contain functional ingredients, thereby promoting their health and well-being (Milner, 1999; Siro et al., 2008). Consequently, research studies in the food industry have concentrated on identifying bioactive ingredients, which provide effective physiological benefits over and above the nutritional value of traditional food products (Bigliardi & Galati, 2013; Goldberg, 2012). These compounds can be added to, or naturally enhanced or derived in variety of foods to provide advantageous health benefits, thus forming the basis of new market food segment so-called “functional foods” (Hasler, 1998; Roberfroid, 2000).
The functional food term was first introduced in Japan, in the 1980s, for food product fortified with, bioactive compounds that possessing positive physiological effects and reducing the risk of diseases, in addition to being nutritious (Doyon & Labrecque, 2008; Hasler, 2002). The functional food can be a natural or processed product that contain one or more biologically active compounds. These foods include: (i) usual foods with naturally occurring bioactive substances (e.g., dietary fibre), (ii) foods supplemented with bioactive substances (e.g., probiotics, antioxidants), and (iii) derived food ingredients (e.g., prebiotics, bioactive peptides) (Roberfroid, 2000; Siro et al., 2008). Thus, together with their acceptance as healthy and nutritious products, functional foods have led to their extended popularity across the world, especially in industrialized countries (Bigliardi & Galati, 2013; Siro et al., 2008). The global market for functional foods has been expected to be worth $305.4 billion by 2020. The faster growing sector within this area corresponds to the dairy products fortified with probiotics and/or prebiotics (Stanton et al., 2005). Their success among functional
5
foods is mainly due to their healthful image among consumers (Granato et al., 2010a). Other potentially functional ingredients such as bioactive peptides and antioxidant compounds have been continuously raising the interest in functional foods (Grajek et al., 2005; Li-Chan, 2015; López‐Barrios et al., 2014; Patel, 2015).
During the last decades, consumers increasingly believe that functional foods significantly contribute to their health and are looking for the bioactive compounds found in them. Indeed, they are demanding for novel, high added value and affordable functional ingredients (Costa & Jongen, 2006). As consequence, the food industry has been focused their efforts to obtain innovative functional products that satisfy this demand (Juriaanse, 2006; Khan et al., 2013). Indeed, one of the promising opportunity in this area lies in development of functional ingredients that offer multiple health benefits in a single food (Teratanavat & Hooker, 2006). The successful innovation in functional products is challenging and complex process highly related to the cost reduction by the incorporation of cheaper raw materials and the use of efficient and adaptive processing technologies (Betoret et al., 2011; Bigliardi & Galati, 2013). Therefore, the cheese whey, a co-product of the dairy industry, potentially rich in lactose, proteins and minerals, is gained increasing attention to be valorized into functional ingredient, which in turn allows avoiding the related environmental problems of whey. Moreover, it is necessary to develop efficient technologies able to reduce the cost of the process, treatment time and the eliminating of the use of reagents, especially when these are toxic. Recent advances in the EA as an emerging technology offers attracting opportunity to promote the development of whey as a functional ingredient in respect of the environmental friendly concept.
Thus, since this review is about the valorization of whey as a functional ingredient with potential prebiotic activity, an overview about probiotics and prebiotics is described. Subsequently, insight into nutritional and biological benefits of whey, the derived bioactive ingredients with special emphasis on lactulose, bioactive peptides and Maillard reaction products, their properties as well as potential uses is given.
6
1.1.1 Probiotics
1.1.1.1 Probiotics concept
Ilya Ilyich Mechnikov, Nobel Prize in Physiology or Medicine 1908, evoked for the first time the concept of «Probiotics» and proposed that yoghurt had beneficial health effects because of the lactic acid bacteria it contains and played a major role in maintaining intestinal health and prolonging life expectancy of Balkans (Anukam & Reid, 2007; Gibson, 1999). The term probiotic comes from the Greek words "pro bios" meaning "for life", and was first proposed in 1965 by Lilly and Stilwell. Later, Fuller and Gibson (1997) focused on viability of probiotics and introduced the idea that they have a beneficial effects on the host (Guarner et al., 2005). The current definition is the one adopted by experts of the commissions' Food and Agriculture Organization United Nations (FAO) and the World Health Organization (WHO) “live microorganisms
which when administered in adequate amounts; exert a beneficial effect on the host's health”(FAO/WHO, 2002). However, the inclusion of the word “live” in definition was
controverted, when positive effects were reported for the same dead cells (Adams, 2010). Recent advances also widened the probiotic to probioactive concept “probiotic-derived bioactive”, there would be to the generation of new bioactive compounds and no further need to have longer shelf-live bacteria. Given these prerequisites, the beneficial health of probiotic bacteria can resulted from the generation of new bioactive compounds derived from both bacterial metabolisms and/or hydrolysis of food matrix (Farnworth & Champagne, 2010). In the International Scientific Association for Probiotic and Prebiotic (ISAPP) consensus meeting stated in October 2013, the expert panels continue to untimely restrict the selection of potential probiotic to viable state and the necessary to obtain claims recognition (Hill et al., 2014; Kumar et al., 2015). Thus, bioactive bacterial products or lysed cells that also reported to modulate the immune response were eliminated from the probiotic concept.
