Thiazide Diuretics Directly Induce Osteoblast
Differentiation and Mineralized Nodule Formation by
Interacting with a Sodium Chloride Co-Transporter in
Bone
Melita M. Dvorak,*†Cyrille De Joussineau,* D. Howard Carter,‡Trairak Pisitkun,§ Mark A. Knepper,§ Gerardo Gamba,储Paul J. Kemp,* and Daniela Riccardi*¶
*Cardiff University School of Biosciences and¶Cardiff Institute of Tissue Engineering and Repair, Cardiff, United Kingdom;†Endocrine Research Unit, Department of Medicine, University of California, San Francisco, California; ‡Turner Dental School, University of Manchester, Manchester, United Kingdom;§Laboratory of Kidney and Electrolyte Metabolism, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland; and储Molecular Physiology Unit, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Instituto de Investigaciones Biomedicas Universidad Nacional Autonoma de Mexico, Mexico City, Mexico
ABSTRACT
Thiazide diuretics are used worldwide as a first-choice drug for patients with uncomplicated hyperten-sion. In addition to their antihypertensive effect, thiazides increase bone mineral density and reduce the prevalence of fractures. Traditionally, these effects have been attributed to increased renal calcium reabsorption that occurs secondary to the inhibition of the thiazide-sensitive sodium chloride cotrans-porter (NCC) in the distal tubule. The aim of the current study was to determine whether thiazides exert a direct bone-forming effect independent of their renal action. We found that the osteoblasts of human and rat bone also express NCC, suggesting that these bone-forming cells may be an additional target for thiazides. In vitro, NCC protein was virtually absent in proliferating human and fetal rat osteoblasts, whereas its expression dramatically increased during differentiation. Thiazides did not affect osteoblast proliferation, but directly stimulated the production of the osteoblast differentiation markers runt-related transcription factor 2 (runx2) and osteopontin. Using overexpression/knockdown studies in fetal rat calvarial cells, we show that thiazides increase the formation of mineralized nodules, but loop diuretics do not. Overall, our study demonstrates that thiazides directly stimulate osteoblast differen-tiation and bone mineral formation independent of their effects in the kidney. Therefore, in addition to their use as antihypertensive drugs, our results suggest that thiazides may find a role in the prevention and treatment of osteoporosis.
J Am Soc Nephrol 18: 2509 –2516, 2007. doi: 10.1681/ASN.2007030348
It has been known for several decades that treat-ment of hypertension with thiazides has the benefi-cial side effect of strengthening bone.1–5 To date, this bone-protective effect has been attributed to thiazides’ acting at the distal nephron to inhibit the Na⫹-Cl⫺ co-transporter (NCC).6 In favor of this hypothesis are two observations. First, patients with Gitelman syndrome and the equivalent murine model, in which NCC is nonfunctional as a result of a mutation in the SLC12A3 gene, exhibit an in-creased bone mineral density.7,8 Second, patients
with pseudohypoaldosteronism type II, who present with an increased NCC activity, exhibit
re-Received March 22, 2007. Accepted May 22, 2007.
Published online ahead of print. Publication date available at www.jasn.org.
M.M.D. and C.D.J. contributed equally to this work.
Correspondence: Dr. Daniela Riccardi, Cardiff University School of Biosciences, Museum Avenue, Cardiff, CF10 3US, UK. Phone: ⫹44-29-20879132; Fax:⫹44-29-20874116; E-mail: riccardi@cardiff.ac.uk Copyright © 2007 by the American Society of Nephrology
duced bone mineral density.9The mechanism by which genetic (Gitelman syndrome) or pharmacologic (thiazide treatment) inhibition of NCC results in enhanced bone mineral density has been hypothesized to be due to increased circulating serum calcium levels.10,11This model proposes that NCC inhibition in the distal tubule evokes hyperpolarization, increased electri-cal driving force for electri-calcium reabsorption, and a subsequent decrease in urinary calcium loss.6However, in patients with Gitelman syndrome, as in patients undergoing thiazide ther-apy, the expected increase in circulating parathyroid hormone levels that should accompany the increase in free ionized plasma calcium concentration is not seen. In fact, plasma para-thyroid hormone levels are either decreased or unchanged, and serum calcium levels remain essentially normal.8,11These ob-servations suggest that the increase in bone mineral density is probably not directly related to the enhanced renal tubular calcium transport but to a direct action of thiazides on bone. Potential mechanisms by which thiazides may exert their effect on bone are via an inhibition of osteoclast-mediated bone re-sorption and/or by an increase in osteoblastic bone formation. Although in vitro studies indicate that thiazides are capable of reducing osteoclastic activity independent of NCC,12,13 ana-bolic effects of thiazides have not been demonstrated. Because NCC mRNA has been previously reported in a bone-derived cell line,14we hypothesized that thiazides increase bone min-eral density by interacting directly with an osteoblast NCC pro-tein. Given the potential therapeutic importance of long-term thiazide treatment in the prevention of age-related osteoporo-sis, it was the objective of this study to investigate the effects of thiazides by establishing NCC expression in human bone and to determine the effects of thiazides on osteoblast prolifera-tion, differentiaprolifera-tion, and mineralized nodule formation in os-teoblast models.
