HAL Id: hal-02947979
https://hal.archives-ouvertes.fr/hal-02947979
Submitted on 19 Nov 2020
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Alejandro Enriquez-Cabrera, Amalia Rapakousiou, Mario Piedrahita-Bello,
Gábor Molnár, Lionel Salmon, Azzedine Bousseksou
To cite this version:
Alejandro Enriquez-Cabrera, Amalia Rapakousiou, Mario Piedrahita-Bello, Gábor Molnár, Lionel Salmon, et al.. Spin crossover polymer composites, polymers and related soft materials. Coordination Chemistry Reviews, Elsevier, 2020, 419, pp.213396. �10.1016/j.ccr.2020.213396�. �hal-02947979�
1
Spin crossover polymer composites, polymers and related soft materials
Alejandro Enriquez Cabrera, Amalia Rapakousiou, Mario Piedrahita Bello, Gábor Molnár,* Lionel Salmon,* Azzedine Bousseksou*
Laboratoire de Chimie de Coordination, CNRS UPR 8241, 205 route de Narbonne, F–31077 Toulouse, France. E-mail: lionel.salmon@lcc-toulouse.fr, gabor.molnar@lcc-toulouse.fr,
azzedine.bousseksou@lcc-toulouse.fr
ABSTRACT. We review the synthesis, properties and applications of spin crossover polymer composites, polymers and some related ‘soft’ materials. These materials have received recently much attention because they provide an efficient way for the processing of spin crossover complexes in various shapes at various size scales and can give rise also to unique physical properties. First, we discuss in detail the state of the art of the elaboration of spin crossover polymer composites, using either inorganic complex precursors in solution or pre-formed spin crossover powder. A particular attention is paid on the influence of the polymer matrix on the spin crossover properties and on the use of ‘active’ polymers for development of synergies between the properties of the matrix and the load. Polymer composite devices for applications in the fields of artificial muscles, energy harvesting and thermochromic sensors are also highlighted. Then, more recent works, in which organic polymeric chains are used as ligands for the transition metal ions are presented. Finally, we overview various related ‘soft’ spin crossover compounds including spin crossover dendrimers, gels, liquid crystals and Langmuir Blodgett films with particular emphasis on compounds with supramolecular interactions of alkyl chains.
Keywords: Spin crossover complexes, composite materials, polymers, soft matter
HIGHLIGHTS
- The article reviews spin crossover polymer composite materials - Recent development in spin crossover polymers
2
CONTENT
1. Introduction ... 3
2. Spin crossover in polymer composites ... 4
2.1. Composites prepared from solution ... 4
2.1.1 Solution casting ... 4
2.1.2 Adsorption into a matrix ... 6
2.1.3 Sol-gel method ... 10
2.1.4 Liophilization ... 12
2.2. Dispersion of SCO particles in polymer matrices ... 13
2.2.1 Drop casting ... 13
2.2.2 Electrospinning ... 16
2.2.3 Spray coating ... 16
2.2.4 In-situ polymerization ... 17
2.2.5 Electrochemically assisted self-assembly (EASA) ... 18
2.2.6 Matrix‐assisted pulsed laser evaporation (MAPLE) ... 19
2.2.7 3D printing ... 20
3. Spin crossover organic polymers ... 24
4. Related compounds ... 27
4.1 SCO dendrimers ... 28
4.2 SCO gels ... 31
4.3 SCO compounds with liquid crystal properties ... 33
4.4 Langmuir-Blodgett films displaying SCO phenomenon ... 34
5. Concluding remarks and prospects ... 35
Acknowledgments ... 36
3
1. Introduction
Polymer composites are multi-phase materials wherein at least one phase is a polymer.[1] The combination of these components results in original physical properties that differ from that of the constituents alone. In the majority of cases, they are composed of organic polymers as matrix and different fillers that act as the reinforcement.[2] Indeed, often the main objective that governs the development of such materials in various fields like construction,[3] aerospace [4] and automotive [5] is the modification of their mechanical and thermal properties. Nevertheless, the scope of advanced polymer composite materials exceeds largely the thermomechanical aspects, providing opportunities to develop a large variety of original physical properties as well as material processing methods.
In the field of spin crossover (SCO) complexes of transition metal ions,[6–11] polymer composites have been developed for several reasons. In the early stages of SCO research, incorporation of SCO complexes into polymer matrixes was carried out in order to make possible some physical characterizations (e.g. photophysical measurements), which were not feasible (or meaningful) using microcrystalline powder samples or liquid solutions.[12,13] To this aim, films or pellets of SCO-polymer composites were fabricated using simple methods such as spin coating or drop casting. Following the visionary ideas of Olivier Kahn in the 90ies,[14,15] the past two decades the SCO research has moved to a considerable extent towards seeking potential technological applications of these smart, multifunctional molecular materials, exhibiting a spectacular change of their magnetic, optical, electrical, thermal and mechanical properties.[16,17] As a result, the need for device integration and processing of SCO materials in different shapes and sizes (from the nanometric to the macroscopic scale) has also significantly increased. This conjuncture has motivated considerable research for the incorporation of SCO materials and nanomaterials [18] into malleable and processable polymer matrices using increasingly sophisticated methods, such as spray coating, matrix-assisted pulsed laser evaporation, electrospinning or 3D printing. However, impacts of the nature and the mechanical properties of the polymer on the spin crossover behaviours, and vice-versa, were clearly evidenced in many cases. These findings generated research for the theoretical modelling of SCO-matrix interactions [19] and, more recently, for the development of more sophisticated SCO polymer composite materials exhibiting synergies between the properties of the SCO particles and the polymer matrix. Notably, strain-coupled electroactive polymer-SCO composites have been developed with promising properties for the development of actuators, sensors and energy harvesters.[16,20,21] Alternative to multi-phase, composite materials, several groups have also undertaken syntheses of ‘spin crossover organic polymers’, i.e. organic polymers functionalized by SCO entities. (N.B. We use the term ‘SCO organic polymers’ to avoid confusion with ‘SCO coordination polymers’, which refer to the well-known SCO coordination networks, such as Fe-triazole chains or Hofmann like clathrates.) The review is organized in three sections. The first section gathers a state of the art, which is aimed to be exhaustive on the synthesis and characterization of the physical properties of spin crossover polymer composites. The second chapter brings together the few reported examples of ‘spin crossover organic polymers’. The last section is a non-exhaustive overview of selected examples of conceptually related ‘soft’ SCO materials, including SCO dendrimers, gels, liquid crystals and Langmuir Blodgett films, which display uncommon properties and allow for easier material processing when compared to ‘conventional’ crystalline SCO materials.
4
2. Spin crossover in polymer composites
Embedding SCO complexes into polymer matrices is a straightforward, yet powerful and generic approach towards processable SCO materials, important for their different applications and integration into devices. From a conceptual point of view, it is interesting to separate these materials into two main categories: composites prepared from solutions (whether using a solubilized SCO complex or the corresponding precursors) and composites prepared from preformed SCO powder. In both cases, a variety of methods of different sophistication were employed, which range from simple casting techniques to state-of-the-art stereo-lithography.
2.1. Composites prepared from solution 2.1.1 Solution casting
Whenever an SCO compound and a polymer have a common solvent, this simple method provides a straightforward means for dispersing the complex within the matrix and process the composite as a film on various substrates. Yet, the resulting morphology and microstructure of the SCO complex is not always trivial to control (e.g. crystalline vs. amorphous, aggregated vs. dispersed, etc.).
Xie and Hendrickson [12] doped the complex [Fe(6-Me-py)2(py)(tren)](ClO4)2
((6-Me-py)2(py)(tren) ligand is a Schiff base) into PSS (polystyrene sulfonate, 6.25 % wt) matrix by
simply dissolving the two compounds in water and allowing the solvent evaporate on a glass substrate. The composite films were then investigated using UV-VIS spectrophotometry, Mössbauer spectroscopy and flash laser photolysis techniques. The authors noticed a change of the SCO behaviour with respect to the perchlorate salt of the complex and they evoked the possibility of specific interactions between the sulfonate groups of the polymer and the SCO cation. One can assume that such interactions can lead to an effective dilution of the complex within the matrix. Hauser et al. [13] conducted similar photophysical studies using the [Fe(2-mephen)3]2+ complex embedded in PVAc (poly-vinyl-acetate) and Nafion films. PVAc was
doped by the SCO cation by co-dissolving the two compounds in methanol and then let the solution dry on a glass slide. The Nafion films were simply immersed in an aqueous solution of the cation. The (very gradual) SCO properties of the complex were found not substantially affected by the polymer matrices, albeit the photo-physical properties of the composites indicated local inhomogeneities.
