HAL Id: hal-01466246
https://hal.archives-ouvertes.fr/hal-01466246
Submitted on 13 Feb 2017
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Ionogel-based solid-state supercapacitor operating over a
wide range of temperature
Léo Nègre, Barbara Daffos, Viviane Turq, Pierre-Louis Taberna, Patrice
Simon
To cite this version:
Léo Nègre, Barbara Daffos, Viviane Turq, Pierre-Louis Taberna, Patrice Simon. Ionogel-based
solid-state supercapacitor operating over a wide range of temperature. Electrochimica Acta, Elsevier, 2016,
vol. 206, pp. 490-495. �10.1016/j.electacta.2016.02.013�. �hal-01466246�
O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 16651
To link to this article : DOI:10.1016/j.electacta.2016.02.013
URL :
http://dx.doi.org/10.1016/j.electacta.2016.02.013
To cite this version :
Nègre, Léo and Daffos, Barbara and Turq,
Viviane and Taberna, Pierre-Louis and Simon, Patrice
Ionogel-based solid-state supercapacitor operating over a wide range of
temperature. (2016) Electrochimica Acta, vol. 206. pp. 490-495.
ISSN 0013-4686
Any correspondence concerning this service should be sent to the repository
administrator:
staff-oatao@listes-diff.inp-toulouse.fr
Ionogel-based
solid-state
supercapacitor
operating
over
a
wide
range
of
temperature
L.
Negre
a,b,
B.
Daffos
a,b,
V.
Turq
a,
P.L.
Taberna
a,b,
P.
Simon
a,b,*
aCIRIMAT,UniversitédeToulouse,CNRS,UT3,118routedeNarbonne,31062ToulouseCedex,France bRéseausurleStockageElectrochimiquedel’Energie(RS2E),FRCNRS3459,France
ABSTRACT
Aninorganicgelpolymerelectrolytebasedontheconfinementofanionicliquidmixture(1:1byweight ormolarratio)ofN-methyl-N-propylpiperidiniumbis(fluorosulfonyl)imide(PIP13FSI)and N-butyl-N-methylpyrrolidiniumbis(fluorosulfonyl)imide(PYR14FSI)intoaSiO2matrixpreparedfromasol-gel
methodwaspreparedandfurtherusedaselectrolyteinanallsolid-statesupercapacitor.Thesynthetized ionogelexhibitsahighionicconductivityoverawidetemperaturerange(from0.2mScm!1at!40"Cup
to10mScm!1at60"C).Theionogel-basedsupercapacitorusingtwoactivatedcarbonelectrodescanbe
operatedover3Vcellvoltagewindow.Moreoverthisallsolid-supercapacitorshowsacapacitanceupto 90Fg!1atroomtemperature.Theseencouragingresultsshowtheinterestofdevelopingsuchdevices,
includingnon-toxicandsaferelectrolytes,packagingissuesandflexibledevicesdevelopment.
1.Introduction
Electrochemicaldoublelayercapacitors(EDLCs),alsoknownas supercapacitors, storeenergy by ion electrosorption onporous electrodes,mostlyactivatedcarbons[1].ThekeyfeatureofEDLCs compared to Li-ion batteries is their highpower delivery (full chargeordischargedcanbeachievedwithinfewsecondsortensof seconds) and high cyclability. Despite abounding research on pseudocapacitive materials with promising performance [2–4], conventionalEDLCsusinghighsurfaceareacarbonelectrodesand non-aqueousliquidelectrolytesrepresentthestateoftheart[5]. However, thecurrent liquid electrolytesusedin supercapacitor devices sufferfromseveral drawbacks.Conventional supercapa-citorsemploytheuseofsolvent-basedelectrolyteswhichallowion conduction at sub-zerotemperatures, e.g NEt4BF4 in propylene carbonate (PC)or acetonitrile (AN). Nevertheless,theseorganic solventsreducethevoltagewindowofthedeviceandANcanpose securityissuesduetoitsflammabilityandlowflashpointof2"C.
