Dissociation
of
increases
in
plasma
insulin-like
growth
factor
I
and
testosterone
during
the
onset
of
puberty
in
bulls
R.
Renaville,
S.
Massart,
M.
Sneyers,
M.
Falaki,
N.
Gengler,
A.
Burny
and
D.
Portetelle
Department
of
MolecularBiology
andAnimalPhysiology;
andDepartment
of
Microbiology,
Faculty
of
Agronomy,
2,Passage
desDéportés
B-5030 Gembloux,Belgium
The
present
study
was conducted to examine therelationship
betweenplasma
concen-trations of testosterone, insulin-likegrowth
factor I(IGF-I)
andIGF-binding
proteins
(IGFBPs)
during puberty,
in male calves treated with GnRH or testosteronepropionate.
Twelve male Holsteincalves
(10
weeksold)
wereassigned
to the control group(n
=6),
the GnRH-treated group(n
=3)
or the testosterone-treated group(n
=
3).
For 8weeks,
theGnRH-treatedgroup receiveda
single
i.v.injection
ofGnRH(0.5
\g=m\g
kg
\m=-\1body
mass)
eachday
while the testosterone-treated group received an i.m.injection
of testosteronepropionate
(0.5
mgkg
\m=-\1body
mass)
twice aday.
The calves werestudied untilthey
were200
days
old. Hormone treatments werestopped
one month afterpuberty
was reached inthe control group. Blood
samples
were collected every 30min for 8 h every thirdday.
Hormone concentrations were determined
by radioimmunoassay.
Westernligand blotting
andimmunoblotting,
using
monoclonal antibodiesagainst
IGFBP-2 andIGFBP-3,
wereusedto characterize the
IGF-binding
proteins.
In the control group,puberty
occurred at about120
days
ofageandwas associated withanincrease inconcentrations of testosterone, IGF-I and IGFBP-3 and a decrease in concentration of IGFBP-2. In the GnRH-treated group,plasma
testosterone remained low until 8 weeks after establishment ofpuberty
in the control group(4
weeks after the endof treatment).
In the testosterone-treated group,testosteronewas
high
during
thetreatmentperiod
and then decreased toprepubertal
values when treatment wasstopped.
Testosterone values increasedagain
to reachpostpubertal
values 5 weeks after the end of hormone treatment.
Nevertheless,
independent
oftestosterone status, the
profile
of IGF-I and the IGFBPs in the GnRH- and testosterone\x=req-\treated groups were
parallel
to thatreported
for the control group with the transitionfromprepubertal
to adult values at about 120days
of age. Inconclusion,
concentrations of testosterone, IGF-I and IGFBP-3 increasetogether,
butprobably independently,
during
theonset of
puberty
in male calves.Introduction
The onset of
puberty
has been shown to be acomplex
synergism
betweensexsteroids and insulin-likegrowth
factorI (IGF-I) in humans(Metzger
et al, 1994),sheep
(Lord
etal,
1991) andin cattle
(Renaville
et al, 1993).During
thisperiod,
testosterone isreleased
episodically
andplasma
concentrationsof IGF-I increase
strikingly,
concomitant with an increase inmean
plasma
concentration of testosterone. Apositive
corre¬lation was noted between the concentrations of these two hormones for bull calves
during
thisphysiological
step
(Renaville
etal,
1993). Moreover, as in humans(Smith
etal,
1993), baboons(Crawford
et al, 1994) andpigs
(Lee etal,
1991a), in
cattle,
the transition fromprepubertal
to maturemale is characterized
by
a marked increase in IGFBP-3concentrations
(Renaville
etal,
1993).However, information on the
possible
link between IGF-Iand testosterone
synthesis
during
thisphysiological
step
iscontradictory. Jasper
(1985), Harrisetal (1985) andAttie etal(1990),inhumans, andLeeelal
(1991b),
incattle,reported
that sex steroids stimulate IGF-Iconcentrationsdirectly
at thetimeof
puberty,
while Crawford et al (1993) concluded that thepubertal
IGF-I surge in mice does notrequire
androgens
ineither the pre- or
postpubertal periods.
In addition,Godfrey
et al (1992) demonstrated that treatment with testosterone
propionate
delays
puberty
andendogenous pulsatile
release of testosterone in bullcalves,
whileRonayme
et al (1993)described an increase in testosterone concentrations in
prepubertal
bulls treatedcontinuously
with GnRH.In the
study
described here,prepubertal
bull calves weretreated with testosterone
propionate
or GnRH to determine whetherIGF-Iisdependent
ontheandrogen
surgeat theonsetof
puberty.
Materials and Methods
Animals
Twelve,
spring-born
(identical
calving day),
Holstein malecalves(10weeks
old)
wereassigned
toeitherthe control group(n=6) or
groups treated with either GnRH (n=3) or testos¬ terone
propionate
(n=3). The 6x3x3experimental design
used in the
present
study
isjustified
by
the fact that all data have been evaluatedby
comparison
with theage of theonsetof
pulsatile
testosterone releaseinthe control group.Thelarge
number of animals in the control group wasjudged
necessarytocharacterize this
physiological
stage
exactly.
Animals were fed with grass
hay
twice aday
and acommercial allmash
containing
130gcrudeprotein
kg
~1.
