The role of lateral septum and anterior hypothalamic area in
mediating the interactive effects of stress and palatable food
Thèse
Arojit Mitra
Doctorat en neurobiologie
Philosophiae doctor (Ph.D.)
Québec, Canada
© Arojit Mitra, 2017Résumé
Le mode de vie stressant et sédentaire associé à un environnement moderne obésogène sont les principales causes des troubles alimentaires et de l’obésité induite par le régime alimentaire. La prise de nourriture hautement savoureuse, ayant une teneur élevée en sucre et en gras, active le circuit de la récompense dans le cerveau, induisant du plaisir et des émotions positives. A l’inverse, un stress aigu évoque principalement l’inconfort ou des émotions négatives, contrecarrant le comportement de recherche de plaisir. En effet, une exposition à un stress aigu entraine une réponse alimentaire anhédonique et anorexique. En revanche, le stress chronique peut induire un comportement de surconsommation, ce qui représente un processus neuroadaptatif. Plusieurs régions cérébrales sont impliquées de façon indépendante dans le traitement du stress, de la prise alimentaire ou de la récompense. Cependant, les régions qui répondent à la fois à un stress et à la récompense alimentaire sont des candidates potentielles dans la coordination de ces réponses comportementales, avec des valeurs translationnelles se rattachant aux anomalies alimentaires induites par le stress. Des études suggèrent que le septum latéral (LS) est sensible au stress et est un centre de régulation de la prise alimentaire dans le cerveau. Cependant, le rôle du LS dans la régulation de la prise alimentaire dans des conditions normales et stressantes n’est pas clair. Dans un premier temps, nous avons examiné le rôle du LS dans la consommation de sucrose chez des rats non stressés. Nous avons démontré que l’inhibition du LS par des agonistes sélectifs des récepteurs GABAA et GABAB
potentialise la prise de sucrose et les paramètres de microstructure des lapements, induisant une motivation amplifiée envers la solution de sucrose chez les rats rassasiés. Ensuite, nous avons développé un modèle de surconsommation de sucrose chez le rat, et nous avons enregistré les changements dans l’activité neuronale du LS lors du passage d’une réponse anorectique induite par un stress aigu à un phénotype de surconsommation du sucrose chez des rats stressés de manière chronique. La diminution de l’expression de l’ARNm c-fos et l’augmentation de la synthèse de GABA dans le LS ont coïncidé avec l’augmentation de consommation de sucrose observée dans ce modèle. Le stress répété a augmenté la proportion de neurones inhibés par le sucrose dans le LS. De plus, l’administration de l’agoniste du récepteur GABAB restaure la diminution de consommation de sucrose induite
l’aire hypothalamique antérieure (AHA) qui a des connexions robustes et réciproques avec le LS et des aires hypothalamiques impliquées dans la régulation de la prise alimentaire et du stress. Dans des conditions non stressantes, la prise de sucrose a suscité des réponses inhibitrices prédominantes dans les neurones de l’AHA. Un stress aigu a augmenté le taux de décharge de ces neurones inhibés par le sucrose, amenant à une réponse anorectique envers le sucrose. Sur la base de ces résultats, nous avons conclu que le stress active, alors que la prise de sucrose inhibe, les neurones de l’AHA et du LS. Par conséquent, le LS et l’AHA sont des régions importantes impliquées dans la régulation du stress et du comportement alimentaire. Une activité neuronale aberrante dans le LS et l’AHA peut être impliquée dans les troubles alimentaires et métaboliques.
Abstract
Stressful and sedentary lifestyle accompanied by modern obesogenic environment, are the leading causes of diet-induced obesity and eating disorders. Intake of highly palatable food, with its high fat and sugar content, activates the reward pathways in the brain inducing pleasure and positive emotion. Conversely, acute stress primarily evokes discomfort or negative emotions counteracting with pleasure-seeking behaviour. In fact, acute stress exposure results in anorexic and anhedonic feeding response. In contrast, chronic stress may induce over-eating behaviour that represents a neuroadaptive process. Multiple brain regions are independently implicated in stress, feeding and reward processing. However, regions co-responsive to stress and food rewards are the candidates for coordinating behavioural responses with translational values pertaining to stress-induced feeding abnormalities. Studies suggest that the lateral septum (LS) is a stress-responsive and feeding regulating center of the brain. However, the role of LS in food intake regulation in normal and stressful conditions is not clear. First, we have investigated the role of LS in sucrose intake in non-stressed rats. We have demonstrated that LS inhibition by selective GABAA and GABAB receptor agonists potentiates sucrose intake and licking
microstructure parameters, resulting in amplified motivation towards sucrose solution in satiated rats. Further, we have developed a sucrose bingeing model in rats, and monitored the changes in LS neural activity during transformation from acute stress-induced anorectic response to sucrose-bingeing phenotype in chronically stressed rats. Decreased c-fos mRNA and increased GABA synthesis in LS coincided with the increased sucrose intake in this model. Repeated stress increased the proportion of sucrose-inhibited neurons in the LS. Supportively, administration of a GABAB receptoragonist rescued an acute stress-induced
decrease in sucrose intake. We also investigated the real-time neuronal responses of the anterior hypothalamic area (AHA) which has robust reciprocal connections with LS and the hypothalamic stress- and food intake-regulating areas. During non-stressful condition, sucrose lick clusters evoked predominant inhibitory responses in AHA neurons. Acute stress increased the firing rate of these sucrose-inhibited neurons, leading to anorectic response towards sucrose. Based on these evidences, we conclude that stress activates, whereas sucrose intake inhibits LS and AHA neurons. Therefore, the LS and AHA are
important brain regions involved in regulation of stress and feeding behaviour. The aberrant neuronal activity in the LS and AHA may be involved in metabolic and eating disorders.
Table of Contents
Resume………..III Abstract……….VI Table of Contents……….VIII List of Figures……….XI List of Tables………XIII List of Abbreviations………..XIV Acknowledgement………..XVI Foreword……….XVII Chapter 1 : General introduction ... 1 1.1 Food intake regulation ... 4 1.1.1 Hypothalamic pathways regulating food intake ... 5 1.1.2 Extra-hypothalamic pathways regulating food intake ... 8 1.2 Sugar: an important ingredient in comfort food ... 9 1.2.1 Central pathway that responds to sucrose ... 13 1.2.2 Why sucrose is rewarding? ... 16 1.2.3 Microstructure of sucrose licking in rats ... 20 1.3 Stress ... 23 1.3.1 Central pathway of stress ... 24 1.3.2 Effect of CRH, ACTH and CORT on feeding behaviour ... 26 1.3.3 Stress responsive extra-hypothalamic brain regions ... 28 1.4 Lateral septum (LS): Role in food intake and stress response regulation ... 29 1.4.1 Morphology of LS neurons ... 29 1.4.2 Connections of the LS ... 30 1.4.3 Role of LS in reward and food intake regulation ... 31 1.4.4 Role of LS in stress response ... 33 1.5 Anterior hypothalamic area (AHA): Role in food intake and stress response regulation ... 35 1.5.1 Morphology of AHA neurons ... 35 1.5.2 Connections of AHA ... 36 1.5.3 Role of AHA in food intake regulation ... 37 1.5.4 Role of AHA in stress response ... 38 1.6 Link between stress and palatable food intake ... 39 1.7 Methodological consideration ... 45 1.7.1 Ten percent sucrose solution as a hedonic high palatable food (HPF) source ... 45 1.7.2 Food deprivation ... 46 1.7.3 Recording licks for qualitative assessment of rodent’s ingestive behaviour ... 47 1.7.4 Foot-shock stress ... 47 1.7.5 Detection of c-fos mRNA expression using in situ hybridization ... 48 1.7.6 Extracellular electrophysiology ... 49 1.8 Objectives ... 52 1.8.1 General objectives ... 52 1.8.2 Objectives and hypothesis of study 1: Activation of GABA-A and GABA-B receptors in the lateral septum increases sucrose intake by differential stimulation of sucrose licking activity. ... 521.8.3 Objectives of study 2: Inhibition in the lateral septum increases sucrose intake and
