Any correspondence concerning this service should be sent to the repository administrator:
staff-oatao@inp-toulouse.fr
DOI:10.1016/j.apsusc.2012.11.098
Official URL:
http://dx.doi.org/10.1016/j.apsusc.2012.11.098
This is an author-deposited version published in:
http://oatao.univ-toulouse.fr/
Eprints ID: 8782
To cite this version:
Giraud, Isabelle and Franceschi-Messant, Sophie and Perez, Emile and
Lacabanne, Colette and Dantras, Eric Preparation of aqueous dispersion of
thermoplastic sizing agent for carbon fiber by emulsion/solvent evaporation.
(2013) Applied Surface Science, vol. 266 . pp. 94-99. ISSN 0169-4332
O
pen
A
rchive
T
oulouse
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
Preparation
of
aqueous
dispersion
of
thermoplastic
sizing
agent
for
carbon
fiber
by
emulsion/solvent
evaporation
Isabelle
Giraud
a,
Sophie
Franceschi-Messant
a,
Emile
Perez
a,∗,
Colette
Lacabanne
b,
Eric
Dantras
baLaboratoiredesI.M.R.C.P.,UMR5623CNRS,UniversitéPaulSabatier,31062ToulouseCedex09,France
bLaboratoiredePhysiquedesPolymères,CIRIMAT,InstitutCARNOT,UniversitéPaulSabatier,31062ToulouseCedex09,France
Keywords: Sizing Carbonfibers Composites Thermoplasticpolymer Aqueousdispersion Emulsion/solventevaporation
a
b
s
t
r
a
c
t
Inthiswork,differentsizingagentaqueousdispersionsbasedonpolyetherimide(PEI)wereelaboratedin ordertoimprovetheinterfacebetweencarbonfibersandathermoplasticmatrix(PEEK).Thedispersions wereobtainedbytheemulsion/solventevaporationtechnique.Tooptimizethestabilityandthefilm formationonthefibers,twosurfactantsweretestedatdifferentconcentrations,withdifferent concen-trationsofPEI.Thedispersionsobtainedwerecharacterizedbydynamiclightscattering(DLS)andthe stabilityevaluatedbyanalyticalcentrifugation(LUMiFuge).Theselecteddispersionsweretestedforfilm formationabilitybyscanningelectronmicroscopy(SEM),andthesizingperformancewasassessedby observationofthefiber/matrixinterfacebySEM.TheresultsrevealedthatanaqueousdispersionofPEI, stabilizedbysodiumdodecylsulfateasthesurfactant,ledtoverystablesizingagentaqueousdispersion withidealfilmformationandbetterinterfaceadhesion.
1. Introduction
Carbonfiberiswidelyusedasareinforcingmaterialin
com-posites,especiallyinadvancedcomposites[1,2].Ascarbonfibers
arebrittle,manyproblems,suchasfilamentbreakageandfluffing,
ariseduetomechanicalfrictionduringthemanufacturingprocess
[3–5].Therefore,carbonfibersaregenerallysizedorcoatedbya
sizinglayeronthesurface,whichisusuallyobtainedfroma
solu-tionoremulsionconsistingofpolymericcomponents[6,7].Sizing
easesfiberhandlingandcanalsoprovideacouplingagentforthe
fiber/matrixbond [8–11]. Thenatureof thesizingis oftenkept
secretbymanufacturersofcarbonfibers.However,sizingischosen
accordingtothenatureofthematrixandisgenerallyapre-polymer
orpolymer.Mostofthecompositesaremadefromepoxyresin,and
sizingagentsareoftenofthesamenature[5,12–14].Thisisa
prob-lemwhenthematrixisahigh-temperaturethermoplasticpolymer
sincethedegradationtemperatureofthistypeofsizingisaround
250◦C [15]. For polyimides, PEEK and other high-temperature
thermoplastic polymers,thefunctional groups provided bythe
traditionalepoxy-compatiblesizingdonotreactchemicallywith
thesepolymersandweakinterfacialshearstrengthsresult[16].
