© Nikunj Gevariya, 2019
Effets des acides gras oméga-3 sur le cancer de la
prostate
Thèse
Nikunj Gevariya
Doctorat en biologie cellulaire et moléculaire
Philosophiæ doctor (Ph. D.)
Effets des acides gras oméga-3 sur le cancer de la
prostate
Thèse
Nikunj Gevariya
Sous la direction de:
Dr. Vincent Fradet, directeur de recherche
Dr. Alain Bergeron, co-directeur de recherche
iii
Résumé
Le cancer de la prostate (CaP) est le cancer le plus fréquemment diagnostiqué chez les hommes canadiens, avec 21 300 nouveaux cas et est la troisième cause de mortalité par cancer au Canada avec 4100 décès en 2017. Des études épidémiologiques ont montré que les populations ayant un régime riche en acides gras oméga (ω) 3 (e.g. les populations asiatiques côtières) ont une faible incidence de CaP alors que les populations des pays occidentaux ayant une diète riche en acides gras ω6 ont une incidence plus élevée (près de 60 fois) de CaP. Les graisses alimentaires influencent de nombreux processus biologiques, dont l'inflammation associée au développement et à la progression du CaP. Notamment, les acides gras ω6 à longues chaînes (LCω6) ont des propriétés pro-inflammatoires et pourraient contribuer à la progression du CaP. À l'inverse, les LCω3, tels que l'acide eicosapentaénoïque (EPA) et l'acide docosahexanénoïque (DHA), ont des propriétés anti-inflammatoires et pourraient inhiber la progression du CaP. J'ai donc émis l'hypothèse que les acides gras ω3 seraient bénéfiques contre la croissance et la progression du CaP principalement par leurs propriétés anti-inflammatoires.
En utilisant le modèle murin de CaP TRAMP-C2, j'ai constaté qu'un régime enrichi en acides gras ω3 réduisait la croissance du CaP par rapport à un régime enrichi en acide gras ω6 chez les souris non-dépourvues et dépourvues d'androgènes, en induisant une réponse immune antitumorale locale de type Th1-, Th2- et associée aux éosinophiles. Dans le but d’étudier plus en détails les effets de différents sous-types d’acides gras ω3, j’ai constaté que la supplémentation alimentaire en monoacylglycéride (MAG)-EPA réduisait la croissance tumorale en inhibant le facteur de croissance endothélial vasculaire (VEGF) et le gène du récepteur 2 de ce facteur de croissance (VEGFR2), ainsi que la taille des vaisseaux sanguins dans les tumeurs
iv
TRAMP-C2. J'ai également observé un effet similaire sur le système vasculaire tumoral des patients participant à un essai clinique visant à analyser les effets de la supplémentation en MAG-EPA sur le CaP avant une prostatectomie radicale. Enfin, dans le but d'analyser l'inflammation et de découvrir des biomarqueurs de la progression du CaP, j'ai analysé les effets d'une intervention alimentaire enrichie en acides gras ω3 versus un traitement avec un inhibiteur de la 5α-réductase (5ARI) sur des patients atteints de CaP de faible risque sous surveillance active. J'ai observé que 6 mois de régime alimentaire riche en acides gras ω3 ont entraîné une réduction des taux plasmatiques de cytokines pro-inflammatoires et pro-angiogeniques, ainsi que des cytokines liées à la réponse immunitaire Th2 et Th17. Afin d'évaluer les effets de ces interventions sur les protéines du tissu prostatique, j'ai effectué des dosages de cytokines et des analyses par spectrométrie de masse (MS) dans l'urine clarifiée obtenue après un toucher rectal (urine TR). Je n'ai pas réussi à identifier des variations significatives dans la teneur en protéines, mais j'ai conclu que l’urine TR non clarifiée, contenait deux fois plus de protéines que l'urine clarifiée, suggérant que ce type d’échantillon devrait être utilisé pour les prochaines analyses. Dans l'ensemble, mes résultats confirment l'importance des acides gras ω3 pour la prévention de la progression du CaP et appuient une étude plus approfondie de leurs effets sur la réponse immunitaire, l'angiogenèse et la progression tumorale chez les patients atteints de CaP.
v
Abstract
Prostate cancer (PCa) is the most frequently diagnosed cancer in Canadian men with 21,300 new cases and is the third-largest cause of cancer mortality with 4100 deaths in 2017. Epidemiological studies have shown that populations with a diet rich in omega (ω)-3 fatty acids (FA) (e.g. coastal Asian populations) have a low incidence of PCa while populations in Western countries with a diet rich in ω6 FA have higher (almost 60 times) incidence of PCa. Dietary fats influence many biological processes including inflammation which is associated with PCa development and progression. Notably, long-chain ω6 FA (LCω6) have pro-inflammatory properties and could contribute to PCa progression. On the opposite, LCω3 such as eicosapentaenoic acid (EPA) and docosahexanenoic acid (DHA) have anti-inflammatory properties and could inhibit PCa progression. I hypothesized that dietary ω3 FA are beneficial against PCa growth and progression mainly via their anti-inflammatory properties.
Using the TRAMP-C2 mouse model of PCa, I found that an ω3 FA-enriched diet reduced PCa growth compared to an ω6 FA-enriched diet in both androgen-deprived and non-deprived mice by inducing local antitumor Th1-, Th2- and eosinophil-related immune response. In the quest to further study the effects of individual ω3 FA subtype, I found that dietary supplementation with monoacylglyceride (MAG)-EPA reduced tumor growth by inhibiting vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor-2 gene (VEGFR2) expression as well as reducing the size of blood vessels in TRAMP-C2 tumors. I also observed that higher level of EPA reduced the size of blood vessels in prostate in a clinical trial testing MAG-EPA supplementation on PCa patients undergoing radical prostatectomy. Finally, I analyzed the effects of an ω3 FA-rich diet intervention versus treatment with a
5α-vi
reductase inhibitor (5ARI) on low-risk PCa patients under active surveillance. I found that 6 months of ω3 FA-rich diet intervention resulted in a reduction of plasma level of pro-inflammatory and pro-angiogenesis cytokines as well as Th2 and Th17 immune response-related cytokines. In order to assess the effects of these interventions on the prostate tissue, I used cytokines assay and mass spectrometry (MS) assay to analyze proteins in clarified urine obtained after digital rectal examination (DRE urine). I did not succeed at identifying significant variation in protein levels following the interventions in this sample type but I found that crude (non-clarified) DRE urine contained twice the amount of proteins compared to clarified urine, suggesting that this type of sample should be used for further analysis. Overall, my results confirmed the importance of ω3 FA for prevention of PCa progression and provide the rationale to further investigate their effects on immune response, tumor vasculature and tumor progression in PCa patients.