With a more technological motivation, Lee et al. [22] reported on processable composites, which consisted of a mixture of PMMA (poly(methyl methacrylate)) and different loadings of the complex [Fe(hptrz)3]X2 (hptrz= 4-heptyl-1,2,4-triazole; X = ClO4−, BF4− or Br−) obtained
from DMF solutions followed by vacuum distillation. Interestingly, the spin transition curves of the complexes in the composites remained relatively abrupt and the hysteresis widths even increased (vs. the bulk microcrystalline powders), despite the fact that x-ray diffraction analysis did not reveal any evidence of crystallinity in the composites. Later, the same authors mixed [Fe(ODT)3](OTs)2 or [Fe(HET)3](OTs)2 (ODT = 4-octadecyl-1,2,4-triazole, HET =
4-(2-hydroxyethyl)-1,2,4-triazole, OTs = p-toluenesulfonate ) with the liquid crystalline polymer poly(oxetane) (POx) in an effort to combine both magnetic and liquid crystal transitions in a multifunctional material.[23] Following vacuum drying of the THF solutions of the materials, they obtained composites where the temperatures of the different transitions (glass transition, spin transition and isotropisation) were lowered in comparison to the bulk materials. However,
5
no interplay between these phenomena was reported. In an interesting study, Chen et al. [24] prepared a composite film by mixing precursors of [Fe(NH2trz)3](ClO4)2 with PVP
(polyvinylpyrrolidone) in ethanol. Due to the strong hydrogen bonds formed in between the amino groups of the complex and the carbonyl groups of the PVP, a microstructure consisting of a regular stripped pattern with 0.3 nm inter-line distance is formed, which can be clearly seen in the transmission electron microscopy images of the composite film (
Figure 1a-b). Based on X-ray diffraction (XRD), infrared (IR) spectroscopy and X-ray photoelectron spectroscopy (XPS) analyses, they concluded that this structure consists of the PVP chains aligned along the Fe-aminotriazole chains in a hexagonal lattice due to the hydrogen bonds between the triazole and the PVP. This microstructure is not observed when the film is prepared with the similar complex with the Htrz ligand for which the hydrogen bonds are not feasible. This suggests that strong hydrogen bond interactions between the polymer and the SCO complex are essential for obtaining a regular structure. Remarkably, the spin transition in the composite (vs. the bulk complex) is significantly shifted towards room temperature from 210 to 250 K and a ca. 6 K-wide hysteresis is also observed (
Figure 1c). These results open up interesting perspectives for the investigation of Fe-triazole compounds in different supramolecular arrangements.
Figure 1. a) Schematic representation of the PVP – Fe-aminotriazole composite where the blue dashed lines represent the hydrogen bonds. b) TEM image of the composite film with regular striped patterns. c) Temperature dependent magnetic behavior of the composite.[24]
Following a ‘polymer science methodology’, Rubio et al. [25] studied the effect on the physical properties of isotactic polystyrene (i-PS) when [Fe(ODT)3](ClO4)2 (0.5 - 5 w%) is dispersed in
it. Blend films of different composition were prepared by mixing homogeneous toluene solution of both polymers at 100°C and evaporating the solvent. Interestingly, the degree of crystallinity of i-PS increases due to the presence of the complex, which indicates that the complex has some nucleating effect for the crystallization of the polymer. This result is important in that it shows that not only the polymer matrix can influence the SCO behavior, but also the SCO complex can significantly alter the physical properties of the polymer, even for such relatively small loads. Unfortunately, the authors do not report on the SCO properties of the composite. In a further study, Echeverria et al. [26] prepared electrospun fibers of atactic polystyrene (a-PS) with [Fe(ODT)3](ClO4)2 for which they observed the formation of ca. 3 m diameter fibers for
loadings of the complex up to 13.5 w%. Yet, for increasing concentration of the complex the appearance of the fibers becomes less uniform. The magnetic behavior of the composite with a 13.5 w% SCO loading is similar to the bulk material and the glass transition temperature of the polymer remain nearly unchanged. The authors report also on structural changes in the SCO
c) c) b) 32 Å 24 Å 40 Å a)
6
complex above room temperature - similar to the behavior previously reported for the isotactic polystyrene blends.[25]
Basak et al. [27] used also PS to embed a micro-patterned film of the SCO complex [Fe(Oct-BPP)2](BF4)2 (4,4″-dioctyl 2’,6’-bispyrazolylpyrine) within a flexible polymer film. First, they
prepared a thin film of PS by drop casting (1.2 cm × 1.5 cm × 5-6 m) and then on top of it a rectangular stripe of the SCO complex was fabricated by lithographic patterning using a PDMS (polydimethylsiloxane) stamp, followed by spin coating a PS solution in top of the SCO layer. This resulted in a flexible polymer film in which the embedded SCO grating shows optical diffraction phenomena (Figure 2). Unfortunately, the authors do not report on the SCO properties of the micro-patterned material.
Figure 2. TEM images of regular stripes of the SCO complex [Fe(Oct-BPP)2](BF4)2 embedded
in a polystyrene film (a,b,c) and photographs showing the flexibility of the thin film.[27] Thin films (5 - 20 micron thick) of PMMA with 2, 4 and 8% wt. loading of the valence tautomeric complex [CoIII(Cat-N-SQ)(Cat-N-BQ)] (Cat-N-BQ = 2-(2-hydroxy-3,5-ditert-butylphenyl-imino)-4,6-ditert-butylcyclohexa-3,5-dienone and Cat-N-SQ is the dianionic radical analogue) were prepared and the spin crossover associated with the valence tautomerism was demonstrated.[28] Compared to a related toluene solution, the best interconversion was obtained with a 2% wt. loading while at 4% wt. the HS state is favored and at 8% only the HS state is observed. This was explained by the crystallization of the complex at concentrations above 2%. An important remark for this system is that when heated above the glass transition temperature (Tg = 395 K), followed by slow cooling, the complex is allowed to crystallize in
the polymer matrix. As the HS isomer is also stabilized in the crystalline form, this results in an irreversible transition. However, if the temperature of the film is kept below Tg, then the
transition is reversible upon several cycles (Figure 3).
Figure 3. a) Flexible PMMA thin film with 2% wt. of [CoIII(Cat-N-SQ)(Cat-N-BQ)]; b) photographs of a thin film representing the reversible and irreversible color change at different temperatures.[28]
7
2.1.2 Adsorption into a matrix
This approach is developed mostly using ionomers, i.e. polymers with ionic properties (often with sulfonate, carboxylate or amino groups), which allow for the immobilization of SCO complexes or their precursors by electrostatic interactions using simple impregnation methods. As mentioned above, Hauser et al. [13] used Nafion in which the sulfonic acids of the Nafion film acted as counter-anions for the [Fe(2-methyl-1,10-phenanthroline)3]2+ complex. Later on,
the group of Kojima carried out a more “material-oriented” investigation of various SCO-Nafion composites.[29–34] By a simple two-step impregnation method (first into the solution of the metal ion and then into the ligand solution), they elaborated FeII(Htrz)3-Nafion films,
providing the polymeric backbone to the final complex as shown in Figure 4a. Nafion is well known to form reverse micelles, that consist of clusters of about 4 nm in diameter and are separated by a distance of about 5 nm.[35,36] It is inside these clusters that the FeII(Htrz)3
species are likely located. The group of Kojima studied the temperature dependence of the magnetic susceptibility for the complexes FeII(Htrz)3-Nafion and FeII(NH2trz)3-Nafion.[29] In
both cases, they observed a very gradual spin crossover below room temperature with half-transition temperatures (T1/2) of 260 K and 198 K, respectively (Figure 4b). Fe K-edge EXAFS
spectra proved the presence of the linear Fe chain structure in the film.[30] They also investigated the photoinduced effects on the FeII(Htrz)3-Nafion film by irradiating it at 514.5
nm, which is close to the 1A1g→1T1g absorption maximum (540 nm). They could observe the
LIESST effect at 4.2 K and provided details on the relaxation kinetics of the photo-induced HS state back to the LS ground state.[30–32] The Mössbauer spectra of the composite films revealed that as much as 40 % HS species remains unconverted even at 10 K. This was attributed to the (presumably) large number of terminal Fe(II) sites in the small oligomers formed in the Nafion clusters.[33] The Debye temperature (D) for the HS and LS states were
estimated from the Mössbauer spectra, resulting in 185 K for the LS and 176 K for the HS forms in the temperature range between 10 and 150 K. Above 150 K, D becomes small (ca. 50 K)
due to the glass transition exhibited by the Nafion at 180 K.[34] Surprisingly, this relatively abrupt change of the Debye temperature does not appear to have an obvious impact on the spin transition properties of the complexes (Figure 4b). Later on, Kamebuchi et al. [37] replaced the iron-triazole complex by FeII(diAMsar) (diAMsar = 1,8-diaminosarcophagine) in the Nafion film, providing a pH dependent SCO complex. Under acidic conditions, the amino groups of diAMsar are protonated resulting in an electrostatic repulsion between the ligand and the iron center leading to the absence of SCO properties. Under basic conditions at room temperature the LS state is stabilized, whereas at pH = 7, the HS state is stabilized.
