HAL Id: hal-02842702
https://hal.inrae.fr/hal-02842702
Submitted on 7 Jun 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Significant proportions of unexpected males in progenies
from single pair matings with sibling sex reversed males
of Oreochromis niloticus
Jean-François Baroiller
To cite this version:
Jean-François Baroiller. Significant proportions of unexpected males in progenies from single pair
matings with sibling sex reversed males of Oreochromis niloticus. The Third International Symposium
on Tilapia in Aquaculture, 41, ICLARM, 574 p., 1996, ICLARM Conference Proceedings,
971-8709-42-8. �hal-02842702�
Slgnlficant Proportions of Unexpected Males
in Progenies from Single Pair Matings with Sibling
Sex Reversed Males of Oreocbromls nllotlcus
J.F. BAROILLE.R•
Programme çquaculture et pêche du Centre de coopération
Internationale en recherche agronomique pour Je développement,
Départment d'élevage et de médecine vétérinaire (CIRAD-EMVT)
BP 5095, 34033 Montpellier Cedex
Of,France
Département piscicole de J'!nstitut des Savanes (J'!DESSA)
BP
62f,Bouake
Of,Côte d'ivoire
BAROILLER. J.F. 1996. Slgnlflcant proportions of unexpected males in progenles from single pair matings with sibling sex reversed males of Oreochromls niloticus, p. 229-237. ln R.S.V. Pullîn,
J.
Lazard, M. Legendre, J.B. Amon Kothias and O. Pauly (eds.) The Third International Symposium on Tilapia in Aquaculture. ICLARM Conf. Proc. 4 t, 575 p.Abstract
The profltablllty of tilapia culture ls dlrectly influenced by the efficient control of lts reproduction through artlfklal me.ans. Thus, many studies have focused on gonadal differentlatlon and sex determinatlon. ln Oreochromis niloticus, XX sex reversed males (produced by hormone treatment and analysîs of the sex ratio of their progenles from single pair matlngs) can be used as a reliable tool to analyze both the efficiency of masculinizing treatments and sex determination. Twenty-two broodfish from Twenty-two sets of sibling sex reversed males were tested through 35 single pair matlngs by microscopie sexing of all 4.546 fry. The 35 crosses ylelded progenies composed of a majority of females, but all-female progenîes were observed in only five reproductions. The other progenles yielded sex ratios slgnificantly different from 1: 1, ranging from 65 to 99% female with an average of 85%. Three unexpected males from these crosses were further tested; their progenîes also lncluded unexpected males. These results cannot be explained by a simple monofactorîal sex determlnlng system. Environmental factors may be involved in the sex determlnlng mechanisms
or
o.
nllot/cus.Introduction
Oreochromis niloticus
is one of the two major cultured specles of tilapias in the world (Pull in 1983). lts ability to reproduce early {Baroîller and jalabert t 989) leads, in c.ontrolled environments, to rapid overpopulation with a tendency to stuntlng. The efficient contrai of tilapia reproduction through artificial means directly influences the profitability of its culture. Sînce growth potential is higher in male tilapias than in females (Hanson et al. 1983), three methods are-Current address: CIRAD-EMVT, Laboratoire de physiologie des poissons, INRA, Campus de Beaulieu, 35042 Rennes Cedex, France.
usually proposed to produce ail-male populations. These are manual sexing. hybridization, and hormonal sex reversai (see reviews of Guerrero 1982: Lovshin
t 982; Wohlfarth and Hulata 1983;
Pandian and Varadaraj 1987). On the average, manual sexing involves 2.
7-10% error, and also requires the elimination of 50% of the population after two to three months of rearing (Baroiller and jalabert 1989). Because of its efficiency and reliability, hormonal sex reversai is today the most widely used method among the three. However, since the metabollsm and the effects on the envîronment of the degradation products of synthetic androgen are not
fully understood in fish, this technique is not authorized in some countries. Hybridization between two parental species of aquaculture interest such as
Oreochromis ni/oticus
andO. aureus
does not generally produce ail-male populations (Majumdar and McAndrew 1983; Wohlfarth and Wedekind 1991 ). The viability of the YY genotype inO. niloticus,
as demonstrated by Baroiller ( 1988b), Baroiller and Jalabert ( 1989) and Scott et al. ( 1989). suggests a fourth, intraspecific approach. lnO. niloticus
where the male is heterogametic, a feminization treatment produces XY sex reversed females which, when crossedwith an XY male, produce a new YY
genotype. Each progeny from crosses between this homogametic YY male and
any homogametic XX female is
theo-retically an all-male population. However, these inter- and intraspecific crosses sometimes yield sex ratios which cannot be explained by a simple XX/ XY monofactorial sex determining mecha-nism in
