O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 19370
To link to this article : DOI:
10.1016/j.corsci.2017.02.022
URL :
http://dx.doi.org/10.1016/j.corsci.2017.02.022
To cite this version :
Gravina, Rosanne and Pébère, Nadine and Laurino, Adrien and
Blanc, Christine Corrosion behaviour of an assembly between an
AA1370 cable and a pure copper connector for car manufacturing
applications. (2017) Corrosion Science, vol. 119. pp. 79-90. ISSN
0010-938X
Any correspondence concerning this service should be sent to the repository
administrator:
staff-oatao@listes-diff.inp-toulouse.fr
ContentslistsavailableatScienceDirect
Corrosion
Science
jo u r n al ho m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / c o r s c i
Corrosion
behaviour
of
an
assembly
between
an
AA1370
cable
and
a
pure
copper
connector
for
car
manufacturing
applications
Rosanne
Gravina
a,b,
Nadine
Pébère
a,
Adrien
Laurino
b,
Christine
Blanc
a,∗aCIRIMAT,UniversitédeToulouse,CNRS,INPT,UPS,ENSIACET,4alléeEmileMonso,CS44362,31030Toulouse,France bLEONIWiringSystemsFrance,5avenuedeNewton,78180Montigny-le-Bretonneux,France
Keywords: A.Aluminium A.Copper B.EIS C.Crevicecorrosion C.Pittingcorrosion
a
b
s
t
r
a
c
t
ThecorrosionbehaviourofanassemblybetweenanAA1370cableandapurecopperconnectorforwiring harnesseswasstudiedinneutralchlorideandsulphatecontainingsolution.Electrochemicalimpedance measurementsshowedthatthecorrosionbehaviourofthecablewascontrolledbytheingressofthe elec-trolyteinsidecablecavities.Further,localimpedancemeasurementswereperformedontwoassembly cross-sections,i.e.withandwithoutcavitiesinthealuminiumcable.Theresultsprovidedevidencefor boththegalvaniccouplingbetweenaluminiumandcopperandthepresenceofcavitiesinthealuminium cableasrelevantexplanationsforthecorrosionbehaviouroftheassembly.
©
1. Introduction
Typicalwiringharnessesintheautomotiveindustryconsistof assemblybetweenCucableandCuconnector.Today,thepressure inenvironmentalregulationsledtosearchforsolutionsgivingrise tofueleconomyandreductioninCO2emission.Inthiscontext,car
manufacturersplannedtoreducebothcostandweightofwiring harnesses.OneinnovativesolutionisthesubstitutionofCubyAl alloys,suchasAA1370,incables.Inthelastyears,severalworks concernedthemanufacturingprocessesofthisnewtypeof assem-bly.Recently,differentmethodshavebeenproposedtoproduceAl strandswithductilityandfatiguecharacteristicsreliablefor auto-motivewiringharnesses[1–4]whileultrasonicweldinghasbeen usedtojoinAlcablewithCuconnector[5,6].However,onemajor problemforwiringharnessesconcernstheircorrosionresistance because,in service,wiring harnesses are exposedto aggressive media,suchasde-icingsalt,whichcangeneratecorrosiondamage. Numerousdataarereportedintheliteratureconcerningthe cor-rosionbehaviourof1xxxAlalloys.Themainresultsconcernedthe influenceofFe-richparticleswhichactascathodicsitesand pro-motefirstdissolutionofthesurroundingmatrixandthen,pitting corrosion[7–9].However,thecorrosionbehaviourofanAlcableis morecomplex.Intheliterature,onlyfewworkshavebeenreported
∗ Correspondingauthor.
E-mailaddress:christine.blanc@ensiacet.fr(C.Blanc).
concerningthecorrosionbehaviourofacable.XuandChen inves-tigatedthecorrosiondamageofwirespecimenspulledfromthe replacedcablesinShimenBridge[10].Theyanalysedthecorroded cablesfromthesheathbreakagetothecablecenterandexplained thattheextentofcorrosionatanypointofacablesectionwas con-trolledbyitsdistancefromthesheathbreakageduetothefluidthat couldpenetrateinsidethecable[10].IshikawaandKawakami[11] showedthepresenceofcavitiesinsideacableduetothe incom-pletepenetrationofthesheathrubber.Then,duringexposuretoan aggressivemedium,thesolutionpenetrateintothecablecavities leadingtotheformationofaconfinedenvironment.Dependingon theconfigurationoftheharness,e.g.numberofstrandarms con-stitutiveofthecableandspacebetweenthem,andtheintrinsic corrosionbehaviourofthestrand,theconfinedelectrolyte com-positionshouldevolverapidly,e.g.,oxygenconcentration,cations concentrationandpH,andtheninduceseverecorrosion phenom-ena,suchascrevicecorrosion[12–14].ChanelandPeberestudied themechanismsgoverningthedegradationofbrass-coatedsteel cords fortyresin a 0.25M Na2SO4 solution incontact withair
andmaintainedat25◦Cbyusingelectrochemicalimpedance
spec-troscopy(EIS)[15].TheyshowedthatEISwasasuitabletechnique toquantifythecorrosionresistanceofacable[15].Moreover,the assemblybetweenanAlcableandaCuconnectorshould gener-ategalvaniccorrosionduetothedifferenceincorrosionpotential valuesbetweenthetwometalsinaqueoussolution[16–21].Khedr andLashieninvestigatedthecorrosionbehaviourofpureAlinCu2+
richsolutionsandshowedtheacceleratingeffectoftheCu2+cations
http://dx.doi.org/10.1016/j.corsci.2017.02.022 0010-938X/©2017ElsevierLtd.Allrightsreserved.
AlcorrosionrateduetoCudepositionandsubsequentgalvanic coupling[17,18].Jorcinetal.[19]showed,foramodelcouple con-stitutedofAlandCu,thechemicaldissolutionofAlduetoapH increaseattheAl/Cuinterface;thisgeneratedanoccludedzonein whichthecompositionoftheelectrolyte(a10−3MNa
2SO4
solu-tionincontactwithairandatroomtemperature)evolvedrapidly. ThegalvaniccouplingbetweenAlandCuwasalsoinvestigatedina 10mMNa2SO4solutionbyusingathin-layercellinordertomodel
theoccludedzoneinthecrevice[20].Jomaetal.performed exper-imentsina0.1MNa2SO4solutionandshowedthatthechemistry
inathin-layercellplaysasignificantroleatleastatalocalscale [21].
The present work contributes to get a better insight of the mechanismsgoverningthedegradationofanAA1370cable/pure Cu assembly with a particular attention to the effect of the electrolyteingressinsidethecable.Conventionalelectrochemical measurements(Ecorrmeasurements,polarisationcurvesand
elec-trochemicalimpedancespectroscopy)werefirstcarriedoutforthe AA1370cableinordertoinvestigatethecorrosionbehaviourofthe cablealone.Giventhatthecablewasmadeofalargenumberof strands,theelectrochemicalmeasurementswerealsoperformed forAA1370strandstohaveabetterunderstandingofthe corro-sionmechanisms.Then,toinvestigatethecorrosionbehaviourof theAA1370cable–Cuconnectorassembly,localelectrochemical impedance(LEIS)measurementswereperformedontwo cross-sectionsof theassembly,onecross-section correspondingtoan AA1370cablewithcavitiesandtheotheronewithoutcavities.LEIS isanon-destructiveelectrochemicaltechniquethathasbeenused inrecentyearstostudylocalizedcorrosiononbimetallicsurface [19,22–26].Itwasusedheretodeterminetheinfluenceofthe cav-itiesintheAA1370cableafterweldingonthegalvaniccorrosion processesoccurringfortheAA1370cable/Cuconnectorassembly.