Electrolyteleaks,whichcanoccurbecauseofinternalgasrelease,is also animportantconcern.Since themaximumspecific energy increases with squared of the voltage window (EmaxðinWhÞ¼1
2&CðinFÞ&DV2maxðinVÞ
.
[1]),thetypeofelectrolyteused is important indetermining theenergydensity andsafety ofa device.Astrategytotackletheseissuesistoreplaceliquidbysolid
–orgelified–electrolytes.Severalmaterialshavebeenutilizedas solidpolymerelectrolytes,suchasNafion[6], poly(etheretherke-tone)[7],polybenzimidazole[8],poly(methylmethacrylate) [9]
andpoly(ethyleneoxide)[10]arepromisingcandidatesofferingas welleasyassembly,lowcost,andflexiblepackaging.However,the operatingvoltageislimitedtolessthan2Vbecauseoftheuseof protonor proton-like conductingpolymers[11].More recently, ionogels,whicharesolidorquasi-solidelectrolytesbasedonthe trappingofroomtemperatureionicliquids(RTILs)inasilicamatrix havebeenproposed[12–14]becausetheycanreachhighvoltage, up to 4V [15]. However, based on ionic liquid salt, the ionic conductivityat low temperatureis still anissuefor developing solid-state supercapacitors operating with a wide range of temperature.
Inthispaper,weproposetodevelopasolid-stateelectrolyte basedonaionicliquidmixturetrappedintoaninorganicsilica network. Following previous work [16], we used an mixture composed of (1:1 by weight or molar ratio) N-methyl-N-propylpiperidinium bis(fluorosulfonyl) imide(PIP13-FSI) and N-butyl-N-methylpyrrolidinium bis(fluorosulfonyl) imide (PYR14-FSI).Inthesemixtures,cationshavethesamemolecularweight andthesamenumberofatomsofthesamenature,withtheonly differencebeingtheircationmolecularstructure:afive-member (piperidinium)orsix-member(pyrrolidinium) heterocycle.Asa result,theincreasingdisorderandasymmetryamongthecations hinderslatticeformation,thustoloweringthemeltingpointwhile maintaininggoodmiscibilityandconductivity[17].Theliquidstate ofthisILmixturecanbemaintainedattemperaturesseveraltens
*Correspondingauthor.
E-mailaddress:simon@chimie.ups-tlse.fr(P.Simon).
ofdegreeslowerthantheindividualILs.Ionicliquidmixture-based ionogelhavebeendesignedforenlargingtheoperating tempera-ture range of these solid state electrolytes, thus offering new opportunitiesforsolid-statesupercapacitors.
2.Experimental
Ionogel synthesis procedure was described elsewhere [14]. Basically,asolidionogel-basedelectrolytewassynthetizedusing two different sol-gel agents: TMOS (TetraMethyl Ortho Silicate 98%,fromSigma–Aldrich)andTEOS(TetraEthylOrthoSilicate98%, fromSigma–Aldrich)underacidicconditions(Methanoicacid98%, fromVWR).TheTEOS:TMOS:MAvolumetric ratiowas 2:2:5.All reactantsweremixedtogetherundermoderatestirring(300rpm)
at40"Cduring18min.Thesecondstepwastheadditionof60%by
volumeof ionic liquidmixture(PIP13-FSI):(PYR14-FSI):50:50 in weight.Comparedtoourpreviouswork using1-ethyl-3-methyl imidazolium bis(trifluoromethanesulfonylimide) EMITFSI-based ionogel[14],ILcontentwasdecreasedfrom70%downto60%vol toimprovethemechanicalpropertiesofthegel.Then,theresulting solutionwascastedinsealedcylindricalcontainersandkeptfor 24hoursatroomtemperatureforgelationandthendriedat50"C
during4 days.Allthecells used forconductivitymeasurement wereassembledina
MBraüngloveboxoperatingunderargonatmosphere(O2and H2Ocontents lower than 0.1ppm) toavoid any contamination. Samplespreparedforfurthermechanicalcharacterizationswere injectedintostainlesssteelmolds,whichensuredgood reproduc-ibilityofmeasurements.