O. niloticus.
To explain these unexpected sex ratios, three other models have been proposed (see reviews of Avtalion and Don 1990; Mair et al. 1991; Wohlfarth and Wedekind 1991 ): a theory combining two alleles from an autosomal locus and two of the three sex chromosomes (Avtalîon and Hammerman 1978; Hammerman and Avtalion 1979): a polygenic mode! (Majumdar and McAndrew 1983}; and finally, a monofactorial sex determination with the possible presence of a rare autosomal gene, epistatic to major genes. However, these three theories do not explain ail the results Found ln the lit-erature.ln order to analyze sex determina-tion in
O. niloticus,
two sets of sibling sex reversed males were pr.oduced and tested in single pair matings.Materlals and Methods
Animais
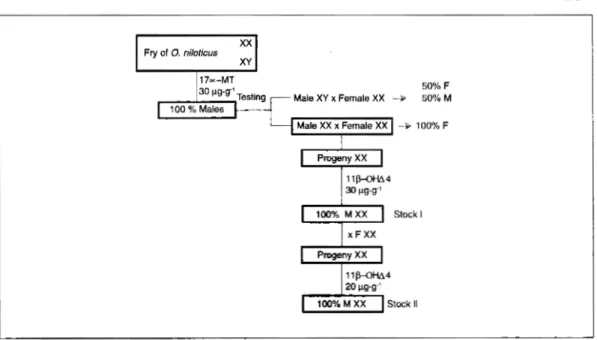
PRODUCTION OF TWO STOCKS OF SEX REVERS ED MALES (FIG. 1)
A progeny of fry from
O. nllotlcus
of the "Bouake" strain (Baroiller et al, in press) was produced by crossîng a male with one of the four females placed in the same 400-1 aquarium. The progeny, removed after 10 days post-fertilization (PF), was exposed to a hormonal sexreversai treatment using 1 7a
methyltestosterone added to the feed at 30 µg·g-1 (Baroiller and Toguyeni, this
vol.). Thirty males from the resulting ail-male population were kept to analyze the sex ratio of their progenies in 400-1 aquaria. Twenty of these males were bred with normal females. Thirty juve-niles of each of these progenles were killed to determine the sex ratio of each population. Sexing by gonadal squash was done after 90 days PF when the his-tological characteristics of differentla-tion were already established for both sexes (Baroiller 1988a and b). The 13 broodfish with progenies showing no significant difference in sex ratio com-pared to a 1: 1 population were elimi-nated (sex ratios ranging from 33.3 to 60% male). The seven other males with all-female progenies (30 females in the sample of 30 juveniles sexed in each progeny) were kept as sex reversed males. One of them was crossed again and its entire progeny was exposed to a hormonal sex reversai treatment us-ing 11 B-OHi.\4 added to the feed at 30 µg·g-1 (Baroiller and Toguyeni, this vol.). The resulting ail-male population was
theoretically an exclusively XX sex
reversed male population; 60 of them constituted Stock 1 and were identified
Fry of 0, nilotious XX ){'(
17oc-MT
30 µg-g'
1 Testing . - Male XY x Female XX -.J>
100%Males ~---1 50%F 50%M 1 Stock 1 100% M XX Stock Il
Fig. 1. Production of two stocks of sex reversed males of Oreochromis niloticus.
by a letter. The progeny of one of them, sex reversed male
J,
was exposed to a hormonal sex reversai treatment using118-0HL14 at 20 µg·g·1 (Baroiller and
Toguyeni, this vol.). The resulting sex reversed ail-male population constituted Stock 2, each broodfish being identi-fied by a number.
