HAL Id: hal-03126997
https://hal.sorbonne-universite.fr/hal-03126997
Submitted on 1 Feb 2021
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Distributed under a Creative Commons Attribution| 4.0 International License
A small-molecule P2RX7 activator promotes anti-tumor
immune responses and sensitizes lung tumor to
immunotherapy
Laetitia Douguet, Serena Janho Dit Hreich, Jonathan Benzaquen, Laetitia
Seguin, Thierry Juhel, Xavier Dezitter, Christophe Duranton, Bernhard
Ryffel, Jean Kanellopoulos, Cecile Delarasse, et al.
To cite this version:
Laetitia Douguet, Serena Janho Dit Hreich, Jonathan Benzaquen, Laetitia Seguin, Thierry Juhel, et
al.. A small-molecule P2RX7 activator promotes anti-tumor immune responses and sensitizes lung
tumor to immunotherapy. Nature Communications, Nature Publishing Group, 2021, 12 (1), pp.653.
�10.1038/s41467-021-20912-2�. �hal-03126997�
A small-molecule P2RX7 activator promotes
anti-tumor immune responses and sensitizes lung
tumor to immunotherapy
Laetitia Douguet
1,15
✉
, Serena Janho dit Hreich
1,2,3,15
, Jonathan Benzaquen
1,2,3
, Laetitia Seguin
1,2
,
Thierry Juhel
1
, Xavier Dezitter
4,5
, Christophe Duranton
6
, Bernhard Ryffel
7
, Jean Kanellopoulos
8
,
Cecile Delarasse
9
, Nicolas Renault
4,5
, Christophe Furman
4,5
, Germain Homerin
4,10
, Chloé Féral
1,2
,
Julien Cher
fils-Vicini
1
, Régis Millet
4,5
, Sahil Adriouch
11
, Alina Ghinet
4,10,12
, Paul Hofman
1,2,13,14
&
Valérie Vouret-Craviari
1,2,3
✉
Only a subpopulation of non-small cell lung cancer (NSCLC) patients responds to
immu-notherapies, highlighting the urgent need to develop therapeutic strategies to improve patient
outcome. We develop a chemical positive modulator (HEI3090) of the purinergic P2RX7
receptor that potentiates
αPD-1 treatment to effectively control the growth of lung tumors in
transplantable and oncogene-induced mouse models and triggers long lasting antitumor
immune responses. Mechanistically, the molecule stimulates dendritic P2RX7-expressing
cells to generate IL-18 which leads to the production of IFN-
γ by Natural Killer and CD4
+T cells within tumors. Combined with immune checkpoint inhibitor, the molecule induces a
complete tumor regression in 80% of LLC tumor-bearing mice. Cured mice are also protected
against tumor re-challenge due to a CD8-dependent protective response. Hence,
combina-tion treatment of small-molecule P2RX7 activator followed by immune checkpoint inhibitor
represents a strategy that may be active against NSCLC.
https://doi.org/10.1038/s41467-021-20912-2
OPEN
1Université Côte d’Azur, CNRS, INSERM, IRCAN, Nice, France.2FHU OncoAge, Nice, France.3Centre Antoine Lacassagne, Nice, France.4Inserm, CHU Lille,
U1286—Infinite—Institute for Translational Research in Inflammation, University of Lille, Lille, France.5Institut de Chimie Pharmaceutique Albert Lespagnol, IFR114, Lille, France.6Université Côte d’Azur, CNRS, INSERM, LP2M, Nice, France.7INEM—UMR7355, Institute of Molecular Immunology and
Neurogenetic, University and CNRS, Orleans, France.8Institute for Integrative Biology of the Cell (I2BC), CEA, CNRS, Université Paris-Saclay, Gif-sur-Yvette Cedex, France.9INSERM, CNRS, Institut de la Vision, Sorbonne Université, Paris, France.10Hautes Etudes d’Ingénieur (HEI), JUNIA, UC Lille, Laboratoire de Chimie Durable et Santé, Lille, France.11Institute for Research and Innovation in Biomedicine, Normandie University, Rouen, France.12Faculty of Chemistry,
‘Al. I. Cuza’ University of Iasi, Iasi, Romania.13Hospital-Related Biobank (BB-0033-00025), Pasteur Hospital, Nice, France.14Laboratory of Clinical and
Experimental Pathology and Biobank, Pasteur Hospital, Nice, France.15These authors contributed equally: Laetitia Douguet, Serena Janho dit Hreich.
✉email:ldouguet@gmail.com;valerie.vouret@univ-cotedazur.fr
123456789
D
espite new biological insights and recent therapeutic
advances, many tumors remain resistant to treatments,
leading to premature death of the patient. This is
parti-cularly true for lung cancer, which is the leading cause of cancer
death for men and women worldwide. The 5-year survival rate for
patients with any type of lung cancer is around 20%, which
dramatically drops to 6% for metastatic lung cancers. Recent
advances in effective therapies such as targeted therapies and
immunotherapies have revolutionized lung cancer treatments
1.
However, it is limited to a small percentage of patients and
alternative approaches are urgently needed to improve patient
outcome.
The P2RX7 receptor (also called P2X7R) is an ATP-gated ion
channel composed of three protein subunits (encoded by the
P2RX7 gene), which is expressed predominantly in immune cells
and in some tumor cells
2. Activation of P2RX7 by high doses of
extracellular ATP (eATP) leads to Na
+and Ca
2+influx, and,
after prolonged activation, to the opening of a larger
con-ductance membrane pore. One consequence of this large pore
opening, a unique characteristic of P2RX7, is to induce cell
death in eATP rich microenvironments. Noteworthy, such high
doses of eATP are present in the inflammatory and tumor
microenvironments (TMEs)
3. P2RX7 functions are largely
described in immune cells, where it is involved in NLRP3
acti-vation to induce the maturation and secretion of 1β and
IL-18 pro-inflammatory cytokines by macrophages and dendritic
cells (DCs)
4. In line, several P2RX7 inhibitors have been
developed with the aim to treat inflammatory diseases. In
addition to its ability to
finely tune the amplitude of the
inflammatory response
5, P2RX7 has been shown to orchestrate
immunogenic cell death (ICD) and to potentiate DC activation
and ability to present tumor antigens to T cells
6. Among
immune cells, regulatory T cells (Treg) are highly sensitive to
P2RX7-induced cell death and, in the presence of eATP, P2RX7
negatively regulates their number and their suppressive
func-tion
7. Such response can participate in P2RX7-dependent
immune surveillance by unleashing the effector functions of
adaptive immune T cells
8. Therefore, P2RX7 has been proposed
to represent a positive modulator of antitumor immune
response. This is in agreement with data from our group
showing that P2RX7-deficient mice are more sensitive to
colitis-associated cancer
9. Also, in this model, we noticed that
trans-planted Lewis lung carcinoma (LLC) tumors grew faster in line
with the
findings of Adinolfi et al. using transplanted B16
melanoma and CT26 colon carcinoma tumors
10. Collectively,
these results support the notion that P2RX7 expression by host
immune cells coordinates antitumor immune response.