8
Figure 4. a) Representation of FeII(Htrz)3-Nafion complex; b)thermal variation of T for
FeII(NH
2trz)3-Nafion (◊) and FeII(Htrz)3-Nafion (●); c) representation of FeII(Htrz)3-Nafion
before and after the glass transition.[30,32,34]
Vishnevskaya et al. [38] studied a FeIII-formazan complex immobilized in the ion-exchange polymer AN-18-10P. They suggested immobilization occurs via ion exchange between the sulfo group of the ligand and the functional groups of the resin. Using EPR spectroscopy, they revealed the gradual spin crossover properties of the composite. Durand et al. [39] prepared mesoporous composite xerogels by immersing a previously prepared monolith of silica in an Fe(BF4)2 solution and then in a solution of the triazole ligand. Nanoparticles of the SCO
complex were formed in the pores of the silica monolith with an average size of 3.2 nm. Remarkably, despite the very small size of the particles, the magnetic behavior of the composite showed a partially reversible spin transition with a wide hysteresis (375 K on heating and 310 K on cooling). The spin transition was confirmed by Mössbauer measurements, which revealed also that at 200 K 32 % of the FeII centers remain in the HS state. Onggo et al. prepared macrofilms (20 cm long, 20 cm wide and 4-5 cm thick) by using ‘nata de coco’ sheets – a food product used in Philippines, in which the major component is cellulose fibers also known as bacterial cellulose. To impregnate the sheets with the SCO complexes [Fe(Htrz)2(trz)](BF4) and
[Fe(NH2trz)3](BF4)2, their synthetic approach was based on immersing the starting films in an
aqueous solution of Fe(BF4)2·6H2O and later into a methanol solution of the corresponding
triazoles. The formation of SCO complexes inside the film, favorized by the hydrophilic properties of ‘nata de coco’, was confirmed by a color change due to the spin transition of the composite. The SCO complexes formed rod-shaped particles with an average diameter of 100 nm and 0.5-1 µm length, which filled the cavities of the sheets. The magnetic behavior of the SCO composites shows an abrupt spin transition, which matches quite well with the bulk SCO materials.[40,41]
a)
b)
9
Figure 5. a) SEM image of a ‘nata de coco’ film containing [Fe(Htrz)2(trz)](BF4) particles (left).
Magnetic measurements of the composite with b) [Fe(Htrz)2(trz)](BF4) and c)
[Fe(NH2trz)2](BF4)2.[41]
Wang et al. [42] prepared nanorods of [Fe(Htrz)2(trz)](BF4) using a cation-exchange polymer
resin, in which the sulfonate groups on the resin served as the nucleation sites for the growth of the anisotropic SCO particles. This was achieved by immersing the resin for different times in a solution of Fe(BF4)2 and then into a solution containing triazole and NaBF4, resulting in
SCO@resin nanorods. The crystalline structure of the bulk SCO was maintained in the nanocomposite as inferred from XRD. The average length of the nanorods was ca. 250 nm for an immersion of 12 h, which increased up to a length of 750 nm after 42 h, reaching a saturation point. On the other hand, as shown in Figure 6, the spin transition behavior does not substantially change with the immersion time, albeit small differences occur. The spin transition temperature of the rods is similar to the bulk material, although less abrupt and with a larger hysteresis loop. The authors speculated the latter might be attributed to the close proximity among the SCO nanorods.
Figure 6. (left panel) SEM images of a) the blank resin and [Fe(Htrz)2(trz)](BF4) nanorod arrays
with immersion times of b) 12, c) 18, d) 24, e) 36 and f) 42 h; (right panel) magnetic measurements of the SCO nanorods for different immersion times.[42]
Larionova et al. [43] reported the use of porous chitosan beads as a polymer matrix to obtain ultra-small nanoparticles (ca. 3.8 nm) of the 3D spin crossover coordination polymer
a) b) c)
a) b)
c) d)
10
[Fe(pz){Ni(CN)4}]. They used a multi-step sequential assembly, which consisted of
consecutive impregnations of the chitosan beads into methanol solutions of Fe(BF4)26H2O,
pyrazine and (N(C4H9)2[Ni(CN)4] as depicted in Figure 7. Remarkably, the ultra-small particles
displayed a well reproducible spin transition with a pronounced hysteresis near room temperature, albeit this transition remained rather incomplete.
Figure 7. a) Schematic representation of the synthesis of the Hoffman-like clathrates [Fe(pz){M(CN)4}] within chitosan beads. Thermal variation of T for the nanocomposite
[Fe(pz){M(CN)4}]-chitosan beads for b) M=Ni; c) M=Pd; d) M=Pt and e) a nanocomposite
film of [Fe(pz){Ni(CN)4}]-chitosan.[44]
Later on, the same authors [44] synthesized a series of nanocomposites using not only chitosan, but also alginate beads for hosting particles of the complexes [Fe(pz){M(CN)4}] (M=Ni, Pd,
Pt). In this case, they dried the chitosan composites either in vacuum to obtain hydrogels or under CO2 supercritical conditions obtaining aerogels, resulting in chitosan composites with an
estimated amount of 35 % wt for Ni, 18 %wt for Pd and 16 % wt for Pt of the immobilized complex and an average particle size of 2.8 nm for chitosan composites and 3.2 nm in the case of alginate. The chitosan beads were then dissolved in water at 70 °C and then placed in a petri dish for slow evaporation of the water resulting in a yellow film. In the nanocomposites, the ultra-small SCO particles are homogeneously distributed within the pores of the beads. In the magnetic measurements for the chitosan composites of Ni and Pd an abrupt spin transition is observed with a smaller hysteresis loop compared to the bulk analogues, whereas for the chitosan composite of Pt and the alginate composite of Ni a gradual and incomplete SCO transition is observed both for the beads and the film samples (Figure 7). Interestingly, Raman microspectroscopy indicated an almost complete spin transition for the chitosan composites with the Ni and Pd based compounds, whereas magnetic and Mössbauer measurements showed considerable residual fractions. This apparent discrepancy was explained by the presence of iron(II) species, which were not incorporated into the main coordination network resulting in an important SCO inactive fraction.
2.1.3 Sol-gel method
The sol–gel process is a widespread method for the fabrication of glassy and ceramic materials. In this process, a colloidal solution (sol) is formed, which evolves gradually towards a gel-like network from which the final form of the material is obtained using various drying and firing approaches. Common precursors are metal alkoxides and metal chlorides, from which the
a)
11
colloid is obtained by hydrolysis and poly-condensation reactions. In the context of SCO research, this method is particularly useful to synthesize SCO-silica composite materials, which can then be used for the fabrication of SCO-active films, coatings and other objects.
Faulmann et al. [45] prepared thin films and monoliths of the SCO complex [Fe(Htrz)2(trz)](BF4) by the hydrolysis of TMOS (tetramethoxysilane) or TEOS
(tetraethoxysilane). After the gelation, the composite was aged during 2 days and then dried over a week at ~40 °C resulting in well dispersed spherical SCO nanoparticles with a size range of 1-5 nm encapsulated in transparent silica films. The magnetic behavior of the composites showed an abrupt spin transition similar to the bulk material.