Feedintake was controlled so that
body
massgain
was about950g
day
~ ; water wassupplied
adlibitum. All animalswerekept
inidentical conditions.Daily
roomtemperature (18°C)
anddaylength
(16hlight:8
hdark)
wereconstant.Initial meanlive masses were 74.4±4.8kg
for the controlgroup, 75.9±3.2kg
for the GnRH-treated group and 73.1±5.9
kg
for thetestosterone-treated group.
Experimental
design
Fora
period
of8weeks, the GnRH-treated group receivedadaily
i.v. injection of GnRH (0.5 µgkg-
body
mass;Sigma,
St Louis, MO) in 0.5ml saline and the testosterone-treated
group was
given
an i.m. injection of testosteronepropionate
(0.5mgkg
~.
body
mass;Sigma)
in 0.5 ml saline twiceaday.
Control calves receivedani.m.injectionof saline(0.5ml)
daily.
Animals were studied between 70 and 200
days
of age(day
0=
birth).
Hormone treatments werestopped
onemonth afterpuberty
wasachievedinthe controlgroup.Puberty
wastaken as the time when more than two successive values of testos¬ terone were greater than 2ng ml~ (Renavilleet al, 1993).Blood
samples
(5ml)
were taken from thejugular
vein viaapolyurethane
catheter intoheparinized
tubes at 30 min inter¬vals from 08:00h to 17:00h every third
day.
Samples
werestored
immediately
at 4°C and thencentrifuged
at 1800 g for10 min.Plasmawas
separated
and storedat—
20°Cuntilitwas
used in the hormone and
binding-protein
assays.Iodination
procedure
The recombinant bovine IGF-I
(rbIGF-1;
batch GTS-1) used as iodinated tracer (inradioimmunoassay
and westernligand
blotting)
and as standard (inradioimmunoassay)
waskindly
donatedby
Monsanto (St Louis, MO). The hormone waslabelled with Na[ I]
by
the chloramine method(Parker,
1990).
I25I-labelled
rbIGF-I wasseparated
from free iodineusing
a Centricon-3microconcentratorasrecommendedby
themanufacturer (Amicon Division,
Beverly,
MA). Thespecific
activity
was 70pCi
pg~.
Tritiated testosterone ([1,2,6,7- Hjtestosterone, batch TRK 402,
specific
activity 80-105 Cimmol~J)
waspurchased
fromAmersham International
(Amersham,
Bucks).
Production
of
monoclonal antibodiesagainst
bovineIGFbinding
protein
(IGFBP)
-2 and-3Anti-bovine IGFBP-2 and IGFBP-3 monoclonal antibodies
were
produced
inourlaboratory
(Porteteile
etal, 1995).Briefly,
ten-week-old female BALB/c mice were immunized with
100 µg recombinant fusion
protein
bovine IGFBP-2 (or-3)-maltose
binding
protein
(MBP) inincomplete
Freund'sadju¬
vant (0.10ml totalvolume)
equally
distributed into the rearfootpads.
Fourteendays
after immunization,micewerebledasrecommended
by
Mirza et al (1987) and thedraining
lymph
nodes(popliteal
andinguinal)
were removed andpooled
for fusionas describedby
Brücketal. (1982)with themodification that NSO murine cells wereused asmyeloma
partner.
Specific antibody-producing
hybridomas
were identifiedby
differential indirect
enzyme-linked
immunosorbent assays(ELISA) tests
using
recombinantproteins
IGFBP-MBPorMBP adsorbed onto the wells(Porteteile
etal,
1989). Positivehybridomas
wereclonedtwiceinsoftagar, grownonPristane-primed
BALB/c mice and thenpurified
by
ion-exchange
chromatography.
In thepresent
study,
the 7B5 anti-IGFBP-3(isotype IgGl)
monoclonalantibody
and the 8G10anti-IGFBP-2
(isotype IgGl)
monoclonalantibody
wereused intheimmunoblot studies.
Hormonedetermination
Testosterone was determined
by radioimmunoassay
asdescribed
by
Renaville et al (1983). The minimum detectable dose of testosterone was 0.1ng ml-1. The intra- and inter¬ assay coefficients of variation were 4.8% and 10.7%, respec¬tively,
for the low standard concentration (0.8ng ml~ ) and2.1% and 6.1%,
respectively,
forhigh
standard concentration(10ng
ml-1).
The
radioimmunoassay
for IGF-I in bullplasma
was per¬ formedaccording
to the method describedby
Lemal et al (1989)andadapted
by
Renavilleetal(1993).Inthismethod,asreported
by
Breier etal (1991),acryoprecipitation step
isusedto eliminate
aggregated
proteins
inplasma
extracts.Briefly,
after acid-ethanol extraction (87.5% ethanol and 12.5% HC1
mol 1~
v/v),
analiquot
of thesupernatant
was neutralizedwith 0.855mol Tris base 1~'
at a ratio of 5:2. The
samples
were storedat
—
20°C for 1hand then
centrifuged
at 3000 gfor30 min at4°C.The
supernatant
wasdecantedintofreshtesttubes and used in the
radioimmunoassay.
The minimumdetectable dose of IGF-I was 1ngml~
. Intra- and interassay coefficients of variation were 12% and 16%,
respectively,
for the low standard concentration (25 ngml-1)
and 6.5% and9%,
respectively,
for thehigh
standard concentration(250ng ml"
x).
