The Effect of Annealing on the Microstructure of Cu-Al-Ni-Mn Shape Memory Alloy Microwires
by Keerti Shukla Submitted to the
Department of Materials Science and Engineering in Partial Fulfillment of the Requirements for the Degree of
Bachelor of Science in Materials Science and Engineering
ARCHNES
at the MASSACHUS-TTS INSTITUTE
OF ELCHNOLOLGY
Massachusetts Institute of Technology
SEP
3
0
2015
June 2015LIBRARIES
0 2015 Keerti Shukla. All rights reservedThe author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or
hereafter created.
Signature redacted
Signature of A uthor...
Keerti Shukla Department of Materials Science and Engineering May 1, 2015
Signature redacted
C ertified by ...Christopher A. Schuh Head of Department of Materials Science and Engineering Danae and Vasilios Salapatas Professor of Metallurgy
Thesis Supervisor
Signature redacted
Accepted by... ... Beah
Geoffrey Beach
The Effect of Annealing on the Microstructure of Cu-Al-Ni-Mn Shape Memory Alloy Microwires
by Keerti Shukla
Submitted to the
Department of Materials Science and Engineering in Partial Fulfillment of the Requirements for the Degree of
Bachelor of Science in Materials Science and Engineering Abstract
Shape memory alloys exhibit superelasticity and the shape memory effect by undergoing a diffusionless phase transformation between the austenite and martensite phases. Nickel-titanium
alloys are currently the most common material used. However, due to their expensive cost, alternatives such as Cu-based alloys have been investigated. Cu-based alloys have exhibited the shape memory effect and have achieved 6-8% strain recovery. This work investigates Cu-Al-Ni-Mn shape memory alloys in the form of microwires with the potential application in smart textiles. Wire microstructure and composition, transition temperatures, and strain recovery were
analyzed after the wires were subjected to varying annealing times and temperatures. These data were used to determine the ideal conditions to achieve the most shape memory and
superelasticity.
Thesis Supervisor: Christopher A. Schuh
Acknowledgements
First and foremost, I would like to thank Nihan Tuncer for advising me and guiding me throughout the entire thesis project. She patiently answered all my questions and was always willing to help out with experiments. This project would not have been possible without her help and support.
I would like to thank Professor Chris Schuh, my thesis advisor, for giving me a change to do my Senior Thesis project in his group. He has not only been a thesis advisor, but also a role model and advisor in my time at in DMSE at MIT.
I would also like to thank Ike Feitler for his immense help with experimentation. Whether it was advice on how to measure something, collecting materials, or machinery expertise, he was
always there to assist. Don Galler also helped me with SEM imaging and EDS data.
In addition to Nihan and Professor Schuh, the other members of the Schuh group have also been kind in welcoming me into the group, keeping me company in lab, and answering any questions I had.
Last but certainly not least, I would like to thank my parents, family, and friends for their constant support, guidance, and genuine interest in my lab work and research. Their excitement helped the sometimes long and stressful days go by quickly and this project would not have been possible without their support and encouragement.
Table of Contents Abstract ... 3 Acknowledge ments ... 5 Table of Contents ... 7 List of Figures ... 9 List of Tables ... 10 List of Equations ... 10 1. Introduction ... 11 1 .1 Sh ap e M em o ry E ffect ... 1 1 1 .2 S u p e re la sticity ... 1 2 1 .3 C ry sta llo g ra p h y ... 1 3 1.4 Transformation Temperatures ... 14
1 .5 M ate ria ls S ele ctio n ... 1 6 1 .6 O b je ctiv e s ... 1 7 2. Experimentation ... 19
2 .1 W ire P ro d u ctio n ... 1 9 2 .2 M icro stru ctu ra l A n aly sis ... 2 1 2.2.1 Encapsulation ... 21
2.2.2 Annealing and Quenching ... 21
2.2.3 M ounting and Polishing ... 22
2.2.4 Etching and Imaging ... 22
2 .3 C o m p o sitio n al A n alysis ... 2 2 2.4 Differential Scanning Calorimetry (DSC) ... 22
2.5 Dynamic Mechanical Analysis (DMA) ... 22
3. Results and Discussion ... 23
3 .1 A n n ealin g C o n d itio n s ... 2 3 3. 1. 1 Inconsistent M icrostructural Results ... 27
3.2 Transition Temperatures ... 29
3 .3 Stra in R eco v e ry ... 3 2 4. Conclusions ... 33
5. Future W ork ... 34
List of Figures
1.1. A hysteretic loop showing the change in length, or strain, as a shape memory material is heated and cooled through its transformation temperatures. ... 12 1.2. A hysteretic loop depicting the recoverable strains of shape memory materials held at a temperature above the austenite finish temperature... 13 1.3. Schematic showing the crystallographic transformations of shape memory materials
undergoing heating, cooling, and mechanical deformation. The horizontal green arrow corresponds to the shape memory effect and the vertical green arrow corresponds to
sup erelasticity . ... 14 1.4. This schematic shows the heat flow of a shape memory material as it is heated and cooled through its transform ation tem peratures. ... 15 1.5. A schematic of a wire with oligocrystalline structure, which consists of columnar grains parallel to the longitudinal axis... 18 1.6. Stress-strain plots showing the amount of recoverable strain for Cu-Al-Ni samples... 19 2.1. This schematic shows the melt-spin technique where the alloy is pushed out of the crucible and rapidly solidified in a water-coated wheel. ... 20 2.2. The microwires obtained after melt-spinning ... 20 2.3. The wire encapsulated inside a quartz tube pressurized with argon... 21 3.1. These plots show the relationship between the aspect ratio and the (a)temperature and