decreases anorectic effects of stress. ... 53
1.8.4 Objectives of study 3: Stress and sucrose intake modulate neuronal activity in the anterior hypothalamic area in rats. ... 55
Chapter 2 Activation of GABAA and GABAB receptors in the lateral septum increases sucrose intake by differential stimulation of sucrose licking activity ... 57 2.1 Résumé ... 58 2.2 Abstract ... 59 2.3 Introduction ... 60 2.4 Materials and methods ... 62 2.4.1 Animals ... 62 2.4.2 Surgery and acclimatization ... 62 2.4.3 Drugs and intra-LS microinjections ... 62 2.4.4 Licking microstructure ... 63 2.4.5 Histology ... 64 2.4.6 Statistical analyses ... 64 2.5 Results ... 65 2.5.1 Sucrose and water intake ... 65 2.5.2 Licking microstructure ... 68 2.5.3 Total licking time and latency to initiate sucrose licking ... 72 2.6 Discussion ... 75 2.7 Conclusions ... 79 2.8 Acknowledgements ... 80 2.9 References ... 81 CHAPTER 3 Inhibition in the lateral septum increases sucrose intake and decreases anorectic effects of stress ... 87 3.1 Résumé ... 88 3.2 Abstract ... 89 3.3 Introduction ... 90 3.4 Materials and Methods ... 92 3.4.1 Animals ... 92 3.4.2 Weekly sucrose regimens and mild foot shock stress sessions ... 92 3.4.3 Brain preparation and in situ hybridisation. ... 92 3.4.4 Direct recording of LSmv neuronal firing ... 94 3.4.5 Intra-LS injections of baclofen ... 98 3.4.6 Statistical Analysis ... 99 3.5 Results ... 100 3.5.1 Body weight and sucrose intake in the first rat cohort ... 100 3.5.2 c-fos, GAD65, and GAD67 mRNAs expression in the LSmv ... 105 3.5.3 Sucrose intake by the second rat cohort ... 110 3.5.4 Neuronal activity in the LSmv ... 114 3.5.5 Administration of baclofen in the medial LS ... 125 3.6 Discussion ... 127 3.7 Acknowledgements ... 133 3.8 References ... 134
CHAPTER 4 Stress and sucrose intake modulate neuronal activity in the anterior hypothalamic area in rats. ... 149 4.1 Résumé ... 150 4.2 Abstract ... 151 4.3 Introduction ... 152 4.4 Materials and Methods ... 154 4.4.1 Animals ... 154 4.4.2 Surgery ... 154 4.4.3 Recording of sucrose-licking events and neuronal activity in the cAHA ... 156 4.4.4 Statistical analysis ... 160 4.5 Results ... 161 4.5.1 Sucrose intake and lick microstructure ... 161 4.5.2 Neuronal firing activity in relation to sucrose licking clusters ... 163 4.5.3 Effects of stress on the firing rate of CS-classified neurons ... 169 4.5.4 Burst firing of cAHA neurons ... 174 4.6 Discussion ... 178 4.7 Acknowledgements ... 185 4.8 References ... 186 Chapter 5 General Discussion and Conclusion ... 192 5.1 LS inhibition facilitates sucrose intake in non-stressful conditions ... 193 5.2 Increased lateral inhibition of LS neurons in sucrose bingeing rats ... 195 5.3 Sucrose intake induces inhibition of LS neurons ... 197 5.4 Acute stress activates sucrose-inhibited neurons in the anterior hypothalamic area ... 199 5.5 Model based on LS, AHA and PVN interconnectivity ... 202 5.6 General conclusions ... 205 5.7 References ... 206 Annex 1: Web links of published papers……….237
List of Figures
Figure 1.1 Homeostatic feeding regulation by AgRP and POMC neurons of the
hypothalamic arcuate nucleus. ... 7
Figure 1.2 Chemical structure of sucrose molecule. ... 10
Figure 1.3 Yearly production and consumption of sucrose from 2009 to 2016. ... 11
Figure 1.4 Sugar consumption and obesity prevalence. ... 11
Figure 1.5 Molecular cascade within a taste receptor cell. ... 14
Figure 1.6 Peripheral and central sucrose sensing and responding pathways. ... 16
Figure 1.7 Schematic showing the differential c-fos mRNA expression in food-anticipating rats. ... 18
Figure 1.8 Dopaminergic reward pathway. ... 19
Figure 1.9 Licking microstructure by a rat during sugar solution consumption. ... 21
Figure 1.10 Internal and external stressors ... 23
Figure 1.11 Hypothalamus-pituitary-adrenal (HPA) axis ... 25
Figure 1.12 Stress-induced reduction in feeding ... 27
Figure 1.13 Anatomical position of lateral septum. ... 29
Figure 1.14 Afferent and efferent connections of the lateral septum ... 30
Figure 1.15 Self-inhibition of lateral septum neurons. ... 31
Figure 1.16 Fear modulation by lateral septum ... 34
Figure 1.17 Anatomical position of anterior hypothalamic area. ... 35
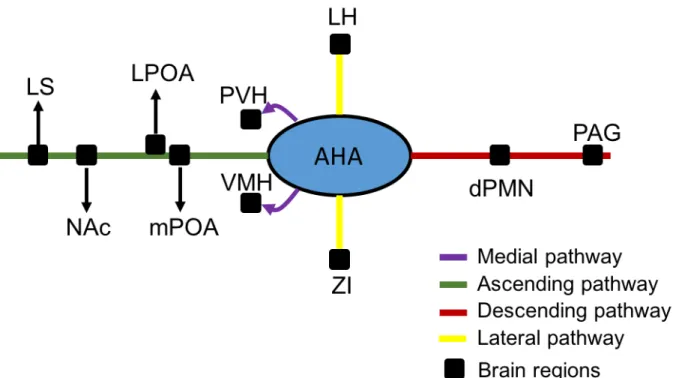
Figure 1.18 Projections from anterior hypothalamic area. ... 36
Figure 1.19 Lateral septum modulation of HPA axis. ... 39
Figure 1.20 Interaction between stress and palatable food intake. ... 41
Figure 1.21 Interaction of stress and feeding circuits in the brain. ... 44
Figure 1.22 Cyclic nature of dieting and palatable food bingeing. ... 46
Figure 1.23 Comparison between few neurophysiological techniques and their spatial-temporal resolution. ... 50
Figure 1.24 Few examples of extracellular recordings and sorted units recorded from awake moving rat. ... 51
Figure 2.1 Histological confirmation of GABA agonist injected site. ... 66
Figure 2.2 The effects of intra-LS injection of muscimol and baclofen on 1-h sucrose intake. ... 67
Figure 2.3 Effect of intra-LS microinjection of muscimol and baclofen on sucrose-lick numbers. ... 71
Figure 2.4 Total licking time assessed by adding the durations of all sucrose licking clusters. ... 73
Figure 2.5 Latency to initiate sucrose licking activity. ... 74
Figure 3.1 The experimental conditions and body weight of the rats subjected to nine weekly cycles of food restriction and foot shock stress. ... 101
Figure 3.2 Nine week dynamics of sucrose intake in 1st cohort rats. ... 103
Figure 3.3 Stress-induced increase in c-fos mRNA expression in the ad libitum-fed rats but not in the food-restricted rats. ... 106
Figure 3.4 Stress-induced decrease in glutamic acid decarboxylase (GAD) 67 mRNA expression in the ad libitum-fed rats but not in the food-restricted rats. ... 108
Figure 3.5 Body weight and histological confirmation of electrodes placement in lateral septum. ... 112 Figure 3.6 Sucrose intake and lick microstructure analysis in 2nd cohort of rats. .... 113 Figure 3.7 Raster of sucrose licking and representative example of perievent histogram of lick-related and –unrelated neuron. ... 117 Figure 3.8 Perievent histograms (PEHs) of CS-excited and CS-inhibted neurons. ... 119 Figure 3.9 Percentage of anticipatory and CS-responsive neurons of lateral septum during control, food restriction and stress conditions. ... 121 Figure 3.10 Responsiveness of CS-excited and CS-inhibited neurons. ... 123 Figure 3.11 Effect of bilateral injection of baclofen in lateral septum (LS) on sucrose intake. ... 126 Figure 3.12 Behavioural effects of a long-term increase in inhibition in the lateral septum. ... 132 Figure 4.1 Histological confirmation of electrodes implantation in central nucleus of anterior hypothalamic area. ... 155 Figure 4.2 A representative example of cluster-start excited neuron of anterior hypothalamic area. ... 164 Figure 4.3 A representative example of cluster-start inhibited neuron of anterior hypothalamic area ... 166 Figure 4.4 Firing rate of cluster-start responsive neurons of anterior hypothalamic area, with or without foot shock stress ... 171 Figure 4.5 Population dynamics of CS-responsive neurons during 1 hour sucrose intake during no-stress and stressed conditions. ... 172 Figure 4.6 Examples of burst firing in anterior hypothalamic area neurons. ... 176 Figure 5.1 LS inhibition by GABA receptor activation increases sucrose intake ... 195 Figure 5.2 Proposed interconnectivity between responsive neurons of LS with CS-responsive neurons of AHA. ... 201 Figure 5.3 Proposed model on LS and AHA dependent sucrose intake in non-stressful and stressful conditions. ... 204