RE.PRODUCTIONS OF SE.X RE.VE.RSE.D MALE.S AND SE.XING OF THE.IR PROGE.Nlf.S
Thlrteen "sex reversed male" broodfish from Stock 1 and nine sex reversed males from Stock 2 were crossed in single pair matings with one or several females in 400-1 aquaria. The water in the spawn-ing aquaria was filtered permanently and maintained at 27°C using a ther-moregulation device. Each animal was tagged in the dorsal musculature. Re-production was detected by the onset of maternai mouthbrooding character-ized by dilation of the female's mouth. As soon as this particular characteris-tic was observed, all other individuals were removed to leave the female mouthbrooding in the aquarium. Five
days after hatching, i.e., nine days PF, the fry were removed from the moth-er's mouth. The entire progeny (or a sam-ple of at least 100 fry from the batch), identified by its fertilization date and the respective tags on the parents, were transferred to 200-1 aquaria. The Fry were fed ad libitum six times a day, seven days a week, with a first-feeding salmonid feed (Aqualim) distributed by automatic feeder du ring a 12-hour photoperiod. The water, aerated and filtered, was kept at 28± 1.5°C. After three weeks of rearing in aquaria, when the gonadal differentiation was already well established (Baroiller t 988a and b), fry were transferred to external 1. S-m3 tanks until sexing and were given the same feed. After 60-90 days PF, when the histological characteristics of the male and female differentiation were already established (Baroiller t 988a and b). all fry were sexed by examination of the gonad squash at x125 magnifi-cation. The presence of previtellogenic or vitellogenic oocytes and the lobular configuration showed the female and male sexes, respectively.
Four sex reversed males from Stock 2 (XX4, XX9, XXt 7 and XX18) were each successively crossed with three or four different females.
Results
The sex ratios of ZZ progenies from sex reversed males were determîned by sexing ail 2,429 fry produced, i.e., ap-proxirnately 110 fish per batch. Four of them, i.e., 18% of the progenies, gave sex ratios of 0: 1 (male:female). ln the 18 other progenies, unexpected males based on a monofactorial sex determî-nation were produced in proportions rangîng from 1 to 40.5% (Table 1 and Fig. t). Only one of these sex ratios (40.5% male) was not signiflcantly dif-ferent from a 1: 1 ratio. Compared to the ratio of an "average" synthetic popu-lation of 5,299 fry from crosses of 42 normal broodfish of the same spedes ( t .39: t ), the 40.5% male showed a hîghly signlficant difference. The male frequency distribution in these progenies was unimodal, around 0-10% male.
Since the progeny of the sex reversed male is theoretic::ally composed of 100% XX individuals, four of the naturally un-expected males from the XXI sex reversed male (Stock 1) were kept until their sexual maturity to analyz.e the sex
ratio of their progenies. Three unex~
pected males (Dl, 02 and 03) naturally originating from XXI sex reversed males were bred with different females (Ta-ble Z). Their progenies, female for most of them included, however, 2.5-9.2% unexpected males. The sex ratios of the progenies of the Dt, 02 and 03 males ail differed significantly from the sex ratios of the progeny of the XXI sex reversed male. There was no signiflcant dlfference between two of the three unexpected male progenies (02 and 03). The XX4 sex reversed male was suc.-çessively bred three times with two
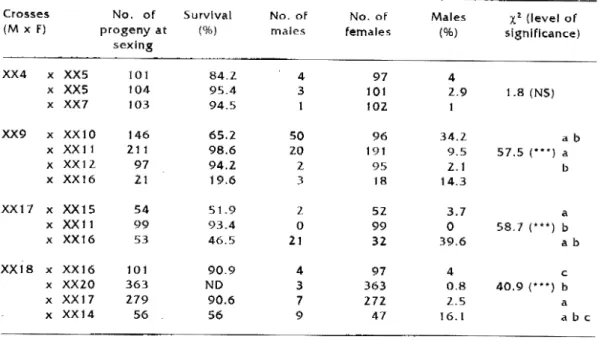
females. There was no sîgniflcant dif-ference between the three sex ratios whether the progenies came from the same pair or only had the same sire (Table 3).