1.1.1.2 Commercially used probiotic foods
Probiotic bacteria are available in the market and consumed as either food products or dietary supplements in the form of tablets or capsules, and they represent important segment in the modern functional foods market (Granato et al., 2010b;
7
Stanton et al., 2005). Probiotics dairy products, especially yogurts and cheese were shown to be the most consumed, since they exhibit good delivery carriers for maintaining the viability of these bacteria (da Cruz et al., 2009; El-Dieb et al., 2012). Moreover, the market is diversified to non-dairy products such as cereals, chocolate bars, biscuits, drinks and even chewing-gum added probiotics (Foligné et al., 2013). The commonly probiotics are from bifidobacteria and lactobacilli genera that considered to be safe based on their historical presence in human gut and foods (Figure
1.1). Other less commonly species belonging to the genera Streptococcus, Escherichia
coli, Lactococcus, Enterococcus and Saccharomyces yeast are also used as probiotics
due to their safety and great health potential (Holzapfel et al., 2001; Kumar et al., 2016).
Figure 1.1: Some most used probiotic species (Holzapfel et al., 2001; Kumar et al.,
8
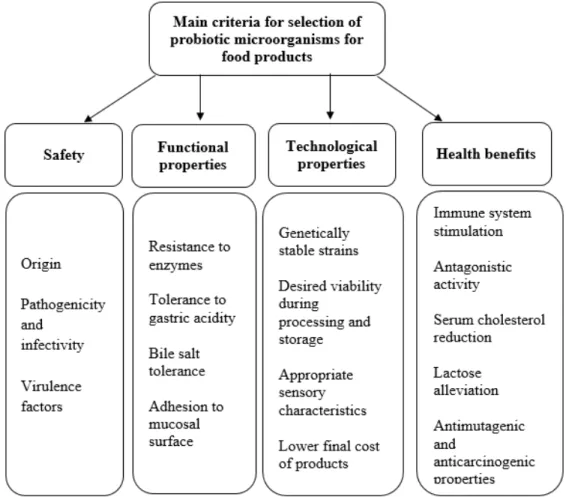
1.1.1.3 Selection of potential probiotics
The appropriate selection of probiotic strains is a curial step to ensure the desirable health benefits and a list of potential criteria appears in Figure 1.2. It must meet security, functionality, good technological properties and the claimed health benefits (FAO/WHO, 2002). The initial screening of probiotics includes the safety of the microorganisms. Lactobacillus and Bifidobacterium strains that generally have the status of "GRAS" (Generally Recognised As Safe) are widely recognized as probiotics. However, the presence of plasmids carrying antibiotic resistance genes and /or virulence factors reduces the security aspects (Saarela et al., 2000; Vesterlund et al., 2007; Zhou et al., 2005).
Figure 1.2: Desirable criteria for the selection of probiotics in commercial applications
9
The viability and metabolic activity are the main features to ensure the functionality of probiotic strains and to finally colonize their specific locations (Mortazavian et al., 2012). Health Canada recommends a daily dose of 109
colony-forming units (CFU) at time of consumption (Champagne et al., 2011). The probiotic orally ingested in foods must remain viable and resist to the harsh conditions encountered during gastrointestinal digestion, in particular amylases, pH acidity of the stomach, pancreatic enzymes and bile salts (Hernandez-Hernandez et al., 2012). The ability of selected probiotic to survive to this stressed environment is species and strains-dependant (Huang & Adams, 2004; Tamime et al., 2005). Charteris et al. (1998) studied the sensitivity of several probiotic strains in the gastrointestinal simulator. These authors found that the majority of strains, except Lactobacillus. fermentum KLD, were susceptible to resist to the gastric environment. The strains of Bifidobacterium and Lactobacillus are mostly resistant to enzymes encountered during intestinal transit.
