Chemical Composition and Acaricidal Properties of Deverra scoparia
Essential Oil (Araliales: Apiaceae) and Blends of Its Major Constituents
Against Tetranychus urticae (Acari: Tetranychidae)
S. ATTIA,1 K. L. GRISSA,2 G. LOGNAY,3 S. HEUSKIN,3 A. C. MAILLEUX,1AND T. HANCE11 Earth and Life Institute, Biodiversity Research Centre, Université Catholique de Louvain, 4-5, Place Croix du sud, B-1348
Louvain-la-Neuve, Belgium.
2 Laboratoire d'Entomologie-acarologie, Institut National Agronomique de Tunisie, 1082 Cité Mahrajène, Tunis, Tunisia. 3 Université de Liège Gembloux Agro-Bio Tech Unité de Chimie analytique. Passage de Déportés, 2, B-5030 Gembloux, Belgium.
ABSTRACT
The essential oil of Deverra scoparia Coss. & Durieu was investigated for its acaricidal activity against the worldwide pest twospotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). The essential oil was analyzed by fast gas chromatography (GC) and GC-mass spectrometry. The activities of its individual and blended constituents were determined. Our study showed that female mortality increased with increasing D.
scoparia oil concentrations, with LD30 and LD90 values at 1.79 and 3.2 mg liter-1, respectively. A reduction in
fecundity had already been observed for concentrations of 0.064, 0.08, and 0.26 mg liter-1 D. scoparia essential
oil. Ten major components, comprising 98.52% of the total weight, were identified; α-pinene was the most abundant constituent (31.95%) followed by sabinene (17.24%) and ∆3-carene (16.85%). The 10 major
constituents of D. scoparia oil were individually tested against T. urticae females. The most potent toxicity was found with α-pinene, ∆3-carene, and terpinen-4-ol. The presence of all constituents together in the artificial mixture caused a significant decrease in the number of eggs laid by females, at 0.26 mg liter-1 (11 eggs),
compared with the control (50 eggs). The toxicity of blends of selected constituents indicated that the presence of all constituents was necessary to reproduce the toxicity level of the natural oil.
KEYWORDS : essential oil, Deverra scoparia, Tetranychus urticae, toxicity, fecundity
Twospotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), is a well-known mite pest worldwide, with a wide range of host plants. It causes serious damage in outdoor crops and greenhouses (Hazan et al. 1974, Goff 1986, Lee et al. 1994, Ho et al. 1997). Synthetic acaricides have been widely used for the control of this pest in greenhouses, orchards and many other cropping systems, a practice that has led to the development of resistance (Sundaram et al. 1995, Van Leeuwen et al. 2006). Indeed, >550 species of insects and mites have developed resistance to one or more classes of synthetic biocides (Van Leeuwen et al. 2009). Consequently, the number of effective chemicals for the control of crop pests and disease vectors is rapidly decreasing (Georghiou 1990). Moreover, some chemical products are known for their negative effects on human health and the
environment (Sathiyamoorthy et al. 1997, Cavalcanti et al. 2010). These concerns have provided the basis for the recent interest in bioactive acaricidal compounds from higher plants. Among other bioactive natural compounds, several plant essential oils have been demonstrated as potential alternative compounds for mite control (Choi et al. 2004, Floris et al. 2004, Calmasur et al. 2006, Pontes et al. 2007, Cavalcanti et al. 2010, Chermenskaya et al. 2010, Han et al. 2010, Neves et al. 2011). Essential oils have a short residual activity and are less persistent than conventional pesticides because they tend to be volatile and are susceptible to temperature and UV light degradation (Miresmailli et al. 2006, Cloyd et al. 2009). Consequently, most of these oils are environmentally nonpersistent and relatively nontoxic to warm-blooded animals and humans (Crowell et al. 1992, Kubo et al. 1994), with some exceptions (Roe 1965, Hjorther et al. 1997).
Essential oils are secondary metabolites that are frequently concentrated in the leaves, bark, or fruit of aromatic plants. The oils are generally obtained by steam distillation and are composed of a complex mixture of
monoterpenes, phenols, and sesquiterpenes (Isman 2000). These constituents are also of interest to industrial markets, especially because of their toxicity to the numerous species of insects that have developed resistance to synthetic biocides (Bekele and Hassanali 2001). Many plants, including garlic (Alliumsativum L.), rosemary (Rosmarinus officinalis L.), cinnamon (Cinnamomum verum J.Presl), citrus (Citrus spp. ), and cedar (Cedrus spp. ), have been used to control a variety of insects (Isman 2004). In this context, Deverra scoparia Coss. & Durieu, an endemic plant of North Africa, is locally known for its pharmaceutical activity (Ben Haj Jilani et al. 2007). Indeed, Ghrabi-Grammar et al. (2009) investigated the acaricidal effect of D. scoparia on the poultry red
mite Dermanyssus gallinae (De Geer), the most economically deleterious parasite of laying hens in Europe. The results showed that D. scopria provides good mortality on D. gallinae (Chauve 1998). So far, no studies of toxicity have been performed on T. urticae with this plant, which is very locally distributed throughout the septentrional ecoregion in the northern region of Algeria (Djeridane et al. 2008).
The first purpose of this study was to evaluate the effect of D. scoparia on the mortality of T. urticae. We measured the acaricidal activity of the essential oil of D. scoparia and the concentrations that killed 50 and 90% of individuals (LD30 and LD90, respectively). The effect of sublethal concentrations on fecundity also was
analyzed by recording the numbers of eggs laid by the treated females.
For a comprehensive evaluation of the potential of D. scoparia essential oil as an acaricide, the essential oil was characterized by gas chromatography (GC) and GC-mass spectrometry (GC/MS). Afterward, we evaluated the effectiveness of the major constituents of D. scoparia oil by spray applications against T. urticae. The aim was to compare the toxicity of the essential oils with that of the major constituents, individually and in selected blends, with the purpose of identifying the compounds conferring the biological activities against T. urticae. This information might help guide the selection and use of appropriate varieties of such plants in traditional postharvest practices (Bekele and Hassanali 2001).