2. Experimental 2.1. Materials
TheassemblywasobtainedbyultrasonicweldingusingaDS 20-IIapparatusbetweenanAA1370(99.7%Al,0.072%Fe,0.0045%Mg, 0.045%Si;wt.%)cableandapureCu(99.90wt.%Cu;200–400ppm O2)connector.TheCuconnectorwasahotstampedCusheet.In
thefollowing,theAA1370cableintheassemblywillbereferred toas‘theAlpart’;theAA1370cable/Cuconnectorassemblywillbe referredtoasAl/Cuassembly.Duringtheweldingprocess,thecable (withoutthepolymershell)waspositionedontheCuconnectorand apressurewasexerted.Then,ultrasonicvibrationswereappliedso thatthecableandtheCuconnectormovedrelativelytooneanother withanoscillatingmovement.Thisledtoafrictionbetweenthetwo metalsandthentotheformationofaweldingzone.Thetotallength oftheweldedzonewasabout12mm.Thequalityofthewelding dependsmainlyontheappliedpressureandontheamplitudeof thevibrations.Therewasashortzone(8mm)wherethestrands constitutiveofthecablewereentirelyweldedtogethersothatthe cabledidnotrevealanycavitiesafterthewelding,thiszonebeing calledasthe‘effectiveweldedzone’.
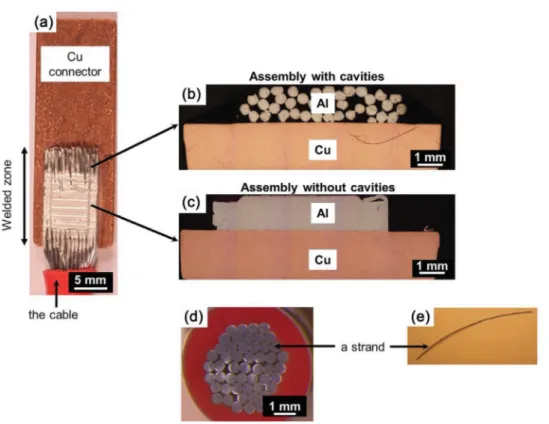
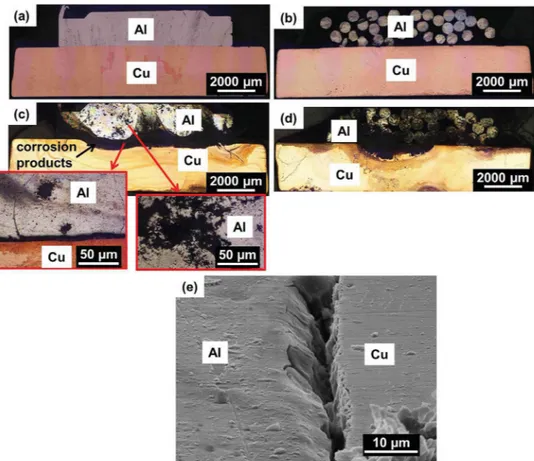
Thegalvanic couplingat theAl/Cu interfacewas studiedby electrochemicalmeasurementsperformedontwocross-sections oftheassembly.Fig.1ashows aglobalpictureoftheassembly. Thetwoselectedcross-sectionsareshowninFig.1bandc.Oneof thecross-sectionswasremovedfromaporouszoneofthecable due tothe fact that thestrands constitutive of the cablewere not entirelyweldedtogether (henceforthcalled ‘assemblywith cavities’)(Fig.1b).Anothercross-sectionwasremovedfromthe effectiveweldedzonewherenocavitieswereobservedbetween theweldedstrands(henceforthcalled‘assemblywithoutcavities’)
(Fig.1c).Beforecorrosiontests,thecross-sectionswereembedded inanepoxy-resinwithoutfillingthecavities.Thiswasachievedby embeddingthecross-sectionsinaparafilmshell.
Fora betterunderstandingofthecorrosionbehaviourofthe assembly, electrochemical measurements were also performed forcross-sectionsofboththeAA1370cablebeforewelding and AA1370strandsconstitutiveofthecable.Thecross-sectionswere perpendiculartothecable/strandaxis.Thecablewasanassembly of50strands(0.52mmdiameter)protectedbyaplastic insulat-ingsleeve.Fig.1dshowsanopticalobservationofacross-section ofacablewhere the50strandscanbeobserved.Itcanbeseen that thespacebetweenthestrands isnot uniform. Toperform theelectrochemicalmeasurements,thecablewasembeddedin anepoxy-resinwithoutfillingthespacebetweenthestrands(see theprocedurefortheassembly).Ifonlythesurface perpendicu-lartothecableaxiswastakenintoaccount,thesurfaceareaof thecross-sectionofthecablewith50strandsexposedtothe elec-trolyteduringtheelectrochemicalexperimentswas0.11cm2.To
studythecorrosionbehaviourofastrand,thedifficultywasits diameter,i.e.0.52mm.Threeelectrodeswerepreparedwiththe cross-sectionsof1strand(S=0.002cm2),4strands(S=0.008cm2)
and22 strands(S=0.047cm2)respectivelyexposed tothe
elec-trolyte.Forthe3electrodes,thestrandswereorganizedtoobtaina regulararrangementoftheircross-sections(theaxisofeach indi-vidualstrandbeingparalleltotheothers)andembeddedtogether inanepoxy-resin.Allthecavitiesbetweenthestrandswerefilled bytheepoxy-resin.Preliminaryexperimentsshowedthatsimilar resultswereobtainedforthe3electrodes.However,the repro-ducibilityofthemeasurementswasbetterfortheelectrodewith 22strandsduetothefactthatthesurfacewasmorerepresentative ofthealloymicrostructure.Inthefollowing,forbrevity,onlythe resultsfortheelectrodewith22strandsarepresented.This elec-trodewasreferredtoas‘strandsample’.Itcouldbenotedherethat thecorrosionbehaviourofthestrandsamplecouldbeconsidered representativeofthebehaviourofacableforwhichthecavities wouldbesealed.Electrochemical measurementswerealso per-formedforaCusampletakenoffaconnectorusedfortheassembly; thesurfaceexposedtotheelectrolytewas0.11cm2.
Beforeallelectrochemicaltests,thesurfaceofthespecimens wasmechanicallyabradedwithsuccessivegritSiCpapers(1200, 2400)andthenpolishedfrom6to1mmgradewithdiamondpaste anddeionisedwateraslubricant.
2.2. Electrochemicalmeasurements
Allelectrochemicalmeasurementswereperformedinsulphate and/orchloride-containingsolutions;theyareassumedtobe rep-resentativeoftheautomotiveenvironments.Severalauthorshave usedsulphateand/orchloride-containingsolutionstostudyAl/Cu galvaniccouplingallowingtheresultsobtainedinthisworktobe comparedwiththeirresults[20,21].Fortheconventional electro-chemicalmeasurements(Ecorrmeasurements,polarizationcurves
andimpedancemeasurements),thecorrosivemedium(pH=6.5) waspreparedfromdeionisedwaterbyadding0.1MNa2SO4 and
asmallconcentrationofchloride(0.001MNaCl).ForLEIS experi-ments,a0.001MNaClsolution(pH=6.5)preparedfromdeionised water was chosen to maintain a low conductivity required to optimize the measurements in the low-frequency range [27]. Theelectrolyte wasmaintained ata temperatureof 25◦C±1◦C
exceptfortheLEISmeasurementsperformedatroomtemperature (22◦C±1◦C).
Fortheconventionalelectrochemicalmeasurements,the exper-imentalset-upconsistedofathree-electrodecell,connectedtoa BiologicVSPapparatus,withalargeplatinumelectrodeusedas counterelectrodeandasaturatedcalomelelectrode(SCE)as ref-erenceelectrode.Specimensusedastheworkingelectrodewere
Fig.1.(a)GlobalviewoftheassemblybetweenAA1370cableandpureCuconnector;cross-sectionsoftheassemblies(b)withand(c)withoutcavities;(d)theAA1370cable and(e)astrand.
thestrandsampleandthecable.Forthetwocross-sectionsofthe assembly,onlyEcorrwasmeasuredforexposuretimestothe
elec-trolyterangingfrom1hto168h.In thefigures,meanvaluesof Ecorr aregivenonthebasisof atleasttenexperimentsforeach
experimentalcondition.Forthepolarizationcurves,thesamples werefirstexposedtotheelectrolyteatEcorrfor3handthen,the
anodicandcathodicpartswereobtainedindependentlyfromEcorr
atapotentialsweeprateof0.07mVs−1.Foreachsample,atleast
threecurveswereplottedtocheckthereproducibility.Impedance measurementswereperformedunderpotentiostaticconditionsat Ecorrwitha15mVpeak-to-peaksinusoidalperturbation.Frequency
wassweptdownwardsfrom65kHzto3mHzwith9pointsper decade.Severalimpedancediagramswererecordedasafunction oftimerangingfrom3hto168h.Allimpedancemeasurements wererepeatedthreetimestocheckthereproducibility.
