Université de Montréal
Chemosensory Perception in Sighted and Blind
Populations
par Simona Manescu
Département de psychologie Faculté des arts et des sciences
Thèse présentée à la Faculté des études supérieurs et postdoctorales en vue de l’obtention du grade de Philosophiae Doctor (PhD)
en psychologie – recherche et intervention option Neuropsychologie clinique
April 2019
Université de Montréal
Département de psychologie, Faculté des arts et sciences
Cette thèse intitulée
Chemosensory Perception in Sighted and Blind Populations
Présentée par
Simona Manescu
A été évaluée par un jury composé des personnes suivantes
Dr. Pierre Jolicoeur Président-rapporteur Dr. Franco Lepore Directeur de recherche Dr. Johannes Frasnelli Codirecteur de recherche Dr. Walter Wittich Membre du jury Dr. Aaron Johnson Examinateur externe
i
Résumé
Comparativement aux autres modalités sensorielles, la recherche au sein du domaine des sens chimiques (système trigéminal et olfactif) a été beaucoup moins développée que celle dans le domaine de la vision ou même de l’audition. Bien que ce champ de recherche soit présentement en émergence, plusieurs questionnements demeurent. Ainsi, le but principal de la présente thèse était d’étudier la façon dont divers facteurs internes parviennent à moduler la perception olfactive, et surtout pour ceux dont la littérature demeure particulièrement peu concluante dans le but de contribuer à l’avancement des connaissances dans le domaine. Pour y répondre, quatre études ont été développées et conduites. L’objectif premier (Étude 1) était de
déterminer l’influence d’une attente sur la perception olfactive. Le deuxième objectif (Étude 2)
visait à explorer la présence de la supériorité d’une narine et son rapport possible avec la préférence manuelle. Étant donné l’importance de l’apport visuel dans la perception olfactive, l’investigation plus particulière de l’influence de la cécité sur les sens chimiques a également été adressée. Le troisième objectif était d’évaluer la catégorisation des odeurs de vins chez les personnes aveugles précoces, une tâche particulièrement complexe et écologique (Étude 3). Le
quatrième et dernier objectif était d’examiner la localisation d’odeurs chez une population non-voyante (Étude 4). À la lumière des résultats de ces études, cela nous a permis de discuter de
leurs divers impacts dans notre compréhension actuelle dans le domaine de la littérature des sens chimiques.
Les résultats de l’Étude 1 ont permis de démontrer que l’étiquetage positif d’odeurs
engendrait systématiquement une évaluation favorable de la comestibilité de celles-ci, et ce en dépit du fait que certaines sources d’odeurs étaient jugées comme étant incomestibles. De plus, il a été relevé que l’étiquetage d’odeurs modulait à la fois l’évaluation de l’intensité ainsi que le temps de réaction vis-à-vis celles-ci. Ces résultats démontrent donc que l’interprétation accordée préalablement à certaines odeurs permet de moduler la perception de celles-ci, soulignant ainsi la présence de mécanismes cérébraux ascendants influençant la perception olfactive.
Dans l’Étude 2, bien que nous sommes parvenus à retrouver partiellement un support
concernant la supériorité de la narine droite chez les droitiers, aucune latéralisation de ce type n’a été observée parmi les individus gauchers. L’hétérogénéité des résultats retrouvés au sein
ii
de notre étude reflète l’état actuel de la littérature scientifique sur le sujet. À cet égard, plusieurs facteurs ont précédemment été soulevés afin d’expliquer la discordance scientifique existante dont la variabilité des méthodologies employées pour évaluer le phénomène ainsi que l’influence du cycle nasal.
Les résultats de l’Étude 3 ont démontré que les personnes aveugles précoces catégorisaient moins bien les odeurs de vins que les participants voyants. En raison de leur privation visuelle, il aurait pu être attendu que les associations visuo-olfactives normalement retrouvées au sein de la population voyante sont compensées par un niveau plus élevé d’associations verbo-olfactives chez les individus atteints de cécité. Toutefois, nos résultats démontrent que la compensation verbo-olfactive ayant pris place suite à la privation visuelle ne semble pas être aussi fonctionnelle que l’association visuo-olfactive retrouvée chez les voyants.
Lors de l’Étude 4, nos résultats ont permis de mettre en lumière que les personnes
aveugles ayant perdu leurs vues à la naissance présentent de meilleures performances de localisation d’odeurs que les individus voyants, de même que les individus aveugles ayant perdu la vision tardivement. Ainsi, l’absence de vision en bas âge semble supporter l’idée que la cécité congénitale occasionne un recours plus important au sens chimiosensoriels afin d’extraire, des odeurs environnantes, des informations spatiales facilitant possiblement la navigation dans l’environnement.
iii
Mots-clés : olfaction, odorat, trigeminal, perception chimiosensorielle, aveugles, aveugles
iv
Abstract
Compared to the other senses, research in the chemical senses (trigeminal and olfactory systems) have received far less attention than other modalities such as vision and audition. Even though it is an emerging field, there are still numerous questions left to answer. The main goal of the present thesis was to further our understanding of the impact of various internal factors on chemosensory perception for which the literature remains particularly inconclusive in hopes to advance our comprehension of this modality. To do so, four objectives were established. The first objective (Study 1) was to determine the impact of an internal state (expectancy) on
olfactory perception using odor labeling. The second objective (Study 2) was to investigate
whether there is a nostril dominance in chemosensory perception and whether it is linked with handedness. Given the importance of visual cues in chemosensory processing, we also set out to investigate the influence of blindness on chemosensory processing. Therefore, the third objective (Study 3) was to determine the impact of blindness on wine odor categorization, a
task particularly complex and ecologic. The fourth and final goal of the present thesis (Study 4)
was to investigate odor localization in blind individuals, a task that has not yet been explored in this population. In light of the results of our four studies, we further discuss their impacts on our current comprehension of the chemical senses.
The results obtained in Study 1 showed that positively labeled odors were systematically
rated as being more edible, and this was true even for odors carrying non-food labels. Positively labeled odors were also rated as being more pleasant than negatively labeled ones, which is in line with previous work. Labels also modulated intensity ratings and reaction times, although to a lesser degree. These results highlight that “top-down” mechanisms can influence olfactory perception, and that previous experiences or different interpretations of a labeled odor can alter our perception of it.
The results acquired in Study 2 found partial support for the presence of a nostril
dominance in right-handed individuals, yet no effect was found for left-handed individuals. The heterogeneity found in these results mirrors the one found in the literature. Methodological differences as well as influences of the nasal cycle could partially explain the heterogeneity in the results.
v
The results from Study 3 displayed worse performance in early-blind individuals
compared to sighted controls on a wine odor categorization task. Since early-blind individuals lacked visual input to make visual-olfactory associations, they could have compensated with a stronger verbal-olfactory association. However, since our results show that blind individuals have a worse performance in wine odor categorization, this suggests that they are penalized for their lack of visual input, and any verbal-olfactory compensation is not enough to match the visual-olfactory association. Put differently; it seems that blind individuals lack mental imagery abilities which are important when undertaking this type of task.
Finally, in Study 4, results show that congenitally-blind individuals outperform
late-blind individuals as well as sighted controls on an odor localization task. This finding provides support for the idea that congenitally-blind individuals rely more on their chemosensory perception to navigate throughout their environment, by retrieving spatial information from the surrounding odors.