vii
Table of contents
Résumé….. ... iii
Abstract…. ... v
Table of contents ... vii
List of figures ... x
List of tables... xii
List of supplementary figures ... xiii
List of supplementary tables ... xiv
List of abbreviations ... xv Acknowledgements ... xxii Préface…… ... xxv Preface…… ... xxviii Chapter 1: Introduction ... 1 1.1 Prostate ...2
1.2 Prostate cancer epidemiology ...2
1.3 PCa diagnosis and risk stratification ...4
1.4 Role of androgens in PCa ...11
1.5 Link between cancer and inflammation ...15
1.6 PCa and inflammation ...21
1.7 ω3, ω6 and ω9 FA ...25
1.8 ω3 FA mechanisms of action: a brief overview ...30
1.9 ω3 FA and PCa ...32
1.10 TRAMP-C2 PCa mouse model ...34
1.11 Clinical challenges of low-risk PCa ...35
1.11.1 Active surveillance for management of low-risk PCa patients ...36
1.11.2 Biomarkers for PCa ...37
1.12 Hypothesis and objectives ...40
Chapter 2: Omega-3 fatty acids decrease prostate cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice ... 41
2.1 Preface ...42
2.2 Résumé ...43
2.3 Abstract ...45
2.4 Introduction ...46
2.5 Materials and Methods ...48
2.5.1 Diets ...48
2.5.2 Cell culture experiments ...48
2.5.3 Mice experiments ...49
2.5.4 FA profiling ...50
2.5.5 Multiplex immunoassay ...51
2.5.6 Flow cytometry analyses ...51
viii
2.6 Results ...53
2.6.1 ω3 FA reduce TRAMP-C2 cell proliferation in vitro independently of androgen levels ...53
2.6.2 Dietary ω3 FA reduce TRAMP-C2 tumor growth in eugonadal and castrated mice ...54
2.6.3 RBCs and TRAMP-C2 tumors incorporate dietary FA ...54
2.6.4 Inflammatory cytokine levels in plasma are unaffected by dietary FA ...55
2.6.5 Dietary ω3 FA modulate cytokines in TRAMP-C2 tumors ...55
2.6.6 Eotaxin-1 secretion by tumor cells is induced by ω3 FA and is regulated by androgens ...56
2.6.7 Dietary ω3 FA marginally modulate eosinophils and immunosuppressive cell infiltration in tumors ...57
2.7 Discussion ...58
2.8 Author contributions ...62
2.9 Acknowledgments ...62
2.10 Figures ...63
2.11 Supplementary figures and table ...70
Chapter 3: Eicosapentanoic acid monoacylglyceride supplementation inhibits prostate cancer by modulating tumor vasculature ... 79
3.1 Preface ...80
3.1 Résume ...81
3.2 Abstract ...83
3.3 Introduction ...85
3.4 Materials and Methods ...87
3.4.1 Cell culture experiments ...87
3.4.2 Mice experiment ...87
3.4.3 FA profiling ...88
3.4.4 Multiplex immunoassays ...88
3.4.5 Gene expression profiling by RNAseq ...89
3.4.6 Gene expression validation by qPCR ...90
3.4.7 Immunohistochemistry (IHC) ...90
3.5 Results ...93
3.5.1 MAG-EPA reduce TRAMP-C2 cell proliferation in vitro and TRAMP-C2 tumor growth in vivo. ...93
3.5.2 FA supplementation modifies the FA profiles in RBCs and TRAMP-C2 tumors ...93
3.5.3 Inflammatory cytokine levels are mostly unaffected by dietary FA supplements ...94
3.5.4 MAG-EPA supplement modulate vascular-related genes in TRAMP-C2 tumors ...95
3.5.5 MAG-EPA supplements reduce vascular-related genes, VEGF level as well as vasculature phenotype in TRAMP-C2 tumors ...96
3.5.6 MAG-EPA supplementation modulates the prostate vasculature in men with PCa ...96
3.6 Discussion ...98
3.7 Acknowledgements ...101
3.8 Figures and Tables ...102
3.9 Supplementary figures and tables ...110
Chapter 4: Analysis of inflammation and discovery of urinary PCa progression biomarkers using samples from low-risk PCa patients under active surveillance participating to a clinical trial comparing ω3 fatty acid-rich diet intervention versus a 5α-reductase inhibitor treatment. ... 116
4.1 Preface ...117
4.2 Introduction ...118
4.3 Materials and Methods ...120
ix
4.3.2 Analysis of cytokines in plasma ...124
4.3.3 Analysis of cytokines in urine ...124
4.3.4 Selection of optimal parameters for MS-based proteomics assay ...125
4.3.5 Analysis of crude DRE-urine ...125
4.3.6 Shotgun MS run data analysis ...126
4.4 Results and Discussion ...127
4.4.1 Effect of ω3 FA-rich diet versus 5ARI on plasma cytokines ...127
4.4.2 Effect of ω3 FA-rich diet versus 5ARI on urinary proteins ...132
4.4.2.1 BioPlex cytokine assay to measure cytokine level in urine: testing the performance of the assay….. ...132
4.4.2.2 MS assay for urine samples: selection of optimal parameters ...135
4.4.3 Analysis of DRE urine compared to pre-DRE urine ...137
4.4.4 Discovery of biomarkers for PCa progression in DRE urine ...142
4.4.5 Alternative strategy for discovery of biomarkers in DRE urine for PCa ...144
4.5 Conclusion, limitation and future directions ...146
Chapter 5: Discussion ... 148
5.1 Immunocompetent mouse models to evaluate the effects of ω3 FA ...149
5.2 Dose of ω3 FA from the ω3 FA-enriched diet and -supplements for PCa ...151
5.3 Mechanisms of action behind the effects of ω3 FA ...154
5.3.1 Effect of ω3 FA on immune cell response in PCa ...154
5.3.2 Effect of ω3 FA on AR pathways in PCa ...158
5.3.3 Effect of ω3 FA on vasculature in PCa ...159
5.3.4 Effect of ω3 FA on gut microbiota and PCa ...161
5.3.5 Effect of ω3 FA on inflammatory metabolites in PCa ...162
5.3.6 Effect of ω3 FA on oxidative stress metabolism in PCa ...163
5.4 Discovery of PCa progression biomarkers in DRE urine: the challenge continues ...165
5.5 General conclusion ...167
Annexes….. ... 168
Annex 1: Cytokine profiles in plasma of low-risk PCa patients under active surveillance randomized to either ω3 FA-rich diet or 5ARI treatment. ...169
Annex 2: Cytokine profile in plasma of radical prostatectomy patients randomized to either MAG-EPA or placebo. ...170
Annex 3. Effect of ω3 FA-enriched diet on enzymatically-derived eicosanoids in androgen-sensitive and -resistant TRAMP-C2 tumors. ...171
Annex 4. Effects of ω3 FA-enriched diet on oxidative-stress derived eicosanoids in androgen-sensitive and -resistant TRAMP-C2 tumors. ...172
Annex 5: Omega-3 fatty acids and high-grade prostate cancer in men under active surveillance ...173
x
List of figures
Figure 1.1: Location of the prostate in the man’s body. ... 2
Figure 1.2: Worldwide incidence of PCa. ... 3
Figure 1.3: Current screening and diagnosis methods for PCa. ... 4
Figure 1.4: Gleason grading for PCa. ... 6
Figure 1.5: Diagram depicting a 12-core needle biopsy of the prostate. ... 7
Figure 1.6: Stages of PCa. ... 10
Figure 1.7: Action of androgens. ... 13
Figure 1.8: Cancer hallmarks and enabling characteristics. ... 16
Figure 1.9: Cancer immunoediting: the 3E hypothesis. ... 17
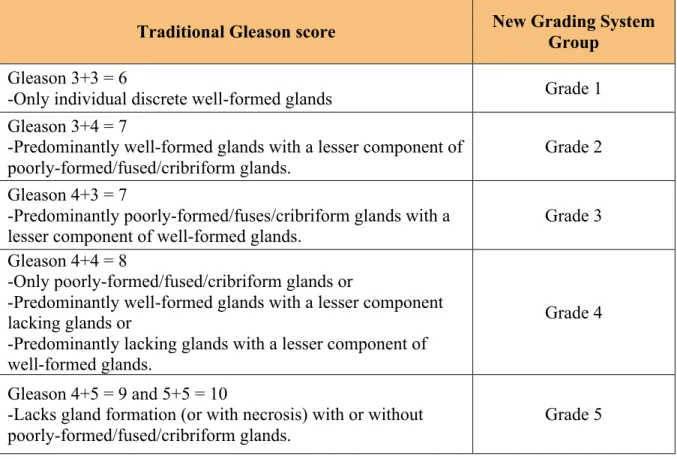
Figure 1.10: Possible causes for inflammation in the prostate. ... 21