The corrosion behaviourof the Al/Cu assembly wasstudied bylocalelectrochemicalimpedancespectroscopy(LEIS).The mea-surementswerecarriedoutwithaSolartron1287Electrochemical Interface,a Solartron1255B frequencyanalyser anda Scanning Electrochemical WorkstationModel 370(Uniscan Instruments). Thismethodusedafive-electrodeconfiguration.Theprobe(i.e., abi-electrodeallowinglocalcurrentdensitymeasurements)was steppedacrossaselectedareaofthesample.Theanalysed part had anareaof 8mm×14mmandthestepsizewas500mmin thexandydirections.Mapswereobtainedatafixedfrequency, choseninthepresentcaseat10Hz,andadmittancewasplotted ratherthanimpedancetoimprovethevisualizationoftheresult [28].Localimpedancediagramswererecordedoverafrequency rangeof65kHz–3Hzwith8pointsperdecade.Spectrawere plot-tedforthe2cross-sectionsoftheassemblyshowninFig.1bandc fromtheCuparttotheAlpartwiththeoriginofaxisbeingtheAl/Cu interface.Thetimetorecordallthelocalimpedancediagramswas lowerthan60min.ForalltheLEISmeasurements,thespatial reso-lutionwasabout1mm2,i.e.theanalysedsurfacewasabout1mm2
whenalocalimpedancediagramwasplotted[27].Fortheassembly
withcavities(Fig.1b),thecavitiescorrespondedto20%ofthe ana-lysedsurfacesothattheeffectivemetallicsurfacewasonly80%of theanalysedsurface(analysisperformedonthecross-section per-pendiculartothecableaxis).Inthefollowing,forLEISresults,the impedancevalues(incm2)arenotcorrected.Therefore,forthe
assemblywithcavities,theimpedancevaluesareoverestimated withanerrorofabout20%ifonlythesurfaceexposedtothebulk solutionistakenintoaccount.
2.3. Surfacecharacterization
AA1370surfaceswereobservedbeforeandafterthe electro-chemicaltestsbyusingaNikonEclipseMA200opticalmicroscope (OM).ALeo435VPscanningelectronmicroscope(SEM)wasused tovisualizetheAl/Cuassemblyafterdifferentexposuretimesto theelectrolyticsolutionandtoobtainabetterdescriptionofthe corrosionmorphology,particularlyattheAl/Cuinterface.Forall theobservations,thesampleswereremovedfromtheelectrolyte aftertheelectrochemicaltests,rinsedwithdeionised waterand thenair-dried.
3. Experimentalresultsanddiscussion
First, the corrosion behaviour of the cable, as compared to the strand sample, was studied by combining stationary and impedancemeasurements.Then,attentionwaspaidtothe corro-sionbehaviouroftheAl/Cuassembly.
3.1. ElectrochemicalbehaviouroftheAA1370cable
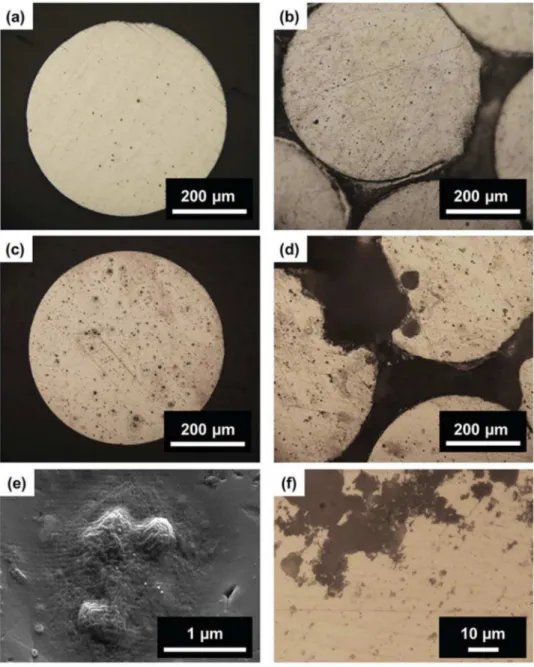
Fig.2showsOMobservationsofastrandandofthecableafter twoexposuretimes(3hand168h)totheaggressivesolutionat theircorrosionpotential.Forbothsamples,aweakdissolutionof thematrixaroundtheFe-richparticles,identifiedasAl3Fe
Fig.2.Opticalmicroscope(OM)observationsofthestrandandofthecableafter:(a,b)3hofimmersionand(c,d)168hofimmersionatEcorrin0.1MNa2SO4+0.001MNaCl.
(a)and(c)correspondtothestrand,(b)and(d)tothecable.(e)SEMobservationofcorrosionphenomenaoccurringattheAl3Feintermetallicparticles.(f)close-uponthe
corrosionfilamentsobserved.
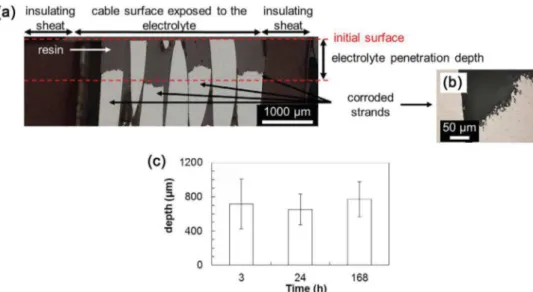
bshowedthatafter3hthematrixdissolutionaroundthe inter-metallicparticleswasmoreextendedforthecablethanforthe strand.After168hofimmersion(Fig.2candd),asignificant dis-solutionofthematrixaroundtheFe-richparticles(Fig.2e)canbe seenonthewholesurfaceforbothsamples,andinaddition,for thecable,largepitssurroundedbyfinefilaments(Fig.2f)werealso observedattheperipheryofthestrands.Thesecorrosionfeatures, inagreementwithliteraturedata,accountedforthepenetration oftheelectrolyteinsidethecableleadingtoaconfinedmedium andcrevicecorrosion phenomenon[10,11].OMobservations of cross-sections parallel to the cable axisafter various exposure timestothechloride-containingsulphatesolutioncorroborated thisassumptionandallowedthedepthofthecorrodedzoneto bemeasured(Fig.3a).Itwasassumedtobeagoodindicationof theelectrolytepenetrationdepth.Inordertomeasurethedepthof thecorrodedzone,thecorrosiondamagewasenhancedby polar-izingat−700mV/SCEfor5minthesamplesaftertheexposureat Ecorr(Fig.3aandb).Theelectrolytepenetrationdepthwasfoundto
becomerapidlyindependentoftheexposuretime,reachingabout 800mmafter3hofexposure(Fig.3c).
Fig.4 reports Ecorr values versus exposuretime tothe
elec-trolyteforthestrandsampleandthecable.Forthestrandsample, Ecorr stabilized rapidly and an almost stationary value(around
−600mV/SCE)wasobtainedafter3hofimmersion.Onthe con-trary, for the cable, Ecorr decreased significantly towards more
negativevaluesduringimmersionwhichwasinagreementwith thedifferencesincorrosionmorphologyobservedbetweenthetwo samples(Fig.2).Fig.5showsthepolarizationcurvesforthestrand sampleandthecableobtainedafter3hofexposuretothe chloride-containingsulphatesolution.Forthecable,thecurrentdensities werecalculatedbytakingintoaccount(i)onlythecross-sections ofthe50 strands constitutiveof thecable,i.e.only thesurface exposedtothebulksolution(S=0.11cm2,nocorrectedsurfacein
Fig.5)and(ii)boththecross-sectionsandthelateralsurfaceof the50strandsona800mmdepth,i.e.boththesurfacesexposed tothebulkand toaconfinedelectrolyte(S=0.76cm2,corrected
Fig.3.(a)OMobservationofacablecross-section(paralleltothecableaxis)after3hofexposuretothe0.1MNa2SO4+0.001MNaClsolutionfollowedbypolarizationfor
5minat−700mV/SCE;(b)close-uponacorrodedstrandinsidethecable;(c)electrolytepenetrationdepthversustheexposuretime.