Keywords : olfaction, odors, trigeminal, chemosensory, blindness, congenitally-blind,
vi
Table of Contents
Résumé ... i
Abstract ... iv
Table of Contents ... vi
List of Tables ... viii
List of Figures ... ix
List of Abbreviations ... x
Acknowledgments... xii
Chapter 1: Thesis overview ... 1
Chapter 2: Chemosensory perception in sighted and slind populations ... 3
2.1. Introduction to the olfactory and trigeminal systems ... 5
2.1.1. Olfactory system ... 5
2.1.2. Trigeminal system ... 8
2.1.3. The interaction between the two systems: Chemosensory perception ... 10
2.2. Chemosensory perception ... 12
2.2.1. Impact of odors on well-being and cognition ... 12
2.2.2. Impact of odors on the autonomic nervous system ... 13
2.2.3. Impact of labels on odor perception ... 13
2.2.4. Impact of individual factors on olfactory processing ... 15
2.2.5. Impact of nostril side of stimulation in chemosensory perception ... 16
2.2.6. Impact of other modalities on olfactory perception ... 18
2.3. Sensory perception in blind populations ... 19
2.3.1. Introduction to the blind population ... 20
2.3.2. Performance in other modalities ... 20
2.3.3. Impact of blindness on chemosensory perception ... 22
2.3.4. The need for more complex tasks to assess chemosensory performance ... 25
2.4. Rationale, objectives, and hypotheses ... 26
vii
Article 2: Objectives and hypotheses ... 30
Article 3: Objectives and hypotheses ... 31
Article 4: Objectives and hypotheses ... 31
Chapter 3: Articles 1 to 4 ... 32
Article 1: Now you like me, now you don’t – Impact of labels on odor perception ... 33
Article 2: Nostril advantage in trigeminal-olfactory perception and its relation to handedness ... 55
Article 3: Early-blind individuals show impaired performance in wine odor categorization81 Article 4: Chemosensory perception in congenitally-blind and late-blind individuals ... 106
Chapter 4: General Discussion... 123
4.1. Findings and General Implications ... 124
4.1.1. Odor evaluation, categorization and the impact of expectancy ... 124
4.1.2. Lateralization ... 129
4.1.3. The impact of vision loss ... 134
4.1.4. Impact of other individual factors ... 141
4.1.5. Clinical implications ... 144 4.2. Limitations ... 145 4.3. Future Directions ... 148 4.4. Summary ... 150 Bibliography ... i Appendices ... i Other Contributions ... i
viii
List of Tables
Article 1: Now you like me, now you don’t – Impact of labels on odor perception
Table 1. Five experimental odors ... 50 Table 2. Label fit measures ... 50 Article 3: Early-blind individuals show impaired performance in wine odor categorization
Table 1. Wine used for the categorization, differentiation and classification tasks ... 101 Appendix - Handbook of odors – Chapter 46 – The intranasal Trigeminal System
ix
List of Figures
Article 1: Now you like me, now you don’t – Impact of labels on odor perception
Schematic view of the protocol... 48
Ratings of odors based on their labels ... 48
Reaction times to labelled odors ... 49
Article 2: Nostril advantage in trigeminal-olfactory perception and its relation to handedness Proportions used as dichorhinic mixtures and physical mixtures ... 71
Schematic view of stimuli administration... 72
Results from Study 1 ... 73
Results from Study 2 ... 74
Mixtures ratings results from Study 2 ... 75
Article 3: Early-blind individuals show impaired performance in wine odor categorization Signal detection theory scores for wine odor categorization task... 97
Signal detection theory scores for wine odor differentiation ... 98
Signal detection theory scores for wine odor classification... 99
Scores for odor identification under the free and cued conditions ... 100
Article 4: Chemosensory perception in congenitally-blind and late-blind individuals Schematic View of the Protocol ... 117
Results for the odor localization task ... 118
Appendix - Handbook of odors – Chapter 46 - The intranasal Trigeminal System Appendix – The trigeminal Pathway ... xxxi
Appendix – Receptive range of four intranasal TRP receptors ... xxxii
Appendix – Procedure of trigeminal ERP recording ... xxxiii
Appendix – Electrophysiological recording sites ... xxxiv
x
List of Abbreviations
ANCOVA: Analysis of Covariance ANOVA: Analysis of Variance
BDI-II: Beck Depression Inventory, 2nd edition
CAPIN: Convergent Active Processing in Interrelated Networks (CAPIN) CB: Congenitally-Blind
CB-C: Congenitally-Blind Control Group CNV: Cranial Nerve 5 – trigeminal nerve CNV1: Cranial Nerve 5 – mandibulary nerve CNV1: Cranial Nerve 5 – ophthalmic nerve CNV2: Cranial Nerve 5 – maxillary nerve CO2: Carbon Dioxide
DTI: Diffusion Tensor Imaging ERP: Event Related Potential
fMRI: Functional Magnetic Resonance Imaging H2S: Hydrogen Sulphide
LB: Late-Blind Control Group LB-C: Late-Blind Control Group ORN: Olfactory Receptor Neuron PEA: Phenyl Ethyl Alcohol SEM: Standard Error of the Mean
STAI: The State-Trait Anxiety Inventory
STAIS: The State-Trait Anxiety Inventory (State)
STAIT: The State-Trait Anxiety Inventory (Trait)
TRP: Transient Receptor Potential
xi
To Cristian, Thank you for everything
xii
Acknowledgments
The achievement of this thesis has been a long process filled with incredible moments as well as difficult ones. I would not have been able to do this alone, and therefore I would like to thank everybody involved who contributed to its completion in one way or another. First and foremost, I would like to thank my research director Dr. Franco Lepore for not only giving me an opportunity to work in his lab, but also for all his support and generosity throughout the years. With your guidance, I was able to acquire extensive knowledge as well as develop great autonomy, which will be immensely valuable in my future career.
I would also like to thank my co-director Dr. Johannes Frasnelli for his support and his incredible efficiency. Despite being a professor at UQTR, he managed to always be present and supportive for any inquiry I would have. For that, I am incredibly thankful to have had you as my co-supervisor. A special thanks for introducing me to the world of odors. Who knew science could be smelly?
I would also like to thank Maria and Stéphane, for always being present and supportive throughout the years. A big thank you to Latifa for all her help regarding the analyses and her caring and supportive nature.
I am also thankful for all the friends that I made throughout this process (Zorina, Christine, Vanessa, Claudia, Valérie, Sabrina, Geneviève, and many others…). It has been a pleasure to exchange on our various research topics as well as non-related ones. To many more outings and unwinding after a hard day’s work!
I’m also very grateful towards my family. Thank you for being there for me throughout the years and giving me the tools, I needed to succeed. A special thanks to my sister for her kindness and support as well as proof-reading my thesis.
I want to thank my husband-to-be; Cristian. Thank you for being there for me, believing in me, reminding me to take breaks and being my timekeeper. Also, thanks to you, I did not have to learn “Photoshop” and for which I am eternally grateful.
Lastly, I want to thank Milo, for always cheering me up with his wagging tail and cookie demands and reminding me that peanut butter is the most essential thing in life.
2
Our nose is more than just for breathing and holding our eyeglasses. This protruding structure in the middle of our face enables us, with every breath we take, to perceive instantly and continuously the chemical constitution of our environment. Although rather unknowingly, our ability to smell various odors strongly influences our behavior. In fact, surrounding odors can not only trigger various specific emotions and memories (Delplanque, Coppin, & Sander, 2017), but they can also influence our autonomic nervous system (He, Boesveldt, Graaf, & Wijk, 2014). To have a better understanding of the various factors that can alter our olfactory perception, we developed four studies. Specifically, we were interested in evaluating how our sense of smell is altered by various internal factors (expectancy when smelling an odor and nostril advantage) in sighted populations. Given the importance of visual cues in olfactory processing, we also wanted to investigate how visual deprivation modulates the sense of smell. In Chapter 2, after briefly describing the anatomy, the functionality of the olfactory and the trigeminal systems as well their interaction with one another, we present some of the factors that can modulate our perception of the odors surrounding us. Specifically, we discuss how various odor qualities influence not only our perception but also our response times to them. We also discuss the impact of various inter-individual features and whether stimulating one nostril over the other influences our perception (nostril advantage). Finally, we address how olfactory perception is modulated by visual input and how visual deprivation influences the remaining intact modalities as well as olfactory perception.