Materials and Methods
Collection and Maintenance of T. urticae. T. urticae were collected from infested plants in citrus orchards in
Tunisia in January 2006 (Tunis, by K.L.-G). They were maintained in a climate room at 26°C, 50-60% RH, and a photoperiod of 16:8 (L:D) h, at the Biodiversity Research Center (Ecology of Interactions and Biological Control team) in the University Catholique of Louvain (Belgium) for >5 yr without any contact with acaricides before the experiments. The strain was reared on bean (Phaseolm vulgaris L.) leaves placed on moistened cotton in petri dishes (Overmeer 1985). Only young female adults (<24 h old) were chosen for the bioassays.
Plant and Essential Oil Extraction. D. scoparia individuals were collected locally in Tunisia (Saddine,
southwest of Kef) in June 2008. This selection was based on previous work and the use of plant products in traditional medicine in Tunisia (Ben Haj Jilani et al. 2007). Its essential oil was obtained through steam distillation of the aerial parts for 4 h by using a Clev-enger-type apparatus and fresh material. The oil yield was 0.51% of the dry weight of D. scoparia.
Identification of Essential Oil Constituents. The essential oil of D. scoparia was analyzed by GC-MS in the
Department of Analytical Chemistry in Gembloux Agro-Biotech, University of Liège, Liège, Belgium. For quantitative analyses (percentage determination), we used a fast GC, which proved to be powerful enough to analyze the essential oil constituents (Bicchi et al. 2004, Heuskin et al. 2009).
GC-MS Analysis. Conventional GC-MS analyses were carried out on a thermo trace MS Finnigan mass-selective
detector equipped with an Optima 5 MS (Macherey-Nagel) capillary column (30m by 0.25mm i.d., 0.25-µm film thickness) and a split/splitless injector (splitless mode) at 250°C The oven temperature was programed from 40 to 210°C Helium was the carrier gas at 1 ml/min. Volatile compounds were identified by comparing the mass spectra obtained with those from the Wiley 275 liters spectral library and with their retention indices. The retention indices were determined relative to the retention times of a series of n-alkane standards (C9-C30, 0.025
µg/µl in n-hexane, Sigma-Aldrich, Bornem, Belgium), measured under the chromatographic conditions
described above, and compared with values in the literature (Adams 2001).
Fast GC Analysis. Fast GC analyses were conducted on a Thermo Ultra-Fast Trace GC operated with a
split/splitless injector and a Thermo AS 3000 autosampler (Thermo Fisher Scientific, Waltham, MA). The GC system was equipped with an ultrafast module (UFM) incorporating a direct resistively heated column (Thermo Fisher Scientific) : UFC-5,5% phenyl (5 m by 0.1 mm i.d., 0.1-µm film thickness). The following
chromatographic conditions were used to obtain a suitable peak resolution. The UFM temperature program was as follows: initial temperature at 40°C, held for 0.1 min, ramp 1 at 30°C min-1 to 95°C, ramp 2 at 35°C min-1 to
155°C, ramp 3 at 200°C min-1 to 280°C, held for 0.5 min. Injection temperature was 240°C; injection volume
was 1 µl; carrier gas was He, at a constant flow rate of 0.5 ml min-1; and split ratio was 1:100. The GC unit had a
high-frequency fast flame ionization detector (300 Hz), at250°C; H2 flow was 35 ml min-1; air flow was 350 ml
min-1; and make-up gas flow (N
2) was 30 ml min-1. Data processing was performed using Chromcard software
version 2.3.3. (Louvain-la-Neuve, Belgium).
Effect of D. scoparia Essential Oil on Mortality. The acute toxicity (measured as mortality at 72 h) of the
essential oil was determined via a spray application method on young females of T. urticae aged 24 h. D.
scoparia essential oil was diluted 1:100 in ethanol. The acaricidal effect of 12 concentrations of essential oil was
investigated which corresponded to 0.064, 0.08, 0.26, 0.66, 1.32, 2, 2.66, 3.32, 4, 4.66, 5.32, and 6 mg liter-1,
A group of 25 young females aged 24 h was randomly selected and transferred to fresh bean leaf discs (diameter, 35 mm) mounted with the adaxial side uppermost on moistened cotton in petri dishes (90 by 15 mm). A Potter spray tower that produced a deposit after a settling time of 1 min 30 s was used to spray the mites on the leaf discs at a pressure 1.4 bar under 20 ± 2°C The distance of the sprayer nozzle from the leaves was 50 cm. The tests were replicated five times per concentration. Each replicate consisted of 25 females of T. urticae, with five mites on each leaf disc. Control tests were performed using water and ethanol. After spraying, the bean leaf discs were maintained at room temperature (26 ± 1°C) 60 ± 10% RH, and a photo-period of 16:8 (L:D) h.
Mites were included in the mortality count 72 h posttreatment if their appendages did not move when prodded with a fine pencil. For each concentration, after using Abbott's correction (Abbott 1925), we calculated the mortality rate according to (mean number of deaths after the treatment — mean number of deaths after the control) /the total number of females at the beginning of the tests. The data obtained in this experiment also were submitted to probit analysis (Finney and Stevens 1948).
Effect of D. scoparia Essential Oil on Fertility. During the mortality experiments (see above), a few dead mites
were found after being sprayed with low concentrations (0.064, 0.08, and 0.26 mg liter-1) of the oil.