Capture of tumor antigens by antigen-presenting DC is a key
step in immune surveillance. Activated DCs present tumor
antigens to naïve T cells leading to their activation and
differ-entiation in effector T cells. Tumor infiltrated effector T cells and
NK cells can recognize and kill tumor cells resulting in the release
of additional tumor antigens and amplification of the immune
response. However, this response is often inhibited by
immuno-suppressive mechanisms present within the TME. Different
mechanisms sustain tumor escape as the reduced immune
recognition of tumors due to the absence of tumor antigens, or
the loss of MHC-I and related molecules, the increased resistance
of tumor cells edited by the immune responses, and the
devel-opment of a favorable TME associated with the presence of
immunosuppressive cytokines and growth factors (such as
VEGF, TGF-β) or the expression of checkpoint inhibitors such as
PD-1/PD-L1
11. Inhibitory checkpoint inhibitors (αPD-1/PD-L1
and anti-CTLA-4) are used in daily practice for the treatment
of advanced malignancies, including melanoma and
non-small cell lung cancer (NSCLC)
12. These antibodies reduce
immunosuppression and reactivate cytotoxic effector cell
func-tions to elicit robust antitumor responses
13,14. High response rate
to
αPD-1/PD-L1 therapy is often associated with immune
inflamed cancer phenotype characterized by the presence in the
TME of both CD4
+and CD8
+T cells, PD-L1 expression on
infiltrating immune and tumor cells and many pro-inflammatory
and effector cytokines, such as IFN-γ
15. Noteworthy, only few
cancer patients achieve a response with anti-immune checkpoint
administered as single-agent
16, suggesting that strategies based on
combined therapies would likely enhance antitumor efficacy and
immunity.
Despite the role of P2RX7 in stimulating antitumor immunity
and the observation that tumor development is more aggressive
in p2rx7-deficient animals
9, it is currently not known whether
P2RX7 activation can modulate tumor progression in vivo. The
purpose of this study is to investigate the effect of a positive
modulator (PM) of P2RX7 on lung tumor fate. To do so, we use
syngeneic immunocompetent tumor mice models and show that
activation of P2RX7 improves mice survival. Mechanistically,
activation of P2RX7 leads to increased production of IL-18 in a
NLRP3-dependent manner, which in turn activates NK and
CD4
+T cells to produce IFN-γ and consequently increases tumor
immunogenicity. Finally, activation of P2RX7 combined with
αPD-1 immune checkpoint inhibitor allows tumor regression,
followed by the establishment of a robust immunological memory
response.
Results
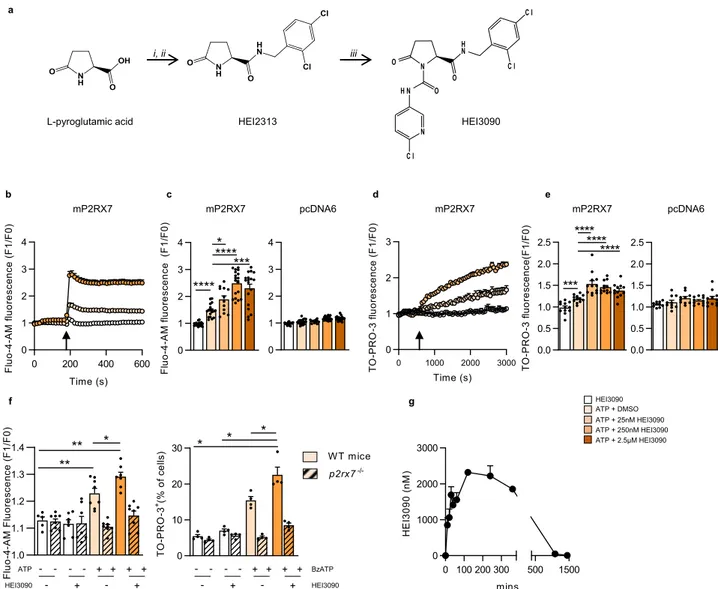
HEI3090 is a positive modulator of P2RX7. In order to identify
positive modulator of P2RX7, 120 compounds from the HEI’s
proprietary chemical library were screened for their ability to
increase P2RX7-mediated intracellular calcium concentration
during external ATP exposure. We
first produced an HEK cell
line expressing the cDNA encoding for P2RX7 from C57BL/6
origin (HEK mP2RX7) and determined the minimal dose of ATP
(333 µM) that should be used to initiate an increase in Ca
2+concentration. We tested
five promising compounds and
identi-fied HEI3090 as a hit (patent WO2019185868A1). HEI3090
corresponds to a pyrrolidin-2-one derivative decorated with a
6-chloropyridin-3-yl-amide in position 1 and with a
2,4-dichlor-obenzylamide moiety in position 5 (Fig.
1
a and Supplementary
Fig. 1). HEI3090 alone showed no toxic activity, was unable to
induce intracellular Ca
2+variation, and required the presence of
eATP to rapidly and dose dependently enhance the
P2RX7-mediated intracellular calcium concentration (Fig.
1
b, c). The
maximum effect of HEI3090 was observed at 250 nM, which is in
the range of doses identified in pharmacokinetic analysis
(Fig.
1
g). HEI3090 action required the expression of P2RX7, since
HEK cells transfected with empty plasmid (pcDNA6) showed no
increase in intracellular calcium concentration (Fig.
1
c). P2RX7
has the unique capacity to form a large pore under eATP
sti-mulation. Large pore opening of P2RX7 was assayed with the
quantification of the uptake of the fluorescent TO-PRO-3 dye. As
expected, HEI3090 alone had no effect and required eATP
sti-mulation to enhance TO-PRO-3 entry within the cells. HEI3090
increased by 2.5-fold the large pore opening (Fig.
1
d). The rapid
uptake of TO-PRO-3 was consistent with direct P2RX7 activation
rather than ATP/P2RX7-induced cell death (Fig.
1
e). We also
tested HEI3090’s effect on splenocytes expressing physiologic
levels of P2RX7 (Fig.
1
f). In these immune cells, HEI3090 alone
did not affect Fluo-4-AM nor TO-PRO-3 uptake. However, in the
presence of eATP, HEI3090 enhanced Ca
2+influx and
TO-PRO-3 uptake. We also showed that HEITO-PRO-3090 required the expression
of P2RX7, since its effect was lost in splenocytes isolated from
p2rx7
−/−mice.
Collectively these results demonstrate that HEI3090 requires
P2RX7 expression to be active and enhances eATP-induced
P2RX7 activation.
HEI3090 inhibits tumor growth and enhances antitumor
effi-cacy of
αPD-1 treatment. We previously suggested that P2RX7
expression might favor the activation of immune responses
9. We
therefore evaluated the immuno-stimulatory effect and antitumor
efficacy of HEI3090 in vivo, hypothesizing that the high level of
eATP contained within the TME
17would be sufficient to stimulate
P2RX7. To do so, we used syngeneic LLC and B16-F10 melanoma
cell lines expressing P2RX7 (Supplementary Fig. 2). Vehicle or
HEI3090 (1.5 mg/kg) was administered concomitantly to LLC
tumor cell injection and mice were treated daily for 11 days. Mice
treated with HEI3090 displayed significantly reduced tumor
growth and more than fourfold decrease in tumor weight (Fig.
2
a
and Supplementary Fig. 3). We next tested the efficacy of HEI3090
to inhibit tumor growth in a therapeutic model, in which
treat-ment started when tumor reached 10–15 mm
2in size. HEI3090
(3 mg/kg) inhibited tumor growth and increased by twofold the
median survival (Fig.
2
b). We also tested the effect of HEI3090 in
the melanoma B16-F10 tumor mouse model and observed the
same efficacy (Supplementary Fig. 4a, b).
Given the efficacy of HEI3090 to inhibit tumor growth, we next
evaluated the combination of HEI3090 and
αPD-1 antibody.