(b)annealing time where the threshold time and temperature is 4 hours and 9000C.. ... 24 3.2. This optical image of the highly oligocrystalline sample annealed at 9000C for 4 hours... 24 3.3. The images above depict the microstructure of three samples annealed at 9500C for (a) 2 hours, (b) 3 hours, and (c) 4 hours... 25 3.4. The images above depict the more oligocrystalline microstructure of three samples annealed
at 950"C for (a) 2 hours, (b) 3 hours, and (c) 4 hours but cooled to 9000C before quenching. .... 26 3.5. A subsequent trial of heat treatment at 9000C for 4 hours produced a non-oligocrystalline
w ire .. ... 2 6 3.6. Plot showing the ternary phase diagram for Cu-Al-Ni alloys at 900'C with low Mn samples m arked at the crosshairs... 29 3.7. These plots show (a) exothermic and (b) endothermic the heat flow of wire samples as they are heated from -800C to 800C and undergo phase transformations... 30 3.8. The microstructures of samples annealed at 9000C for (a) 2 hours, (b) 3 hours, and (c) 5 h o u rs... 3 1 3.9. A stress-strain plot that shows strain recovery of the sample in Figure 3.2 which underwent heat treatm ent at 9000C for 4 hours.. ... 33
List of Tables
2.1. The various combinations of annealing times and temperatures tested in various trials... 21
3.1. The atomic percent of each element in the Cu-Al-Ni-Mn alloys shown in Figures 3.2, 3.3,
3 .4 , an d 3 .5 ... 2 8
3.2. A chart showing the transformation temperatures and aspect ratio for each sample shown in
F ig u re 3 .8 . ... 3 2
List of Equations
I Introduction
Shape memory materials have been used for a wide variety of military, medical, and device applications due to their controlled and specific temperature and stress transitions. These materials can be seen in cell phone antennas, automotive actuators for transmission fluid control, and micro-electromechanical devices. They have even been used in eyeglass frames and in the underwire of women's brassieres'. The wide variety of applications for these materials has led to the increase in interest and research.
Shape memory properties arise from a diffusionless solid-to-solid reversible phase transformation between the strong, high temperature austenite phase and the weak, low temperature martensite phase. This phase transformation can be thermally induced or stress
induced under isothermal conditions. 1.1 Shape Memory Effect
The thermally induced phase transformation is depicted in Figure 1.1. Shape memory materials elongate and contract as they are heated and cooled through the transformation
temperatures. As the material is cooled, the sample transforms from austenite to martensite. This process occurs over temperatures ranging from the martensite start (Ms) to the martensite finish
(Mf) temperatures. When heated, the material transforms from martensite to austenite over temperatures ranging from the austenite start (As) to the austenite finish (Af) temperatures2. This
I
AT'
MI ~ Me AsA
Figure 1.1. A hysteretic loop showing the change in length, or strain, as a shape memory material is heated and cooled through its transformation temperatures2 3.
1.2 Superelasticity
The stress-induced phase transformation under isothermal conditions is shown in Figure 1.2. The sample must be at a temperature higher than the austenite finish temperature ensuring that it is completely in the austenite phase. As the strain on the sample increases the material transforms to martensite over a range of stresses from the martensite start stress (am,) to the martensite finish stress (amf). As the sample is unloaded, it returns back to the austenite phase over a range of stresses from the austenite start (aA,) to the austenite finish (A f). As long as the
operating temperature is above Ar, the stable phase is austenite and the sample will transform to austenite when unloaded. Since these materials can recover strains of up to 10% this effect is called superelasticity2,4,5.
100 a 80 S 60-20
C~
0 1 2 3 4 5 6 7 8Msreuto Stubl
4%)
Figure 1.2. A hysteretic loop depicting the recoverable strains of shape memory materials held at a temperature above the austenite finish temperature2.
1.3 Crystallography
From a crystallographic perspective, the austenite and martensite phases have different volumes and orientations. Figure 1.3 shows the shape memory effect and superelasticity from an atomistic perspective. The red lattice represents the austenite phase and the blue lattices represent the martensite phases. The four diagonal black lines represent each of the transformation
temperatures. The horizontal lines represent the transformation stresses.
For the shape memory effect, the sample follows the horizontal green arrow and is first cooled from austenite to the twinned martensite phase, which is represented by the zig-zag structure in the bottom left corner of Figure 1.3. There is no macroscopic volume change to the sample in this transformation. This process occurs by nucleation and growth of the martensite crystals. When deformed, the martensite de-twins and the atomic planes reorient without causing slip or permanent deformation. Then, when the sample is heated above Af, it returns to original the austenite phase4.