Moreover,forcompositesmoldedwithpolyimidesorPEEK,high
processingtemperaturesduringmanufactureandcontinuoususe
∗ Correspondingauthor.
E-mailaddress:perez@chimie.ups-tlse.fr(E.Perez).
inhigh-temperatureenvironmentsdegradetheepoxysizingand,
consequently,weakenthefiber/matrixinterface,producingvoids
anddelaminations[17–19].Alloftheseobservationsunderlinethe
importanceofhavingsizingthatissuitableforhigh-temperature
thermoplasticmatrices.Fromapracticalpointofview,thesizing
formulationshouldbeeasytouse,non-toxicandenvironmentally
friendly.Inthispaper,wereportthefirstexampleofpreparation
anaqueousdispersionofathermoplasticsizingagentforcarbon
fiberbyemulsion/solventevaporation.
2. Experimental
2.1. Materials
ThepolyetherimidePEI(Ultem1000)wasobtainedfromSabic®.
Thesodiumdodecylsulfate(SDS)andthechloroformwere
pro-videdbySigma–Aldrich,thebenzalkoniumchloride(BC)wasfrom
Fluka (C12 60%, C14 40%). The AS4 carbon fiber tow, provided
byHexcel,wastreatedunsizedandcontained12,000fibers.The
polyetheretherketone(PEEK)providedbyVictrexwasa 100mm
thickfilm.TheremoldingagentwasCIREX041WBfromSICOMIN.
2.2. PreparationofPEIdispersionsbyemulsion/evaporation
In order to reduce thetoxicity and to respect the
environ-ment,organicsolventsmustbeavoidedinthefinalsizingagent
formulation.Forthesereasons,wedecidedtoelaborateaqueous
Fig.1.Schematicrepresentationofthepharmaceuticalemulsification/evaporationprocess.
dispersions.Thesecanbemadebyavarietyofmethods[20–23]
leading,attheend,tostablehydrophobicparticlesinwater.The
preparationprocessdescribedherewaslargelyinspiredby
emul-sion/solventevaporation,anencapsulationtechniqueusedinthe
pharmaceuticalindustrytoprepareaqueousdispersionsof
poly-mernanoparticlesormicrospheres.
Emulsion/solvent evaporation involves a two-step process
(Fig.1):theemulsificationofa polymersolutioncontainingthe
encapsulatedsubstance,followedbyparticlehardeningthrough
solventevaporationandpolymerprecipitation.Duringthewater
emulsification, the polymer in solution in the volatile,
water-immisciblesolventisbrokenintomicrodropletsbytheshearstress
producedbyeitherahomogenizerorasonicatorinthepresenceof
asurface-activeagentuntilthepolymerprecipitates[24–27].
Thismethodwasusedtoprepareastableaqueousdispersionof
PEIasthesizingagent.Weusedtwodifferentsurfactants,sodium
dodecylsulfate(SDS)andbenzalkoniumchloride(BC)atdifferent
concentrations(0.3%,0.5%and1wt%).Thefinalconcentrationsof
PEIwere0.1%,0.3%,0.5%and1wt%.ThePEIdispersionat0.5wt%ina
0.5wt%surfactantsolutionwaspreparedasfollows.Ina5-mLflask,
0.1005gofPEIwasdissolvedin2mLofchloroform.Thissolution
waspouredintoanotherflaskcontaining20mLofthesurfactant
solution.Themixturewasemulsifiedbyultrasoundshearing(Vibra
Cell,BioblockScientific600W,20Hz).Theshearinglasted5minat
power4.Awaterbathwasusedtomaintainthesolutionatroom
temperature.Then,magneticstirringoftheemulsionat1200rpm
for12hallowedtotalevaporationofthechloroform.