Fig. 1 HEI3090 enhances ATP-induced receptor channel activity. a Representation of HEI3090’s synthesis steps. b Modulation of ATP-induced intracellular Ca2+variation (F1/F0) in HEK293T-mP2RX7 cells (C57Bl/6 origin). After ten baseline cycles, ATP (333µm) and HEI3090 (250 nM) were injected. Error bars are mean ± SEM (n = 3 independent experiments, 6 replicates). c Average Fluo-4-AM fluorescence intensities in HEK293T mP2RX7 or control HEK pcDNA6 measured 315 s after stimulation with ATP (333µM) and HEI3090 at concentrations of 25, 250, and 2.5 µM, as indicated in the color code. Data are presented as scatter dot plots ± SEM (n = 3 independent experiments and 6 replicates, two-tailed Mann–Whitney test). d Modulation of ATP-induced TO-PRO-3 uptake in HEK293T mP2RX7 cells (F1/F0) in cells treated with ATP and HEI3090 (25 nM). Error bars are mean ± SEM (n = 3 independent experiments, 4 replicates, two-tailed Mann–Whitney test). e Average fluorescence intensities in HEK293T mP2RX7 or control HEK pcDNA6 measured 10 min after stimulation with ATP and HEI3090 at concentrations of 25, 250, and 2.5µM, as indicated in the color code. Data are presented as scatter dot plots ± SEM (n = 3 independent experiments and 4 replicates, two-tailed Mann–Whitney test). f Left: average Fluo-4-AM fluorescence intensities in WT orp2rx7−/−splenocytes stimulated with 50µM ATP measured at the plateau i.e., 10 min after stimulation. Data are presented as scatter dot plots ± SEM (n = 2 independent experiments and 4 replicates). Right: graph represents the percentage of TO-PRO-3 positive cells in splenocytes isolated from naïve WT orp2rx7−/−mice. Data are presented as scatter dot plots ± SEM (n = 3 independent experiments in duplicate, two-tailed Man–Whitney test). g Pharmacokinetic analysis of HEI3090 intraperitoneally injected in WT mice. Error bars are means ± SEM (n = 3 independent experiments in duplicate). Bars are mean ± SEM.p values: *p < 0.05, **p < 0.01 ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
After tumor inoculations, mice were treated daily with HEI3090
or vehicle and
αPD-1 was administered at days 4, 7, 10, 13, and
16. While only 1 mouse out of the 16 mice treated with the
αPD-1
alone showed a tumor regression, 13 out of the 16 mice treated
with HEI3090
+ αPD-1 were tumor-free, suggesting that this
molecule increased the efficacy of immune checkpoint inhibitor
to induce effective antitumor immune responses and tumor
regression (Fig.
2
c). Importantly, only the combo treatment
allows a long-lasting improved survival of at least 340 days
(Fig.
2
c, right panel). The combo treatment also increased the
survival of mice grafted with B16-F10 tumors (Supplementary
Fig. 4c). As illustrated in Fig.
2
d, we tested the combo treatment
on the KRAS-driven lung cancer (LSL Kras
G12D) model, which
leads to adenocarcinomas 4 months after instillation of
adenoviruses expressing the Cre recombinase
18. Whereas
αPD-1
treatment tends to reduce the number of ADC (Fig.
2
d), HEI3090
was able to enhance
αPD-1’s effects in this mouse model. Indeed,
tumor burden in mice treated with the combo treatment is
reduced by 60% compared to mice treated with
αPD-1 alone.
Accordingly, the cell number per mm
2and the number of cells
positively stained for the proliferation marker Ki67 were
decreased by 50% in lesion areas (Supplementary Fig. 4d). One
mouse out of the six treated with HEI3090 and
αPD-1 was
protected against adenocarcinoma formation.
DCs mediate the antitumor effect of HEI3090. LLC tumor cells
express an active P2RX7, since the presence of high doses of
eATP leads to an increase in intracellular Ca
2+concentration,
which is blocked by the GSK1370319A P2RX7 inhibitor
19(Sup-plementary Fig. 2a). To functionally investigate which cells are
targeted by HEI3090, we inoculated LLC in p2rx7
−/−mice and
treated them with HEI3090. Whereas HEI3090 efficiently blocked
LLC tumor growth in WT mice (Fig.
2
a), the same treatment was
inefficient in p2rx7
−/−mice, as tumor growth was
indis-tinguishable in treated or untreated groups (Fig.
3
a). This result
suggests that HEI3090 requires P2RX7 expression by mouse host
cells to inhibit tumor growth. The importance of immune cells
was further confirmed by the demonstration that the antitumor
efficacy of HEI3090 was restored after adoptive transfer of WT
splenocytes into p2rx7
−/−mice. DCs express P2RX7 and
orchestrate antitumor immunity. Purified DC from WT spleens
transferred into p2rx7
−/−mice were able to restore the antitumor
effect of HEI3090 (Fig.
3
b). This experiment was further
sup-ported by the fact that phagocytic cells (macrophages and DC)
were required for HEI3090’s antitumor effect (Supplementary
Fig. 5a) and that macrophages are less implicated in HEI3090’s
effect in vivo since HEI3090 is still able to inhibit tumor growth
in p2rx7
fl/flLysM mice (Supplementary Fig. 5b).
Flow cytometry analyses revealed that the TME of mice treated
with HEI3090 were more infiltrated by immune cells than control
mice (Fig.
3
d). An increased infiltration of CD8
+T cells was also
observed in the LSL-Kras
G12Dlung tumor mouse model (Fig.
3
e).
Furthermore, we showed that HEI3090-treated mice showed
higher levels of P2RX7 on DC (Supplementary Fig. 5c). Deeper
characterization of immune cell infiltrate in the LLC tumor model
revealed that anti-CD3 staining of tumors from HEI3090-treated
mice contained four times more CD3
+T cells than tumors from
vehicle-treated mice (Fig.
3
f). Whereas the proportion of
CD4
+FOXP3
+Treg cells was comparable between treated or
untreated mice (Fig.
3
g), we found fewer myeloid derived
suppressor cells (PMN-MDSCs) after HEI3090 therapy (Fig.
3
h)
and higher NK/PMN-MDSC and CD4/PMN-MDSC ratios
(Fig.
3
i) but the treatment failed to consistently increase the
CD8/PMN-MDSC ratio. We also showed that HEI3090 targets
immune cells in the low immunogenic B16-F10 melanoma
syngeneic mouse model, where it was able to increase antitumor
effector cells and decrease M-MDSCs infiltration (Supplementary
Fig. 6).
P2RX7 expressed by DC has been shown to link innate and
adaptive immune responses against dying tumor cells upon
chemotherapy-induced ICD and facilitate tumor antigens
pre-sentation to T cells
6. We evaluated the capacity of HEI3090
treatment to kill tumor cells and concomitant stimulation of DC
maturation. Our results showed that HEI3090 is not an ICD
inducer (Supplementary Fig. 7).
The two tumor cell lines used in this study express different
levels of P2RX7 (Supplementary Fig. 2), yet HEI3090 required
P2RX7’s expressing immune cells to inhibit tumor growth in both
tumor mouse models. These results demonstrate that HEI3090
controls tumor growth by recruiting and activating
P2RX7-expressing immune cells, especially DC, within the TME to
initiate an effective antitumor immune response.
