Decreased Corticosensitivity in
Quiescent Crohn’s Disease
An Ex Vivo Study Using Whole Blood Cell Cultures
DENIS FRANCHIMONT, MD, EDOUARD LOUIS, MD, PIERRE DUPONT, MD, YVONNE VRINDTS-GEVAERT, WALTHERE DEWE, BSc, GEORGE CHROUSOS, MD,
VINCENT GEENEN, MD, PhD, and JACQUES BELAICHE, MD
Corticose nsitivity in¯ uence s the degree and the duration of an in¯ ammatory reaction by alte ring target cell response s to endoge nous and/or exoge nous glucocorticoid s. Indeed, different clinical response s to glucocorticoid s have be en observe d among patie nts with Crohn’ s disease , sugge sting different de gre es of corticose nsitivity in the se subje cts. The purpose of this study was to compare the corticose nsitivity of patie nts with quie scent Crohn’ s dise ase to that of he althy subje cts (HS). Nine teen patie nts with quie scent Crohn’ s disease and 14 HS were studie d; all patie nts were steroid-fre e for at least six months; 7 of the 19 were corticoste roid-de pende nt (CSD) and treate d with nonglucocortic oid immunosupp ressants at the time of the study. Corticose nsitivity was measure d by the inhibition of LPS-induce d cytokine secretion in whole blood cell culture s treate d with incre asing conce ntrations (102 9 to 102 6M) of de xame thasone . Tumor-ne crosis factor-a (TNF-a ), interle ukin-6 (IL-6), and interleukin-1b (IL-1b ) were measure d using spe ci® c immunoassays. Crohn’ s dise ase patie nts had a marke dly decrease d dexamethasone -mediate d inhibition of TNF-a (P, 0.01), IL-6 (P , 0.001) , and IL-1b (P , 0.01) compare d to he althy subje cts, with a shift of the dexame thasone dose ± response curve to the right. No signi® cant differences in the basal and LPS-stimulate d secretion of the thre e cytokine s were obse rved between CSD and non-CSD patie nts, and both subgroups of patie nts had similar de gre es of dexame thasone -mediate d cytokine inhibition. We conclude that patie nts with Crohn’ s disease have a signi® cant decrease in the corticose nsitivity of the ir le ukocyte s. This may be relate d to a spe ci® c ge netic/constitutio nal background and/or could be acquire d, due to in¯ ammation-re lated endocrine and/or immune factors.
KEY WORDS: glucocorticoids; cytokines; corticosensitivity; Crohn’s disease .
Incre asing evide nce supports an important role of the hypothalamic± pituitary± adre nal (HPA) axis in the
pathoge nesis and course of in¯ ammatory diseases (1). The end-effector of the HPA axis, glucocorticoids , restrain the immune and in¯ ammatory response s (2, 3). Thus, the in¯ ammatory response of in¯ ammatory disease-susceptible Lewis and -resistant Fische r rats is inve rsely relate d to the magnitude of the ir HPA axis response to in¯ ammatory mediators (4, 5), while the glucocorticoid receptor antagonist RU 486 rende rs Fischer rats susceptible to streptococcus cell wall-induce d arthritis (4).
Glucocorticoids are use d as ® rst line antiin¯
amma-Manuscript re ce ived July 13, 1998; re vised manuscript re ce ived January 5, 1999; accepte d January 7, 1999.
From the De partme nt of Medicine, Division of Gastroente rology, Laboratory of RadioImmunology and Ne uroe ndocrine -Immunology, Unive rsity Hospital of LieÁ ge , Belgium; and De velop-mental Endocrinology Branch, National Institutes of Child Health and Human Deve lopme nt, National Institutes of Health, Bethesda, Maryland.
Address for re print re que sts: Dr. Jacque s Belaiche , Institute of Pathology CHU-B35, De partme nt of Gastroente rology, LieÁge Uni-versity Medical School, 4000 Lie ge 1 - Sart Tilman, Be lgium.
tory and immunosuppre ssive drugs in many in¯ am-matory and autoimmune dise ase s (6). Different clin-ical response s to glucocorticoids have be en observe d among patie nts suffering from such dise ase s, and pa-tie nts with Crohn’ s disease have be en classi® ed as corticoste roid-se nsitive (when a good response to treatment is obse rve d) , corticoste roid-de pe nde nt (CSD) (when glucocorticoids are ne ede d to ke ep the in¯ ammatory disease quie scent), and corticoste roid-resistant (CSR) (whe n the y are not responsive to glucocorticoid treatme nt). The pre vale nce of cortico-steroid de pende ncy in Crohn’ s disease is about 15± 35% (7± 9), while corticoste roid resistance is observe d in less than 10% of the patie nts (9, 10).