For the superelastic effect, the sample starts at a temperature above Af. It follows the vertical green arrow and is loaded above am-f. The sample transforms completely to the martensite phase. When the sample is unloaded, it returns back to the original austenite phase that is stable above Af4.
S t
r
S OM 'aA. Defor Martt No volumetric change Twinned MartensiteMMA,
A,
Tm
p
rator.
Figure 1.3. Schematic showing the crystallographic transformations of shape memory materials undergoing heating, cooling, and mechanical deformation. The horizontal green arrow corresponds to the shape memory effect and the vertical green arrow corresponds to
4
superelasticity .
1.4 Transformation Temperatures
Transformation temperatures are a key property of shape memory materials that affect the potential applications. For superelasticity, the operating temperature must be higher than Af. For
med ensite
shape memory, the transition temperatures determine how much the sample must be heated to return back to its original "parent" shape.
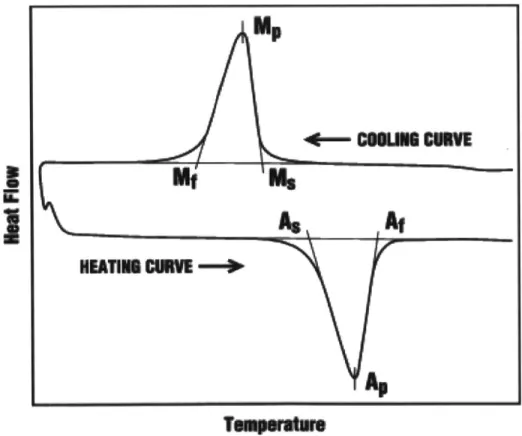
To accurately determine the transformation temperatures Differential Scanning
Calorimetry (DSC) is used. This technique measures the amount of heat given off or absorbed by a sample as it is heated and cooled through its transformation temperatures. A generic output for a shape memory material is show in Figure 1.4. The top curve shows the endothermic reverse transformation as the sample is cooled from the austenite phase to the martensite phase. The bottom curve shows the exothermic forward transformation as the sample transforms from the martensite phase to the austenite phase2
I
MITI C
MEWOP
Figure 1.4. This schematic shows the heat flow of a shape memory material as it is heated and cooled through its transformation temperatures2
Often times, alloys with Al will depict multiple jagged DSC peaks due to the presence of martensite variants. Each variant has their own stacking sequence period and structure symmetry. Two common martensites seen are in Cu-based alloys with Al are 2H and 18R where the H stands for hexagonal and R stands for rhombohedral. The 2H martensite is common in sections of the sample with high Al content and the 18R martensite is more common in sections with lower Al content. The 18R phase will produce smoother peaks in DSC experiments similar to what is show in Figure 1.4. The 2H phase will show multiple sharper peaks.
1.5 Materials Selection
Nitinol, a nickel-titanium alloy, is the most commonly used shape memory alloy in industry due to its biocompatibility and robustness. Nitinol has been used in stents, sutures, and orthodontic brackets'. However, Nitinol is costly to produce7. Cu-based alloys are cheaper and have shown shape memory properties that are on par with Nitinol. Furthermore, Cu-based alloys can be welded and have high thermal and electronic conductivities .
Cu-Al binary systems were used because they have several ordered phases which allow for shape memory properties. But Cu-Al alloys also have high elastic anisotropy leading to poor mechanical properties, easy intercrystalline failure, and short fatigue life cycle9. The addition of manganese and decrease of aluminum content increases the ductility, shape memory effect, and long-range order. It has also been found that the addition of nickel, an element soluble in the Cu-Al matrix, decreases the martensite-austenite transition temperatures, which is a key
transformation in superelasticityl 12. For these reasons, Cu-Al-Mn-Ni alloys were investigated
1.6 Objectives
Using Nitinol as the standard for shape memory materials, Cu-Al-Ni-Mn alloys in the
form of microwires were analyzed. Microwires are advantageous for their potential application 13
in smart textiles such as shirts that un-wrinkle with when exposed to body heat .Microwires have a large surface area to volume ratio. This allows for quick martensite-austenite phase transformations due to rapid heat transfer. For shape memory to occur, the microwires need to be austenite at room temperature as described in Section 1.1. The rapid heat transfer is critical in
allowing the complete transformation to the austenite phase.
Additionally, the microstructure of the wires can greatly affect the shape memory and superelasticity. Single crystal Cu-based shape memory materials have shown strain recovery of
~10%5 . The absence of grain boundaries makes the martensite-austenite phase transformation easier. However, single crystal materials are expensive to produce thus rendering single crystal
Cu-based alloys useless replacements for Nitinol.
In polycrystalline materials, grain boundaries resist the grown of the high-volume
martensite phase. Martensite grows via nucleation and growth and grain boundaries inhibit the
austenite to martensite transformation. Also, stress concentrations near grain boundaries and triple junctions lead to slow transformations and early failure5. In addition, polycrystalline Cu-based shape memory allows are also too brittle to be cold-worked which nullifies their used as
cyclic shape memory materials.