Fig.4.VariationofEcorrduringimmersionin0.1MNa2SO4+0.001MNaClforthe
strandsampleandthecable.
Fig.5.Polarizationcurvesofthestrandsampleandthecableobtainedafter3h ofimmersionatEcorrin0.1MNa2SO4+0.001MNaCl.Scanrate=0.07mVs−1.For
thecable,thecurve‘nocorrectedsurface’onlytakesintoaccountthecablesurface exposedtothebulk(S=0.11cm2);the‘correctedsurface’curvetakesintoaccount
boththesurfaceexposedtothebulkandthatexposedtotheconfinedmedium insidethecavities(S=0.76cm2).
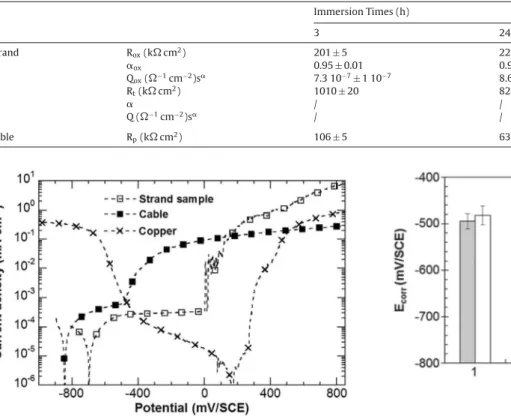
surfaceinFig.5).Forboththestrandsampleandthecable,the cathodicbranch,correspondingmainlytotheoxygenreduction, wassimilar.Forthestrandsampleandthecable,Ecorrvalueswere
−580mV/SCEand−730mV/SCErespectively.Theanodicdomain wascharacterizedforthestrandsample bya passivityplateau, withpassivecurrentdensitiesofabout4.10−4mAcm−2,followed
byabreakdownpotentialat500mV/SCEassociatedwith,first,a slowincreaseoftheanodiccurrentdensitiesandthenasharper one.Thiscouldbeassociatedto,first,anincreaseofthe dissolu-tionrateofthematrixsurroundingtheFe-richparticlesandthen topittingcorrosion.Besides,thesignificantcurrentfluctuations observedafterthesharpincreaseofthecurrentdensities consti-tutesacharacteristicfeatureofthepittingcorrosion.Forthecable, ashortpseudo-plateauwasobservedandtheanodiccurrent densi-tieswereabout2.10−3mAcm−2(correctedsurface).Whateverthe
surfacetakenintoaccount,thecurrentdensitieswerehigherforthe cable,ascomparedtothestrandsample;thiscouldbeassignedto adissolutionprocessratherthanapassivityprocess.Abreakdown potentialwasthenobservedat−550mV/SCEfollowedbyasharp increaseoftheanodiccurrentdensities.Alltheresultsshoweda lowercorrosionresistanceforthecableascomparedtothestrand sample.Thedifferencesincorrosionbehaviourbetweenthetwo sampleswererelatedtothepenetrationoftheelectrolyteinsidethe cablecavitiesleadingtotheformationofaconfinedandthenmore aggressiveelectrolyte.Thisconfirmed,forthecable,theoccurrence ofbothpittingcorrosiononthesurfaceexposedtothebulk solu-tionandcrevicecorrosiononthesurfaceexposedtotheconfined mediuminside thecavities. Thehigheranodiccurrentdensities observedforthecablewererelatedtotheprogressive modifica-tionoftheelectrolytetrappedinsidethecavitiesandsubsequent dissolutionphenomenon.
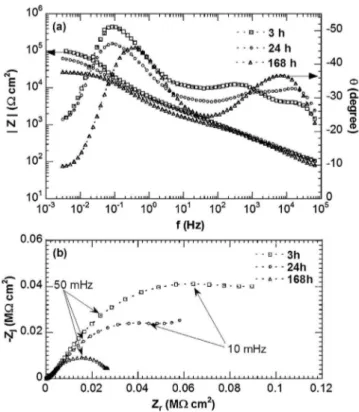
Impedance measurements were performed for the strand sampleandthecableafterdifferentexposuretimestothe chloride-containingsulphatesolution.Fig.6showsthediagramsinBode (Fig.6a) and Nyquist (Fig. 6b) coordinates for thestrand sam-ple. After3hand 24h of immersion, the impedance diagrams arecharacterizedbytwotimeconstants.Thefirsttimeconstant (60kHz–1Hz)wasassociatedtotheresponseofthepassivefilm, whilethesecondtimeconstant(1Hz–100mHz)wasattributedto thechargetransferprocessand totheoxygenreductiononthe passivefilminagreementwithliterature[27].Impedance mea-surementsperformedatEcorrinadeaeratedelectrolyte(resultsnot
Fig.6.Electrochemicalimpedancediagramsobtainedforthestrandsample–(a) Bodeand(b)Nyquistcoordinates–afterdifferentexposuretimesatEcorrto0.1M
Na2SO4+0.001MNaCl.
at low frequencies and no modification in the high frequency domain.Thisconfirmedtheattributionofthelowfrequencytime constanttotheoxygenreduction.After168hofimmersion,the impedancediagramshowedonlyonetimeconstantwithalower resistancewhichcouldbeassociatedtothecorrosionofthealloy, i.e.tothechargetransferprocess.Suchanevolutionofthediagrams wasinagreementwiththeOMobservations(Fig.2c),i.e.the disso-lutionofthematrixsurroundingtheFe-richparticlesduetooxygen reductionontheintermetallicsand subsequentalcalinizationof theelectrolyte for increasingimmersion times. The impedance diagramsobtainedforthecable(Fig.7)wereclearlymodifiedas compared tothose obtainedfor the strandsample. Theresults arepresented by takinginto account thetotal surface exposed totheelectrolyte(the surfaceexposedtothebulksolutionand thatexposedtotheconfinedmediumtrappedinsidethecavities). TheNyquistdiagram(Fig.7b)wasconstitutedbyastraightlinein thehigh-frequencydomainfollowedbyasemicircle.Thistypical shapecanbeassociatedtotheoxygendiffusioninaporoussystem [30–33]inagreementwiththepenetrationoftheelectrolyteinside thecablecavities.Theflattenedsemicirclemayoriginatefromthe cavityshape [34].Theimpedancevalues forthecablewereten timessmallerthanthoseobtainedforthestrandsamplein agree-mentwiththedifferencesincorrosiondamagebetweenthetwo sampleswhichhighlightedtheinfluenceoftheelectrolyte pene-trationthroughthecavitiesofthecable.For168hofimmersion,a decreaseoftheimpedancewasobservedand,inthehigh-frequency domain,atimeconstantappearedwhichcouldbeassociatedtothe corrosionofthealloyintheconfinedmedium[35,36].Additional experiments(results notshown) wereperformedwitha cross-sectionofthecableforwhichthecavitieshadbeensealedwitha resinbyforcingtheresintopenetrateintothecavitieswitha vac-uumsystem.Theimpedancediagramobtainedforthecablewith sealedcavitiesafterimmersionintheaggressivesolutionandthat ofthestrandsampleweresuperimposed.Thiswasinagreement withcommentsintheexperimentalpartandconfirmedthatthe corrosionbehaviourofthecablewascontrolledbythepenetration oftheelectrolyteinsidethecable.
Fig.7.Electrochemicalimpedancediagramsobtainedforthecable–(a)Bode and(b)Nyquistcoordinates–after differentexposuretimesatEcorr to0.1M
Na2SO4+0.001MNaCl.Theimpedancevaluestakeintoaccountboththesurface
exposedtothebulkandthatexposedtotheconfinedmediuminsidethecavities (S=0.76cm2).