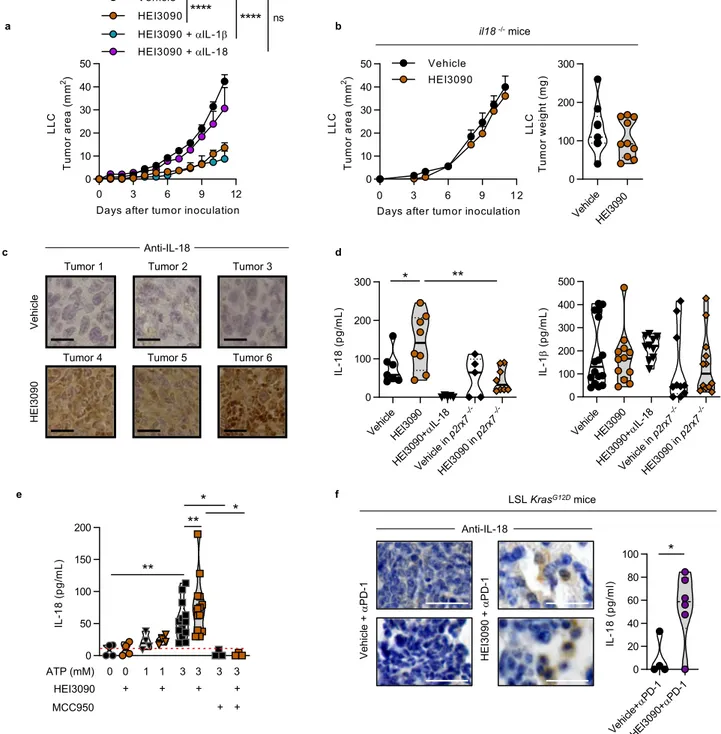
IL-18 is produced in response to HEI3090 treatment and is
required to mediate its antitumor activity. We then investigated
how the activation of P2RX7 enhanced antitumor immune
responses. In addition to increasing intracellular Ca
2+con-centration and stimulating the formation of a large membrane
pore (see Fig.
1
), P2RX7’s activation is also known to activate the
NLRP3 inflammasome that leads to the activation of caspase-1
and consequently to the maturation and release of the
pro-inflammatory cytokines IL-1β and IL-18. We showed that
HEI3090 enhanced caspase-1 cleavage (Supplementary Fig. 8).
Whereas neutralization of IL-1β did not impact HEI3090’s
anti-tumor activity, neutralization of IL-18 suppressed the antianti-tumor
effect of HEI3090 (Fig.
4
a). This result was confirmed using
il18
−/−mice in which HEI3090 had no impact on tumor growth
(Fig.
4
b). IHC staining of LLC tumors from HEI3090-treated
mice showed a significant intratumor amount of IL-18 compared
to mice treated with the vehicle (Fig.
4
c), whereas staining of
tumors from il18
−/−mice revealed no staining (Supplementary
Fig. 9a). Concordantly, serum levels of IL-18 were statistically
more abundant in mice treated with HEI3090 than in vehicle
mice (Fig.
4
d), and no IL-18 was detected in the serum of mice
that received IL-18 neutralizing antibody. In addition, HEI3090
was unable to modulate the levels of IL-18 in p2rx7
−/−mice.
Moreover, HEI3090-treated WT and p2rx7
−/−mice show indeed
a significant difference in the release of IL-18. Finally, primary
peritoneal macrophages and bone-marrow-derived dendritic cells
(BMDC) from WT mice-cultured ex vivo with ATP and HEI3090
produce more IL-18 than cells cultured with ATP and vehicle
(Fig.
4
e and Supplementary Fig. 10c). IL-18 release by HEI3090
required the NLRP3 inflammasome, since its production is
inhibited by the NLRP3 inflammasome-specific inhibitor
(MCC950) (Fig.
4
e). Moreover, we showed that HEI3090
enhanced caspase-1 cleavage (Supplementary Fig. 8) meaning
that HEI3090 was able to increase IL-18 production by enhancing
the activation of the NLRP3 inflammasome. Activation of P2RX7
by HEI3090 in macrophages from p2rx7
−/−mice failed to
increase IL-18 secretion (Supplementary Fig. 9a) and no staining
was observed in LLC tumors from HEI3090-treated p2rx7
−/−mice (Supplementary Fig. 9b). In agreement with the observation
Fig. 2 HEI3090 inhibits tumor growth and combined with immunotherapy ameliorates mice survival. a Prophylactic administration. Average tumor area and weight of LLC allograft after daily treatment with HEI3090. Curves showed mean tumor area in mm2± SEM (vehiclen = 28, HEI3090 n = 32, two-way
Anova test, left panel) and graph showed tumor weight the day of sacrifice. Data are presented by violin plots showing all points with hatched bar corresponding to median tumor weight (vehiclen = 28, HEI3090 n = 32. Two-tailed Mann–Whitney test, right panel). b Therapeutic administration. Average tumor area and survival curves of LLC allograft. HEI3090 started when tumors reached 10–15 mm2of size. Curves showed mean tumor area in
mm2± SEM (n = 12, two-way Anova test, left panel and Mantel Cox test, right panel). c Combo treatment. Average tumor area of LLC allograft after
HEI3090 andαPD-1 treatment. Spaghetti plots and survival curves of animals are shown (n = 16, Mantel Cox test). d Schematic illustration of treatment given to LSL-KrasG12Dmice. Representative images showing lung tumor burden (Bar= 2 mm upper panel and 500 µm lower panel) with tumor histopathology (n = 4). Average tumor burden of LSL-KrasG12D mice in response to treatments were studied as the number of ADC per mouse (untreated, n = 4, vehicle + αPD-1, n = 4, HEI3090 + αPD-1, n = 6; two-tailed Mann–Whitney test) and the surface of ADC lesions per lung. Each point represents one lesion, all lesions are shown to illustrate the heterogeneity (untreated and vehicle+ αPD-1, n = 4 mice, HEI3090 + αPD-1, n = 6, Two-tailed
Mann–Whitney test). p values: *p < 0.05, **p < 0.01 ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file. AD adenoma, ADC adenocarcinoma.
that HEI3090 retained its antitumor activity in mice treated with
IL-1β neutralizing antibody, HEI3090 did not modify IL-1β
protein levels in serum (Fig.
4
d) and did not modulate IL-1β
secretion in macrophages cultured ex vivo (Supplementary
Fig. 9c, d).
Increased production of IL-18 was also observed in the
LSL-Kras
G12Dlung tumor mouse model. Indeed, cells within lesions of
mice treated with HEI3090 combined with
αPD-1 expressed more
IL-18 than mice treated with
αPD-1 alone (Fig.
4
f). IL-18 protein
levels in serum of mice that received the combo treatment were
also increased by sixfold (Fig.
4
f and Supplementary Fig. 9e). As
described with the LLC tumor model, HEI3090 did not impact
the levels of IL-1β in this in situ genetic tumor mouse model
(Supplementary Fig. 9f).
Collectively, these results demonstrate that the antitumor effect
of HEI3090 is highly dependent on P2RX7 expression and on its
capacity to induce the production of mature IL-18 in the presence
of eATP.
IL-18 is required to increase antitumor functions of NK and
CD4
+T cells. To identify which immune cells were involved in
the HEI3090-induced antitumor response, we performed
antibody-specific cell depletion experiments. While NK and
CD4
+T cells depletions prevented HEI3090 treatment from
inhibiting tumor growth (Fig.
5
a, b), CD8
+T cells depletion had
no impact on HEI3090 treatment efficacy (Fig.