RESULTS
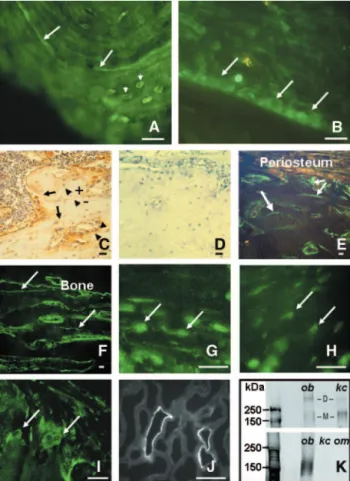
For the immunologic detection of NCC expression in skeletal tissue, we used freshly frozen rat femur; freshly frozen human mandible; and EDTA-decalcified, wax-embedded rat femur. At low magnification, we observed NCC immunoreactivity in human and rat cortical and trabecular bone, in both undecal-cified, frozen (Figure 1, A and B) and decalundecal-cified, paraffin wax– embedded human (data not shown) and rat bone (Figure 1C). No nonspecific immunoreactivity was detected when the pri-mary antibodies were omitted (Figure 1D). NCC immunore-activity was also observed in snap-frozen sections of rat femur (Figure 1, E through I). With the use of two different antibod-ies raised against two different epitopes of either human or rat proteins, NCC-specific immunoreactivity was localized to cells of the osteoblastic lineage (particularly osteoblasts) in both human and rat bone (Figure 1, A, G, and top of K). NCC protein was also present in some but not all osteocytes (Figure 1, A, C, and H), perhaps indicating that cells at different stages of differentiation display differential expression of this protein.
Occasionally, osteoclasts in the rat cryosections (Figure 1I) but not those in sections of decalcified bone (data not shown) also Figure 1. Sodium chloride co-transporter (NCC) is expressed in bone. Using human NCC antibodies, specific immunofluores-cence is evident in undecalcified frozen human (A) and rat (B) sections. NCC is expressed in reversal lines (A; arrows), in osteo-cytes (A; small arrows), and lining osteoblasts (B; arrows). (C) Bright-field photomicrograph of EDTA-decalcified paraffin sec-tion of rat femur shows NCC immunoperoxidase activity (brown) in reversal lines (arrows), osteoblasts (arrowheads), and some osteocytes (arrowhead⫹). A proportion of osteocytes are nega-tive for NCC (arrowhead⫺). (D) Omission of primary antibodies does not result in staining. (E through I) Photomicrographs of rat femur cryosections show that NCC immunofluorescence is in reversal lines (E and F; arrows), osteoblasts (G; arrows), osteocytes (H; arrows), and some osteoclasts (I; arrows). (J) NCC-specific, distal convoluted tubule staining is present in the rat kidney. Bar⫽ 20m throughout. (K, top) NCC immunoreactivity in os-teoblast-derived MG63 cells (ob; 30 g of total homogenate loaded) is comparable to that detected in human kidney cortex sample (kc; 30g), with predicted sizes for the NCC monomer (M) and dimer (D) of approximately 160 and 320 kD, respectively. (K, bottom) Expression of the ubiquitously expressed type 1 Na⫹ -K⫹-2Cl⫺co-transporter (NKCC1) is also expressed in MG63 cells using an anti-rat NKCC1 antibody (ob, 30g of total homogenate loaded). The figure also shows that, as expected, NKCC1 is low abundant in human kidney cortex and outer medulla. om, kidney outer medulla, 10g.