In Chapter 3, we present the four articles of the manuscript. In the first article, we assess the impact of positively or negatively labeling odors on chemosensory perception. In the second article, we examine the influence of nostril dominance and its relationship with handedness. In the third article, we evaluate the influence of complex odors such as wine odors on olfactory processing in early-blind individuals. In the fourth and final article, we investigate odor localization performance in congenitally-blind and late-blind individuals.
In Chapter 4, we conduct a general discussion where we reflect on the influence of our results and on their various implications and limitations in regard to the literature on the subject. Finally, we address possible future directions in the hope of contributing towards the advancement of the field.
Chapter 2: Chemosensory perception in sighted and blind
populations
4
The sense of smell (olfaction), along with the trigeminal and gustatory systems, constitute the chemical senses, or chemosensory systems (Pickenhagen, 2017). Evolutionarily speaking, olfaction is considered to be the most primitive and oldest of the senses in the development of life on earth and developed long before species presented any form of brain development with cognitive abilities (Pickenhagen, 2017). Today, it plays a vital role in detecting and identifying food, selecting potential mating partners, navigating within our surroundings, to name only a few of its functions (Sarafoleanu, Mella, Georgescu, & Perederco, 2009). In humans, on top of contributing to the pleasure derived from eating and drinking, olfaction is also used to assess potentially dangerous situations (e.g., fumes that could indicate a fire), to determine the edibility of certain foods (e.g., nutritious vs. poisonous foods), and to a lesser extent, it contributes to social interactions (e.g., detection of potential mating partners; Hornung, 2013; Stevenson, 2009).
The sense of smell has some very unique features. Humans can discriminate a countless number of odors, although the exact number is difficult to estimate (Gerkin & Castro, 2015; Meister, 2015). One report suggests that we can differentiate at the utmost one trillion odors (Bushdid, Magnasco, Vosshall, & Keller, 2014), but the actual number is most likely in the thousands (Malnic, Hirono, Sato, & Buck, 1999). Furthermore, olfactionis the only sensory system in which neurons regenerate throughout life (Welge-Luessen, Leopold, & Miwa, 2013). Despite predating all the other senses, the chemosensory senses have received significantly less attention compared to other modalities, and much is still unknown.
The trigeminal system is a third chemical sense, next to smell and taste, and is responsible for providing various chemosensory information from the mouth and nose (sensations of warmth, coldness, heat, etc., Frasnelli & Manescu, 2017). However, this system received even less attention compared to the olfactory system, and it is only in the last years that literature in the domain has expanded. Furthermore, it is also an important system since it provides us with valuable information about odors and certain foods that we eat (ex: spiciness). More importantly, it also gives us information about the location of a potentially dangerous situation (e.g., smoke fumes would irritate our nose and inform us that a fire might be near and that we should retrieve to safety).
5
In this Chapter, we first present the olfactory and the trigeminal systems as well as how they interact with one another. Then, we discuss the various factors that can modulate chemosensory perception and what are the current questions that remained to be answered. Finally, we discuss how these questions led to the conception of our four articles. It should be noted that although the chemical senses also include the gustatory system, the focus of the current work was solely based on the first two systems.
2.1. Introduction to the olfactory and trigeminal systems
In contrast to the other senses which are triggered by signals of physical forces, e.g., the visual system where photons stimulate receptor cells in the retina and elicit a visual perception or pressure waves stimulate internal hair cells in the inner ear (Pickenhagen, 2017), the chemical senses are stimulated by a direct contact between the source substance and the receptors. Pure odorants will stimulate the olfactory system whereas trigeminal or irritant stimuli will stimulate the trigeminal system (Frasnelli & Manescu, 2017). Despite a handful of exceptions, most volatile substances (i.e., odors) will stimulate both, depending on the concentration. We will briefly describe these two systems and how they interact with one another.
2.1.1. Olfactory system
In this section, we present the path taken by the olfactory information from the odor molecules in the air, to our perception of that odor.
2.1.1.1. The periphery
Odorants from the air stream enter the nasal cavity during inspiration via the nostrils. Although most of the air stays in lower sections of the nasal cavity, some smaller amounts reach the upper portions of the nasal cavity up to the olfactory cleft. Here the airflow gets in contact with the olfactory epithelium which is distinct from the respiratory epithelium of the rest of the nasal cavity (Cowart & Rawson, 2008). The odorants may also enter the nasal cavity via the mouth and the nasopharynx, which is called the retronasal pathway as opposed to the orthonasal described above; (Cowart & Rawson, 2008). Retronasal olfaction is the main contributor to flavor perception, although most studies focus on orthonasal odorant delivery.
6
The olfactory epithelium has a total surface area of around 3-5cm2 and contains about 6-10 million olfactory receptor neurons (ORN; Leinders-Zufall & Ma, 2009). The olfactory epithelium lines the mucosa right below the cribriform plate, and it is composed of ORN and other types of cells (supporting cells, basal cells, and Bowman’s glands; Freiherr, 2017). The ORN are bipolar cells, and their dendritic extensions carry on its surface several cilia; where the olfactory receptors are located. The binding of an odorant molecule to the olfactory receptors activates its associated ORN and generates an action potential, initializing the first steps in olfactory processing.
Humans possess approximately 340-400 different functional olfactory receptor genes coding for ORN, and it is the most significant gene superfamily in the human genome (Olender, Lancet, & Nebert, 2008). However, each ORN expresses only one type of odorant receptor, and therefore each receptor is specialized for a small number of odorants (Buck & Axel, 1991). Perception of smells is done via the recognition of activation patterns across receptor types and thus enabling us to smell a quasi-unlimited number of odors.
2.1.1.2. The central section of the olfactory system
The ORN axons converge into the olfactory nerves which pass through the small holes of the cribriform plate and project ipsilaterally to the glomeruli in the olfactory bulbs and within the glomeruli to the mitral/tufted cells (Huart, Eloy, & Rombaux, 2013). The ORN carrying the same type of receptor send their axons to the same glomerulus in the olfactory bulbs. Therefore, we find a high level of convergence from thousands of neurons onto a few glomeruli which, by means of combinational code (or in a spatial pattern), make it possible for thousands of different odorant molecules to be recognized with a relatively low number of receptors (Malnic et al., 1999). Additionally, the olfactory bulb also contains periglomerular neurons and interneurons which transmit inhibitory signals to the neighboring glomeruli and the contralateral olfactory bulb leading to contrast enhancement. Those cells also receive retrograde inhibitory signals from higher order brain areas. This mechanism enhances the contrast between olfactory stimuli and background noise resulting in the amplification of olfactory information which contributes to the sensitivity of the system (Freiherr, 2017). In other words, this amplification allows us to detect odorants at low intensities.
7
A small note should be made about the olfactory bulb. Although in humans, the relative size of the olfactory bulb is small compared to the rest of the brain (0.01%; Kavoi & Jameela, 2011), whereas the relative size is larger in other species (e.g., 2% in mice Baron, Frahm, & Stephan, 1988). However, its absolute size is bigger compared to other animals (humans: 60mm3; Kavoi & Jameela, 2011vs. rats; 27mm3: Hinds & McNelly, 1981). Yet, the number of neurons within the olfactory bulb is surprisingly similar across species (McGann, 2017), indicating that human show excellent olfactory abilities comparable to other mammals. In contrast to popular belief, humans are similarly sensitive as dogs, rabbits, and monkeys are to specific odorants (McGann, 2017).
The axons of the mitral cells from the olfactory tract; via this tract olfactory pathways lead to the primary (anterior olfactory nucleus, piriform cortex, amygdala, and the entorhinal cortex) and secondary olfactory cortex (orbitofrontal cortex, insula, ventral striatum, cerebellum, brainstem, hippocampus, and hypothalamus (Freiherr, Wiesmann, & Witt, 2014)). It is important to point out that these processing structures are not exclusively dedicated to olfaction; unlike the other sensory systems. Next, there is no thalamic relay, which is another particularity of the olfactory system. Finally, the projections of the olfactory cortex are mainly ipsilateral, but there are also few contralateral connections via the anterior commissure (Lascano, Hummel, Lacroix, Landis, & Michel, 2010).