List of Tables
Table 1.1 Stress statistics on U.S. population ... 4 Table 1.2 Sucrose concentration (in grams) of some of the processed food that are
commonly consumed worldwide. Source USDA nutrient list.3 ... 13 Table 1.3 Lick parameters and its physiological meaning. ... 21 Table 1.4 Sucrose concentration-dependent changes in lick microstructure in rats. .. 22 Table 2.1 The effects of muscimol and baclofen on the sucrose licking microstructure
during 1 h following intra-LS injections. ... 70 Table 3.1 Baseline firing rate and percentage of lick-related and lick-unrelated
neurons in the medioventral part of the lateral septum (LSmv) recorded during 1-h access to sucrose. ... 118 Table 4.1 Sucrose intake and licking microstructure during the 1-h access in rats in
non-stressful conditions and after acute foot shock stress. ... 162 Table 4.2 Number (n) and percentage (%) of cAHA neurons classified according to
the response to the sucrose licking cluster start (CS). ... 168 Table 4.3 Burst parameters of the cAHA neurons classified according to the responses
to cluster start (CS) of sucrose licking during 1-h access to sucrose in
List of Abbreviations Acb: nucleus accumbens
AcbSh: nucleus accumbens shell ACTH: Adrenocorticotrophic hormone ADX: Adrenalectomy
AgRP: Agouti related peptide AHA: anterior hypothalamic area ALC: ad libitum chow-fed rats ALS-NS: non-stressed ALS rats ALS-SS: stressed ALS rats
ALS: ad libitum chow- and sucrose-fed rats AMY: Amygdala
ARC: Arcuate nucleus
ANOVA: analysis of variance AP: area postrema
ATP: Adenosine triphosphate AVP: Arginine-vasopressin BE: burst end
BNST: bed nucleus of the stria terminalis BS: burst start
CA: control access to sucrose
cAHA: central nucleus of the anterior hypothalamic area cc: corpus callosum
CE: licking cluster end CORT: Corticosterone
CRH: Corticotropin-releasing hormone
CRH-R1: Corticotropin-releasing hormone receptor type 1 CRH-R2: Corticitropin-releasing hormone receptor type 2 CS: licking cluster start
CSF: cerebrospinal fluid
DMHc: compact part of the dorsomedial hypothalamic nucleus DMHv: ventral part of the dorsomedial hypothalamic nucleus FAA: food-anticipatory activity
FDA: U.S. Food and Drug Administration FEO: food-entrainable oscillator
FRS: food-restricted rats FRS-NS: non-stressed FRS rats FRS-SS: stressed FRS rats
GABA: gamma-aminobutyric acid
GABAA: type A receptor of gamma-aminobutyric acid
GABAB: type B receptor of gamma-aminobutyric acid
GAD65: glutamic acid decarboxylase 65 GAD67: glutamic acid decarboxylase 67 Glu: Glutamate
HPF: Highly palatable food ICI: inter-cluster intervals IL: infralimbic cortex ILI: inter-lick interval ISI: inter-spike interval
LEO: light-entrainable oscillator LH: lateral hypothalamus
LHa: Anterior lateral hypothalamus LHA: lateral hypothalamic area LiCl: lithium chloride
LS: lateral septum
LSmv: medioventral part of the lateral septum LSv: ventral part of the lateral septum
MEG: Magnetoencephalography MR: Mineralocorticoid receptor MS: medial septum
NAc: Nucleus accumbens NPY: Neuropeptide Y
NTS: nucleus of the solitary tract OD: optic density
OFC: orbitofrontal cortex oz: Ounce
PAG: Periaqueductal grey PBN: Parabrachial nucleus PEH: perievent histogram
PET: Positron emission tomography PFC: Prefrontal cortex
POMC: Proopiomelanocortin
PVN: Paraventricular hypothalamic nucleus SCN: suprachiasmatic nucleus
SHi: septohippocampal nucleus SON: supraoptic nucleus T1R: Type 1 taste receptor
T1R2: Type 1 taste receptor subunit 2 T1R3: Type 1 taste receptor subunit 3 TCA: Tricarboxylic acid
TRPM5: Transient receptor potential M5 taste channel VMH: ventromedial hypothalamic area
VPM: ventero-postero-medial thalamic nucleus VTA: ventral tegmental area
ZT: Zeitgeber time CS: Cluster-start CE: Cluster-end
Acknowledgement
This thesis is a collective sum of lab work and stimulating interactions with various mentors, friends and lab colleagues. Here I would like to write a few lines to thank those who have directly and/or indirectly helped and supported me in my doctoral studies. Firstly, I would like to express my deepest gratitude to my mentor Prof. Elena Timofeeva for giving me a golden opportunity to work in her lab, and trusting me with enormous resources and time. Her endless patience, professional and personal availability have deeply helped me to achieve my doctoral goals. I am indebted by her efforts to integrate me in Quebec, and helping me out in various academic and non-academic situations. I could not have wished for a better supervisor.
I am very thankful to Genevieve Guèvremont, the research professional of our lab who patiently taught me various lab techniques, including animal surgeries. I admire her ethical and professional integrity, along with a contagious compassion towards animals. She, on various occasions, has helped me with fine tuning of my projects and troubleshooting various logistical issues. Thank you Genevieve for being there for me.
I am thankful to my doctoral advisory committee members Dr. Denis Richard, Dr. Didier Mouginot and Dr. Guy Drolet for their time and constructive comments on the projects. I also owe a warm thanks to Dr. Yves Deshaies for helping me with recommendations and support for various applications. Conversations with you have always been stimulating and uplifting.
I am also very thankful to Prof. Igor Timofeev, who was generous enough to provide me with his valuable time and lab resources, as and when needed. Special thanks to Josee, Sylvain and Sara for helping me out on numerous occasions, and for their valuable suggestions. Thank you Josee and Igor, for teaching me in-vitro slice electrophysiology. I want to express my deep appreciation to Mr. Sergiu Fitomov, for helping me with logistics and making several modifications to behavioural setup. Thank you for your electrical, electronic and hardware skills in the design of several useful gadgets that helped me to realize the full potential of the projects that I was entrusted with.
I am fortunate to have worked and interacted with a very understanding and compatible group of people of our lab and animal facility: Jessica, Dr. Christophe Lenglos, Dr. Juliane Calvez, Dr. Olga Bukhtiyarova, Yavar, Dr. Sandrine Chomettone, Camila, Richard, Zhifei, Louis-Olivier, Justin, Sebastian, Nicolas, Audrey, Erika. Special thanks to Sandrine for her time and efforts for French translation of chapter’s abstracts. Thanks for all the scholarly suggestions and extending your valuable help on a daily basis. It was an honor to have worked by your side. You have elevated the threshold of compatibility, assistance and companionship for me to adhere to.
I would like to share the credit of my work with Dr. Christophe Lenglos, a friend and colleague, who wrote useful computer codes for time-series analysis leading to accelerated analysis of data. His interest and skill in biostatistics helped me immensely to understand and interpret our data. Thanks Chris!
I would like to thank the funding agencies- CIHR, NSERC, FRSQ, and CFI, for supporting our lab and for providing me generous opportunity to attend local, national and
I would like to acknowledge the guidance and efforts of my previous mentors- Prof. Birendra Nath Mallick (who taught me extracellular electrophysiology), Late Vijay Kumar Sharma (interpretation of behaviour), Prof. Ishan Kumar Patro (fundamentals of neuroscience), Dr. Nisha Patro (fundamentals of neuroscience), Prof. Atanu Kumar Pati (introduced me to the circadian rhythm and scientific research) and Prof. Arti Parganiha (actigraphy and statistical interpretation of time series data), for developing and nurturing my interest in behavioural neuroscience. Without your passion for science, my passion and interest for science would have been non-existent.