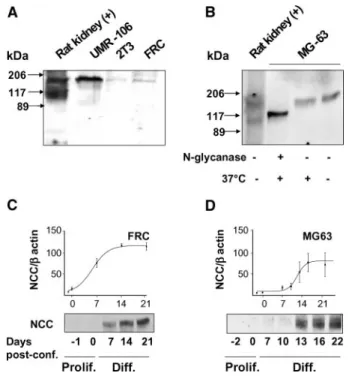
expressed NCC; immunostaining in osteoclasts was absent in human freshly frozen and paraffin sections (data not shown). Furthermore, no NCC immunoreactivity was detectable in ei-ther human or rat cartilage (data not shown). Positive control experiments carried out on rat kidney cryosections (Figure 1J) confirmed the expression of NCC exclusively in the distal con-voluted tubule,1thus demonstrating the specificity of the anti-body. To investigate the size of human NCC protein in osteo-blasts, we performed Western analysis on crude membrane-enriched fractions from human osteoblast-derived MG63 cells. Human kidney cortex was used as the control tissue. Re-sults showed comparable immunoreactivities of the expected molecular weights for the NCC monomer and dimer (Figure 1K, top). Figure 1K also shows that MG63 cells expressed the type 1 Na⫹-K⫹-2Cl⫺ co-transporter (the ubiquitously ex-pressed form NKCC1; Figure 1K, bottom),1whereas they lack the kidney-specific isoform of the type 2 Na⫹-K⫹-2Cl⫺ co-transporter (NKCC2; data not shown).1Western analysis was performed on homogenates of established osteoblast cellular models after they had reached confluence. These were rat os-teosarcoma UMR-106 cell line, mouse osteoblast-derived 2T3 clonal cell line, and fetal rat calvaria (FRC). To varying degrees, all of these osteoblast-derived lines expressed immunoreactiv-ity of the expected size for NCC (Figure 2A). After enzymatic deglycosylation with N-glycanase, the molecular weight of the NCC monomeric protein in MG63 cells was reduced from ap-proximately 160 kD to apap-proximately 120 kD, a size close to the unglycosylated core protein (Figure 2B). It is interesting that Western analysis also showed that NCC protein expres-sion levels in FRC cells (Figure 2C) and in MG63 cells (Figure 2D) were virtually undetectable in proliferating cells (up to 10 d in culture). In contrast, in postconfluent, differentiating FRC and MG63 cells, NCC protein expression levels increased up to 15 d after confluence.
It is conceivable that the osteoanabolic effects of metola-zone could be ascribed to stimulation of cell proliferation. In line with the absence of NCC expression in preconfluent, pro-liferating osteoblasts, FRC cell proliferation was not affected by metolazone (1 to 100M) for up to 2 wk in culture (Figure 3A, three independent experiments performed in triplicate). Type I collagen is the first marker of osteoblast differentiation.15Our data show that neither metolazone nor chlorothiazide had any effect on the production of type I collagen (Figure 3B, three independent experiments performed in triplicate). In contrast, metolazone produced a concentration-dependent increase in the expression of later osteoblast differentiation markers runt-related transcription factor 2 (Runx2; Figure 3, C and D, five independent observations from three independent cell iso-lations) and osteopontin (Figure 3, E and F, five indepen-dent observations from three indepenindepen-dent cell isolations) in both FRC (Figure 3, C and E) and MG63 (Figure 3, D and F) cells. In addition, chronic metolazone treatment per se in-creased NCC expression in a dosage-dependent manner (data not shown).
The effect of thiazides and of loop diuretics on mineralized
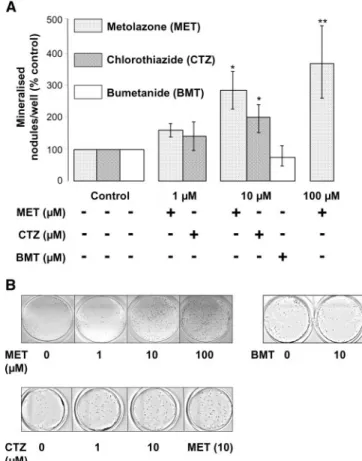
nodule formation was tested in postconfluent FRC cells kept in culture up to 3 wk, and mineralization was visualized using von Kossa staining. Figure 4 shows that both metolazone and chlo-rothiazide treatment of FRC cells induced a dramatic, concen-tration-dependent increase in mineralized nodule formation. Figure 4 also shows that, under the same experimental condi-tions, the loop diuretic bumetanide did not increase mineral-ization of FRC cells. These results indicate that thiazides in-crease mineralization and strongly suggest that this effect is dependent on NCC expression.