Corticose nsitivity depe nds not only on the glu-cocorticoid receptor (GR) number and af® nity, but also on pre - and postreceptor mechanisms of GR activation, including the inte ractions of GR with cy-toplasmic and nucle ar factors and spe ci® c DNA-responsive ele ments (11, 12) . Only a few clinical stud-ies have be en carrie d out on corticose nsitivity and none in Crohn’ s dise ase . Familial glucocorticoid re-sistance was studie d by Chrousos et al (13) and Lam-berts et al (14) and was shown to be of ge netic etiology and related to a de crease of GR af® nity for its ligand or to a de crease in GR numbe r pe r cell (13, 14) . Sher et al (15) de scribed two populations of corticoste roid-re sistant (CSR) asthma patie nts: type 1 is de ® ned by de crease d GR af® nity binding, and type 2 is de ® ne d by decreased GR number per cell. Type 1 CSR asthma was shown to be reve rsible and second-ary to in¯ ammation, while type 2 CSR asthma ap-pe are d to be gene tically and/or constitutionally de ter-mined (15) . Finally, Schlaghe cke et al reported a 50% de crease of GR number/cell in patie nts with rheuma-toid arthritis (16) ; howeve r, inexplicably, this did not appe ar to in¯ ue nce the in vitro corticose nsitivity of these patie nts (17).
Whole blood cell culture is an appropriate ex vivo technique to analyze corticose nsitivity within a con-trolle d environme nt, by studying the inhibition of cytokine secretion by grade d concentrations of glu-cocorticoids (18) . The aim of this work was to com-pare corticose nsitivity between patie nts with quie s-cent Crohn’ s disease and he althy control subje cts.
MATERIALS AND METHODS
Patients an d Con trols. Patie nts with Crohn’s disease (10
women and 9 men) and healthy volunteers (7 women and 7 me n) serve d as blood donors. The me an age was 35 years in patients with Crohn’s disease (range : 20 ± 53 years) and 33 ye ars in controls (range: 24 ± 44 ye ars). The clinical
charac-teristics of patients with Crohn’s disease are summarized in Table 1. All patients with quiescent Crohn’s disease were followed in the Gastroenterology Division at the University Hospital of LieÁge , Belgium. The diagnosis of Crohn’s dis-e asdis-e was maddis-e by classical clinical, radiological, and dis-e ndo-scopic criteria. Seven of the 19 patie nts were corticosteroid-dependent. The corticoste roid dependency was de® ned e ither by two successive relapses following glucocorticoid discontinuation or by two successive relapses at dose tape r-ing afte r a successful treatme nt of a ¯ are-up with glucocor-ticoids (7, 8, 19) . Treatme nt with immunosuppressive drugs (azathioprine or me thotrexate ) allowed the complete with-drawal of glucocorticoids in these patie nts. Nine non-CSD patients were treated with 5-aminosalicylic acid (5-ASA). All patients studied were in clinical remission and cortico-steroid-free for at least six months. Three groups were de® ned: non-corticosteroid-dependent (non-CSD) patients; corticosteroid-dependent (CSD) patients; and healthy con-trols (HS). Data from the interleukin-1b (IL-1b ) assay of four CSD patients and two non-CSD patie nts were not included. Similarly, data from the tumor necrosis factor-a (TNF-a ) assay of one CSD patient and one non-CSD patient were not included because of absence of stimulation by LPS.
Wh ole Blood Cell Cultures. The blood was tre ate d as
previously described (18) . Blood samples were collected in apyroge nic he parinized tubes provided by Biosource / Medge nix (Fleurus, Belgium). The white blood cell count was in the normal range in CD patients. The blood was processed imme diate ly and diluted with 1/10 RPMI 1640 supplemente d with 2 mM glutamine, 100 IU/ml penicillin, 100m g/ml streptomycin (Biowitthaker), and was then dis-tributed in 2-ml wells. RPMI 1640 medium was tested for apyroge nicity. LPS (endotoxin from Salmonella enteritidis; Sigma, St. Louis, Missouri) was added at a ® nal concentra-tion of 25 pg/ml. A synthetic glucocorticoid, dexame
tha-TABLE1. CLINICALCHARACTERISTICS OFCORTICOSTEROID -DEPENDENT(CSD)ANDNON-CORTICOSTEROID-DEPENDENT
(NON-CSD) PATIENTS WITHCROHN’SDISEASE Non-CSD patients
(N5 12)
CSD patients
(N5 7)
Mean age [yr (range) ] 37( 20± 53) 31( 24± 48)
Fe male /male 6/6 4/3
Dise ase duration [months
(me an, range) ] 144( 8± 284) 96( 30± 156) Crohn’s disease activity
index , 150 , 150
Dise ase location
Small bowel only 2/12 0/7
Colonic only 1/2 5/7
Ileocolonic 9/12 2/7
Dise ase type
In¯ ammatory 4/12 7/7 Fibrostenotic 8/12 1/7 Fistulizing 1/12 0/7 Tre atme nt 5-ASA 9/12 0/7 Azathioprine (2 mg/kg/day) 0/12 6/7 Me thotrexate (25 mg/week) 0/12 1/7
sone, was added at a ® nal concentration ranging from 102 9 to 102 6M in separate wells. Plates were incubated at 37°C in a 5% CO2 athmosphere; the incubation time was 24 hr
for LPS-stimulated whole blood cell cultures (18, 20). The contents of the wells were the n collected and centrifuged at 900g for 10 minutes. Supernatants were recovered and frozen at2 20°C. Blood was drawn from women during the proliferative phase of the ir cycle.