2.1.Supercapacitorcellassembly
Activematerialwasmadeof95wt%ofPICACTIFSC,astandard highspecificsurfaceareaactivatedcarbon(2300m2g!1)[18],and 5wt%ofPTFE(60wt%PTFEinwaterDuPontNemours,France)[19]. Carbonelectrodesweredriedoutinavacuumovenduring2hours then 250
m
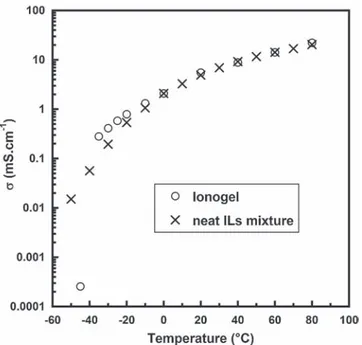
L of silane-ILmixture was spread ontoan electrode whichwaslaidinanappropriatemould,thenanotherelectrodeFig.1.ConductivityvariationversustemperatureofneatIlsmixture(PIP13-PYR14/ FSI)andionogelbetween!50"Cand80"C.Conductivitieswerecalculatedfrom
high frequency intercept with real axis of the Nyquist plotsobtained from ElectrochemicalImpedanceSpectroscopy(EIS)measurements.
wasdepositedontothesurfaceoftheliquidSilane-ILsmixture.The gel formation was obtained following procedure previously described. The resulting Carbon-Ionogel-Carbon sandwich was placed between two gold current collectors in a RHD cell for furtherelectrochemicaltests.Thistechniqueoffersawaytowork
with a large amount of active material (from few mg up to 15mgcm!2).
Electrochemicaltestswerecarried outusingan Autolab PG-STAT128N(Metrohm,Switzerland).ARHDCell(RHD,Germany) wasusedforprecisecontrolofthecelltemperaturefrom!40"Cto
+60"C.Prioranymeasurement,thecellwasmaintainedattheset
temperature for 2h until equilibration. After sandwiching the samplesbetweentwogoldcurrentcollectors,ionicconductivity measurementsoftheionogelwereperformedbyElectrochemical ImpedanceSpectroscopy(EIS)for each temperature;a biascell voltageof0Vwithafrequencyrangefrom25kHzdownto0.01Hz wereapplied.Conductivitieswerecalculatedfromhighfrequency interceptwiththerealaxisoftheNyquistPlots.Allelectrochemical testsof the supercapacitors wereperformed withan electrode massloadingof5mgcm!2.
Mechanical characterizations of theionogel wereconducted using an UltraNanoHardness Tester from CSM Instruments (Switzerland).Young’smodulusEandhardnessHandpercentage of elastic recovery were measured with a modified Berkovich three-sidedpyramiddiamondindenter.Themaximalnormalforce was 200
m
N; the maximum force was keptduring 60s before discharge.Theloadingratewas400m
Nmin!1andthemaximum penetrationdepthwas12m
m(valuelowerthanthetenthofthe sample thickness (1.5mm) thus the mechanical answer of the substratewasneglected).ThePoissonratioofthehybridcoatings wastakenatamediumvalueofy
=0.3(valuebetweenthePoisson ratioofamorphoussilica(0.17)andthatofconventionalpolymeric materials(0.5)).Experimentswereperformedatambient temper-ature.InallCSMnano-indentationtests,atotalofthreeindents wereaveragedtodeterminethemeanYoung'smodulusand nano-hardness.Theanalysis proceduresuggestedbyOliver andPharr[20]wereusedtocalculatethehardnessandelasticmodulus. 3.Resultsanddiscussions
3.1.Ionogelconductivity
Fig.1shows thechangeof theionogelandneat ILsmixture conductivitieswithtemperature(Arrheniusplot) between80"C
and!50"C.AscanbeseeninFig. 1,theionogelconductivityisclose
tothatof theneatIL mixturein the!20"C +60"C temperature Fig.3. CyclicVoltammetryofan ionogel-basedsupercapacitorusingactivated
carbonelectrodes,at5mVs!1atvarioustemperatures(from
!40to60"C).
