Biochem. J. (1974) 143, 241-249 Printed inGreat Britain
Binding of
(-Lactam
Antibiotics to
the
Exocellular
DD-Carboxypeptidase-Transpeptidase of Streptomyces R39
By JEAN-MARIE
FRLRE,
JEAN-MARIE GHUYSEN,* PETER E. REYNOLDStand RAMON MORENO
ServicedeMicrobiologie, Faculte de Medecine, Institut de Botanique, Universite deLiege, SartTilman,4000 Liege,Belgium
and HAROLD R. PERKINS
National Institute
for
MedicalResearch,
MillHill,
London NW71AA,
U.K. (Received10April1974)Benzylpenicillin and cephaloridine reacted with theexocellular
DD-carboxypeptidase-transpeptidase
from Streptomyces R39 toformequimolarand inactiveantibiotic-enzyme complexes. At saturation, the molar ratio ofchromogenic cephalosporin 87-312 to enzyme was 1.3:1, but thisdiscrepancy mightbedueto alackofaccuracy in the measure-mentoftheantibiotic. Spectrophotometric studies showedthatbindingof cephaloridine andcephalosporin87-312 totheenzymecausedopening of their/J-lactam
rings. Benzyl-penicillin and cephalosporin 87-312 competed for the same site on the free enzyme, suggestingthatbinding ofbenzylpenicillinalsoresulted intheopening of its 8-lactam ring. InTris-NaCl-MgCI2
bufferatpH7.7 and 37° C, the rate constants for the dissocia-tion of theantibiotic-enzyme complexes were 2.8x10-, 1.5x10-6 and 0.63x106s1 (half-lives70, 130and300h) for benzylpenicillin,cephalosporin 87-312and cephalorid-inerespectively. Duringthe
process, the protein underwent reactivation. The enzyme that was regenerated from its complex with benzylpenicillin was as sensitive to fresh benzylpenicillinas thenativeenzyme.With['4C]benzylpenicillin,
thereleased radioactive compound was neither benzylpenicillin nor benzylpenicilloic acid. The Streptomyces R39 enzyme thusbehavedasa,8-lactam-antibiotic-destroying
enzyme butdidnotfunction as a,8-lactamase.
Incubationat37° Cin0.01M-phosphate
buffer,pH7.0,and inthe same buffersupplemented with sodiumdodecyl sulphatecaused a morerapid reversionof the['4C]benzylpenicillin-enzyme
complex. Therateconstants were 1.6x10-5s-1 and0.8x10-4s-1 respectively. Under these conditions, however, there was no concomitant reactivation of the enzyme and the released radioactive compound(s) appeared not to be the same asbefore.The Streptomyces R39 enzyme and the exocellular DD-carboxy-peptidase-transpeptidasefrom Streptomyces R61 appeared to differ from each other with regardto thetopography of their penicillin-binding site.
On the basis of its specificity profile for donor andacceptorsubstrates, the exocellular DD-carboxy-peptidase-transpeptidaseofStreptomyces R39 might be a soluble form of the membrane-bound
trans-peptidase which catalyses peptide cross-linking duringthe last stages ofthe wallpeptidoglycan bio-synthesis (Leyh-Bouilleetal., 1972; Ghuysenetal.,
1973, 1974; Perkins etal., 1973). The Streptomyces R39enzyme wasinhibitedbyverylow concentrations
ofbenzylpenicillin and the interaction between the proteinand the antibioticappeared tobereversible
at least with regard to theenzyme activity (Leyh-*To whomreprintrequests should besent.
tPermanent address: University of Cambridge, DepartmentofBiochemistry, Sub-Departmentof Chemi-cal Microbiology, Tennis Court Road, Cambridge
CB2IQW,U.K.
$Permanent address: Universidad de los Andes,
Facultad deCiencias, Merida,Venezuela.
Bouilleetal., 1972). The binding ofbenzylpenicillin and of various cephalosporins to theStreptomyces R39enzyme andthe dissociation of the complexes
thus formed were further studied. The results of
theseinvestigationsarepresentedinthepresentpaper.
Materials and Methods
DD-Carboxypeptidase-transpeptidase from
Strepto-mycesR39
The enzyme used in most experiments was 90%
pureand hadaspecific activity of 17.1units/mgof
protein as determined in the DD-carboxypeptidase
assay (Ac2§-L-Lys-D-Ala-D-Ala+H20 -OD-alanine
+Ac2-L-Lys-D-Ala) (preparation A after step 4; Frere etal., 1974). In some experiments, a 100 %-pureenzyme (specific activity 19.8units/mgof
pro-tein;Frereetal., 1974)wasused.
§Abbreviation:Ac,acetyl.
J.-M. FRtIRE, J.-M.GIAuYSEN, P. E.REYNOLDS, R. MORENO AND
14.
R.PERKINS,B-Lactamase
Thisenzyme was purchased from Riker Labora-tories,Loughborough,Leics.,U.K.One penicillinase unithydrolysed1
pmol
ofbenzylpenicillinto benzyl-penicilloicacid/minatpH 7.0 and25° C. The concen-tration ofbenzylpenicillinwas 1mm.Antibiotics
Benzylpenicillin was purchased from Rhone-Poulenc, Paris, France, cephaloridine from Glaxo Research Ltd., Greenford, Middx., U.K., and
[I4C]benzylpenicillin
(45mCi/mmol) from The Radiochemical Centre, Amersham, Bucks., U.K. Chromogenic cephalosporin 87-312 [i.e. 3-(2,4- dinitrostyryl)-(6R-7R)-7-(2-thienylacetamido)-ceph-3-emA-carboxylicacid, E-isomer]
was agift
from Dr.O'Callaghan,GlaxoResearchLtd.(O'Callaghan et al., 1972). Solutions of cephalosporin 87-312 (usually about 0.1mM) weremadebydissolvingthe antibiotic in 1ml ofdimethylformamide
and the volume of thesolution wasadjusted to250ml with theTris-NaCl-MgCl2
buffer, pH7.7 (see below). The finalconcentrationwasestimatedby measuring the extinctionat386nmandbyusingamolar coeffi-cient8"'of17500(O'Callaghan
etal., 1972).[44C]Benzylpeniciloic
acid["4C]Benzylpenicilloic
acid was preparedby
incubationof[14C]benzylpenicillin
withfl-lactamase.