2.3. CharacterizationofPEIdispersions 2.3.1. Particlesizeanalysis
Dynamiclightscattering(DLS)wasperformedusingaMalvern
InstrumentsNanoZSwithaHe–Nelaser(633nm)atascattering
angleof173◦andat25±1◦C.Thehydrodynamicmeandiameterof
thenanoparticleswasdeterminedusingthesoftwareprovidedby
MalvernInstruments.TheContinmodelwasappliedtoobtainsize
data.Alltheauto-correlationfunctionfitswerecheckedandfound
tobeinaccordancewiththeexperimentaldata.Fivemeasurements
weremadeoneachsamplewithanaccuracyofabout2nm.
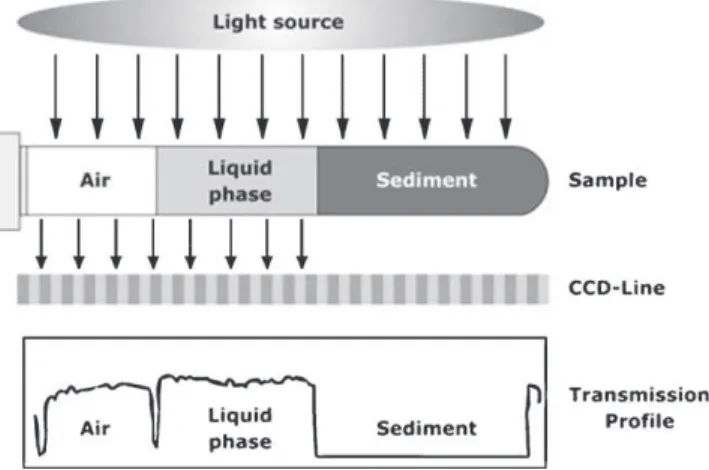
2.3.2. Evaluationofstabilityusinganalyticalcentrifugation
Aseparationanalyzer(LUMiFuge,L.U.M.Berlin,Germany)was
usedtodeterminetheseparationbehaviorofdispersionsunderthe
influenceofvariouscentrifugalforces(5–1000×g).Thisapparatus
isbasedona low-speedcentrifugecombinedwithan
optoelec-tronicmeasuringsystemthatrecordsthelighttransmissionover
theentire sample cuvette (Fig. 2). The cuvettes containingthe
suspensionare positioned in thehorizontal planeon therotor
ofthecentrifuge.Duringcentrifugationalightsourcepositioned
abovetherotoremitsradiation(near-infrared)ontothesample.
TransmittedlightisdetectedbyaCCDlinesensorbelowtherotor
planeandisanalyzedbyamicrocontroller,whichgeneratesa
light-transmissionprofileof thesampleareafor everymeasurement
step.
This technique is very appropriate for the study and
opti-mizationofverystableaqueousdispersions.Thecentrifugalforce
acceleratesthedestabilizationofthedispersionandrapidly
deter-minestheshelflifeofthedispersion[28].Moreover,thepossibility
ofstudying8samplesatthesametimeenablesdifferent
formu-lationstobecomparedimmediately[29,30].Thedispersionsare
naturallystableover6monthssowechosetosimulate3yearsof
aging.Thedataacquisitioncorrespondedto255profilesrecorded
everyeverysecondsat4000rpm.Thetemperaturewas20◦C.
2.4. Sizingtreatmentofcarbonfiberandcompositepreparation 2.4.1. Sizingofcarbonfiber
Differentmethodscanbeusedtosizecarbonfibers,such as
electrodeposition[31,32]orelectropolymerization[33,34],butthe
mostcommonisbathcoating.Wetestedthesizingatlaboratory
scaleso,inthiscase,themostsuitabletechniquewastospraythe
dispersiondirectlyontothefibersurface.Anunsizedfibertowwas
strainedbyaweighttokeepitvertical,allowinguniformspraying
ofthesizingatthefibersurface(Fig.3).Aftersizing,thefiberswere
driedatroomtemperature.
Fig.3. Schematicrepresentationofthesizingprocess.