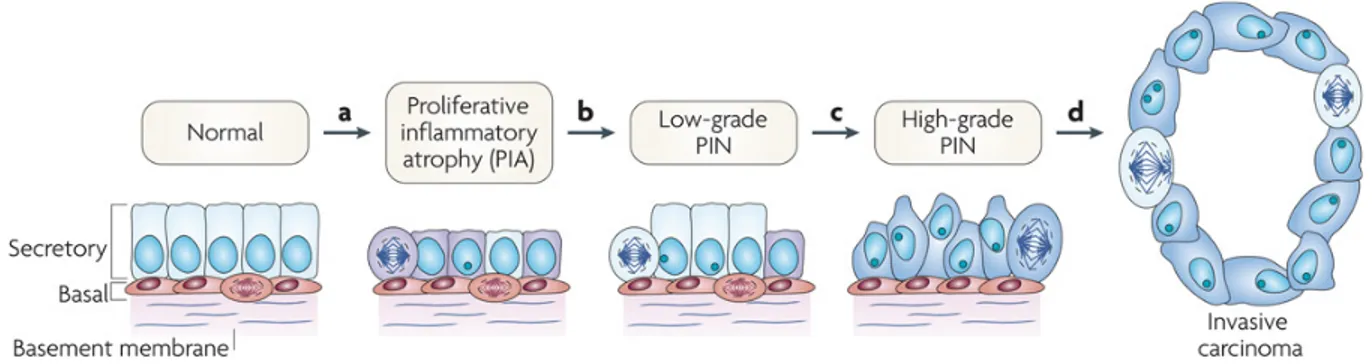
Figure 1.11: Cellular and molecular model of early prostate neoplasia progression. ... 22
Figure 1.12: Chemical structures of key ω3, ω6 and ω9 FA. ... 25
Figure 1.13: Metabolism of ω3 and ω6 FA. ... 28
Figure 1.14: History of human FA (ω6, ω3, trans and total) intake. ... 29
Figure 1.15: FA mechanisms of actions. ... 31
Figure 1.16: DRE urine collected after DRE examination. ... 38
Figure 2.1: Effect of ω3 FA and ω6 FA as well as androgen on TRAMP-C2 tumor cells. ... 63
Figure 2.2: Effect of ω3 FA- versus ω6 FA-enriched diet on TRAMP-C2 tumor growth in eugonadal mice. ... 64
Figure 2.3: Effect of ω3 FA- versus ω6 FA-enriched diet on TRAMP-C2 tumor growth in castrated mice. ... 65
Figure 2.4: Fatty acid (FA) profiles in red blood cells (RBC) and tumors. ... 66
Figure 2.5: Cytokine expression in tumors. ... 67
Figure 2.6: Eotaxin-1 regulation in TRAMP-C2 tumor cells. ... 68
Figure 2.7: Infiltration of immune cells in tumors of eugonadal mice. ... 69
Figure 3.1: Effects of purified MAG-FA supplements on TRAMP-C2 tumor cells in vitro and flowchart of the experiment in mice. ... 102
Figure 3.2: Effects of purified FA supplements on TRAMP-C2 tumor growth and survival in mice. ... 103
Figure 3.3: FA profiles in RBC and tumors. ... 104
Figure 3.4: Principle component analysis (PCA) of gene expression. ... 105
Figure 3.5: Venn diagram of differentially expressed genes in tumors of MAG-EPA supplemented mice. ... 107
Figure 3.6: Validation of the effects of MAG-EPA on the vasculature in TRAMP-C2 tumors. 108 Figure 3.7: Effects of MAG-EPA or placebo on the vasculature of the prostate of men with PCa. ... 109
Figure 4.1: Flowchart of the phase IIb clinical trial. ... 121
Figure 4.2: Selection of plasma samples for analysis. ... 123
Figure 4.3: Selection of DRE urine samples for analysis. ... 123
Figure 4.4: Effect of ω3 FA-rich diet and 5ARI on plasma cytokines after a 6-month intervention. ... 128
Figure 4.5: Effect of urine pH on cytokine measurements. ... 134
xi
Figure 4.7: Number of proteins detected in pre-DRE and DRE urine samples. ... 138
Figure 4.8: Volcano plot for level of proteins in DRE urine compared pre-DRE urine. ... 139
Figure 4.9: Venn diagram representing common protein hits found in Principe et al. study and our experiment. ... 140
Figure 4.10: Volcano plot of P-value versus fold change. ... 143
Figure 4.11: Evaluation of crude versus clarified DRE urine by shotgun MS assay. ... 145
xii
List of tables
Table 1.1. The new ISUP 2014 PCa Grading System. ... 8
Table 1.2. TNM system for PCa staging. ... 9
Table 3.1: Number of genes with differential expression in tumors of specific FA-supplemented mice. (Complete list of gene is in Table S3.2) ... 106
Table 3.2: Gene enrichment analysis of differentially expressed genes in MAG-EPA supplemented mice. ... 106
Table 4.1: List of studies evaluating the effects of dietary ω3 FA on plasma cytokines in humans. ... 129
Table 4.2: Variation in pH of urine samples collected from 10 different men. ... 133
Table 4.3: Enrichment of DRE urine with prostatic fluid compared to pre-DRE urine. ... 139
xiii
List of supplementary figures
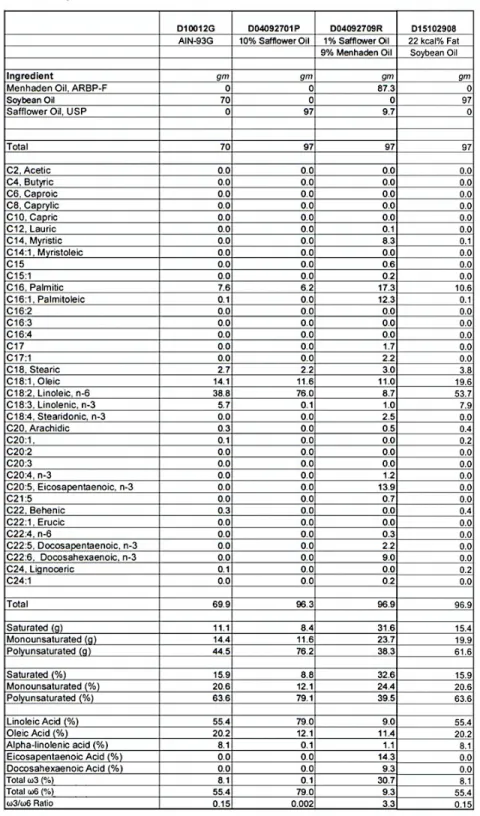
Figure S2.1: Weight of mice fed with FA-enriched diets over time. ... 71
Figure S2.2: Cytokine profiles in the plasma of eugonadal mice. ... 72
Figure S2.3: Cytokine profiles in the plasma of castrated mice. ... 73
Figure S2.4: Immune cell infiltration in tumors of eugonadal mice. ... 74
Figure S2.5: Immune cell infiltration in tumors of castrated mice. ... 75
Figure S2.6: Complete tumor cytokine profile in tumors of eugonadal mice. ... 76
Figure S2.7: Complete tumor cytokine profiles in tumors of castrated mice. ... 77
Figure S2.8: Immune cell infiltration in eugonadal versus castrated mouse tumors. ... 78
Figure S3.1: Cytokine profiles in the plasma of mice supplemented with purified FA. ... 114 Figure S3.2: Cytokine profile in TRAMP-C2 tumors of mice supplemented with purified FA. 115
xiv
List of supplementary tables
Table S2.1: Composition and fatty acid profile for control balanced, ω3 FA- and ω6 FA-enriched diets. ... 70 Table S3.1: Composition and FA content of low-fat diet (LFD). ... 110 Table S3.2: Differentially expressed genes in tumors of MAG-EPA supplemented mice. ... 111
xv
List of abbreviations
5ARI 5 alpha-reductase inhibitor(s)
AA Arachidonic acid
ADT Androgen deprivation therapy
ALA Alpha-linoleic acid
AMACR Alpha-methylacyl-coenzyme A-racemase
AR Androgen receptor
ARE Androgen response element
ARR2PB Two-component androgen responsive region of probain promoter
ATCC American type culture collection
bFGF Basic fibroblast growth factor
BPH Benign prostatic hyperplasia
cAMP cyclic adenosine monophosphate
CCL Chemokine (C-C motif) ligand
CCR4 CC chemokine receptor 4
CD Cluster of differentiation
CDU Collagenase digestive units
COX Cyclooxygenase
CPa Cancer de la prostate
CRPC Castration resistant prostate cancer
CSF Colony stimulating factor
CT scan Computed tomography scan
DHA Docosahexaenoic acid
DHT Dihydrotestosterone
DMEM Dulbecco's modified eagle medium
DMSO Dimethyl sulfoxide
DOC Deoxycholate
DRE Digital rectal examination
xvi
EDTA Ethylenediaminetetraacetic acid
EGF Epidermal growth factor
EP G-protein-coupled prostaglandin E2 receptor
EPA Eicosapentaenoic acid
FA Fatty acid(s)
FBS Fetal bovine serum
FDA Food and drug administration of United States
FFQ Food frequency questionnaire
FO Fish oil
G-CSF Granulocyte-colony stimulating factor
GC-MS Gas chromatography-mass spectrometry
GM-CSF Granulocyte-macrophage colony-stimulating factor
GnRH Gonadotropin releasing hormone
GO Gene ontology
GPR120 G-protein coupled receptor 120
GST Glutathione S-transferase
HBSS Hank's balanced salt solution
HDHA Hydroxy-docosahexaenoic acid
HEPA Hydroxyl-eicosapentaenoic acid
HOSO High oleic sunflower oil
IFN Interferon
IHC Immunohistochemistry
IL Interleukin
IP-10 Interferon gamma-induced protein 10
isoP Isoprostane(s)
ISUP International society of urological pathology
IkBα Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor- alpha
KLK Kallikrein
LA Linoleic acid
xvii
LFQ Label free quantification
LH luteinizing hormone
LHRH Luteinizing hormone-releasing hormone
LNCaP Lymph node carcinoma of the prostate cell line
LOX Lipoxygenase(s)
LT Leukotriene(s)
M1 Macrophage 1
M2 Macrophage 2
MAG Monoacylglyceride
MCP Monocyte chemoattractant protein
MDSC Myeloid-derived suppressor cell(s)
mi-R Micro-RNA
MIC1 Macrophage inhibitory cytokine 1