5
a). To further
study the effect of HEI3090 treatment on these subsets, we
assessed their cytokine production within the TME. Analyses of
tumor infiltrating immune cells first revealed that HEI3090
treatment significantly increased their capacity to produce IFN-γ
(Fig.
5
c). To precisely evaluate which cells in the TME produce
IFN-γ, we studied the TIL subpopulation and determined the
ratios of IFN-γ to IL-10 production in each subset (Fig.
5
d). NK
and CD4
+T cells were more biased to produce IFN-γ than the
IL-10 immunosuppressive cytokine. CD8
+T cells were relatively
less prone to modification in this cytokine ratio profile upon
HEI3090 treatment. In addition, twofold more NK cells from
mice treated with HEI3090 degranulate after ex vivo
restimula-tion with LLC compared to NK from control mice (Fig.
5
e),
confirming their activation state, while no effect was noticeable
on CD8
+T cells. These phenotypic and functional analyses of
intratumor immune infiltration suggested furthermore that
treatment with HEI3090 stimulates CD4
+T cells and NK cells’
activation in the TME. Importantly, IL-18 neutralization
abro-gated the increase of the IFN-γ/IL-10 ratio by CD4
+T cells and
NK cells (Fig.
5
f), suggesting that its production is a direct
con-sequence of IL-18 release and signaling. We showed that DC and
IL-18 were necessary for HEI3090’s activity (Figs.
3
b and
4
a, b).
In vitro stimulation of splenocytes treated with BzATP and
HEI3090 did not increase IFN-γ production by T cells and NK
cells indicating that its higher production in the tumor of treated
mice is rather an indirect consequence of the therapy
(Supplementary Fig. 10a). Concordantly, CD45
+cells, CD8
+T cells, and NK cells in the TME of p2rx7
−/−mice supplemented
with WT DC showed an increase in the IFN-γ/IL-10 ratio in the
HEI3090-treated mice (Supplementary Fig. 10b). This result
indicates that WT DC were able to produce IL-18 after the
adoptive transfer, since antitumor effector cells were more prone
to produce IFN-γ than the IL-10 immunosuppressive cytokine.
Finally, we uncovered that HEI3090 treatment of LLC tumor
bearing mice in vivo increased the expression of MHC-I and
PD-L1 by 2.2-fold (Fig.
5
g). However, when LLC cells were treated
in vitro with HEI3090, neither MHC-I nor PD-L1 expression
were increased. By contrast, IFN-γ induced the expression of
these two proteins (Supplementary Fig. 10d). Taken together, our
results suggest that the in vivo increase of MHC-I and PD-L1
expression is a consequence of IFN-γ upregulation driven by
IL-18. Finally, using the LSL-Kras
G12Dtumor mouse model, we
showed that tumor cells from mice that received both HEI3090
and
αPD-1 expressed more PD-L1 than tumor cells from mice
treated with
αPD-1 only (Fig.
5
h). Altogether, our results indicate
that HEI3090 increases IL-18 production allowing the
recruit-ment and activation of NK and CD4
+T cells and the production
of IFN-γ. In turn, IFN-γ stimulates expression of MHC-I and
PD-L1 on cancer cells, leading to an increased-tumor
immunogeni-city and an increased sensitivity to anti-immune checkpoint
inhibitors.
Combined with
αPD-1 antibody, HEI3090 cures mice carrying
LLC tumors and allows memory immune response. Combined
with an
αPD-1 antibody, HEI3090 cured 80% of
LLC-tumor-bearing mice (Fig.
2
d). To determine whether cured mice
devel-oped an antitumor immune memory response, they were
rechallenged with LLC tumor cells 90 days after the
first
inocu-lation and were maintained without any therapy as illustrated in
Fig.
6
a. All long-term-recovered mice were protected from LLC
rechallenge, whereas all age-matched control mice developed
tumors (Fig.
6
b). The rechallenged mice were still alive 150 days
after the initial challenge (Fig.
6
c), sustaining the hypothesis that
combo treatment effectively promoted an efficient antitumor
memory immune response. Our results suggested that CD8
+T cells are not directly involved in the primary antitumor effect of
HEI3090 (see Fig.
5
a). Nevertheless, it is well-characterized that
these cells play a pivotal role in the host’s ability to mount an
Fig. 3 Immune cells mediate the antitumor activity induced by HEI3090. a Average tumor area of LLC allograft inp2rx7-deficient mice (p2rx7−/−) after daily treatment with HEI3090 or after adoptive transfer of WT splenocytes and daily treatment with HEI3090. Curves showed mean tumor area in mm2±
SEM (vehiclen = 13, HEI3090 n = 16 mice, vehicle + WT spleno n = 11, HEI3090 + WT spleno: n = 12, two-way Anova test). b Average tumor area of LLC allograft inp2rx7-deficient mice (p2rx7−/−) after adoptive transfer of WT DCs and daily treatment with HEI3090. Curves showed mean tumor area in mm2± SEM (n = 8, two-way Anova test). c Tumor weight of animals from the study shown in a and b. Data are presented by violin plots showing all points
with hatched bar corresponding to median tumor weight (vehiclen = 8, HEI3090 n = 10 mice, vehicle + WT spleno n = 10, HEI3090 + WT spleno n = 12, vehicle+ WT DC n = 13, HEI3090 + WT DC n = 12 mice, two-tailed Mann–Whitney test). d Characterization of immune infiltrate at day 12. Percentage of CD45+analyzed byflow cytometry among living cells within TME. Data are presented by violin plots showing all points with hatched bar corresponding to median tumor weight (vehiclen = 7, HEI3090 n = 8, two-tailed Mann–Whitney test). e Representative picture of CD8+cells recruitment in LSL-KrasG12D mice over six mice studied (bar= 100 µm) and quantification. Data are presented by violin plots showing all points with hatched bar corresponding to median CD8+T cells (four tumors per mouse,n = 4 mice per group, two-tailed Mann–Whitney test). f Representative images of CD3 staining in LLC tumors over six mice studied (bar= 100-µm upper panel and 50-µm lower panel) and quantification data are presented by violin plots showing all points with hatched bar corresponding to median CD8+T cells (n = 6 per group, two-tailed Mann–Whitney test). g Percentage of regulatory T cells determined byflow cytometry as FOXP3+CD4+among CD3+within LLC tumors. Data are presented by violin plots showing all points with hatched bar corresponding to median FOXP3+cells of CD3 cells (n = 8 per group, two-tailed Mann–Whitney test). Gating strategy is presented in Supplementary Fig. 12. h Proportion of PMN-MDSC among CD45+within LLC tumors. Data are presented by violin plots showing all points with hatched bar corresponding to median PMN-MDSC cells among CD45+cells (n = 7, per group. Two-tailed Mann–Whitney test). Full gating strategy is presented in Supplementary Fig. 12. i Gating strategy (left panel) and ratio of NK, CD4+, or CD8+T cells on PMN-MDSC within LLC tumors (right panel). Data are presented by violin plots showing all points with hatched bar corresponding to median of indicated cells (n = 8 per group, Two-tailed Mann–Whitney test). p values: *p < 0.05, **p < 0.01, ****p < 0.0001. Source data are provided as a Source Data file. Spleno Splenocytes, DC dendritic cells, PMN-MDSC poly morpho nuclear-myeloid-derived suppressor cells.