Separation of
[14C]benzylpenicillin
and[14C]benzyl-penicilloicacid
This wasdoneasdescribedby Marquet etal.(1974) byt.l.c. on silica-gel plates with butan-l-ol-water-ethanol-acetic acid
(10:4:3:3,
by vol.) as solvent, and by paper electrophoresis at pH6.5 (collidine-aceticacid-water, 9.1:2.65: 1000,by vol.)for 60min at 60V/cm. TheRF
values were 0.82±0.03 for[14C]benzylpenicillin
and 0.67±0.03for[14C]benzyl-penicilloic acid.
The electrophoretic migrations toward theanodewere16cmfor [14C]benzylpenicillin
and
22cmfor
[14C]benzylpenicilloic
acid.
Spectra
Spectrawere determinedwith a Cary 17 double-beam recording spectrophotometer with automatic slit-width adjustment.
Titration and kinetic data
From the plotted data, all the lines were drawn accordingtothe bestfitfrom least-squares regression
analyses
(P<0.001).Tris-NaCI-MgCl2
buffer
This buffer was0.1M in Tris, 0.2Min NaCl and 0.05M in MgCI2; adjusted to pH7.7 with HCI. Inthisbuffer, the Streptomyces R39 enzyme was very
stable. No loss ofactivitywasobserved after 100h of incubationat37° C (enzyme concentration 0.2mgof protein/ml).
Measurement of the Streptomyces R39 enzyme concentration
This was done on the basis of aE}4m value at 280nm of9.7
(Frere
etal.,
1974).
Measurementof radioactivity onchromatograms and electrophoretograms
The radioactive compounds on t.l.c. plates and electrophoresis papers were located by using a Packard Radiochromatogram Scanner. The radio-activity wasdetermined by cutting or scraping the radioactive areaswhichwerecounted ina Packard Tri-arbliquid-scintillationspectrometer.
Results
Binding of benzylpenicillin tothe R39enzyme Binding of benzylpenicillin to the exocellular DDD-carboxypeptidase-transpeptidase from Strepto-myces R61 caused quenching of the fluorescence ofthe enzyme and affected its near-u.v. circular-dichroism (Nieto etal., 1973). In marked contrast with these observations, the fluorescence emission andcircular-dichroism spectra ofthe Streptomyces R39enzyme were not
modified
by benzylpenicillin.Binding
ofbenzylpenicillin
totheStreptomyces R39 enzyme wasstudied by
measuring
theinhibition oftheDD-carboxypeptidase activity
caused by the anti-biotic.Samples
(1-S5#1)
of a 5uM-benzylpenicillin solution inTris-NaCl-MgCG2
buffer were added stepwise to1104u1
of a solution containing 15,ug of the Streptomyces R39 enzyme (100% purity) inthe samebuffer.After
eachaddition, the solution was mixed, maintained at room temperature for 5min and asample(2-10#1)
wasremovedandused for the measurement of theDD-carboxypeptidase
activity(on
Ac2-L-Lys-D-Ala-D-Ala).
After correction forthedecrease inthe amount ofenzymeowing to theremovalofthesamples, the end-point for titra-tion oflS,ug
ofStreptomyces R39 protein, on the basis of thedisappearance ofthe enzyme activity, occurred after the addition of 58.5,u1 ofthe anti-bioticsolution (i.e. 0.294 nmol ofbenzylpenicillin).
Assuming that onebenzylpenicillin-binding
site occurred per molecule ofprotein, the above result gave a molecularweight of 50500 for the Strepto-myces R39 enzyme. From this value and from those obtained by physical methods (Frere et al., 1974), an average molecular weight of 53300±1700 was assigned to the enzyme. From this average value, theend-point for titrationof the Streptomyces R39 enzyme in the aboveexperimentoccurredatamolar ration ofbenzylpenicillintoenzyme of1.04+0.03:1(Fig. 1).
PENICILLIN BINDING TODD-CARBOXYPEPTIDASE-TRANSPEPTIDASE
50
\
0~~~~~~~~~n
0 0.5 I.O 1.5
Molar ratio (benzylpenicillin/enzyme)
Fig. 1. Titration of the Streptomyces R39protein with
benzylpenicillin,
basedontheinhibitionof
enzymeactivity
o,Experiment carried out withpurified enzyme (100% purity); *, experiment carried out with partially purified enzyme (90% purity). For conditions see the text. The molar ratios werecalculated on the basis of an average molecular weight for the Streptomyces R39 protein of 53300.
In a second experiment, the Streptomyces R39 enzymepreparation used was at 90% purity and the time of incubation with benzylpenicillin was prolonged. Samples containing0.0119nmol of pro-tein were incubated at room temperature with increasing amounts of benzylpenicillin in
30,lu
(final volumes) ofTris-NaCl-MgCl2 buffer. After 60min,AC2-L-Lys-D-Ala-D-Ala
was added to each sample and the DD-carboxypeptidase activity was neasured. On the basis of an average molecular weight of 53300,completeinhibition of the enzyme ocurredatamolar ratio ofbenzylpenicillin to enzyme of 0.91±0.02:1 (oraftercorrection for the presence of about 10% impuritiesinthe enzyme preparation, at amolar ratiovirtually equal to 1:1; Fig. 1). Reversion ofthe ['4C]benzylpenicillin-Streptomyces R39 enzymecomplexReversion of the complex was studied under conditions inwhich,intheabsence of the antibiotic, the enzyme was perfectly stable (i.e. in the Tris-NaCl-MgCI2 buffer)and underconditionswhere it underwent spontaneous inactivation.