MIP Macrophage inflammatory protein
MMP Matrix metalloproteinase(s)
MRI Magnetic resonance imaging
MRM Multiple reaction monitoring
MS Mass spectrometry
MUFA Monounsaturated fatty acid(s)
MYC Myelocytomatosis
NCCN National comprehensive cancer network
NFkB Nuclear factor kappa-light-chain-enhancer of activated B cells
NO Nitric oxide
NOS Nitric oxide synthase
NSAID Non-steroidal anti-inflammatory drug(s)
OA Oleic acid
OCT Optimal cutting temperature compound
p53 Tumor protein p53
PAMP Pathogen-associated molecular pattern(s)
PC3 Human prostatic carcinoma cell line 3
xviii
PCA3 Prostate cancer antigen 3
PCPT Prostate Cancer Prevention Trial
PDGF Platelet-derived growth factor
PG Prostaglandin(s)
PGE Prostaglandin E
PGF Prostaglandin F
PGI Prostaglandin I
PhiP 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine
PIA Proliferative inflammatory atrophy
PIN Intraepithelial neoplasia
PPARg Peroxisome proliferator-activated receptor gamma
pRb Retinoblastoma protein
PSA Prostate specific antigen
PSA-TRICOM Phase II randomized controlled trial of a poxviral-based prostate specific
antigen (PSA)-targeted vaccine
PSMA Prostate-specific membrane antigen
PTEN Phosphatase and tensin homolog
PUFA Polyunsaturated fatty acid(s)
qPCR Quantitative polymerase chain reaction
RANTES Regulated on activation, normal T cell expressed and secreted
RBC Red blood cells
REDEEM trial Reduction by dutasteride of clinical progression events in expectant
management trial
REDUCE trial Reduction by dutasteride of prostate cancer events trial
RNA Ribonucleic acid
ROS Reactive oxygen species
rPB Rat probasin promoter
RPMI Roswell park memorial institute medium
rs Spearman correlation
RV Resolvins
xix
SR1001 Small molecule inhibitor of Th17 cells
SV40-Tag Simian virus 40 large T-antigen
TAM Tumor-associated macrophage
Th1 T helper 1 cell(s)
Th2 T helper 2 cell(s)
TLR Toll-like receptors
TMA Tissue microarrays
TMPRSS2:ERG Transmembrane protease serine 2: v-ets erythroblastosis virus E26
oncogene homolog
TMPRSS2:ETS Transmembrane protease serine 2: E twenty-six transcription factors
TNF Tumor necrosis factor
Tr1 Type 1 regulatory cell(s)
Tr2 Type 2 regulatory cell(s)
TRAMP Transgenic adenocarcinoma of the mouse prostate
Treg Regulatory T cell(s)
TRUS Trans rectal ultrasound
TX Thromboxane(s)
VEGF Vascular endothelial growth factor
VEGFR-2/KDR Vascular endothelial growth factor receptor-2
ω3 Omega-3
ω6 Omega-6
xx
Dedicated to humanity and its future
To me, my thesis is a snapshot of the current knowledge available to humankind and
experimenting with it to add a new dot in this exciting journey we share on the only home so far ‘the earth!’. I stand on all the advancements made by everyone ever lived here, my purpose of this work was to advance our continuous expedition.
xxi
When something is important enough, you do it even if the odds are not in your favor. -Elon Musk
You can’t connect the dots looking forward; you can only connect them looking backwards. -Steve Jobs
xxii
Acknowledgements
This journey to pursue Ph.D. for the last 6 years has been life-changing. All my accomplishments during this journey wouldn’t have been possible without support from colleagues, friends and family members.
I would like to first thank my Ph.D. supervisor Dr. Vincent Fradet for giving me an opportunity to join the lab. I appreciate the chances given by him to explore new techniques, let me attend workshops and present scientific work at many national and international meetings and conferences. I admire his desire to do divers scientific work as well as friendly personality, although his availability remained limited during my Ph.D.
I want to convey immense appreciation to my co-director, Dr. Alain Bergeron. A person who has always been there for help and direct me to a right path. True breath that you bring to the whole team is quite astonishing. You have always been encouraging person during all my stay in the lab. Sincerely, without your support, I would not have been able to achieve this milestone. I will remember all the late-night drop-by talks and attending a scientific meeting with you in Toronto! I would like to direct a very special thanks to Dr. Karine Robitaille. I truly admire your sound mind in the projects. I really appreciate your high standards for figures and data. I love your creative mind as well as a wonderful understanding personality. Thank you for being very kind and supportive in my ups and downs. I must say that it was quite an emotional journey with you for the last three years. “Wow, this is cool results… you are going to be famous” (with your dancing moves!) these cheerful words are in my memory forever!
I also thank Dr. Yves Fradet. It has been my true honor to be part of the team that you have passionately built in the last 35 years. Your enthusiasm as well as collaborative mind is amazing. Thank you for being such a wonderful mentor.
I also thank Dr. Caroline Léger for guidance and support. I had a very good time scientifically and personally during her stay in the laboratory.
Special thanks to all the present and past students with whom I shared a wonderful time in the lab specifically Lisanne Beaudoin, Sébastien Le Batteux, Clovis Boibessot, Lamoussa Diabaté, Audrey Champagne, Pallavi Jain, Hanane Moussa, Oscar Eduardo Molina, Janie Allaire, Marjorie Besançon, Lauriane Velot, Siham Berra and many more.
I thank all current and past members of the lab who directly or indirectly helped to complete my Ph.D. Valérie Picard, thanks for teaching me different techniques and providing all technical support whenever I needed. Dr. Laurence Bettan, your corridor talks and cheerful laughs were lovely, you are an amazing person. Dr. Moliere Nguile Makao, thanks for cracking many French jokes (and its humble translation!) as well as helping me out with statistics whenever I needed. Jean-Francois Pelletier, thanks for your support especially with bio-banking samples for my experiments, which would not have been possible without it. I also express my sincere gratitude to Denise St-Onge, Dr. Bertrand Neveu, Claire Ménard, Vanessa Bussières, Dr. Mélanie
xxiii
Rouleau, Helene Hovington, Hervé Brisson, Benjamin Vittrant, Dr. Fanny Gaignier, France-Helene Joncas, Dr. Marie-Hélène Guertin, Dr. Stéphanie Bégin, Dr. Xavier Moreel and Marilyn Savard.
Thanks to uro-oncologists of the team for their support in many instances. Dr. Louis Lacombe, Dr. Frédéric Pouliot, Dr. Paul Toren, Dr. Michele Lodde.