Fig. 4 HEI3090-induced IL-18 production is required to inhibit tumor growth. a Average tumor area of LLC allograft in WT mice injected with IL-1β and IL-18 neutralizing antibodies and daily treatment with HEI3090. Curves showed mean tumor area in mm2± SEM (vehiclen = 28, HEI3090 n = 32,
HEI3090+ IL-1β treated n = 6, HEI3090 + IL-18 n = 8. Two-way Anova test). b Average tumor area and tumor weight of LLC allograft in il-18-deficient mice (il-18−/−) and daily treatment with HEI3090. Curves showed mean tumor area in mm2± SEM (vehiclen = 9, HEI3090 n = 10. Two-way Anova test,
left panel) and graph showed tumor weight the day of sacrifice. Data are presented by violin plots showing all points with hatched bar corresponding to median tumor weight (vehiclen = 9, HEI3090 n = 10. Two-tailed Mann–Whitney test, right panel). c Representative images of IL-18 staining in LLC tumors of six mice studied. Bar= 50 µm. d Production of IL-18 and IL-1β in serum of treated mice determined by ELISA. Data are presented by violin plots showing all points with plain bar corresponding to median cytokine concentration (IL-18 production: vehiclen = 7, HEI3090 n = 8, HEI3090 + αIL-18 n = 6, vehicle p2rx7−/−n = 5, HEI3090 p2rx7−/−n = 8, (IL-1β production: vehicle n = 13, HEI3090 n = 12, HEI3090 + αIL-18 n = 10, vehicle p2rx7−/−n = 10, HEI3090
p2rx7−/−n = 12. Two-tailed Mann–Whitney test). e Ex vivo production of IL-18 in primary peritoneal macrophages. Data are presented by violin plots
showing all points with hatched bar corresponding to median cytokine concentration (no treatmentn = 4, HEI3090 n = 4, ATP 1 mM n = 4, ATP 1 mM + HEI3090n = 4, ATP 3 mM n = 13, ATP 3 mM + HEI3090 n = 13, ATP 3 mM + MCC950 n = 3, ATP 3 mM + HEI3090 + MCC950 n = 3. Two-tailed Mann–Whitney test). f Representative images of IL-18 staining in lung tumor lesions from LSL-KrasG12Dmice over four mice studied (bar= 100 µm) and production of IL-18 in serum of LSL-KrasG12Dmice. Data are presented by violin plots showing all points with hatched bar corresponding to median IL-18 concentration (vehicle+ αPD-1, n = 4, HEI3090 + αPD-1, n = 6. Two-tailed Mann–Whitney test). p values: *p < 0.05, **p < 0.01, ****p < 0.0001. Source data are provided as a Source Datafile.
antitumoral adaptative immune response
20. To evaluate the
involvement of secondary memory CD8
+T cells response in
these mice, we sorted CD8
+cells from age-matched naïve mice or
5 months (day 150) surviving rechallenged mice (see Fig.
6
a) and
injected them to naïve mice prior to inoculation of LLC tumor
cell in a 1/1 ratio. No treatment was given to mice. In this
experimental condition, tumor growth was reduced by twofold in
mice that received CD8
+T cells isolated from cured mice
(Fig.
6
d), indicating that the combo therapy promoted a
func-tional immune memory response that partly depends on CD8
+T cells.
We next characterized the mice that were cured for a very long
period (300 days), as illustrated in Fig.
6
e. First, to discriminate
between dormancy and eradication of tumor cells, we depleted
CD8
+T cells from 300-day-old cured mice and followed mice
welfare in the absence of treatment (Fig.
6
f). In this condition, no
tumor relapse was observed during the 40 days of the experiment
and the weight of the mice remained constant, revealing that the
combo treatment efficiently eliminated tumor cells. Second, since
circulating CD8
+T cells are actively involved in the immune
memory response
20and participated in the HEI3090-induced
antitumor response (see Fig.
6
d), we investigated their
involve-ment in the long-term memory immune response. To do so,
340-day-old cured or age-matched naïve mice were inoculated with
LLC tumor cells in the absence of CD8
+T cells. Both naïve- and
cured-age-matched mice developed tumors (Figs.
6
g, h).
How-ever, the tumor growth was significantly reduced in cured mice
and three out of the six mice did not have tumors (Fig.
6
g, right
panel). Cured mice survival was also significantly increased in
comparison to naïve mice (Fig.
6
i). Collectively, these results
suggest that circulating CD8
+T cells participate in the antitumor
immune response induced by HEI3090.
P2RX7 is positively correlated with high infiltration of
anti-tumor immune cells in NSCLC patients. Using the lung
ade-nocarcinomas (LUAD) TCGA dataset, we analyzed the effect of
P2RX7 expression levels on the recruitment of cytotoxic immune
cells. We clustered tumors of 80 patients with all stage (I-IV) of
lung adenocarcinoma according to P2RX7 expression and showed
that high levels of P2RX7 expression correlated with an increased
immune response in LUAD patients, characterized by a high
mRNA expression of CD274 (PD-L1), IL1B, IL18, a signature of
primed cytotoxic T cells (defined by CD8A, CD8B, IFN-G,
GZMA, GZMB, PRF1) (Fig.
7
a). Accordingly, Gene set
enrich-ment analysis (GSEA) demonstrated a positive correlation
between high P2RX7 expression and the well-characterized
established signatures of
“adaptive immune response,”
“T-cell-mediated immunity,” “cytokine production” (Fig.
7
b).
Further-more, high P2RX7 expression is correlated with high levels of
CD274 (PD-L1), independently of the stage of the disease
(Fig.
7
c). Consistently, a significant reduced overall survival is
observed for P2RX7 hi, CD274 hi, and P2RX7 hi
+ CD274 hi
LUAD patients (Fig.
7
d), suggesting that high expression levels of
P2RX7 is sufficient to bypass immune responses in the presence
of high levels of CD274. Such a situation is considered to benefit
from anti-checkpoint blockade and/or strategies aiming to
reac-tivate immune responses, e.g., with an activator of P2RX7.
Indeed, only few cancer patients achieve a response with
anti-immune checkpoint administered as single-agent and combined
therapies to enhance antitumor immunity and bring a clinical
benefit for patients are actively tested. We showed in this study
that the combination of HEI3090 and
αPD-1 is more efficient to
inhibit lung tumor growth than
αPD-1 alone (see Fig.
2
c).
Discussion
We demonstrated in this study that activation of the purinergic
P2RX7 receptor represents a promising strategy to control tumor
growth. We developed a positive modulator of P2RX7, called
HEI3090, that stimulates antitumor immunity. HEI3090 induces
production of IL-18 by P2RX7-expressing immune cells, by
mainly targeting DC. IL-18 drives IFN-γ production to increase
tumor immunogenicity and reinforces NK and CD4
+T cells
immune responses and generates protective CD8
+T cells
responses from recidivism. Noteworthy, therapeutic association
of HEI3090 with
αPD-1 antibody synergizes to cure mice in the
LLC syngeneic model of lung cancer and elicits an antitumor
immunity. We also observed that the combo treatment is more
efficient than αPD-1 alone to inhibit tumor growth in the
LSL-KRas
G12Dlung tumor genetic mouse model. Lung tumor
regression correlates with an increased immune cell infiltration,
more secretion of IL-18 within the TME and higher expression of
PD-L1 by tumor cells. Furthermore, this mode of action was
confirmed using the B16-F10 melanoma tumor model
(Supple-mentary Fig. 6). Collectively these results demonstrate that the
antitumor activity of HEI3090 follows the same rules in all tumor
models tested and highlight the strength of HEI3090 to reactivate
antitumor immunity.