Fig.4.EISplotof(A)supercapacitorusingtwocarbonglasselectrodesandanionogelsoakedseparatorbetween1MHzand200Hz(B)supercapacitorusingtwoactivated carbonelectrodesandanionogelsoakedseparatorbetween1MHzand0.1Hz.BiasVoltage:0V.Signalamplitude:5mVRMS.
Table1
Gravimetricandarealcapacitancecalculatedfromthe5mVs!1CVsduringthe
dischargeatdifferenttemperatures.
Temperature !40"C !20"C 0"C 20"C 40"C 60"C
Gravimetriccapacitance(Fg!1) 34 68 89 91 94 96
range,whichevidencesasmallimpactofthepresenceofthesilica matrix.Theroomtemperatureconductivity(20"C)wasmeasured
at5.5mScm!1forboththeneatILmixtureandtheionogel,which iscomparabletoconventionalpropylenecarbonate-basedliquid electrolytes[21]andmatcheswiththestandardsneededforuseas electrolytein supercapacitordevices. However,for temperature below!20"C,thelimitedmobilityoftheelectrolyteduetothe
confinementeffectinsidethesilicamatrixisassumedtolimitthe conductivity.A conductivity upto0.2 mS.cm!1was still main-tained at !40"C. Such high conductivity values at such low
temperature,toourknowledge,haveneverbeenreportedinthe literature for ionogel electrolyte. Nevertheless, these kind of electrolytes have been intended to suit to mechanical stress experiencesinceagoodmechanicalstabilityhastobe.Mechanical
tests were carried out using a nano-indentation technique to assessthemechanicalpropertiesofasmadeionogels.
3.2.Mechanicalcharacterizations
Mechanical properties of ionogels were measured by nano-indentation.Fig.2showstheloadasafunctionofthepenetration depth plotfor a 1.5mm-thick ionogelpellet usinga Berkovitch indenter.IonogelsexhibitratherlowvaluesofbothVickersHardness (0.11' 0.02MPa) and elastic modulus (0.935'0.114 MPa) as comparedto other gelelectrolytes: 26MPafor PMMA [22] and 35–50MPaforPVdF-basedpolymer[23].Theelasticcontributionto theloadingcanbeobtainedfromFig.2,bysubtractingtheplastic displacement to the total displacement normalized to the total
Fig.5.EISPlotofionogel-basedsupercapacitoratdifferenttemperature(a)!40"C,!20"Cand0"C(b)20"C,40"Cand60"C.Biasvoltage:0V.Signalamplitude:150mVRMS.
Fig.6.Evolutionofcapacitanceversuspotentialscanrateofionogel-basedsupercapacitorwithin3Vatdifferenttemperatures(A)!40"C,!20"Cand0"C(B)20"C,40"Cand
displacement.Anelasticrecoveryof45%wasmeasured,thathasto becomparedto14%forpureTEOSandTMOSsilicagel.Thetrapped ionicliquidisactingasaplasticizerintheresultingionogel. 3.3.Ionogelassolid-stateelectrolyteforsupercapacitor
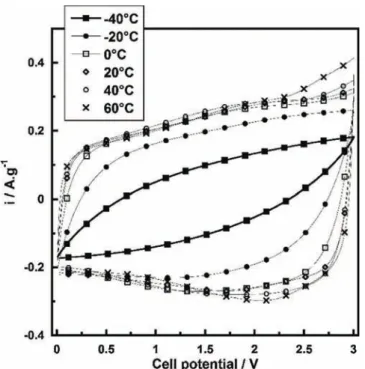
An all-solid supercapacitor was assembled using porous PICACTIF activated carbon based electrodes stacked with the ionogelaselectrolyte(seeexperimental).InFig.3arepresented CyclicVoltammogramsobtained(CV)at5mVs!1between0and 3Vatvarioustemperatures(from!40to60"C).Downto!20"C
theelectrochemicalsignaturesarequietdecentsincea rectangu-lar-shape characteristicwas achievedwhich was expectedfora capacitive behaviour based on charging/discharging of the electricaldoublelayer.