[14C]Benzyl-penicillin-Streptomyces
R39 enzyme complex (5.5nmol;0.28pCi;
4lmCi/mmol; in lml of Tris-NaCl-MgCI2 buffer) was prepared by mixing the Streptomyces R39 enzyme with a twofold excess, on amolar basis, of['4C]benzylpenicillin
and was separated from the unbound[(4Cjbenzylpenicillin
by filtration on a 25ml column (O.8cmx13 cm) of SephadexG-25
equilibrated
againstthesameTris-NaCI-MgC12
buffer. The solution containing the isolated[(4C]benzylpenicillin-enzyme
complex was then maintained at 37° C for4 days. Atincreasing time-intervals during the incubation, samples wereremoved which showed a progressive recovery of the enzyme activity (DD-carboxypeptidase assays) and a parallel release of the radioactivity (as revealed by both t.l.c. and paper electrophoresis; see theMaterials and Methodssection). In both these latter tests, the enzyme-bound radioactivity remained at the origin. On the basis of the enzyme activity recovered and the radioactivity released, the half-lives of the complex were almost identical, 65 and 73h respectively (Fig. 2). The reactivated enzyme
(after 90h of incubation at 37° C; 63
%
recovery) was identical withthe native onewithregard both to the Km value for Ac2-L-Lys-tl-Ala-D-Ala (iD-carboxypeptidase assay) and to the rates of concomitant hydrolysisand transfer reactions that'. 404 bo 0 40 Time(h)
Fig. 2. Dissociation of the
[14C]benzylpenicillin-Strepto-myces R39 enzymecomplex
(a) Time-course of the recovery of enzyme activity in Tris-NaCl-MgCl2 buffer.[EP]o=initialconcentrationof the inactive complex;
[EP],
=residual concentration of the inactive complex after time t. [EP], is equal tolEP]o
-[E1t
where[E]t
is the concentration ofactiveenzyme attimet.Squares and circles referto twodistinctexperi-ments.Forconditionsseethe text.(b) Time-course of the disappearance of the
[14C]benzylpenicillin-enzyme
com-plex as revealed by t.l.c. (0; [E; a; v) and by paper
electrophoresis(0;*; w; v). Incubations of thecomplex
werecarriedoutinTris-NaCl-MgCI2buffer(I),in 0.01
M-sodiumn
phosphate buffer, pH 7.0(II andIII) and in thesamebuffercontainingi%(w/v)sodiumdodecyl sulphate
(IV). [EP3, and
[EP]J
=radioactivityattheoriginof thechromatogramns
and electrophoretograns. For otherconditionsseethetext.
J.-M.
FRkRE,
J.-M. GHUYSEN, P. E. REYNOLDS,R. MORENO AND H. R. PERKINS it catalysed(transpeptidase
assay, withAc2-L-Lys-D-Ala-D-Ala as donor and
meso-diaminopimelic
acidas acceptor;Frere etal., 1973). Moreover, the regenerated enzyme reacted with fresh benzyl-penicillin as did the native one and formed an equimolar and inactive antibiotic-enzyme
complex
which did notmigrate
on t.l.c. and paper electrophoresis.Reversion of the
[14C]benzylpenicillin-enzyme
complexwas also studied in 0.01M-sodium phos-phate, pH7.0, under the following conditions: (1) at
370C
and in the absence of sodium dodecyl sulphate (Fig.2); (2)at37° C and in thepresenceof sodiumdodecyl sulphate(Fig.
2)
and(3)
at 100° C. Under these conditions, the freeenzyme either ex-hibited a low stability (at 37° C) or was very rapidlydestroyed (at 100° C).Onthebasis of release of the radioactivity, the half-lives of the complex were 11-14h, 147min and less than 5min respective-ly. In all cases, recoveryofenzymeactivity didnot occur.Natureofthe radioactivecompound(s) releasedfrom the["4C]benzylpenicillin-StreptomycesR39complex
Dissociation of
the
complex was carried out at370C
eitherin Tris-NaCl-MgCl2 bufferorin0.01 M-sodiumphosphatebuffer,pH7.0.Controlexperiments werecarriedoutwith free["4C]benzylpenicillin
and free["4C]benzylpenicilloic
acid. After 100h of incubation in both buffers,['4C]benzylpenicilloic
acid had unchangedRp
and electrophoretic-mobility values (see the Materials and Methods section). After 40h ofincubation
in phosphate buffer,[14C]benzylpenicillin
wasdegraded
intoacompound
which behaved as[(4C]benzylpenicilloic
acid on both chromatography and electrophoresis. After 15h of incubation inTris-NaCl-MgCl2
buffer,[I4C]benzylpenicillin
gave rise to twocompounds:
a main one (80% of the original radioactivity) which was less anionic than
['4C]benzylpenicillin
andaminorone(20%) which had thesame electro-phoretic mobility
as.['4C]benzylpenicilloic
acid(Fig.