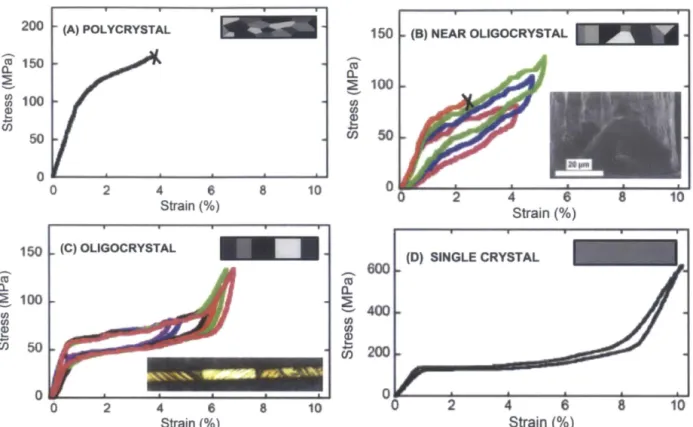
It has been shown that wires with oligocrystalline structures, shown in Figure 1.5, behave 5
Figure 1.5. A schematic of a wire with oligocrystalline structure, which consists of columnar grains parallel to the longitudinal axis.
Figure 1.6 shows the stress strain curve for polycrystalline, near oligocrystalline, oligocrystalline, and single crystal Cu-based shape memory alloy samples. The brittle
polycrystalline materials fail at low strains and do not show any recoverable strain. However, as the amount of oligocrystallinity increases and nears single crystal behavior, the amount of recoverable strain increases. The columnar grain structure in oligocrystalline materials
minimizes the grain boundary resistance during phase transformations. While single crystal materials still show better strain recovery, the cost and difficulty of producing them makes oligocrystalline materials a more viable option. The polycrystalline microwires were subjected to varying annealing times and temperatures to determine the ideal conditions to achieve grain growth into oligocrystalline structures with 6-8% recoverable strain.
200 (A)POLYCRYSTAL
co
150 a_ 6 ~ 100 ~ Ci5 50 2 4 6 Strain(%) 150 (C) OLIGOCRYSTALco
a_ 6 100"'
"'
~ Ci5 50 8 10m-
a..e,
en en ~ Ci3 2:.
'
·. . '\'t
'I, E:J!!ill I -4 6 8 10 Strain(%)- eOO (D) SINGLE CRYSTAL
<O a..
6
"'
"'
~ Ci5 0 0 2 4 6 8 10 2 4 6 8 10 Strain(%) Strain(%)Figure 1.6. Stress-strain plots showing the amount of recoverable strain for Cu-Al-Ni samples5.
2 Experimentation 2.1 Wire Production
The wires were produced using a rapid solidification technique known as melt-spinning shown in Figure 2.115• The Cu-Al-Ni-Mn alloy melt is heated in a crucible with induction coils
and held at a temperature of 1100 °C. The alloy is drawn out of a nozzle with a diameter of 200 µm. The molten alloy flows into a spinning wheel below that contains the water as the cooling medium. The wheel continuously spins at 325 rpm until the melt is depleted and the wire is completely drawn. Parameters such as cooling medium, ejection temperature, wheel velocity, and nozzle diameter all affect the final wire microstructure, diameter, and mechanical properties.
Figure 2.1. This schematic shows the melt-spin technique where the alloy is pushed out of the crucible and rapidly solidified in a water-coated wheel'.
Figure 2.2. The microwires obtained after melt-spinning.
P Gas pressure Crucible
*
0
Induction ci M 0 --- -- Molten alloy 0 diameter Cooling medium Liquid jetI hEP
V Wheel velocity2.2 Microstructural Analysis 2.2.1 Encapsulation
Before heat treatment, the microwires were cut into 1-2 inch pieces and placed in hollow Quartz tubes. These tubes are pressurized and with argon. The ends of the quartz tubes are sealed using an oxyhydrogen torch. The final sealed tube encapsulated the microwire sample is shown
in Figure 2.3.
Figure 2.3. The wire encapsulated inside a quartz tube pressurized with argon. 2.2.2 Annealing and Quenching
The encapsulated wire samples were heated and in a furnace. The furnace takes 0.5 hours to ramp up to the final annealing temperatures. Various samples and combinations of annealing times and temperatures were tested as show in Table 2.1 below. Some samples underwent through a multi-step annealing process. After annealing, the samples were quenched in ice water
at approximately -4 "C.
Table 2.1. The combinations of annealing times and temperatures tested.
Time (hours) Temperature (UC)
2 950 2 900 3 850 3 950 3 900 4 850 4 900 5 850
Time Temperature Time Temperature (hours) 1 ("C) (hours) 2 (*C) 2 900 N/A N/A 3 900 N/A N/A 4 900 N/A N/A 2 950 1 900 3 950 1 900 4 950 1 900
2.2.3 Mounting and Polishing
The annealed and encapsulated wires were removed from the quartz tubing and mounted in ConduFast using a hot press mounting system. These mounted samples were grinded on silicon carbide paper with varying gratings from 800 to 4000 pm. The samples were then polished with I ptm A1203 followed by a colloidal silica suspension.