Aspecificcapacitanceof91Fg!1(pergramofactivatedcarbon) havebeenobtainedat20"Cwithacellvoltageof3V,whichisvery
similar to that was measured in conventional organic liquid electrolyte [19]. Table 1 summarizes gravimetric and areal capacitancescalculatedfromtheCVsatapotential scanrateof 5mVs!1between0and3Vfrom!40to60"C.
Asobservedinthistable,thecapacitivebehaviouriskeptdown totemperatureaslowas!20"C;withonly25%capacitancelossat
!20"C.TheseresultsareconsistentwithFig.1thatshowedthat
ionogelionicconductivitywasstilldecent(0.2mScm!1)atsuch lowtemperature.At!40"C,thecrystallizationoftheILconfinedin
pores of thecarbon structure is assumed to occur. Despite an electrochemicalkineticsmainlycontrolledbyohmicdrop,40%of thecapacitanceobtainedat20"Cwaspreserved.Interestinglythe
electrochemicalbehaviourofall-solidsupercapacitorwasfoundto beveryclosetoliquid-basedsupercapacitors.Suchperformance couldbeexplainbyawettingcarbonmicroporebytheILthatcan movefreelywiththesiliciumnetwork.Tocheckthehypothese,we carriedouttodesignanadditionalexperimentusinganon-porous carbon(carbonglass)forcomparisonprupose.
Intheexperiment,an(EMITFSI)-basedionogelsoakedseparator wasusedtoobservethecarbon/ionogelinterface;EISmeasurements were madeeach30minbetween 1MHz–200Hz to measurethe change of theimpedance with time. Fig. 4A shows thesoaked separatorbetweentwocarbonglasselectrodes.Sameexperiment wasconductedwithtwoactivatedcarbonelectrodeplacedbetween thesameseparatorsoakedwithionogel(Fig. 4B).
While the impedance is kept constant for the non porous electrode(Fig.4B),therealpartoftheimpedanceincreaseswith timewhenusingporouscarbonelectrode.Thisisassumedtobe linked tothehydrophobicityof thecarbonwhichattracts ionic liquid (hydrophobic) from the silica host network (which is hydrophilic)intothecarbonpores;besides,capillarityforcecan alsocontributetoattractILinthepores.Thisbehaviourallowsa (partial) filling of the carbon microporous structure with ions leadingtosuchhighcapacitance.
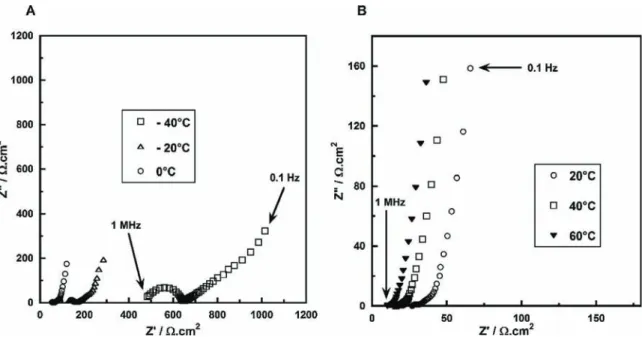
In Fig.5 arepresentedtheNyquist plotsof acarbon-carbon ((PIP13-FSI)0.5(PYR14-FSI)0.5) ionogelbased supercapacitorata biascellvoltageof0V.