The experimental method was similar to that used to test the lethal toxicity described previously. A group of 25 young females aged 24 h were randomly selected and transferred to fresh bean leaf discs on moistened cotton in petri dishes. These females were sprayed with a Potter spray tower. The tests were replicated five times per concentration. Each replicate consisted of 25 females of T. urticae, with five mites in each leaf disc. Control tests were performed using water and ethanol. After spraying, each female was transferred to bean leaf discs
(diameter, 15 mm), which had been completely cleaned, to check fecundity. These bean leaf discs were maintained at room temperature (26 ± 1°C), 60 ± 10% RH, and a photoperiod of 16:8 (L:D) h.
To test the effect of the sublethal concentrations of the essential oil on fecundity, the numbers of eggs laid by the treated females was recorded daily for 12 d, the point when maximum fecundity was expressed (Hance and Van Impe 1999), and the eggs were destroyed after counting. The fecundity of each female treated with the three solutions (0.064, 0.08, and 0.26 mg liter-1) and with the control solution (water and ethanol) was compared. Effect of Constituents of D. scoparia Essential Oil on Mortality and Fecundity. The toxicity experiments
were repeated with commercially available constituents of the essential oil and with blends of these at their natural proportions (GC-MS; Table 1) in the oil and in amounts present in the minimum 100% lethal dose of the oil (3.42 mg liter-1).
Pure compounds were purchased from Sigma-Al-drich: α-thujene, α-pinene, sabinene, myrcene, Δ3-carene, ocimene, terpinene-4-ol, pulegone, eugenol, and β-eudesmol. Purities of the compounds varied from 95 to 99%. To identify the relative contribution of each constituent to the toxicity of the oil, we tested each of these constituents individually. We then tested a blend of all major constituents, called a full mixture (TFM). Finally, we tested the toxicity of three selected blends: TM1 was composed of α-pinene, Δ3-carene, and terpinene-4-ol, three constituents that were found to be highly toxic to T. urticae (i.e., that were lethal to at least 65.6% of the individuals). TM2 was composed of eugenol, pulegone, α-thujene, β-eudesmol, sabinene, and ocimene, six moderately active constituents with mortality rates between 23 and 65.6%. TM3 was composed of myrcene, an inactive constituent with a mortality rate <23%. This classification of toxicity was similar to that used by Miresmailli et al. (2006).
Finally, we tested the effects of different blends of the constituents on fecundity: PM1 was composed of myrcene, eugenol, and ∆3-carene, three constituents that were found to be highly active on the fecundity of T.
urticae (the number of eggs laid never overpasses 30 eggs per female. PM2 was composed of terpinene-4-ol,
ocimene, sabinene, and α-thujene, four moderately active constituents with laying rates between 30 and 34 eggs. PM3 was composed of α-pinene, β-eudesmol, and pulegone, three inactive constituents with a laying rate >34 eggs. The results were compared with the results obtained from the complete essential oil.
Statistical Analysis. All the data were corrected by using Abbott's formula (Abbott 1925). Probit analysis was
used to determine the LD50 and LD90 values using the StatPlus program (AnalystSoft Inc., www.
analystsoft.com).
Regarding mortality, the data also were analyzed using Prism version 5.01 for Windows (GraphPad Software, San Diego, CA) for analysis of variance (ANOVA). All tests were applied under two-tailed hypotheses and the significance level P was set at 0.05. Tukey's test was used to compare means.
In the fecundity experiments, the cumulative number of eggs was best-fitted to a sigmoidal curve (Prism) using the formula Y = B + (A - B)/(1 + 10^( (LT50 - X))). In this formula, Y represents the value of the cumulative
number of eggs, B is the minimum number of eggs (constrained with a minimal value of 0), A is the maximum number of eggs (plateau value), and LT50 is the period of time (days) that provided a response halfway between
zero and the maximum of eggs (plateau), and X is the period of time of the experiment (days).
Table 1. : Major constituents of D. scoparia essential oil and their relative proportions in the pure oil
No. Component Retention time (min) Retention
index (measured) % 1 α-Thujene 5.62 930 13.71 2 α-Pinene 5.85 939 31.95 3 Sabinene 6.91 975 17.24 4 Myrcene 7.43 991 3.46 5 Δ-3-Carene 8.75 1031 16.85 6 Ocimene 13.20 1144 9.75 7 Terpinene-4-ol 14.66 1177 2.84 8 Pulegone 17.27 1237 0.16 9 Eugenol 22.70 1359 1.80 10 β-Eudesmol 34.79 1651 0.76
Identification with retention index was obtained by GC-MS. Percentage were obtained by Fast-GC-FID.
Results
Identification of Essential Oil Constituents. Table 1 showed the composition of D. scoparia essential oil. Ten
components were identified (electron impact-mass spectra and retention index comparison) by GC-MS comprising 98.52% of the total weight; α-pinene was the most abundant compound (31.95%) followed by sabinene (17.24%) and ∆3-carene (16.85%). The essential oil was then analyzed by fast GC on a UFM column of the same polarity as in GC-MS (apolar stationary phase). The retention times of the components of interest from the essential oil were compared with those of the reference compounds.
Effect of D. scoparia Essential Oil and Its Constituents and Blends on Mortality. Toxicity of Essential Oil.
All mites (females) in the control group survived 72 h. A low mortality rate (<20%) was observed at 0.064, 0.08, and 0.26 mg liter-1. The LD
50 of D. scoparia was 1.79 mg liter-1 for the adult female spider mites (with a SE of
0.123 and a β value of 0.84). The LD90 and LD100 values for the mites were obtained at 3.16 and 3.42 mg liter-1,
respectively.