3). Chromatographically, however,
the main degra-dationcompoundwasindistinguishable from intact[14C]benzylpenicillin
whereas the minor one still behaved as[14C]benzylpenicilloic
acid. Both com-pounds appeared to be degradation end-products, since their relativeproportion
after 96h ofincubation was the same as after 15h. Although it was not identified, the main breakdown compound was probably caused byaminolysis ofthebenzylpenicillin moleculeby
theTris buffer, i.e.a benzylpenicilloyl-Tris derivative(Schwartz, 1969).Dissociation of the
[14C]benzylpenicillin-enzyme
complex in Tris-NaCl-MgCl2 buffer, i.e. under conditions wherethe freeenzyme was verystable and where recovery of the enzyme activity from the inhibited complex occurred,gaveriseto acompound which behavedchromatographicallyas
['4C]benzyl-penicillin (Fig. 4)
andelectrophoretically
as[14C]ben-penicilloic acid and thus,
clearly,
was neither ben-zylpenicillinnorbenzylpenicilloic acidnor aproduct arising from a spontaneous degradation of free benzylpenicillin in Tris-NaCl-MgCl2 buffer.Dissociation of the complex in phosphate buffer, i.e. under conditionswhere recoveryof theenzyme
0 1-: E 0. -I 01 -5 0 5 10 15 Distancemigrated (cm)
Fig. 3. Co-electrophoresis at pH6.5 of intact
[14Clbenzylpenicillin
andofits degradation products that appeared after incubationoffree[L4C]benzylpenicijjin
inTris-NaCl-MgCI2
bufferfor15h at 37° CFromleft to right, the compounds were: at -3 cm, impurity present in the
[14C]benzylpenicillin;
at +12cm, main degradationproduct (thiscompound behaved as
[14C]benzylpenicillin
ont.l.c.);
at+16cm,intact [14C]benzylpenicillin (control); at +22cm,minor degradation product with the same mobility as[(4C]benzylpenicilloic
acid (this compound also behaved as[1C]benzylpenicilloicacid ont.l.c.). Scanning conditions: 3000c.p.m. atfull scale; 0.5cm/min; time constant, lOs; slit width,0.5cm.
PENICILLIN BINDING TODD-CARBOXYPEPTIDASE-TRANSPEPTIDASE (a) 15001 E 75 a c) 0
15C
c C5 75 iO ~0 1--I! (b)ho
-0* 1 0 0 5 1015~~~~~~I
Distancemigrated(cm)Fig. 4.T.l.c.ofthe['4C]benzylpenicillin-StreptomycesR39 enzyme complex after incubation in Tris-NaCl-MgCI2
bufferfor 60h at 37° C
(a) Direct chromatography of the incubated solution. From left to right: at Ocm, enzyme-bound radioactivity; at 9 and12cm, degradation products. The compound at 12cmhad exactly the same RF value as
['4C]benzylpeni-cillin. (b) Co-chromatography of the same solution and of
[14C]benzylpenicilloic
acid (at 8.5cm). Arrows: solvent fronts.activity did not occur, gave rise to a compound which behavedas
['4C]benzylpenicilloic
acid on both chromatography and electrophoresis. Dissociation of the complex in the same phosphate buffer but inthe presenceof 1 % (w/v) sodium dodecyl sulphate was alsostudied.Chromatographically,the observed increase in the released['4C]benzylpenicilloic
acid-likecompound was equivalent to the decrease in the radioactivity remaining at the origin of the plates. Electrophoretically, however, the [14C]benzylpeni-cilloic acid-like compound represented only a frac-tion of the total released radioactivity whereas various compounds of slower velocity on electro-phoresis progressively accumulated (Fig. 5). Effects ofthiol-group reagents on enzyme activity and[14C]benzylpeniciffin
bindingIodoacetate. Incubation of the Streptomyces R39 enzyme (0.lOnmol) in 100lul of Tris-NaCl-MgCI2 buffer containing 1mM-iodoacetate for 16h at 37° C had no effect on the enzyme activity or on the ability of theproteintobindbenzylpenicillin.
5,5'-Dithiobis-(2-nitrobenzoic acid). Samples
(40p1l)
ofa 1mM-5,5'-dithiobis-(2-nitrobenzoicacid) solution in
Tris-NaCl-MgC92
buffer were added stepwise and at roomtemperature to 0.4ml ofasolutioncontaining 4.5nmol of Streptomyces R39 enzyme protein (90% purity)in the same buffer. Thesame experiment Vol.was carried outin the presenceof
5M-guanidinium
chloride (previously neutralized with 5M-NaOHto pH7.5). Finally, controls consisted of the same mixtures as above but without the Streptomyces R39 enzyme. Assuming a molarextinction coefficient at 420nm of 13.6x 103 for the thiol-5,5'-dithiobis-(2-nitrobenzoicacid) derivative (Umbreit&Strominger, 1973)and that two free thiol groups occur perprotein molecule (amino acid analyses had revealed the presence oftwo half-cystine residues; Frere et al., 1974),theaboveconditionsweresuch that areaction would havecaused an increasein theE420 of 0.25. Infact, no differences between thesamples and the controlswereobservedevenwhen theexperimentwas carriedout in thepresence ofguanidinium chloride. Moreover, the5,5'-dithiobis-(2-nitrobenzoic
acid)-treated Streptomyces R39protein (inthe absenceofguanidinium)
exhibited the same enzymeactivity
and the samebenzylpenicillin-binding capability
as thenativeenzyme.
Binding ofcephalosporin 87-312 to theStreptomyces R39 enzyme
When exposed to theStreptomyces R39 enzyme, a solution of
cephalosporin
87-312 turned red immediately. This change of colour was observed even whenbothreagentswerecooled to0° C
before being mixed. Since thehydrolysis
of the,B-lactam
ring ofcephalosporin
87-312wasknown tocause ashift ofthe
absorption
maximum from 386to482nm1-*5 ._ 0 cd s5 3 Time(h) 5
Fig.5.Time-courseofthedegradation ofthe
[14Clbenzyl-penicillin-Streptomyces R39 enzyme complex at 370Cin
0.01M-sodiumphosphate buffer, pH7.0andinthepresence'
of
1%Y
(w/v)sodiumdodecyl sulphateSeparationsof residualcomplexanddegradation products were carried out by paper electrophoresis at pH6.5. *, Residual
['4C]benzylpenicillin-Streptomyces
R39 enzymecomplex(attheoriginoftheelectrophoretograms);o, radioactive
compound migrating
as[14C]benzyl-penicilloic acid; V, sum of all radioactive compounds exhibiting intermediate mobilities.
.- . -j- -%~x 0
1
J.-M.