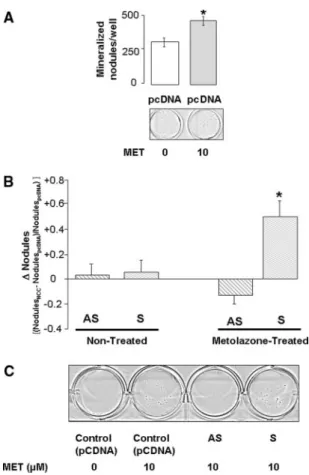
To confirm that NCC is required for the thiazide-evoked mineralization, we genetically manipulated NCC expression in postconfluent FRC cells. First, to rule out nonspecific effects, we transfected postconfluent FRC cells with an empty vector (pcDNA3.1) and measured mineralization in the presence or absence of concentrations of metolazone known to evoke sta-tistically significant effects in nontransfected cells (i.e., 10M; see Figure 4). Figure 5A shows that the effects of metolazone Figure 2. NCC is expressed in differentiating osteoblasts. (A) Western analysis of protein homogenates extracted from estab-lished models of osteoblasts (human UMR-106, murine 2T3, and fetal rat calvaria [FRC]) reveals that NCC immunoreactivity is of comparable size to that observed in rat kidney. (B) Enzymatic deglycosylation of protein extracts from the human osteoblast-derived cell line MG63 indicates a reduction in the NCC mono-mer molecular weight from approximately 160 kD to approxi-mately 120 kD. Control protein exposed to 37°C for 60 min without N-glycanase showed presence of the fully glycosylated protein. (C and D) NCC protein expression is absent in precon-fluent, proliferating FRC (C) and MG63 cells (D). In both models, NCC expression increases as the differentiation process progresses and peaks at approximately 2 wk after confluence. Note that 0 d after confluence represents the beginning of dif-ferentiation.
were maintained even after plasmid delivery. Indeed, 10M metolazone increased the number of mineralized nodules per well from 285.9⫾ 16.3 to 470.2 ⫾ 18.6 (n ⫽ 9 observations from three independent cell isolations; P⬍ 0.05). In the ab-sence of metolazone treatment, neither overexpression nor an-tisense knockdown evoked significant changes in mineralized nodule formation (Figure 5B). In contrast, overexpression of
NCC increased metolazone-dependent mineralized nodule formation by approximately 50% (49.3⫾ 13.0%; n ⫽ 12 rep-licates from four independent cell isolations), an effect that was completely prevented by NCC knockdown (⫺13 ⫾ 8.7%; n ⫽ 12 replicates from four independent cell isolations; P⬍ 0.05).
DISCUSSION
Long-term thiazide treatment is associated with a reduction in the risk for hip and wrist fractures in postmenopausal women and elderly men.1–5This bone-sparing effect is thought to oc-cur through blockage of the renal sodium chloride co-trans-porter NCC and subsequent reduction in urinary calcium ex-Figure 3. Metolazone (MET) does not affect proliferation but
stimulates the expression of osteoblast differentiation markers. (A) FRC cell proliferation (for up to 2 wk in culture) is not affected by increasing dosages of the thiazide-like metolazone. (B) Con-sistent with the lack of NCC expression at days 1 to 3 after confluence (see Figure 2, C and D), the activity of collagen 1A (coll1A), the major structural component of the organic matrix and early differentiation marker, is not affected by 48-h treatment with MET (10M) or with chlorothiazide (CTZ; 10 M). (C through F) Western analyses show increased expression of osteoblast mark-ers runt-related transcription factor 2 (Runx2; C [*P⬍ 0.05 at 10 M, ANOVA, Tukey post hoc test] and D [*P ⬍ 0.01, unpaired t test]) and osteopontin (E [*P⬍ 0.05, ANOVA, Tukey post hoc test] and F [*P⬍ 0.05, unpaired t test]) in rat (C and E) and human (D and F) osteoblast models in response to increasing concentra-tions of metolazone (7 d of treatment). Histograms represent mean band densities and indicate that the levels of expression of Runx2 or osteopontin, normalized for the levels of expression of -actin in the same samples, are significantly increased.
Figure 4. Thiazides stimulate mineralized nodule formation by FRC cells. (A) Continuous treatment of FRC cells with MET (1 to 100M) and CTZ (1 and 10 M) for 10 to 21 d after confluence induces dosage-dependent increases in mineralized nodule for-mation (MET: n⫽ 3; *P ⬍ 0.05, **P ⬍ 0.01; CTZ: n ⫽ 3; *P ⬍ 0.05). The loop diuretic bumetanide (BMT; 10M) does not significantly affect nodule formation. (B) Representative von Kossa staining of FRC cells (15 d after confluence) treated with MET (1 to 100M) shows a concentration-dependent increase in the number of min-eralized nodules (top), whereas no effects on FRC cell mineraliza-tion are seen in the presence of BMT (10 M). (Bottom) The number of mineralized nodules is comparable for MET- and CTZ-treated postconfluent FRC cells (representative of three indepen-dent cell isolations).
cretion. Whether thiazides directly effect new bone formation independent of their renal action has never been demon-strated. Here, we show that NCC is expressed in freshly frozen and decalcified sections of human and rat bone; in cells of the osteoblast lineage, particularly osteoblasts; and, to a lesser ex-tent, in the osteocytes, in both rat and human bone.