Lactobacillus acidophilus La-5 as well as Lactobacillus. johnsonii La-1 were more
resistant than Lacobacillus. casei strain and Lactobacillus. rhamnosus GG to bile salts. In addition, there is an induction of resistance of Lactobacillus. rhamnosus GG to the gastrointestinal environment with certain ingredients of the food matrix (Sumeri et al., 2008). The probiotics adhesion capacity to the intestinal mucosa is also required to guarantee the survival of the probiotics into the gut and then to exert their beneficial action (Ouwehand & Salminen, 2003). Some probiotic strains demonstrated to have the ability to exclude the pathogenic microbiota through a competition for the same site adhesion at the intestinal mucosa (Collado et al., 2007). Further functional properties such as their ability to grab nutrients, lactic acid production, and secretion of antimicrobial metabolites such as bacteriocins and organic acids are required to the selection of suitable probiotic strains (Saad et al., 2013; Saarela et al., 2000).
Moreover, for their industrial applications, probiotics must demonstrate their technological ability to grow at large scale, remain stable at high survival levels during processing and storage (Champagne et al., 2011; Saarela et al., 2000). It is important that the selected strains involved in improving the organoleptic and textural qualities of the final probiotic product (Champagne et al., 2005; Granato et al., 2010a). The acidifying activity of probiotics exerts a protective effect against the spoilage flora and
10
improves its sensory qualities (Donkor et al., 2006). The production of exopolysaccharides by some probiotic strains improves the rheological properties of yoghurt and reduces syneresis (Badel et al., 2011). The ability of probiotic bacteria to release bioactive molecules such as conjugated linoleic acid (CLA), γ-aminobutyric acid (GABA), bioactive peptides and other molecules is very suitable from the industrial point of view (Coakley et al., 2003; Gobbetti et al., 2010; Stanton et al., 2005). In the processing of fermented dairy products, selecting the most biocompatible combination of starter and probiotic cultures has to be chosen in the regard of the strains growth and the final quality of the product (Gardner-Fortier et al., 2013; Vinderola et al., 2002).
1.1.1.4 Mechanisms of action of probiotics
Probiotics have demonstrated their potential as therapeutic agents for a variety of illnesses but the mechanisms associated with the probiotic activity have not been fully elucidated yet. Several operating mechanisms have been highlighted and are schematically summarized in Figure 1.3 (O'Toole & Cooney, 2008). Probiotics mainly act in the gut homeostasis by excluding the pathogens invasion by competition with them for limiting nutritional resources, reduction luminal pH or production of antimicrobial substances and receptor adhesion in mucosal and epithelial cells (Ng et al., 2009; Sarkar & Mandal, 2016). It has been reported that one of the efficient mechanisms of bifidobacteria in the gut is by sequestering iron, thus causing deprivation for enteropathogenic bacteria involved in infectious diseases (Vazquez-Gutierrez et al., 2015). Selective fermentation of dietary carbohydrates by intestinal probiotics resulted in metabolites of physiological importance such as short chain fatty acids (SCFAs), acids and carbon dioxides which play role in the reduction of luminal pH, consequently inhibiting the growth of acid-sensitive pathogens (Fukuda et al., 2011; Yasmin et al., 2015). Probiotics also release antibacterial products referred to as bacteriocins and de-conjugated salts leading to intestinal homeostasis (Bermudez-Brito et al., 2012; Ng et al., 2009). In addition to direct antibacterial mechanisms, probiotics contribute to enhance the epithelial barrier by competitive exclusion of pathogens and toxins trough expression of the analogue trans-membrane or establishing biofilms and
11
surfactants (Oelschlaeger, 2010; Sherman et al., 2009). Probiotics promote intestinal epithelial cell survival and restore barrier integrity after damages by enhanced the expression and the redistribution of tight junction proteins (Bermudez-Brito et al., 2012). Probiotics can also mediate their positive immunomodulatory effects by enhancing and modulating pathogen induced inflammation related in inflammatory bowel diseases like ulcerative colitis and Crohn’s disease (Chong, 2014). Probiotics exert immunomodulatory effects through the expression of signaling molecules, which interact with the immune cells by pattern recognition receptors (PRRs) like the well-known toll-like receptors (TLRs). Thus, immune response may be even achieved by probiotics-derived signaling molecules such as exopolysaccharides, teichoic acids, peptidoglycan fragments or DNA-like peptidoglycan, and (Oelschlaeger, 2010; Sarkar & Mandal, 2016). Moreover, probiotics may regulate the immune responses by enhancing the innate immune molecules including mucins, trefoil factors and defensins (Sherman, Ossa et al. 2009). Current reach also suggests that bacteria in the gut could have a direct effect on the central nervous system via the vagus nerve, even in the absence of an immune response (Gayathri & Rashmi, 2017) . For example, in the mouse, the consumption of Lactobacillus. rhamnosus JB-1 modulate the central GABA receptors and reduced the anxiety (Bravo et al., 2011). Thus, the understand of the mechanisms of action and the appropriate probiotics enhances their effectiveness application for the prevention and treatment of a certain diseases (Bermudez-Brito et al., 2012).