Various structures of the olfactory pathway get activated depending on the nature of undergoing tasks. For instance, the anterior portion of the piriform cortex is involved in basic or low olfactory processing such as passive smelling and encoding of the chemical or molecular structure of an odorant (Gottfried, 2010). The posterior portion is responsible for the higher order olfactory processing such as hedonic quality evaluation or discrimination and encoding of the perceptual character of an odorant (Gottfried, Winston, & Dolan, 2006; Howard, Plailly, Grueschow, Haynes, & Gottfried, 2009; Lundstrom, Boesveldt, & Albrecht, 2011). The piriform cortex is also involved in olfactory memory and multisensory integration (Gottfried & Arnold, 2006). The amygdala, however, gets activated by intensity or valence of stimuli (Winston, Gottfried, Kilner, & Dolan, 2005), but is also involved in olfactory memory (Gottfried & Arnold, 2006). Since the entorhinal cortex has robust projections to the hippocampal formation, it is not surprising that it is also involved in olfactory memory processes (Zald & Pardo, 2000).
8
Although the role of the insula in olfactory processing is somewhat unclear, it seems to play a vital role during the integration of olfaction with trigeminal and gustatory modalities (Lundstrom et al., 2011). High-order processing takes place within the orbitofrontal cortex which is involved in the integration of olfaction with other sensory modalities (Ongür & Price, 2000). It also seems to be involved in odor discrimination processes as well as recognition and reward value of an odor (Rolls, 2004b, 2004a). Finally, hemispheric differences are dependent on the task at hand. For instance, the left hemisphere is more engaged in the processing of familiar odors, whereas the right hemisphere is more implicated in the processing of unpleasant odors (Savic, 2002).
Overall, olfactory processing seems to be initially localized ipsilaterally in the primary olfactory cortex (piriform cortex, ehrorhinal cortex, amygdala, parahippocampal gyrus, and insula) followed by an activation of these structures contralaterally, followed by an activation of the bilateral frontal areas (Lascano et al., 2010). To sum, cortical olfactory processing follows a hierarchical and parallel pathway, and numerous factors can influence which regions get activated (i.e., type of task, nature of the stimuli, unilateral vs. bilateral stimulation, etc.).
There are a handful of pure odorants that will stimulate the olfactory system with little to no co-stimulation of the trigeminal system, such as phenyl ethyl alcohol (PEA, a rose-like odorant), vanillin (a vanilla-like odorant), hydrogen sulphide (H2S; rotten egg-smell; Jacquot, Hidalgo, & Brand, 2010; Kobal, Van Toller, & Hummel, 1989) and decanoic acid (a rancid-like odorant; Doty et al., 1978). However, it is argued that even some of the pure odorants can co-activate the trigeminal system at high enough concentrations (Frasnelli, Hummel, Berg, Huang, & Doty, 2011), and that most odorants, if not all, can co-activate the trigeminal system (Doty et al., 1978).
2.1.2. Trigeminal system
This system relies on the trigeminal nerve, which is the fifth and thickest cranial nerve (CN V) due to its three branches composition: the ophthalmic nerve (CN V1), the maxillary nerve (CN V2) and the mandibulary nerve (CN V3) (Huff & Daly, 2018). It is mainly responsible for facial sensation such as touch, pain, heat, and pressure but is also responsible for
9
biting and chewing. On top of innervating facial skin, it also innervates the mucosa of the nose, mouth, and eyes which contributes to chemosensory/trigeminal perception.
At the receptor level, the trigeminal system is mainly composed of ion channel-based receptors belonging to the subfamily of the transient receptor potential (TRP), although there is also evidence of non-TRP channels which get activated by nicotine (nicotinic acetylcholine receptors) and acids (proton-gated ion channels; Thuerauf, Kaegler, Dietz, Barocka, & Kobal, 1999; Waldmann, Champigny, Bassilana, Heurteaux, & Lazdunski, 1997). What is particularly interesting about the TRP receptors is that they are polymodal nociceptors because they respond to both thermal and chemical stimuli (Boonen, Startek, & Talavera, 2017). When these receptors get activated, they give rise to various types of sensations such as cooling, burning, stinging and tingling (Sell, 2014). Although there is much less receptor diversity compared to the olfactory nerve, the activation of the trigeminal system does lead to various sensations such as cooling, stinging, tingling, burning, etc. (Frasnelli, 2013). One important note is that once the inhaled air enters our nasal airways, it gets heated up to 30-32oC (Keck, Leiacker, Riechelmann, & Rettinger, 2000). Therefore, any deviation from these temperatures could be perceived on a continuum from painfully cold to noxiously hot. Specifically, to name a few receptors, TRPA1 receptor gets activated by cold temperatures below 17oC as well as by compounds in mustard oil and various irritant gases, giving a dull and painful sensation (Bandell et al., 2004). The TRPM8 receptor, however, gets activated by mainly cool temperatures between 15-28oC, as well as cooling compounds like menthol and eucalyptol (McKemy, Neuhausser, & Julius, 2002). On the warmth sensations spectrum, the TRPV3 receptor gets activated at warm temperatures between 33-35oC and is also activated by compounds in spices like thyme and oregano, and gives rise to sensations of warmth (Peier et al., 2002; Xu, Delling, Jun, & Clapham, 2006). Finally, the TRPV1 receptor gets activated by high temperatures above 43oC, and by the compounds in spices and cloves (capsaicin and eugenol) as well as various acids (Caterina et al., 1997; Jordt et al., 2004; Yang et al., 2003), which leads to sensations of tingling that can become sharp, painful and give a burning sensation at higher intensities (Frasnelli, Albrecht, Bryant, & Lundstrom, 2011).
From these receptors, the signal travels through the fibers of the trigeminal nerve, passes through the epithelium of the nasal cavity and then converges on the trigeminal ganglion,
10
although no information is transferred at this stage, as these are pseudo-unipolar neurons similar to the ones found in spinal nerves (Frasnelli & Manescu, 2017). From there, the nerve fibers project to the brainstem where the first relay takes place and continues to the second relay located in the thalamus and a third and final relay project to the somatosensory areas. As all somatosensory projections, the trigeminal pathway is crossed; however, some authors also suggest additional ipsilateral projection sites, much like the olfactory pathways (Brand, 2006). It is important to note that throughout this pathway, the fibers remain arranged in a somatotopic fashion (i.e., branches of the ophthalmic nerve are placed above those of the maxillary nerve; Borsook, Burstein, Moulton, & Becerra, 2006). The trigeminal system not only activates the somatosensory structures but also activates the brainstem, ventrolateral posterior thalamus, anterior cingulate and precentral gyrus (Albrecht et al., 2010).
One particularity of the trigeminal system is that unlike pure odorants, chemosensory stimuli which activate the trigeminal system can be localized by humans (Frasnelli, Charbonneau, Collignon, & Lepore, 2009). In other words, humans can determine the stimulated nostril only if the stimuli contain a trigeminal quality.