Voisin et al. [46] prepared a composite gel of sulfonate-functionalized SiO2 nanoparticles with
the SCO complex [Fe(NH2trz)3](SO4)by suspending the nanoparticles in a mixture of ethylene
glycol : water (9:1) solution to which the iron salt and the ligand was added. After 20 h, gelation occurred resulting in a white gel, which turned pink later. SEM images showed a good dispersion of the SiO2 nanoparticles in the matrix of the SCO gel, which is mostly composed
of needles (Figure 8). Using freeze-fractured TEM, particle imprints are seen, indicating that the silica surface is physically interacting with the SCO coordination chains. Rheological studies conducted on the composite aged for 9 days, resulted in an average storage modulus (G’) of 5-8 kPa and a loss modulus (G’’) of one order of magnitude less. The magnetic behavior of the composites showed a reversible and abrupt spin transition that is shifted to higher temperatures when compared to the pure SCO gel. In addition, a broadening of the hysteresis loop occurs. The authors observed that when drying both the SCO gel and the composite their SCO properties become comparable (T1/2↑=343 K, T1/2↓=329 K), but the spin transition shifts
to higher temperatures when compared to their solvated counterparts. These results emphasize the sensitivity of the spin transition on multiple factors in such complex, multiphase systems.
Figure 8. Schematic representation of a) the particle free suspension, b) composite gel NP-SiO2@Fe/NH2trz/SO4, c) dry particle free system. Composite gel NP-SiO2@Fe/NH2trz/SO4
imaged by d) SEM-FEG and e) cryo-TEM; f) magnetic measurements of the composite gel NP-SiO2@Fe/NH2Trz/SO4 and the particle free suspension.[46]
Later on, the same authors [47] used the composite gel NP-SiO2@Fe/NH2Trz/SO4 [46] and
casted it onto a PTFE (polytetrafluoroethylene) mold (50×25×2 mm), which was exposed to an alkoxysilane vapor in a closed desiccator to obtain a flexible macroporous hybrid silica network (Si@Si-SCO), which was eventually coated with PDMS (PDMS@Si@Si-SCO) (Figure 9).
a) b) c) d)
e)
12
This way they obtained a flexible polymer nanocomposite material with PDMS protection, which provided long-term stability to the material (> 80 days), prevented its decomposition when immersed in hot water and allowed to preserve its SCO properties over >15 thermal cycles. Two parameters were essential to obtain a material with optimal mechanical properties: the nature of the alkoxysilane and the exposure time. Indeed, TMOS and TEOS produced a brittle solid, while DMDS (dimethyldimethoxysilane) or BTESE (1,2-Bis(triethoxysilyl)ethane) or BTEB (1,4-Bis(triethoxysilyl)benzene) resulted in a viscous liquid. The best result was obtained for MTEOS (methyltriethoxysilane) for which a flexible solid was obtained. In addition, shorter or longer times of exposure resulted in inhomogeneous fragile gels that in some cases were oxidized with time. For more than 5 days of exposure, a homogeneous gel is obtained, with the best results after ca. 7 days of exposure. However, after 28 days, the material evolved in a brittle solid and the pink colour faded away, probably due to solvent expulsion, which could be prevented by coating it with PDMS. SEM images of the Si@Si-SCO composites showed needles and spherical nanoparticles located in the cavities of the macroporous silica matrix (Figure 9).
Figure 9. a) Scheme of the processing steps of PDMS protected silica-SCO nanocomposite material within silica network (PDMS@ Si@Si-SCO); b) SEM image of Si@Si-SCO aged for 14 days; c) scheme of the structure of Si@Si-SCO where the grey background represents the organosilica matrix, the yellow disks the silica NPs, the dark blue needles the crystalline SCO objects and the light purple lines the amorphous SCO network.[47]
2.1.4 Liophilization
Kuroiwa et al. combined a cobalt(II)-terpyridine [48] as well as the [Fe(ppi)2(NCS)2] (ppi=
N-phenyl-2-pyridinalimine) [49] SCO complex with diblock copolypeptydes amphiphiles composed only of glutamic acid and leucine, in which the carboxylate in the glutamic residue is the counter-anion of the SCO complex (Figure 10). They were prepared by simply mixing the copolypeptide and the desired complex in solution, following a precipitation and lyophilization.
a)
13
Figure 10. a) Copolypetides used as counter-anions for b) cobalt(II)-terpyridine and c) [Fe(ppi)2(NCS)2].[48,49]
For the terpyridine complex, structures with width of 0.5 to 2 m were observed by TEM except when using the polypetide without leucine. Interestingly, all the terpyridine complexes showed reverse spin transition after annealing (T1/2↓≈260 K, T1/2↑≈345 K) – indicative of a structural
phase transition. When using the FeII complex, a gradual SCO behavior that was completely
reversible only for one of the copolypeptides was observed by UV-Vis in water solution. It was suggested that the composites in solution are composed of nanostructures consisting in cylindrical and spherical structures at 5 °C, and lamellar structures at 60 °C. The authors proposed a packing of 1D or 2D sheets among the metallic complexes, which is in agreement with previous crystallographic data obtained for both complexes and the supramolecular arrangement of both the glutamic acid and leucine in their corresponding -sheets forms.
2.2. Dispersion of SCO particles in polymer matrices
Mixing polymers with preformed SCO powders (including both micro- and nanocrystals, nanorods, etc.) provides obviously the advantage of better control over particle morphology and SCO properties. In addition, feebly soluble SCO compounds can be also processed. On the other hand, particle aggregation may be an issue in particular for their integration and their homogeneous distribution in the composite materials.
2.2.1 Drop casting
Drop casting has been employed at several instances as a simple means to obtain functional SCO particle – polymer composites, which can be used as mechanical actuators, optical sensors or smart papers.
In 2013, Shepherd et al. reported on a SCO-polymer composite based bilayer mechanical actuators of PVP and PMMA, the latter with different SCO particle loadings.[50] It was shown, for the first time that the strain associated with the spin transition can be used to produce useful mechanical work. Later, the same group investigated more in detail PMMA [Fe(trz)(Htrz)2](BF4) (10-50% wt.) composite films in combination with a silver-based
conducting polymer composite layer. The latter film was used for the electrothermal actuation of the device. Upon Joule-heating, a switching from the LS to the HS state was observed accompanied by the bending of the bilayer cantilever with frequencies up to a few Hz over a few hundreds of actuating cycles without noticeable fatigue (Figure 11). This ‘artificial muscle’ device showed a volumetric work density of ca. 150 mJ.cm-3.[51]
14
Figure 11. Schematic representation of a bilayer SCO-polymer composite cantilever with electrothermal actuation and the experimentally observed variation of the cantilever tip position (in ambient conditions) upon the application of an alternating current.[51]
Using a similar approach, Chen et al. [52] prepared a bilayer device consisting of a [Fe(Htrz)2(trz)](BF4)/Polycarbonate (PC) composite layer and a piezoresistive layer. The
mechanical stress induced by the SCO particles in the device gave rise to a change in the electrical resistance, which was detected through a Wheatstone bridge (Figure 12). Another example of the exploitation of the volume change associated with the spin transition in a polymer composite was described by Rat et al. [16] They dispersed nanoparticles (≈20 nm) of the SCO complex [Fe{(Htrz)2(trz)}0.9(NH2trz)0.3](BF4)1.1 in the ferroelectric poly(vinylidene
fluoride-co-trifluoroethylene) P(VDF-TrFE) 70-30% copolymer matrix. In this composite, the large strain associated with the spin transition is expected to give rise to an electrical response (voltage or current) due to the piezoelectric properties of the polymer matrix. Macroscopic freestanding films of the composite were first poled and then thermally cycled under short-circuit conditions. Around the spin transition temperatures, they displayed current discharge peaks, showing the effective coupling between the SCO and piezoelectric properties. Further work [20] allowed for tuning concomitantly the spin transition temperature of the filler and the Curie temperature of the copolymer in such a way that the piezoelectric effect from the spin transition and the intrinsic pyroelectric response of the polymer could be concomitantly observed (Figure 13), providing prospects for thermal energy harvesting applications.