Im m un oassays. TNF-a , IL-1b , and IL-6 were me asured with speci® c immunoassays from Biosource/Medgenix with-out any cross-reactivity. Immunoassays were performed ac-cording to the manufacturer’s directions. The dete ction limit was 3 pg/ml for TNF-a , 2 pg/ml for IL-1b , and 2 pg/ml for IL-6. Plate s were read at 460 nm by a microplate ELISA reade r from Medge nix Diagnostics. Absorbancy was trans-formed to cytokine concentration using a standard curve computed by Medgenix ELISA software. Plasma cortisol (RIA, Immunotech, Marseille, France) and cortisol-binding globulin (RIA, Radim, Liege, Belgium) were me asured with the routine laboratory methods. The blood was col-lected from each individual betwe en 3 and 6 PM. The blood was then processed immediately for whole blood cell cul-tures. Plasma obtained at the same time was frozen at
2 20°C until assay. Once all the plasma samples from the patients and the healthy subjects were collecte d, cortisol and cortisol binding globulin concentrations were me asured in a single batch.
Statis tical Analyses. Statistical analyse s were carried out
using the SAS software package (SAS Institute Inc., Cary, North Carolina). The relative changes in cytokine produc-tion were computed for e ach dose of dexame thasone. A log transformation was used for both the absolute values of cytokine production and for the percentage of the response in each individual to normalize the distribution of the data. A ge neralized linear mixe d model with random effe cts (SAS Proc Mixed) was ® tted to study the dexame thasone ge neral e ffe ct and dose effect on cytokine secretion. All results were considered to be signi® cant at the 5% critical level (P, 0.05). The SAS Proc Mixe d is an ANOV A for repeated me asures, as is appropriate where the observations in the same subject are not independent of e ach other. This me thod adjusts the standard error as a function of the covariance structure of the observations.
The ED5 0 was calculated as the dose of dexame thasone
necessary to produce a 50% inhibition in the secretion of e ach of the three cytokines for e ach individual, using the SAS Proc Mixed program. The statistical comparisons were
betwe en the me ans of the ED5 0s in the three groups of
subjects with the Kruskal-Wallis and the Wilcoxon tests. Othe r statistical comparisons within the same group or betwe en the three groups were performed using nonpara-me tric (Kruskal-Wallis and Wilcoxon tests) and paranonpara-metric tests (ANOV A and Student t tests afte r a logarithmic transformation).
RESULTS
Cytokin e Secretion in Wh ole Blood Secretion from CD Patien ts an d Health y Subjects
The re was a signi® cant difference in cytokine secre-tion between basal and LPS-induce d le ve ls of cyto-kine s in the non-CSD patie nts (P, 0.001 for TNF-a , IL-6, and IL-1b ), in CSD patie nts (P , 0.001 for TNF-a and IL-6, P, 0.01 for IL-1b ), and in he althy subje cts (P , 0.001 for TNF-a , IL-6, and IL-1b ) (Table 2).
Basal cytokine leve ls in whole blood cell culture s were similar in both groups of patie nts with Crohn’ s disease and he althy subje cts. LPS-stim ulate d IL-6 and TNF-a leve ls were signi® cantly different between CD patie nts and he althy subje cts (P, 0.05 and P , 0.05, respective ly) and be tween CSD patie nts and he althy subje cts (P , 0.05 and P , 0.05, respectively). No difference was obse rved be tween non-CSD patie nts and he althy subje cts (Table 2).
Inh ibition of Cytokin e Secretion by Dexam eth ason e in Wh ole Blood Cell Cu ltu res from CD Patien ts an d Health y Su bjects
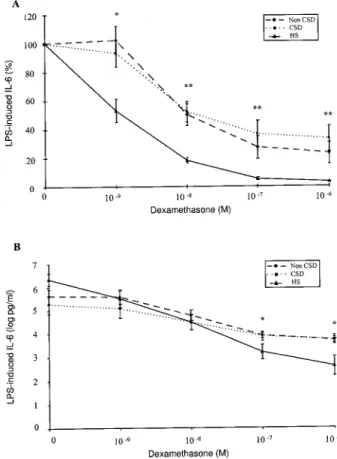
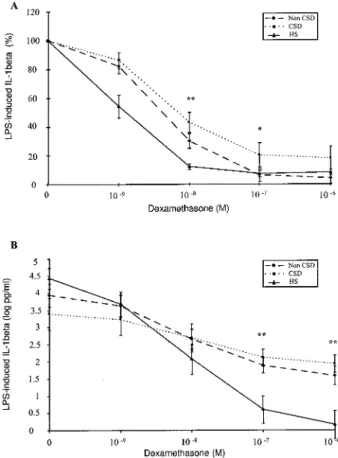
The results of the conce ntration-de pe ndent effect of de xame thasone on TNF-a , IL-6, and IL-1b secre-tion in whole blood cell culture s in the thre e groups are shown in Figure s 1± 3. In the top pane ls of the se ® gure s, the mean percentage s of inhibition of cyto-kine production are pre sented, while in the bottom pane ls the means of the logarithm ically transforme d absolute cytokine leve ls are shown.