Western
ligand blotting
Western
ligand blotting
wasperformed
to evaluateplasma
IGFBPs
following
the method describedby
Renaville et al(1993).
Briefly,
1µ
of SDS-denaturedplasma
wasapplied
to a4%
stacking gel
andelectrophoresis
wasperformed
through
a12.5%
polyacrylamide gel.
PrestainedI4C-labelled
molecularweight
markers(Amersham)
were runinparallel
lanes.Thegels
were then soaked in Towbin buffer (25 mmol Tris 1~\
192mmol
glycine
1\
20%(v/v)
methanol,pH
8.3) andproteins
were blotted onto nitrocellulose sheets(Hybond-C,
Amersham).
Electrophoresis
and electrotransfer were per¬ formedusing
theMini-Protean II system(Bio-Rad, Richmond,
CA). After saturation, membranes were incubated with
I25I-labelled
rbIGF-I (4000 c.p.m. cm "2
blot)
overnight
at4°C.The membranes were then
washed,
air-dried andexposed
toKodak X-Omat AR films
(Rochester,
NY) for 2-4days
at
-70°C.
Autoradiograms
werescannedusing
aSharp
JX325 scanner and band intensities wereanalysed
with imagemaster id soft¬ ware(Pharmacia,
Uppsala).
Aplasma pool
wasusedas internalstandard.
Immunoblotting
Plasma
samples
(1.5µ )
were denaturedby
SDS withdi-thiothreitol as
reducing
agent
and thenapplied
to a 4%stacking gel;
electrophoresis
wasperformed through
a 12.5%SDS-polyacrylamide
gel,
and blotted onto Immobilon sheet.Electrophoresis
and electrotransfer wereperformed
using
theMini-ProteanII
System
(Bio-Rad).
Membranesweresaturated for1hatroom
temperature
with a 10% defatted milk solution in Tris saline buffer (10mmol Tris-HCl 1"
\
150mmol NaCl\
pH
7.4),and then incu¬
bated
overnight
at 4°C with monoclonalantibody
(mAb)
7B5(2.4pg
mP1)
or mAb 8G10 (2.1pg ml":)
diluted in Trissaline buffer
containing
0.1%(v/v)
Tween20.Membraneswerewashed threetimesinTris saline buffer
containing
0.1%Tween20
(v/v),
followedby
incubation for 1h at roomtemperature
with alkaline
phosphatase
coupled
goat-anti-mouse
antibody
(0.133pgml" ;
Promega,
Leiden)
inTris saline buffercontain¬ing
0.1%Tween 20(v/v).
Membraneswerewashed four timesin Tris saline buffer
containing
0.1% Tween 20, andalkaline-phosphatase
activity was revealedusing
NBT/BCIP substrate(KPL,
Gaithersburg,
MD).Immunoblot membraneswere scanned
using
aSharp
JX325scanner and band intensities were
analysed
with imagemasterid software. A
plasma
pool
was used as the internalstandard.
Statistical
analyses
Valuesare
presented
as means±sd.Between-group
signifi¬
cancefor
parameters
wasassessedby analysis
ofvarianceusing
the GLM
procedure
of sas software(SAS®,
1991). Thefollowing
model wasused:#
=µ+ ,
+,
+( ),>
+^where
Y¡jk
=observations fordependent
variables oftreatmenti
during period
jonanimalk;
µ=overallmean;T¡
=treatmenteffect
(i
=1, 2, 3);P¡
=period
effect(j
=1, 2, 3.41, 42, 43);( )1(
=fixed effect associated with interaction between treat¬ment i at
period
;',·
andeik=random residual effect for the ithtreatment and the
jth
period.
Significant
differencesamongmeans weredeterminedby
theFtest at 5%
probability.
Results
Changes
in theconcentrationsof
hormonesduring
puberty
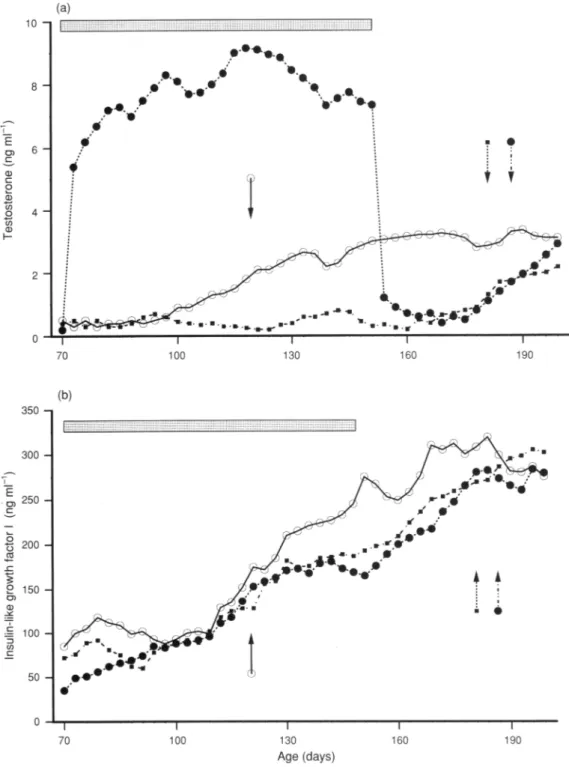
In thecontrol group, mean
plasma
concentrations oftestos¬ terone increasedsignificantly
(P<0.001) from 105 ±5days
old (mean value: 0.7+0.2 ng
ml"1)
to 120±10days
(2.1±0.3ngml~
J)
(Fig.
la).During
thisperiod,
release ofthehormone became
pulsatile
(values ranging
from 0.2±0.1 ngml~
1
to 4.3± 1.9ng ml~
:), indicating
that
puberty
had been reached. Mean concentrations of testosterone then increasedprogressively
today
160(meanvalue:3.1±0.8ngml~:),
afterwhich
they
remainedrelatively
constantup to the end of theexperimental period.