2.4.2. Compositepreparation
WealsopreparedPEEK/unidirectionalcarbonfibersamplesat
laboratoryscale.Thesampleswerepreparedbyhotpress
mold-ing.ThepressusedwasaCarver4128CEequippedwithheating
plates.Theprocessingtookplaceinseveralsteps.Thesamplewas
firstprepared,thenmoldedinthehotpressandfinallycooledand
remolded.Inordertokeepallthecarbonfibersinthesame
direc-tionduringthedifferentsteps,thestrandsofcarbonfiberwere
insertedinafoldedPEEKfilm(Fig.4).
The sample was then placed in an aluminum mold
previ-ouslycoatedwiththeremoldingagent.Thenthemoldwasplaced
betweenthetwoplates,previouslyheatedto400◦C,andkeptin
contactfor15mintoallowthePEEKtomeltuniformly.6MPaof
pressurewasthenappliedfor30stoletthePEEKimpregnatethe
fibers.Finally,thesamplewasaircooledandremoldedatroom
temperature.Thefinalsamplecontained30wt%ofcarbonfibers.
2.5. CharacterizationofPEIfilmsandcomposite 2.5.1. Scanningelectronmicroscopy(SEM)analysis
Thedifferentsampleswereexaminedusingascanningelectron
microscope(JEOLJSM6700F)withanacceleratingvoltageof5kV.
Thefilmsobtainedafternaturaldryingatroomtemperaturewere
mountedonaluminumstubsand sputtercoatedwithgold.The
compositeswerefreezefracturedinordertoobservetherupture
faces.
3. Resultsanddiscussion
3.1. Sizingagentformulation 3.1.1. Stabilitystudy
We selected PEIas thesizing agent becauseit is a
thermo-plastic polymer with high heat resistance [35], miscible with
polyetheretherketone(PEEK)[36],andsolubleinchlorinated
sol-ventslikechloroform.Severalfactorsinfluencethestabilityofthe
dispersion,suchasthenatureandtheamountofsurfactant.
Usu-ally,thechoiceofsurfactantdependsonthenatureoftheparticles
and,in particular,theirsurface charge.SincePEIhasnospecial
charge,thesurfactantcanbeanionicorcationic.Itisalsoimportant
Fig.4. Schemeofthesamplepreparation.
Fig.5.Influenceofthenatureandconcentrationofthesurfactantonthemean particlediameter([PEI]=0.5wt%).
todeterminetherightquantityofsurfactanttomaintainastable
dispersion.PEIconcentrationisalsoanimportantparameter.The
emulsion/evaporationmethodisnotsuitableforthepreparation
ofconcentrateddispersionsbut,inthecaseofsizing,thisisnota
limitationbecausetheconcentrationofpolymerdoesnotexceed
1wt%[37,38].
First,westudiedtheinfluenceofthenatureandthe
concentra-tionofthesurfactant,andalsotheinfluenceofthePEIconcentration
onthecharacteristicsofthesizingdispersions.
Thefirstparametertobeconsideredwastheparticlesizeas
itiswellknownthatthesmallertheparticlesare,themore
sta-blethedispersionwillbe.Dynamiclightscatteringmeasurements
(DLS)wereperformedonallthedispersions.Theinfluenceofthe
surfactantonthemeandiameterofparticlescanbeseeninFig.5.
Themeandiametersoftheparticleswerelessthan100nmand
favored stabledispersions.The natureofthesurfactantdidnot
haveasignificanteffectontheparticlesizeeventhoughthe
par-ticlesseemedsmallerwiththeBCsurfactant.Ontheotherhand,
thediametersvariednoticeablywiththesurfactantconcentration.
Thehighertheconcentrationwas,thesmallerweretheparticles.
Atlowconcentration,therewasnotenoughsurfactantto
main-tainsmalldropletsofchloroformandthisdeterminedthefinalsize
oftheparticles.Althoughthesmallestparticleswereobtainedfor
1wt%,theconcentrationof0.5%waspreferredinordertominimize
theamountofsurfactantinthefinalformulation.
Thesamestudywasperformedtoobservetheinfluenceofthe
PEIconcentration(Fig.6).Theparticlesizeincreasedquitelinearly
withtheconcentrationforbothsurfactantsbutthemean
diam-eterremainedunder 100nm.This resultwasrelated toseveral
factors.Thefirstwastheratiobetweentheconcentrationof
surfac-tantandtheamountofchloroformphasecontainingthedissolved
PEI[23].Thesecondwastheviscosityoftheorganicphase[23].