TABLE2. BASAL ANDLPS-STIMULATEDCYTOKINELEVELS INCORTICOSTEROID-DEPENDENT(CSD)AND
NON-CORTICOSTEROID-DEPENDENT(NON-CSD) PATIENTS WITHCROHN’SDISEASE ANDHEALTHY
SUBJECTS*
Cytokine (pg/m l)
Non-CSD patients CSD patients Healthy subjects
TNF-a basal 606 24 (N5 11) 1046 84 (N5 6) 356 11 (N5 14) TNF-a after LPS 2046 65 (N5 11) 2486 160 (N 5 6)a 4206 123 (N 5 14) IL-6 basal 1886 56 (N5 12) 3446 260 (N 5 7) 1026 42 (N5 14) IL-6 after LPS 4176 106 (N 5 12) 6586 447 (N 5 7)a 8156 178 (N 5 14) IL-1b basal 426 13 (N5 10) 1226 98 (N5 3) 176 7 (N5 14) IL-1b after LPS 876 23 (N5 10) 1676 124 (N 5 3) 1356 33 (N5 14) * Data expressed as me an6 SEM. CSD patients vs HS:aP, 0.05.
TNF-a . There was a signi® cant inhibition of TNF-a
secretion by dexamethasone from 102 8to 102 6M in he althy subje cts (P , 0.001) , in non-CSD patie nts (P, 0.001) , and in CSD patients (P , 0.001). While there was a signi® cant inhibition of TNF-a secretion by dexame thasone at 102 9M in he althy subje cts (P, 0.001) , no inhibition was observe d in non-CSD or CSD patie nts.
IL-6. The re was a signi® cant inhibition of IL-6
secretion by dexamethasone from 102 8to 102 6M in he althy subje cts (P, 0.001) and in non-CSD (P , 001) and in CSD patie nts (P, 0.001) . While there was a signi® cant inhibition of IL-6 secretion by de xa-methasone at 102 9M in he althy subje cts (P, 0.001) , no inhibition was obse rved in non-CSD or CSD pa-tie nts.
IL-1b . The re was a signi® cant inhibition of IL-1b
secretion by dexamethasone from 102 8to 102 6M in
he althy subje cts (P, 0.001) and in non-CSD (from
P, 0.01 to P , 0.001) and in CSD patients (from P ,
0.06 to P , 0.001) . While there was a signi® cant inhibition of IL-1b secretion by de xame thasone at 102 9M in he althy subje cts (P, 0.05), no inhibition was observe d in non-CSD or CSD patie nts.
Plasma Cortisol an d Cortisol-Bin din g Globu lin Levels in CD Patien ts an d Health y Subjects
Plasma cortisol (112.66 6.5 vs 120.6 6 12.2m g/dl, respective ly) and cortisol-binding globulin (28.16 1.3 vs 25 6 1.3 m g/ml, respe ctive ly) leve ls were similar in CD patie nts and healthy subje cts. Plasm a cor-tisol (120 6 10.1 vs 104.5 6 7.5 m g/dl, respe ctive ly) and cortisol-binding globulin (25.3 6 2.0 vs 31.3 6 2.8 m g/ml) were also similar in non-CSD and CSD patie nts.
Fig 1. Effect of de xame thasone on LPS-induced TNF-a secretion in whole blood cell cultures from 17 patients with Crohn’s disease (11 non-CSD and 6 CSD) and 14 HS. Statistical analyses are de scribed in Results. Data are e xpresse d as the mean 6 SEM: inhibition is pre sente d as mean pe rce nt base line (A, top pane l) or in mean of logarithmically transformed absolute cytokine levels (B, bottom pane l). HS: healthy subjects; CSD: corticosteroid-depende nt; Non-CSD: non-corticosteroid-depe nde nt. CD patients vs HS: *P, 0.05, **P, 0.01.
Fig 2. Effect of dexamethaso ne on LPS-induced IL-6 secretion in whole blood ce ll cultures from 19 CD patients (12 non-CSD and 7 CSD) and 14 he althy subjects. Statistical analyse s are describe d in Re sults. Data are expressed as the me an 6 SEM: inhibition is pre sente d as mean pe rce nt base line (A, top pane l) or in mean of logarithmically transformed absolute cytokine levels (B, bottom pane l). HS: healthy subjects; CSD: corticosteroid-depende nt; Non-CSD: non corticosteroid-dependent. CD patients vs HS: *P, 0.01, **P, 0.001.
Assessment of Corticosen sitivity in CD Patien ts an d Health y Su bjects
The top pane ls of Figure s 1± 3 show the mean pe rcentage of inhibition of cytokine production by de xame thasone in the three groups, taking into ac-count the O-dexamethasone conce ntration (cytokine leve l after LPS stimulation) , which is the reference for each individual (Figure s 1A, 2A, and 3A).
TNF-a . A signi® cant difference be tween all
pa-tie nts with Crohn’ s disease and controls was observe d in the gene ral pattern of TNF-a inhibition by de xa-methasone (P , 0.01). A signi® cant difference also was obse rved between all patie nts and controls in the rate of TNF-a inhibition by de xamethasone at 1 nM (P, 0.05), 10 nM (P , 0.01) and 100 nM (P , 0.05). There was a slight, but nonsigni® cant, difference at 1
m M of dexame thasone be tween all patie nts and con-trols (P, 0.052) (Figure 1A).
A difference was obse rved in the rate of TNF-a inhibition between non-CSD patie nts and controls by de xame thasone at 1 nM and 10 nM (P, 0.01). A similar difference was observe d in the rate of TNF-a inhibition between CSD patie nts and controls by de xame thasone at 1 nM and 10 nM (P , 0.01) and from 100 nM to 1 m M (P, 0.05) (Figure 1A).