12
Figure 1.3: Schematic diagram illustrating potential or known mechanisms whereby
probiotic bacteria might affect the microbiota. These mechanisms include (1) competition for dietary ingredients as growth substrates, (2) bioconversion of, for example, sugars into fermentation products with inhibitory properties, (3) production of growth substrates, for example, EPS or vitamins, for other bacteria, (4) direct antagonism by bacteriocins, (5) competitive exclusion for binding sites, (6) improved barrier function, (7) reduction of inflammation, thus altering intestinal properties for colonization and persistence within, and (8) stimulation of innate immune response. IEC: epithelial cells, DC: dendritic cells, T:T-cells (O'Toole & Cooney, 2008).
1.1.1.5 Beneficial health effects of probiotics
A wide array of health benefits have been attributed to the consumption of probiotics in adequate amount, including improvement of intestinal disorders, enhancement of the immune response, alleviation of lactose intolerance and reduction of serum cholesterol as well as prevention of colon cancer (Agarwal et al., 2016; Gill & Prasad, 2008; Kechagia et al., 2013). Figure 1.4 summarizes the multiple benefits of probiotics on human health (Parvez et al., 2006). Health attributes of probiotics can be exerted locally in the gastrointestinal gut or systemically thought out the body (Kumar et al., 2016). There are a documented benefits of Lactobacillus. rhamnosus GG
13
in treating several forms of diarrhea, including rotavirus diarrhea, travelers’ diarrhea, relapsing Clostridium difficile diarrhea as well as to prevent antibiotic associated diarrhea in children (McFarland, 2006; Szajewska et al., 2013). Other probiotics such as Lactobacillus. reuteri DSM 17938 and Saccharomyces boulardii have been shown to be effective for the treatment of acute gastroenteritis (Guandalini, 2008). The consumption of some probiotic yogurts was related to improve lactose digestion and to lower serum cholesterol levels through de-conjugation of bile salts (Shah, 2007). There are also agreements relating to the effectiveness of Lactobacillus. rhamnosus GG and
Bifidobacterium. animalis subsp lactis Bb12 to reduce the severity of atopic eczema
(Doege et al., 2012; Isolauri et al., 2000). While some of the health benefits are well established, others require additional studies in human to substantiate these benefits (Rijkers et al., 2011). Promising applications of probiotics include the prevention of respiratory and urogenital infections, prevention of dental caries and treatment of inflammatory bowel diseases (Anderson et al., 2017; Chapman et al., 2011; Gille et al., 2016; Stamatova & Meurman, 2009). More studies are anticipated for future applications of probiotics in the treatment of rheumatoid arthritis, management of depression, prevention of cancer diseases, and treatment of diabetes as well as using as vaccine adjuvants (Gayathri & Rashmi, 2017; Sun & Buys, 2016; Tonucci et al., 2017; Zoumpopoulou et al., 2017). These health properties are highly species and strain specific and impacted by the various mechanisms of action mentioned above (Hill et al., 2014; Sánchez et al., 2016). The convincing dose therapeutic effect is not precisely defined because the strains do not survive in the same level to different stresses encountered during their manufacturing and through the gastrointestinal passage (Reid 2008). Many of these probiotics are being delivered through dairy products, of which yogurt is the most deliverer carrier used (Marco et al., 2017; Pei et al., 2017; Reid, 2015).
14
Figure 1.4: Health benefits from probiotic consumption (Parvez et al., 2006).