Fig.7.EffectofthePEIconcentrationontheclarificationkineticsfordispersionsat0.3%BC.
IncreasingthePEIconcentrationinchloroformincreasedthe
vis-cosityofthesolution.Giventhattheshearforceswerealwaysthe
same,whentheconcentrationofPEIwastoohigh,therewasnot
sufficientenoughavailabletocreatesmalldropletsofchloroform.
Theparticlesizeforthelowestconcentrationswasverysmall,
lead-ing,inprinciple, tothemoststable dispersions.However,for a
sizingformulation,thedispersionsmusthaveaminimumof0.5%
or1wt%ofPEI.
An interesting stability analysis consisted in determining a
destabilization velocity by accelerating the gravitation by
cen-trifugation. This kind of analysis could be performed withthe
“LUMiFuge”apparatus.Thistechniqueissuitabletooptimizevery
stabledispersions(stableformorethan6months).
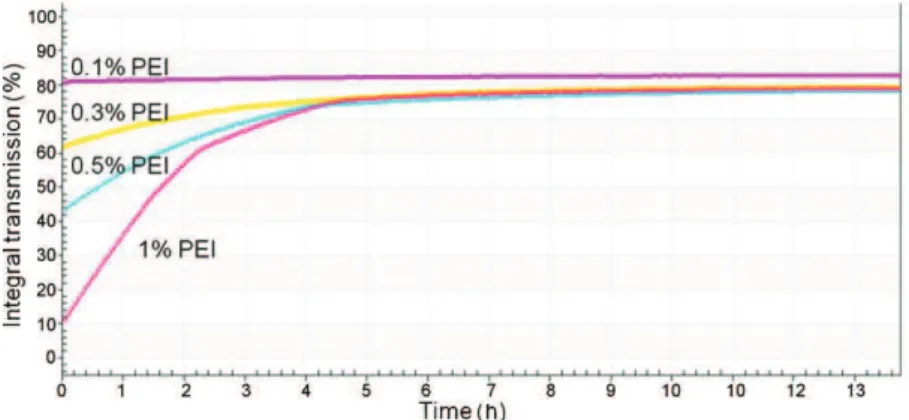
Fromtheprofiles,anintegraltransmissionwascalculatedasa
functionoftime.Forinstance,theinfluenceofthePEIconcentration
onthestabilitycanbehighlightedimmediately(Fig.7).
Fromthisgraph,aclarificationvelocity,correspondingtothe
slopeofthefirstlinearpartofthecurves,wascalculatedbythe
“SEPView”software.Thesteepertheslope,themoreunstablethe
dispersion.Theclarificationvelocitywascalculatedforthedifferent
dispersionsandcomparedsoastohighlighttheeffectofdifferent
parameters.
AsshowninFig.8,thenatureofthesurfactantdidnothavea
significantinfluenceonthevelocity,exceptfor0.1wt%PEIsolution,
whereBCwaslessefficient.Consideringthesurfactant
concentra-tion,itseemsthat,from0.5wt%,thestabilityreachesaplateau.This
resultindicatesthatitisnotnecessarytousemorethan0.5wt%
surfactantsolutionstoincreasethestability.
As expected, the PEI concentration had a major impact on
thedispersionstability (Fig.9).Theclarificationvelocitytripled
between0.5%and1wt%.Althoughtheshelflifecannotbe
deter-mineddirectly from theclarification velocity, the real stability
Fig.8. Influenceofthenatureandconcentrationofthesurfactantontheclarification velocity([PEI]=0.5wt%).
periodextrapolatedfromthedatawasestimatedtobearound6
monthsforthe1%PEIdispersion.
Consideringtheaboveresults,boththetestedsurfactantswere
usable.However,benzalkoniumchloridemightbemoreinteresting
becauseofitsantimicrobialandlowfoamingproperties.