Figure 12. a) Schematic structure of the bilayer SCO-piezoresistive cantilever and (b) photos of the prefabricated polyimide/constantan alloy/polyimide stress-sensitive plate before (left) and after (right) drop-casting of the SCO-active composite. c) Bistable voltages of the Wheatstone bridge upon 4 thermal cycles.[52]
a)
b)
15
Figure 13. (a) Photographs of a freestanding SCO-P(VDF-TrFE) composite film. (b) Pyroelectric discharge cycle for a polarized SCO- P(VDF-TrFE) composite film (25 w%) when short-circuited through an electrometer. The arrows indicate heating and cooling. Dotted lines show the SCO-related discharge peaks for cooling (left) and heating (right).[16,20]
Thermochromic thin films of [Fe(NH2trz)3](BF4)2 particles in different polymer matrices were
studied by Lapresta-Fernández et al.[53,54]. They evaluated the effect of the interaction of the particles with the polymer matrix via the color change of the composite. Significant differences were observed in terms of aggregation and clustering when using solvents and polymers of different polarity. For example, in the case of PMMA composites, the size of the clusters are reduced when they are prepared in THF, whereas aggregation of the nanocrystals is observed when they are prepared in toluene. In contrast, in the case of hydrophilic polymers such as Nafion or polyurethane-D6, fiber-shaped particles are observed. Notably, in the case of polyurethane-D6 the particles are needle-shaped with a width of 250-500 nm and several
micrometers in length and differ with the large clusters of ca. 500 nm observed for the bulk sample. Such evolution of the material morphology in the different matrixes can explain the change in the SCO properties and was used as a tool to develop a colorimetric sensor array based on a photographic digital camera.[55] Vinogradova et al. [56] used [Fe(NH2trz)3](NO3)2
particles embedded in polystyrene to prepare films with a thickness above 10 m. The resulting composites retained the SCO properties of the bulk material showing different spin transition temperatures depending on the water content.[57] Figure 14 shows the magnetic properties of the composite in different experimental conditions: either in vacuum (i.e. dehydrated composite) where the spin transition shows a hysteresis of 27 K, or after being exposed to ambient air for 15 min and measured in a sealed capsule, where the spin transition shows a hysteresis of 2 K. The vapochromic behavior of these films were tested, by exposing them to HCl, HBr + Br2, HBr, HNO3 and NH3 vapors resulting in drastic color changes (implying the
16
Figure 14. Magnetic properties of a PS - [Fe(NH2trz)3](NO3)2·0.5H2O composite (left) and
vapochromic effect in the composite films (right).[56]
Nagy et al. [58] prepared linter cellulose nanocomposites from [Fe(hptrz)3](OTs)2 nanoparticles
doped with acridine orange dye, resulting in a thermochromic and thermofluorescent material. The SCO nanoparticles were randomly dispersed along the surface of the cellulose fibers -stabilized electrostatically due to the interaction between the hydroxyl groups of the cellulose and the metal cations of the SCO complex. It is noteworthy that the nanocomposites were stable under air for several months and that no thermal quenching or photobleaching of the fluorescence was observed. Later on, the same group reported cellulose and [Fe(Htrz)2(trz)](BF4) nanocomposite sheets of 15 mm width and about 0.7 mm thickness.[59]
These composites show better elongation, lower elastic modulus and higher resistance to stress when compared to normal cellulose sheets. They were able to print white patterns in the violet background of the composite sheets by heating it locally either with a focused laser beam (photothermal effect) or by a hot tip. Similarly, they could print violet patterns in the white background by local cooling. These patterns could be recycled (by erasing/rewriting through heating or cooling) over more than 100 times and they are stable upon storage at ambient conditions for long periods of time (at least 9 months) – providing scope for applications in rewritable papers. These cellulose composites with a 50% wt. of the SCO complex were also investigate using dynamical thermomechanical analysis (DMA) and a ca. 10% variation of the storage modulus between the LS and HS states could be detected [60].
2.2.2 Electrospinning
Electrospinning is an electrostatic (nano) fiber fabrication method, which uses polymer solutions, suspensions or melts. A simple setup consists of syringe needle, connected to a high-voltage power supply, a syringe pump and a grounded collector. This technique has been used to produce SCO-polymer composite fibers either from solutions [26] (see section 2.1.1) or from particle-polymer suspensions. The latter approach was used by Dreyer et al. [61] who prepared electrospun fibers from polylactic acid and [Fe(Htrz)2(trz)](BF4) or [Fe(NH2trz)3](BF4)2
particles using a SCO/polymer ratio of 5% wt. They tested the SCO behavior of the composite fibers by Mössbauer spectroscopy showing that the spin crossover behavior was preserved in the final material, for which the LS state is stabilized at room temperature, unlike the bulk material.
2.2.3 Spray coating
Spray coating consists of depositing microdroplets (i.e., an aerosol) of the desired material on a surface by forcing the dissolved (or dispersed) material through a nozzle. This versatile
17
method allows for fabricating smooth, homogeneous coatings with well-defined compositions, and geometries on various substrates.
Hellel et al. [62] spray coated [Fe(NH2trz)3]Br23H2O and [Fe(NH2trz)3](NO3)2H2O particles
with ~1 m diameter on a polyester film. They then successfully induced the spin transition from the LS to the HS state of the SCO complex via IR laser-induced heating of the matrix by means of a CO2 laser (=10.6 m). With this technique, they were able to write the letters
“CNRS” in the thin film in less than 1 s at room temperature. As long as the temperature of the film was kept within the hysteresis loop (10 °C < T < 45 °C) the stored information persisted. Spray coating was also used by Manrique-Juarez et al. to deposit a composite consisting of [Fe(H-trz)2(trz)](BF4) nanoparticles (ca. 85 nm) in an epoxy-based photoresist (SU-8). The
composite was spray-coated over silicon micro-cantilevers (MEMS) and crosslinked by UV light exposure and consequent baking steps. Intriguingly, the spin transition in this composite showed a large hysteresis of 59°C (T1/2↑=113°C and T1/2↓=54°C), more than twice than that of
the bulk material (T1/2↑≈105°C and T1/2↓≈82°C). Thermally-driven actuation of the cantilevers
in the MEMS was observed, resulting in an abrupt bending at the temperature of the spin transition (Figure 15d) with a stable amplitude of actuation. The actuating behavior of these SCO-MEMS devices was evaluated in static as well as in dynamic modes and a considerable change of the resonance frequency was detected at the SCO. This composite was also spray-coated over a free-standing polyester film, to develop a macroscopic bilayer actuator as shown in Figure 15e-f. Six flower-petals were cut from the bilayer, in which a reversible opening and closing of the flower together with a color change was observed by thermal cycling.[63]
Figure 15. a) Scheme of spray-coating of an SCO-polymer composite on silicon microcantilevers, b) SEM image of the resulting MEMS, c) variable temperature UV absorption at 310 nm of the SCO/SU-8 composite, d) variable temperature actuation amplitude in the MEMS, e-f) macroscopic SCO/SU-8 actuator in the LS and HS states.[63]
2.2.4 In-situ polymerization
Suzuki et al. [64] studied the effect of PVA (poly(vinyl alcohol)) and SLS (sodium lauryl sulfate) as additives in the polymerization of TFEMA (trifluoroethylmethacrylate) mixed with
c)
d)
e)
18
1% wt of a Fe(II)-triazole SCO complex. Microscopy analysis showed that the nanodispersed SCO complex, displaying bistability, was incorporated into the cores of polymer particles covered with PVA shells. SCO/ppy (polypyrrole) composite films with [Fe(H-trz)2(trz)](BF4)
and [Fe(NH2trz)3](SO4)2 were reported by Koo et al. [65] The composite materials were
prepared by a two-step synthetic method. First, a chemical oxidation of the py monomer takes place in the presence of the polycrystalline powder SCO complex resulting in ppy-covered SCO particles. Then, the particles are sintered into a thick film (ca. 60 µm) with an isostatic pressure of 0.62 GPa. The magnetic properties of the complexes in the composite film were identical to those in the bulk. Remarkably, a pronounced coupling between the conductivity of the polymer matrix and the SCO properties were observed in the composite films (Figure 16), with a thermal hysteresis loop of the electrical conductivity. A dynamic widening of the hysteresis at faster scan rates, without a significant fatigue after several cycles as well as a difference between HS
and LS of about 60% (for BF4) and 50% (for SO4) were also reported. The coupling was
attributed to a piezoresistive effect due to the volume change of the SCO complex that takes place during the spin transition. Different parameters, such as the PPY/SCO ratio, the pressure applied during the sintering process, the thickness of the film etc. were shown to have an impact on the HS/LS ratio. This work is the first example for strain-coupling of SCO to electroactive
polymers (EPA) in a composite materials, with interesting perspectives for the development of sensors, actuators and energy harvesting devices.
