OATAO is an open access repository that collects the work of Toulouse
researchers and makes it freely available over the web where possible
Any correspondence concerning this service should be sent
to the repository administrator:
tech-oatao@listes-diff.inp-toulouse.fr
This is an author’s version published in:
https://oatao.univ-toulouse.fr/18287
To cite this version:
Bres, Lucie and Gherardi, Nicolas and Naudé, Nicolas and Rives,
Bertrand Remote atmospheric pressure plasma for improving
acid-base surface properties of PEEK polymer: relevance to coating
adhesion. (2018) In: ISPC23 (23rd International Symposium on
Plasma Chemistry), 30 July 2017 - 4 August 2017 (Montréal,
Canada).
Open Archive Toulouse Archive Ouverte
Official URL :
Remote atmospheric pressure plasma for improving acid-base surface
properties of PEEK polymer: relevance to coating adhesion
L. Brès1,2, N. Gherardi2, N. Naudé2 and B. Rives1
1I.R.T Saint-Exupéry, Toulouse, France
2LAPLACE, Université de Toulouse, CNRS, INPT, UPS, Toulouse, France
Abstract: Our study focuses on a remote atmospheric pressure plasma for the improvement of polymer surface reactivity. From the perspective of linking surface modifications to adhesives performances, wettability measurements are made using two models (Owens-Wendt-Rabel-Kaelble and Van Oss-Good-Chaudhury). This study is a contribution to correlate wettability with adhesion results obtained with three-point bending test. Acid-base properties of the treated polymeric surfaces are discussed according to the Lewis’s theory. Keywords: APPJ, polymer, acid-base interactions, wettability, adhesion improvement. 1. Introduction
The increasingly widespread use of Carbon Fiber Reinforced Polymers (CFRP) in structural engineering could be explained by their low weight coupled with high mechanical properties [1]. Poly-EtherEtherKetone (PEEK) matrix composites are particularly appreciate in aeronautic applications because of their high thermal stability and chemical resistance associated to a good ability to withstand high mechanical loads [2]. However, literature shows several studies aiming to reduce surface energy to obtain high and durable adhesive properties [3– 5]. In this context, knowledge about adhesion mechanisms is a fundamental point for the control of the interface and the future paint interphase which depends on the surface quality of materials and the bonding conditions.
In this study, industrial PEEK composites are characterized prior to and after activation by a remote atmospheric pressure cold plasma. Wettability modifications are studied using two different models as OWRK (Owens-Wendt-Rabel-Kaelble) and VOG (Van Oss-Good-Chaudhury). Physicochemical properties of plasma-treated substrates are discussed with respect to adhesion performances of a PEEK/coating interface. The aim is to characterize effect of process parameters on the acid-base properties and hence, to forecast the strength of the interface.
2. Experimental part
The substrate studied is a CFRP with a PEEK matrix. The composite shows a visually planar surface, slightly rough and is around 2 mm thick. The samples are cleaned three times by moistened wipes before any treatment or characterization. The coating used in this study is an industrial polyurethane paint employed in external aircrafts. It is a bicomponent product applied with traditional spraying techniques. Dry thickness measured after complete polymerization is around 40 ± 7 µm.
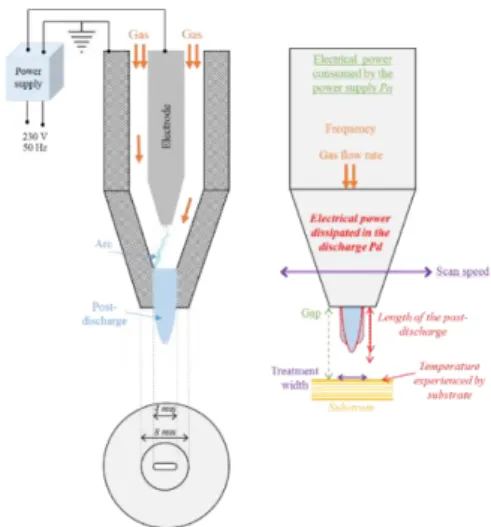
Fig. 1. Schematic of the experimental device UL-SCAN and process parameters.
A remote atmospheric pressure cold plasma (UL-SCAN) from AcXys Technologies is used to activate the CFRP substrates [6]. The process is operated with either dry air or pure nitrogen (Alphagaz 1 from Air Liquide) at a flow rate ranging from 30 to 60 slm. The system is driven by an alternating current power supply with frequency ranging from 80 to 200 kHz. Created arc between the inner and the coaxial outer electrode is blowing out from the torch. An afterglow region over a few tens of millimetres, namely the post-discharge, is created and contains reactive and excited species (metastables, neutrals, and radicals). Displacement of the torch is ensured by a directional nozzle mounted on a 3-axes robot that scans the surface to be treated line by line. Activation performances can be promoted by tuning different parameters listed in Fig. 1. They allow operator maximizing the effect of the treatment while preventing some degradations on the sample surface.