2.2.4 Etching and Imaging
The polishing microwire samples were etched with a 50% nitric acid solution that has been proven to adequately show the grains of Cu-based alloys. Post-etching, the samples were
imaged using an optical microscope to determine the size and arrangement of the grains. 2.3 Compositional Analysis
To determine the elemental make-up, the samples were placed in a scanning electron microscope (SEM) under vacuum. Energy dispersive x-ray spectroscopy (EDS) was used to determine the elemental composition. The wires were irradiated with x-rays at 15-20kV to determine the elemental composition of Cu, Al, Ni, and Mn.
2.4 Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC) was used to determine the four transition temperatures of the microwires. DSC testing measures the heat flow in the sample over a range of temperatures from -80 0C to 80 'C. The DSC output produces two curves as seen in Figure
1.4. The upper curve is endothermic and represents the martensite to austenite transformation. The lower curve is exothernic and represents the austenite to martensite transition.
2.5 Dynamic Mechanical Analysis (DMA)
Dynamic mechanical testing is the process of loading and unloading the material. This measures the percent of strain recovery a material can undergo and still return to its original
shape when unloaded. Strain recovery is a key feature of shape memory alloys and is a measure of the amount of superelasticity. Figure 1.6 depicts data collected using DMA.
3 Results and Discussion 3.1 Annealing Conditions
The microstructure of the microwires was analyzed to determine the ideal time and temperature conditions for annealing to achieve the most oligocrystalline structure.
Samples were annealed at varying times and temperatures as shown in Tables 2.1 (a) and (b). Aspect Ratio was used to measure and compare the "amount of oligocrystallinity" a wire. The aspect ratio was used in conjunction with visual observations of the optical images of the microstructure. The width and length of the grains of each sample were measured and the aspect
ratio was calculated as shown in Equation 3.1.
grain length (parallel to axis of wire) grain width (perpendicular to axis of wire)
The arrows in Figure 3.2 show the width and length used to measure the aspect ratio of a grain. The width of the grains often equaled the width of the wires. Therefore, a high aspect ratio was a good indicator of rectangular grains and high oligocrystallinity. It directly compared the grain lengths of varying samples.
Figure 3.1 shows the average aspect ratio plotted versus annealing time and temperature. These data show that an annealing temperature of 9000C and time of 4 hours produced the largest and most oligocrystalline microstructure. This is corroborated by the optical microscopy image of the grains shown in Figure 3.2.
Temperature vs. Aspect Ratio
25 -20 -10 S5-0 8 (b) Temperature (2C)Annealing Time vs. Aspect Ratio
-I
6
Annealing Time
1 4
(hours)
Figure 3.1. These plots show the relationship between the aspect ratio and the (a)temperature and (b)annealing time where the threshold time and temperature is 4 hours and 9000C.
Figure 3.2. This optical image of the highly oligocrystalline sample annealed at 900'C for 4 hours. p3 (a)_
I
40 860 25 20 -20 15 0 -5 880 900 920 940 960 2 3 5 IAnnealing at 900°C showed good results; however, there is clearly large variability in the grain sizes per sample. Since grain growth is a thermally activated process, 950°C were expected to produce more oligocrystalline structures in less time. Figure 3.3 shows the microstructure of three wires annealed at 950°C for 2, 3, and 4 hours.
Figure 3.3. The images above depict the microstructure of three samples annealed at 950°C
for (a) 2 hours, (b) 3 hours, and (c) 4 hours.
The sample shown in Figure 3.3(a) is highly oligocrystalline and similar to the sample shown in Figure 3.2. However, increasing annealing time at 950°C did not increase the amount of oligocrystallinity. However, at the elevated annealing temperatures, the alloy could lie within a two phase region. To mitigate this issue, samples were annealed at 950°C, then cooled to 900°C before quenching. Figure 3.4 shows three samples annealed at 950°C for 2 hours, 3 hours, and 4 hours and then cooled to 900°C before quenching.
Figure 3.4. The images above depict the more oligocrystalline microstructure of three
samples annealed at 950°C for (a) 2 hours, (b) 3 hours, and (c) 4 hours but cooled to 900°C before quenching.
The microstructures of the samples in Figure 3.4 are single phase and more
oligocrystalline than samples quenched from 950°C. However, the largest annealing time did not produce the most oligocrystalline structure. The wire annealed at 950°C for 3 hours produced the sample with the highest aspect ratio. Furthermore, in subsequent trials of 900°C for 4 hours, wires produced non-oligocrystalline multiphase microstructures as shown in Figure 3.5.
Figure 3.5. A subsequent trial of heat treatment at 900°C for 4 hours produced a
non-oligocrystalline wire.
These data were not consistent with the predicted thermally activated grain growth. In conducting microstructural analysis, it was assumed that the wires were all homogeneous and identical to start. These inconsistencies may disprove this assumption that all the wire segments
had identical microstructures. Further experiments were conducted to find the cause of these inconsistencies.