The design of P2RX7’s modulators was based on a ligand-based
approach allowing the generation of a pharmacophore model.
One hundred and twenty compounds were generated and were
tested for their ability to enhance P2RX7’s activities; five of them
were able to do so. HEI3090 was the most promising and effective
compound of the
five and was therefore chosen for our study.
Other natural or synthetic molecules have been described to
facilitate P2RX7 response to ATP
21–23. P2RX4 is another member
of the P2X family that is described to regulate P2RX7’s activities
in macrophages. Recently Kawano et al. have shown that a
positive modulator of P2RX4, the ginsenoside CK compound
24,
calibrates P2RX7-dependent cell death in macrophages
25.
Therefore, we checked whether HEI3090 modulates P2RX4’s
activities, which is not the case (Supplementary Fig. 11).
Until now, neither of these molecules has been tested in cancer
models. Moreover, attempt to facilitate P2RX7 activation in the
field of oncology has been limited by the finding that P2RX7
variants expressed by some tumor cells may sustain their
pro-liferation and metabolic activity
2. To explore this question, we
analyzed P2RX7’s functional features in ex vivo lung cancer
Fig. 5 HEI3090 triggers antitumor responses mediated by IL-18-induced NK and CD4+T cells. a Average tumor weight of LLC allograft in WT mice injected with depleting antibody and daily treated with HEI3090. Data are presented by violin plots showing all points with plain bar corresponding to median tumor weight (vehiclen = 28, HEI3090 n = 32, HEI3090 + αCD8 n = 8, HEI3090 + αCD4 n = 8, HEI3090 + αNK1.1 n = 8. Two-tailed Mann–Whitney test). b Spaghetti plots of LLC allograft in WT mice injected with depleting antibody and daily treated with HEI3090. vehicle n = 28, HEI3090n = 32, HEI3090 + αCD4 n = 8, HEI3090 + αNK1.1 n = 8. c Average of IFN-γ+cells among CD45+cells in LLC tumors. Data are presented by violin plots showing all points with plain bar corresponding to median of IFN-γ+cells among CD45+cells (vehiclen = 5, HEI3090 n = 8. Two-tailed Mann–Whitney test). d Representative dot plots of IFN-γ and IL-10 staining on TILs (left panel) and ratios of IFN-γ on IL-10 in the same positive cells of each TILs (right panel). Data are presented by violin plots showing all points with plain bar corresponding to median of the cytokine ratio vehiclen = 5, HEI3090n = 8. Two-tailed Mann–Whitney test). e Ex vivo degranulation assay of splenocytes from LLC tumor bearing mice. CD107a+cells in NK, CD4+, and CD8+T cells are shown. Data are presented by violin plots showing all points with plain bar corresponding to median % of CD107a+cells (vehiclen = 10 vehicle and HEI3090n = 8. Two-tailed Mann–Whitney test). f Ratios of IFN-γ on IL-10 in the same positive cells of each TILs of IL-18 neutralized mice. Data are presented by violin plots showing all points with plain bar corresponding to median of the cytokine ratio (vehiclen = 12, HEI3090 n = 6. Two-tailed Mann–Whitney test). g Flow cytometry analyses of MHC-I and PD-L1 expression on CD45−cells in LLC tumors. Data are presented by violin plots showing all points with plain bar corresponding to median of positive cells over CD45 cells (vehiclen = 8, HEI3090 n = 4. Two-tailed Mann–Whitney test). h Representative images of PD-L1 staining in cancer lesion of LSL-KRasG12Dmice representative of six mice studied. (Bar= 100 µm) and quantification. Data are presented by violin plots showing all points with hatched bar corresponding to median of positive cells over total cells (vehiclen = 7, HEI3090 n = 8. Two-tailed Mann–Whitney test). p values: *p < 0.05, **p < 0.01 ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
samples
26and showed that P2RX7 is functional in leukocytes
whereas it is nonfunctional in tumor cells. Considering that
P2RX7 is a pro-apoptotic receptor, it makes sense that tumor cells
express a nonfunctional receptor. Whether this nonfunctional
receptor
corresponds
to
the
non-conformational
P2RX7
(nfP2RX7), described to be expressed by tumor cells
27, remains to
be determined as well as the effect of HEI3090 on nfP2RX7.
Despite the
finding that P2RX7 expression by immune cells
restrains tumor growth
9,10, the use of specific P2RX7 antagonists
has been promoted to treat cancers on the basis that inhibition of
tumor cell proliferation would be more efficient
28,29.
Consider-able effort has been made to engineer-specific P2RX7
antago-nists
30and two of them (A74003 and AZ10606120) inhibited B16
tumor growth in immunocompetent mice
10. However, to our
knowledge, these compounds have not been tested to treat cancer
and have failed in the
first clinical trials to treat inflammatory and
pain-related diseases
30. In addition, in the preclinical mouse
model, we were unable to inhibit LLC and B16-F10 tumor growth
when we tested the GSK1370319A compound, a
well-characterized P2RX7 antagonist
19. In line with this
finding, our
present results suggest that facilitation of P2XR7 is associated
with efficient antitumor immunity in two different models of
transplantable tumor (expressing moderate or higher level of
P2RX7) as well as in the LSL-KRas
G12Dgenetic lung cancer
mouse model. These results illustrate the view that P2RX7
acti-vation, rather than inhibition, represents a promising strategy in
cancer immunotherapy to unleash the immune responses,
nota-bly in conjunction with anti-checkpoint blockade. Therapeutic
antibody represents another promising
field of investigation to
treat cancer. In particular, Gilbert et al. described an antibody
against a nonfunctional P2RX7 variant that is promising to treat
basal cell carcinoma
31. It would be interesting to combine
HEI3090 with the therapeutic P2RX7 antibody and assay the
efficacy of this combo treatment.
Antineoplastic action of eATP was previously explored using
ATP administration in cancer patients and abandoned for lack of
convincing results
32,33. Extracellular ATP is naturally degraded to
adenosine by ectoenzymes, and adenosine is an
immunosup-pressive molecule
34. To inhibit the production of adenosine,
blocking antibodies against CD39 and CD73 ectoenzymes were
produced and tested in mouse cancer models but also in ongoing
clinical trials (NCT03454451). This strategy seems to be
pro-mising, at least, in mice tumor models. In a
first study, Perrot
et al. showed that antibodies targeting human CD39 and CD73
promoted antitumor immunity by stimulating DC and
macro-phages which, in turn, restored the activation of effector T cells
35.
The authors also reported that the combination of anti-CD39
monoclonal antibody with oxaliplatin increased the survival of
tumor bearing mice, at least for 50 days. In a second study, an
independent anti-CD39 antibody was generated and tested on
different mouse tumor models. This antibody alone dampened
tumor growth and when combined with
αPD-1, it further slowed
tumor progression and 50% of the mice showed a complete
rejection
36. Mechanistically, the anti-CD39 antibody treatment
led to an increased eATP levels via the P2RX7/NLRP3/IL-18 to
stimulate myeloid cells. Next, the authors demonstrated that
anti-CD39 antibody sensitized
αPD-1 resistant tumors by increasing
CD8
+T cells infiltration. Our results confirm these findings but
also bring additional highlights. First, we showed that activation
of the eATP/P2RX7/NLRP3/IL-18 pathway by HEI3090 increased
long-lasting immune responses when combined with
αPD-1
antibody. Second, we demonstrated that the endogenous eATP
levels present in the TME were sufficient to enhance P2RX7’s
activation in the presence of HEI3090. These conditions are ideal
to allow P2RX7 activation where it is needed and avoid the
possible adverse effects associated with a systemic increase of
ATP levels, such as the one observed in response to anti-CD39
and -CD73 antibodies.