Immunoblotting performed on crude membrane extracts of freshly isolated (FRC), osteosarcoma-derived (UMR-106) and virally transformed (2T3) cells confirmed that NCC in osteoblasts is of an equivalent molecular mass as its renal coun-terpart. The renal NCC contains two N-linked glycosylation
sites that are important for sensitivity to thiazides.16Enzymatic deglycosylation of NCC in MG63 cells demonstrated the pres-ence of carbohydrate residues in the osteoblast protein. Taken together, these observations show that NCC is expressed in bone and suggest that the osteoblast NCC, like the kidney NCC, may also be a target for thiazide diuretics. Thus, the bone-protective effects of thiazides may be due to their direct interaction with this protein in the osteoblasts.
Bone formation is characterized by a distinctive sequence of events beginning with the commitment of mesenchymal cells to osteoblast lineage, followed by osteoblastic proliferation and differentiation. This sequence of events culminates in the for-mation of mineralized extracellular matrix by terminally dif-ferentiated osteoblasts.15Previous studies have ruled out an effect of hydrochlorothiazide on human bone marrow stromal cells, suggesting that the thiazide-dependent enhanced bone mineral density is not due to an increase in osteoblast progen-itors.17However, the effects of thiazides on osteoblast prolifer-ation are controversial, with either an increase or no change in proliferation having been reported,13,18depending on species. Therefore, we investigated the effects of thiazides on osteoblast proliferation, differentiation, and mineralization to ascribe a potential role of NCC in each of these events. To exclude the possibility that the effects of thiazides could be indirect (i.e., a consequence of their renal actions), we carried out these stud-ies in vitro, using both primary and established models of os-teoblasts of either rat or human derivation, namely FRC and MG63 cells, respectively. First, we tested the effects of metola-zone and of chlorothiazide on FRC cells and found that neither of these compounds affected proliferation rates for up to 10 d in culture. Consistent with a lack of effect of thiazides on os-teoblast proliferation, NCC protein expression levels in FRC cells and in MG63 cells were negligible during the proliferative phase (i.e., up to 10 d in culture). At this point, FRC cells had stopped proliferating and begun their differentiation process, evident from increased expression of alkaline phosphatase (data not shown) and aggregation of cells into nodular areas. In postconfluent, differentiating osteoblasts, NCC protein ex-pression levels in both FRC and MG63 cells gradually rose and peaked at approximately 2 wk after confluence, indicating that NCC acts as a novel potential osteoblast differentiation marker. Having ascertained the presence of NCC in postcon-fluent, differentiating osteoblasts, we then tested the effects of thiazides on the expression levels of known osteoblast markers. Type I collagen is the major structural component of the or-ganic matrix of bone and one of the earliest marker of osteo-blast differentiation.15,19Our data show that, at a stage when NCC protein expression is absent (i.e. 2 d after confluence), neither metolazone nor chlorothiazide had any effect on the production of type I collagen. In contrast, metolazone pro-duced a concentration-dependent increase in the expression of Runx2, a master osteoblast-specific transcription regulator, in both FRC and MG63 cells. The ability of thiazides to regulate the osteoblast differentiation process was tested by measure-ment of the levels of osteopontin, a soluble, secreted phospho-Figure 5. MET-induced mineralization is mediated by NCC. (A)
MET treatment evokes a significant increase in mineralization in postconfluent FRC cells transiently transfected with empty vector (pcDNA3.1; *P⬍ 0.05; n ⫽ 9 from three independent cell isola-tions). (B) MET-dependent (10M) mineralization in postconflu-ent FRC cells is dramatically enhanced by NCC overexpression (sense [S]), and this is significantly blunted by NCC knockdown (antisense [AS]; P⬍ 0.05; n ⫽ 12 observations made from four independent cell isolations). Note that NCC overexpression in-creases mineralization of postconfluent FRC cells only after MET treatment. Data are expressed as change in number of nodules per well, defined as [(NodulesNCC ⫺ NodulespcDNA
)/Nod-ulespcDNA], where NodulespcDNA is the number of mineralized
nodules in cells transfected with empty vector and NodulesNCCis
the number of mineralized nodules in cells transfected with NCC sense or antisense, as indicated below bars. (C) Representative von Kossa staining of FRC cells after the treatments indicated below each well.
protein that is a component of the bone mineralized extracel-lular matrix (also called bone sialoprotein I, secreted phosphoprotein, 2ar, and bp69). In both FRC and MG63 cells, metolazone significantly increased osteopontin production. These data, taken together with our observations that metola-zone was ineffective during stages when NCC expression was undetectable and upregulated NCC expression in MG63 cells, suggest that thiazides act directly on differentiating osteoblasts through NCC.