I am indebted by the everlasting support of my wife, Tulika. Thank you for your understanding and support through numerous weekends and late-evening experiments. It is in your trust and love that I found solutions to most complex things in life. Thank you for adding colors to the posters, presentations, and in my life.
Words would never be enough to thank my spiritual parents Mr. Timir Baran Mitra and Mrs. Kakoli Mitra who showed me the light and path when I was completely lost. Their everlasting support and trust provided me with an academic direction and hope in life. Special thanks to Kantadi and Mitco, who made things simple just by their presence. Thanks for lending your ears, time, culinary skills and efforts to help me in your own unique way. Thanks for taking us out, on several occasions, to appreciate the scenic beauty of Quebec’s countryside, national parks and cities. Thanks for all the fun, memories and foremost, for providing and introducing us to wonderful YOU. You will be deeply missed and will be forever admired.
Thanks to my badminton friends in Quebec for giving me their valuable time to vent out the sporadic frustration of non-yielding lab hours. Thank you Elaine, Ranjan, Prakash, Jayesh, Khai, Pallavi, Priyanka, Kallolda, Chris, Nikunj, Needhesh, Abhishek, Senthil, David, Christian, Juliane, Aurelien and Gowshika. Sincere thanks to friends and lab colleagues working in nearby labs at IUCPQ and IUSMQ– Mihaela, Yves, Dana, Sophie, Stephanie, Catherine, Wei, Boris, Alex, Damian, Joulie, Anastasia, Marie-Claude, Piere, Damien, Renato, Fernando, Blandine, Sandrine, Patrick and Jean. Thanks to Bubai, Ratul, Budu, Kshitij, Sunny, Arpan, Tony, MAK, and Harshit for their everlasting support and friendship.
Thank you Mamuni (mother), Bapin (Father), Amma (grandmother), Mashi (aunt), Dada (elder brother) and Pixie (sister-in-law), for trusting me with my academic interest and helping me to make decisions that, at times, were too difficult for me to make. I could only hope that I have not let you down by my conscious choices and deeds. An honorable mention to my nephew ‘Tupai’ for his adorable smile and ipad ‘high fives’, that at times, helped me with the ‘writer’s block’ while writing this thesis.
Thanks to entire team of IUCPQ, INAF, IUSMQ, residence Alphonse-Marie-Parent and University Laval for your hospitality, generosity and providing me with a harmonious and safe environment to work.
Lastly, thanks to LIFE itself for letting me experience the wonderful people, things and places around.
Foreword
Chapter 2, 3 and 4 are published manuscripts. Chapter 2:
Arojit Mitra, Christophe Lenglos, Elena Timofeeva. Activation of GABAA and GABAB
receptors in the lateral septum increases sucrose intake by differential stimulation of sucrose licking activity. Behavioural Brain Research (2014) 273:82-88.
In this paper, AM and ET were responsible for generation of all the data to figures 2.1-2.5. CL wrote MATLAB programming to analyse lick-related data presented in figure 2.4. AM and ET wrote the paper.
Chapter 3:
Arojit Mitra, Christophe Lenglos, Elena Timofeeva. Inhibition in the lateral septum increases sucrose intake and decreases anorectic effects of stress. European Journal of Neuroscience (2015) Feb; 41(4): 420-433.
In this paper, AM and ET were responsible for generation of all the data to figures 3.1-3.12. CL wrote MATLAB programming to analyse lick-related data presented in figure 3.6 using which we were able to perform analysis of data presented in figures 3.8-3.10. AM, CL and ET wrote the paper.
Chapter 4:
Arojit Mitra, Genevieve Guèvremont and Elena Timofeeva. Stress and sucrose intake modulate neuronal activity in the anterior hypothalamic area in rats. PLOS ONE (2016) May; 11(5): e0156563.
In this paper, AM and ET were responsible for generation of all the data to figures 4.1-4.6 and tables 4.1-4.3. GG performed animal surgeries and conducted post-operative care. AM and ET wrote the paper.
Every living system is adapted to extract energy from its surroundings in order to sustain vital cellular processes. In mammals, energy derivation from surroundings is through eating (ingestion of solid food by chewing and swallowing, or swallowing without chewing) and/or drinking (ingestion of liquid) which are important variables of feeding behaviour (Logue, 1991, John Blundell, 2009). Feeding behaviour is a highly complex, stereotyped conduct exhibited to replenish the energy reservoir for an optimum cellular efficiency. Selection pressure to procure and assimilate energy, through eating and proper food choice, would have been of paramount importance, at par to reproduction, for existence (Roper, 1986, Vaesen, 2012). Feeding behaviour is therefore not just a response to our physical world, but an internally driven state where energy requirement of entire organ system is collectively expressed as hunger, representing motivation to eat (Dagher, 2009, Berthoud, 2011, Burnett et al., 2016, Ferrario et al., 2016). Feeling of hunger creates a negative emotional state (Betley et al., 2015, Chen et al., 2016) which is defended by the central homeostatic mechanism, generating 1) motivation or desire to eat (Gutierrez et al., 2011); 2) goal-directed motor movements for surveillance and search of food (O'Connor et al., 2015, Nieh et al., 2016, Padilla et al., 2016); 3) a proper motor response depending upon the physical nature of food (Morquette et al., 2012, Stanek et al., 2014, Rossi et al., 2016) and, 4) a stop signal or feeling of satiety derived from peripheral and central feedback (Batterham et al., 2002, Murphy and Bloom, 2006, Alhadeff and Grill, 2014, Campos et al., 2016).
A negative emotional state is created not just by disturbances in feeding homeostasis, but homeostasis in general. Stress, which imposes an immediate threat to the homeostatic system, also invokes negative emotional state (Feldman et al., 1999, Ulrich-Lai et al., 2015). Indisputably, stressful life style has become an emergent phenomenon, mounting from socio-economic and political framework of human society. Subjective stress experienced by an individual in modern society is on a rise, and so are stress-induced complexities such as depression, diabetes, cardiovascular complications, eating disorders and obesity (Mooy et al., 2000, Polivy and Herman, 2002, Jiang et al., 2008, Lo Sauro et al., 2008, Bose et al., 2009, Scott et al., 2012, Steptoe and Kivimaki, 2012, Wiegner et al., 2015, Razzoli and Bartolomucci, 2016) (Table 1.1). Competition, financial difficulties, and professional pressure to perform within or beyond expectations are few of the stressors that
are inevitable and cosmopolitan. However, an individual’s physiological and psychological reaction towards stress is highly subjective, and depends on various environmental and genetic factors (Schneiderman et al., 2005, Franklin et al., 2012). In situations where stressors are inescapable, including metabolic stressors such as self-imposed dieting, homeostatic system triggers a counter regulatory response by dealing with the negative emotional state induced by stress, via overriding the feeding homeostasis and eliciting maladaptive feeding behaviour (Patterson and Abizaid, 2013, Hardaway et al., 2015). Thus, stress could perpetuate feeding response. Feeding response elicited in sated state is non-homeostatic eating or ‘hedonic hunger’, which is essentially directed towards highly palatable food such as desserts that we are fond of eating when we are almost full or at the end of the meal (Corwin and Hajnal, 2005, Lowe and Butryn, 2007). It is difficult to eat another steak at the end of a meal, but a scoop of ice-cream or a piece of cake is still tempting and ingested due to its sensory rewarding properties derived by high concentration of fat and sugar. Another interpretation for stress-induced hedonic feeding behaviour has designated the high palatable food as ‘comfort food’, as it mitigates the discomfort of stress (Tomiyama et al., 2011, Weltens et al., 2014). Studies have shown that both animals and humans resort to palatable food consumption when repeatedly stressed or experiencing negative emotions (Groesz et al., 2012, Handy et al., 2016). Modern obesogenic environment including sedentary and stressful lifestyle inflicts a dilemma to the fundamental homeostatic brain system evolved in environment of food scarcity, demanding enormous energy expenditure (Berthoud, 2012).