1.1.1.6 Factors affecting the survival of probiotics in dairy products
Dairy products are considered the most suitable carriers for delivering probiotic cultures to the human gastrointestinal tract (Granato et al., 2010a; Mortazavian et al., 2012; Ranadheera et al., 2010). There are mainly incorporated into products such as cheese, yogurt, beverage, ice cream, and other dairy desserts (Ranadheera et al., 2010). The application of these cultures in dairy products represents a huge challenge.The probiotics may lose their viability and metabolic activity due the unfavorable environment conditions during production and over storage period (Champagne, 2009; Ross et al., 2005). Thus, factors such as chemical composition of product where they are added, milk solid content, addition of salts, sugars and sweeteners, properties of strains used and their interactions with the starter cultures, moment and proportion of inoculation, content and availability of nutrient, temperature and duration of
15
fermentation, redox potential and dissolved oxygen, pH and titrable acidity, concentration of final metabolites as well as storage temperature might significantly affect the viability of probiotic cultures (Dave & Shah, 1997b; Kneifel et al., 1993; Lourens-Hattingh & Viljoen, 2001; Mattila-Sandholm et al., 2002; Mortazavian et al., 2011; Shah, 2000; Talwalkar & Kailasapathy, 2004; Vinderola et al., 2002). Probiotic cultures, in particular, L. rhamnosus and bifidobacteria usually exhibited weakly growth in milk due to their slow proteolytic activity and lack of some vitamins, as well as to their low lactase levels (Gaudreau et al., 2005; Roy, 2005). During fermentation and storage of bio-yogurt, these microorganisms are considerably affected by the decrease of pH which may reach values as low as 3.6 (Lankaputhra et al., 1996). Lactobacilli are generally more acid tolerant than bifidobacteria since their growth is inhibited below pH value of 4.6 (Lee & Salminen, 2009; Shah et al., 1995). Moreover, during the refrigerated storage, uncontrolled growth of some L. delbrueckii ssp.
bulgaricus at low pH results in excessive production of organic acids termed
post-acidification that may further affect the viability of acid-sensitive probiotics (Donkor et al., 2006; Shah & Ravula, 2000). Another main stress that probiotic encountered in fermented dairy products is their exposition to oxygen toxicity (Dave & Shah, 1997b; Talwalkar & Kailasapathy, 2004). It is well known that oxygen easily penetrates and dissolves during production, in addition to oxygen permeation through the packaging (Lourens-Hattingh & Viljoen, 2001; Shah, 2000). Lactobacilli are aerotolerant or anaerobic, while bifidobacteria are strictly anaerobic, thus the oxygen content and the redox potential have significant importance in maintaining their survival, when the oxygen-scavenging system in these bacteria is either reduced or completely absent (Holzapfel et al., 2001). Consequently, when water is present, oxygen is reduced to form toxic oxygenic metabolites such as hydrogen peroxide (H2O2), superoxide anion
(O2-) and the highly toxic hydroxyl radical (OH-) which lead to the cell death
(Talwalkar & Kailasapathy, 2004). Moreover, in the presence of oxygen, some L.
delbrueckii ssp. bulgaricus can produce H2O2 and may indirectly affect the viability of
probiotic bacteria during storage (Shah, 2000). The conservation of probiotic dairy products at low temperature is also a matter of concern. Cold temperatures reduce the membrane fluidity as well as influence the strain multiply and increase sensitiveness
16
toward sodium chloride, which may cause perturbation in the membrane integrity (Corcoran et al., 2008).
Thus, many alternatives have been adopted to enhance the viability of probiotics, the most commonly being stress adaptation, two step-fermentation, microencapsulation as well as fortification of milk with different growth and promoting factors such as hydrolyzed protein, whey derivatives, amino acids, with recently emphasis on prebiotics and antioxidant compounds (Champagne, 2009; Dave & Shah, 1998; Dave & Shah, 1997a; Gouin, 2004; Mattila-Sandholm et al., 2002; Mohammadi & Mortazavian, 2011; Mortazavian et al., 2012; Romano et al., 2015).