Hormone treatments had different effects on
plasma
testos¬ terone concentrations. Injections of testosteronepropionate
immediately
inducedasignificant
(P<0.001)increase inplasma
testosterone;
however,
therewas nodaily
episodic
release,andconcentrationsremained
higher
(7—9ngml~ ; <0.001) thanin the control group
during
the treatmentperiod
(Fig.
la).When testosterone treatmentwas discontinued
(day
150;onemonth after the onset of
puberty
in the controlgroup),
meanplasma
concentrations fell toprepubertal
values (0.5±0.2ngml
~ )in
one
week,
and werelower than inthe control group(P< 0.001)
(Fig.
la). Itwasonly
at 187±5days
(33± 7days
after the end of treatment) that a
pulsatile
release ofendog¬
enous testosteronewasosbervedin thisgroup and meandaily
concentrations reached similarvalues to those inthe controls.
In the GnRH-treatedgroup,a
daily
injectionofGnRH affected the release of testosterone. Fromday
70 today
110, meanvalues were low
(range
values: 0.3—0.7ngml-1)
and nosignificant
differences wereobserved with the control group. Afterday
110, whiletestosterone concentrationsinthecontrol groupincreased,valuesintheGnRHgroup,remained low untilday
163 ± 6(3.1 ±0.8ngml~~ inthe controlgroupcompared
with 0.2±0.1 ng ml"1 in the GnRH group; <0.001), fromwhich time concentrations
slowly
increased,finally
exhibiting
pulsatile
release and similar mean values to the control group(P>0.05)at
day
181±4.During
theexperimental period,
meanplasma
concentrationsofIGF-I exhibited asimilar patterninall groups. From
day
70 today
110, meanplasma
IGFT values wererelatively
constant and nosignificant
differences (P>0.05) were observedbetween the
experimental
groups and the control group, with mean values for theperiod
as follows: control group: 93+ 14ngml"1;
testosterone-treated group:76±17ng
ml":
and GnRH-treated group: 71±19ng ml"1
(Fig.
lb).
From
day
110 today
130, asharp
increase (P<0.001) inplasma
concentrations was observed in all groups:182±35ng ml~
:
in the control group; 156±24ng ml~
:
in
the testosterone-treated group and 163+34ng ml
~ in the
GnRH-treated group. IGF-I concentrations in the control
group then continued to increase, to reach values of about
250-300ng ml~
l
at the end of the
experiment.
In the twotreatment groups, IGF-I concentrations increased more
slowly
and were below values for the control group betweendays
145 and 155 (P<0.01). A second increase was observed between
days
160 and 185 in the two treatment groupswhen values similar to those seen in the controls were reached.
Fig. 1.Mean
plasma
concentrations of(a) testosteroneand(b) insulin-likegrowth
factorIin(O) control, (·)testosterone
propionate-treated
and ( ) GnRH-treated bullsduring
the onset ofpuberty.
(D) Theperiod
oftreatment.Arrowsindicate theonsetof
puberty
ineachgroup: (O) control; (·)testosteronepropionate-treated
and ( ) GnRH-treated groups.
Western
ligand blotting
of
IGFBPsWestern
ligand blotting
of bovineplasma
revealed four distinct bands thatspecifically
bound125I-labelled
IGF-I.Their molecularmasseswere,respectively,
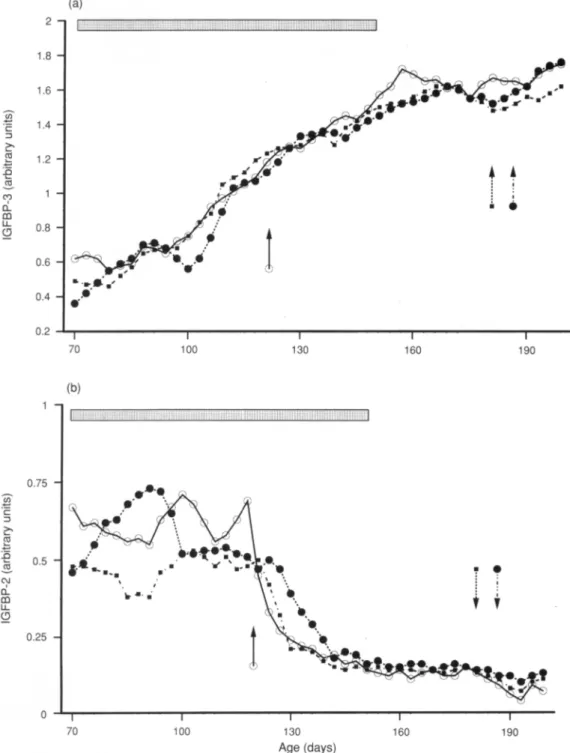
45-54, 38,28and24kDa.Inthecontrolgroup,theonsetof
puberty
wascharacterizedby
an increase fromday
100 (0.69+0.14 units) today
150(1.72±0.21 units) (P<0.001) in the
intensity
of the doublet45—54kDa band, which was identified as IGFBP-3, after which it remained constant until the end of the
experiment
(Fig.