The neural mechanism and substrate of stress-induced increase in palatable food intake are at their infancy, and understanding them would certainly help in designing better therapeutic or behavioural interventions to treat stress-induced eating disorders, such as anorexia nervosa and binge eating disorder. This thesis has been conceived to test whether specific extra-hypothalamic and hypothalamic stress-responsive regions in the brain could independently and sufficiently trigger a maladaptive feeding response towards sucrose solution, a high palatable food substitute in rodent model. Using rat as an animal model and inescapable electrical shock as a stressor, both anorexic and sucrose bingeing phenotypes would be investigated. It would also generate essential data on how intake of sucrose
U.S. Stress Statistics Data
Percent of people who regularly experience physical symptoms
caused by stress 77%
Regularly experience psychological symptoms caused by stress 73%
Feel they are living with extreme stress 33%
Feel their stress has increased over the past five years 48% Cited money and work as the leading causes of their stress 76%
Reported lying awake at night due to stress 48%
Stress Impact Statistics
Percent who say stress has a negative impact on their personal and
professional life 48%
Employed adults who say they have difficulty managing work and
family responsibilities 31%
Percent who cited jobs interfering with their family or personal time
as a significant source of stress 35%
Percent who said stress has caused them to fight with people close
to them 54%
Reported being alienated from a friend or family member because of
stress 26%
Annual costs to employers in stress related health care and missed
work $300 Billion
Percent who say they are "always" or "often" under stress at work 30%
Table 1.1 Stress statistics on U.S. population
A survey result conducted in United States of America revealing the subjective stress felt by the general population in past 5 years and some of its direct negative impacts that affect daily life. Retrieved from Statistic Brain, Statistic Brain Research Institute, American Institute of Stress, 2016; http://www.statisticbrain.com/stress-statistics/.
1.1 Food intake regulation
Requirement of energy to fuel cellular processes, explains ‘why’ food intake is such a ubiquitous behaviour in living organism. However, decision of ‘when’, ‘where’, and ‘what’ to feed on resides within individual and originates as a crosstalk between peripheral and central components.
Early during the last century, observations that intravenous glucose relieves whereas insulin aggravate hunger-induced stomach contractions, fueled ‘peripheral theory’ in which stomach’s musculature was assumed to be the seat of hunger and food intake (Anand, 1961). Intriguingly, removal of stomach (Wangensteen and Carlson, 1931), denervation hampering hunger-induced stomach contraction or eliminating its conscious sensation (Grossman et al., 1947, Grossman and Stein, 1948) failed to abolish food intake or feeling of hunger indicating an extra-gastric center for feeding and hunger regulation.
Obesity due to hypothalamic lesion in human, dogs and rodents, implicated hypothalamus to be important for feeding regulation (Brobeck et al., 1943, Heinbecker et al., 1944, Thaler et al., 2012). There are now sound evidences that appetite is regulated by the hypothalamus (Kennedy, 1953, Kalra et al., 1999, Cowley et al., 2001, Williams et al., 2001, Cowley et al., 2003, Neary et al., 2004). Bilateral lesion and stimulation studies have generated strong evidences of ventromedial hypothalamus (VMH) being satiety center and lateral hypothalamus (LH) being feeding or hunger center in the brain (Delgado and Anand, 1953, Parkinson and Weingarten, 1990, Stanley et al., 1993). These two opposing features of the two structures or nuclei of the hypothalamus define the “set point” for metabolic need vs. expenditure which consequently controls a stable body weight.
In addition to hypothalamus, various other extra-hypothalamic regions have been implicated in feeding and energy homeostasis. Especially the neural substrates for reward-related behaviour such as the nucleus accumbens, prefrontal cortex, lateral septum and VTA assign reward and motivational values to ingested food (Waterson and Horvath, 2015, Olivo et al., 2017). Feeding on highly palatable food acts on these extra-hypothalamic areas generating pleasure and thus, leading to positive reinforced behaviour of seeking and procuring such foods (Epstein et al., 2007).
1.1.1 Hypothalamic pathways regulating food intake
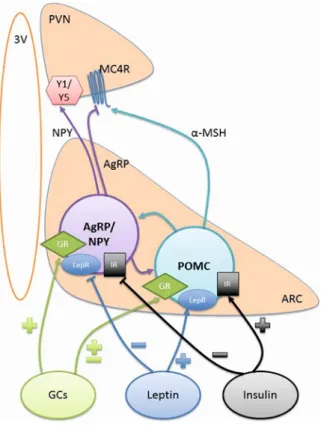
Neurons in hypothalamic arcuate nucleus (ARC) expressing agouti related peptide (AgRP) and neuropeptide Y (NPY) or proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) are key regulators of homeostatic feeding
with absence of blood brain barrier provides an ideal anatomical location to surveillance the humoral factors such as level of glucose, insulin, leptin, ghrelin, and glucocorticoids (Gali Ramamoorthy et al., 2015). Specific ablation of AgRP neurons using diphtheria toxin lead to lethal outcome in mice due to hypophagia (Gropp et al., 2005). Contrarily, optogenetic (Aponte et al., 2011) and chemogenetic (Krashes et al., 2011) activation of AgRP neurons lead to voracious appetite in sated animals. The hunger hormone ghrelin excites (Tong et al., 2008) whereas leptin hyperpolarizes (van den Top et al., 2004) the AgRP neurons resulting in decrease in food intake. Similarly, post-prandial increase in insulin level inhibits AgRP neurons (Konner et al., 2007). AgRP neurons strongly project to the paraventricular hypothalamic nucleus (PVN) and receives excitatory inputs from PVN’s thyrotropin-releasing hormone and pituitary adenylate cyclase-activating polypeptide expressing neurons. Activating these neurons elicits robust feeding in sated mice. These evidences suggest that AgRP neurons activation represents a potent orexinergic signal.
On the contrary, POMC knockout mouse develops obese phenotype due to persistent hyperphagia (Yaswen et al., 1999). Similarly, knocking out its receptor MC3R and MC4R in mice also results in obesity in mice (Girardet and Butler, 2014). Ghrelin inhibits POMC neurons indirectly by activating AgRP GABAergic neurons (Cowley et al., 2003), whereas leptin administration activates POMC neurons (Cowley et al., 2001). This observation strongly suggests that POMC neurons activation represent positive metabolic state of the body and lead to satiety response. Surprisingly, long (24 h) but not short-term optogenetic stimulation of POMC neurons are required to have negative effects on food intake (Aponte et al., 2011). This could be due to rather heterogeneous nature of POMC neurons expressing either GABA or glutamate (Hentges et al., 2009, Aponte et al., 2011) as opposed to relatively homogenous GABA expression in AgRP/NPY neurons (Wu and Palmiter, 2011). Interestingly, AgRP is an endogenous antagonist of POMC receptors (Ebihara et al., 1999) and therefore, AgRP neurons activation could alleviate the tonic satiety state imposed by POMC neurons. Thus, activation of AgRP/NPY neurons promotes whereas activation of POMC/CART neurons inhibits feeding.
The ventromedial hypothalamic nucleus (VMH) was considered to be a satiety center in the brain because absence of this nucleus by lesion induces hyperphagia and obesity (Hetherington and Ranson, 1940, Shimizu et al., 1987). The VMH innervate the hypothalamic arcuate nucleus and activates POMC neurons leading to reduced food intake. In fasted animals the activation strength of VMH neurons over POMC neurons diminishes, permitting transient increase in food intake (Sternson et al., 2005). Supportively, anorexinergic leptin activates VMH neurons which in turn could lead to POMC neurons activation in the arcuate nucleus resulting in satiety response (Dhillon et al., 2006). Interestingly, arcuate AgRP/NPY neurons projects to VMH and inhibits the excitatory neurons in the VMH raising a possibility of the net orexinergic effect due to inactivation of POMC neurons (Fu and van den Pol, 2008). Conclusively, the VMH interacts with orexinergic AgRP and anorexinergic POMC neurons to regulate feeding responses in mammals.