1.1.2 Prebiotics
1.1.2.1 Prebiotics concept
Currently, there is a great deal of interest in the use of prebiotics as functional food ingredients as they combine health benefits and interesting technological properties (Al-Sheraji et al., 2013; Charalampopoulos & Rastall, 2012; Roberfroid, 2002). The concept of prebiotics was introduced at the end of the 20th century and
originally was defined as "selectively fermented ingredient that induces specific
changes in the composition and/or the intestinal flora, conferring benefits to health and well-being of the host" (Gibson & Roberfroid, 1995). Based on this definition, the most
known prebiotics are carbohydrates such as fructooligofructoses (FOS), inulin, galactooligosaccharide (GOS) and lactulose, which resist digestion and can, metabolized by health promoting colonic microflora, especially by bifidobacteria and lactobacilli (Fuller & Gibson, 1997; Gibson, 2004; Gibson et al., 2004). At the 6th ISAPP Meeting in 2008, the more recently definition of “dietary prebiotics” was updated as “a selectively fermented ingredient that results in specific changes in the
composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health”. The most important emphasis is toward the extension of
the physiological benefits dominated by the gastrointestinal health to more targeted sites such as the oral cavity, skin and the urogenital tract (Gibson et al., 2010). Moreover, in the light of the current knowledge on the ecological and the functional features of microbiota, Bindels et al. (2015) widen the concept of prebiotics and
17
included more compounds as potential prebiotic candidates such as resistant starches, pectin, arabinoxylan, whole grains and non-carbohydrate compounds such as polyphenols and bioactive peptides.
1.1.2.2 Criteria of prebiotics
There are many candidates for prebiotic name, however it must fulfilled some criteria: (i) Resistance to gastric activity and resistance to hydrolysis by mammalian enzymes, (ii) No absorption in the upper gastrointestinal tract, (iii) Fermentation by the intestinal microbiota and (iv) Selective stimulation of the growth and/or activity of intestinal bacteria believed to be beneficial to the host (Gibson et al., 2004). The selectivity is the most difficult criterion to demonstrate. It is generally accepted that prebiotics have potentially selective effect on bifidobacteria and lactobacilli genus said to be beneficial for health, whilst decreasing bacteroides, clostridia and fusobacteria branded detrimental (Rastall & Gibson, 2015). However, the selectivity criterion is not assumed as previously when strains belonging the pointing detrimental groups such as
Faecalibacterium prausnitzii and Akkermansia muciniphila have shown to be
beneficial and to metabolize specific prebiotics (Hutkins et al., 2016). Prebiotics are also appreciated for their technological properties (Huebner et al., 2007; Wang, 2009). Thus, their stability during the product processing and their contribution to improve organoleptic and functional properties of the foods is another potential prebiotic selection criterion (Al-Sheraji et al., 2013; Charalampopoulos & Rastall, 2012).
1.1.2.3 Sources, production of prebiotics
The most food-grade utilized prebiotics are carbohydrates with different degree of polymerisation. These include (FOS), inulin type-fructans, (GOS) and lactulose (Gibson, 2004). Moreover, these is an emerging list of a potential prebiotics including lactosucrose, isomalto-oligosaccharides (IMO), xylo-oligosaccharides (XOS), resistant starch and soybean oligosaccharides (SOS) (Charalampopoulos & Rastall, 2009). Some prebiotics are naturally present in common foods including milk, vegetables and fruits such as asparagus, chicory, leek, banana, wheat and soybean (Fu & Wang, 2013). However, their content is generally low to engender the desirable bifidogenic effect
18
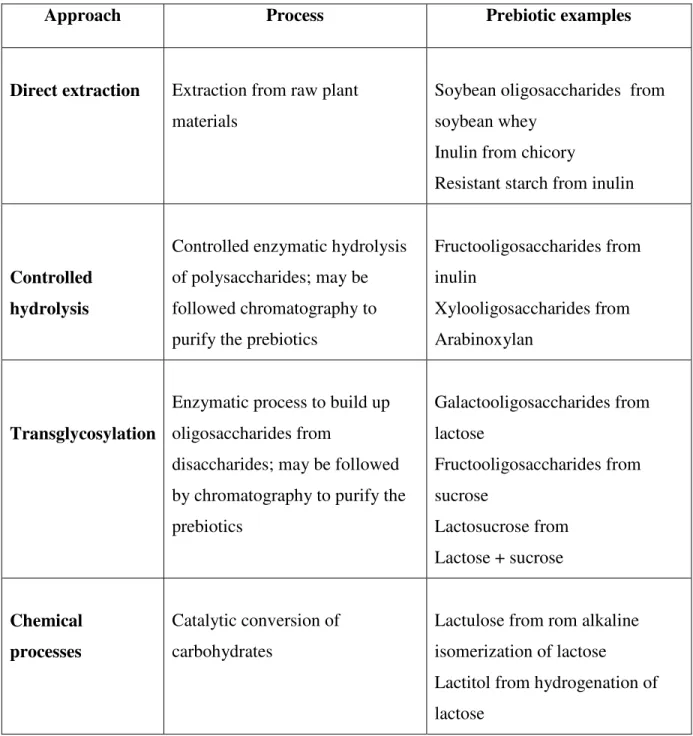
and need more subsequent manufacturing steps (Michalak et al., 2014). The usually used methods for prebiotics production are illustrated in Table 1.1. For examples, inulin and soybean oligosaccharides are produced by direct extraction in aqueous media. The FOS are manufactured from the partial enzymatic hydrolysis of inulin or synthesised from sucrose using a fructosyltransferase enzyme. Industrially, lactulose is mainly manufactured from lactose trough chemical isomerisation using alkaline catalysts. The GOS and lactosucrose are another prebiotic compounds produced from lactose by transglycosylation with ß-galactosidase and ß-fructofuranosidase enzymes, respectively. These prebiotics are available in the market with different purity degree as powders or syrups and formulated as supplements or incorporated as a functional ingredients in various food products (Charalampopoulos & Rastall, 2009).