Equivalent electrical circuits are frequently used to extract parametersassociatedwiththeimpedancediagrams.Inthepresent study,thedifferentsetsofimpedancemeasurementsperformed allowedtheinterpretationofthedifferenttimeconstantsin agree-mentwithliteraturedataandrelevantparametersweredefined. Forthestrandsample,relevantparametersweretheoxidefilm resistance(Rox)andthechargetransferresistance(Rt).Theywere
directlymeasuredontheimpedancespectra.Further,among rel-evantparameters,aconstant phaseelement(CPE)isoftenused insteadofa capacitance totakethenon-ideal behaviourofthe interfaceintoaccount.TheCPEisgivenby:
ZCPE=
1
Q (jω)˛ (1)
where ais related to theangle ofrotation of a purely capaci-tivelineonthecomplexplaneplotsandQisin−1cm−2s˛.In
thepresentstudy,aandQweredeterminedusingthegraphical methodproposedbyOrazemetal.[37].Forthecable, consider-ingthecomplexityofaporoussystem,thepolarizationresistance (Rp)wasassumedtoallowthecorrosionresistanceofthecableto
becomparedtothatofthestrandsample.Fig.7ashowedthatthe impedancemoduluswasquitestableforfrequencieslowerthan 10mHz;therefore,theimpedanceatlowfrequency(3mHz)was measuredandusedtoevaluatethecorrosionresistanceofthecable. TheimpedanceparametersarereportedinTable1.
Forthestrandsample,duringthefirst24hofimmersion,aox
remainedconstantandequal to0.95. ThevaluesofQox slightly
increasedfrom7.310−7−1cm−2s0.95to8.610−7−1cm−2s0.95.
Theaox andQox valuesaccountedforthepresenceofapassive
layer.ThevariationofQoxwithincreasingimmersiontimecould
berelatedtotheevolutionofthethicknessand/orofthe chemi-calcompositionofthepassivefilminrelationwiththedissolution ofthematrixaroundtheintermetallics.Duringthefirst24h,the
85 Table1
Parametersobtainedfromtheimpedancediagramsforthestrandsampleandforthecableafterdifferentimmersiontimesin0.1MNa2SO4+0.001MNaClsolution.Forthe
cable,theimpedancevaluestakeintoaccountboththesurfaceexposedtothebulkandthatexposedtotheconfinedmediuminsidethecavities(S=0.76cm2).
ImmersionTimes(h) 3 24 168 Strand Rox(kcm2) 201±5 220±5 / aox 0.95±0.01 0.95±0.01 / Qox(−1cm−2)sa 7.310−7±110−7 8.610−7±110−7 / Rt(kcm2) 1010±20 821±10 324±5 a / / 0.94±0.01 Q(−1cm−2)sa / / 4.2510−6±110−6 Cable Rp(kcm2) 106±5 63±5 27±2
Fig.8. PolarizationcurvesoftheAA1370strandsample,theAA1370cableandthe pureCuconnectorafter3hofimmersionatEcorrin0.001MNaClsolution.
corrosionbehaviourofthestrandsamplewascontrolledbythe presenceof theoxidefilmwithhighvaluesof bothRox andRt
while,for168hofimmersion,theelectrochemicalbehaviourwas controlledbythecorrosionprocesses(decreaseoftheRtvalues).
Forthecable,thelowRpvalueswereinagreementwiththelow
corrosionresistanceofthecableascomparedtothestrand sam-ple;moreover,thedecreaseofRpwithincreasingimmersiontime
wasinagreementwiththeextentofthecorrosiondamagerelated tothepenetrationoftheelectrolyteinsidethecableandtothe crevicecorrosionphenomenaonthesurfaceexposedtothe con-finedmediumwhilepittingoccurredonthesurfaceexposedtothe bulk.
3.2. Electrochemicalbehaviouroftheassembly 3.2.1. Preliminaryexperimentsandobservations
Apreliminarystudywasperformedin0.001MNaClto charac-terizeseparatelythecorrosionbehaviourofAl(i.e.AA1370)andCu inthesamesolutionasthatusedfortheLEISmeasurements.First, theanodicbranchofthepolarizationcurvesfor theAlsamples (strandsampleandcable)andthecathodicandanodicbranches forpureCuwereobtained(Fig.8).ComparisonofFigs.5 and8 showedthattheglobalshapeofthecurvesfortheAlsampleswas thesameindependentlyoftheelectrolyte.Forthestrandsample, a600mV-longplateaucorrespondingtoapassivityplateauwith lowanodiccurrentdensities(3.10−4mAcm−2)wasobservedand
followedbyanabruptincreaseofthecurrentassociatedtopitting corrosion.Forthecable,aplateauwasalsoobserved;itwas bet-terdefinedthaninsulphateandchloride-containingsolutionsbut, again,correspondedtohigheranodiccurrentdensitiesthanforthe strandsampleandwasconsideredasapseudo-passivityplateau. Itwasrapidlyfollowedbyasharpincreaseoftheanodiccurrent densitiesassociatedtobothpittingandcrevicecorrosionas
previ-Fig.9. VariationofEcorrduringimmersionin0.001MNaClsolutionfortheAl/Cu
assemblieswithandwithoutcavities.
ouslyexplained.Asobservedinthechloride-containingsulphate solution,thebreakdownpotentialassociatedtotheincreaseofthe anodiccurrentdensitieswasmorenegativeforthecable(pitting andcrevicecorrosion)thanforthestrandsample(pitting corro-sion) whichshowedthelowercorrosionresistanceof thecable ascomparedtothestrandsample.Inthe0.001MNaClsolution, thebreakdownpotentialwas10mV/SCEand−500mV/SCEforthe strandsampleandthecable,respectively.Then,itcanbeseenfrom Fig.8thatthecorrosionpotentialofpureCuwasmorepositive (200mV/SCE)thanthoseofthestrandsample(−700mV/SCE)and thecable(−850mV/SCE)showingasexpectedthat,intheAl/Cu assembly,pureCuwillbethecathodeandtheAlsampleswillbe theanode[20,21,38–40].FromtheanodiccurvesoftheAlsamples andthecathodiccurveofCu,thecommonpotentialmeasured,in thecaseofagalvaniccouplingbetweenthetwometalswiththe samesurfacearea,wouldbeabout−400mV/SCEforanAlstrand sample/Cuassembly,and−500mV/SCEforanAlcable/Cu assem-bly.Itwasworthnoticingthat,fortheassembliesinvestigatedhere andasshowninFig.1,theratioSCu/SAl(SCuandSAlarethesurface
areasexposedtotheelectrolyteintheassemblyrespectivelyfor CuandAl)wassignificantlyhigherthan1.Therefore,thecommon potentialshouldbeshiftedtowardsmorepositivevaluesthanthose previouslyestimated(withthesamesurfacearea).FromFig.8,it canbeclearlyseenthat,in0.001MNaCl,thecommonpotentialwas inthelocalizedcorrosion(pittingandcrevicecorrosion)regimefor thecable,whereas,dependingontheratioSCu/SAl,itcouldbeinthe
passiveregimeforthestrandsample.Thismeantthatasevere cor-rosiondamagewasexpectedforthecablewhencoupledwithCu. Thissuggestedalsothatdifferencesshouldbeobservedbetween thetwotypesofassembly,i.e.withandwithout(allthestrands con-stitutiveofthecablebeingweldedtogether)cavities,asdescribed inFig.1.
Fig.10.OpticalmicroscopeobservationsofthecorrosiondamagefortheAl/Cuassembliesafter(a,b)3hand(c,d)168hofimmersionatEcorrina0.001MNaClsolution:
assembly(a,c)withoutand(b,d)withcavities.(e)SEMmicrographoftheAl/Cuinterfaceoftheassemblywithoutcavitiesafter24hofimmersionin0.001MNaClsolution (tiltof70◦).
Fig.9showedEcorr valuesversusimmersiontime in0.001M
NaClforthetwotypesofassembly.Asexpected,Ecorrvalueswere
betweenthoseoftheAlcableandCu.Forbothassemblies,Ecorr
valuesdecreasedwithincreasingimmersiontimewhichcouldbe relatedtocorrosiondamage.Duringthefirst24h,Ecorrvalueswere
thesameforthetwotypesof assembly;theydecreasedslowly from−500mV/SCEto−600mV/SCE.After24hofimmersion,Ecorr
oftheassemblywithoutcavitiesstabilizedaround−600mV/SCE, whereas,fortheassemblywithcavities,Ecorrmovedtowardsmore
negativevaluesandreached−770mV/SCEafter168hof immer-sion.ThisvariationofEcorrsuggestedthat,foranimmersiontime
longerthan24h, thecorrosiondamage wasmoreextended for theassemblywithcavitiesandunderlined,asalreadydiscussed, thatthepresenceofcavitiesinthecablemodifiedthecorrosion processes.