In the 20"C ! 60"C temperature range (Fig. 5A), the high
frequencyseriesresistance,whichistheionicbulkresistance,was measured at 20
V
cm2 for thewholecell at roomtemperature(20"C), which is comparable to our previous work [14]. As
expected,whenthetemperatureincreasesfrom0"Cupto60"C,
thehighfrequencyresistancedecreasesfrom52to9.2
V
cm2.The sharp increase of the imaginary partof theimpedance at low frequencies ischaracteristic ofthesystem capacitivebehaviour. Evenfor sub-zerotemperatures,EIS measurementsstill showa capacitivebehaviour,despitethepresenceofagrowingsemi-circle athighfrequency,attributedtotheonsetofgelationthatisrelatedto decreased mobility of the electrolyte ions at such low temperatures.
Fig. 6showsthechangeofthearealandgravimetriccapacitance (derivedfromtheslopeoftheQ-Vcurveduringcelldischarge)of theactivatedcarbonelectrodesversusthepotentialscanrateat various temperatures. The capacitance of the ionogel-based supercapacitor decreases with increasing scan rate, since the ohmicdropincreaseswiththescan rate. Temperatureplaysan importantrole,sincealreadymentionedseriesresistanceincrease at low temperaturewhile a fastdecrease of thecapacitance is observedathigherscanrate.
Inhere,itwasexpectedsincecapacitancelimitationispurely due to ohmicdrop. The effective accessible surface is strongly dependentonthescanrate[17].
4.Conclusion
Thepresentpaperpresentssomeresultsobtainedwitha carbon-carbonsolid-statesupercapacitorusing((PIP13-FSI)0.5(PYR14-FSI) 0.5)-basedionogelasbothelectrolyteandseparator.Thankstohigh ionicconductivityoftheionogel,ionsfromthegelareabletofulfil porosityofthecarbonelectrodesleadingtosuchhighcapacitance (95Fg!1at room temperature). Anall solid supercapacitor with attractiveareal capacitance up to 100mFcm!2 per electrodeat
!40"Cfor5mVs!1scanrateupto 3Vcellvoltagecouldbewas
assembled.Suchdevicescouldefficientlymatchmultiplecriteria (high capacitance, good power density and high cell potential window)neededforsolidsupercapacitorapplications.
References
[1]P.Simon,Y.Gogotsi,Materialsforelectrochemicalcapacitors,Nat.Mater.7 (2008)845–854.
[2]P.Simon,Y.Gogotsi,B.Dunn,WhereDoBatteriesEndandSupercapacitors Begin,Science343(2014)1210–1211.
[3]V.Augustyn,P.Simon,B.Dunn,Pseudocapacitiveoxidematerialsforhigh-rate electrochemicalenergystorage,EnergyEnviron.Sci.7(2014)1597. [4]T.Brousse,P.-L.Taberna,O.Crosnier,R.Dugas,P.Guillemet,Y.Scudeller,etal.,
Long-termcyclingbehaviorofasymmetricactivatedcarbon/MnO2aqueous electrochemicalsupercapacitor,J.PowerSources173(2007)633–641. [5]J.R.Miller,P.Simon,MaterialsScience:ElectrochemicalCapacitorsforEnergy
Management,Science321(2008)651–652.
[6]B.C.Kim,J.S.Kwon,J.M.Ko,J.H.Park,C.O.Too,G.G.Wallace,Preparationand enhancedstabilityofflexiblesupercapacitorpreparedfromNafion/polyaniline nanofiber,Synth.Met.160(2010)94–98.
[7]P.Sivaraman,V.R.Hande,V.S.Mishra,C.S.Rao,A.B.Samui,All-solid supercapacitorbasedonpolyanilineandsulfonatedpoly(etheretherketone), J.PowerSources.124(2003)351–354.
[8]D.Rathod,M.Vijay,N.Islam,R.Kannan,U.Kharul,S.Kurungot,etal.,Designof anallsolid-statesupercapacitorbasedonphosphoricaciddoped
polybenzimidazole(PBI)electrolyte,J.Appl.Electrochem.39(2009)1097– 1103.