2a). Theintensity
of the IGFBP-2 (38kDa)
band wasmarkedly
reduced fromday
120(0.67±0.18 units)today
160(0.17±0.08 units) (P<0.001) and remained low until the
end of the
experiment
(Fig.
2b).
During
thisperiod,
noFig.2.Mean
plasma
concentrationof(a)insulin-likegrowth
factor-binding
protein-3 (IGFBP-3)and(b)IGFBP-2in(O)control, (·)testosterone
propionate-treated
and( ) GnRH-treatedgroupsduring
theonsetofpuberty.
( ):The
period
oftreatment.Arrowsindicate theonsetofpuberty
ineach group:(O)control;(·)testosteronepropionate-treated
and( ) GnRH-treated groups.corresponding
toIGFBP-1 andIGFBP-4,respectively
(data
notshown).
Daily
injections oftestosteronepropionate
orGnRH hadno effecton meanplasma
concentrations ofIGFBP-2andIGFBP-3.Indeed,
theprofile
of these twoproteins
was similar to thatreported
for the controlgroup, with aprogressive
increase inIGFBP-3 fromabout
day
100 and asharp
decreasein IGFBP-2 afterday
120 in both groups. Nosignificant
difference(P> 0.05)wasobserved onany
day
of collection between the values in controland treated groups.Immunoblotting
of
IGFBP-2 and IGFBPSThe immunoblot
method, using
monoclonal antibodiesagainst
bovine IGFBP-2 and IGFBP-3,showedasimilarpattern
Fig. 3. (a) Plasma insulin-like
growth
factorbinding
protein-3 (IGFBP-3) and(b) IGFBP-2 inanindividual bull calf3 weeks before(lanes1-3) and three weeks after(lanes5-7)puberty.
Lane4 representsthe weekduring
which the animal attainedpuberty
(pulsatile
release of testosterone).The proteins were revealedusingmonoclonalantibodies against bovine IGFBP-3 or IGFBP-2.
for these two
proteins
throughout
theexperimental period
(Fig.
3)andwasinagreement
with dataobtainedusing
westernligand blotting.
Discussion
The
study
reported
herewasconducted to define the relation¬ship
betweenplasma
concentrations oftestosterone and IGF-Iduring
puberty,
by
treating
male calves with GnRH or testo¬sterone
propionate.
In the control group, theonset ofpuberty
in bull calves was identified
by
concomitant rises ofplasma
concentrations of testosterone and IGF-I, as described
by
Ronge
and Blum (1989a,b)
and Renaville et al (1993). It hasbeen
suggested by
previous
studies,particularly
in humans(Kerrigan
andRogol,
1992; Zachmann, 1992;Metzger
etal,
1994),that these coordinatedhormonalincreasesaredriven
by
growth
hormone. Indeed, data from the control group in thepresent
study
suggests
that thepuberty-associated
increase inplasma
concentrations of IGF-I could result from the stimula¬toryeffect oftestosteroneonthe
somatotrophic
axis,indirectly
mediatedvia the
oestrogen
receptor
after thearomatizationoftestosterone to oestradiol. Schwarz et al. (1992)
reported
thatgonadectomy
incattlecauses afall inplasma
concentrationsofGH, testosterone and IGF-I and Metzer and
Kerrigan
(1994) showed thatblocking
theoestrogen
receptor
with tamoxifen results indecreased 24h meanserum GH andplasma
IGF-I inhumans. However,
Metzger
et al. (1994) concluded that it isdifficult to differentiate between the testosterone effects because the hormone can act either
directly
through
theandrogen
receptor
orindirectly
through
theoestrogen
receptor
once it is aromatizedtooestrogen.
Under the
experimental
conditions in thepresent
study,
hormonal treatment ofprepubertal
bulls inhibitedendogenous
testosterone
production.
Thepartial
suppression
ofgonadal
steroidogenesis
by
GnRH (aprepubertal
levelwasmaintained)
observedin thisstudy
are inagreement
withprevious reports
in men (Harris et al, 1985;Styne
et al, 1985), rams(Lincoln
et
al,
1986) andstags (Lincoln,
1987) but are in conflict withstudiesin maturebulls
(Rechenberg
etal, 1986;Ronayme
etal,
1993). Mannet al. (1984)
reported
that chronicadministration ofagonist
analogues
ofGnRH inducedadesensitization ofthepituitary
gonadotroph
in rhesusmonkeys.