3.1.1 Inconsistent Microstructural Results
The inconsistent microstructural data seen in heat treatment experiments is due to varying initial microstructures and compositional differences. It is possible that all the wire segments did not have the same microstructure post melt-spinning. Since grain growth is a thermally activated process, the initial microstructure can greatly affect the post heat treatment microstructure. A sample starting off with many small grains will require more heat and more time to produce an oligocrystalline structure. It is difficult to capture the starting microstructure of the wire segment. To see the microstructure, the wire segment has to be mounted, polished, etched and imaged. By the end of this process the wire is unable to be removed from the mount and heat treated. The assumption that all the samples were homogeneous was made for simplicity in the
experimentation process, but given the data, this assumption is most likely not valid. Another cause for the inconsistencies is the compositional variation among the wire segments. Table 3.1 shows the composition for the wire segments shown in Figures 3.2, 3.3, 3.4, and 3.5. The composition data in conjunction with the optical microscopy images of the grains
shows a correlation between elemental composition and microstructure.
Decreased composition of Al and Mn leads to poor grain growth, low oligocrystallinity, and low resulting aspect ratios. The lowered compositional values also increase brittleness of the wires, inhibit grain growth, and slow grain boundary movement. All of these contribute to the lack of oligocrystalline structures observed in the wires with lowered composition of Al and Mn.
Table 3.1. The atomic percent of each element in the Cu-Al-Ni-Mn alloys shown in Figures 3.2, 3.3, 3.4, and 3.5.
Sample Annealing %CU %AI %Ni %Mn Aspect
Conditions Ratio Figure 3.2 9000C - 4 hours 69.7 24.1 2.6 3.6 10.93 Figure 3.5 900'C - 4 hours 75.27 20.7 3.3 0.7 1.77 Figure 3.3 9500C -2 hours 70.7 23 2.6 3.6 7.96 (a) Figure 3.3 9500C -3 hours 89 7.4 3.3 0.4 4.94 (b) Figure 3.3 9500C -4 hours 89.2 8.2 2.3 0.2 2.74 (c) Figure 3.4 9500C - 2 hours;
Fge quenched from 66.2 27.27 3.37 3.2 5.94
(a)
9000C
Figure 3.4 950'C - 3 hours; (b) quenched from 65.63 27.73 2.63 3.97 2.92 (b)__9000
C
Figure 3.4 9500C - 4 hours; Fgr quenched from 64.95 27.85 3.25 3.95 5.84(c)
9000
C
1_1_1 _ 1The varied composition also affects the phases present at the annealing temperatures. Currently, no phase diagram exists for Cu-Al-Ni-Mn alloys. But the Cu-Al-Ni phase diagrams can be used to analyze samples with less than 1% Mn as they are essentially Cu-Al-Ni alloys. Figure 3.6 shows the phase diagram for Cu-Al-Ni alloys at 900C 1 6. The crosshairs represent approximately where the samples with low Mn content lie on the phase diagram. All three
samples lie within a two phase region which leads to the non-oligocrystalline multiple phase structure we see in Figures 3.5, 3.5(b), and 3.5(c).
Cu A / 10 2 30 0 0 60 70 80 90 9000C (1173K) 90 z + z ~40 30 20 10 10 20 30 40 50 60 70 80 90 Al Ni2A3 at.% Ni Ni
Figure 3.6. Plot showing the ternary phase diagram for Cu-Al-Ni alloys at 900'C with low
Mn samples marked at the crosshairs6.
It seems that the addition of Mn modifies the phase diagram such that at 900'C, the
sample is not within the two phase region. For Cu-Al-Ni-Mn alloys, there is an ideal composition
range where the sample will likely be oligocrystalline when annealed at 900'C for 4 hours of
approximately 65-70% Cu, 25% Al, 3% Ni, and 3.5% Mn. Outside of this ideal composition
range, either alternate annealing conditions must be used or the sample will not be shape memory
due to presence of two phases.
3.2 Transition Temperatures
Differential Scanning Calorimetry (DSC) was used to determine the Ms, Mf, As, and Af
temperatures for each of the samples. Due to time constraints, only samples from one experiment
were tested in the DSC. Of those samples, only wire segments annealed at 900'C showed peaks.
80
70
\6
Figure 3.7 below shows the (a)forward and (b)reverse transformations for samples annealed at 900'C for 2, 3, 4, and 5 hours.
(a)
0.1
Forward Transition for 900'C samples
2 2 hours -3 hours 4 hours 5 hours -0.5 -0.6 -07 0.8 -90 -40 10 60 110 160 Temperature ("C) (b)
0.6
Reverse Transition for 900'C samples
2 hours 0.5 3 hours 0.4 4 hours 5 hours 0.2 0.1 0 -80 -60 -40 -20 0 20 40 60 80 Temperature ("C)Figure 3.7. These plots show (a) exothermic and (b) endothermic the heat flow of wire samples as they are heated from -80'C to 80'C and undergo phase transformations.
The sample annealed at 900°C for 3 hours showed no peaks within this temperature range. Either the transformation occurs at temperatures higher than 80°C or lower than -80°C, or the sample is simply not shape memory and will not have phase transformations.
The microstructure of these same samples are shown in Figure 3.8. The sample annealed at 900°C for 4 hours was lost during the grinding and polishing however the samples annealed at 2, 3, and 5 hours are showing in Figure 3.8(a)-(c) respectively.