I also thank collaborators and co-authors: Dr. Gabriel Lachance, Florence Roux-Dalvai, Dr. Frédéric Fournier, Dr. Pierre Julien, Dr. Mickael Leclercq, Dr. Charles Joly-Beauparlant, Dr. Samuel Fortin, Dr. Jean-François Bilodeau, Dr. André Marette, Dr. Nicolas Bisson, Dr. Steve Bilodeau and Dr. Arnaud Droit.
Apart from lab life, I had a true privilege of being surrounded by amazing friends. Niraj, my life in Quebec would not have been the same without meeting my bro like you. We had lots of wonderful memories and lot more yet to come in life. A very special thanks to my friend Emilie. I am truly blessed to have wonderful and creative person like you in my life. Lillian Lee, my pal “such a beautiful life we have!” Our friendship lives up to this sentence. You are a truly gifted human. Seb, thanks for not just sharing desk side by side in the lab but being supporting every time in person and professional front. We have countless memories from crazy lab life, amazing sci-fi talks as well as joyful camel and motorbike rides in India. David Auclair, you are truly passionate individual, I thank you for wonderful time together. Supreet Wantamutte, thank you for sharing many funny and enjoying moments during your stay in Quebec.
I thank my friends Praveena, Chandu, Surya, Asmita, Sidharth, Manasa, Hemanta and Anandita all of your friendship means a lot to me and I have no words to express the magnitude of the same. This beautiful journey would have been different and difficult without your company. Thanks for countless joyful moments at dinners, parties, game nights that are permanently imprinted in my memories.
I am delighted to share lifetime memories with Jean-Clément Mars, Justin Sitz, Salar Ahmad, Maë Va, Anthony Couturier, Vero, Chelsea Shannon, Gabriel Bossé, and Sara Banerjee, Maria Zuzu, Ramesh, Sai and many other friends with whom I had a wonderful time.
Anil, my tech buddy and passionate human being & Deepika, a beautiful hearted person, meeting with you was like triumph. Also, Ela and Jia, and late Dr. Purshotam Joshi thank you for a wonderful time together.
Amita, Praful, Simal, Milly and Raj and all Manek family, Dr. Girish Shah, Rashmi Shah, Jay and Dipti Manek, Preetesh and Mulji Kaka & heavenly Joti aunty with all of you around I found my home here far from India. You all remind me ≪Jya Jya Vase Gujarati, Tya Tya Sadakal Gujarat ≫. I am immensely thankful for the time we spent together.
My parents Rasikbhai, Kantaben and grandparents Gordhanbhai and Parvatiben. Thank you for fostering love, trust and vision in me. My beloved sisters Sudhaben, Sumitaben, Sangita and Ranjan, you all are my treasure of life. I can proudly say that the person who I am today is because of you and your constant support and unconditional love. I also thank Shaileshjiju,
xxiv
Vipuljiju, Pradipjiju, Krishnajiju and my nephews Dhrumil, Dasrhit, Shlok, Manav, Hetansh, Kalp, Krishiv and niece Ishani for the love.
Countless thanks to my Purohit parents: Rohini & Kaushik Purohit for great support, love and encouragement. I am also thankful to Meet as well as all Purohit Family for kind love.
My highest thanks to my beloved wife, Nupur for always being supportive throughout the challenging times. You have always pushed me to go further to achieve my goals. You make my life lively and cheerful, a one that is worth living! Words will never be enough to express just how thankful I am.
Also, I would like to thank organizations CHU de Québec – Université Laval, Prostate Cancer Canada, MITACS as well as Canadian Urology Association (CUA). Their funding and scholarship have contributed to my graduate studies and work of this thesis.
Finally, Thanks to the Jury of this thesis, composed of Dr. David Labbe, Dr. Caroline Diorio, Dr. Dimcho Bachvarov, Dr. Alain Bergeron and Dr. Vincent Fradet for having accepted to review my thesis.
xxv
Préface
Les découvertes scientifiques présentées dans cette thèse sont le fruit de six années de travail en tant qu'étudiant au doctorat dans l'équipe de recherche du Dr Vincent Fradet au Laboratoire d'Uro-Oncologie Expérimentale (LUOE). Au cours de ma thèse, j'ai étudié les effets des acides gras ω3 sur le CaP dans le cadre de projets de recherche précliniques et cliniques. Ci-dessous, j’introduis brièvement le contenu de chaque chapitre ainsi que ma contribution aux différents projets de la thèse. Plus de détails sont fournis dans la préface de chacun des chapitres.
Le chapitre 1 présente une introduction portant sur le CaP ainsi que sur les défis
cliniques associés au CaP développés dans les chapitres subséquents. Il présente également l’état des connaissances actuelles sur le lien entre l'inflammation et le cancer en général, ainsi que plus spécifiquement entre l’inflammation et le CaP. Il comprend également une introduction sur les acides gras ω3 et ω6 et leur importance dans plusieurs mécanismes physiologiques, notamment l'inflammation. Enfin, ce chapitre présente une brève introduction sur le modèle de CaP murin TRAMP-C2 utilisé dans les expériences des chapitres 2 et 3, ainsi que les défis cliniques du CaP à faible risque rencontrés dans le cadre de l'essai clinique de phase IIb présenté au chapitre 4.
Le chapitre 2 est le premier article dont je suis premier auteur qui s’intitule « Omega-3
fatty acids decrease prostate cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice », publié dans la revue The Prostate en 2018. Cet article présente les effets de diètes enrichies en acides gras ω3 et ω6 sur les tumeurs TRAMP-C2 chez les souris eugonadiques (non castrées) et castrées. J'ai fait la majeure partie du travail et j'ai écrit le manuscrit qui a été édité par les autres co-auteurs.
xxvi
Le chapitre 3 est le second manuscrit dont je suis premier auteur, intitulé «
Eicosapentanoic acid monoacylglyceride supplementation inhibits prostate cancer by modulating tumor vasculature ». Cet article présente les résultats des effets d’une supplémentation en acides gras ω3 purifiés tels que l’acide eicosapentaénoïque, l’acide docosapentaénoïque et les acides gras ω6 tel que l’acide arachidonique sur les tumeurs TRAMP-C2. J'ai fait la majorité des analyses et j'ai écrit le manuscrit qui a ensuite été édité par les autres co-auteurs.
Le chapitre 4 est un projet de recherche en cours visant à évaluer les effets des acides
gras ω3 provenant de l’alimentation sur l'inflammation, chez des patients atteints de CaP. Pour les expériences de ce projet, j'ai utilisé des échantillons issus d'un essai clinique de phase IIb effectué chez des patients atteints d'un CaP à faible risque sous surveillance active, comparant une alimentation riche en acides gras ω3 à une intervention avec un inhibiteur de la 5α-réductase. Je présente également les résultats pour découvrir des biomarqueurs non-invasifs de la progression du CaP dans l'urine recueillie après un toucher rectal. J'ai généré tous les résultats présentés et écrit ce chapitre en format classique (pas sous forme de manuscrit) avec la contribution critique des co-auteurs.
Le chapitre 5 est la discussion finale intégrant et discutant de façon globale les résultats
présentés dans les chapitres 2, 3 et 4. J'ai aussi donné quelques points forts des expériences en cours présentées dans les annexes. Enfin, j'ai inclus une conclusion générale et les orientations futures pour le travail suite à cette thèse.
La thèse contient également une série d'annexes. Celles-ci présentent les résultats de certaines expériences qui ne sont pas présentés dans les chapitres principaux de la thèse. J'ai également inclus une copie du manuscrit intitulé « Moussa et al. Omega-3 fatty acids and risk of prostate
xxvii
cancer progression during active surveillance » présentant des résultats de l'essai clinique lié au chapitre 4, auquel j'ai contribué en partie, en participant activement à la construction de la biobanque, en gérant les bases de données et en optimisant les protocoles pendant la première année et demi de mon doctorat. Le premier auteur, Hanane Moussa, est une étudiante au Ph.D. dans mon équipe de recherche.
xxviii
Preface
The scientific findings presented in my thesis are the results of six years of work as a graduate student in the research team of Dr. Vincent Fradet at the Laboratoire d'Uro-Oncologie Expérimentale (LUOE). During my Ph.D., I have evaluated the effects of ω3 FA on PCa in preclinical and clinical research projects. Below I briefly mention the content of each Chapter and my contribution to the projects. More details are provided in the preface of each chapter.