IL-6. A signi® cant difference between all patie nts
and controls was observe d in the gene ral patte rn of IL-6 inhibition by de xame thasone (P , 0.001) . A signi® cant difference in IL-6 inhibition also was ob-served be tween all patie nts and controls at 1 nM (P, 0.01) and from 10 nM to 1 m M of de xame thasone (P, 0.001) (Figure 2A).
The re was a difference in the rate of IL-6 be tween non-CSD patie nts and controls by dexame thasone at 1 nM (P , 0.01) and from 10 nM to 1 m M (P , 0.001) . A difference also was obse rve d be tween CSD patie nts and controls by de xame thasone at 1 nM (P, 0.05) and from 10 nM to 1m M (P, 0.001) (Figure 2A).
IL-1b . A signi® cant difference be tween all patie nts
with Crohn’ s dise ase and controls was obse rved in the gene ral patte rn of IL-1b inhibition by de xame thasone (P , 0.01) . A difference between all patie nts and controls also was observe d in the rate of IL-1b inhi-bition by de xame thasone at 10 nM (P, 0.01) and at 100 nM (P, 0.05). There was a slight, but nonsignif-icant, difference at 1 nM between all patie nts and controls (P5 0.092) (Figure 3A).
A signi® cant difference in the rate of IL-1b inhibi-tion was obse rved be tween non-CSD patie nts and controls by de xame thasone at 10 nM (P , 0.01) . There was a signi® cant difference in the rate of IL-1b inhibition by dexame thasone be tween CSD patie nts and controls at 10 nM (P, 0.01) and at 1m M (P, 0.05) . The re was a slight, but nonsigni® cant, differ-ence at 1 nM of de xamethasone between non-CSD patie nts and CSD patie nts versus controls (P5 0.15 and P5 0.16, respectively) (Figure 3A).
Inte restingly, no signi® cant diffe rence be twee n non-CSD and CSD patie nts was obse rved in the de xa-methasone gene ral and dose effect on cytokine secre-tion (Figure s 1A, 2A, and 3A).
The IC50s of dexamethasone effects on the secre-tion of the three in¯ ammatory cytokine s are reporte d on Table 3. The re was a global signi® cance be tween patie nts with Crohn’ s disease and HS for TNF-a (P, 0.01) , IL-1b (P, 0.01), and IL-6 (P , 0.001) . There were no signi® cant difference s between non-CSD patie nts and CSD patie nts for each of the thre e cytokine s.
Fig 3. Effect of dexamethaso ne on LPS-induce d IL-1b secretion in whole blood ce ll cultures from 13 CD patients (10 non-CSD and 3-CSD) and 14 healthy subjects. Statistical analyse s are described in Results. Data are e xpresse d as the mean 6 SEM: inhibition is pre sente d as mean pe rce nt base line (A, top pane l) or in mean of logarithmically transformed absolute cytokine levels (B, bottom pane l). HS: healthy subjects; CSD: corticosteroid-depende nt; Non-CSD: non corticosteroid-dependent. CD patients vs HS: *P, 0.05, **P, 0.01.
The bottom pane ls of Figure s 1± 3 show the means of logarithmically transforme d absolute cytokine le v-els in the thre e groups. A shift to the right of the sigmoidal dose ± response curve of cytokine inhibition by dexamethasone was obse rved in Crohn’ s dise ase patie nts in comparison to healthy subje cts (Figure s 1B, 2B, and 3B).
The re was no signi® cant difference in the inhibition of TNF-a secretion by dexame thasone between CD patie nts and he althy subje cts or be tween the thre e groups (Figure 1B). The re was a signi® cant difference in the inhibition of IL-6 secretion by de xame thasone at 100 nM and 1 m M be tween CD patie nts and he althy subje cts (P, 0.01 and P , 0.01, respectively). A signi® cant difference in the inhibition of IL-6 se-cretion also was obse rved at 1m M of de xame thasone between non-CSD and he althy subje cts and be tween CSD patie nts and healthy subje cts (P, 0.01) (Figure 2B). The re was a signi® cant difference in the inhibi-tion of IL-1b secretion by dexame thasone at 100 nM and 1m M between CD patie nts and he althy subje cts (P, 0.01 and P , 0.01, respectively). A signi® cant difference also was obse rved at 100 nM and 1 m M between CSD patie nts and healthy subje cts (P, 0.05 and P, 0.01, respectively) (Figure 3B).