Figure 16. Variable temperature magnetic susceptibility and electrical conductivity data for ppy/SCO composites with a-b) [Fe(H-trz)2(trz)](BF4) and c-d) [Fe(NH2trz)3](SO4)2.[65]
2.2.5 Electrochemically assisted self-assembly (EASA)
Ahoulou et al. [66] observed that if the SCO complex Fe(Htrz)3 is used during the preparation
of mesoporous silica thin films by EASA, it can act either as an additive when used at low
a)
b)
c)
19
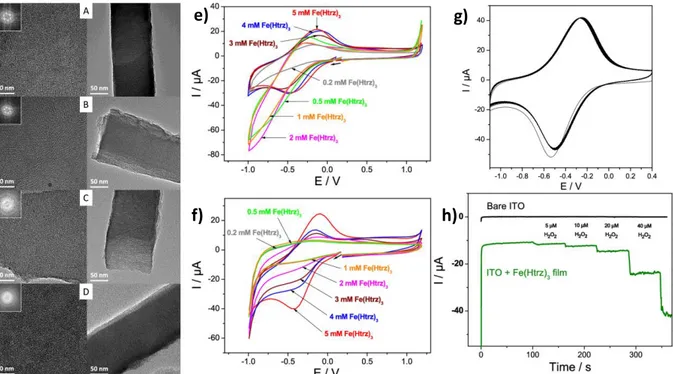
concentrations (≤3 mM) or as a template in higher concentrations (5 mM). As a result, one may obtain either a vertically aligned mesostructure where the complex is incorporated with the surfactant species (low concentration) and can be removed by solvent extraction or a worm-like mesoporous film filled with the complex immobilized in the silica matrix (high concentration). This was confirmed by the cyclic voltammograms and from the XPS spectra of the mesoporous silica films before and after treatment with HCl to remove the complex. Interestingly, the film thickness is independent of the concentration of the complex. As observed from the TEM images (Figure 17), the films consisted of well-organized mesopore channels hexagonally packed and vertically aligned onto the electrode surface. Interestingly the film obtained at 5 mM showed no change in the cyclic voltammogram even after 20 consecutive cycles. Through the integration of the peak currents, the authors estimated that there are ca 110-120 complex units per mesopore in the film. As a potential application, they use the composite for the amperometric detection of hydrogen peroxide (Figure 17h), for which a linear response was observed in the concentration range of 1-160 mM.
Figure 17. TEM images (top views on the left and cross-sectional views on the right) of the mesoporous silica films in the presence of increasing concentrations of Fe(Htrz)3 complex: 0.5
mM (A), 1 mM (B), 3 mM (C), and 5 mM (D); Cyclic voltammograms of the mesoporous silica film-modified ITO electrodes prepared from a starting sol containing increasing concentrations of Fe(Htrz)3 complex before (e) and after (f) film treatment in 0.1 M HCl in ethanol; g) the 20
consecutive cycles for the film prepared with 0.5 mM; h) Amperometric responses of increasing concentrations of H2O2 for bare ITO and the film electrode prepared from 5 mM Fe(Htrz)3.[66]
2.2.6 Matrix‐assisted pulsed laser evaporation (MAPLE)
MAPLE is a variant of pulsed laser deposition, which was developed to deposit thin films of soft and fragile materials. In the MAPLE approach, the target is a frozen solution or suspension of the desired material. Sawczak et al. [67] used the MAPLE technique to deposit nanocrystalline thin films of the SCO complex [Fe(pz)Pt(CN)4] impregnated with polyethylene
glycol (PEG). They used a cryogenically cooled suspension of nanocrystals of the SCO
e)
f)
g)
20
complex in a mixture of 1,1‐dichloroethane and the polymer. The resulting 150-200 nm thick films exhibited cooperative spin transition with hysteresis centered around 155 K evidenced by variable temperature Raman measurements. In contrast to other technics, such approach seems to preserve the crystallinity of the compound.
2.2.7 3D printing
Three-dimensional (3D) printing refers to a family of methods, which allow for making objects from a 3D computer model by joining materials, i.e. by additive manufacturing as opposed to subtractive manufacturing approaches. Recently, much attention has been focused on the possibility of 3D printing smart, actuating materials able to produce a shape change with time. The term ‘4D printing’ is often used for this process, wherein the 4th dimension refers to time.
Whereas 4D printing has been achieved mostly using shape memory polymers, elastomers and hydrogels, SCO materials represent also an appealing scope in this context. The 3D printability of SCO materials can be most conveniently attained using polymer composites. Notably, in a recent work Piedrahita-Bello et al. [68] used a stereolithographic approach to fabricate various shaped objects from the SCO complex [Fe(NH2trz)3]SO4 embedded in a commercial photoresist
with sizes up to several cm and structural details down to the 80 m scale (Figure 18). Besides monolithic objects, bimorph actuators were also 3D printed and their actuation performance evaluated. The key interest of this approach is the possibility to create arbitrary planar and three-dimensional geometries, which are otherwise not accessible using spin crossover complexes.
Figure 18. 3D printed objects of polymer composites of the SCO complex [Fe(NH2trz)3]SO4.[68]
21
Table 1. List of the most relevant SCO polymer composites and their main characteristics.
Polymer SCO Load
(% wt)
SCO measurement SCO
dissolved Preparation ref T1/2↑ (°C) T1/2↓ (°C) ΔT1/2 (°C) PMMA [Fe(hptrz)3](ClO4)2 only SCO -38 -46 8 --- --- [22] 10 -37 -51 14
yes Solution casting (drop casting) 25 -36 -45 9 [Fe(hptrz)3](BF4)2 only SCO -30 -40 10 --- --- 10 -24 -45 21
yes Solution casting (drop casting) 25 -25 -38 13 [Fe(hptrz)3](Br)2 only SCO 59 57 2 --- --- 10 57 46 11
yes Solution casting (drop casting) 25 56 52 4 [Fe(Htrz)2(trz)](BF4) only SCO 123 70 53 --- --- [65] 10-50 ca. 123 ca. 70 ca. 53 no
Solution casting (dip
coating) [51] POx [Fe(ODT)3](OTs)2 only SCO 37.6 28.2 9.4 --- --- [23] 25 22.2 16.8 5.4 [Fe(HET)3](OTs)2 only SCO 25 10.6 14.4 --- --- 25 17.9 1.6 16.3
Polypyrrole [Fe(Htrz)2(trz)](BF4) 250 123 70 53 no In situ polymerization [65] [Fe(NH2trz)3](SO4) 72 59 13
PTFEMA [Fe(Htrz)
3-3X(NH2trz)3X](BF4)2 1 ---- no In situ polymerization [64]
Chitosan [Fe(pz){(MCN)4}] (M=Ni,
Pd, Pt) NA --- --- --- yes
Multilayer sequential assembly
[43,4 4]
22
Alginate [Fe(pz){(NiCN)4}] NA --- --- ---
AN-18-10P (ion exchange polymer) Fe(formazan) NA ca. 123 --- yes Adsorption into matrix [38] Dowex 50WX4 hydrogen form (ion
exchange polymer) [Fe(Htrz)2(trz)](BF4) NA 119 69 50 yes Adsorption into matrix [42] PVP [Fe(NH2trz)3](ClO4)2
only
SCO -63 0 --- --- [69]
59 -19 -25 6 yes Solution casting (dip
casting) [24]
Polystyrene
[Fe(ODT)3](ClO4)2
only
SCO -60 -60 0 --- --- [25]
13.5 -60 -60 0 yes Electrospun from
solution [26] [Fe(NH2trz)3](NO3)2·0.5H2O only SCO 55 52 2 --- --- [57] 16-17.5 53 51 2
no Solution casting (drop
casting) [56] 70 43 27 Cellulose [Fe(hptrz)3](OTs)2 only SCO 37 38 1 --- --- [70] NA 54 51 3
no Adsorption into matrix
[58] NA 46 40 6 [Fe(Htrz)2(trz)](BF4) 25 116 85 31 [59] 50 110-115 80 30-35 [60] [Fe(NH2trz)3]Br2 only SCO 44.6 32.8 15 ---- --- [71]
30 32 25 7 no Adsorption into matrix [59] Polyester [Fe(NH2trz)3](NO3)2·H2O NA ~ 73 ~ 48 ~ 25 no Solution casting (spray
coating) [62] [Fe(NH2trz)3]Br2·3H2O NA ~ 48 ~ 9 ~ 39