Chapter 1 provides an introduction about PCa and its clinical challenges in the context
of the next chapters. It also presents current understanding of the link between inflammation and cancer in general and in particular with PCa. It also includes an introduction on ω3 and ω6 FA and their importance in several physiological mechanisms and especially in inflammation. A brief introduction on the TRAMP-C2 PCa mouse model that was used in experiments of Chapters 2 and 3 as well as on the clinical challenge of low-risk PCa in the context of a phase IIb clinical trial described in Chapter 4 is also presented.
Chapter 2 is my first author article entitled “Omega-3 fatty acids decrease prostate
cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice” that has been published in the journal The Prostate in 2018. This article presents the effects of ω3 FA- and ω6 FA-enriched diets on TRAMP-C2 tumors in eugonadal and castrated mice. I did the major part of the work and I wrote the manuscript which was edited by the other co-authors.
Chapter 3 is my second first author manuscript entitled “Eicosapentanoic acid
xxix
intend to publish in a peer-reviewed journal. This article presents the effects of individual purified ω3 FA subtype such as eicosapentaenoic acid, docosahexaenoic acid and ω6 FA such as arachidonic acid on TRAMP-C2 tumors. I did most of the analyses and wrote the manuscript which was later edited by the other co-authors.
Chapter 4 is an ongoing research project evaluating the effects of dietary ω3 FA on
inflammation in PCa patients. In this project I have used samples from a phase IIb clinical trial comparing ω3 FA-rich diet versus 5α-reductase inhibitor intervention in low-risk PCa patients under active surveillance. I have also presented the results to find non-invasive biomarkers of PCa progression in the urine collected after digital rectal examination. I generated all the results and wrote the chapter in classical format (not a manuscript) with critical input from co-authors.
Chapter 5 is the final discussion providing an overview of findings presented in chapters
2, 3 and 4. I also gave some highlights of the ongoing experiments presented in annexes. Finally, I have also included an overall conclusion and future directions.
My thesis also contains a series of Annexes. These annexes present results of some experiments that are related to but not presented in the main chapters. Annex 5 is a manuscript ‘Moussa et al. Omega-3 fatty acids and risk of prostate cancer progression during active surveillance’ presenting some findings from the clinical trial (related to chapter 4) to which I have contributed by performing bio-banking of samples, database management and optimization of protocols during the first one and half year of my Ph.D. The first author Hanane Moussa is a PhD student in my research team.
1
2
1.1 Prostate
The prostate (meaning in Greek ‘one who stands before’, ‘protector’, ‘guardian’) is a walnut-sized exocrine gland of the male reproductive system. It is located between the bladder and the penis, right in front of the rectum (Figure 1.1). It is surrounding the urethra, which runs from the bladder, through the prostate to the penis. The prostate produces fluid components of semen for nourishing and protecting the sperms. The muscles of the prostate help to propel seminal fluid into the urethra during ejaculation [1].
Figure 1.1: Location of the prostate in the man’s body.
(Figure was taken and modified from book: Prostate Cancer Treatment (PDQ®) [2].)
1.2 Prostate cancer epidemiology
Prostate cancer (PCa) is a major health problem worldwide and the most common non-cutaneous malignancy diagnosed in men particularly in Western countries [3,4]. PCa
3
substantially carries clinical and economic burdens [5,6] with a total cost of care estimated at $9.76 billion annually in Canada alone [7]. PCa also carries an important individual and social burden. In 2017, it was estimated that one in seven Canadian men have been diagnosed with PCa and one in 29 men died of the disease [8]. The occurrence of PCa varies largely worldwide with more than 60-fold difference among geographical populations. The incidence is the highest in African-Americans in the USA, whereas it is the lowest among Japanese and Chinese in their own countries [9]. Interestingly, Asian men migrating to western countries and adopting western habits have shown a significant increase of PCa [10] (Figure 1.2). Moreover, PCa incidence was found to be raised among people in Asian countries who have adopted western diet and lifestyles [11-13]. These epidemiological observations suggest that incidence of PCa varies a lot around the world, nevertheless the widespread screening and diagnosis methods for PCa have not changed much for decades.
Figure 1.2: Worldwide incidence of PCa.
PCa incidences on world map and its changes with migration. (Figure by Nikunj Gevariya and
4
1.3 PCa diagnosis and risk stratification
There are three gold-standard tests used for PCa screening, diagnosis and progression tracking: prostate-specific antigen (PSA), digital rectal examination (DRE) of prostate and histopathological examination of prostate biopsies [15] (Figure 1.3).
Figure 1.3: Current screening and diagnosis methods for PCa.
There are three main screening and diagnosis tests for PCa (A) PSA test, (B) DRE and (C) histopathological analysis for cancer cell detection in prostate biopsies. (Figure was taken and
modified from book: Prostate Cancer Treatment (PDQ®); [2]).
Prostate-specific antigen (PSA): PSA blood test (measurement of PSA level in blood)
and DRE are routinely used to screen men for PCa. PSA is a gamma-seminoprotein, also known as kallikrein (KLK)-3. It is a glycoprotein enzyme encoded by the KLK3 gene. It is mainly secreted by the glandular epithelial cells of the prostate. Function: PSA is secreted in the ejaculate where it liquefies semen in seminal coagulum and allows sperm to swim freely. It helps to dissolve cervical mucus to allow sperm to enter the uterus [16]. PSA is usually present in small quantities (≤4 ng/mL) in the serum of men with healthy prostate, but it is frequently
5
elevated in presence of PCa or other prostate related issues. The PSA test is often used to monitor response to cancer treatment, to monitor disease recurrence and progression. However, use of the PSA test for screening asymptomatic man is highly variable and may lead to overtreatment of many PCa patients. Therefore, this test is one of the highly debatable issues of PCa screening in recent years [17-19].
Digital Rectal Examination (DRE): A DRE is also routinely done by a physician to feel
the prostate through the anterior rectal wall. The dorsal and peripheral region of the prostate, adjacent to the rectal wall, is a region where usually tumors develop [20]. Nodular/hard lobe or asymmetry in this region indicates the possibility for the presence of PCa.
Histopathological examination of prostate biopsies: Patients with abnormal PSA
and/or DRE report are further advised to undergo biopsies for histopathological examination of prostatic tissues. For this procedure, a thin and hollow needle is inserted into the prostate via the rectal wall. When the needle is pulled out, it removes a small part of prostate tissue in cylindrical shape called a biopsy core. Pathologists evaluate cross-section of a biopsy core under the microscope and assign the histologic grade using numbers from 1 to 5 based on the degree of cell differentiation and architectural patterns called Gleason grade (Figure 1.4). If cells and glands look much like normal/well differentiated, a histologic grade of 1 is assigned. If cells and glands look highly irregular in shape and organization, a histologic grade of 5 is assigned. In between, level of irregular shape of the gland and cellular organisation is assigned as 2 to 4. This system of grading, called the Gleason system, was developed by Donald Gleason and colleagues at Minneapolis Veterans Affairs Hospital, MN, USA in the 1960s [21]. Prostate tumors often show multiple ‘patterns’ within a biopsy core. To account for this, two Gleason grades are assigned for each biopsy core. A primary grade is the predominant grade given to describe the cells that make
6
up the largest area of the tumor and the secondary grade is given to describe the cells of the second largest area. The Gleason score (commonly reported as Gleason) is then displayed as the sum of both. For example, a Gleason 7 (4+3) in a biopsy core corresponds to a most common pattern of 4 and a second most common pattern of 3. Given this, individual Gleason grade ranges from 1 to 5 and Gleason score can range from 2 (1+1) to 10 (5+5). Nowadays in the clinic, Gleason score usually range from 6 (3+3) to 10 since grades 1 and 2 are not anymore assigned after the revision of Gleason system in 2005 [22]. According to this revised system, only Gleason score 6 and above are considered as PCa.