Concern-ingthedifferentconcentrations,agoodcompromiseseemstobe
0.5wt%ofsurfactantand0.5wt%ofPEI.
3.1.2. Filmformation
Forsizing,thecoating,andconsequentlytheformationofafilm,
isaveryimportantproperty.Alltheaqueousdispersionsprepared
wereabletoformfilmsafterwaterevaporation.Toensurethe
qual-ityofthefilm,twochosendispersionswereobservedbySEM.One
wasmadewithSDSandtheotherwithBC,andbothcontained
0.5wt%ofsurfactantand0.5wt%ofPEI.
ThesurfaceaspectofthePEIfilmobtainedwithSDSwasvery
homogeneous(Fig.10).Thecrackswereduetouncontrolled
evapo-ration.Thisparameterwillneedtobetakenintoaccountforfurther
applications.Themagnificationofthisfilmshowspartiallyfused
PEIparticles(Fig.11).Thisobservationistypicaloflatexfilm
for-mation,andisidealforahomogeneouscoating.
ThePEIfilmformedbytheBCdispersionwasverydifferent.
Fig.12isanSEMobservationofthisfilmshowingaheterogeneous
surface.Themagnificationshowsthat,infact,theparticlesformed
agglomeratesbutdidnotfuse(Fig.13).Thedifferenceinfilm
forma-tioncouldbeexplainedbytheabilityofthesurfactanttobedrained
outoftheevaporatingfilm[39–41].Wehavetoconsiderthe
affin-ityofthesurfactantwiththesurfaceofthePEIparticlestoexplain
thisbehavior.Itseemsthat,comparedtoBC,SDShasalower
affin-itywiththesurfaceoftheparticlesandismainlydrainedoutofthe
film,leadingtothefusionoftheunprotectedparticlesandfinally
toahomogeneousfilm.
Fig.9. InfluenceofthePEIconcentrationontheclarificationvelocity ([surfac-tant]=0.5wt%).
Fig.10.SEMobservationofthefilmfromtheSDSdispersion.
Fig.11.MagnificationofFig.10.
Fig.12.SEMobservationofthefilmfromtheBCdispersion.
Fig.13.MagnificationofFig.12.
Consideringthesefilmformationresults,thebestdispersions
forasizingapplicationseemtobethoseobtainedwithSDSasthe
surfactant.Itisveryimportanttoobtainahomogeneouscoatingon
thecarbonfibers.
3.2. Sizingevaluation
Theaimofthisstudywastoelaborateastableaqueous
disper-sionusableasathermoplasticsizingformulationforcarbonfibers.
Thesizing hasvariousroles,suchasfacilitating thehandlingof
fibersandimprovingtheinteractionsbetweenthematrixandthe
fibers.
Toevaluatetheeffectofthisnewsizing,PEEK/carbonfiber
com-positesweremade;onewithunsizedcarbonfibersandanother
withPEIsizedcarbonfibers.Thechosensizingwastheaqueous
dispersionwith0.5wt%ofPEIand0.5wt%ofSDS.Thebestwayto
highlighttheinfluenceofthesizingwastoobservethefiber/matrix
interface.Forthatpurpose,thecompositeswerefreezefractured
transversallyandobservedbyscanningelectronmicroscopy.
Fig.14correspondstoanunsizedcarbonfibercompositeand,as
wecansee,therearevoidsandnointeractionsbetweenthePEEK
matrixandthecarbonfibers.Incontrast,theinterfacebetweenthe
Fig.15. PEIsizedcarbonfibercomposite.
compositeandthePEIsizedcarbonfibersiscontinuous(Fig.15).
Inthiscase,thereisarealbondbetweenthePEEKandthecarbon
fibers.Theseobservationsconfirmnotonlythatthesizingremains
duringthecompositeprocessingbutalsothatthematrixandthe
carbonfibersareconnectedbythesizingagent.