Four progenies were produced by crossing an XX9 sex reversed male with four different females. Three progenies (XX4 male x XX11, XX12 and XX16 fe-males) out of four yielded sex ratios which did not differ significantly among themselves. ln contrast, the cross with the XX 10 female gave a progeny with a sex ratio differing significantly from that of the progenies from the XX 11 and
XX
12 females.The same applied to the sex ratios of progenies from XX 18 sex reversed males as three of them did not show any significant difference (progenies from XX16, XXl 7 and XX20 females).
ln the XX 17 male, there was no sig-nificant difference between the propor-tions of unexpected males in two out of the three crosses (XX 17 x XX t 5 and XX11 females). However, only the XX.17
x XX 11 cross produced an
all-female population. The progeny from the same male crossed with the XX t 6 female gave 39.6% males. Therefore, the same XX 1 7 male successively produced 0 and 39.6% unexpected males with two different females.
Finally, a diallel cross experiment was made involving two sex reversed males and two females (Table 4). A maternai Influence was observed in the two prog-enies from XX9 sex reversed males. ln contrast, a paternal influence was ob-served between the two progenies from the XX 16 female.
Discussion
ln species such as O.
niloticus
where the female ls considered homogametic, the progeny from a sex reversed male ls theoretlcally all-female, based on aTable 1. Single pair matings of sex reversed males from Stocks and Z of Oreochromls nlloticus
with XX females.
Crosses No. of Survival No. of No. of Males
x
2 (level of(Mx F) progeny at (%) males females (%) sîgniflcance)•
sexing 1 :1 t .39: t XXA x XXA t 16 55 47 69 40.5 2. t (NSJ 6.9(**) XX7 x XX7 179 89.5 63 116 35.2 8 ( .. ) XX9 x XXtO 146 65.2 50 96 34.2 7.4 ( .. ) XXD x XXD 149 ND 38 1 1 t 25.5 t 8.9( .. *) XXE x XXE 141 47 36 105 25.5 t 7 .9( ... ) XXIO x XX20 89 ND 21 68 23.6 13.3( ... ) XXF x XXF 199 ND 45 154 2Z.6 3Z.Z (* .. ) XXG x XXG 152 ND 34 118 22.4 25. t ( .. ") XXH x XXH 27 ND 6 21 ZZ.3 4.44 (*) 7. 7(**) XXI x XXI 175 54.2 35 140 20 34.5 ('**) XX8 X XX8 85 85 15 70 17.6 19.8 (* .. ) XX) x XX) 70 ND 1 1 59 15. 7 18.6('*') XXK x XXK 119 95.2 14 105 11.8 40.6 ('*') XXL x XXL 76 ND 6 70 7.9 32. 7 (' **) XX18 x XX16 101 95.3 4 97 4 54.2 ( .. ') XX17 X XX15 54 51.9 2 52 3.7 29.5 ('") XX4 X XX7 103 94.5 102 1 65.1 ( ... ) XXI x XX1 96 90.6 95 1 60.5 (*") XXC X XXC 74 66.1 0 74 0 74 (***) XXB x XXB 86 43 0 86 0 86 (***) XXM x XXM 32 ND 0 32 0 32 ( ... , XXI 1 x XXl 1 160 93.6 0 160 0 160 (**•)
•When
x!
is low.er than or close to the level of significance vis-à-vis a 1: 1 population, sex ratios are tested against a 1.39: 1 sex ratio which is the mean sex ratio (min.: 15.8% males; max.: 77. t %)from ail 42 single pair matlngs of XY males, i.e., 5,299 sexed fry. Levels of significance were: '=p<0.05; "=p<0.01; and •••=p<0.001.
ND = No data on survival: the initial number of fry was not recorded.
Table 2. Crosses of a sex reversed male Oreochromis niloticus with three of the unexpected males found in its progeny: progenies from the same sex reversed male characterized with the same lower case letter (a, b or c) show significant dffferences (p•0.05) in their sex ratios; •• = p<0.01. Crosses (Mx f) Sire XXI x XXI Progenles Dt x XX21 02 X XXll 03 x XX23 No. of Survival progeny at (%) sexing 175 317 314 102 54.2 92.9 89.4 86.4 No. of males 35 8 29 8 No. of females 140 309 285 94 Males % 20 2.5 9.2 7.8
xi
(level of significance) ab ab 12.9('*) a bTable 3. Successive matings of four sex reversed Oreochoromls niloticus males: progenles from the same sex reversed male charaderlzed wlth the same lower case Jetter (a, b or c) show signlficant dlfferences (p~0.05) ln thelr sex ratios; NS •• t.