FRiRE,
J.-M.GHUYSEN,
P. E.REYNOLDS,
R. MORENO AND H. R. PERKINS 0.30 0.20 0.10 0.06 0.04 0.02 0 -0.02 300 400 500 600 Wavelength(nm)Fig. 6. Absorption spectrumof cephalosporin87-312after treatment with the Streptomyces R39 enzyme and with
fi-lactamase
-,Solutioncontaining 15.5 nmol of Streptomyces R39 protein (100% purity) in 0.4m1 of Tris-NaCl-MgCl2
buffer was mixed with 0.2ml of a 67.7,M solution of cephalosporin 87-312. The mixture was incubated for 5min at room temperature before the absorption spectrum was determined. ----, Solution containing 6 units of f-lactamase in 0.4ml of Tris-NaCl-MgCk2
buffer was mixed with 0.2ml of the same solution of
cephalosporin 87-312 as above. The mixture was incubated at370Cuntil the absorbance at 482nm was a maximum (less than 5min). The broken linerepresents the difference spectrum between cephalosporin treated
withStreptomyces R39 enzyme andcephalosporintreated
with/-lactamase.Notethe change of scale.
(O'Callaghan et al., 1972), the above observation suggestedthat the exposure of cephalosporin 87-312 to the Streptomyces R39 enzyme resulted in the openingof its /-lactamring.Inagreement with this conclusion, theabsorption spectrum of the Strepto-myces R39 enzyme-treated cephalosporin 87-312 wasvirtually identical withthat of the f-lactamase-treatedcephalosporin87-312(Fig. 6).Hence
cephalo-sporin87-312 allowed thetworeagentsinvolvedin thereactiontobetitratedsimultaneously, the protein
by
estimation of its enzyme activity and the f8-lactam antibiotic by measurement of the change in the absorptionspectrum. Portions ofa58.8,pM-cephalo-sporin 87-312 solution in Tris-NaCl-MgCl2 buffer were added stepwise and at room temperature to
0.4ml of a solution containing 13.3 nmol of Streptomyces R39
protein (90%
purity) in thesamebuffer. After each addition, the extinction of the solution wasmeasured both at 386nm
(to
measure intactcephalosporin)
and at 482nm(to
measure modified cephalosporin) and the enzyme activity wasmeasuredin a2,u1 sample. Theend-points
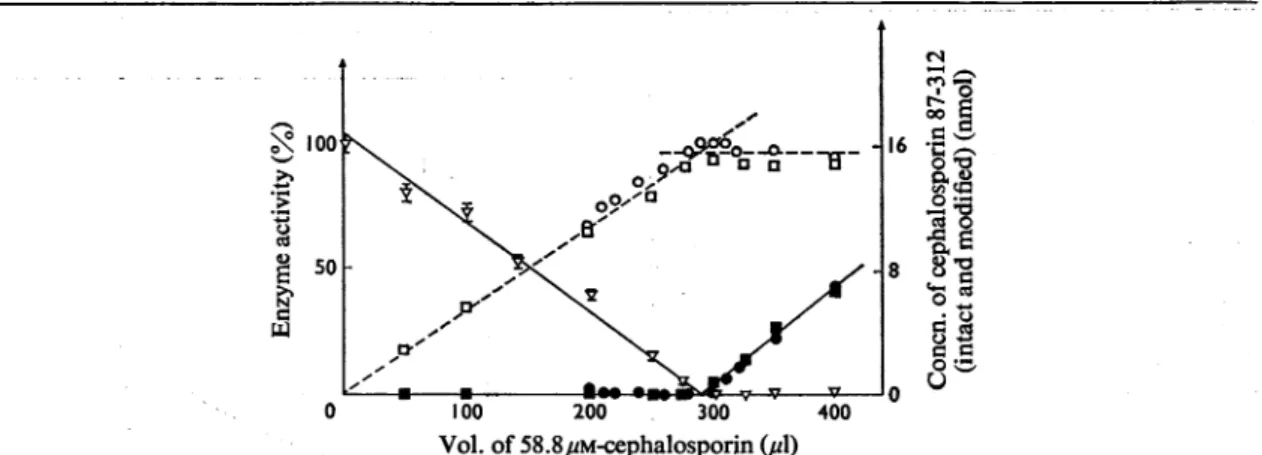
ofthe titration as revealed by the three procedures used occurred at amolar ratio ofcephalosporin 87-312 to Streptomyces R39 protein of 1.27:1 (Fig.7).