Bacterial cellulose (“nata de coco”) [Fe(Htrz)2(trz)](BF4) NA 112 72 40 yes Adsorption into matrix [41] [Fe(NH2trz)3](BF4)2 NA 95 60 35
Polycarbonate [Fe(Htrz)2(trz)](BF4) 50 97 59 38 no Solution casting (drop
23 --- [Fe(NH2trz)3](BF4)2 only SCO 3.1 -3.8 6.9 --- --- [53] Nafion NA 32.4 19.8 12.6 no
Solution casting (dip coating) Polyurethane D6 NA 6.7 0.2 6.5 no PMMAtoluene 50 3.6 -5.6 9.2 no PMMATHF 50 0.7 -6.3 7.0 no PVCTHF 66 1.4 -9.3 10.6 no PSTHF 45 -0.1 -9.6 9.5 no SU-8 (3050) [Fe(Htrz)2(trz)](BF4) only SCO 105 82 23 --- --- [63] 30 113 54 59 no Solution casting (spray
coating)
P(VDF-TrFE) 70-30% mol [Fe{(Htrz)2(trz)}0.9(NH2trz)0.3 ](BF4)1.1
only
SCO 66 58 8 --- ---
[16] ≈25 67 54 13 no Solution casting (drop
casting) Diblock copolypeptide Co(MeO-terpy)2 --- 72 -13 85 yes Solution casting
(lyophilization)
[48]
Fe(ppi)2(NCS)2 --- --- [49]
Polylatic acid [Fe(Htrz)2(trz)](BF4) 5 --- no Electrospun from
solution [61] [Fe(NH2trz)3](BF4)2 ---
SiO2
[Fe(Htrz)2(trz)](BF4) 1 108 87 21 No Sol-gel [45]
[Fe(Htrz)2(trz)](BF4) NA 102 37 65 yes Adsorption into matrix [39]
Fe/NH2trz/SO4 only
SCO 49 38 11
no
suspension NP-SiO2@Fe/NH2trz/SO4
NA
57 43 14 Sol-gel [46]
Si@NP-SiO2
@Fe/NH2trz/SO4
60 43 23
Sol-gel and coating [47] PDMS@Si@ NP-SiO2
@Fe/NH2trz/SO4 55 39 16
24
3. Spin crossover organic polymers
Fundamentally, we can divide ‘organic polymer SCO complexes’ into two basic categories based on the way the polymer is attached to the SCO complex: a) when the polymer is attached by supramolecular interactions to the SCO complex, for example representing the counter-anion of the complex (polymer backbone) such as in Nafion-SCO composites and b) when the polymer is covalently attached to the SCO ligands of the SCO complex. In this section, we focus exclusively on the second approach. (The supramolecular approach was discussed in section 2.12.) The primary interests of ‘SCO organic polymers’ with respect to SCO-polymer composites is that phase separation, inherent to composites, is avoided and a better dispersion of the SCO centers can be achieved. On the other hand, the price to pay is that the SCO in these systems is usually not cooperative and therefore the spin crossover behavior is usually gradual an incomplete. A notable exception is the case of Fe(II)-triazole based SCO polymers, which can display rather abrupt spin transition owing to the multinuclear (chain-type) character of these complexes.
Maeda et al. [72] were the first to use ligands covalently attached to a polymer to obtain SCO complexes. They obtained different amorphous iron(III) complexes from copolymers of PVP (poly(4-vinylpyridine)) or poly(1-vinylimidazole) and various Schiff base ligands like H2Salten
(Figure 19d). A color change observed at 78 K allowed to visually corroborate the existence of SCO in the polymers, which was also confirmed by variable-temperature Mössbauer and EPR spectroscopies. As it can be expected in such materials with “diluted” iron centers, magnetic measurements revealed that the spin crossover is very smooth, spans over a broad range of temperatures and remains largely incomplete. Later on, Davidson et al. attached terpyridine to polyphosphazenes (PP) and synthesized the corresponding iron(II) complex, resulting either in a soluble polymer with intramolecular loops (Figure 19a) or in an insoluble pink solid with probably a high degree of cross-linked polymerization.[73] The Mössbauer spectra showed the presence of a mixture of iron species, FeII-Terpy
2 in the LS and HS states with a paramagnetic
impurity consistent with a FeIII species in the HS state. However, no spin crossover could be detected in these samples between 10 - 300 K. Wang’s group reported Fe(II) SCO complexes using PGMA (poly(glycidyl methacrylate)) [74] and MPEG-750 (methoxy polyethylene glycol) [75] based PGMA-trz and MPEG-trz ligands (Figure 19b). The variable temperature magnetic susceptibility measurements showed that, in the case of PGMA, the complex is in the HS state at room temperature and the spin crossover is gradual along a vast range of temperature (mainly 150 - 250 K), which eventually never reaches a full LS state. The most abrupt transition was obtained for the complex [FeII(MPEG-trz)1.5(NH2trz)1.5](BF4)2 in which the transition
temperatures are T1/2↓=248 K and T1/2↑=251 K denoting a small hysteresis (ΔT = 3 K) as shown
in Figure 19c. The group of Jäjkle reported two different supramolecular polymer complexes consisting of polystyrene terminated in either one or both sides of the polymer chain with tris(1-pirazolyl)borate (Tp).[76] The Fe(II) complexes obtained from these polymers (Figure 19e-f) turned out to be pink powders with an UV-Vis absorption at 530 nm, which corresponds to the
1A
1g→1T1g transition of the LS ferrous ion. However, no magnetic measurements were
performed in order to investigate if the compounds display SCO behavior. Schwarzenbacher et
al. [77] prepared the ligand 11-(4H-1,2,4-triazol-4-yl)-undecylmethacrylate to form the
complex (FeL3)(BF4)2, which was later polymerized in situ, obtaining an oligomer that contains
25
was followed by IR spectroscopy. The magnetic behavior of the polymerized SCO complex reveals a gradual, but fairly complete spin crossover, which remains similar to the starting monomeric complex. This similarity provides an additional proof for the lack of influence of the polymerization reaction on the SCO active part of the system. The authors attributed this successful synthesis to the relatively large distance between the groups involved in the polymerization reaction and the SCO.
Figure 19. a) [FeII(Terpy2-PP)](ClO4)2; b) PGMA-trz and MPEG-trz; c) T for [FeII
(MPEG-trz)1.5(NH2trz)1.5](BF4)2; d) H2salten ligand ; e) and f) polystyrene-Tp FeII complexes.[72–76]
An iron(III) Schiff base with pendant thienyl groups were prepared by Lemaire and Djukic.[78] A film of around 130 nm thickness was obtained by in situ electropolymerization of a solution of the Schiff base complex (Figure 20a) on an ITO-coated glass. The magnetic behavior of both the monomer and the polymer are similar, showing a smooth decrease of the magnetic moment when the temperature is decreased (Figure 20b). Remarkably, these systems not only display SCO, but they exhibit also a high electrical conductivity (Figure 20c). However, no clear correlation between the temperature dependence of the magnetic moment and that of the conductivity could be established. Later on, aerobic oxidation in solution allowed the formation of SCO-polymer microspheres of a similar thienyl iron (III) Schiff base,[79] which shows similar magnetic behavior to the parent monomer.