Toxicity of Single Constituents. A significant difference was found in the lethal toxicity of the single
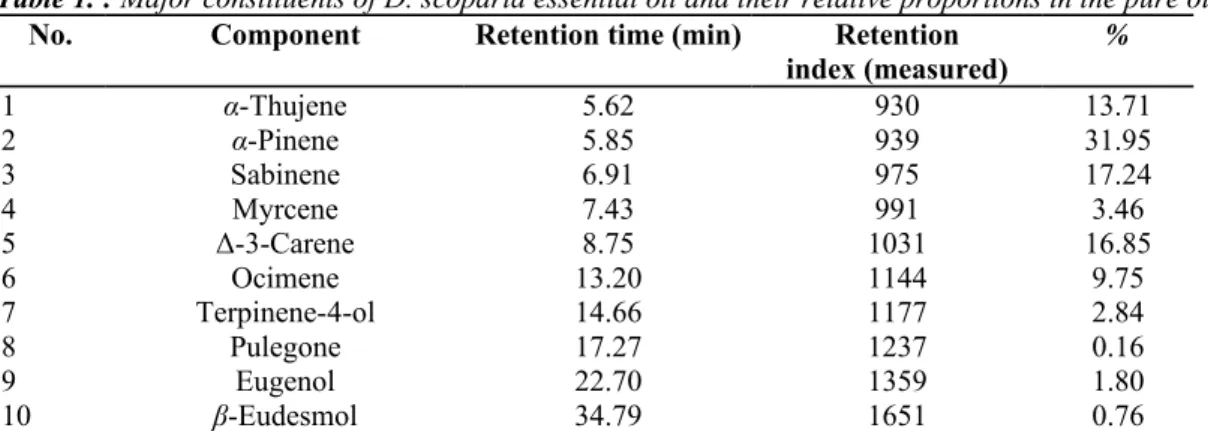
constituents of D. scoparia when all treatments were compared (including the essential oil and the control treatments, one-way ANOVA; F = 88.91; df = 12,104; P < 0.001) (Fig. 1). Indeed, post hoc Tukey's tests comparing the toxicity of single constituents revealed that three constituents (α-pinene, ∆3-carene, and terpinene-4-ol) were similarly and highly toxic, but they were not as toxic individually as the essential oil (Fig. 1). Six constituents (eugenol, pulegone, α-thujene, β-eudesmol, sabinene, and ocimene) were slightly toxic, whereas the remaining constituent (myrcene) was not toxic to T. urticae and did not differ significantly (post hoc Tukey's tests) from the mortality rate found in the control test (Fig. 1).
Toxicity of Recomposed Oil. Based on the 100% lethal concentration LD100 = 3.42 mg liter-1 and following the
natural composition of the oil as indicated by GC-MS (Table 1), bioassays with the recomposed oil (α-thujene,
α-pinene, sabinene, myrcene, ∆3-carene, ocimene, terpinene-4-ol, pulegone, eugenol, and β-eudesmol) showed
that the greatest mortality rate was obtained when all 10 constituents were present (full mixture). This was significantly higher than the mortality rates for the three most toxic constituents (α-pinene, ∆3-carene, and terpinene-4-ol) when tested individually (Fig. 1). The mortality rate caused by the full mixture (a blend of all 10 major constituents) was significantly lower than the mortality caused by pure D. scoparia oil (post hoc Tukey's tests) (Fig. 1).
Bioassay of Selected Blends. The mortality rates caused by the selected blends differed with the blend (one-way
ANOVA; F = 248,2; df = 5, 54; P < 0.001) (Fig. 2). The mortality rate caused by all of the selected blends (TM1, TM2, TM3, TM1 + 2 = TM1+TM2) was significantly lower than the mortality rate caused by pure D.
scoparia oil (Figs. 1 and 2).
The mortality rates of TM1, TM2, and TM3 were lower than the mortality rate of the full mixture of the 10 major components (Fig. 2), the mortality rate of TM1 + 2 was not significantly different from that of the full mixture (Fig. 2), and TM3 did not cause any significant mortality in T. urticae.
Effect of D. scoparia Essential Oil and Its Constituents and Blends on Fecundity. Effect of Essential Oil.
Fecundity was affected by the concentration of the essential oil (Fig. 3). The experimental data were correlated with the theoretical data and followed a sigmoid model. The maximum values of the cumulative numbers of eggs
were significantly different between the control experiments and the treatments (Fig. 3).
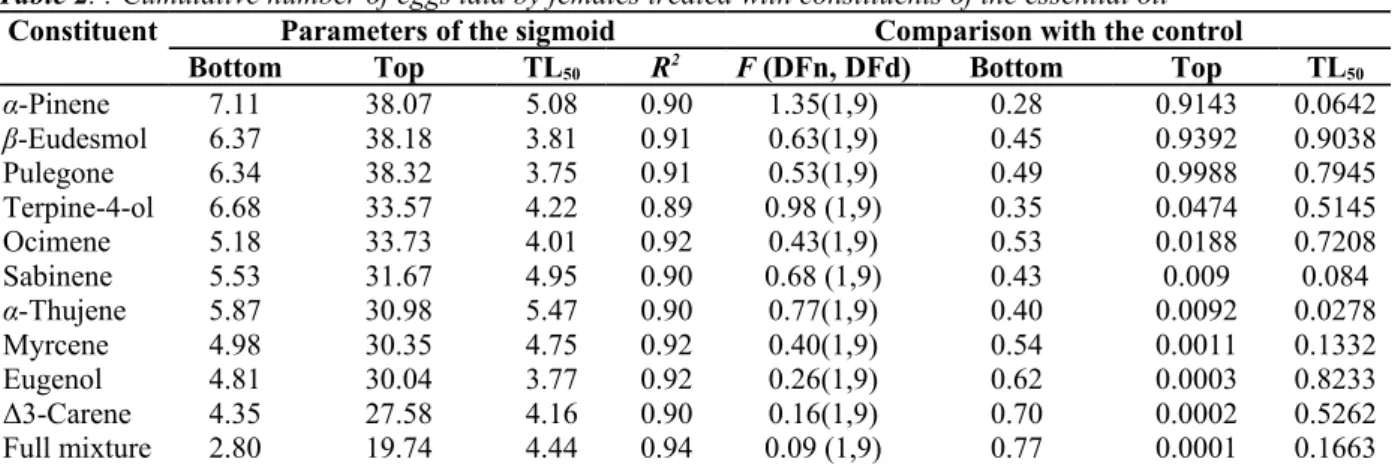
Effect of Single Constituents. The maximum values of the cumulative numbers of eggs obtained for α-pinene,
β-eudesmol, and pulegone did not differ significantly from that obtained for the control (Table 2). The maximum values of the cumulative numbers of eggs obtained for the remaining constituents (ter-pinene-4-ol, ocimene, sabinene, α-thujene, myrcene, eugenol, and ∆3-carene) were significantly lower than those obtained by the control and the other constituents.