Acid-base surface properties are explored by contact angle measurements using static sessile drop. In the
OWRK theory [7], surface free energy γS is expressed as
follow:
𝛾𝐿(1 + cos 𝜃𝑎) = 2√𝛾𝑆𝑑𝛾𝐿𝑑+ 2√𝛾𝑆𝑝𝛾𝐿𝑝 (1)
with γSD and γSP respectively the dispersive and the polar component.
In the VOGC theory [8], the surface free energy is decomposed as follow:
𝛾𝐿(1 + cos 𝜃𝑎) = 2√𝛾𝐿𝐿𝑊𝛾𝑆𝐿𝑊+ 2√𝛾𝐿+𝛾𝑆−+ 2√𝛾𝐿−𝛾𝑆+ (2)
with γSLW, γSAB, γS+ and γS- respectively the Lifshitz-Van Der Waals (LW), Acid-Base (AB), acidic and basic components.
The surface free energy components in both theories are determined using several reference liquids of known γLD,
γLLW, γS+ and γS- (see table 1). For each individual sample,
repeatability is checked by analysing 3 drops with each liquid at room temperature, 5 seconds after deposition of the drop.
γL γLLW γAB γ+ γ
-Water 72 8 21 8 51 25 5 25 5
Diiodomethane 50.8 50.8 0 0 0 Formamide 58 39 19 2.28 39.6 Table 1. Wettability characteristics of liquids used in the study in (mN/m) [9].
Adhesion performances of the coating/substrate interface prior to and after plasma activation are evaluated by three-point bending test. According to the standard NFT 30-010 [10], test specimens are made up of PEEK composite coated with an industrial polyurethane external paint. An epoxy stiffener is formed on the latter in order to create a discontinuity of the strains and focus the failure initiation along the stiffener (see fig 2). Bold black arrow represents the bending stress of the specimens between supports (in blue). The strength of the tested system is achieved under the conditions of 0.5 mm/sec loading rate, 35mm distance between supports, and with a 5 kN load sensor. The maximum tensile strength value of each interface is calculated from the average of 8 specimens.
Fig. 2. Diagram of the specimen’s position during three-point bending test.
3. Results and discussions
At initial state, PEEK composites used in this study have a poor total surface free energy (53mN/m±) associated to a rather high water contact angle (68°±). This is in accordance with other authors who consider that PEEK matrix is quite inert when applying a coating without activation [11-12]. Surface free energy γS of
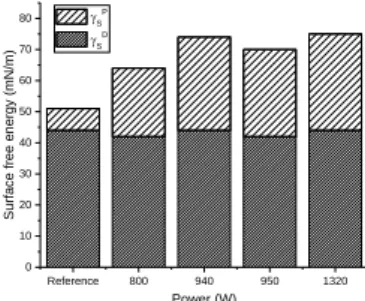
PEEK substrates activated by air plasma according to the device power are shown in Fig. 3. It is calculated from the water and diiodomethane contact angle using the OWRK theory. This approach reflects both dispersive interactions as the Van Der Waals ones through γSD and polar
interactions through γSP. One can see a significant
increase of the polar component γSP after air plasma while
no changes in the dispersive component γSD. This give
rise to the total surface free energy γS which indicate a
more wetting property of the PEEK surface. Previous chemical analyses made on the top layer of the PEEK matrix (not shown here) are in accordance with this results since they notice a greater oxygen grafting ratio. This tendency is also observed in the literature [12-13]. Despite the increase of the surface polarity after air plasma, Fig. 3 illustrates that it is impossible to provide a significant trend about the influence of the device power.
Fig. 3. Surface free energy discriminates in polar and dispersive components according to OWRK theory. Although the OWRK is a widespread method to calculate the surface free energy, it seems to present some shortcomings for this materials. Indeed, Lavielle also noticed that this method is not sufficient to explain the whole surface interactions when a droplet is deposited on a substrate [14]. She attributed these observations to the polar initial character of her substrate before treatment. In order to overcome this problem, she used the VOGC method that consider interactions as electron exchanges between an acidic substrate and a basic liquid or vice-versa. The two components γS+ and γS- in (2) express this
acid-base potential.
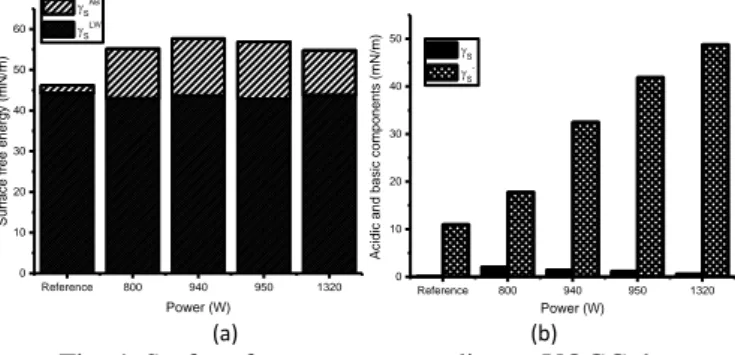
Values of surface free energy included AB and LW components are summarized in Fig. 4(a) whereas basic γS
-and acidic γS+ components are illustrated in Fig. 4(b). As
can be seen, untreated substrate present a basic monopolar character whereas plasma activation makes it an amphoteric character, i.e. both acidic and basic properties. The decomposition of the AB component indicates an
Reference 800 940 950 1320 0 10 20 30 40 50 60 70 80 Surfa ce f re e e ne rg y (m N/m) Power (W) S P S D
increase of basic properties of treated surface through γS-.