Crosses No. of Survival (Mx f) progeny at (%) sexing XX4 X XX5 101 84.Z X
xxs
104 95.4 X XX7 103 94.5 XX9 X XX10 146 65.2 X XXl 1 211 98.6 X XX1Z 97 94.Z X XX16 21 19.6 XX17 X XX.15 54 51.9 X XXl 1 99 93.4 X XX16 53 46.5 XX18 X XX16 101 90.9 X XX20 363 ND X XX17 279 90.6 X XX14 56 56 No. of No. of males females 4 97 3 101 IOZ 50 96 zo 191 2 95 3 18 l 5Z 0 99 21 32 4 97 3 363 7 272 9 47 Males (%) 4 l.9 34.2 9.5 2.1 14.3 3.7 0 39.6 4 0.8 Z.5 16.1x
1 (level of signlflcance) 1.8 (NS) ab 57.5 ( ... , a b a 58.7 ('**) b ab c 40.9 (··-i b a a b c:Table 4. Sex ratio (d :9 and % of males) in a diallel cross expîrement involving two sex reversed males and two females: progenies from the same sex reversed male characterized wlth the same lower case (a, b or c) show significant differences (p=0.05) in thelr sex ratios.
MXX17 FXX11 0:99 (0) Q FXX16 21:32 (39.6)
monofactorial system. Out of 35 crosses of sex reversed males conducted in the present study, only five progenies, i.e., 14.3%, correspond to the monofactorial theoretical model. ln the 31 other progenies, 1-40.5% of unexpected males were observed. These males dld not result from sexing errors during micros-copie examination of gonads and showed normal fertility: three of them bred. for
a b a d MXX9 20:191 a (9.5) 3:18 ( 14.3) b
O.
ni/oticus,
three other studies have analyzed the sex ratios of progenies from sex reversed males. ln the first study of eight male progenies produced by masculinlzing treatment, four progenies were all-female, one showed a sex ratio not significantly different from t: 1 and three ylelded unexpected high propor-tions of females in the range 65.2-81.8% (Jalabert et al. t 974). However, it isimpossible to attribute the paternity of these unexpected sex ratios to sex reversed males rather than to XY males, considering the large range of sex ratios that these XY males show in O.
ni/ot/cus
(Shelton et al. 1983; Wedekind 1987 cited by Wohlfarth and Wedekind 1991 : Lester et al. 1989). ln the second study (Calhoun and Shelton 1983), sex reversed males, produced by exposing the progenies of a sex reversed broodfish to a masculinizing treatment, were mass-bred (22 per experiment) with two batches of females and a sample of 1OO
fry was sexed. ln one of the female batches, one progeny out of nlne yielded a 99% female sex ratio. ln the other batch, eight progenies out of nine yielded 87 -99% females, and only one progeny (i.e., t t %) was a real all-female population and therefore corresponded to the monofactorial XX/XY theoretical model. As in the present study, one of the batches produced a majority of progenies with unexpected sex ratios. Finally, in the third study, four sex reversed males were identifled by the sex ratio of their progenies in 11 single pair matings. Two progenies out of 1 1 yielded unexpected sex ratios, with 6. 7 and t 7 .4% males. Nine other progenies were consîdered all-femal e. However. the number of sexed fish was still very low, with an average of 20 fry per batch (min.: 6; max.: 43), and low percentages of unexpected males may therefore have escaped notice.
These four different studies show sex ratios that cannot be explained by a
monofactorial sex determination al~
though they can constltute 50 (Calhoun and Shelton 1983) to 88% (thls study) of the progenies. However, as the number of these unexpected proportions of males was often fairly low, they may have been masked, at least partially, in other studies based on a very lim-ited sample size.
235
The percentage of unexpected males in successive progenles from the same sex reversed male differs from progenies to progenies; a paternal and maternai effect may be involved. A maternai in-fluence is also suggested by the differ-ences in the proportions of unexpected males observed in the mass breeding of sex reversed males depending on the batch of females used (Calhoun and Shelton 1983). ln diallel crosses involving five pairs of broodfish of O. nilot/cus, there is no such maternai or paternal influence (Mair et al. 1991 ).