2.1.3. The interaction between the two systems: Chemosensory perception
As previously mentioned, only a handful of odorants will solely activate the olfactory system, indicating that most compounds, will eventually recruit both the trigeminal and the olfactory systems (Doty et al., 1978; Frasnelli, Hummel, et al., 2011). Therefore, there is no surprise that these two systems interact by suppressing and enhancing each other (Hummel & Livermore, 2002).An interesting way to assess the interaction between these two systems is to evaluate individuals with olfactory dysfunction and its impact on trigeminal perception or vice versa (Frasnelli & Hummel, 2007). For instance, individuals with anosmia (individuals who have lost their ability to smell) remain able to detect many volatile chemicals suggesting that stimuli perception is processed by the trigeminal nerve (Doty et al., 1978). Furthermore, individuals with anosmia have a lower trigeminal sensitivity compared to individuals who have a healthy olfactory system, highlighting the interdependence of the two systems. Put differently, the optimal functionality of one system is depended on the other (Gudziol, Schubert, & Hummel,
11
2001; Hummel et al., 1996). Indirect interactions also exist where trigeminal reflexes would alter the mucus patency in the olfactory epithelium, and thus alternating olfactory processing (Finger, Getchell, Getchell, & Kinnamon, 1990). The inverse is also true, where odorants can trigger the release of various peptides and analgesic effects from the trigeminal fibers (Brand, 2006), once again influencing olfactory perception.
Finally, there are central interactions where both olfactory and trigeminal stimuli coactivate the same brain regions. Namely, 10% of the piriform cortex gets activated by both systems along with the orbitofrontal cortex, the rostral insula and superior temporal gyrus (Hummel, Boyle, Heinke, Gerber, & Frasnelli, 2007). Additionally, chemosensory regions (orbitofrontal cortex) and regions of multisensory integrations (intraparietal sulcus) were more strongly activated when a mixture of CO2 (trigeminal stimuli) and PEA (pure odorant) was presented compared to their activation when both stimuli were presented independently (Boyle, Frasnelli, Gerber, Heinke, & Hummel, 2007); supporting the enhancing and interconnectivity properties of both systems.
Even though the trigeminal pathway is crossed, as is the case with all somatosensory projections, some authors also suggest additional ipsilateral projection sites, much like the olfactory pathways (Brand, 2006). Possibly as a consequence, mixed trigeminal/olfactory stimuli appear to be predominantly processed ipsilaterally and is thus closely interconnected with the olfactory pathways (Hummel et al., 2009). Furthermore, right-hemisphere lateralization predominance was shown in the processing of not only relatively pure trigeminalstimuli (Hari, Portin, Kettenmann, Jousmaki, & Kobal, 1997), but also predominantly olfactory stimuli (Yousem et al., 1997) as well as mixed trigeminal/olfactory stimuli (Savic & Gulyas, 2000; Zatorre, Jones-Gotman, Evans, & Meyer, 1992).
To sum, it is undeniable that the olfactory and the trigeminal systems are closely intertwined, and generally, the activation of one often modulates the activation of the other. It should be noted that for simplicity reasons, within the literature, we often refer to the olfactory system even when the trigeminal system is implicated as well. Similarly, we often refer to odors as the percept evoked by olfactory stimulations (i.e., what we smell) and odorant as the stimuli that activate the olfactory system but can also activate the trigeminal one as well. Although more exact terms could be used such as chemosensory, chemical senses, chemicals or irritants, we
12
will maintain the same terminology as throughout the literature for simplicity, readability and uniformity reasons.
2.2. Chemosensory perception
Beyond the unique mechanisms of the olfactory and trigeminal systems, the perception of odors is influenced by the subjective experience of an individual. For instance, a rose odor could trigger a positive and vivid memory of one’s childhood and consequently ignite a feeling of well-being (Chu & Downes, 2002). However, this chain of events is unique to each persons’ subjective experience and their perception of that odor may be very different from someone else’s (i.e., a person who fell into a rosebush when they were young, might have an aversive reaction to a rose odor; Toffolo, Smeets, & van den Hout, 2012). In this section, we first discuss the impact of odor valence on well-being, cognition, and chemosensory perception, Then, we address the influence of odor labeling on an internal state (expectancy) which in turn influences olfactory perception. Finally, we discuss other internal factors (nostril advantage and handedness) and their impact on chemosensory perception.
2.2.1. Impact of odors on well-being and cognition
Pleasant and unpleasant odors have a significant impact on our mood and well-being in various ways, which can explain some of the popularity and benefits of aromatherapy (Nibbe & Orth, 2017). For instance, pleasant odors such as orange and peppermint tend to improve mood and calmness, as well as reduce anxiety (Lehrner, Eckersberger, Walla, Potsch, & Deecke, 2000; Moss, Cook, Wesnes, & Duckett, 2003; Moss, Hewitt, Moss, & Wesnes, 2008; Schifferstein, Talke, & Oudshoorn, 2011; Schiffman, Suggs, & Sattely-Miller, 1995). On the other hand, unpleasant odors, (e.g., pyridine; which has a fishy-rotten smell), decrease mood and increase anxiety (Schiffman, Miller, Suggs, & Graham, 1995; Villemure, Slotnick, & Bushnell, 2003).
Pleasant odors not only improve our mood but also affect cognitive performances as measured by neuropsychological tests. Pleasant rosemary odors enhance memory performance on a battery of tests (Moss et al., 2003), and pleasant fragrances (lemon and floral scents) led to better performance on an anagram task (Baron & Thomley, 1994). Pleasant odors also increase performance on visual and sustained attention task (Warm, Dember, & Parasuraman, 1991).
13
Additionally, even merely suggesting the presence of a pleasant ambient odor (when in fact no odor was present), led to reports of better moods as well as fewer physical symptoms (such as back pain, throat problems, irritated eyes) compared to when an unpleasant ambient odor was suggested (Knasko, Gilbert, & Sabini, 1990). These findings suggest that the valence of the odors we smell or that we think we smell influences not only our perception of those odors but also our cognition and our well-being.
2.2.2. Impact of odors on the autonomic nervous system
Along with the effects of odor valence on the aforementioned subjective measures, odor valence can also impact various psychophysiological measures. For instance, unpleasant odors trigger faster reaction times (Jacob & Wang, 2006), especially if they are unpleasant edible odors (i.e., smell of rotten fish), versus any other type of odor (i.e., musky odor or pleasant edible odor of strawberries; Boesveldt, Frasnelli, Gordon, & Lundstrom, 2010). Unpleasant odors also increase skin conductance (Brauchli, Ruegg, Etzweiler, & Zeier, 1995), heart rate (Alaoui-Ismaili, Vernet-Maury, Dittmar, Delhomme, & Chanel, 1997; Bensafi et al., 2002; Brauchli et al., 1995) and the startle reflex (Miltner, Matjak, Braun, Diekmann, & Brody, 1994), whereas pleasant odors will decrease all these measures. Additionally, participants who perceive androsterone as a negative odor (i.e., “ruinous” or “sweaty”) exhibit larger event-related potential (ERP) amplitudes than those who perceive it as a positive odor (“wooden” or “floral”; Lundström, Seven, Olsson, Schaal, & Hummel, 2006) suggesting once again a stronger reaction to negative odors. This is believed to be an evolutionary survival mechanism to protect us from rotten foods that would most likely make us ill if ingested.