4. Conclusions
Theanalysesperformedonthedifferentaqueousdispersions
revealedthatthequantityofparticleswasasignificantfactorfor
stability.TheparticlesizeincreasedgreatlywiththePEI
concentra-tion,whichtendedtodecreasethestabilityofthedispersion.The
LUMiFugestudyconfirmedthisresult.ThebestPEIconcentration
obtainable bytheemulsion/evaporation technique was0.5wt%.
Thedispersions werestable1 yearat 0.5wt%,nevertheless the
dispersionat1wt%remainedstablefor3months.Concerningthe
natureofthesurfactant,benzalkoniumandSDSallowedstable
dis-persionstobeobtained.Nevertheless,thebenzalkoniumdispersion
didnotformahomogeneousfilmandsowasnotsuitablefor a
sizingapplication.Incontrast,withSDSdispersion,thefilmwas
reallyuniformandweobservedacoalescencephenomenon
typi-caloflatexfilmformation.Theconcentrationofthesurfactantalso
hadaninfluenceontheparticlesizeandstability,and0.3%wasnot
enoughtoobtainagooddispersion.Thegaininstabilityobtained
at1%didnotjustifytheuseofsuchaconcentrationconsidering
thattherewasonly0.5wt%ofPEI.Sothebestconcentrationof
surfactantwas0.5wt%.
Consideringalltheresults,thechosendispersionforsizingwas
0.5%PEIand0.5%SDS.Theefficiencyofthisnewsizingagent
aque-ousdispersionwasappreciatedthroughSEMobservations,which
showedacontinuousinterfacebetweenthecarbonfibersandthe
PEEKmatrix.
Acknowledgements
WethankMrJ.M.Bergerat,AIRBUSIndustry,ToulouseFrance,
for useful discussions and advice. The financial support of FUI
INMAT2andAIRBUSisgratefullyacknowledged.WealsothankMr
D.KemmishandMrA.WoodfromVictrexInc.,fortheirinsightful
commentsandfruitfuldiscussions.
References
[1] P.Morgan,CarbonFibersandTheirComposites,CRCPressTaylor&Francis Group,NewYork,2005.
[2] D.D.L.Chung,CarbonFiberComposites,Butterworth-Heinemann,Newton, 1994.
[3] B.Fernandez,A.Arbelaiz,A.Valea,F.Mujika,I.Mondragon,PolymerComposites 25(2004)319–330.
[4]W.Chen,Y.Yu,P.Li,C.Wang,T.Zhou,X.Yang,CompositesScienceand Tech-nology67(2007)2261–2270.
[5] A.Paipetis,C.Galiotis,CompositesPartA:AppliedScienceandManufacturing 27(1996)755–767.
[6] N.Dilsiz,J.P.Wightman,Carbon37(1999)1105–1114. [7] R.B.Guan,Y.G.Yang,J.T.Zheng,Fibercomposites1(2002)23. [8] T.Q.Li,M.Q.Zhang,H.M.Zeng,Polymer40(1999)4307–4313.
[9] S.-L.Gao,J.-K.Kim,CompositesPartA:AppliedScienceandManufacturing32 (2001)775–785.
[10]L.T.Drzal,M.J.Rich,M.F.Koenig,P.F.Lloyd,TheJournalofAdhesion16(1983) 133–152.
[11]Z.Dai,F.Shi,B.Zhang,M.Li,Z.Zhang,AppliedSurfaceScience257(2011) 6980–6985.
[12] Z.Dai,B.Zhang,F.Shi,M.Li,Z.Zhang,Y.GU,AppliedSurfaceScience257(2011) 8457–8461.
[13]P.Ren,G.Liang,Z.Zhang,PolymerComposites27(2006)591–598.
[14]V.S.Mironov,M.Park,J.Kim,S.H.Lim,C.R.Choe,JournalofMaterialsScience Letters20(2001),1211–1211.
[15]A.Bledzki,E.Fabrycy,A.Kwasek,JournalofThermalAnalysisandCalorimetry 29(1984)989–994.
[16]D.M.Blackketter,D.Upadhyaya,T.R.King,J.A.King,PolymerComposites14 (1993)430–436.