AgRP/NPY and POMC neurons express receptors for various peripheral factors that convey information related to metabolic state of the body. Activation of AgRP neurons is orexinergic whereas activation of POMC neurons is anorexinergic. ARC, arcuate nucleus; AgRP, agouti-related peptide; NPY, neuropeptide Y; POMC, proopiomelanocortin; GC, glucocorticoid; GR, glucocorticoid receptor; LepR, leptin receptor; IR, insulin receptor; Y1/Y5, NPY receptors; a-MSH, alpha melanocortin stimulating hormone; MC4R, melanocortin receptor 4; PVN, paraventricular nucleus; 3V, 3rd ventricle; +, activation; -, inhibition. Adapted from Ramamoorthy et al. (Gali Ramamoorthy et al., 2015)
Another population of neurons in the lateral hypothalamic area (LHA) expressing orexin neuropeptide is important regulator of feeding. Orexin neurons extensively projects to the arcuate nucleus and innervate both AgRP and POMC neurons (Horvath et al., 1999, Muroya et al., 2004). Orexin activates AgRP neurons (Yamanaka et al., 2000) whereas inhibits POMC neurons (Ma et al., 2007) representing its orexinergic behavioural effects. Orexin-positive neurons in LHA are inhibited by high concentration of glucose in extracellular fluid and therefore, could act as direct sensor of metabolic state of the body (Burdakov et al., 2005, Burdakov et al., 2006).
Feeding regulating orexin, AgRP/NPY and POMC/CART neurons projects to and receives inputs from multiple hypothalamic and extra-hypothalamic regions (Peyron et al., 1998, Sakurai et al., 2005, Wang et al., 2015). This gives rise to both monosynaptic and multi-synaptic network of interconnected neurons that fine tune the feeding response.
1.1.2 Extra-hypothalamic pathways regulating food intake
The choice on the menu cart seems not to be a prerogative of the hypothalamus. Scrupulous examination of various cases of local brain damage revealed that dysfunctions in the hypothalamic regions may simply change appetite, while disturbances in the prefrontal cortex (PF) can lead to development of so called ‘gourmand syndrome’: an overwhelming increase in food preoccupation (Beatty and Schwartzbaum, 1968, Regard and Landis, 1997, Cockrell, 1998, Uher and Treasure, 2005). The PF is traditionally included in the
reward circuitries; as its projection fields include the ventral tegmentum area (VTA), nucleus accumbens shell (AcbSh) and lateral septum (LS) which in turn are interconnected, are key brain regions of the brain reward system (Sesack et al., 1989, Staiger and Wouterlood, 1990, Knutson et al., 2001, Gabbott et al., 2005, Staudinger et al., 2011, Welberg, 2011). It has been suggested that the PF is involved in anticipation of reward as its neurons change firing activity during reward seeking before the actual consumption of food (Staudinger et al., 2011). The induction of expression of c-fos mRNA, a marker of persistent neuronal activation, in the PF, LS and AcbSh was seen in rats anticipating highly palatable food but not the regular chow (Mitra et al., 2011). Conversely, anticipation of regular chow activated the regions of the medial hypothalamus, but not the PF, LS and AcbSh (Poulin and Timofeeva, 2008, Mitra et al., 2011). The AcbSh and LS seem to play a pivotal role in food anticipation, stress modulation and natural reward conditioning and hence these regions may be involved in stress-induced bingeing and reward-induced dampening of stress effects (Meredith et al., 1993, Will et al., 2003, Khamassi et al., 2008, Gutierrez et al., 2010, Krause et al., 2010, Mitra et al., 2011). Both the AcbSh and LS sends axonal projections to the LHA (Heimer et al., 1991, Zahm and Brog, 1992, Kirouac and Ganguly, 1995). Because these projections are exclusively GABAergic (Maldonado-Irizarry et al., 1995, Sweeney and Yang, 2016), they could inhibit the LHA neurons to bring about the stress-induced anorexic effects (Stratford and Kelley, 1999). Dopaminergic innervations to the PF, LS and AcbSh from the VTA play a role in inducing an addiction-like behaviour on natural rewards with an intermittent exposure to rewarding substances (Johnson and Kenny, 2010).
1.2 Sugar: an important ingredient in comfort food
The best example of ‘free sugar’ is sucrose, commonly known as table sugar. Sucrose is a white crystalline, brittle and hygroscopic organic compound with an intense sweet taste evoking pleasure when ingested (Levine et al., 2003). It is a disaccharide consisting of glucose and fructose in a 1:1 ratio joined together by an acetal glycosidic bond (Figure 1.2). It is catalyzed by sucrase enzyme in intestine and broken down into fructose and glucose. A part of glucose remains in systemic circulation while rest of it including fructose is
metabolized to glycogen in liver or enters tricarboxylic acid (TCA) cycle (Timofeeva and Mitra, 2013).
Figure 1.2 Chemical structure of sucrose molecule.
Adapted from http://www.nutrientsreview.com/carbs/disaccharides-sucrose.html
‘Free or pure sugar’ terminology stems from the fact that it is chemically refined to an extent that it is completely devoid of any other macro- or micro-nutrients other than carbohydrate that its parent raw material, such as sugar cane or sugar-beet, would have contained. Therefore, it is a pure source of carbohydrate, that in relatively less processed form aids in proper metabolic usage, storage or disposal (Gondal et al., 1996, Harish Nayaka et al., 2009). Due to inadequate scientific and political consensus, it is an ingredient in food product’s ‘nutritional fact’ label which is expressed in weight (g) opposed to other ingredients that are expressed as percent daily value recommended by FDA (U.S. Food and Drug Administration) for a person on 2000 calorie diet (Erickson and Slavin, 2015). Its abundance, affordability and pleasant sweet taste fuel its voracious consumption in processed food products such as desserts, sweetened beverages and confectionaries (Berthoud, 2012) (Table 1.2). Owing to its demand, global production of sugar had been exponentially increased from 8 million tons in 1900 to 110 million tons by the end of 20th
century (Johnson et al., 2007). Estimated global consumption of sugar in its free, added or processed form was 173.4 million tons in 2015-2016 which was higher than the estimated production of sugar worldwide (U.S. Department of Agriculture, 2015) (Figure 1.3). In
conclusion, humans have transformed themselves from casual to voracious consumers of sugar, which has given rise to various diet-related health complications including obesity (Ludwig et al., 2001) (Figure 1.4).
Figure 1.3 Yearly production and consumption of sucrose from 2009 to 2016.
Worldwide statistics on yearly escalation of sucrose consumption and its production from 2009 to 2016. Notice that in year 2009-10 and 2015-16, the demand of sucrose has exceeded the production prediction for the corresponding year. Retrieved from USDA report, 2015;
http://usda.mannlib.cornell.edu/usda/fas/sugar/2010s/2015/sugar-05-21-2015.pdf
A dual-axis graph showing an increase in escalation of sugar consumption (left y-axis) by the beginning of last century coincides with increased obesity prevalence (right y-axis) in UK and USA. Adapted from Johnson et al. (Johnson et al., 2007).
FOOD (serving) SUCROSE (g)
Tonic water (12 oz, 355 mL) 32
Pie, fruit, baked (1 piece, 4.8 oz, 135 g) 29
Fondant (1 oz, 28 g) 27
Ice tea (12 oz, 355 mL) 27
Coffee liqueur (2.5 oz, 75 mL) 22
Root beer (12 oz, 355 mL) 20
Cake, chocolate (1 piece, 65 g) 20
Ice cream, pudding chocolate, soft (1 cone, 3.5 oz, 100 g) 17
Pudding, chocolate (4 oz, 110 g) 17
Shake (1 cup, 240 mL) 17
Fudge, chocolate (1 oz, 28 g) 16
Pickled cucumbers (2 oz, 57 g) 16
Coconut cream, sweetened (1 oz, 28 g) 14
Syrups (1 tbsp, 20 g): liquid sucrose , maple 13
Cider, certain varieties (12 oz, 355 mL) 13
Yogurt, berry (5.3 oz, 150 g) 13
Milk chocolate (1 oz, 28 g) 12
Chocolate milk (1 cup, 240 mL) 12
Energy drink (4 fl. oz, 120 mL) 11
Strawberry jam (1 tbsp, 20 g) 10
Ice lolly (3.5 oz, 100 g) 10
Ginger ale (12 oz, 355 mL) 7
Granola bar (1 oz, 28 g) 5
Salad dressing, French (2 oz, 57 g) 3
Crackers (1 cup, crushed, 50 g) 3
Chewing gum (1 stick, 3 g) 2
Infant formula (1 fl. oz, 30 mL) 1
Table 1.2 Sucrose concentration (in grams) of some of the processed food that are commonly consumed worldwide. Retrieved from USDA nutrient list, 2016;
https://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0 &nutrient1=210&nutrient2=&nutrient3=&subset=0&fg=&sort=c&measureby=m.