The ultimate osteoblast differentiation marker is the forma-tion of new bone, and FRC cells in culture form calcified nod-ules that can be visualized by von Kossa staining. Metolazone treatment of postconfluent FRC cells induced a dramatic, con-centration-dependent increase in mineralized nodule forma-tion. This effect was specific to thiazide diuretics because it could be mimicked by chlorothiazide but could not be emu-lated by the loop diuretic bumetanide, even though our results show that osteoblast models express NKCC1, the molecular target for loop diuretics.
As proof of concept, we used plasmid delivery of NCC an-tisense and sense cDNA in FRC cells and assessed the effects of metolazone on mineralized nodule formation. Overexpression of NCC resulted in a significant increase in metolazone-in-duced mineralized nodule formation. This increase was com-pletely prevented by NCC knockdown with the antisense con-struct. This effect is even more striking when one considers that the efficiency of transfection of the plasmid in primary cells is only approximately 10% (estimated with co-transfec-tion with a fluorescence reporter; data not shown). The evi-dence that the increase in mineralization is observed only in the presence of thiazides suggests the possibility that NCC might act as receptor for thiazides, rather than a co-trans-porter, although such an interpretation would not explain why patients with Gitelman syndrome exhibit an increased bone mineral density.
Finally, it has been suggested that thiazides prevent bone loss because they reduce acid production by inhibiting car-bonic anhydrase activity in osteoclasts.20It is interesting that we demonstrate NCC immunostaining in some osteoclasts of cryoprepared rat femora but not human bone. Given that os-teoclast staining was observed in five different preparations, with the appropriate positive and negative controls, we believe that NCC immunofluorescence in a subpopulation of oste-oclasts is real. This observation opens the possibility that thia-zides might affect osteoclastic function through NCC in addi-tion to creating alkalinizaaddi-tion of the resorpaddi-tion milieu. The dual action of thiazide drugs on both osteoblast and osteoclast function could account for the observed reduced remodeling in patients taking such drugs in the absence of changes in plasma circulating parathyroid hormone levels.
The main finding of this study is the demonstration that thiazides directly stimulate osteoblast differentiation and min-eral production independent of their renal action. This effect of thiazides is concentration dependent, is not mimicked by loop diuretics, is not due to increased osteoblast proliferation, and is
enhanced by NCC overexpression. Together with the observa-tions that thiazide treatment and inactivating mutaobserva-tions of NCC are associated with an increased bone mineral density in humans and in knockout murine models, our findings support a pivotal role for the osteoblast NCC in mediating thiazide-induced bone formation. Thiazide diuretics are inexpensive and exhibit a good safety profile. Our findings suggest that it might be possible to develop osteoblast-specific thiazides as part of osteoporosis prevention and therapeutic programs.
CONCISE METHODS Animals
Sprague-Dawley rats (Charles River Laboratories, Wilmington, Kent, UK) were killed by cervical dislocation and used in accordance to the UK Animals Scientific Procedures Act of 1986.
Cell Culture
The human osteoblast cell line MG-63 was cultured as described pre-viously.18FRC cells were isolated as described previously15; FRC, rat
UMR-106, and mouse osteoblast-derived 2T3 cells were cultured as described previously.19Metolazone (Sigma-Aldrich, Poole, Dorset,
UK) was dissolved at 37°C for 2 h in the culture medium before being added to the cells. This procedure was repeated every 3 d. From con-fluence onward, the media were supplemented with ascorbic acid (284M for MG-63, UMR-106, and 2T3 and 568 M for FRC cells; Sigma-Aldrich) and-glycerophosphate (3 mM; Sigma-Aldrich).