DISCUSSION
The corticose nsitivity of patie nts with Crohn’ s dis-ease and control subje cts was evaluate d in this study by the de gre e of inhibition of cytokine secretion in whole blood cell culture s by grade d conce ntrations of de xame thasone . The data sugge sted a signi® cant de -crease in the corticose nsitivity of patie nts with Crohn’ s dise ase . The re was no difference be tween CSD patie nts and non-CSD patie nts, howeve r. All of our patie nts in this study were in clinical remission (Crohn’ s disease activity inde x, 150) and corticoste-roid-fre e for at le ast six months. Pre vious studie s on
steroid-se nsitive and steroid-re sistant asthma were carried out in patie nts concurre ntly receiving glu-cocorticoid the rapy (15, 21, 22) . Glucocortic oids nor-mally induce a down-re gulation of GR, and this could lead to a decrease in corticose nsitivity. Thus, a study of corticose nsitivity in patie nts treate d with exoge -nous glucocorticoid s may re¯ ect treatme nt rathe r than disease effect. The comple te abstention from glucocorticoid s for at least six months in non-CSD patie nts and in CSD patie nts treate d with immuno-suppre ssants allowe d us to avoid this pote ntial pitfall. Inte rle ukin-1b , IL-6, and TNF-a are important cy-tokine s of innate immunity and mediate both speci® c and nonspe ci® c in¯ ammatory response s. These cyto-kine s are also important mediators of in¯ ammation in the inte stinal mucosa of patie nts with in¯ ammatory bowe l diseases. Enhance d secretion of such cytokine s by periphe ral blood mononucle ar cells (PBMCs) was reporte d in patie nts with Crohn’ s dise ase earlie r (23) . We did not con® rm this, but rathe r de monstrate d that the basal production of in¯ ammatory cytokine s ex
vivo was not different from that of controls and that
afte r LPS stimulation, the production of TNF-a and IL-6 was, in fact, decrease d in CSD patie nts com-pared to those of he althy subje cts. This could be explaine d by the different methodologie s use d and the type of subje ct populations studie d. All the CSD patie nts were treate d with immunosuppre ssants.
The de crease d PBMC corticose nsitivity of patie nts with Crohn’ s disease might be related to the in¯ am-matory state itse lf. Inde ed, corticose nsitivity was ear-lie r shown to be modulate d by cytokine s (24) . Some proin¯ ammatory cytokine s, such as IL-1b , IL-6, TNF-a and interferon-g (IFN-g ), increased (24 ± 26) , while othe r mostly antiin¯ ammatory cytokine s, such as IL-4 and IL-13, decreased sensitivity to glucocor-ticoids, possibly glucocorticoid receptor numbe r and af® nity change s (27, 28) . Glucocorticoids are potent inhibitors of NF-k B, a pivotal transcription factor for the expression of many cytokine ge nes in chronic in¯ ammatory dise ase s (29, 30) . NF-k B functions as an intrace llular ampli® cation factor that exace rbate s chronic in¯ ammatory proce sses and itse lf inhibits the activity of the ligand-bound glucocorticoid receptor (31) . Exce ssive NF-k B induction thus could pre vent glucocorticoid suppre ssion and could contribute to a de crease in corticose nsitivity (32) .
The susce ptibility to de ve lop an in¯ ammatory dis-ease could also be relate d to a gene tically and/or constitutionally de termined de crease in corticose nsi-tivity. Type 2 CSR asthma was shown to be related to a primary decrease of GR numbe r pe r cell (15) .
TABLE3. COMPARISON OFIC50OFDEXAMETHASONE INHIBITION ONTHREEPROINFLAMMATORY CYTOKINESBETWEEN
CORTICOSTEROID-DEPENDENT(CSD)ANDNON-CORTICOSTEROID -DEPENDENT(NON-CSD) PATIENTS WITHCROHN’SDISEASE AND
HEALTHYSUBJECTS* IC5 0 Non-CSD patients (102 9M) CSD patients (102 9M) Healthy subjects (102 9M) TNF-a 86 6.5a 6.36 2.3a 2.76 2.4 IL-6 546 138.4b 25.46 28.9a 2.96 2.6 IL-1b 12.26 17.8a 17.26 19.4a 2.76 2.3 * Data are expressed as me an6 SD.aP, 0.001;bP, 0.001 vs
Hypose nsitivity could le ad to an hype rimmune state and to susceptibility to in¯ ammatory and autoim-mune dise ase s (11, 12) . A primary decrease in corti-cose nsitivity could result in a relative inability of endoge nous glucocorticoids to modulate the mucosal immune response to bacte rial or alime ntary luminal antige ns, superantige ns, and nonspe ci® c immuno-stimulants. This study sugge sts that decrease d corti-cose nsitivity in patie nts with quie scent Crohn’ s dis-ease might be a factor favoring further relapse s.
A potential effect of the treatme nt with 5-ASA or azathioprine could not be exclude d. Whether such a treatment could directly in¯ ue nce corticose nsitivity is, howe ver, uncle ar. The patte rn and degree of de xa-methasone -mediate d inhibition were the same in qui-escent CSD and quie scent non-CSD patie nts. On one hand, this could mean that corticoste roid depe nde ncy in Crohn’ s disease is not relate d to a decrease of corticose nsitivity. On the othe r hand, the same corti-cose nsitivity in both groups might be due to the immunosupp ressants improving the corticose nsitivity of CSD patie nts with Crohn’ s disease (33) .