1.2.1 Central pathway that responds to sucrose
Sucrose, like any other food ingredient, enters our body through eating or drinking. After the visual confirmation of its presence in added form, the second sensory domain directly sampling its sweet and nutritious property are the taste receptors of the tongue. The taste receptors acknowledging the sweet and pleasant nature of sucrose are the members of type 1 taste receptor (T1R) family of receptors present on the taste bud (Li, 2009). T1R2 and T1R3 form a heterodimer G-protein coupled receptor, which when encounters sugar molecule as a ligand undergoes a conformational change resulting in activation of alpha-gustducin (Stone et al., 2007). This activates phospoholipase C and adenylate cyclase, resulting in increase in cytosolic concentration of calcium ions released by intracellular sources (McCaughey, 2008) (Figure 1.5). The non-selective cation-channel, transient receptor potential channel M5 (TRPM5), exhibits a calcium-dependent opening that leads to influx of cations such as Na+ ions leading to depolarization and release of ATP as a neurotransmitter (Huang and Roper, 2010). ATP acts on post-synaptic sensory fibers of vagus (X), facial (VII) and glossopharyngeal (IX) cranial nerve terminals that innervate tongue, and transduce the information of ‘sweetness’ to the brain (Finger et al., 2005). These three cranial nerves ascend and innervate the rostral half of the nucleus of the solitary tract (NTS) in hind brain, which also receives taste information from gastrointestinal tract through enteric nervous system via vagus relay (Berthoud, 2008, Gutierrez and Simon, 2011). Almost all types of T1R and its heterodimers are reported in the inner lining of stomach, duodenum, ileum, jejunum and colon (Hofer et al., 1996, Dyer et al., 2005, Bezencon et al., 2007). Taste receptors present in gastrointestinal tract could independently sense presence of sweet and nutritious meal, as demonstrated by increased
dopamine secretion in dorsal and ventral striatum following intra-gastric injection of glucose or artificial sweetener (Tellez et al., 2016). Hence, detecting taste is not just a mandate of the tongue but also of the gastrointestinal tract. Glucosensing molecular machinery that could act as a proxy detector for ingested sucrose, readily detects glucose in extracellular space, are expressed in hepatic portal mesenteric vein (Donovan, 2002), blood vessels in the brain (Gerhart et al., 1992) and neurons of the ventromedial hypothalamus (VMH), lateral hypothalamus (LHA) and arcuate hypothalamic nucleus (Levin et al., 2004, Burdakov et al., 2005, Burdakov et al., 2006, Marty et al., 2007, Baik, 2013).
Figure 1.5 Molecular cascade within a taste receptor cell.
Schematic of intracellular molecular cascade triggered by presence of sugar that binds to apical T1R2-T1R3 taste receptor, leading to depolarization of the cell. Depolarization could be achieved either by inhibiting exit of potassium ions by PKA-mediated mechanism or by calcium-dependent opening of non-selective cationic channel TRPM5 leading to influx of
positive ions such as sodium, potassium or calcium ions. Adapted from Stuart A. McCaughey et al. (McCaughey, 2008).
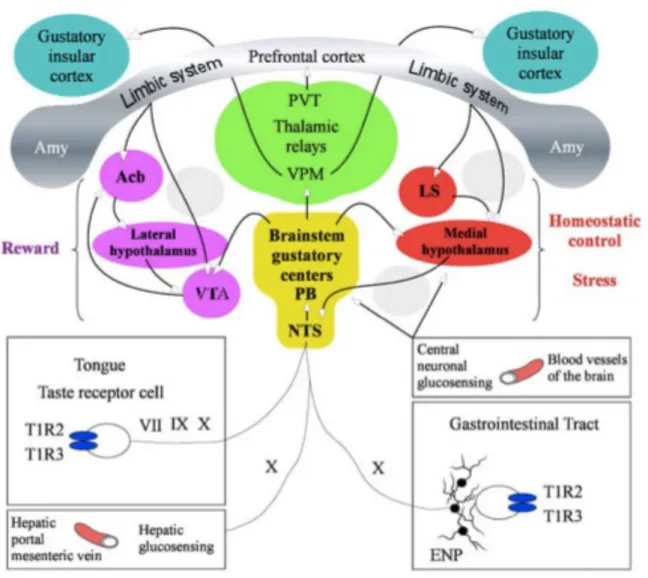
In rodents, the NTS further projects to the ipsilateral central lateral sub-nucleus of pontine para-brachial nucleus (PBN) (Small and Scott, 2009), which consecutively relays the gustatory information to the ventro-postero-medial (VPM) thalamic nucleus, that finally sends efferent to agranular subregion of the primary gustatory insular cortex of forebrain (Oliveira-Maia et al., 2011). Additionally, PBN projects directly to the paraventricular thalamic nucleus (PVT) which sends efferent to the prefrontal cortex (PFC). Forebrain gustatory insular cortex and hindbrain gustatory regions, NTS and PBN, could independently give rise to perception of sweet taste and associated mimetic responses (Yamamoto et al., 1994, Accolla et al., 2007, Hajnal et al., 2009, Chen et al., 2011, Peng et al., 2015). Auxiliary taste-related pathways originating at the level of PBN reach the VTA and medial hypothalamic region, thus merging the reward aspects of taste with the homeostatic feeding response (Fulwiler and Saper, 1984) (Figure 1.6). The circuit comprising of reciprocal connections between the PFC, lateral septum, nucleus accumbens, lateral hypothalamus and VTA encodes for the reward attributes of ingested food products, and reinforces the seeking and feeding of such food products (Sheehan et al., 2004).
Figure 1.6 Peripheral and central sucrose sensing and responding pathways.
Schematic of the peripheral and central pathways that detect the presence of sugar in oral cavity or gastrointestinal tract, and relay this information through cranial nerves to the gustatory centers in brainstem and cortex. The regions in pink play a role in reward aspects of sugar, whereas areas in red are the regions that play a role in homeostatic control and stress response. Adapted from Timofeeva and Mitra (Timofeeva and Mitra, 2013).
1.2.2 Why sucrose is rewarding?
Sucrose broadly has two sensory properties – its sweet taste or palatability and its nutritional or calorific value. Intra-gastric delivery of sugar solution, that overcomes the
conscious perception of its sweetness, or access to artificial sweeteners, that overcomes nutritional attributes of sugar, have failed to mitigate the rewarding properties of sweet tastes (Sclafani, 2001, Tellez et al., 2016). The preference for sweet tasting sucrose is innate, as demonstrated by hedonic responses in rat pups and human infants (Hall, 1980, Ayres et al., 2016). Interestingly, recent studies that employ TRPM5 knockout that essentially renders mice to be taste-blind, fails to affect their preference or avoidance towards sweet or bitter solution (Damak et al., 2006). This persistence of sucrose preference without sweet taste sensing cellular machinery, reveals that caloric value of sucrose could independently and sufficiently evoke feeding preferences (de Araujo et al., 2008). Therefore, palatability and nutrition can independently drive the sucrose preference. Intriguingly, this raises the question whether sweet taste and nutritious attributes of sucrose affect the overlapping or distant neuronal network in the brain. Interestingly, sucrose-naïve, food-deprived, and chow-anticipating rats show elevated c-fos mRNA expression in the paraventricular (PVN) and dorsomedial (DMH) hypothalamic regions, the homeostatic medial hypothalamic centers of the brain (Poulin and Timofeeva, 2008, Mitra et al., 2011). Conversely, in sucrose-anticipating rats, the metabolic negative state and food anticipation triggered expression of c-fos mRNA expression in the prefrontal cortex, anterior lateral hypothalamus, accumbens shell and lateral septum, with little or no activation in the medial hypothalamic structures (Mitra et al., 2011) (Figure 1.7).
In other words, hunger for mundane chow evokes physiological activation of homeostatic hypothalamic feeding centers that reflect the ‘need’ of the system for metabolic replenishment. On the contrary, in sucrose-experienced animals, sucrose anticipation evokes activation in reward and limbic brain regions, but not in the hypothalamic homeostatic centers, representing that ‘want or liking’ could override the metabolic ‘need’ and could lead to maladaptive inclination towards palatable sucrose. Indeed, animals given equal opportunity to eat chow and sucrose make a poor dietary judgment by escalating the intake of sucrose, and neglecting the nutritionally-balanced and often calorically superior chow (Bell et al., 2002, Pecoraro et al., 2004, Mitra et al., 2011).