Western Blotting
SDS-PAGE immunoblotting of MG-63, UMR-106, 2T3, and FRC cells (whole-cell lysates) and human and rat kidney was performed as de-scribed previously,21,22using the following primary antibodies:
Affinity-purified rabbit anti-human NCC polyclonal antibodies (1:1000),22
affin-ity-purified rabbit anti-rat NCC polyclonal antibodies (1:5000),23
affinity-purified rabbit anti-rat NKCC1 (a gift of Dr. R. James Turner, National Institute of Dental and Craniofacial Research, Bethesda, MD; 1:1000), affinity-purified rabbit anti-rat NKCC2 polyclonal antibodies (1:1000),24mouse anti-human Runx2 mAb (a gift of Dr. Andre von
Wi-jnen, University of Massachusetts, Worcester, MA; 1:4000), mouse anti-rat osteopontin mAb (Iowa Hybridoma Bank, Iowa City, IA; 1:4000), and mouse anti–-actin mAb (Abcam, Cambridge, Cambridgeshire, UK; 1:10,000). For SDS-PAGE, samples were heated to 60°C for 10 to 15 min in a 5⫻ Laemmli sample buffer, in the presence of dithiothreitol (30 mg/ml) or-mercaptoethanol (143 mM). Proteins were resolved by SDS-PAGE and transferred onto nitrocellulose membranes before block-ing (30 min; PBS containblock-ing 5% semiskim milk powder or Odyssey blocking buffer; Li-Cor, Lincoln, NE) and antibody incubations (1 to 12 h). Membranes were washed in Tween-Tris– buffered saline (15 mM Tris [pH 8], 150 mM NaCl, and 0.1% [vol/vol] Tween 20). Antibody binding was visualized by an enhanced chemiluminescence system (Am-ersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK). Hu-man kidney samples for immunoblotting were obtained from the unaf-fected portion of a kidney that had been resected because of a renal tumor
(approved as exempt from review by Office of Human Subjects Re-search).
Immunohistochemistry
Undecalcified, snap-frozen rat femora (2 mo) and human mandible resections and EDTA-decalcified rat femora were prepared and used for immunofluorescence and immunoperoxidase experiments as de-scribed previously.19Anti-human and rat NCC polyclonal
anti-bodies (see previous section) were used at 1:10 and 1:100 dilutions, respectively. The human mandible tissue was from neck resections from patients with squamous cell carcinoma invading bone (ethical approval and signed informed patient consent were obtained).
Effect of Metolazone on Osteoblast Proliferation
Cells were plated in 12-well plates at a density of 5000 cells/cm2and
were treated with metolazone (1 to 100M) for up to 2 wk. At each time point, triplicate wells were trypsinized and the cells were counted with a Coulter counter (Beckman Coulter, High Wycombe, Bucking-hamshire, UK).
Effect of Metolazone on Collagen 1A Content
The effects of metolazone on collagen 1A content were quantified as measurements of the hydroxyproline content in FRC cells after 48 h of treatment. After HCl digestion for 24 h at 110°C, the samples were freeze-dried to remove the acid, diluted in distilled water, and oxi-dized with chloramine followed by coupling with dimethylamino benzaldehyde at 70°C for 10 to 20 min. The colored product was measured at 550 nm. Standards of 1 to 10g/ml hydroxyproline were used to calculate the standard curve.
Effect of Metolazone on Osteoblast Differentiation
FRC and MG63 cells were cultured in 35-mm dishes (20,000 cells/cm2)
and treated with metolazone or chlorothiazide, for 2 d after confluence for early differentiation experiments or for 7 to 14 d after confluence for mineralization experiments. For immunoblotting, the cells were washed in PBS and lysed in RIPA buffer as described previously.21
Semiquantita-tive changes in Runx2 and osteopontin immunoreactivities were nor-malized for the levels of-actin (mouse monoclonal; Abcam, Cam-bridge, Cambridgeshire, UK).
Effect of Thiazides on Mineralized Nodule Formation
FRC cells were treated from confluence up to 3 wk. Mineralized nod-ules were visualized by von Kossa staining, as described previous-ly.19,21The images of mineralized nodules were captured on a flatbed
scanner, and image analysis software (Scion Image, Frederick, MD) was used to count the number of mineralized nodules.
Statistical Analyses
The statistical significance was assessed by one-way ANOVA with the Tukey post hoc test or with the unpaired t test, as appropriate. Observa-tions were considered to be statistically significant different at Pⱕ 0.05.
ACKNOWLEDGMENTS
This work was funded by the Arthritis Research Campaign (R0626 to D.R. and D.H.C.) and The Wellcome Trust (CRIG 070159 to D.R. and G.G.).
We thank Prof. V. Duance and Dr. S. Gilbert, Cardiff University, for the help with the collagen I assay; Dr. A. von Wijnen, University of Massachusetts, for the gift of the Runx2 antibody; Dr. A. Mee, Uni-versity of Manchester, UK, for the gift of MG-63 cells; N. Vasquez, National University of Mexico, for technical help; and the Iowa Hy-bridoma Bank for the osteopontin antibody.
DISCLOSURES
None.