Therefore, deviations from the theo-retical mode! may be found in high pro-portions and seem to be incompatible with rare autosomal factors. The con-ditions under which these reversais occur in relation to the expected phenotype have not yet been dearly established. The hypothesis of genetic contamina-tion seems unlikely since Majumdar and McAndrew ( 1983) demonstrated the presence of unexpected sex ratios in isolated natural populations. More com-plex, polygenic genetic models could be lnvolved. However, the absence of sex-specific genetic markers in tîlapias renders difficult genetic analyses such as those conducted on platyfish
(Xiphophorus maculatus)
(Kallman 1984). The deviations observed could also re-sult from the potential effects of external factors on differentiatîon. Depending on the genotype, one or more external factors could influence the phenotypic sex of the fry during the gonadal dif-ferentiation of sex. This hypothesis, far easier to test than a complex sex determining mechanism, could explain some unexpected results such as the presence of males in progenies from sex reversed males as well as the presence of a natural XY sex reversed female in O.niloticus
(Scott et al. 1989; Mairet al. 1991 ). Consequently, a study on the effects of temperature on the sex ratiowas conducted on progenies from these sex reversed males (Baroiller et al., this vol.).
References
Avtallon. R.R. and l.S. Hammerman. 1978. Sex determlnatlon in Sarotherodon {tilapia). 1. Introduction to a theory of autosomal in-fluence. Bamidgeh 30:110-115.
Avtalion. R.R. and J. Don. 1990. Sex determin-ing genes ln tllapla: a mode! of genetic recombination emerging from sex ratio results of three generations of diploid gynogenetic Oreochromis aureus. J. Fish Bioi. 37: 167-173.
Baroiller, J.F. 1988a. Etude corrélée de l'apparition des critères morphologiques de la différenciation de la gonade et de ses potentialités stéroïdogènes chez
Oreo-chromis ni/oticus. Université Pierre et Marie
Curie, Paris, 89 p. Ph.D. dissertation. Baroiller, J.F. 1988b. Etude des processus
morphologiques et endocrinologiques de la différenciation naturelle du sexe chez
Oreochromis niloticus, appliquée à la
pro-duction de populations monosexes : intérêts et perspectives, p. 256-266. ln G.M. Bernacsek and H. Powles {eds.) Aquaculture systems research in Afrlca. Proceedings of a Work5hop held in Bouake, Côte d'ivoire, 14-17 November 1988. International De-velopment Research Centre, Ottawa. Canada. Baroiller. J.F. and B. Jalabert. 1989.
Contribu-tion of research in reproductive physlol-ogy to the culture of tilapias. Aquat. Liv-ing Resour. 2:105-116.
Baroiller. J.F .. X. Rognon, V.C. Yapi and A. Cissé. Genetie characterization and sex determi-natlon in tilapia in Côte d'ivoire. ln A. Eknath and B. Acosta {eds.) Present progress, fu-ture direction and needs ln flsh genetics research in Asia Pacifie and Africa. Man il a, Philippines. {ln pre5s.)
Calhoun, W.E. and W.L. Shelton. 1983. Sex ra-tios of progeny from mass spawning of sex reversed broodstock of Tilapia nilotlcà.
Aquaculture 33:365-371.
Guerrero, R.D. 1982. Control of tllapia repro-duction, p. 309-316. ln R.S.V. Pullin and R.H. Lowe-McConnell {eds.) The biology and culture of tilapias. ICLARM Conf. Proc. 7. 434p.
Hammerman, l.S. and R.R. Avtallon. 1979. Sex determinatlon in Sàrotherodon {tilapia). Part Il. The sex ratio a5 a tool for the determl-nation of genotype - a mode! of autosomal and gonosomal Influence. Theor. Appl.
Genet. 55:177-187.
Hanson, T.R .• R.D. Smitherman, W.L. Shelton and R.A. Dunham. 1983. Growth comparlsons of monosex tilapia produced by separation of sexes, hybridization and sex reversai, p. 570-579. ln L. Fishelson and Z. Yaron {comps.) Proceedings of the First Interna-tional Symposium on Tilapia in Aquaculture. Tel Aviv University, Tel Aviv, Israel. Jalabert, B .. J. Moreau, P. Planquette and R. Billard.