Figure 1.4: Gleason grading for PCa.
The histopathology of a prostate cancer biopsy is analyzed to determine the Gleason score.
7
Additionally, PCa is frequently ‘multicentric’, meaning it has multiple foci. To survey the prostate for possible cancer, multiple biopsy cores (usually 12) are taken to improve the accuracy of grading results. Trans rectal ultrasound (TRUS) is used to guide the systematic 12-core biopsy of the prostate. All biopsies are centrally analyzed by a pathologist for grading PCa [24] (Figure 1.5). In recent years, a new grading system for PCa grading has been introduced by the International Society of Urological Pathology (ISUP) 2014 (Table 1.1) [23,25-27]. However, this system is not yet well implanted and the traditional Gleason system is still widely used.
Figure 1.5: Diagram depicting a 12-core needle biopsy of the prostate.
Two biopsies from each of the six prostate regions are taken for diagnosis of PCa. A. Diagrammatic representation of systematic 12-core biopsy procedure sampling some tissue with cancerous regions. B. Diagrammatic representation showing limitation of systematic 12-core biopsy procedure by possibilities of missing cancerous regions. (Figure was produced by Nikunj
Gevariya). Left Right Base Middle Apex
A
Left Right Cancerous regionsB
Base Middle Apex Biopsy cores Urethra Prostate Prostate8
Table 1.1. The new ISUP 2014 PCa Grading System.
Table by Nikunj Gevariya, Information source from Jonathan I. Epstein et al. [26].
Traditional Gleason score New Grading System
Group
Gleason 3+3 = 6
-Only individual discrete well-formed glands Grade 1
Gleason 3+4 = 7
-Predominantly well-formed glands with a lesser component of poorly-formed/fused/cribriform glands.
Grade 2 Gleason 4+3 = 7
-Predominantly poorly-formed/fuses/cribriform glands with a
lesser component of well-formed glands. Grade 3
Gleason 4+4 = 8
-Only poorly-formed/fused/cribriform glands or
-Predominantly well-formed glands with a lesser component lacking glands or
-Predominantly lacking glands with a lesser component of well-formed glands.
Grade 4
Gleason 4+5 = 9 and 5+5 = 10
-Lacks gland formation (or with necrosis) with or without poorly-formed/fused/cribriform glands.
Grade 5
The results of DRE, PSA testing and Gleason grading help to determine whether X-rays, bone scans, CT scans or MRI are also needed. If scans are needed, they add more information to help the urologist to figure out the clinical stage. Cancer staging system called TNM (T for tumor, N for node and M for metastasis) is widely used for PCa. It describes how far cancer has spread within the prostate or body. The TNM system is based on size and location of the primary tumor (T); whether or not it has spread to nearby lymph nodes (N); and the presence or absence of distant metastasis (M). Specific information for ‘clinical-stage’ is listed in Table 1.2 and representative spread of PCa is shown in Figure 1.6.
9
Table 1.2. TNM system for PCa staging.
Staging system to determine clinical stage of PCa. (Table by Nikunj Gevariya, Information from
American Society of Clinical Oncology ® Cancer.Net, Doctor-Approved Patient Information; Approved by the Cancer.Net Editorial Board, 01/2017).
Tumor (T)
T0 There is no evidence of a tumor in the prostate.
T1
The tumor cannot be felt during a DRE and is not seen during imaging tests. It may be found when surgery is done for another reason, usually for BPH or an abnormal growth of noncancerous prostate cells.
T1a: The tumor is in 5% or less of the prostate tissue removed during surgery. T1b: The tumor is in more than 5% of the prostate tissue removed during surgery. T1c: The tumor is found during a needle biopsy, usually due to elevated PSA level. T2
The tumor is detectable only in the prostate, not other parts of the body. It is large enough to be felt during a DRE.
T2a: The tumor is in one-half of one side of the prostate.
T2b: The tumor is in more than one-half of one side of the prostate but not both sides. T2c: The tumor has grown into both sides of the prostate.
T3
The tumor has grown through the prostate on one side and into the tissue just outside the prostate.
T3a: The tumor has grown through the prostate either on one or both sides of the prostate. This called extraprostatic extension (EPE).
T3b: The tumor has grown into the seminal vesicle(s), the tube(s) that carry semen. T4 The tumor is fixed, or it is growing into nearby structures other than the seminal vesicles, such as the external sphincter, the part of the muscle layer that helps to control
urination; the rectum; the bladder; levator muscles; or the pelvic wall.
Nearby (regional) lymph nodes (N)
NX The regional lymph nodes cannot be evaluated.
N0 The cancer has not spread to the regional lymph nodes. N1 The cancer has spread to the regional (pelvic) lymph node(s).
Distant Metastasis (M)
MX Distant metastasis cannot be evaluated. M0 The disease has not metastasized.
M1
The disease has distant metastasis.
M1a: The cancer has spread to non-regional, or distant, lymph node(s). M1b: The cancer has spread to the bones.
M1c: The cancer has spread to another part of the body, with or without spread to the bones.
10
Figure 1.6: Stages of PCa.
PCa progresses from Stage I to Stage IV, the cancer cells grow within the prostate through the outer layer of the prostate, into nearby tissue and then to lymph nodes or other parts of the body. (Figure was taken and modified from book: Prostate Cancer Treatment (PDQ®) [2]).
Based on PCa stage, Gleason grade and PSA along with age and other clinical factors such as family history and life expectancy, the urologist estimates the risk of PCa. The risk stratification helps patients to choose the most appropriate option for treatment. Of note, for PCa patients who undergo surgery to remove the cancerous prostate (called radical prostatectomy), information found during surgery plus the pathology laboratory results of the prostate and lymph nodes (also removed during surgery) analyses provide more information for ‘pathological staging’ of PCa. This will help to choose treatment option after the surgery.
11
Risk stratification of PCa: According to the guideline from National Comprehensive
Cancer Network (NCCN), there are five risk-group categories based on PSA level, prostate size, needle biopsy findings and stage of cancer. (1) Very low-risk: tumor cannot be felt during a DRE and is not seen during imaging tests but PCa was found during needle biopsy (T1c); PSA < 10 ng/mL; Gleason score ≤ 6; cancer found in less than three biopsy cores taken during a biopsy session; the cancer is found in half or less of any biopsy core. (2) Low-risk: tumor is classified as T1a, T1b, T1c, or T2a; PSA < 10 ng/mL; Gleason score ≤ 6. (3) Intermediate-risk: tumor has two or more of these characteristics: classified as T2b or T2c; PSA between 10-20 ng/mL; Gleason score of 7. (4) High-risk: tumor has two or more of these characteristics: classified as T3a; PSA > 20 ng/mL; Gleason score of 8-10. (5) Very high-risk: tumor is classified as T3b or T4; histologic grade is 5 for the main pattern of cell growth; more than four biopsy cores with Gleason scores between 8 and 10.
1.4 Role of androgens in PCa
Androgens are important steroid hormones mainly produced by the testes and less abundantly by adrenal glands. They are crucial for the development and function of the prostate. Testosterone is one of the main type of androgens. Production of testosterone is regulated by luteinizing hormone-releasing hormone (LHRH) also known as gonadotropin-releasing hormone (GnRH) released by the hypothalamus. LHRH activates the synthesis of luteinizing hormone (LH) by the pituitary gland. LH travels to the testes and induces synthesis of the testosterone by Leydig cells. Most of the testosterone in serum is binds to the sex hormone binding globulin, whereas 1% to 2% exists as free testosterone. In prostate cells, free testosterone is converted into a more active form called dihydrotestosterone (DHT) by the 5α-reductase enzyme. DHT binds
12
androgen receptors (AR) with almost five-fold high affinity than testosterone and induces AR to undergo dimerization and trans-phosphorylation. Active DHT/AR complexes translocate inside the nucleus and bind to androgen response elements (ARE) on DNA. Binding to ARE of target genes affects recruitment and crosstalk of transcription factors and subsequently, regulate gene expression and downstream pathways to mediate the effects on cell growth and survival [28] (Error! Reference source not found.).