Unexpectedly, this valuewasmorethan30% higher
than that obtained whenbenzylpenicillin
reacted with thesame(90%-purity)
enzymepreparation (see
C.-)
Ce
4)'
Li~
100 200 300
Vol. of
58.8,pM-cephalosporin
(,ul)
_ _0
9
l IC x o * -4Fig.7. Titrationofthe Streptomyces R39 protein with cephalosporin 87-312, based on the inhibition ofenzyme activity and on thealteration of theantibioticmolecule
v,Residualenzymeactivity;0and
[l,
determination of modified cephalosporin 87-312 (two experiments); 0 andm(twoexperiments),
determinationof intact cephalosporin 87-312. For conditions see the text. The extinction values at 386 and 482nm weretransformed into concentrations (and from these intonmol)ofintact and modified cephalosporin 87-312 fromthe equations:
8386 =17500[intactcephalosporin]+7700[modifiedcephalosporin];
8482
=2600[intact
cephalosporin]+16700 [modified cephalosporin]wherethevalues 17500and 2600 were the molar extinction coefficients of intact cephalosporin 87-312 and the values
7700 and 16700werethemolarextinction coefficients of modified cephalosporin 87-312 at the relevant wavelengths. The
8366
valuefor intactcephalosporin87-312wasthatgivenbyO'Callaghan etal. (1972). The other coefficients weredeter-niined experimentally.The
8386
and 8482values for the modified cephalosporin 87-312 were determined after completehydrolysis of cephalosporin87-312 with
fi-lactamase
and were assumed to be identical with those of the cephalosporin 87-312treated with theStreptomycesR39enzyme. The validity ofthis assumption was supported by the data of Fig. 6.PENICILLIN BINDING TO DD-CARBOXYPEPTIDASE-TRANSPEPTIDASE2
above). At present, the reason for this discrepancy isnotunderstood since, as shown in the next para-graph,
benzylpenicillin
and cephalosporin 87-312 were found to compete for the same site on the enzyme.Competitionbetween
('4C]benzylpenicillin
andcephalo-sporin87-312for the Streptomyces R39 enzymeCephalosporin87-312(26nmol) andStreptomyces R39 protein (15.5nmol; 90% purity) were mixed together (final volume
300pul
ofTris-NaCl-MgCl2
buffer).After 5min at room temperature, ['4C]benzyl-penicillin (55nmol; 2.5puCi) was added to the mixture, thesolution incubated at 37° Cfor 20min and then filtered at room temperature on a 25ml column (0.8cmxl3cm) of Sephadex G-25 equili-brated against the same Tris-NaCl-MgCl2 buffer. Asshown in Fig. 8, all themodified cephalosporin 87-312 waseluted withtheprotein
atthevoid volume ofthecolumn, whereas thesmallamount ofradio-0 0 '4) 04-'0 10 CU 10 15 Fractionno. Is i 10
D
C) >b 0n:4
x 5 0IFig. 8. Competition between [14C]benzylpenicillin and
cephalosporin 87-312 for the Streptomyces R39 protein
Asolution of Streptomyces R39enzymethat had been
treatedfirst with aslight excessof cephalosporin87-312
and then withalargeexcessof['4C]benzylpenicillin was
chromatographedonacolumn of SephadexG-25 (0.8cm x13cm). v, Radioactivity; *, e482; 0, protein concen-tration.Volume ofeach fraction, 1.3ml.Forother
con-ditionsseethetextandthe Materialsand Methods section.
Radioactivitywasdeterminedon0.1ml samples whichwere
addedto lOml ofBray'sliquid scintillator (Bray, 1960) and counted ina Packard Tri-Carb liquid-scintillation
spectrometer.
activity foundassociated with these fractiois corre-sponded to less than 0.01 mol of benzylpenicillin/mol of enzyme. In another experiment, benzylpenicillin (lOnmol) and Streptomyces R39 enzyme (Snmol) were mixed together (final volume 300,1 of Tris-NaCl-MgCl2buffer). After 10min at 37° C, 90,1 of a 80pM solution ofcephalosporin 87-312 in the same buffer was added. Since no variations in the
E3o0/E482
ratio occurred, thefi-lactam
ring of the cephalosporin 87-312 must haveremainedintact. Binding of cephaloridine to the Streptomyces R39 enzymeHydrolysis ofthe ,B-lactam ring of cephaloridine caused a70% decrease of the absorbanceat250nm (O'Callaghan et al.,
1969).
Hencecephaloridine
offered another possibility to study the interaction between the Streptomyces R39 enzyme and acephalosporin-type antibiotic by measuring the alterationsundergone by bothreagents.Samples of 0.5mM-cephaloridine in
Tris-NaCI-MgCl2
buffer were addedstepwise
and at room temperature to0.4ml ofasolution
containing
6.5nmol of Strepto-myces R39protein(90% purity)in thesamebuffer. After each addition, the extinction of the solution wasmeasuredat250nmand theenzymeactivity
was measured in a2,1
sample.
Aplot
oftheincreased extinctionofthe solutionat250nmas afunctionof the amount ofcephaloridine
addedyielded
twostraight lines
intersecting
at apoint.
Onthebasis of thesespectrophotometric
data and the determina-tions ofenzymeactivity,theend-points
ofthe reaction occurredatmolar ratios ofcephaloridine
toenzyme of0.85 and 0.98respectively (Fig. 9).
Theaverage value of 0.915 was identical with that obtained whenbenzylpenicillin
reacted withthesameenzyme preparation and was about30%
lower than that obtained withcephalosporin
87-312.Reversion of the
cephalosporin 87-312-Streptomyces
R39 enzyme and
cephaloridine-Streptomyces
R39 enzymecomplexesTheexperiments werecarriedout in Tris-NaCl-MgCl2 buffer and at
37° C.
The antibiotics left in excess afterreaction
with the enzyme weredestroyed by
,B-lactamase
andthedissociation of theantibiotic-enzyme
complexes
was followedby
measuring the recovery of the enzymeactivity.
A controlexperiment
withbenzylpenicillin
showed that the rate constantfor the dissociation was identical with that obtained when thebenzylpenicillin-enzyme
complex
wasseparated
from the excess ofbenzylpenicillin
by
chromatography
onSephadex
G-25. Hence the presence of
,B-lactamase during
dissociation of the
complex
had no effect on the kinetics oftheprocess.Enzyme(90 %purity;
2.5nmol)
and lOnmol of eithercephalosporin
87-312 orcephaloridine
in100l1
(final volumes)
of Tris-247J.-M.
FRtRE,
J.-M. GHUYSEN, P. E.REYNOLDS,R. MORENOAND H. R. PERKINS 0.7 ~~~~~~~~~~~0.6 ~50 0.5 0 4 12 20 28Vol.of50mM-cephaloridin(,ul)
Fig. 9. Titration of the Streptomyces R39 protein with cephaloridine, basedon the inhibition ofenzyme activity
andonthealterationofthe antibiotic molecule
o, Enzymeactivity (% of untreated control); 0, E250.
For conditionsseethetext.The observedpoint of inter-section occurring at saturation of the enzyme by the
antibiotic couldbeexplained by assuming that binding of thecephaloridinetotheenzymeresulted intheopening of the,B-lactam ringand thatboth thecephaloridine treated with theStreptomycesR39enzymeand thecephaloridine
treated with fl-lactamase had the same e250. After saturation of the enzyme, the added cephaloridine remained intact and the slope of the line increased accordingly.