These authors alsoreported
that infusion of a GnRHagonist
in newborn rhesusmonkeys
suppressed
theexpected prepubertal
increase in LHand testosterone (Mann et al, 1989). In rats, the
postnatal
increase in testosteronecould be abolished
by
treatment witheither GnRH
agonist
orantagonist (Kolho
and Huhtaniemi,1989). Several studies have also
emphasized
therelationship
between testosteroneand avariety ofsteroid
growth
promot¬
ers.Itwasobserved
(Fabry
etal,
1983, 1984;Jonesetal,
1991;Godfrey
et al, 1992) that thepulsatile
nature of testosteroneprofiles
wasabolished, testosterone secretionmarkedly
reduced(but
notsuppressed)
andpuberty
delayed
aftertreatment withandrogen,
progestagen
oroestrogen
aloneor in combination.In the
present
study,
thesharp
testosteronedecrease observed in the testosterone-treated group after the end of treatmentconfirmed that
endogenous
testosteronesynthesis
was at aprepubertal
level.There are few studies that have examined the effects of
testosterone or GnRH administration on the
production
ofIGF-Iin
prepubertal
males. Schwarzetal.(1992)observedno difference inplasma
IGF-Iprofiles
between bulls castratedbefore
puberty (gonadectomy
at 108days
ofage)
and intactbulls until
approximately
250days
ofage. In thepresent
trial,although
major inhibition of the secretion ofendogenous
testosteronetook
place
during
both hormonaltreatments,there was no major alteration in IGF-Iprofiles during
the onset ofpuberty
in thetwo treatmentgroupswhencompared
with thecontrolgroup. The aim of the
present
study
was to determineby
whichpathways
IGF-Iwasmodifiedby
GnRH ortestoster¬one treatment. The first observation in the
present
trial wasthat, contrary to the situation in castrated
animals,
the inhi¬ bition ofendogenous
testosteronesynthesis
in theGnRH- and testosterone-treated groups was notcomplete.
Testosteronethe IGF-I surge at
puberty.
However, since testosteronetreatmentdidnotinducean increaseinIGF-I,it is
possible
thattheenzymesnecessary to aromatizetestosterone to
oestradiol,
which is known to induce a
growth
hormone-induced IGF-Iresponse in cattle (Breier et
al,
1988), areonly
present
atpuberty.
Anotherpossibility
wassuggested
by
Hobbs et al (1993) who observed different effects of treatment with nan-drolone and testosterone enanthate on IGF-Isynthesis
by
aqualitative
difference inandrogenic
potency
attheneuroendo¬crine
level,
particularly
if local 5 -reduction of theandrogen
occurs. However, Tenover and Matsumoto (1992) found no
change
in eithergrowth
hormone or IGF-I values in youngadult men treated with testosterone enanthate for 12 weeks.
Low IGF-I values from
day
145 today
155 in the treatedgroups could also indicate that testosterone
synthesis
wasinsufficient to sustain normal IGF
production
viaoestrogen
receptors
and stimulationby
growth
hormone, asreported
in steersby
Schwarz et al. (1992). Thishypothesis
is alsosupported
by
the IGF-I increase noted in thepresent
study
after the
physiological
induction of apulsatile
testosteronerelease in both treatmentgroups.
IGFs in
plasma
and mostbody
fluids arecomplexed
withhigh-affinity binding
proteins
(IGFBPs). Numerous studies on nutritional status or treatment withgrowth
hormone haveclearly
demonstrated thatIGFBP-3, thepredominant
IGFBP inplasma,
isrelevantto thepharmacokinetics
andactionofIGF-I,while there is a
negative
correlation between IGFBP-2 andIGF-I concentrations
(Cohick
etal,
1992; Thissenetal,
1994).Taking
into account thisinformation,
the results of ourprevious
study
(Renaville
etal,
1993)andthe data observedby
Lee et al
(1991b)
inpigs,
the transition frompuberty
tomaturityin bullcalves maybe identified
by
amarkeddecreasein IGFBP-2 and
by
an increase in IGFBP-3.This is the first
report
of the use ofspecific
monoclonalantibodies
against
bovine IGFBP-2 and IGFBP-3 in immuno¬blotting
to confirm thechanges
inplasma
concentrations ofIGFBP-2andIGFBP-3 observed
by
westernligand blotting.
Aswasobserved forIGF-I,
plasma
concentrationsofIGFBP-2andIGFBP-3intreatedanimalsfollowed the
profile
reported
forthe control group andappeared
unaffectedby
testosterone status. In conclusion, ourstudy
shows that the abolition of thepubertal
rise inplasma
testosterone,by
treatment with GnRHortestosterone
propionate,
does not have a marked effectonIGF-Ioronits
plasma-binding
proteins
(IGFBP-2andIGFBP-3) in male calves. However, our resultssuggest that testosteronecan still stimulate IGF-I
synthesis,
as observed in both treat¬ ment groups betweendays
180 and 190, whenendogenous
plasma
concentrationsoftestosteroneare increased.Wethere¬ fore consider: (1) that thepubertal
androgen
surge is not themajorfactor
responsible
forthesimultaneous increase in IGF-I and (2), that testosterone and IGF-I values increase simultane¬ously,
butindividually,
attheonsetofpuberty
inbulls. Furtherinvestigations
are needed to understand the mechanismby
which thesynthesis
of IGF-I and testosterone are induced atpuberty.