[9]M.Schroeder,P.Isken,M.Winter,S.Passerini,A.Lex-Balducci,A.Balducci,An InvestigationontheUseofaMethacrylate-BasedGelPolymerElectrolytein HighPowerDevices,J.Electrochem.Soc.160(2013)A1753–A1758. [10]C.V.SubbaReddy,G.P.Wu,C.X.Zhao,Q.Y.Zhu,W.Chen,R.R.Kalluru,
CharacterizationofSBA-15doped(PEO+LiClO4)polymerelectrolytesfor electrochemicalapplications,J.Non-Cryst.Solids353(2007)440–445. [11]A.A.Łatoszy!nska,G.Z. _Zukowska,I.A.Rutkowska,P.-L.Taberna,P.Simon,P.J.
Kulesza,etal.,Non-aqueousgelpolymerelectrolytewithphosphoricacid esteranditsapplicationforquasisolid-statesupercapacitors,J.PowerSources 274(2015)1147–1154.
[12] D.Membreno,L.Smith,K.-S.Shin,C.O.Chui,B.Dunn,Ahigh-energy-density quasi-solid-statecarbonnanotubeelectrochemicaldouble-layercapacitor withionogelelectrolyte,Transl.Mater.Res.2(2015)015001.
[13]M.Brachet,T.Brousse,J.LeBideau,AllSolid-StateSymmetricalActivated CarbonElectrochemicalDoubleLayerCapacitorsDesignedwithIonogel Electrolyte,ECSElectrochem.Lett.3(2014)A112–A115.
[14]L.Negre,B.Daffos,P.L.Taberna,P.Simon,Solvent-FreeElectrolytesfor ElectricalDoubleLayerCapacitors,J.Electrochem.Soc.162(2015)A5037– A5040.
[15]M.Armand,F.Endres,D.R.MacFarlane,H.Ohno,B.Scrosati,Ionic-liquid materialsfortheelectrochemicalchallengesofthefuture,Nat.Mater.8(2009) 621–629.
[16]R.Lin,P.-L.Taberna,S.Fantini,V.Presser,C.R.Pérez,F.Malbosc,etal., CapacitiveEnergyStoragefrom!50to100"CUsinganIonicLiquidElectrolyte, J.Phys.Chem.Lett.2(2011)2396–2401.
[17]W.-Y.Tsai,R.Lin,S.Murali,L.Zhang,J.K.McDonough,R.S.Ruoff,etal., Outstandingperformanceofactivatedgraphenebasedsupercapacitorsin ionicliquidelectrolytefrom!50to80"C,NanoEnergy2(2013)403–411.
[18]P.L.Taberna,P.Simon,J.F.Fauvarque,ElectrochemicalCharacteristicsand ImpedanceSpectroscopyStudiesofCarbon-CarbonSupercapacitors,J. Electrochem.Soc.150(2003)A292.
[19]J.Gamby,P.L.Taberna,P.Simon,J.F.Fauvarque,M.Chesneau,Studiesand characterisationsofvariousactivatedcarbonsusedforcarbon/carbon supercapacitors,J.PowerSources101(2001)109–116.
[20]W.C.Oliver,G.M.Pharr,Animprovedtechniquefordetermininghardnessand elasticmodulususingloadanddisplacementsensingindentation experiments,J.Mater.Res.7(1992)1564–1583.
[21]M.Ue,Conductivitiesandionassociationofquaternaryammonium tetrafluoroboratesinpropylenecarbonate,ElectrochimicaActa39(1994) 2083–2087.
[22]S.M.Zebarjad,S.A.Sajjadi,T.E.Sdrabadi,S.A.Sajjadi,A.Yaghmaei,B.Naderi,A StudyonMechanicalPropertiesofPMMA/HydroxyapatiteNanocomposite, Engineering03(2011)795–801.
[23]B.Mohammadi,A.A.Yousefi,S.M.Bellah,Effectoftensilestrainrateand elongationoncrystallinestructureandpiezoelectricpropertiesofPVDFthin films,Polym.Test.26(2007)42–50.