Figure 1.7 Schematic showing the differential c-fos mRNA expression in food-anticipating rats.
Two groups of rats were maintained on scheduled sucrose + chow (pink) or only chow (green) diet. Notice that during food-anticipating period, the rats maintained on sucrose + chow diet showed activation in the prefrontal cortex, anterior lateral hypothalamus (LHa), lateral septum (LS) and accumbens shell (AcbSh), while rats on mundane chow showed activation in the medial hypothalamic structures such as the paraventricular (PVH) and dorsomedial (DMH) hypothalamic nuclei. Adapted from Mitra et al. (Mitra et al., 2011).
Dopaminergic system originating from the VTA, plays an important role in shaping motivated behaviour such as feeding and addiction (Baik, 2013) (Figure 1.8). Sucrose and saccharine intake elevates extracellular dopamine concentration in nucleus accumbens due to activation of VTA dopaminergic neurons (McCutcheon et al., 2012a). However, release of dopamine following sucrose intake or in response to sucrose-associated cues, is significantly higher compared to saccharine or saccharine-associated cues, suggesting synergistic effects of taste and nutrition values of sucrose on dopamine release. Similarly, increase in dopamine in striatum has also been observed in other pleasure-arousing
behaviours such as administration of drugs of abuse, sexual interaction, exercise, meditation and gambling (Fiorino and Phillips, 1999, Kjaer et al., 2002, Petzinger et al., 2007, Linnet et al., 2010, Willuhn et al., 2010). A recent study by Tellez et al. (Tellez et al., 2016) has demonstrated that dopamine concentration was differentially regulated in dorsal and ventral striatum in response to palatability and nutritional aspects of sugar. Specifically, oral administration of sucralose with concomitant intra-gastric release of either sucralose or glucose increased the dopamine concentration in the ventral striatum, but not the dorsal striatum. Conversely, intra-gastric injection of glucose, but not sucralose, elevated dopamine concentration in the dorsal striatum, but not in the ventral striatum.
Figure 1.8 Dopaminergic reward pathway.
Schematic of central dopamine pathways (red) originating from ventral tegmentum area (VTA) and projecting to the striatum, hippocampus and frontal cortex. Release of dopamine in aforementioned brain regions (blue) plays important role in reward learning and instinctive motivated behaviour. Adapted from http://organicallyintune.com/the-organic-effects-of-dopamine-and-the-brains-reward-system.html
Intriguingly, LiCl injection following sucrose intake, leads to taste aversion on subsequent exposure to sucrose, and decreases dopamine release in the nucleus accumbens (Roitman et al., 2010). Also, ingestion of bitter compounds such as quinine (Roitman et al., 2008, McCutcheon et al., 2012b) or denatonium benzoate (Tellez et al., 2016) reduces the concentration of extracellular dopamine in the nucleus accumbens. Dopamine release and extracellular concentration were also elevated in the prefrontal cortex following food reward in food-deprived rats (Feenstra and Botterblom, 1996). Conclusively, dopamine release in the nucleus accumbens and other reward brain regions, reflects the reward value of sucrose that evokes pleasure and reinforces sucrose seeking and intake.
1.2.3 Microstructure of sucrose licking in rats
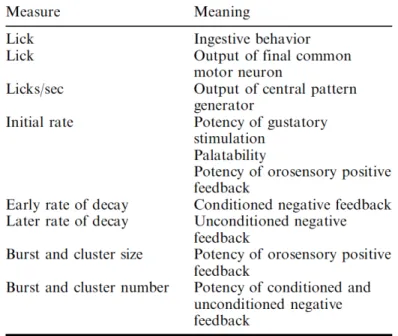
Liquid ingestion in rodents happens through rhythmic tongue protrusions making recurrent contacts with a liquid source such as bottle spout (Travers et al., 1997). This rhythmic protrusion and retraction of tongue happens at a frequency of 3-7 Hz and is assumed to be regulated by central pattern generator in hypoglossal nucleus of brainstem (Wiesenfeld et al., 1977, Davis and Smith, 1992). Duration between two licks is termed as inter lick interval (ILI). Groups of ILIs could be temporally associated into burst and clusters. Lick bursts are group of licks with an ILI of ~150 ms, with an interburst interval (IBI) between 300 ms and 500 ms. ILI of ≥500 ms had been conventionally classified as intercluster interval (ICI) and this relatively long pause between two licks give rise to lick clusters. Thus, clusters are collection of high frequency licks (3-7 Hz) temporally arranged as one or more lick bursts (Davis and Smith, 1992) (Figure 1.9). Licks and its temporal microstructures in rodents had been extensively studied by John Davis and specific physiological meaning (Table 1.3) had been attributed to its parameters (Smith, 2001).
Figure 1.9 Licking microstructure by a rat during sugar solution consumption.
This schematic represents licks as vertical lines. The duration between two licks are called inter lick interval (ILI). Burst is a collection of licks with an ILI of ~150 ms. A pause between two bursts is represented as inter burst interval (IBI). Lick cluster is made up of one or multiple lick bursts with an inter cluster interval (ICI) of ≥ 500 ms. Adapted from Davis and Smith (Davis and Smith, 1992).
Table 1.3 Lick parameters and its physiological meaning. Adapted from Smith (Smith, 2001).
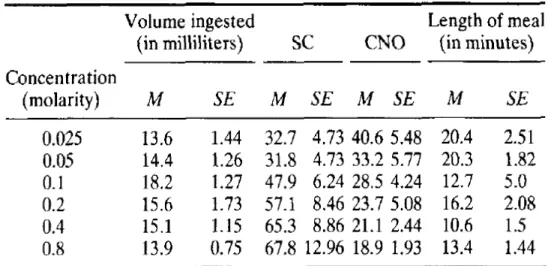
Using variable concentration of sucrose solution, Davis and Smith (Davis and Smith, 1992) had shown distinct features of lick microstructure (Table 1.4). In Table 1.4, low concentration of sucrose solution (0.025 to 0.05 M) results in low consumption with relatively long meal length (sum total of cluster duration) but smaller size of clusters (SC; number of licks in cluster) and less number of clusters (CNO). On the contrary, the medium concentration (0.1 to 0.2 M) stimulated largest intake with relatively smaller meal size but increased cluster size and highest number of clusters among all the concentration used. Further increase in sucrose concentration (0.4 to 0.8 M) attenuated the intake by decreasing meal length and number of clusters but with further increase in size of clusters. Thus, an increase in cluster size and a decrease in cluster number represent the increased sucrose concentration in ingested liquid in rats. Similarly, lick microstructures have also been shown to be modulated by strain (Boughter et al., 2007, Marco et al., 2009), age (Marco et al., 2009), sex (Marco et al., 2009), metabolic state (Grigson et al., 1993) and stress (Vajnerova et al., 2003, Dwyer et al., 2012).
Table 1.4 Sucrose concentration-dependent changes in lick microstructure in rats.
Effect of increasing concentration of sucrose on volume ingested, size of licking cluster (SC), number of clusters (CNO) and length of meal. M, mean; SE, standard error of mean. Adapted from Davis and Smith (Davis and Smith, 1992).
1.3 Stress
According to Hans Selye (Selye, 1936), stress is defined as “a syndrome produced by nocuous agents”. These nocuous agents are also known as stressors, which could be of physical and psychological nature, or both. Additionally, stress could be initiated by both external, and internal stressors (Ginty and Conklin, 2011). For e.g. tissue damage after an accident or foot shock in rodents are externally inflicted stress; whereas an entrance examination or public speaking is an internally perceived stress (Figure 1.10).
Figure 1.10 Internal and external stressors
Different forms of external and internal stressors that evoke the central stress response. Adapted from https://zteaminternational.com/2016/04/07/blog-post-title-5/
Stressors shift the physiological set-points such as heart and respiratory rate or extent of vigilance to a resource-hungry unstable state in order to trigger an appropriate fight or flight response (Wray et al., 2002). Hence any threat to homeostasis or situation where the feeling of well-being is challenged, triggers an adaptive and beneficial stress response (McEwen, 2004).
However, chronic stress often leads to secondary complexities such as depression (van Praag, 2005), cardiovascular diseases (Dimsdale, 2008, Steptoe and Kivimaki, 2012),