e) f) a) b) 0 50 100 150 200 250 300 350 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 Temperature/K XM T/cm 3K mo l -1 c c) d)
26
Figure 20. a) Electropolymerization of the Schiff base ligand; b) magnetic and c) conductivity properties of the SCO polymer film at variable temperature [78]
A similar approach of electropolymerization, using thiophene derivatives, was used by the same group [80] and more recently by the group of Lescouezec [81] in combination with a valence tautomeric cobalt bis(semiquinone) complex and {Fe4Co4} cages, respectively. Both types of
polymers were shown to display charge transfer-induced spin transition upon temperature variation or light irradiation. Overall, this approach of electropolymerization not only affords for processing the compound as smooth, ultrathin films, but also provides potentially an interesting means to develop synergies between the magnetic switching behavior, inherited from the metal complex, and electroactive properties arising from the polythiophene part. An original synthetic approach was developed by the group of Weber [82], leading to materials, which can be considered either as SCO-polymers or SCO-polymer composites. Indeed, the SCO complex is chemically attached to the polymer backbone, yet phase separation occurs with well-defined crystalline SCO domains within block copolymer micelles. In a first publication, the authors used a poly(4-vinylpyridine) (P4VP) matrix in which the 1D coordination polymer [FeIIL(bipy)] (L= Schiff base ligand, bipy= 4,4’-bipyridine) was systematically grown by repetitive synthetic cycles.[83] This procedure resulted in microcrystals of controlled size within the polymer matrix. By increasing the number of synthetic cycles, the magnetic behavior of the polymer approaches more and more the behavior of the bulk material: after 5 cycles, an abrupt and almost complete transition with T1/2↓=226 K and T1/2↑= 239 K (i.e. a hysteresis of
13 K) takes place.[83] By changing the initial matrix to the block copolymer polystyrene-block-poly(4-vinylpyridine) (PS-b-P4VP), Klimm et al. [84] found out that not only they can grow a 1D coordination network, but that in this case well-defined spherical nanoparticles of ca. 50 nm are formed (Figure 21). Their core size is independent of the quantity of coordination polymer used and is only determined by the size of the starting block-copolymer micelle. The observed
a)
27
magnetic behavior showed a gradual and incomplete spin transition. Controlling the reaction conditions can improve the crystallinity of the complex, in which case after 5 synthetic cycles a full HS state is attained at room temperature with T1/2↓=162 K and T1/2↑= 170 K (as shown
in Figure 21), resembling to the bulk system. A further study on the influence of the rigidity of the axial ligand in the formation of the nanoparticles and their SCO behavior was performed by alternating the bipy to bpea, bpee and bpey (see Figure 21).[85] The introduction of different axial ligands did not change the core-size, but did increase the solubility of the coordination complex resulting in the formation of microcrystals outside the micelle. This is also favored by changing to a solvent in which the complex is more soluble (toluene in this case). The general result is that the magnetic behavior of these polymers shows drastic differences with their corresponding bulk SCO complexes, due to the crystallization motif of the block copolymer. It is also interesting to note that the use of blockcopolymers allows an additional functionalization of the obtained nano-objects by variation of the polymer blocks. More recently, the same group reported the crystallization of smaller SCO nano-particles of ca. 15 nm in the micellar confinement ([FeIIL(bipy)]@PS-b-P4VP).[86] When the material is heated above the glass transition temperature of the PS shell, a significant improvement of the SCO properties is observed (decrease of the residual HS fraction at low temperature and wide hysteresis loop) with an increase of the transition temperatures.
Figure 21. a) Schematic representation for the synthesis of the SCO polymer using P4VP and PS-b-P4VP as matrix and b) magnetic measurement of [FeIIL(bipy)]@PS-b-P4VP after 5
synthetic cycles; TEM photograph of c) ([FeLeq(bpey)]n@BCP particles after 5 cycles and d)
[FeIIL(bipy)]@BCP.[82–85]
4. Related compounds
In this section, we review different classes of ‘soft’ SCO compounds, which are, strictly speaking, not polymeric and cannot be classified either as polymer composites, but are closely related to them. These include SCO dendrimers, gels, liquid crystals and Langmuir-Blodgett films. The common feature of these materials that they display properties, which are typical of ‘soft matter’, such as liquid crystal properties, viscoelasticity and large deformability. As such,
a)
28
they allow for ‘unconventional’ properties in combination with the SCO phenomenon as well as easier processing with respect to ‘conventional’ polycrystalline SCO materials. Since these ‘soft’ SCO materials have been already reviewed,[87] we restrict our discussion to the most prominent and/or most recent examples.
4.1 SCO dendrimers
Dendrimers are globular-shaped, highly branched macromolecules with controlled molecular weight, size and number of functional groups, due to their well-defined synthetic method based on iterative reactions. Their structure consists of three distinct parts: a central core, the layers of branched repeating units (interior dendritic structure) and an exterior outer layer with functional surface groups. A generation number Gn is increased when every branched unit is
multiplied, usually by 2 (1 → 2 connectivity) or 3 (1 → 3 connectivity). Their construction starts from the focal core following either a divergent or a convergent way.[88] The convergent synthesis of dendrimers is greatly used providing a stepwise structural control and synthetic versatility. On the same line, dendronized objects can benefit from enhanced solubility and processability. It is worth mentioning that dendrimers or dendronized objects are regarded as molecular micelles or reversed micelles when the core is hydrophobic and the surface end-groups hydrophilic or vice-versa, making them ideal candidates for nanomedicine, notably for drug encapsulation.[89,90] The multiple reactive sites for molecular attachment such as the periphery, core, branching points or cavities in coordination with their micellar properties make dendritic molecules very promising for various applications.[91,92] Indeed, the combination of variable size, topology, structure and conformation of dendrimers, accompanied by the resultant physical and supramolecular properties, opened the path for different research directions towards a variety of applications, such as molecular electronics, photonics, nanomedicine, sensing and catalysis.[93,94] Although a previous assessment of SCO in dendritic systems was done by Gaspar and Seredyuk in 2014,[87] we have decided to incorporate here a short, updated section of dendritic SCO complexes.
Three generations Gn (n=0-2) of poly(benzyl ether) dendrons with a triazole focal group
coordinated to Fe(II) form discotic columnar core-shell assemblies of ([Fe(Gntrz)3](MeSO3)2·2H2O) (Figure 22.a).[95] They display a change of spin state of iron
ions above room temperature, albeit this change is irreversible after the first heating process in all three dendritic Fe(II) complexes, most likely due to the irreversible loss of water molecules ‘bonded’ to the dendritic ligands (through hydrogen bonding etc.). It is noteworthy that the temperature of water loss upon heating - erroneously described by the authors as “spin-transition behavior” - depend on the generation number. Indeed, the dehydration temperature (Tc = 335 → 315 → 300 K) is inversely dependent on the increase of generation number (n = 0 → 1 → 2). On the other hand, the larger the dendron, the more restricted is the supramolecular polymerization, therefore the degree of polymerization was calculated to decrease (D.p. = 20 → 10 → 3) upon the increase of dendron generation (Gn, n = 0 → 1 → 2). Despite the claims
of the authors, this trend cannot be correlated with the cooperativity of the spin transition as none of the compounds display spin transition in the investigated temperature range (240 – 350 K). Interestingly, the self-organized (G1trz)Fe showed extraordinary large ΔH and ΔS values in
comparison with the other generations, which indicates a coupled structural transition upon the dehydration.

 2 embedded in a polystyrene film (a,b,c) and photographs showing the flexibility of the thin film.[27]](https://thumb-eu.123doks.com/thumbv2/123doknet/13638560.427183/7.893.139.761.387.554/figure-regular-stripes-complex-embedded-polystyrene-photographs-flexibility.webp)
![Figure 7. a) Schematic representation of the synthesis of the Hoffman-like clathrates [Fe(pz){M(CN) 4 }] within chitosan beads](https://thumb-eu.123doks.com/thumbv2/123doknet/13638560.427183/11.893.108.798.285.512/figure-schematic-representation-synthesis-hoffman-clathrates-chitosan-beads.webp)




 and c-d) [Fe(NH 2 trz) 3 ](SO 4 ) 2 .[65]](https://thumb-eu.123doks.com/thumbv2/123doknet/13638560.427183/19.893.183.717.586.1005/figure-variable-temperature-magnetic-susceptibility-electrical-conductivity-composites.webp)
![Figure 18. 3D printed objects of polymer composites of the SCO complex [Fe(NH 2 trz) 3 ]SO 4 .[68]](https://thumb-eu.123doks.com/thumbv2/123doknet/13638560.427183/21.893.285.611.593.942/figure-printed-objects-polymer-composites-sco-complex-fe.webp)