The corrosiondamages for thetwo types of assembly were observedbyOMandSEMaftershortimmersiontimes(3hand24h) andlongimmersiontime(168h)in0.001MNaCl(Fig.10;results areonlygivenfor3hand168h).Independentlyoftheimmersion time,AlpartwascorrodedwhiletheCusurface exposedtothe bulksolutionremainedsafe.Forashortimmersiontime,no signif-icantdifferencewasobservedbetweenthetwotypesofassembly (Fig.10aandb).After168hofimmersion,significantdifferences wereobservedfortheAlpartdependingonthepresenceornot ofcavities(Fig.10candd).Fortheassemblywithoutcavities,OM observationsshowedthepresenceofpitsandnumerousfine fila-mentsaroundthepitsfortheAlpart(Fig.10c).Fortheassembly withcavities,aseverecorrosiondamageoftheAlpartwasobserved withnot onlypitsobservedonthesurfaceexposedtothebulk solutionbutalsosomestrandsconstitutiveofthecablecompletely dissolvedoverseveralhundredsofmicrometersindepth.These
corrosionfeatureswereinagreementwithpittingcorrosion phe-nomenaoccurringonthesurfaceexposedtothebulksolutionwhile crevicecorrosionoccurredinthecavities[38].Moredetailed atten-tionwaspaidtotheAl-Cuinterface.Forbothassemblies,corrosion products(blackareasinFig.10candd)wereclearlyobservedatthe Al/CuinterfaceandspreadallovertheAlpartfortheassemblywith cavities(Fig.10d).Fig.10eshowsaSEMmicrographoftheAl/Cu interfaceafter24hofimmersionfortheassemblywithout cavi-ties.AttheAl/Cuinterface,acrevicewasclearlyvisibleandalsothe Aldissolution.Suchacrevicewasalsoobservedfortheassembly withcavities:itwasevenmorevisible.Similarfeaturewasalready observedforaAl/Cumodelcouple[19].ThisshowedthatforCu, eventhoughnocorrosiondamagewasobservedonthesurface exposedtothebulksolution,corrosionphenomenaoccurredon theverticalwallinsidethecrevice,referringtocrevicecorrosion processes[20,21].Thus,forbothassemblies,theinitialstepofthe corrosionprocesswasthedissolutionoftheAlpartattheAl/Cu interfaceduetothealkalinisationlinkedtotheoxygenreduction reactionontheCupart[19].Theelectrochemicalreactionsthat justifythealuminiumcorrosionwereasfollows[38,40–42]:
Cathodic(ontheCusurfaceexposedtothebulksolution):
O2+2H2O+4e−→4OH− (2)
Anodic:
Al→ Al3++3e− (3)
2Al+6H20 →2Al(OH)3+3H2 (4)
Al(OH)3+OH−→ [Al(OH)4]− (5)
With increasing immersion time, in the crevice, hydrolysis of cationscan beassumed and asa consequencethe chemical
Fig.11. Admittancemapsobtainedafter24hofimmersionatEcorrin0.001MNaCl
solutionat10HzabovetheAl/Cuassemblies:(a)withoutcavitiesand(b)with cav-ities.Admittancevaluesarecalculatedtakingintoaccountananalyzedsurfaceof 1mm2.
compositionoftheelectrolyteattheAl/Cuinterfaceshouldvary significantlywithadecreaseofpHasshownbyShietal.[40].This canbeascribedtothereactionofAldissolutionandhydrolysis[40]: Al+nH2O→Al(OH)n3−n+nH++3e−,n=1–3 (6)
Therefore,inthecreviceformedattheAl/Cuinterface,thepH gotmore acidicwhichshouldpromoteacrevicecorrosion phe-nomenonforCu.Ontheopposite,farfromtheAl/Cuinterface,Cu wasincontactwiththebulksolutionwhereoxygenispresent[21] leadingtoanon-corrodedsurface.FortheAlpart,fortheassembly withoutcavities,pittingcorrosionwasobserved.Concerningthe assemblywithcavities,pittingcorrosionwasobservedontheAl surfaceexposedtothebulksolutionandcrevicecorrosionoccurred inthecavitiesexistingbetweenthestrandsduetothe penetra-tionoftheelectrolyteinsidethecavities.Therefore,thecorrosion productsobservedshouldincludebothcopperoxides,aluminium oxidesandhydroxides[20,38,40,42].Håkanssonetal.showedthat themaincorrosionproductsformedduringgalvaniccorrosionof aluminium/carbonsystemsin0.6MNaClwasAl(OH)3,presented
asagelatinoussubstanceinthecrevicesbetweentheAlwires[43]. TheseauthorsalsoshowedthatAl(OH)3shouldcontroltherateof
thecorrosionprocesseswhichshouldexplaintheplateauobserved onthepolarizationcurvesforthecableafterthebreakdown poten-tial(Fig.8).
3.2.2. LEISstudyoftheassemblies
LEISwasfirstusedinmappingmode.Mapsobtainedat10Hzfor theassemblieswithandwithoutcavities,after24hofexposurein a0.001MNaClsolutionareshowninFig.11.Forbothsamples,the admittancevaluesarehigher(i.e.,lowerresistance)ontheAlpart whichwasduetothegalvaniccouplingbetweenAl(anode)and Cu(cathode).Moreover,heterogeneousvaluesoftheadmittance
wereobservedontheAlpartinaccordancewithpittingcorrosion processes,aspreviouslyshown.ComparisonofFig.11aandbdid notshowsignificantdifferencesbetweenthetwotypesofassembly inagreementwithEcorrmeasurements(Fig.9).
Then, localimpedance spectrawerecollected after24hand 168hofimmersionforthetwotypesofassembly(Fig.12,Nyquist coordinates).
First,itcanbeseenthatimpedancevaluesontheAlpartfor theassemblywithcavitiesarehigherthanthosefortheassembly withoutcavities.Asexplainedintheexperimentalpart,impedance valuesontheAlpartareoverestimatedfortheassemblywith cavi-ties.Theerroronthemeasurementswasestimatedtobeabout20% byconsideringonlythesurfaceexposedtothebulksolution.Ifthe surfaceexposedtotheconfinedelectrolyteinthecavities (consid-eringthesurfaceintheplaneparalleltothecableaxis)istakeninto account,thiserrorshouldbereducedbecausetheexposedsurface wouldbeincreased.Nevertheless,despitetheseinaccurate mea-surements,itwashelpful,forbothAl/Cuassemblies,tostudythe changesobservedforthelocalimpedancediagramsversus immer-siontimeandtocomparetheelectrochemicalresponseoftheAl partandtheCuparttodescribetheinfluenceofthecavitiesonthe corrosionbehaviouroftheAl/Cuassembly.
Then,forbothassemblies,theimpedancevaluesafter24hof immersion (Fig. 12a and b) were higher on the Cu part, then decreasedwhentheprobemovedtowardstheAl/Cuinterface,and decreasedagainwhentheprobewasovertheAlpart.Suchan evo-lutionoftheimpedanceasafunctionofthepositionoftheprobe wasinagreementwiththegalvaniccouplingbetweenAlandCu. After24hofimmersion,pittingcorrosionoccurredontheAlpart (Fig.10).Fortheassemblywithcavities,thediagramsplottedfor theCupart(redcurves inFig.12)aresignificantlymodified in thelowfrequencyrange ascomparedtothoseobtainedforthe assemblywithoutcavities.Theirglobalshapesuggesteda diffusion-controlledelectrochemicalprocess inrelationwiththecathodic reactionofoxygenreductionontheCupart.After168hof immer-sion,thediffusion-controlledprocesswasevenmoremarkedon thediagramsfortheassemblywithcavities(Fig.12d)andcouldbe alsonotedfortheassemblywithoutcavities(Fig.12c).This conclu-sioncouldberelatedtothoseofHåkanssonetal.whoshowedthat, foraAl/carboncouple,theratecontrollingmechanismwassolely themasstransportofoxygenthroughthegelatinousAl(OH)3inside
thecrevice[43].