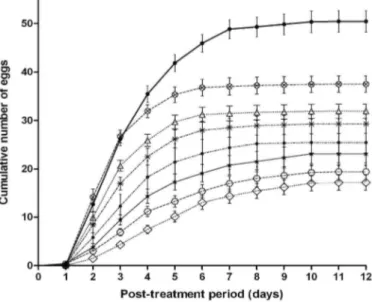
Effect of Selected Blends. For PM2 + PM3, the maximum value of the cumulative number of eggs was 32,
which was significantly different to the 17 eggs obtained with the full mixture PFM (Fig. 4). Removal of the mixture containing the moderately active constituents (PM2) increased the maximum value of the cumulative number of eggs to 24 compared with the full mixture, and removal of the inactive constituents (PM3) increased the maximum value of the cumulative number of eggs to 19 compared with the full mixture (17 eggs).
Fig. 1. Mortality caused by the oil, full mixture, and individual constituents to T. urticae when applied at levels
equivalent to those found in the 100% lethal concentration of the pure oil (LD100 = 3.42 mg liter-1). Values are
mean ± SE of five replicates with 25 adult female mites per replicate. Treatments with different letters are significantly different from each other (Tukey's test).
Fig. 2. Mortality caused by selected blends of active and inactive constituents of D. scoparia oil when applied at
levels equivalent to those found in the 100% lethal concentration of the pure oil (LD100 = 3.42 mg liter-1) Values
are mean ± SE of five replicates with 25 adult female mites per replicate. Treatments with different letters are significantly different from each other (post hoc Tukey's tests). TM1 (active), α-pinene + ∆3-carene + terpinene-4-ol; TM2 (moderately active), eugenol + pulegone + α-thujene + β-eudesmol + sabinene + ocimene; and TM3 (inactive), myrcene.
Fig. 3. Mean + SE cumulative number of eggs laid by female T. urticae after treatment with a 0.064, 0.08, or
0.26 mg liter-1 Deυerra oil, or after treatment with a control (water and ethanol) solution.
Effect of Recomposed Oil. The combination of the active constituents with either the moderately active (PM1 +
PM2) or inactive constituents (PM1 + PM3) restored a level of toxicity that was consistent with the full mixture of 10 compounds (Fig. 4). The absence of PM1 constituents from the mixture (PM2 + PM3) caused a significant increase in egg production (P < 0.05).
In our study, the lowest concentrations did not affect female survival but they did affect female fecundity in comparison with the control group. Our results showed that females laid eggs during similar periods of time but that the laying rate was decreased by treatment with D. scoparia essential oil. The total number of eggs was lower (38, 34, and 11 eggs per female for 0.064, 0.08, and 0.26 mg liter-1; 14 compared with 50 eggs in the
control group) at the end of the laying period.
Although it only represented 16.85% of D. scoparia oil, ∆3-carene was the major contributor to the decrease in the maximum cumulative number of eggs obtained after 12 d. Similarly to the toxicity experiments, individual constituents in the fecundity inhibition assays showed that the absence of certain constituents (myrcene, eugenol, and ∆3-carene) in the artificial mixture caused a significant increase in the cumulative number of eggs laid by the females.
Here, we showed that the artificial blend TM1 (myrcene, eugenol, and ∆3-carene) was responsible for decreasing the cumulative number of eggs to 25 eggs per female compared with 50 eggs per female in the control, whereas myrcene, eugenol, and Δ3-carene decreased the cumulative number of eggs to 30, 30, and 27 eggs per female, respectively, allowing us to conclude that the active constituents had synergistic effects because the number of eggs laid was significantly higher when the constituents were tested individually rather than when they were in a mixture with other active constituents.
Comparison between the maximal numbers of eggs laid by the females treated with the pure oil, full mixture, individuals constituents of D scoparia and with the control solution when applied at levels equivalent to those found in 3.42 mg liter-1. LT
50 is the period of time (days) that provided a response halfway between zero and the
maxiumum of eggs (plateau), and X is the period of time of the experiment (days). Values are mean of n = 5 replicates with 25 adult female mites per replicate (df = 9 for all).