[17]T.K. O’Brien, Journal of Reinforced Plastics and Composites 7 (1988) 341–359.
[18]P.Davies,W.J.Cantwell,H.H.Kausch,JournalofMaterialsScienceLetters9 (1990)1349–1350.
[19]A.Todoroki,H.Kobayashi,CompositesScience andTechnology52(1994) 551–559.
[20]S.Freitas,H.P.Merkle,B.Gander,JournalofControlledRelease102(2005) 313–332.
[21]C.Zhang,G.Zhang,V.Ji,H.Liao,S.Costil,C.Coddet,ProgressinOrganicCoatings 66(2009)248–253.
[22]P.R.Nepal,M.-K.Chun,H.-K.Choi,InternationalJournalofPharmaceutics341 (2007)85–90.
[23] M.Li,O.Rouaud,D.Poncelet,InternationalJournalofPharmaceutics363(2008) 26–39.
[24]T.R.Tice,R.M.Gilley,JournalofControlledRelease2(1985)343–352. [25] K.S.Soppimath,T.M.Aminabhavi,A.R.Kulkarni,W.E.Rudzinski,Journalof
Con-trolledRelease70(2001)1–20.
[26]S. Freiberg, X.X. Zhu,International Journal of Pharmaceutics 282 (2004) 1–18.
[27] Z.ElBahri,J.-L.Taverdet,JournalofAppliedPolymerScience103 (2007) 2742–2751.
[28]M.Kuentz,D.Röthlisberger,EuropeanJournalofPharmaceuticsand Biophar-maceutics56(2003)355–361.
[29] T.Sobische,H.Heb,H.Niebelschütz,U.Schmidt,ColloidsandSurfacesA: PhysicochemicalandEngineeringAspects162(2000)1–14.
[30]T.Sobische,D.Lerche,ColloidsandSurfacesA:Physicochemicaland Engineer-ingAspects331(2008).
[31]A.Bismarck,A.F.Lee,A.S.Sarac,E.Schulz,K.Wilson,CompositesScienceand Technology65(2005)1564–1573.
[32]A.S.Sarac,E.A.Parlak,E.Sernath,T.Cakir,JournalofAppliedPolymerScience 104(2007)238–246.
[33] B.Lin,R.Sureshkumar,J.L.Kardos,ChemicalEngineeringScience56(2001) 6563–6575.
[34]C.Dalmolin,S.C.Canobre,S.R.Biaggio,R.C.Rocha-Filho,N.Bocchi,Journalof ElectroanalyticalChemistry578(2005)9–15.
[35]R.Torrecillas,A.Baudry,J.Dufray,B.Mortaigne,PolymerDegradationand Sta-bility54(1996)267–274.
[36]G.Crevecoeur,G.Groeninckx,Macromolecules24(1991)1190–1195. [37]R.L.Zhang,Y.D.Huang,L.Liu,Y.R.Tang,D.Su,L.W.Xu,AppliedSurfaceScience
257(2011)3519–3523.
[38]D.D.L. Chung,CarbonFiberComposites,Butterworth-Heinemann,Newton, 1994,pp.91–93.
[39]E.Kientz,Y.Holl,ColloidsandSurfacesA:PhysicochemicalandEngineering Aspects78(1993)255–270.
[40] J.Mallégol,J.-P.Gorce,O.Dupont,C.Jeynes,P.J.McDonald,J.L.Keddie,Langmuir 18(2002)4478–4487.
[41]A.Tzitzinou,P.M.Jenneson,A.S.Clough,J.L.Keddie,J.R.Lu,P.Zhdan,K.E. Treacher,R.Satguru,ProgressinOrganicCoatings35(1999)89–99.
![Fig. 5. Influence of the nature and concentration of the surfactant on the mean particle diameter ([PEI] = 0.5 wt%).](https://thumb-eu.123doks.com/thumbv2/123doknet/3579942.104967/4.892.456.823.920.1125/fig-influence-nature-concentration-surfactant-mean-particle-diameter.webp)