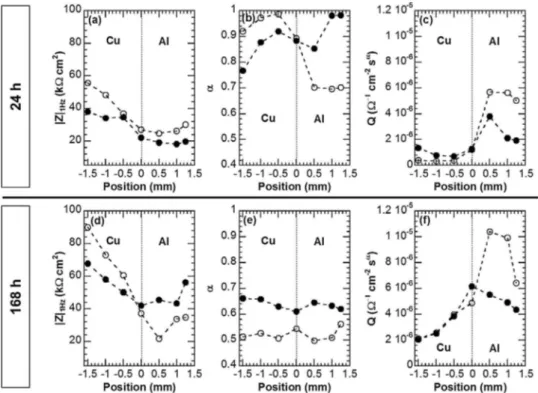
In order to quantitatively analyse the LEIS diagrams, three parametersweregraphicallyextracted:theCPEvalues(aandQ) andthevaluesoftheimpedancemodulusat1Hz(|Z|1Hz).Fig.13
showsthevariationoftheseparametersforeachdiagramobtained overtheAlandCuparts.Independentlyoftheassemblyandof theimmersiontime,|Z|1HzvaluesfortheCupartwerehigherthan
thosefortheAlpart,whichconfirmedthedifferencesin reactiv-ity betweenthetwo metals whentheywerecoupled(Fig.13a, d).On theCupart,thelow frequency rangewasrelated tothe diffusionprocesslinkedtotheoxygenreduction(Fig.12). What-evertheassembly,theincreaseof|Z|1Hzbetween24hand168hof
immersionwasingoodagreementwiththeshiftofthecorrosion potentialsmeasuredfortheassembliestowardsmoreandmore negativevaluesfrom24hto168hofimmersion(Fig.9):thiswas linkedtoamoreandmoresignificantcathodicpolarizationforthe Cupart.Whentheimmersiontimeincreased,inagreementwith thecorrosionpotentialvalues measuredforthetwoassemblies, thedifferencesin|Z|1HzvaluesbetweentheAlandCupartswere
evenmoremarkedfortheassemblywithcavities.After168hof immersion,|Z|1HzvaluesmeasuredontheAlpartfortheassembly
withcavitieswerelowerthanthoseoftheAlpartforthe assem-blywithoutcavities.Thiswasexplainedbytakingintoaccountthe increaseoftheelectrochemicalprocessesina confinedmedium previouslyshowedforthecable:theeffectofthegalvaniccoupling
Fig.12.Localimpedancespectraobtainedafter(a,b)24hand(c,d)168hofimmersionatEcorrin0.001MNaClsolutionfordifferentpositionsofthebi-electrodeabovethe
Al/Cuassemblies:(a,c)withoutcavitiesand(b,d)withcavities.Impedancevaluesarecalculatedtakingintoaccountananalyzedsurfaceof1mm2.
Fig.13. Variationoftheparameters((a)and(d)|Z|1Hz,(b)and(e)aand(c)and(f)Q)obtainedfromthelocalimpedancespectraafterdifferentexposuretimesin0.001M NaClandfordifferentpositionsofthebi-electrodeovertheAl/Cuassemblies:(d)withoutcavitiesand( )withcavities.Impedancevaluesarecalculatedtakingintoaccount ananalyzedsurfaceof1mm2.
wasmoreandmoremarkedwithincreasingimmersiontimefor theassemblywithcavities.
ThedifferencesinreactivitybetweenCuandAlfromonepart, andbetweenthetwoassembliesfortheotherpart,wereconfirmed bythevariationofaandQwithincreasingimmersiontime.After 24hofimmersion,thestrongreactivityattheAl/Cuinterfacewas
showedwithboththedecreaseofaandtheincreaseofQinthezone whereSEMmicrographies(Fig.10e)showedacreviceontheAlpart. Thisevolutionwasmoremarkedforanassemblywithcavitiesin relationwithalowercorrosionresistanceoftheAlpartduetothe penetrationoftheelectrolyteinsidethecavitiesofthecable.After 168hofimmersion,aandQvaluesconfirmedtheincreaseofthe
89 galvaniccouplingeffect.Between24hand168hofimmersion,a
valuessignificantlydecreasedinagreementwithanincreaseofthe diffusionprocessesontheCupartandastrongercorrosionforthe Alpart.Moreover,itcanbeseenthatafter168hofimmersion, avaluesarelowerforboththeCuandAlpartsfortheassembly withcavitiesascomparedtotheassemblywithoutcavities.The resultcouldbelinkedtoastrongerincreaseofQvaluesattheAl/Cu interfaceobservedfortheassemblywithcavitiesascomparedto theassemblywithoutcavities.Theresultswereingoodagreement withthepresenceoftheconfinedelectrolytewhichamplifiedthe effectofthegalvaniccouplingwhentheimmersiontimeincreased. Therefore,theLEISmeasurementsprovidedevidencethatthe corrosionbehaviouroftheAl/Cuassemblywasexplainedbyboth thegalvaniccouplingbetweentheAlandCuparts,whichenhanced thecorrosionoftheAlpart,andthepenetrationoftheelectrolyte insidethecavitiesobservedintheAlpartoftheassembly,which ledtoamoreandmoreconfinedandaggressiveelectrolytewith increasingimmersiontimesothatitenhancedthereactivityofthe AlpartandthusthegalvaniccouplingwithCu.
4. Conclusions
ThestudywasfirstfocussedonthecorrosionbehaviourofanAl (AA1370)cableascomparedtoastrand.
1.OMobservationsafterexposuretoachloride-containing sul-phatesolutionshowedamoreextendedcorrosiondamagefor thecablethanforthestrand.
2.Electrochemical measurements showed for the cable: (i) a strongerdecreaseofthecorrosionpotentialwithtimethanfor thestrand,(ii)anincreaseoftheanodiccurrentdensity associ-atedwithapseudo-passivityplateauandamorenegativepitting potentialascomparedtothestrandand(iii)impedancediagrams characteristicofaporoussystem.
3.ThecorrosionbehaviouroftheAlcablewascontrolledbythe penetrationoftheelectrolytebetweenthestrandsconstitutive ofthecable.Suchaphenomenonledtoamoreaggressive con-finedmediumwhichexplainedthelowercorrosionresistance ofthecableascomparedtothestrand.
4.Then,thecorrosionbehaviouroftheassemblybetweenanAl cableandaCuconnectorwasinvestigatedbyelectrochemical techniques,andinparticularlocalimpedancemeasurements. 5.Aldissolution occurreddue togalvaniccouplingwiththeCu
connectorandcrevicecorrosionwasobservedontheCuwalls perpendicularlytotheAl/Cuinterfaceexposedtotheelectrolyte. 6.LocalimpedancedatashowedthatthecavitiesoftheAlcable significantlyinfluencedthecorrosionbehaviourofthe assem-bly:thereactivityattheAl/Cuinterfaceandtheextentofthe corrosiondamageontheAlpartwerestrongerfortheassembly withcavities.
References
[1]Y.Yamano,T.Hosokawa,Developmentofaluminumwiringharness,SEITech. Rev.73(2011)73–80.
[2]A.Laurino,E.Andrieu,J.P.Harouard,J.Lacaze,M.C.Lafont,G.Odemer,C. Blanc,Corrosionbehaviorof6101aluminumalloystrandforautomotive wires,J.Electrochem.Soc.160(2013)5069–5575.
[3]K.Yoshida,K.Doi,Improvementofductilityofaluminumwireforautomotive wiringharnessbyalternatedrawing,ProcediaEng.81(2014)706–711. [4]S.Koch,H.Antrekowitsch,AluminumAlloysforwireharnessesinautomotive
engineering,BergHuettenmaennMonatsh152(2007)62–67.
[5]S.-I.Matsuaka,H.Imai,Directweldingofdifferentmetalsusedultrasonic vibration,J.Mater.Process.Technol.209(2009)954–960.
[6]J.W.Yang,B.Cao,X.C.He,H.S.Luo,Microstructureevolutionandmechanical propertiesofCu–Aljointsbyultrasonicwelding,Sci.Technol.Weld.Join.19 (2014)500–504.
[7]R.Rambat,A.J.Davenport,G.M.Scamans,A.Afseth,Effectofiron-containing intermetallicparticlesonthecorrosionbehaviourofaluminum,Corros.Sci. 48(2006)3455–3471.
[8]J.O.Park,C.H.Paik,R.C.Alkire,ScanningMicrosensorsformeasurementof localpHdistributionsatthemicroscale,J.Electrochem.Soc.143(1996) 174–176.