At initial state, the basic monopolar character is due to the presence of oxygen atoms in the PEEK monomer which are Lewis-base [15]. The constant increase of γS- with the
power arises from the constant oxygen incorporation onto the surface, not fully understood with the OWRK theory. These data predict a strong interaction with a Lewis-acid, as OH- molecules, NH
3 or polyurethane product [16].
(a) (b)
Fig. 4. Surface free energy according to VOGC theory and decomposition of acid-base component into acidic
and basic parts.
Improvement of the surface reactivity through the oxidation previously highlighted results in a better strength of the interface between the polyurethane paint used in this study and PEEK substrates. Indeed, Fig. 5 show the fracture face of an untreated (a) and an air plasma-treated (b) specimen obtained after a three-points bending test. In the first case, failure is clearly adhesive which means that there is no adhesion between the untreated PEEK and the polyurethane coating. In contrast, after air plasma and whatever the conditions (all the failure faces not shown here), the breaking is of cohesive type in parallel of the improvement of the strength’s interface.
Fig. 5. Fracture faces of an untreated (a) and an air plasma-treated (b) specimen associated to their maximal
tensile strength (N). 4. Conclusions
The study focuses on the influence of atmospheric pressure plasma process parameters on the wettability of a PEEK based CFRP. This paper is a contribution to the correlation of the surface reactivity according to the Lewis theory with mechanical behaviour of the new interphase, characterized by bending three points.
To do that, the VOGC coupled with the acid-base approach are chosen because they seem to give valuable
chemical information about the surface interactions, attractive as repulsive ones, through both components γS
-and γS+. Bending three-point test is also a meaningful way
to get both qualitative and quantitative characterization of the interface through the maximal tensile strength and optical analysis of the failure face.
This approach seems to be suitable for better understanding of adhesion phenomena and interface ageing mechanisms.
5. Acknowledgments
This research is supported by Airbus Helicopters Company within the SURFINNOV IRT Saint-Exupéry contract project.
6. References
[1] C. Soutis, Mater. Sci. Eng. A, vol. 412, no. 1–2, pp. 171–176, (2005).
[2] A. A. Collyer, A Practical Guide to the Selection
of High-Temperature Engineering Thermoplastics,
Elsevier, (1990).
[3] P. B. . Comyn J, Mascia L, Xiao G, Int. J. Adhes.
Adhes., vol. 16, no. 2, pp. 97–104, (1996).
[4] A. Dupuis, T. H. Ho, A. Fahs, et al, Appl. Surf.
Sci., vol. 357, pp. 1196–1204, (2015).
[5] B. R. K. Blackman, a. J. Kinloch, and J. F. Watts,
Composites, vol. 25, no. 5, pp. 332–341, (1994).
[6] “AcXys Technologies,” 2016. [Online]. Available: http://www.acxys.com/fr/.
[7] D. K. Owens and R. C. Wendt, J. Appl. Polym.
Sci., vol. 13, no. 8, pp. 1741–1747, (1969).
[8] C. J. Van Oss, M. K. Chaudhury, and R. J. Good,
Chem. Rev., vol. 88, no. 6, pp. 927–941, (1988).
[9] C. J. Van Oss, Interfacial Forces in Aqueous
Media, Second edi. Londres, (2006).
[10] “NF T 30-010, Collection AFNOR, (1992). [11] S. Jha, S. Bhowmik, N. Bhatnagar, et al, J. Appl.
Polym. Sci., vol. 118, pp. 173–179, Nov. (2010).
[12] E. Occhiello, M. Morra, G. L. Guerrini, et al,
Composites, vol. 23, no. 3, pp. 193–200, (1992).
[13] H. Luo, G. Xiong, K. Ren, et al, Surf. Coatings
Technol., vol. 242, pp. 1–7, (2014.
[14] L. Lavielle, Ann. Phys. Fr., vol. 14, pp. 1–48, (1989).
[15] A. Bismarck, M. E. Kumru, and J. Springer, J.
Colloid Interface Sci., vol. 217, no. 2, pp. 377–
387, (1999).
[16] J.-C. Joud and M.-G. Barthés-Labrousse,
Physico-chimie des surfaces et acido-basicité. ISTE
Edition, 2015). Reference 800 940 950 1320 0 10 20 30 40 50 60 Surfa ce f re e e ne rg y (m N/m) Power (W) S AB S LW Reference 800 940 950 1320 0 10 20 30 40 50 Acidic an d b asic co mp on en ts ( mN/m) Power (W) S S
-Reference Mirror po ish 200 250 300 350 400 450 500 550 875 1050 1225 1400 U.A (N)
No activation Air activation