Recently, the activity of the HPA axis itse lf was shown to be alte red in in¯ ammatory diseases such as rhe umatoid arthritis (34) . Chikanza et al reporte d that rhe umatoid arthritis patie nts had normal cortisol leve ls and a normal response to exoge nous cortico-tropin rele asing factor but a reduce d response to increase d leve ls of circulating IL-1 and IL-6 induce d by surgical stress, compare d to patie nts with oste oar-thritis and chronic oste omye litis also unde rgoing a major operation (34) . Similarly, early untre ated pa-tients with rhe umatoid arthritis had paradoxically normal ACTH and cortisol le vels, which would not have been pre dicted by the ir pain, feve r, and high leve ls of in¯ ammatory cytokine s (35) . In our patie nts, hype rcortisolism could be associate d with a de crease in corticose nsitivity via homologous down-re gulation of the GR. The HPA axis of patie nts with Crohn’ s disease has not been studie d exte nsive ly as ye t; how-ever, our normal base line measure ments of cortisol and cortisol-binding globulin in this study do not corroborate the ide a of a major change in the activity of the HPA axis in Crohn’ s disease, ye t further studie s ne ed to be done to rule out subtle or dynamic change s of the ir axis.
In conclusion, a de crease of corticose nsitivity in whole blood cell culture s was obse rved in patie nts with Crohn’ s disease compared to he althy controls. The alte ration of corticose nsitivity in Crohn’ s dise ase could be ge ne tic/constitutio nal and/or acquire d in response to endocrine and/or immune factors relate d
to the in¯ ammatory dise ase . Furthe r studie s are ne ede d to characte rize the complex inte ractions be -tween the HPA axis and the immune syste m in in-¯ ammatory bowe l dise ase s.
ACKNOWLEDGMENTS
Statistical analyse s were performed by W. De we at the Division of Biostatistics of LieÁge University Me dical School (Pr. A. Albert). D. Franchimont is Re search Assistant of the Belgian National Foundation for Scienti® c Re search (NFSR) and is supported by the Leon Fredericq Founda-tion. E. Louis is supported by a grant of the NFSR. V . Gee nen is Senior Re search Associate of the NFSR.
REFERENCES
1. Chrousos GP: The hypothalamic± pituitary± adre nal axis and immune -mediate d in¯ ammation. N Engl J Me d 20:1351± 1362, 1995
2. Wilder RL: Neuroendocrine-immune syste m interactions and autoimmunity. Ann Re v Immunol 13:307± 338, 1995
3. Besedovsky HO , De l Rey A: Immune -neuro-endocrine inter-actions: Facts and hypothese s. Endocr Re v 17:64 ± 102, 1996 4. Sternbe rg E M, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ,
Gold PW, Wilder RL: In¯ ammatory me diator-induced hypo-thalamic± pituitary± adre nal axis activation is de fective in stre p-tococcal cell wall arthritis-susce ptible Lewis rats. Proc Natl Acad Sci USA 86:2374 ± 2378, 1989
5. Sternbe rg E M, Scott Young W, Bernardini R, Caloge ro AE, Chrousos GP, Gold PW, Wilder RL: A central nervous system defe ct in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci USA 86:4771± 4775, 1989
6. Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE : Glucocorticoid therapy for immune-mediated diseases: Basic and clinical correlate s. Ann Intern Me d 119:1198 ± 1208, 1993 7. Landi B, N’Guyen Anh T, Cortot A, Soule JC, Rene E , Ge ndre JP, Boiries P, See A, Metman EH, Flore nt C, Lere bours E, Mary JY, Modigliani R, GE TAID: Endoscopic monitoring of Crohn’s disease treatme nt: A prospe ctive, randomize d clinical trial. Gastroe nterology 102:1647± 1653, 1992
8. Modigliani R, Colombel JF, Dupas JL, Dapoigny M, Costil V, Ve yrac M, Duclos B, Soule JC, Gendre JP, Galmiche JP, Danne O, Guillaume C, Lamouliatte H, Belaiche J, Mary JY, GETAID: Mesalamine in Crohn’s disease with steroid-induced remission: Effect on steroid withdrawal and remission mainte -nance . Gastroe nterology 110:688 ± 693, 1996
9. Munkholm P, Langholz E, Davidsen M, Binder V: Freque ncy of glucocorticoid re sistance and depe nde ncy in Crohn’s dis-ease . Gut 35:360 ± 362, 1994
10. Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Ge ndre JP, Rene E, GETAID: Clinical, biological and endoscopic picture of attacks of Crohn’s disease . Gastroente rology 98:811± 818, 1990
11. Chrousos GP, Castro M, Leung DM, Webster E , Kino T, Bamberger C, Elliot S, Stratakis C, Karl M: Mole cular me ch-anisms of glucocorticoid re sistance and hypersensitivity. Am J Crit Care Me d 154:S39 ± S44, 1996
12. Bamberger CM, Schulte HM, Chrousos GP: Mole cular De ter-minants of glucocorticoid receptor. Function and tissue se nsi-tivity to glucocorticoids. Endocr Re v 17:245± 261, 1996 13. Chrousos GP, Dete ra S, Karl M: Syndroms of glucocorticoid