19
Table 1.1: Main approaches used for the production of prebiotic carbohydrates (Playne
& Crittenden, 2009).
Approach Process Prebiotic examples
Direct extraction Extraction from raw plant materials
Soybean oligosaccharides from soybean whey
Inulin from chicory
Resistant starch from inulin
Controlled hydrolysis
Controlled enzymatic hydrolysis of polysaccharides; may be followed chromatography to purify the prebiotics
Fructooligosaccharides from inulin
Xylooligosaccharides from Arabinoxylan
Transglycosylation
Enzymatic process to build up oligosaccharides from
disaccharides; may be followed by chromatography to purify the prebiotics Galactooligosaccharides from lactose Fructooligosaccharides from sucrose Lactosucrose from Lactose + sucrose Chemical processes Catalytic conversion of carbohydrates
Lactulose from rom alkaline isomerization of lactose
Lactitol from hydrogenation of lactose
20
1.1.2.4 Beneficial health effects of prebiotics
The most health promoting effects of prebiotics are believed to come through the modulation of the gut microbiota towards a more beneficial microbiota composition and their fermentation metabolites (Verbeke et al., 2015; Verspreet et al., 2016). The beneficial effects of prebiotics include an increase of good bacteria and a decrease of detrimental bacteria in the gut, alleviation of constipation, treatment of hepatic encephalopathy, prevention of infections, increased absorption of minerals, regulation of blood lipids and reduced of cancer risk (Al-Sheraji et al., 2013; Roberfroid et al., 2010; Slavin, 2013). FOS and inulin prebiotics have shown to stimulate bifidobacteria growth and to reduce harmful bacteria in the human colon (Gibson & Roberfroid, 1995). Prebiotics have shown protective effects against infections by exerting anti-adhesive activity and inhibit binding of pathogens to the intestinal cells (Rastall et al., 2005). FOS can directly stimulate bacteriocin production from Lactobacillus and
Lactococcus strains (Chen et al., 2007). Indeed, the fermentation of prebiotics in colon
leads to the increase in the production of short-chain fatty acids (SCFAs) with various physiological effects. They low the pH of the colon making the growth difficult for some pathogenic bacteria (Wang & Gibson, 1993). In addition, the acidification of the colon leads to increased mucin production and reinforced the mucosal barrier and in this way decreasing translocation of pathogen bacteria. The low pH also reduces the formation of toxic compounds such as ammonia, biogenic amines, and phenolic compounds (Lomax & Calder, 2009). The SCFAs including acetate, propionate and butyrate exert important physiological effects on colonic epithelium as a source of energy for the colonocytes as well as for immune system cells (Bermudez‐Brito et al., 2015). Prebiotics are also known to interact with carbohydrate receptors on immune cells in Peyer’s patches, to increase the cytotoxicity of natural killer cells and to increase the production of IgA and various interleukins (Tuohy, 2008). Increasing SCFAs has been also associated with improving availability of minerals in the colon, which can be beneficial in preventing osteoporosis and avoiding diet-related anaemia. Calcium and magnesium absorption has been found to be increased following ingestion of GOS (Chonan et al., 2001). Indeed, inulin has been proved to reduce the plasma levels of cholesterol and triacylglycerols and different mechanisms on lipid metabolism