Pseudo-bimolecular [2+2] cycloaddition studied by time-resolved photoelectron spectroscopy
Texte intégral
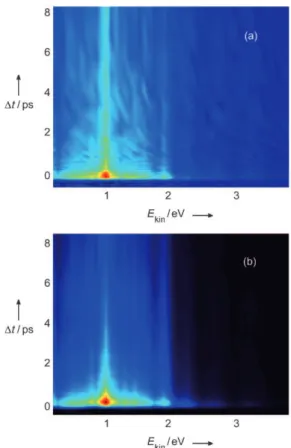
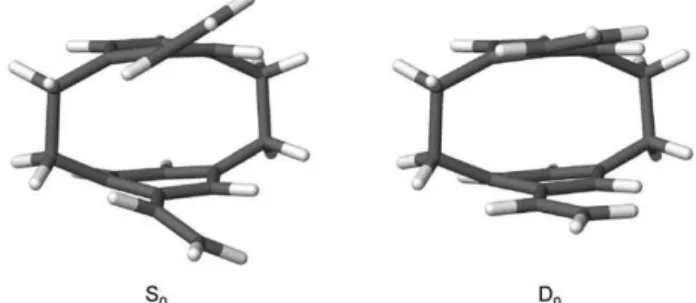
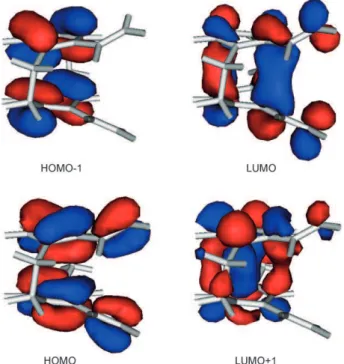
Figure


Documents relatifs
Abstract: Investigations based on NMR, MS and DFT studies shed light on the metallic species generated in the rhodium- catalyzed asymmetric [2+2+2]
We wondered about the regioselectivity observed in these reactions, especially for cases involving tertiary propargyl alcohols, insofar as the coor- dination of the free hydroxyl
In addition, Li and Luo have developed the first hetero [5 + + 2] cycloadditions of 2-(2-amino- ethyl)oxiranes with alkynes promoted by a combina- tion of FeCl 3 and BF 3 (Et 2 O)
CY 1'a can participate in polar 32CA reactions as a consequence of its high electrophilic and nucleophilic character, the low electrophilic and nucleophilic power of
Les cétènes ont largement démontré leur versatilité dans les réactions de cycloaddition [2+2] et [4+2] réagissant avec une grande diversité de substrats. Le
The reaction mixture was concentrated under reduced pressure and the crude product was purified by silica gel chromatography with ethyl acetate in pentane (1:9) to afford 3.38
The key-strategy underlines a highly chemo- and regioselective rhodium-catalyzed [2+2+2] cyclotrimerization between appropriately tailored yne-ynamides and
9 With the less aromatic indole ring, we show herein that 3-cyanoindoles behave again as C=C dipolarophiles 10 and undergo fast dearomatization through