resistance . Ann Intern Med 19:113± 1124, 1993
14. Lamberts SWJ, Poldermans D, Zwee ns M, de Jong FH: Fa-milial cortisol re sistance : Differential diagnostic and therapeu-tic aspects. J Clin Endocrinol Me tab 63:1328 ± 1333, 1986 15. She r ER, Le ung DYM, Surs W, Kam JC, Z ieg G, Kamada AK,
Harbeck R, Sze ¯ e r SJ: Steroid re sistant asthma: Cellular me chanisms contributing to inadequate response to glucocor-ticoid therapy. J Clin Inve st 93:33± 39, 1994
16. Schlaghecke R, Korne ly E, Wollenhaupt J, Spe cker C: Glu-cocorticoid receptors in rheumatoid arthritis. Arthritis Rheum 35:740 ± 744, 1994
17. Schlaghecke R, Beusche r D, Kornely E, Spe cker C: Effects of glucocorticoids in rhe umatoid arthritis. Arthritis Rhe um 37:1127± 1131, 1994
18. Franchimont D, Louis E, Martens H, De Groote D, Be laiche J, Gee nen V: Effects of de xame thasone on the pro® le of cytokine se cre tion in human whole blood cell cultures. Re gul Pe pt 73:59 ± 65, 1998
19. Franchimont D, Louis E, Croes F, Belaiche J: Clinical pattern of corticosteroid-dependent Crohn’s disease . E ur J Gastroe n-terol He patol 10:821± 825, 1998
20. De Groote D, Z ange rle PF, Gevaert Y, Fassote MF, Be guin Y, Noizat-Pirenne F, Pire nne J, Gathy R, Lopez M, Dehart I, Igot D, Baudrihaye M, De lacroix D, Franchimont P: Direct stimu-lation of cytokine s in whole blood. Comparison with isolated PBMC stimulation. Cytokine 4:239 ± 248, 1992
21. Corrigan CJ, Brown PH, Barnes NC, Sze¯ e r SJ, Tsai JJ, Frew AJ, Kay AB: Glucocorticoid re sistance in chronic asthma. Am Re v Respir Dis 144:1016 ± 1025, 1991
22. Alvarez J, Surs W, Leung DYM, Ikle D, Gelfand E W, Sze¯ e r SJ: Steroid-resistant asthma: Immunologic and pharmacologic features. J Allergy Clin Immunol 89:714 ± 721, 1992
23. Mazlam MZ , Hodgson HJF: Pe ripheral blood monocyte cyto-kine production and acute phase response in in¯ ammatory bowel disease. Gut 33:773± 778, 1992
24. Costas M, Trapp Thorsen, Pere da MP, Saue r J, Rupprecht R, Nahmod VE, Re ul J MHM, Holsboer F, Artz E: Molecular and functional e vide nce for in vitro cytokine e nhance me nt of human and murine target cell se nsitivity to glucocorticoids. J Clin Invest 98:1409 ± 1416, 1996
25. Rakasz E, Gal A, Biro J, Balas G, Falus A: Modulation of glucocorticosteroid binding in human lymphoid, monocytoid and he patoma cell lines by in¯ ammatory cytokine s IL-1b , IL-6, and TNF-a . Scand J Immunol 37:684 ± 689, 1993
26. Salkowski CA, Vogel SN: IFNg mediates increase d glucocor-ticoid receptor e xpre ssion in murine macrophage s. J Immunol 148:2770 ± 2777, 1992
27. Kam JC, Sze ¯ er SJ, Surs W, Sher ER, Le ung DY: Combina-tion of IL-2 and IL-4 reduce s glucocorticoid re ce ptor-binding af® nity and T cell response to glucocorticoids. J Immunol 7:3460 ± 3466, 1993
28. Spahn DS, Sze ¯ er J, Surs W, Doherty DE, Leung DY: A novel action of interleukin-13; induction of diminished monocyte glucocorticoid receptor binding af® nity. J Immunol 157:2654 ± 2659, 1996
29. Ray A, Pre fontaine KE: Physical association and functional antagonism be tween the p65 subunit of transcription factor NFkB and the glucocorticoid re ce ptor. Proc Natl Acad Sci USA 91:752± 756, 1994
30. Auphan N, Didonato JA, Rosette C, He lmberg A, Karin M: 1995 Immunosuppre ssion by glucocorticoids: Inhibition of NF-kappaB activity through induction of I kappa B synthe sis. Science 270:283± 286, 1995
31. Barnes PJ, Karin M: Nucle ar Factor-kB: A pivotal transcrip-tion factor in chronic in¯ ammatory diseases. N Engl J Med 336:1066 ± 1071, 1997
32. Van der Burg B, Liden J, Okret S, De launay F, Wissink S, V an der Saag PT, Gustafsson JA: Nucle ar factor-kB re pression in anti-in¯ ammation and immunosuppre ssion by glucocorticoids. Tre nds Endocrinol Metab 8:152± 157, 1997
33. Pearson DC, May GR, Fick GH, Sutherland LR: Azathioprine and 6-mercaptopurine in Crohn’s disease . A meta-analysis. Ann Intern Med 122:132± 142, 1995
34. Chikanza IC, Petrou P, Kingsle y G, Chrousos GP, Panayi GS: Defe ctive hypothalamic re sponse to immune and in¯ ammatory stimuli in patients with rheumatoid arthritis. Arthritis Rheum 35:1281± 1288, 1992
35. Crofford LJ, Kaloge ras KT, Mastorakos G, Magiakou MA, Wells J, Kanik KS, Gold PW, Chrousos GP, Wilder RL: Circadian re lationships be tween interleukin 6 and hypothalam-ic± pituutary± adre nal axis hormones: Failure of IL-6 to cause sustained hype rcortisolism in patients with e arly untreate d rheumatoid arthritis. J Clin Endocrinol Me tab 82:1279 ± 1283, 1997