Financialsupport for this
study
wasprovided
bytheInstitut pourl'Encouragement
de la RechercheScientifique
dans l'Industrie etl'Agriculture
(research grants 5512 A and 5645 A) andby
Pôle d'Attraction Interuniversitaire research grant PAI 15. The authorswish to thank Y. Braet, R-M. Maistriaux and G. Tavernierfor their technical assistanceandJ.Moremanfor her
help
inthepreparationof the manuscript.References
AttieKM, RamirezNR, Conte FA,KaplanSL and Grumbach MM (1990)The
prepubertal growthspurtineightpatientswithtrueprecociouspubertyand
growthhormonedeficiency:evidenceforadirectrole ofsexsteroidsJournal
ofClinicalEndocrinologyand Metabolism71 975—983
BreierBH,Cluckman PD and BassJJ (1988)Thesomatotrophicaxis inyoung
steers: influence of nutritional status and oestradiol-17ß on hepatic high
and low-affinity somatotrophic binding sitesJournal ofEndocrinology 116 169-177
Breier BH, Gallaher BW and Gluckman PD (1991) Radioimmunoassay for insulin-likegrowthfactor-I: solutionstosomepotentialproblemsandpitfalls
Journal ofEndocrinology 128 347-357
Brück C, MathotS,Portetelle D,BerteC,Franssen JD,Herion andBurnyA
(1982) Monoclonal antibodies defineeightindependentantigenicregionson
the bovine leukemiavirus(BLV)envelopeglycoproteingp51 Virology122 342-352
CohickWS,McGuireMA,ClemmonsDRand BaumanDE (1992)Regulationof
insulin-likegrowthfactor-bindingproteinsinserumandlymphoflactating cowsbysomatotropinEndocrinology 130 1508-1514
Crawford BA, Singh J, Simpson JM and Handelsman DJ (1993) Androgen regulationofcirculatinginsulin-likegrowthfactor-Iduringpubertyinmale
hypogonadalmiceJournal ofEndocrinology13957—65
CrawfordBA,SimpsonJMand HandelsmanDJ (1994)Regulationofcirculating IGF-I levels during puberty in male baboons (Papio hamadryas) Growth
Regulation4 (Supplement1) 111 (Abstract)
FabryJ,RenavilleR,Halleux V andBurnyA (1983)PlasmatestosteroneandLH responses to LHRH in doubled-muscled bulls treated with trenbolone
acetateandzeranolJournal ofAnimalScience 57 1138—1145
FabryJ, Renaville R and BurnyA (1984)Blood plasma LH and testosterone concentration inanabolized bullsAnimalProduction39 345-354 GodfreyRW,LunstraDDandSchanbacherBD (1992)Effectofimplantingbull
calves with testosterone propionate, dihydrotestosterone propionate or
oestradiol-17ß prepubertally on the pituitary—testicularaxis and onpost¬
pubertalsocial and sexual behaviourJournal ofReproductionandFertility94 57-69
HarrisDA, VanVlietG, Egli CA, Grumbach MM, KaplanSL, Styne DM and Vainsel M (1985)Somatomedin-Cinnormalpubertyandin trueprecocious
puberty before and after treatment with a potent luteinizing
hormone-releasinghormone agonist Journal ofClinicalEndocrinologyandMetabolism
61 152-159
HobbsCJ, Plymate SR,Rosen CJ andAdler RA (1993)Testosterone adminis¬
tration increasesinsulin-likegrowthfactor-I levelsinnormalmenJournal of
ClinicalEndocrinologyand Metabolism 77776-779
Jasper HG (1985) Somatomedin response to testosterone stimulation in
children with male pseudohermaphroditism, cryptorchidism, anorchia, or micropenisJournal ofClinicalEndocrinologyandMetabolism60 910—913
Jones SJ, JohnsonRD,CalkinsCRand DikemanME (1991)Effects of trenbolone
acetateon carcasscharacteristicandserumtestosteroneand Cortisolconcen¬
trations inbulls and steerson differentmanagementand implant schemes
JournalofAnimal Science69 1363—1369
Kerrigan JR and RogolAD (1992) The impactof gonadalsteroid hormone
action on growth hormone secretion during childhood and adolescence
EndocrineReviews 13 281—298
Kolho KL and Huhtaniemi I (1989)Suppressionofpituitary-testisfunctionin ratstreatedneonatallywithagonadotrophin-releasinghormoneagonistand
antagonist:acuteand long-termeffectsJournal ofEndocrinology 123 83—91
LeeCY,BazerFW,Etherton TD andSimmenFA (1991a)Ontogenyof insulin¬ likegrowthfactors (IGF-Iand IGF-II)andIGF-binding proteinsinporcine
serumduringfetal andpostnataldevelopmentEndocrinology128 2336-2344
Lee CY, HuntDW, Gray SL and Henricks DM (1991b) Secretory patterns of
growthhormone andinsulin-likegrowthfactor-Iduring peripubertalperiod
in intactand castrated male cattleDomesticAnimalEndocrinology8 481-489
Lemal D,RenavilleR, ClaesV,RuelleL,FabryJ, BurnyA,Underwood LEand
KetelslegersJM (1989)Effectofpituitary somatotropininjectionsonplasma
insulin-likegrowthfactor-I andgrowthhormoneprofilesingrowingheifers
Lincoln GA (1987) Long-term stimulatoryeffects ofacontinuousinfusion of
LHRHagonistontesticular functioninmale red deer[Cervuselaphus)Journal
ofReproductionandFertility80 257—261
LincolnGA,FraserHM and AbbottMP (1986)BlockageofpulsatileLH, FSH