Table 2. : Cumulative number of eggs laid by females treated with constituents of the essential oil
Constituent Parameters of the sigmoid Comparison with the control
Bottom Top TL50 R2 F (DFn, DFd) Bottom Top TL50
α-Pinene 7.11 38.07 5.08 0.90 1.35(1,9) 0.28 0.9143 0.0642 β-Eudesmol 6.37 38.18 3.81 0.91 0.63(1,9) 0.45 0.9392 0.9038 Pulegone 6.34 38.32 3.75 0.91 0.53(1,9) 0.49 0.9988 0.7945 Terpine-4-ol 6.68 33.57 4.22 0.89 0.98 (1,9) 0.35 0.0474 0.5145 Ocimene 5.18 33.73 4.01 0.92 0.43(1,9) 0.53 0.0188 0.7208 Sabinene 5.53 31.67 4.95 0.90 0.68 (1,9) 0.43 0.009 0.084 α-Thujene 5.87 30.98 5.47 0.90 0.77(1,9) 0.40 0.0092 0.0278 Myrcene 4.98 30.35 4.75 0.92 0.40(1,9) 0.54 0.0011 0.1332 Eugenol 4.81 30.04 3.77 0.92 0.26(1,9) 0.62 0.0003 0.8233 Δ3-Carene 4.35 27.58 4.16 0.90 0.16(1,9) 0.70 0.0002 0.5262 Full mixture 2.80 19.74 4.44 0.94 0.09 (1,9) 0.77 0.0001 0.1663
Essential oil 1.87 13.69 5.14 0.93 2.30(1,9) 0.16 0.0001 0.0093
Fig. 4. Mean + SE cumulative number of eggs laid by the female T. urticae treated with the full mixture, and
blends of constituents of D. scoparia essential oil when applied at levels equivalent to those found in 0.26 mg liter-1. Value are mean of n = 5 replicates with 25 adult female mites per replicate. PM1 (active), ∆3-carene +
eugenol + myrcene; PM2 (moderately active), terpinene-4-ol + ocimene + sabinene + α-thujene; PM3 (inactive), α-pinene + /3-eudesmol + pulegone; and PFM, PM1 + PM2 + PM3.
Discussion
This study is the first to qualitatively show how the concentration of D. scoparia essential oil varies in its efficacy against the twospotted spider mite.
Mortality. Our results clearly indicated that the essential oil of D. scoparia is toxic to T. urticae females with
LC90 and LC100 values of 3.2 and 3.42 mg liter-1, respectively. We identified 10 components in the essential oil
that had differing levels of toxicity: α-pinene, the major constituent (31.95%) of D. scoparia oil, was the major contributor to the toxicity of the artificial blend, followed by ∆3-carene (16.85%) and terpinene-4-ol (2.84%). Myrcene was inactive on its own. However, the toxicity of our artificial mixture only reached 83% mortality when the blends of the active constituents were mixed with myrcene (inactive). The highest mortality rate was obtained when all of the constituents were present in the mixture, but the mortality caused by the artificial mixture containing all of the constituents remained significantly lower than that caused by the natural essential oil. This suggests that unknown constituents comprising 1.5% of the oil made a significant contribution to its overall toxicity, for example, through synergy.
One interesting aspect of our study was the difference found in the role of the major constituents in a mixture as opposed to their individual toxicities. The artificial blend TM1 (α-pinene, ∆3-carene, and ter-pinene-4-ol) was responsible for 65% mortality, whereas α-pinene, ∆3-carene, and terpinene-4-ol caused 68, 65.2, and 63.2% mortality, respectively, when tested individually. Consequently, our results strongly suggest that the active constituents had an antagonistic effect (or at least no additional effect) on each other, because their toxicity level was significantly higher when tested individually than when they were in a mixture with the other active constituents.
Fecundity. We found that the presence of all of the constituents together in the artificial mixture caused a
significant decrease in the number of eggs laid by females, at 0.26 mg liter-1 (11 eggs), compared with the
control (50 eggs). Similarly, the highest mortality rates was obtained when all of the constituents were present in the mixture at 3.42 mg liter-1 (83.6%).
Another interesting aspect of our study was that our results strongly suggested that D. scoparia oil might have more than one mode of action (fecundity and mortality). Indeed, three major constituents (myrcene, eugenol, and ∆3-carene) strongly decreased fecundity but only ∆3-carene had a strong effect on mortality. Moreover, the most toxic constituent was α-pinene, but this had no effect on fecundity. Our results confirm those of a previous report
(Miresmailli et al. 2006) where the absence of α-pinene from the artificial mixture caused a significant decrease in toxicity (80%), which tempted us to conclude that this constituent is the major contributor to the toxicity of the essential oil.
We found that the absence of some blends from the artificial mixture caused a significant decrease in toxicity and an increase in the number of eggs laid by the females. This indicates that the presence of all of the constituents was necessary to achieve full toxicity.
Our study shows that terpinen-4-ol can be considered as being an important contributor to the toxicity against T.
urticae, with a 63.2% mortality rate. Our results confirm a previous report (Kouninki et al. 2007) that found that
terpinen-4-ol is responsible for 50% of the mortality against Sitophilm zeamais Motschulsky adults.
Another previous report showed that 1,8-cineole is responsible for the major toxicity of rosemary oil and Listea
pungens Hemsl. against T. urticae and Trichopulsiani (Hübner), respectively (Miresmailli et al. 2006, Jiang et al.
2009).
A few plant-derived essential products are effective at controlling arthropods (Cloyd et al. 2009). Among these, some are prohibited because of their potential phytotoxicity (Cloyd et al. 2009). Previous studies have reported that the acaricidal effects of plant essential oils are related to their chemical compositions (Isman et al. 2001, Singh et al. 2001). Mansour et al. (1986) pointed out that besides toxicity, the residues of essential oils of some Labiatae species are repellent and strongly reduce the fecundity of Tetranychm cinnabarinus (Boisduval) females.
The octopaminergic nervous system is considered to be the site of action of essential oils in the American cockroach (Enan 2001), but this may not be the case for the twospotted spider mite, and there is also the possibility that essential oils have more than one site of action, because they are complex mixtures (Miresmailli et al. 2006).
A further step after this study might be to test the phytotoxicity of D. scoparia essential oil on vegetables and herbaceous and foliar plant material (Cloyd et al. 2009) However, the extent of plant injury can be dependent on numerous factors, including the precise concentration a plant was exposed to during applications. Consequently, it is necessary to know the precise effective concentration of an essential oil; this underlines the importance of efficient analyses over a wide range of concentrations. The present results strongly suggest that D. scoparia might add to the arsenal of methods for controlling mites in greenhouses and orchards. However, further studies are needed to evaluate the cost, efficacy, and safety of the oil. Knowing the role of each constituent in the toxicity and effects on fecundity of this oil would provide an opportunity to create an artificial blend of different constituents on the basis of their activities against different pests. Indeed, several constituents are commercially available at a reasonable purity (95%), and essential oil producers and suppliers can often provide chemical specifications for even the most complex oils (Isman 2000). Current information indicates that they are safe to the user and the environment, with few qualifications. The exemption of some essential oils from registration in the United States has stimulated their development for use as commercial insecticides (Isman 2000).