1974. Déterminl5me du sexe chez Tilàpia
màcrochir et Ti/ap/à nllot/cà : action de la
méthyltestostérone dans l'alimentation des alevins sur la différenciation 5exuelle; pro-portion des sexes dans la descendance des mâles Inversés. Ann. Biol. Anim. Bloch. Blophys. 14{4B):7Z9-739.
Kallman. K.D. 1984. A new look at sex deter-mination in poeclliid fishes. p. 95-171. ln
B.J. Turner (ed.) Evolutlonary genetics of Fishes. Plenum Press, New York. New York. Lester, L.J .. K.S. Lawson, T.A. Abella and M.S. Palada. 1989. Estimated heritability of sex ratio and sexual dimorphism in tilapia. Aquacult. Fish. Manage. 20:369-380. Lovshin, L.L. 1982. Tilapia hybridization, p.
279-308. ln R.5.V. Pullin and R.H. Lowe-McConnell (eds.) The blology and culture of tilapias. ICLARM Conf. Proc. 7. 434 p. Mair, G.C., A. Scott, D.J. Penman. J.A. Beardmore
and D.O.F. Skibinski. 1991. Sex reversai. gynogenesis and triploidy in O. niloticus
{L.). Theor. Appl. Genet. 82:144-152. Majumdar, K.D. and McAndrew. 1983. Sex
ra-tios from interspecific crosses within the tilapias. p. 261-269. ln L. Fishelson and Z. Yaron {comps.) Proceedings of the First International Symposium on Tilapia ln Aquaculture. Tel Aviv University, Tel Avlv. Israel.
Pandian. T.J. and K. Varadaraj. 1987. Techniques to regulate sex ratio and breeding in tllapia. Curr. Sei. 56(8):337-343.
Pull in, R.S.V. 1983. Choice of tilapia species for aquaculture. p. 64- 76. ln L. Flshelson and
z.
Yaron (comps.) Proceedings of the First International Symposium on Tilapla ln Aquaculture. Tel Aviv University, Tel Aviv, Israel.Scott, A.G .. D.J. Penman. ].A. Beardmore and D.0.F. Sklbinski. 1989. The YY supermale ln O. niloticus {l.) and its potential in aquaculture. Aquaculture 78:237-251. Shelton. W.S., F.H. Meriwether, K.J. Semens and
W. E. Calhoun. 1983. Progeny sex ratios from lnterspecific pair spawnings of Tilaplà àUrt!à
and T. niloticà, p. 2 70-280. ln L. Flshelson
and Z. Yaron (comps.) Proceedings of the First International Symposium on Tilapia in Aquaculture. Tel Aviv University, Israel.
Wohlfarth, G.W. and G. Hulata. 1983. Applled genetlcs of tllapla, 2nd edltlon. ICLARM Stud. Rev. 6, 26 p.
237
Wohlfarth, G.W. and H. Wedekind. 1991. The heredi.ty of sex determination in tilapias. Aquaculture 92: 143-1 56.;
I"
International Center for Living Aquatic
Resources Management Centre de recherches
era
oceanologiques.
)
I
r";f~~~31
L'lnstitut fran!;ais de recherche scientifique
pour Ie developpement en cooperation
With the cooperation of
c!
Centre de cooperation internationale en recherche agronomique pour Ie developpelTlent
Cooperation francaise
'fbA
Centre technique
de cooperation agricole et rurale '
;
So-, =.
11.::1
~- - . -
-~
~-Tl;IÉ THIRD INTERNATIONAL SYMPOSIUM
ON TILAPIA IN AQUACULTURE
lnte(n;ttl<intll Cootcr fot L.i'IÎf"Q Aquatic ResourcN Management
r.,;5;.::1
L' Mafltut frun~Î$ d• r«t'M:f'çhc $<:Ï4ntlflqu•
pour le dëveloppement en COOl)êl"aU<W\
1
EHTERED IN NÂGA ]
Edited
by
R
.
S.V. P)lll.IN
J.LAZill
1\1. LEGENDRE
J.B. AMON
KOTHJAS
D.PAULY
Tnutslarlnn.• byC.
LHOMMF.-BlNUDlN
1996
•
~trG Cie coopération JMtrnttionMç Ct'I teeh•n:he agro1'omlque pour le df}w)foppemenl
With
the ç
oop
er
ation
of
Centre technique