NaCl-MgCl2 buffer were incubated together for
10minatroomtemperature.
,B-Lactamase
(6 units)wasaddedtothe mixtureswhichweremaintainedat37° C for several days. On the basis ofenzyme recovery,
the rate constants for the dissociation of the
antibiotic-enzyme complexes were 1.5x10-6S-I
(half-life 130h) with cephalosporin 87-312 and
0.63 x10-6s-' (half-life 300h) with cephaloridine. Discussion
Saturation of the Streptomyces R39enzyme with
benzylpenicillin and cephaloridine occurred when 1mol ofantibiotic was added to mol of protein
(mol.wt. 53 300). Thesameconclusioncanprobably
be extended tochromogenic cephalosporin 87-312,
althoughin thiscasesaturation occurredatamolar
ratio ofantibiotic to enzyme of about 1.3:1. One
possible explanation for this discrepancy might be
thattheamountsof cephalosporin87-312usedwere
estimated from extinction values of the solutions
by using a
8"m
value of17500 (O'Callaghan etal.,1972). An underestimation of this coefficient
would ofcourse result inan overestimation ofthe
amountofantibiotic requiredtosaturatetheenzyme.
The titrationof theStreptomycesR39 enzyme with the three antibiotics yieldedvery clear-cutend-points. Theseexperimentsareincontrastwith thosecarried out with the
purified
DD-carboxypeptidase from Bacillus subtilis (Umbreit & Strominger,1973),
inwhich about20%
ofthe enzymeactivityremained detectable after the addition of 2-3 times the theoretical amount ofbenzylpenicillin.Thepresent results also offer an explanation for the unusual kineticspreviously observedfor the inhibition of the Streptomyces R39 enzymeby benzylpenicillin (Leyh-Bouilleetal., 1972). It isnowclear that the benzyl-penicillin concentrations usedin thesepreliminary
studieswerevery closetothat of the enzymeitself, so that theupward concavity
exhibitedby
the Dixon plots of1/v versus[benzylpenicillin]
simply
reflected the decrease in the concentration of the residual freeenzyme.The isolated f-lactam antibiotic-Streptomyces R39 enzyme complexes, when incubated under conditions where the free enzymeitselfwas
stable,
underwent dissociation withtheconcomitant reacti-vation of the enzyme. Hence, and aspreviously
observed (Leyh-Bouille et al.,1972),
inhibition of the Streptomyces R39 enzymeby
fl-lactam
antibiotics wasreversibleatleast withregard
tothe enzyme itself.Spectrophotometric studies, however, gave direct evidence that binding ofcephaloridine
andcephalosporin 87-312 to the StreptomycesR39 enzymeresultedintheopening ofthe,B-lactam ring.
Since binding ofcephalosporin
87-312 and benzyl-penicillin were mutually exclusive, there was very little doubtthat bothantibiotic moleculescompeted
forthesamesite on the free enzyme, andtherefore it seemed reasonable to assume that binding of benzylpenicillintotheStreptomyces R39 enzymealso resultedintheopeningof itsf6-lactam
ring.The nature ofthe chemical alteration undergone by the antibiotic molecule during its interaction withthe Streptomyces R39protein remainsamatter for speculation. The following observations made with
[14C]benzylpenicillin
are important for the final elucidation of the problem. (1) Reversion of the[14C]benzylpenicillin-enzyme
complex inTris-NaCl-MgCl2
buffer, under conditions where con-comitant reactivation of the enzyme occurred, yielded areleasedradioactive compoundwhich was not benzylpenicillin, nor benzylpenicilloic acid, nor aproduct which could have arisen from a spon-taneous degradation of free['4C]benzylpenicillin.
Hence the Streptomyces R39 enzyme, although behavingas afi-lactam-antibiotic-destroying
enzyme, did not function toward benzylpenicillin as a (primitive)6i-lactamase.
Moreover the regenerated enzyme was as sensitive to fresh benzylpenicillin as the native one, hence excluding the theoretical possibilitythat thereleasedpenicillin derivativewas carrying with it some labile part of the protein. 248PENICILLINBINDING TODD-CARBOXYPEPTIDASE-TRANSPEPTIDASE 249
(2) Reversion of the complex in 0.01 M-phosphate buffer without concomitant reactivation of the enzyme yielded a radioactive compound which behaved as benzylpenicilloic acid. However, when sodium dodecyl sulphate was present during the dissociation of the complex, the benzylpenicilloic acid-like compound behaved as an unstable inter-mediate, decaying into several other compounds. Hence the conditions which prevailed during the dissociation of the [14C]benzylpenicillin-enzyme complex were importantforthe fate of the antibiotic molecule(asthey were for the enzyme). Thechemical alterations undergone by the antibiotic molecule appeared to depend onwhether or not the enzyme wasreleasedin an active form.