Consequently, despite the fact that this oil provides a good level of T. urticae mortality and inhibition of female fecundity, if it was to be developed for use in pest management it might be necessary to look toward more efficient means of oil extraction.
Acknowledgments
We are very grateful to Vincent Hote for useful discussions about chemical compositions (Université de Liège Gembloux Agro-Bio Tech Unité de Chimie analytique) and to Ghrabi Gammar Zeineb for providing the D.
scoparia samples. We also indebted to Wallonies-Bruxelles International. This work was presented at the
Journée scientifique annuelle de la société royale de chimie-Chimie verte., held 14 October 2010 in Belgium. This work was supported by funding from the Institut d'encouragement de la Recherche Scientifique et de l'Innovation de Bruxelles (to A.CM.) and the National Fund for Scientific Research (Belgium) (to T.H.). This is publication BRC 190 of the Biodiversity Research Centre at Université Catholique de Louvain.
References Cited
Abbott, W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol 18: 265-267.
Adams, R. P. 2001. Identification of essential oil components by gas chromatography quadrupole mass spectroscopy. Allured Publishing, Carol Stream, IL.
Bekele, J., and A. Hassanali. 2001. Blend effects in the tox-icity of the essential oil constituents of Ocimum kilimand-scharicum and Ocimum kenyense (Labiateae) on two post-harvest insect pests. Phytochemistry 57: 385-391.
Ben Haj Jilani, I., Z. Ghrabi-Gammar, and M. Zouaghi. 2007. Valorisation de la biodiversité en plantes médicinales et étude ethnobotanique de la flore du Sud-Ouest du Kef Ethnopharmacologia 39: 36-43.
Bicchi, C, C. Brunelli, C. Cordero, P. Rubiolo, M. Galli, and A. Sironi. 2004. Direct resistively heated column gas chromatography (Ultrafast module-GC) for high-speed analysis of essential oils of differing complexities. J. Chro-matogr. 1024: 195-207.
Calmasur, O., I. Asian, and F. Sahin. 2006. Insecticidal and acaricidal effect of three Lamiaceae plant essential oils against T. urticae Koch and Bemisia tabaci. Genn. Ind. Crops Prod. 23: 140-146.
Cavalcanti, S.C.H., E. Dos Niculau, A. F. Blank, C.A.G Câmera, I. N. Araujo, and P. B. Alves. 2010. Composition and acaricidal activity of Lippia sidoides essential oil against two-spotted spider mite (Tetranychus urticae Koch). Bioresour. Technol. 101: 829-832.
Chauve, C. 1998. The poultry red mite Dermanyssus gallinae (De Geer 1778) : current situation and future prospects for control. Vet. Parasitol. 79: 239-245.
Chermenskaya, T. D., E. A. Stepanycheva, A. V. Shchenikova, and A. S. Chakaeva. 2010. Insectoacaricidal and deterrent activities of extracts of Kyrgyzstan plants against three agricultural pests. Ind. Crops Prod. 32: 157-16.
Choi, W. I., S. G Lee, H. M. Park, and Y. J. Ahn. 2004. Toxicity of Plant essential oils to Tetranychus urticae ( Ac-ari: Tetranychidae) and Phytoseilus persimilis (Acari: Phytoseiidae). J. Econ. Entomol. 97: 553-558.
Cloyd, R. A., C. L. Galle, S. R. Keith, N. A. Kalscheur, and K E. Kemp. 2009. Effect of commercially available plant-derived essential oil products on arthropod pests. J. Econ. Entomol. 102: 1567-1579.
Crowell, L. P., S. W. Kennan, D. J. Haag, S. Alomad, E. Vedejs, and N. M. Gould. 1992. Chemoprevention of mammary carcinogenesis by hydroxylated derivatives of d-limonene. Carcinogenesis 13: 1261-1264.
Djeridane, A., J. M. Brunei, N. Vidal, M. Yousfi, E. H. Ajan-douz, and P. Stocker. 2008. Inhibition of porcine liver carboxylesterase by a new flavone glucoside isolated from Deverra scoparia. Chem. Biol. Interact. 172: 22-26.
Enan, E. 2001. Insecticidal activity of essential oils: octo-paminergic site of action. Comp. Biochem. Physiol. 130C: 325-327.
Finney, D. J., and W. L. Stevens. 1948. A table for the calculation of working probits and weights in probit analysis. Biometrika 35: 191-201. Floris, I., A. Satta, P. Cabras, V. Garau, and A. Angioni. 2004. Comparison between two thymol formulations in the control of Varroa destructor: effectiveness, persistence, and residues. J. Econ. Entomol. 97: 187-191.
Georghiou, G P. 1990. Overview of insecticide resistance. Am. Chem. Soc. Symp. Ser. 421: 18-41.
Ghrabi-Gammar, Z., D. R. George, A. Daoud-Bouattour, I. Ben Haj Jilani, S. Ben Saad-Limam, and O.A.E. Spara-gano. 2009. Screening of essential oils from wild-growing plants in Tunisia for their yield and toxicity to the poultry red mite, Dermanyssus gallinae. Ind. Crop. Prod. 30: 441-443.
Goff, L. 1986. Spider mites (Acari: Tetranychidae) in the Hawaiian Islands. Int. J. Acarol. 12: 43-49.