andtestosterone secretion inramsbyconstantinfusionofanLHRHagonist Journal ofReproductionandFertility77 587—597
Lord APD,MartinAA,WaltonPE,BallardFJand Read LC (1991) Insulin-like
growth factor-binding proteins in tissue fluids from the lamb Journal of
Endocrinology129 59-68
Mann DR, Gould KG and Collins DC (1984) Influence of continuous gonadotropin-releasinghormone (GnRH) agonist treatment on luteinizing
hormone and testosterone secretion, the response to GnRH, and the testicular response to human chorionic gonadotropin in male rhesus
monkeysJournal ofClinicalEndocrinologyand Metabolism58 262-267
Mann DR, Gould KG, Collins DC and Wallen (1989) Blockage of neo¬ natal activation of the pituitary—testicular axis: effect on peripubertal
luteinizinghormone and testosterone secretionandontesticulardevelop¬
ment inmalemonkeysJournal ofClinicalEndocrinologyandMetabolism60 600-607
MetzgerDLandKerriganJR (1994)Estrogenreceptorblockade with tamoxifen diminishes growthhormonesecretion in boys: evidence fora stimulatory
role ofendogenous estrogens duringmale adolescenceJournal ofClinical
Endocrinologyand Metabolism 79513-518
Metzger DL, Kerrigan JR and Rogol AD (1994) Gonadal steroid hormone
regulationofthesomatotropicaxisduringpubertyinhumans: mechanisms ofandrogen andestrogenactionTrendsin EndocrinologyandMetabolism 5 290-296
MirzaI,WilkinT,Cantarmi M and Moore (1987)Acomparisonofspleenand
lymphnode cellsasfusionpartnersfor theraisingofmonoclonalantibodies
after differentroutesofimmunization Journal ofImmunologyMethods 105 235-243
Parker CW (1990) Radiolabelling of proteins Methods in Enzymology 182
721-737
PortetelleD,DandoyC,BurnyA,ZavadaJ,SiakkouH,Gras-MasseH,Drobecq
andTartarA (1989)Syntheticpeptides approachtoidentification ofepitopes
onBovineLeukemiaVirusenvelope glycoproteingp51 Virology169 34-41
PortetelleD,MassartS,SneyersM,BurnyAand RenavilleR (1995) Monoclonal
antibodies to bovine insulin-like growth factor-binding protein-2 and -3:
productionand preliminarycharacterizationSymposiumonHormoneBinding
Proteins: Physiology and Clinical Implications. 78° Journées Internationales
Henri-PierreKlotzd'EndocrinologieClinique,Parisp58(Abstract)
RechenbergWV, SandowJand Klatt (1986) Effect oflong-terminfusion ofan
LH-releasing hormone agonist on testicular function in bulls Journal of
Endocrinology 109R9-R11
RenavilleR,FabryJ,HalleuxVandBurnyA (1983)Testosteroneplasma profiles,
as afunction ofagein youngbulls fromthe bovinedouble-muscledBelgian
White Blue breed.Apreliminaryreport Theriogenology19 159-167
RenavilleR,DevolderA,MassartS,SneyersM,BurnyAand Portetelle D (1993)
Changes in the hypophysial-gonadal axisduringthe onset ofpubertyin
youngbullsJournalof ReproductionandFertility99 443-449
RonaymeE,EnrightWJand RocheJF (1993) Effects ofcontinuousadministration ofgonadotropin-releasinghormone(GnRH)or apotentGnRHanalogueon bloodluteinizing hormoneand testosterone concentrations inprepubertal
bullsDomesticAnimalEndocrinology 10 179-189
RongeH and BlumJW (1989a) Insulin-likegrowthfactor-1 during growthin
bullsReproductionNutritionDéveloppement29 105-111
RongeHand BlumJW (1989b)Insulin-likegrowthfactor-Ibindingproteinsin
dairycows, calves andbulls ActaEndocrinologica 121 153—160
SAS (1991)SAS User'sGuide:Statistics(Version6),SASInstitute, Inc.Cary,NC SchwarzFJ,RöpkeR,SchamsDandKirchgessnerM (1992) Effects ofsexand
growthonplasma concentrations ofgrowthhormone, insulin-likegrowth
factor-I andinsulininfatteningsimmentalcattleJournal ofAnimalPhysiology
andAnimalNutrition68 263-271
SmithWJ,NamTJ,UnderwoodLE, BusbyWH, CelnickerAand Gemmons DR
(1993) Use of insulin-like growth factor-binding protein-2 (IGFBP-2),
IGFBP-3,andIGF-Ifor assessinggrowthhormonestatus inshort children
Journal ofClinicalEndocrinologyandMetabolism 77 1294—1299
StyneDM, Harris DA, Egli CA,Conte FA, Kaplan SL,Rivier J,Vale Wand
Grumbach MM (1985)Treatment oftrueprecociouspubertywithapotent
luteinizing hormone-releasing hormone agonist: effect of growth sexual
maturation,pelvicsonographyand thehypothalamic—pituitary—gonadalaxis Journal ofClinicalEndocrinologyand Metabolism65 142—149
TenoverJS and Matsumoto AM (1992) Effect of testosterone (T)on growth
hormone(GH)and insulin-likegrowthfactor-I levelsinhealthy elderlyand
youngadult men. 74th AnnualMeeting ofthe EndocrineSociety page 367 (Abstract)
ThissenJP,KetelslegersJMand UnderwoodLE (1994)Nutritionalregulationof the insulin-likegrowthfactors EndocrineReviews15 80—101
Zachmann M (1992) Interrelations between growth hormone and sex hor¬
mones: physiology and therapeutic consequences. Hormone Research 38