13
Figure 1.7: Action of androgens.
The major androgen (male sex hormone) is testosterone. It is secreted primarily by the testes. In the circulation, it is bound to albumin and sex-hormone-binding globulin (SHBG), with a small fraction freely dissolved in the serum. Free testosterone enters prostate cells and is converted to dihydrotestosterone (DHT) by the enzyme 5α-reductase. Binding of DHT to the androgen receptor (AR) induces its dissociation from heat-shock proteins (HSPs) and receptor phosphorylation. The AR dimerizes and translocate and then bind to androgen-response elements in the promoter regions of target genes. Activation (or repression) of target genes leads to biological responses including growth, survival and the production of prostate-specific antigen (PSA). (Figure was taken from Feldman et al. 2001 [29]).
14
Reduction of DHT synthesis from testosterone using 5ARI (dutasteride and finasteride) is a key treatment for benign prostatic hyperplasia (BPH). 5ARI reduces the size of prostate and improve BPH-related symptoms. However for PCa, reduciton of testosterone synthesis by the testes is targeted since PCa is dependent of androgens in its initial phase. Surgical castration (also known as orchiectomy) is a procedure to quickly reduce testosterone level in blood and control PCa growth. However, removing testicles is not a common practice in clinic. Hormone-related chemical drugs are actually the main way to reduce androgen levels in men, this line of treatment is called androgen-deprivation therapy (ADT). ADT is a critical treatment for men with PCa. Common methods of ADT include chronic administration of LHRH agonists and antagonists. They inhibit the synthesis of LH by the pituitary gland. LHRH agonists produce a sudden increase of the testosterone level followed by a huge falling, whereas LHRH antagonists decrease directly the amount of testosterone.
ADT suppresses most PCa long enough to sustain an asymptomatic life for majority of patients. However, some high-risk PCa patients will gradually progresses to castration-resistant PCa (CRPC), which can grow in absence of androgens. Mechanisms underlying for the development of CRPC include 1) point mutation of AR, 2) AR amplification, 3) changes in androgen biosynthesis, 4) changes in AR co-factors, and 5) expression of AR splice variants. Second generation of nonsteroidal anti-androgens are now commercialiszed and others are currently being developed. These drugs bind to the AR ligand binding domain with greater affinity as well as inhibit its nuclear localization and DNA binding. However, PCa cells acquire resistance to these treatments as well and invariably lead to mortality of patients [30].
Alterations acquired by PCa cells to develop resistance to these treatments could be in part due to chronic inflammation in prostate. In this context, reports have shown that
15
inflammatory cytokines may activate alternative pathways for development of CRPC [31]. Inflammation in the prostate can be caused or aggravated by environmental factors [32-34]. Although the underlying mechanisms remain vague, these studies gained attention towards prostate chronic inflammation as a potential cause of PCa development and progression in men [35-37].
1.5 Link between cancer and inflammation
Inflammation is the first line of defense against a threat caused by an infection, a trauma, an allergen or damaged/tumor cells. It is a protective response that eliminates harmful stimuli and helps to restore tissue homeostasis. The relationship between cancer and inflammation was first documented by the German pathologist Rudolf Virchow in 1860s. He observed inflammatory cells in biopsies of tumor tissue, which later gained strong observational and mechanistic support from many other studies [38,39]. Tumor-associated inflammation was recognized as one of the cancer hallmarks 150 years later (Figure 1.8) [40]. Many years of research in animal models and various observations in patients together indicated that a process of cancer immune-surveillance does exist. However, it has also become clear that in certain conditions, the immune responses do facilitate tumor progression. This dual role of the immune system became the genesis of the 3E hypothesis of cancer immunoediting theory – Elimination, Equilibrium & Escape (Figure 1.9). At first, the immune system recognizes tumor cells and fight to Eliminate them. But, because tumor cells are genetically unstable and rapidly mutating, some tumor cells develop resistance under the selective pressure of the immune response and continue to proliferate and establish an Equilibrium with the immune system. Finally, tumor cells acquire new properties that allow them to totally Escape the immune system and grow aggressively [41].
16
Figure 1.8: Cancer hallmarks and enabling characteristics.
The hallmarks of cancer comprised six biological capabilities acquired during the multistep development of cancer. These are i) sustaining proliferative signaling, ii) evading growth suppressors, iii) resisting cell death, iv) enabling replicative immortality, v) inducing angiogenesis and vi) activating invasion and metastasis [42]. More recently two emerging hallmarks have been added i) deregulating cellular energetics, i.e. the capability to modify or reprogram cellular metabolism in order to effectively support neoplastic proliferation and ii) avoiding immune destruction, i.e. the capability to avoid immunological destruction particularly by T- and B- lymphocytes, macrophages and natural killer cells. Additionally, two consequential characteristics were also introduced i) genomic instability and mutation which result in cancer cells genetic alterations that drive tumor progression and ii) tumor promoting inflammation, i.e. the immune cells designed to fight infections and heal wounds can instead result in the promotion of tumor cell growth. (Figure was taken modified from Hanahan et al. 2011 [40]).
Sustaining proliferative signaling Evading growth suppressors Resisting cell death Enabling replicative immortality Inducing angiogenesis Activating invasion and metastasis
17
Figure 1.9: Cancer immunoediting: the 3E hypothesis.
Cancer immunoediting consists of three sequential phases: Elimination, Equilibrium and Escape. The immune system destroys developing tumor cells before they become clinically apparent. This initial step is called the Elimination phase. However, mutations in cancer cells lead to the development of rare cancer cell variants that are not destroyed by the immune system but still their outgrowth is prevented by immunologic mechanisms. This step is called the Equilibrium phase. At this stage, T cells, Interleukin (IL)-12 and Interferon (IFN)-γ (an adaptive immune response) are required to maintain tumor cells in a state of functional dormancy. The editing of tumor immunogenicity occurs in the Equilibrium phase. Constant immune selection pressure placed on genetically unstable tumor cells may lead to the development of tumor cell variants that are no longer recognized by adaptive immunity or become insensitive to immune effector mechanisms. This induces an immunosuppressive state within the tumor microenvironment and cancer enter the Escape phase. At this stage of cancer, cells can outgrowth and cause clinically apparent disease. (Figure was taken and modified from Schreiber et al. 2011 [43]).
C C C C C C
18
The protective role of inflammation against cancer suggested that the immune system can induce rejection of tumors. Indeed, tumor-infiltrating lymphocytes in colorectal cancer and ovarian were shown to inhibit tumor growth [44-47]. High densities of (cluster of differentiation molecules = CD) CD3+CD8+CD45RO+ cells, which are a type of memory T cells, were strongly associated with a long-term disease-free survival in different cancers [48]. In addition, the extensive infiltration of natural killer (NK) cells in human gastric or colorectal carcinomas were shown to be associated with a favorable prognosis [44,45]. Apart from immune cells, cytokines − key mediators of the immune system for cell communication − also exert anti-tumor effects by modifying the behaviour of immune as well as non-immune cells. Cytokines can suppress growth of tumor cells by arresting cell-cycle, stimulating apoptosis and senescence as well as by inhibiting motility [49,50]. Cytokines’ broad range of anti-tumor activities has been translated into a number of cytokine-based approaches for cancer therapy. Indeed, cytokines such as GM-CSF (Granulocyte macrophage colony stimulating factor), IL(Interleukin)-12, IL-15 and IL-21 have been tested in clinical trials for patients with advanced cancer [51,52]. This support the above notion for cancer surveillance and elimination by the immune system.
After eradication of cancer, resolution of acute inflammation is necessary for tissue homeostasis. One of the key transition towards resolution of inflammation is the replacement of polymorphonuclear leucocyte (PMNs) by monocytes and phagocytosing macrophages at inflamed site. Clearance of PMNs is mediated by recirculation, local death by apoptosis and phagocytosis by macrophages. These macrophages leave the inflamed site by lymphatic drainages. However, macrophages may live for a long-time and phagocytized cell debris may remain indigestible inside vesicles of macrophages for long-periods [53]. Macrophages are avid phagocyte and they may continue to engulf material even they cannot digest. When the stimulus