2.2.3. Impact of labels on odor perception
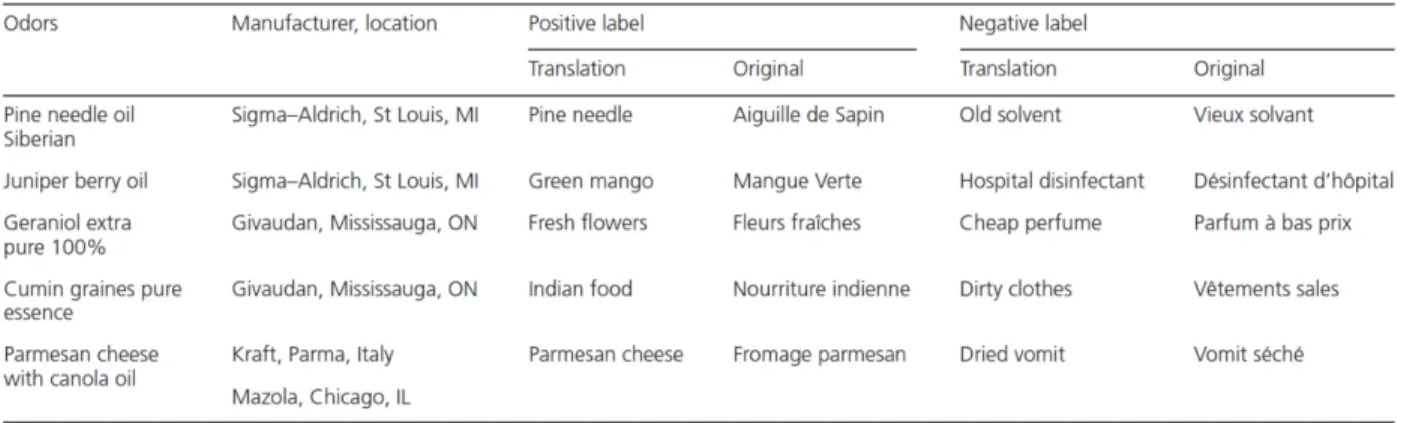
On top of odors modulating our reaction times, merely labeling an odor differently influences our perception of that odor (Bensafi et al., 2012; Djordjevic et al., 2008; Herz & von Clef, 2001). Specifically, having a positive label (such as parmesan cheese) of an odor makes us perceive it as more pleasant (Ayabe-Kanamura, Kikuchi, & Saito, 1997; Bensafi, Rinck, Schaal, & Rouby, 2007; De Araujo, Rolls, Velazco, Margot, & Cayeux, 2005; Djordjevic et al., 2008) and more arousing (Djordjevic et al., 2008) compared to the same odor presented under
14
a negative label (such as dried vomit). On the other hand, negatively labeled odors are perceived as more intense than positively labeled odors (Chen & Dalton, 2005; Djordjevic et al., 2008).
This odor bias is seen best when the odors are ambiguous (Bensafi et al., 2007; Herz & von Clef, 2001). In this context “ambiguous odors” can be either perceived as positive or negative depending on the context. For instance, the smell of cheese is not particularly a good smell, but when presented in a restaurant, it will more likely be perceived as a positive odor. However, if the same smell is present in a dark alley, the odor will more likely be perceived as a foul odor. Furthermore, these findings appear to be true mainly if the labels are congruent with the corresponding odors (Herz & von Clef, 2001). More specifically, the odors not only have to be ambiguous enough so that they can be interpreted as either positive or negative, but their associated labels have to “fit” accordingly (i.e., “parmesan cheese” for the positively labeled odor and “dry vomit” for the negatively labeled one). In other words, labeling parmesan cheese as “rose” will not be a good label fit and thus weakens the labeling effect on our perception of that odor.
Finally, although the impact of labels on intensity, pleasantness, and arousal was previously reported (Djordjevic et al., 2008), the impact of labels on the edibility of ambiguous odors has yet to be studied. Since previous reports showed a positive correlation between pleasantness and edibility evaluation of various odors (Ayabe-Kanamura et al., 1998), one may expect a similar link and evaluation of differently labeled odors. Furthermore, as previously reported, some researchers investigated the impact of odor valence on physiological measures such as reaction times, yet none have evaluated the impact on reaction times of differently labeled odors of positive and negative valence.
To sum, odor valence or the simple suggestion of the presence of an odor, as well as the context in which they are presented, have not only the ability to influence our interpretation of these odors but also how quickly we respond to them. This led to the conception of our first study which evaluated the impact of negatively and positively labeling odors on reaction times and their subjective ratings (see “Article 1: Objectives and Hypothesis” section for more detail).
15
2.2.4. Impact of individual factors on olfactory processing
We have previously addressed how a factor such as odor labeling can influence our olfactory perception, yet other internal factors can also affect odor perception. For instance, anxious and neurotic males detect odors faster compared to controls, suggesting that personality or individual characteristics can modulate reaction times (Chen & Dalton, 2005). In fact, high levels of anxiety are associated with a lower olfactory threshold (Rovee, Harris, & Yopp, 1973), suggesting that they are more sensitive to detect odors. However, patients in the acute phase of major depression exhibit significantly higher olfactory thresholds but do recover to their initial olfactory threshold after completing their depression treatment (Pause, Miranda, Goder, Aldenhoff, & Ferstl, 2001).
Gender and age also affect olfactory performance. Women not only have a better sense of smell (Brand & Millot, 2001) and detect pleasant and unpleasant odors faster than men (Jacob & Wang, 2006) but they are also able to increase their sensitivity via olfactory training whereas men could not (Dalton, Doolittle, & Breslin, 2002). Additionally, women seem to be more sensitive to odors in the fertile phases of their menstrual cycle than in the non-fertile phases (Nováková, Havlíček, & Roberts, 2014). Numerous reports confirmed that olfactory performance diminishes with age and that a decreased olfactory function is found in over half of the population between the ages of 65-80 (for a review see Doty & Kamath, 2014).
Smokers also have a decreased olfactory function, and, when controlling for age, their performance worsens with each year that they continue to smoke (Katotomichelakis et al., 2007). Various medication intake can also modulate our olfaction, yet it is not uncommon that the underlying medical condition for which the prescribed medication was given (i.e., epilepsy, migraines, hypothyroidism, schizophrenia, infections, etc.) is also responsible for olfactory impairment or distortion (Doty & Bromley, 2004). Finally, recent literature highlights that olfactory dysfunction seems to be an early sign in a neurodegenerative disorder such as Alzheimer’s (Zou, Lu, Liu, Zhang, & Zhou, 2016) and Parkinson’s disease (Fullard, Morley, & Duda, 2017). Consequently, these factors should be considered or at least controlled for in chemosensory studies, as we did in the research presented in this manuscript.
16
2.2.5. Impact of nostril side of stimulation in chemosensory perception
Another internal factor that could influence chemosensory perception is which nostril is stimulated. Humans can localize auditory sources by comparing simultaneous inputs from both ears (Ahveninen, Kopčo, & Jääskeläinen, 2014), and the same process can be applied to olfaction (Porter et al., 2007). In contrast to the prevailing notion, each nostril inspires air from distinct, non-overlapping regions in space (Porter et al., 2007). In other words, humans can compare inputs from both of their nostrils and be able to track or localize a scent, much like dogs are able to. Porter at al. (2007) also showed a natural asymmetry in the airflow of each nostril, which could suggest that one nostril may be more dominant than the other and thus receive additional olfactory information resulting in increased olfactory performance. Put differently, much like we have a hand preference (left vs right-hand), it could be theorized that such a preference can be found within the olfactory system (e.g., nostril dominance).
Although some studies failed to find nostril sensitivity differences with regards to relatively pure odorants (Betchen & Doty, 1998; Frasnelli, Livermore, Soiffer, & Hummel, 2002; Zatorre & Jones-Gotman, 1990) and trigeminal stimuli (Stuck et al., 2006), a right-nostril dominance was reported for mixed trigeminal/olfactory stimuli (Frasnelli et al., 2009; Youngentob, Kurtz, Leopold, Mozell, & Hornung, 1982). On top of the significant variations in terms of which type of odors were used (pure odorants, trigeminal or mixed), within these studies there was also significant variation in terms of methodology (monorhinal consecutive stimulation, passive vs. active smelling, etc.). Therefore, to truly evaluate nostril dominance, our research team had previously developed a squeeze-bottle paradigm which allowed for simultaneous nostril stimulation which can more accurately assess nostril dominance compared to evaluating each nostril separately (Frasnelli et al., 2009). From this study, it was concluded that there seems to be a right-nostril advantage independent of handedness and that pure odorants (e.g., phenyl ethyl alcohol; rose-like odor) were not localizable (e.g., unable to accurately state which nostril received the odor), which led us to focus on mixed trigeminal/olfactory stimuli.