[9]O.Seri,M.Imaizumi,ThedissolutionofFeAl3Intermetalliccompoundand
depositiononaluminuminAlCl3solution,Corros.Sci.30(1990)1121–1133.
[10]J.Xu,W.Chen,Behaviorofwireinparallelwirestayedcableundergeneral corrosioneffects,J.Constr.SteelRes.85(2013)40–47.
[11]Y.Ishikawa,S.Kawakami,Effectsofsaltcorrosionontheadhesionofbrass platedsteelcordtorubber,RubberChem.Technol.59(1986)1–15. [12]J.J.Perdomo,I.Song,Chemicalandelectrochemicalconditionsonsteelunder
disbondedcoatings:theeffectofappliedpotential,solutionresistivity, crevicethicknessandholidaysize,Corros.Sci.42(2000)1389–1415. [13]S.H.Zhang,S.B.Lyon,Anodicprocessesonironcoveredbythin:dilute
electrolytelayers(I)-anodicpolarisation,Corros.Sci.36(1994)1289–1307. [14]R.Oltra,B.Malki,F.Rechou,Influenceofaerationonthelocalizedtrenching
onaluminumalloys,Electrochim.Acta55(2010)4536–4542.
[15]S.Chanel,N.Pébère,Aninvestigationonthecorrosionofbrass-coatedsteel cordsfortyresbyelectrochemicaltechniques,Corros.Sci.43(2001)413–427. [16]D.Wong,L.Swette,Aluminumcorrosioninuninhibitedethyleneglycol-water
solutions,J.Electrochem.Soc.126(1979)11–15.
[17]M.G.A.Khedr,A.M.S.Lashien,Theroleofmetalcationsinthecorrosionand corrosioninhibitionofaluminuminaqueoussolutions,Corros.Sci.33(1992) 137–151.
[18]M.G.A.Khedr,A.M.S.Lashien,Corrosionbehaviorofaluminuminthepresence ofacceleratingmetalcationsandinhibition,J.Electrochem.Soc.136(1989) 968–972.
[19]J.-B.Jorcin,C.Blanc,N.Pébère,B.tribollet,V.Vivier,Galvaniccoupling betweenpurecopperandpurealuminum,J.Electrochem.Soc.155(2008) C46–C51.
[20]C.Blanc,N.Pébère,B.Tribollet,V.Vivier,Galvaniccouplingbetweencopper andaluminuminathin-layercell,Corros.Sci.52(2010)991–995. [21]S.Joma,M.Sancy,E.Sutter,T.T.M.Tran,B.Tribollet,Incongruentdissolution
ofcopperinAl-Cualloys:influenceoflocalpHchanges,Surf.InterfaceAnal. 45(2013)1590–1596.
[22]P.DeLima-Neto,J.P.Farias,L.F.G.Herculano,H.C.DeMiranda,Determination ofthesensitizedzoneextensioninweldedAISI304stainlesssteelusing non-destructiveelectrochemicaltechniques,Corros.Sci.50(2008) 1149–1155.
[23]G.Baril,C.Blanc,M.Keddam,N.Pébère,Localelectrochemicalimpedance spectroscopyappliedtothecorrosionbehaviorofanAZ91magnesiumalloy, J.Electrochem.Soc.150(2003)488–493.
[24]S.Marcelin,N.Pébère,Synergisticeffectbetween8-hydroxyquinolineand benzotriazoleforthecorrosionprotectionof2024aluminiumalloy:alocal electrochemicalimpedanceapproach,Corros.Sci.101(2015)66–74. [25]M.Mouanga,M.Puiggali,B.tribollet,V.Vivier,N.Pébère,O.Devos,Galvanic
corrosionbetweenzincandcarbonsteelinvestigatedbylocalelectrochemical impedancespectroscopy,Electrochim.Acta88(2013)6–14.
[26]D.Sidane,E.Bousquet,O.Devos,M.Puiggali,M.Touzet,V.Vivier,A. Poulon-Quintin,Localelectrochemicalstudyoffrictionstirweldedaluminum alloyassembly,J.Electroanal.Chem.737(2015)206–211.
[27]J.-B.Jorcin,M.E.Orazem,N.Pébère,B.Tribollet,CPEanalysisbylocal electrochemicalimpedancespectroscopy,Electrochim.Acta51(2006) 1473–1479.
[28]J.-B.Jorcin,E.Aragon,C.Merlatti,N.Pébère,Delaminatedareasbeneath organiccoating:alocalelectrochemicalimpedanceapproach,Corros.Sci.48 (2006)1779–1790.
[29]N.A.Belov,D.G.Eskin,A.A.Aksenov,MulticomponentPhaseDiagrams: ApplicationsforCommercialAluminiumAlloys,firstedition,ElsevierLtd, Oxford,2005.
[30]D.D.Macdonald,Reflectionsonthehistoryofelectrochemicalimpedance spectroscopy,Electrochim.Acta51(2006)1376–1388.
[31]C.Gabrielli,M.Keddam,N.Portail,P.Rousseau,H.Takenouti,V.Vivier, Electrochemicalimpedancespectroscopyinvestigationsofamicroelectrode behaviorinathin-layercell:experimentalandtheoreticalstudies,J.Phys. Chem.110(2006)20478–20485.
[32]H.-K.Song,H.-Y.Hwang,K.-H.Lee,L.H.Dao,Theeffectofporesize distributiononthefrequencydispersionofporouselectrodes,Electrochim. Acta45(2000)2241–2257.
[33]A.Lasia,Impedanceofporouselectrodes,J.Electroanal.Chem.397(1995) 27–33.
[34]C.Hitz,A.Lasia,Experimentalstudyandmodellingofimpedanceoftheheron porousNielectrodes,J.Electroanal.Chem.51(2001)213–222.
[35]S.Marcelin,N.Pébère,S.Régnier,Electrochemicalinvestigationsoncrevice corrosionofamartensiticstainlesssteelinathinlayercell,J.Electroanal. Chem.737(2015)198–205.
[36]M.Keddam,A.Hugot-Le-Goff,H.Takenouti,D.Thierry,M.C.Arevalo,The influenceofathinelectrolytelayeronthecorrosionprocessofzincin chloride-containingsolutions,Corros.Sci.33(1992)1243–1252. [37]M.E.Orazem,N.Pébère,B.Tribollet,Enhancedgraphicalrepresentationof
electrochemicalimpedancedata,J.Electrochem.Soc.153(2006)B129–B136. [38]R.Vera,P.Verdugo,M.Orellana,E.Mu ˜noz,Corrosionofaluminiumin
copper–aluminiumcouplesunderamarineenvironment:influenceof polyanilinedepositedontocopper,Corros.Sci.52(2010)3803–3810.
R.Gravinaetal./CorrosionScience119(2017)79–90 [39]L.B.Coelho,M.Mouanga,M.-E.Druart,I.Recloux,D.Cossement,M.-G.Olivier,
ASVETstudyoftheinhibitiveeffectsofbenzotriazoleandceriumchloride solelyandcombinedonanaluminium/coppergalvaniccouplingmodel, Corros.Sci.110(2016)143–156.
[40]H.Shi,E.-H.Han,F.Liu,T.Wei,Z.Zhu,D.Xu,Studyofcorrosioninhibitionof coupledAl2Cu–AlandAl3Fe–Albyceriumcinnamateusingscanningvibrating
electrodetechniqueandscanningion-selectiveelectrodetechnique,Corros. Sci.98(2015)150–162.
[41]N.Murer,R.Oltra,B.Vuillemin,O.Néel,Numericalmodellingofthegalvanic couplinginaluminiumalloys:adiscussionontheapplicationoflocalprobe techniques,Corros.Sci.52(2010)130–139.
[42]J.Bernard,M.Chatenet,F.Dalard,Understandingaluminumbehaviorin aqueousalkalinesolutionusingcoupledtechniques:partI.Rotatingring-disk study,Electrochim.Acta52(2006),86–93.
[43]E.Håkansson,J.Hoffman,P.Predecki,M.Kumosa,Theroleofcorrosion productdepositioningalvaniccorrosionofaluminum/carbonsystems, Corros.Sci.114(2017)10–16.