Figure 3.8. The microstructures of samples annealed at 900°C for (a) 2 hours, (b) 3 hours,
and ( c) 5 hours.
As seen with many other samples, the microstructural results are inconsistent with the expected thermally activated growth of grains. The sample annealed for the shortest time shows the most oligocrystalline structure. However, the relationship between aspect ratio of the grain sizes and transformation temperatures is consistent with the expected trend. As the aspect ratio increases, there are fewer grain boundaries. The lower the grain boundary surface area, the lower the resistance for the martensite-austenite transformation, and the easier it is for the phase
transformation to occur. Table 3.2 shows the transformation temperatures and aspect ratios of each sample. As the aspect ratio of the sample increases, the Ms temperature decreases. This
martensite phase is favorable due to less friction and resistance by the gran boundaries.
Similarly, the higher the aspect ratio, the lower the As temperature. As in, the sample does not have to be heated as much before the high-temperature austenite phase is favorable.
Table 3.2. A chart showing the transformation temperatures and aspect ratio for each sample shown in Figure 3.8.
Transformation Temperatures
Aspect
Samples
M_
Mf
As
Af
Ratio
9000C -2
-19.719 -40.407 -70.244 -45.429 6.56
hours
9000C-3 N/A N/A N/A
N/A 3.91 hours 9000C - 5 -7.456 -53.957 -76.73 -42.602 4.98 hours 3.3 Strain Recovery
Dynamic mechanical analysis (DMA) was used to determine the amount of strain the sample could withstand and still return to its original shape when unloaded. Due to time
constraints, only the highly oligocrystalline samples were tested. The stress-strain curve for the sample annealed at 9000C for 4 hours is shown in Figure 3.9 The single phase, highly
oligocrystalline microstructure is shown in Figure 3.2. This sample falls within the ideal compositional range, as shown in Table 3.1, and has an oligocrystalline microstructure. This sample exhibits -5.2% strain recovery which is on par with Nitinol. This shows that when produced and heat treated correctly, Cu-Al-Ni-Mn alloy microwires could be a viable cheaper alternative to Nitinol with respect to strain recovery.
250
-
900LC for 4 hours (Figure 3.2)
200
-150 -
-e
100
-50
-1 0 1 2 3 4 5 6 StrainFigure 3.9. A stress-strain plot that shows strain recovery of the sample in Figure 3.2 which underwent heat treatment at 9000C for 4 hours.
4 Conclusions
Cu-Al-Ni-Mn is a viable cheaper alternative to Nitinol. Cu-based shape memory alloys also have high thermal and electrical conductivities which allows for a broader range of applications as compared with Nitinol. This study showed that melt-spinning could rapidly produce long shape memory microwires and with post-production heat treatment, these wires can exhibit shape memory and superelasticity.
In this study, the aspect ratio became the metric used to quantify the "amount of
oligocrystallinity" in the sample. The optical microstructure images were used to corroborate the aspect ratio calculations. Initial microstructural analysis showed a threshold annealing time and temperature of 4 hours and 900'C to obtain the largest aspect ratio. However, further analysis
generally valid when the composition of Al is approximately 25 atomic percent and Mn is approximately 3.5 atomic percent. The heat treatment time could be shortened by increasing the annealing temperature from 900"C to 950"C. Those annealing conditions can achieve nearly oligocrystalline microstructure wire segment if the sample is cooled to 900"C before quenching. The ideal annealing time at 950'C will be dependent on composition as well as starting
microstructure.
The differential scanning calorimetry testing showed that as the aspect ratio increases the samples have to be cooled/heated less to undergo a phase transformation. There may be
compositional affects on transition time and temperature as well since composition affects the microstructure.
The dynamic mechanical testing proved that Cu-Al-Ni-Mn alloys can achieve strain recovery of ~5.2% which is on par with Nitinol. When these alloys fall within the ideal
composition range and are treated to produce the oligocrystalline structure, they are viable cheaper shape memory alternatives to Nitinol.
5 Future Work
This study has flagged many different aspects of shape memory microwire production, treatment, and analysis that should be investigated further to understand all the potential applications of Cu-based shape memory alloys.
While the melt-spinning technology allows for rapid production of long microwires, the resulting wires are neither homogeneous in composition nor microstructure. It has been shown that the melt-spinning technique reduces the grain size Cu-based alloy microwires . In knowing this as a side effect of the production technique, other parameters can be tuned to minimize the reduction in grain size. Producing more consistent wires with this methodology would allow for
a better understanding of the direct effect of annealing conditions. A study examining the effects of cooling medium, cooling medium temperature, ejection temperature, wheel velocity, and nozzle diameter should be conducted to allow for a more homogeneous production of the microwires.
Another challenge is the inability to compare a wire segment to itself. In this research study, it was assumed that all wire segments from the same batch were homogeneous and identical in microstructure. However, this may not be an acceptable assumption due to the variability in the wire production process. Electropolishing the wires before encapsulation and heat treatment can show the grain boundaries on the surface of the wires. This could provide a
better insight into the initial microstructure of the wires. Annealing conditions could be
correlated with the change in aspect ratio or delta in microstructure allowing for a more accurate understanding of the grain growth and grain boundary movement in Cu-Al-Ni-Mn alloys.