The nature of the bond formed between the -lactam antibiotics and the Streptomyces R39 enzymealso remainsa matterofspeculationexcept that no thiolgroup appearedto be involvedin the binding.This situation iscomparable withthat pre-viously observed for the exocellular DD-carboxy-peptidase-transpeptidase from Streptomyces R61 (Nieto et al., 1973) and stands in marked contrast with theDD-carboxypeptidase ofB.subtilis(Umbreit &Strominger, 1973),inwhichcase athioesterbond was apparently formed on reaction with benzyl-penicillin. In Tris-NaCl-MgCI2 buffer, i.e. under conditions wherereversion of the benzylpenicillin-Streptomyces R39 enzyme complex was accom-panied bythereactivation ofthe enzyme, the half-life of the complex was about 70h at 37° C. This valuecorrespondedto afirst-orderrateconstant for thedissociationof 2.8x10-6s-1.In0.01M-phosphate buffer reactivation of the enzyme did not occur, and parallel to this, the rate constant for the dis-sociation considerably increased to 1.6x 10-5s-1. Addition of sodiumdodecyl sulphatetothemixture caused,inturn,anadditionalincrease of the constant value to 0.8x10-4s-1. Theseresultssuggest that the bond formed between the
f,-lactam
antibiotics and the Streptomyces R39 enzyme, whatever its exact nature, isprobablyburied withintheprotein molecule and that denaturation of the protein greatly facili-tates the exposure of the bond to the solvent and the subsequent release of the modified antibioticmolecule.The exocellular DD-carboxypeptidase-transpep-tidase from Streptomyces R61 differs from the Streptomyces R39 enzyme in that the complex formed with benzylpenicillin is very unstable even underconditions where reactivation of the enzyme occurred. Strikingly, the rate constant for the dissociation(1x
10-4s-1;
Nietoetal.,1973)is almost identicalwith the rate constant for the dissociation of the benzylpenicillin-Streptomyces R39 enzyme complex in the presence of sodium dodecylsulphate (0.8x10-4s-'). The Streptomyces R61
enzyme alsoaltered the ['4C]benzylpenicillin mole-cule and the released radioactive compound was neither benzylpenicillin nor benzylpenicilloic acid (J. M. Frere, M. Leyh-Bouille, J. M. Ghuysen & H. R. Perkins, unpublished work). Hence a major difference between the Streptomyces R61 and R39 enzymes might reside in thetopography of the site onwhich binding of the f-lactam antibiotics occurs and which would make the bond thus formed more or less accessible to the action of thesolvent. Itmight also be possible that the different molecularweights of the two enzymes (38000 for the R61 enzyme; 53300 for the R39 enzyme) are relevant to this problem.
Thework in Liegewassupportedin partbythe Fonds
National de la Recherche Scientifique, the Fonds dela
Recherche Fondamentale Collective, Brussels, Belgium
(contractno.1000)andbytheInstitut pour
l'Encourage-mentdela RechercheScientifiquedans l'Industrie et l'Agri-culture, Brussels, Belgium (contractno.1699). J. M. F. is
Charg6de recherches du Fonds National dela Recherche
Scientifique, Brussels, Belgium, and is indebted to the European Molecular Biology Organization for a short-term fellowship duringa 3-month visit at the National Institute for Medical Research, Mill Hill, London, U.K.
References
Bray,G.A. (1960) Anal. Biochem. 1, 279-283
Fr6re, J. M.,Ghuysen, J. M., Perkins, H. R. & Nieto, M. (1973) Biochem. J. 135, 483-492
Frere, J. M., Moreno, R.,Ghuysen, J. M., Perkins, H. R., Delcambe, L. & Dierickx, L. (1974) Biochem. J. 143,233-240
Ghuysen, J. M., Leyh-Bouille, M., Campbell, J. N., Moreno, R., Fr6re, J. M., Duez, C., Nieto, M. & Perkins, H. R.(1973) Biochemistry 12,1243-1251 Ghuysen, J. M.,Reynolds, P. E., Perkins,H.R.,Fr6re,
J. M. & Moreno, R. (1974) Biochemistry 13, 2539-2547
Leyh-Bouille, M., Nakel, M., Fr6re, J. M., Johnson, K., Ghuysen, J. M., Nieto, M. & Perkins, H. R. (1972) Biochemistry 11, 1290-1298
Marquet,A.,Dusart, J.,Ghuysen,J. M. &Perkins,H.R. (1974) Eur. J. Biochem. 46, 515-523
Nieto,M.,Perkins,H. R.,Frere, J. M. &Ghuysen,J.M. (1973)Biochem.J.135, 493-505
O'Callaghan, C., Muggleton, P. W. & Ross, G. W. (1969) Antimicrob. Ag. Chemother. 57-63
O'Callaghan,C., Morris, A., Kirby, S. A. & Shingler,
A. H.(1972)Antimicrob. Ag. Chemother. 1, 283-288 Perkins, H. R.,Nieto, M., Frere,J.M.,Leyh-Bouille, M.
&Ghuysen, J. M.(1973) Biochem. J. 131,707-718
Schwartz, M. A.(1969)J.Med. Chem. 12, 36-38 Umbreit,J.N. &Strominger,J. L.(1973)J.Biol.Chem.
![Fig. 2. Dissociation of the [14C]benzylpenicillin-Strepto- [14C]benzylpenicillin-Strepto-myces R39 enzyme complex](https://thumb-eu.123doks.com/thumbv2/123doknet/5956993.147294/3.757.394.695.369.704/dissociation-benzylpenicillin-strepto-benzylpenicillin-strepto-myces-enzyme-complex.webp)
![Fig. 3. Co-electrophoresis at pH6.5 of intact [14Clbenzylpenicillin and of its degradation products that appeared after incubation offree [L4C]benzylpenicijjin in Tris-NaCl-MgCI2 bufferfor 15h at 37° C](https://thumb-eu.123doks.com/thumbv2/123doknet/5956993.147294/4.757.156.610.654.855/electrophoresis-clbenzylpenicillin-degradation-products-appeared-incubation-benzylpenicijjin-bufferfor.webp)
![Fig. 4. T.l.c. ofthe ['4C]benzylpenicillin-Streptomyces R39 enzyme complex after incubation in Tris-NaCl-MgCI2](https://thumb-eu.123doks.com/thumbv2/123doknet/5956993.147294/5.757.65.337.104.367/ofthe-benzylpenicillin-streptomyces-enzyme-complex-incubation-tris-nacl.webp)
![Fig. 8. Competition between [14C]benzylpenicillin and cephalosporin 87-312 for the Streptomyces R39 protein A solution of Streptomyces R39 enzyme that had been treated first with a slight excess of cephalosporin 87-312 and then with a large excess of ['4C]](https://thumb-eu.123doks.com/thumbv2/123doknet/5956993.147294/7.757.58.350.414.769/competition-benzylpenicillin-cephalosporin-streptomyces-protein-solution-streptomyces-cephalosporin.webp)