Han, J., B. R. Choi, S. G. Lee, S. I. Kim, and Y. J. Ahn. 2010. Toxicity of plant essential oils to acaricide-susceptible and -resistant Tetranychus urticae (Acari: Tetranychidae) and Neoseiulus californicus (Acari: Phytoseiidae). J. Econ. Entomol. 103: 1293-1298. Hance, T., and G. Van Impe. 1999. The influence of initial age structure on prey-predator interaction. Ecol. Model. 114: 195-211. Hazan, A., U. Gerson, and A. S. Tahori. 1974. Spider mite webbing. I. The production of webbing under various environmental conditions. Acarologia 16: 68-84.
Ho, C. C, C. C. Lo, and W. H. Chen. 1997. Spider mite (Acari: Tetranychidae) on various crops in Taiwan. J. Agric. Res. China 46: 333-346. Heuskin, S., B. Godin, P. Leroy, Q. Capella, J. P. Wathelet, F. Verheggen, and E. Haubruge. 2009. Fast gas chromatography characterisation of purified semiochemicals from essential oils of Matricaria chamomilla L. (Aster-aceae) and Nepeta cataria L. (Lamiaceae). J. Chromatogr. 1216: 2768-2775.
Hjorther, A. B., C. Christophersen, B. M. Hausen, and T. Menne. 1997. Occupational allergic contact dermatitis from carnosol, a naturally-occurring compound present in rosemary. Contact Dermatitis 37: 99-100.
Isman, M. B. 2000. Plant essential oils for pest and disease management. Crop Prot. 19: 603-608.
Isman, M.B. 2004. Plant essential oils as green pesticides for pest and disease management, pp. 41-51. In W. M. Nelson (ed.), Agricultural Applications in green chemistry. Am. Chem. Soc. Symp. Ser. 887. American Chemical Society, Washington, DC.
Isman, M. B., A. J. Wan, and C. M. Passreiter. 2001. Insecticidal activity of essential oils to the tobacco cutworm, Spodoptera lituta. Fitoterapia 72: 65-68.
Jiang, Z., Y. Akthar, R. Bradbury, X. Zhang, and M. B. Isman. 2009. Comparative toxicity of essential oils of Litsea pun-gens and Litsea cuheha and blends of their major constituents against the cabbage looper, Trichoplusia ni. J. Agric. Food Chem. 57: 4833-4837. Kouninki, H, T. Hance, A. Noudjouf, G. Lognay, F. Malaisse, B. Ngassoum, M. Mapongmetsemp, S. T. Ngamol, and E. Haubruge. 2007. Toxicity of some terpenoids of essential oils of Xylopia aethiopica from Cameroon against Sitophilus zeamais Motschulsky. J. Appl. Entomol. 131: 269-274.
Kubo, I., H. Muroi, and A. Kubo. 1994. Naturally occurring antiacne agents. J. Nat. Prod. 57: 9-17.
Lee, M. H, K. M. Choi, J. Han, S. B. An, S. H. Lee, J. Y. Choi, and D. N. Choi. 1994. Illustrated insect pests on medicinal crops. Agric. Sci. Inst. RDA, Korea.
Mansour, F., U. Ravid, and E. Putievsky. 1986. Studies on the effects of essential oils isolated from 14 species of Labiatae on the carmine spider mite, Tetranychus cinna-harinus. Phytoparasitica 14: 137-142.
constituents against Tetranychus urticae Koch (Acari: Tetranychidae) on two different host plants. Pest Manag. Sci. 62: 366-371.
Neves, I. de A., C.A.G. da Camara, J.C.S. de Oliviera, A. V. de Almeida. 2011. Acaricidal activity and essential oil composition of Petiveria alliacea L. from Pernambuco (northeast Brazil). J. Essent. Oil Res. 23: 23-26.
Overmeer, W.P.J. 1985. Alternative prey and other food resources, pp. 131-139. In Spider mites: their biology, natural enemies and control in world crop pests. Elsevier, Amsterdam, The Netherlands.
Pontes, W.J.T., J.C.S. Olivera, C.A.G. Camara, A.C.H.R. Lopes, M.G.C. Gondim, J. V. Oliveira, and M.O.E. Schwartz. 2007. Acaricidal activity of the essential oils of leaves and fruits of Xylopia sericea St. Hill. On the two spotted spider mite (Tetranychus urticae Koch). Quim. Nova 30: 838-841.
Roe, F.J.C. 1965. Chronic toxicity of essential oils and certain other products of natural origin. Food Cosmet. Toxi-col. 33: 311-324. Sathiyamoorthy, P., H. Lugasi-Evgi, P. Van-Damme, A. Abu-Rabia, J. Gopas, and G. Goldhirsh. 1997. Larvicidal activity in desert plants of the negev and bedouin market plant products. Int. J. Pharmacog. 35: 265-273.
Singh, U. P., B. Prithiviraj, B. K. Sarma, M. Singh, and A. B. Ray. 2001. Role of garlic (Allium sativum L.) inhuman and plant diseases. Indian J. Exp. Biol. 39: 310-322.
Sundaram, KM.S., R. Campbell, L. Sloane, and J. Studens. 1995. Uptake, translocation, persistence and fate of azadirachtin in aspen plants (Populus tremuloides Michx. ) and its effect on pestiferous two-spotted spider mite (Tetranychm urticae Koch). Crop Prot. 14: 415-421. Van Leeuwen, T., S. V. Pottelberge, and L. Tirry. 2006. Biochemical analysis of a chlorfenapyr selected resistant strain of Tetranychus urticae Koch. Pest Manag. Sci. 62: 425-433.
Van Leeuwen, T., J. Vontas, A. Tsagkarakou, and L. Tirry. 2009. Mechanisms of acaricide resistance in the two-spotted spider mite Tetranychus urticae. Am. Chem. Soc. Sym. Ser. (DOI: 10.1007/978-90-481-2316-2_14).