Further studies should be conducted to better understand the affect of composition on microstructure and transformation temperatures. This study could elucidate whether the shape memory wires must be within a very specific range of composition to achieve superelasticity and shape memory or if the post production heat treatment conditions can increase the allowable range of compositions. It would also be helpful to find the ideal annealing conditions for different compositional ranges and the maximum and minimum allowable compositions to ensure shape memory properties. Since composition and temperature are interconnected via the phase diagram, it is also essential to gain a better understanding of the Cu-Al-Ni-Mn phase diagram.
It has also been shown in some literature that using high temperature annealing to increase the grain size can result in an increase in both the M, and A, temperatures of some
rapidly solidified Cu-based shape memory alloys7. Lower annealing temperatures and longer annealing times may produce wires with lower transformation temperatures. For certain smart textile applications, this increase in transformation temperature may not be desirable. A study could be performed to corroborate this effect of heat treatment on the Cu-Al-Ni-Mn alloys. Further analysis could be done to tune the annealing process at 900"C to lessen the effects of high temperature heat treatment.
Nihan Tuncer, a member of the Schuh Group, works on the Cu-based shape memory microwires as well. Nihan had noticed that wire sample size can affect the microstructure and compositional make-up post heat treatment. The decrease on Mn composition is not seen as frequently when the samples are annealed in large sizes. This suggests that Mn and possibly Al, may be diffusing out of the sample or reacting with impurities in the air during the heat treatment process. In this study, no experiments were conducted to investigate the effects of sample size. However, for textile, medical devices, and other applications, the effects of sample size are important and should be further studied.
The dynamic mechanical testing conducted in this study showed -5.2% strain recovery. This is fairly comparable to Nitinol which means Cu-based alloy shape memory wires area viable alternative to Nitinol2. However, more comprehensive mechanical tests should be conducted on all the samples to correlate microstructure and composition with percent of strain recovery.
Lastly, smart textiles such as wrinkle-free shirts pose an interesting challenge for microwires I,13. The mechanical properties of wires woven together can be tested to better understand how such a structure would behave in the presence of heat or strain.
6 References
1. Wu, M. H. & Schetky, L. M. Industrial Applications for Shape Memory Alloys. Int. Conf
Shape Mem. Superelastic Technolgies 171-182 (2000).
2. Matthey, J. Nitinol Alloys. (2015). at <http://jmmedical.com/> 3. Funakubo, H. Shape Memory Alloys. (University of Tokyo, 1984).
4. Gager, M. Cu-based Shape Memory Microwires Towards Complex Structures. (2014). 5. Ueland, S. M., Chen, Y. & Schuh, C. A. Oligocrystalline shape memory alloys. Adv.
Funct. Mater. 22, 2094-2099 (2012).
6. Sari, U. & Aksoy, 1. Electron microscopy study of 2H and 18R martensites in Cu-l 1.92 wt% Al-3.78 wt% Ni shape memory alloy. J. Alloys Compd. 417, 138-142 (2006). 7. Wilkes, K. E., Liaw, P. K. & Wilkes, K. E. The fatigue behavior of shape-memory alloys.
Jom 52, 45-51 (2000).
8. Cederstrom, J. & Humbeeck, J. Van. Relationship Between Shape Memory Material Properties and Applications I .Shape memory properties and materials 2 .Shape Memory Applications. 5, (1995).
9. Aron, H. B. & Aaronson, H. I. Growth of grain boundary percipitates in Al-4% Cu by interfacial diffusion. Acta Metall. 16, 789-798 (1968).
10. Canbay, C. A., Genc, Z. K. & Sekerci, M. Thermal and structural characterization of Cu-Al-Mn-X (Ti, Ni) shape memory alloys. Appl. Phys. A Mater. Sci. Process. 115, 371-377 (2014).
11. Suresh, N. & Ramamurty, U. Effect of aging on mechanical behavior of single crystal Cu-Al-Ni shape memory alloys. Mater. Sci. Eng. A 454-455, 492-499 (2007).
12. Lara-Rodriguez, G. a., Gonzalez, G., Flores-Zn'iiga, H. & Cortds-Perez, J. The effect of rapid solidification and grain size on the transformation temperatures of Cu-Al-Be melt
spun alloys. Mater. Charact. 57, 154-159 (2006).
13. Wan, T. & Stylios, G. K. Shape Memory Training for Intelligent Fabrics. Rjta 11, 11 (2007).
14. Sutou, Y., Omori, T., Wang, J. J., Kainuma, R. & Ishida, K. Effect of grain size and texture on superelasticity of Cu-Al-Mn-based shape memory alloys. J. Phys. IVFr.
511-15. Ochin, P. et al. Shape memory thin round wires produced by the in rotating water melt-spinning technique. Adca Mater. 54, 1877-1885 (2006).
16. International, A. Alloy Phase Diagram Database. (2006). at <www1.asminternational.org> 17. Wood, J. V. & Shingu, P. H. The effect of processing conditions and subsequent heat
treatment on the transformation behavior of some rapidly solidified copper-base shape memory alloys. Metall. Trans. A 15, 471-480 (1984).