REFERENCES
1. Gamba G: Molecular physiology and pathophysiology of electroneu-tral cation-chloride cotransporters. Physiol Rev 85: 423– 493, 2005 2. Wasnich RD, Benfante RJ, Yano K, Heilbrun L, Vogel JM: Thiazide
effect on the mineral content of bone. N Engl J Med 309: 344 –347, 1983
3. LaCroix AZ, Wienpahl J, White LR, Wallace RB, Scherr PA, George LK, Cornoni-Huntley J, Ostfeld AM: Thiazide diuretic agents and the incidence of hip fracture. N Engl J Med 322: 286 –290, 1990 4. Jones G, Nguyen T, Sambrook PN, Eisman JA: Thiazide diuretics and
fractures: Can meta-analysis help? J Bone Miner Res 10: 106 –111, 1995
5. Brickman AS, Massry SG, Coburn JW: Changes in serum and urinary calcium during treatment with hydrochlorothiazide: Studies on mech-anisms J Clin Invest 51: 945–954, 1972
6. Gesek FA, Friedman PA: Mechanism of calcium transport stimulated by chlorothiazide in mouse distal convoluted tubule cells. J Clin Invest 90: 429 – 438, 1992
7. Cruz DN: The renal tubular Na-Cl co-transporter (NCCT): A potential genetic link between blood pressure and bone density? Nephrol Dial
Transplant 16: 691– 694, 2001
8. Nicolet-Barousse L, Blanchard A, Roux C, Pietri L, Bloch-Faure M, Kolta S, Chappard C, Geoffroy V, Morieux C, Jeunemaitre X, Shull GE, Meneton P, Paillard M, Houillier P, De Vernejoul MC: Inactivation of the Na-Cl co-transporter (NCC) gene is associated with high BMD through both renal and bone mechanisms: Analysis of patients with Gitelman syndrome and Ncc null mice. J Bone Miner Res 20: 799 – 808, 2005
9. Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z: Pseudohypoaldosteronism type II: Marked sensitivity to thiazides, hy-percalciuria, normomagnesemia, and low bone mineral density. J Clin
Endocrinol Metab 87: 3248 –3254, 2002
10. Kamel HK: Low-dose thiazide and bone density. Ann Intern Med 136: 252–253, 2002
11. Van Den Berg CJ, Tucker RM, Dousa TP: Idiopathic hypercalciuria: Hydrochlorothiazide decrease urinary calcium without altered renal response to parathyroid hormone. J Clin Endocrinol Metab 55: 23–26, 1982
12. Hall TJ, Schaueblin M: Hydrochlorothiazide inhibits osteoclastic bone resorption in vitro. Calcif Tissue Int 55: 266 –268, 1994
13. Lalande A, Roux S, Denne MA, Stanley ER, Schiavi P, Guez D, De Vernejoul MC: Indapamide, a thiazide-like diuretic, decreases bone resorption in vitro. J Bone Miner Res 16: 361–370, 2001
14. Barry EL, Gesek FA, Kaplan MR, Hebert SC, Friedman PA: Expression of the sodium-chloride cotransporter in osteoblast-like cells: Effect of thiazide diuretics. Am J Physiol 272: C109 –C116, 1997
15. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB: Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol 143: 213– 221, 1990
16. Hoover RS, Poch E, Monroy A, Vazquez N, Nishio T, Gamba G, Hebert SC: N-Glycosylation at two sites critically alters thiazide binding and activity of the rat thiazide-sensitive Na(⫹):Cl(⫺) cotransporter. J Am
Soc Nephrol 14: 271–278, 2003
17. Kim CH, Kim SW, Kim GS: Effects of hydrochlorothiazide and furo-semide diuretics on human bone marrow stromal osteoprogenitor cells. Metabolism 49: 17–21, 2000
18. Aubin R, Menard P, Lajeunesse D: Selective effect of thiazides on the hu-man osteoblast-like cell line MG-63. Kidney Int 50: 1476–1482, 1996 19. Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth
EF, Riccardi D: Physiological changes in extracellular calcium con-centration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci U S A 101: 5140 – 5145, 2004
20. Raisz LG, Simmons HA, Thompson WJ, Shepard KL, Anderson PS, Rodan GA: Effects of a potent carbonic anhydrase inhibitor on bone resorption in organ culture. Endocrinology 122: 1083–1086, 1993 21. Dallas SL, Park-Snyder S, Miyazono K, Twardzik D, Mundy GR,
Bone-wald LF: Characterization and autoregulation of latent transforming growth factor beta (TGF beta) complexes in osteoblast-like cell lines. Production of a latent complex lacking the latent TGF beta-binding protein. J Biol Chem 269: 6815– 6821, 1994
22. Biner HL, Arpin-Bott MP, Loffing J, Wang X, Knepper M, Hebert SC, Kaissling B: Human cortical distal nephron: Distribution of electrolyte and water transport pathways. J Am Soc Nephrol 13: 836 – 847, 2002 23. Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA: The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 95: 14552–14557, 1998
24. Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA: Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle’s loop. Am J Physiol 276: F96 –F103, 1999