Further, chemosensory stimuli presented to the right nostril are not only rated as more pleasant (Herz, McCall, & Cahill, 1999), as more intense (Thuerauf et al., 2008), as more familiar (Hudry et al., 2014), as easier to discriminate (Hummel, Mohammadian, & Kobal,
17
1998; Zatorre & Jones-Gotman, 1990; Savic & Berglund, 2000), and as easier to detect (Toulouse & Vaschide, 1899; Youngentob, Kurtz, Leopold, Mozell, & Hornung, 1982) but also generate faster reaction times (Kéïta, Frasnelli, La Buissonnière-Ariza, & Lepore, 2013). Altogether, these results indicate that a right-nostril sensitivity might exist although this finding is not always systematic.
Additionally, since pure olfactory and mixed/olfactory stimuli are predominantly processed ipsilaterally, and a right-hemisphere lateralization predominance was shown in the processing of chemosensory stimuli (Hari et al., 1997; Savic & Gulyas, 2000; Zatorre et al., 1992), a right-nostril stimulation could be advantageous in terms of chemosensory processing and thus could explain the tendency for a right-nostril advantage.
Furthermore, previous reports established that handedness has a clear effect on language lateralization; right-handed individuals had stronger left hemisphere lateralization, contrasting left-handed individuals who have stronger right hemisphere lateralization (Knecht et al., 2000). One report showed that unpleasant odors activated the left ventral insula in right-handers, whereas these odors activated the right ventral insula in left-handers, confirming that hemispheric lateralization of odor processing is influenced by handedness, at least to some extent (Royet, Plailly, Delon-Martin, Kareken, & Segebarth, 2003). Therefore, based on the lateralization of chemosensory perception and the hemispheric differences between right and left-handers, one may expect that handedness could also influence this potential nostril advantage.
In another report, right-handed participants performed significantly better with their right nostril whereas left-handed participants performed significantly better with their left nostril on olfactory discrimination (Hummel et al., 1998) and threshold (Youngentob et al., 1982), suggesting a link with handedness. However, other reports indicate that the right nostril advantage may be due to an advantage of this particular nostril, independent of handedness (Bensafi et al., 2003; Frasnelli et al., 2009). The right-nostril advantage could soley be found in right-handed individuals (Zatorre & Jones-Gotman, 1990). However, some report fails to find any effect of handedness (Hebbal & Mysorekar, 2003). For instance, there was no effect of handedness on the amplitude or latency of olfactory event-related potentials while actively smelling pure odorants (Gottschlich & Hummel, 2015), or passively smelling them (Lübke, Gottschlich, Gerber, Pause, & Hummel, 2012a).
18
Overall, the literature on the subject suggests that if this effect exists, it is yet to be understood. The current literature proposes that a right nostril advantage may be due to either an advantage of this particular nostril, independent of handedness (Bensafi et al., 2003; Frasnelli et al., 2009), or it could be linked to handedness (Hummel et al., 1998; Youngentob et al., 1982), or simply that a right-nostril advantage can solely be found in right-handers. Since little is known about the impact of handedness on nostril dominance, this led to the conception of our second study, where we evaluated nostril dominance and whether this effect is present and whether it is linked with handedness (see “Article 2: Objectives and Hypothesis” section for more detail).
2.2.6. Impact of other modalities on olfactory perception
On top of all the factors above, olfactory processing is also influenced by cues from other sensory modalities, resulting in multifaceted processing (Seo & Hummel, 2017). For instance, when eating a piece of bacon, we receive various sensory cues such as the smell of it (olfactory), its color and shape (visual), its texture (tactile), its crispiness (auditory), and finally its taste (gustatory). However, if one of these cues is altered in an unexpected manner (ex: a green bacon strip instead of a golden crispy brown), it will impact our olfactory interpretation of that item; it will be perceived as less flavorful/pleasant. Therefore, it is not surprising that cues from other modalities influence olfactory processing.
Specifically, research showed an increase in olfactory sensitivity in the presence of a congruent taste (i.e., saccharine presented with cherry odor) and a decrease in olfactory sensitivity when presented with an incongruent taste (i.e., monosodium glutamate – savory/umami taste presented with a cherry odor; Dalton, Doolittle, Nagata, & Breslin, 2000). A similar effect was found, when an odor (i.e., cinnamon) was judged as more pleasant when presented with a congruent song (i.e., Christmas carol) compared to when it was presented with an incongruent song (i.e., pop music; (H. S. Seo, Lohse, Luckett, & Hummel, 2014)). Similarly, with regard to the olfactory-tactile cross-modality, one study found that participants rated an inodorous creamy custard more congruent with vanilla or buttery odors and incongruent with the other aromas (rubbery, chocolate and lavender; Harthoorn et al., 2008). In sum, gustatory, auditory and to a lesser extent tactile, are all modalities that can influence olfactory perception.
19
Vision also largely influences olfactory perception. As previously mentioned, merely labeling an odor differently altered expectancies which in return affected the perception of the odorant (Djordjevic et al., 2008). Although unknowingly to us, odor identification is often difficult, and that stems from the fact that we often are presented with visual cues when smelling odors (i.e., the smell of coffee is often co-presented with a cup of coffee (Seo & Hummel, 2017)). However, when these cues are not present, labeling an odor is quite difficult. A recent review showed how congruent visual cues (i.e., colors, pictures. symbols, etc.) increases response times, accuracy, intensity, and strengthens the hedonic value of the presented odor (i.e., a congruent visual cue would increase odor pleasantness for pleasant odors, and would increase unpleasantness for unpleasant odors (Seo & Hummel, 2017)). The influence of visual-olfactory congruency has also been highlighted in imaging studies where the orbitofrontal cortex and insular cortex activated progressively and proportionally more as the perceived congruency increased (Osterbauer et al., 2005).
These studies highlight the importance of visual input in olfactory processing, which raises the question of how olfactory perception is modulated in the case of visual deprivation. Aside from olfactory perception, we also integrate visual, and auditory cues to explore and navigate throughout our surroundings (Millar, 2008). Therefore we might expect that blind individuals have a worse performance not only on all olfactory tasks but also in all the other senses. However, this does not seem to be the case since reports showed a supra-performance in blind individuals in auditory (Gougoux et al., 2004) and tactile tasks (Norman & Bartholomew, 2011). Therefore this led to investigations on how visual deprivation impacts the remainder of the intact modalities, and particularly its impact on chemosensory processing.
2.3. Sensory perception in blind populations
Anecdotal evidence supports the idea that losing one’s sight could enhance the remainder of the other functions. For instance, Stevie Wonder and Ray Charles, whom both became blind at an early age, could have benefited from a heightening of the remainder of their senses, namely auditory, which helped them in their music career. Although this remains anecdotal, sensory compensation was reported repeatedly for blind individuals. Therefore, evaluation of blind individuals could provide interesting information about the chemosensory processing and how
20
it can adapt, and possibly become enhanced in the presence of a sensory loss. In the present section, we elaborate on how an internal factor such as blindness can influence chemosensory perception.
2.3.1. Introduction to the blind population
Brain plasticity, or neuroplasticity, is the brains’ ability to adapt and change throughout the lifespan (Oberman & Pascual-Leone, 2013). Extensive pioneer work has shown the presence of a critical, early-period of postnatal life where the brain is most receptive to change (Oberman & Pascual-Leone, 2013), and this is especially true for visual development (Hooks & Chen, 2007). In that sense, early visual deprivation compared to late visual deprivation triggers a systematic whole-brain reorganization of cortical networks (Hasson, Andric, Atilgan, & Collignon, 2016). Therefore, a great deal of research evaluates the performance of blind individuals in different modalities in order to have a better understanding of how the brain adapts to visual loss. Not surprisingly, the age of blindness onset is an important factor when evaluating its impact on brain plasticity. Therefore, blind subjects are usually classified into three categories; the congenitally blind who have lost their vision at birth or soon after, the early-blind who lost their vision within the two-three years of their life and late-blind who have lost their vision during adulthood (Burton, 2003). Since the occipital lobe is responsible for visual processing and perception composes around 20% of the whole brain (Krug, 2012), this whole region could potentially be undertaken and rehabilitated by other modalities via neuroplasticity mechanisms.
2.3.2. Performance in other modalities
With regards to the auditory modality, compared to controls, blind subjects are better at judging a change in pitch directionality (Gougoux et al., 2004), accurately mapping their auditory environment and localizing sounds monaurally (sounds which are presented with one ear blocked). Age of blindness onset is an important factor in auditory localization performance since the earlier they lost their vision, the better they performed (Gougoux et al., 2004). Furthermore, blind people with residual peripheral vision localized sounds less accurately than controls (Lessard, Pare, Lepore, & Lassonde, 1998a), indicating that a total loss of vision is better compensated for than a partial loss (for example those who have residual vision). Even a
21
short and transient period of visual deprivation (9 to 238 days of age) was enough to trigger cross-modal changes within the occipital cortex (Collignon et al., 2015). Actually, functional magnetic resonance imaging (fMRI) studies promote the idea that the recruitment of additional areas such as the occipital cortex (Burton, 2003) could explain the increase in auditory processing. Namely, pre-existing pathways linking the auditory and the visual cortex would be unmasked and strengthened in the absence of vision; thus contributing to a heightened auditory perception (Burton, 2003).
In terms of the tactile modality, blind subjects are better at discriminating grating orientations as well as 3-D shapes compared to sighted controls (Norman & Bartholomew, 2011). However, for the 3-D shapes, early-blind and late-blind subjects show better discrimination compared to congenitally blind, indicating that some degree of vision is required to process 3-D shapes accurately. Higher tactile acuity in blind subjects was also found in grating orientation tasks even when different covariates such as the degree of vision during childhood, light perception level and Braille reading expertise were taken into account (Goldreich & Kanics, 2003).
Although few studies have examined the gustatory modality in blind subjects, some propose a superior taste sensitivity in the blind (Terner, Nagy, & Jávor, 1987; and Mahner, 1909 as cited by Gagnon, Kupers, & Ptito, 2013) although others fail to support such finding (Gagnon et al., 2013; Smith, Doty, Burlingame, & McKeown, 1993). In fact, congenitally blind subjects seem to have increased taste thresholds – meaning lower sensitivity - which could be explained by an underexposed gustatory system due to visual loss limitations (Gagnon et al., 2013).
Another body of literature also found recruitment of the occipital cortex of congenitally blind and early-blind subjects during higher cognitive tasks such as attention and memory (Simon-Dack, Rodriguez, & Teder-Salejarvi, 2008), verbal memory (Amedi, Raz, Pianka, Malach, & Zohary, 2003) and verb generation tasks (Amedi et al., 2003; Burton et al., 2002). Despite a few studies promoting cross-modality plasticity of the occipital cortex, some studies challenged the idea by suggesting that the occipital cortex is recruited in auditory and tactile tasks due to higher cognitive demands rather than the direct recruitment of those modalities (Roder, Rosler, Hennighausen, & Nacker, 1996; Simon-Dack et al., 2008). Furthermore, task difficulty also modulated occipital activation independent of stimulus modality (Roder et al., 1996). Anatomical studies showed the presence of direct projections from the auditory cortex
22
to the visual cortex in healthy adult monkeys, highlighting that not only such a pathway could be present in healthy humans (Collignon, Lassonde, Lepore, Bastien, & Veraart, 2007; Rockland & Ojima, 2003), but these projections could also extend from the visual cortex to the somatosensory cortex as well (Ptito, Schneider, Paulson, & Kupers, 2008). A diffusion direction analysis study also found support for neural reorganization promoting functional adaptation to visual loss during the early development period suggesting the development of “non-traditional” pathways in blind subjects (Park et al., 2007). Although the neuroplastic mechanisms underlying crossmodal recruitment of the visual cortex following visual deprivation remains unclear (Bavelier & Neville, 2002), these studies do provide some evidence for the existence of “masked” multi-modal cortical pathways which could hypothetically increase performance in other modalities. Therefore, one can hypothesize that similar “masked” multi-modal cortical pathways could also be present for chemosensory processing, which are unveiled in blind individuals.
Although few studies have shown that blind subjects do have superior sensory functions compared to sighted subjects in different modalities (Burton, 2003), far less is known about the impact of blindness on chemosensory abilities. Based on the concept of brain plasticity and performance in the other modalities, we can speculate that vision loss could also enhance their chemosensory abilities. In that way, blind individuals can help us better understand the impact of an internal factor such as vision loss and its repercussion on chemosensory performance.
2.3.3. Impact of blindness on chemosensory perception
To assess olfactory function in blind subjects, researchers usually look at different tasks, which have varying levels of peripheral and central involvement. These tasks are odor detection thresholds (sensory level), odor discrimination (basic level of perception) and identification (higher order of perceptual processes). Therefore, this could lead to the emergence of behavioral adjustments in which compensatory mechanisms enhance the intact modalities at any of these levels.
The olfactory threshold is the lowest odorant concentration at which someone can detect an odor. It is usually assessed by standard olfactory tests like the Sniffin’ Sticks olfactory threshold test (Hummel, Sekinger, Wolf, Pauli, & Kobal, 1997) which use pen-like odorants of n-butanol. When investigating olfactory thresholds, some found no differences between blind
23
and control subjects (Schwenn, Hundorf, Moll, Pitz, & Mann, 2002), and others found that blind subjects had higher thresholds compare to control subjects (Murphy & Cain, 1986). Other studies found a lower olfactory threshold in congenitally blind subjects (Beaulieu-Lefebvre, Schneider, Kupers, & Ptito, 2011) as well as in early-blinds (Cuevas et al., 2010), however this was not replicated in early-blind children (Rosenbluth, Grossman, & Kaitz, 2000; Wakefield, Homewood, & Taylor, 2004), and no difference was found between early-blind, late-blind and controls in a recent review (Sorokowska, Sorokowski, Karwowski, Larsson, & Hummel, 2018).
Odor discrimination is defined by the ability to differentiate between odors and is also assessed by olfactory tests such as the Sniffin’ Sticks test (Hummel et al., 1997). In one study early-blind subjects showed superior odor discrimination compare to controls (Cuevas et al., 2010). However, this finding was not replicated in the congenitally blind (Beaulieu-Lefebvre et al., 2011) and neither in the early and late-blind subjects (Schwenn et al., 2002) and could not be confirmed in a recent metanalysis (Sorokowska et al., 2018).
Odor identification is the ability to name different odors. It can be assessed either with cued identification in which an odor is given to the subject, and they must choose the correct answer between four choices of odorants, (cued-recall) or without any cues given (free-recall). Several studies failed to show significant differences in cued odor identification between early-blind, late-blind and controls (Beaulieu-Lefebvre et al., 2011; Cuevas et al., 2010; Rosenbluth et al., 2000; Schwenn et al., 2002; Smith et al., 1993). The picture is however different for free-recall identification where early-blind children and adults outperformed sighted controls (Cuevas, Plaza, Rombaux, De Volder, & Renier, 2009; Rombaux et al., 2010; Rosenbluth et al., 2000; Wakefield et al., 2004). This study was also replicated in early and late-blinds in which they had to identify 80 everyday odors in a free-recall manner (Murphy & Cain, 1986).
Odor localization is the ability to determine if an odorant is administrated in the right or left nostril. Odor localization can also be used for extracting spatial information from chemosensory clues (Koutsoklenis & Papadopoulos, 2011). For instance, detecting the source of an odorant can guide us to its location (i.e., a fish shop). Although healthy subjects are not able to localize pure odorants like (PEA), one report found this to be possible after four training sessions (Negoias, Aszmann, Croy, & Hummel, 2013). However, localization of chemosensory stimuli containing a trigeminal component like eucalyptol (Frasnelli, La Buissonniere Ariza,