It was shown that eATP attracts DC precursors toward the
TME and promotes their activation state and their capacity to
present antigen
37,38. During this study, we showed that HEI3090
targets P2RX7-expressing immune cells, especially phagocytic
cells, such as macrophages and DCs (Supplementary Fig. 6a).
Between macrophages and DCs, DCs were the most promising
candidate; they express high levels of P2RX7, they are able to
release IL-18, and they are professional antigen-presenting cells
able to induce a potent antitumor immune response. We
there-fore tested their involvement by doing an adoptive transfer of WT
DC in p2rx7
−/−mice. Doing so, we restored responsiveness to
HEI3090 (Fig.
3
b). We also observed that cDC CD4
+from mice
treated with HEI3090 expressed higher levels of P2RX7
(Sup-plementary Fig. 4c). Collectively, these results demonstrated that
DCs mediate HEI3090’s antitumor activity, but macrophages may
have a secondary role in this effect.
Intriguingly, we did not observe an enhanced production of
mature IL-1β in mice treated with HEI3090 (Fig.
4
d). This was
unexpected as secretion of mature IL-1β depends on the ATP/
P2RX7-induced NLRP3 inflammasome activation as well
39.
However, unlike IL-1β, the inactive precursor form of IL-18 is
constitutively expressed in most human and animal cells.
Whe-ther this explanation is sufficient to account for this differential
IL-1β/IL-18 production is currently not known.
Whereas IL-1β is described to induce immune escape
40, IL-18
is involved in Th1 polarization and NK cell activation. We
showed here that IL-18 produced in response to HEI3090
treat-ment orchestrated the antitumor immune response by driving
IFN-γ production by NK and CD4
+T cells. This is in line with
the well-known IFN-γ stimulating activity of IL-18 (originally
designated as IFN-γ-inducing factor), and with its Th1 and NK
cells stimulating activity
41,42. Protective effect of IL-18, but also
the activation of NLRP3, have been previously reported in various
mouse cancer models
43,44NLRP3 activation in DCs as well as
IL-18 have been linked to better prognosis, to drive antitumor
immunity and to enhance the efficacy of immunotherapies in
different tumor models
45,46. In fact, when we combined HEI3090
with an
αPD-1 antibody, we observed that the combo therapy
efficiently controlled tumor burden in the three cancer models
studied. Notably, the combo treatment cured 80% of LLC tumor
bearing mice and very interestingly, cured mice developed an
antitumor memory response.
CD8 memory T cells, comprising the circulating memory pool
—composed of effector memory (T
EM) and central memory
(T
CM) cells—and the tissue resident (T
RM) pool, play crucial roles
in antitumor memory responses
47. We showed that circulating
CD8
+T cells participated in cancer immunosurveillance after
HEI3090 treatment (Fig.
6
d). However, this CD8
+T cells pool
cannot be responsible for the entire response, since antitumor
responses were still effective when CD8
+T cells were depleted
(Fig.
6
g). These results suggest that other immune cells participate
in local cancer surveillance. Possible candidates are the
non-recirculating CD8
+T
RMcells. The persistence of T
RMcells in
tissues has been shown to depend on signaling programs driven
by TGFβ and Notch-dependent signaling signature. Whether
HEI3090 directly stimulates those programs remains to be
Fig. 6 HEI3090 combined withαPD-1 induces antitumor memory immune response. a Schematic illustration of treatments with transfer of CD8 cells. b Average tumor area of LLC allograft in 90-day-old WT and 90-day-old cured mice in absence of treatment. Curves showed mean tumor area in mm2±
SEM (n = 7 per group, two-way Anova test). c Survival curves of animals from the study shown in b. Curves showed survival (n = 7 per group, Mantel Cox test).d Average tumor area of LLC allograft in WT mice injected with CD8+T cells isolated from rechallenged cured mice as shown ina. Curves showed mean tumor area in mm2± SEM (n = 4 per group, two-way Anova test). e Long-lasting antitumor immune response: schematic illustration of treatments.
f Mouse body weight follow up of 300-day-old cured mice injected with anti-isotype (black circle) or depletingαCD8 antibodies (blue circle). Each curve represents one mouse (n = 6 per group). g Individual survival curves of 340-day-old WT and cured animals injected with anti-CD8 antibody and rechallenge with LLC in absence of treatment. (n = 6 per group). h Average tumor area from animals shown in g. Data are presented by violin plots showing all points with hatched bar corresponding to median of tumor area (n = 6 per group. Two-tailed Mann–Whitney test). i Survival curves from animals shown ing. (n = 6 per group, Mantel Cox test). p values: *p < 0.05, **p < 0.01 ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Datafile.
determined but we observed, using HEK mP2RX7 cells, that
HEI3090 enhanced ATP-stimulated ERK pathways. Our results
are also compatible with a role for CD4
+T memory cells and the
setup of a humoral response, in which B lymphocytes produce
antibody against tumor cells.
Therapy with different
αPD-1/PD-L1 antibodies was approved
in NSCLC in the
first- and second-line settings. However, a
significant fraction of patients does not benefit from the
treat-ment (primary resistance), and some responders relapse after a
period of response (acquired resistance)
48. Expression of PD-L1
per se is not a robust biomarker with a predictive value since the
αPD-L1 response has also been observed in some patients with
PD-L1-negative tumors. Improvement of patient management for
immunotherapy undoubtedly relies on the identification of such
Fig. 7 P2RX7 expression in LUAD is associated with“hot” immunophenotype signature. a Association of P2RX7 mRNA expression with a cluster of inflammatory genes (heatmap). Expression values are represented as colors, where the range of colors (red, pink, light blue, dark blue) shows the range of expression values (high, moderate, low, lowest). Rawp values (Linear models for microarray analysis, Limma) are shown. b Gene set enrichment analysis (GSEA) plot associatingP2RX7 high mRNA levels from LUAD patients (TCGA) with three inflammatory signatures. The enrichment score is shown as a green line, and the vertical black bars below the plot indicate the positions of specific inflammatory signature-associated genes, which are mostly grouped in the fraction of upregulated genes. For each signature, normalized enriched score (NES),p values (bilateral Kolmogorov–Smirnov), and false discovery rate (FDR) are shown.c. Correlation curves ofP2RX7 and CD274 expression from LUAD patients (TCGA) of all stage (left panel), low stage (middle panel), and high stage (right panel).r, r2, andp values are shown in each panel, (Person correlation and t test). d Kaplan–Meyer plot (http://kmplot.com) showing
survival curves ofP2RX7 high vs. P2RX7 low patients (left panel), CD274 high vs. CD274 low (middle panel), and P2RX7 high or low vs. CD274 high or low (right panel). For all panels, the optimal cutoff is determined on KMplot. Thep value (log-rank, Mantel Cox test), the hazard ratio, and number of patients are indicated. Source data are provided as a Source Datafile. ADC adenocarcinoma, HR hazard ratio.
predictive markers. Using TCGA dataset, we uncovered that
P2RX7 expression is correlated to CD274 (PD-L1) expression and
“hot” immunophenotype signatures in NSCLC patients. In
addition, patients with high P2RX7 and low CD274 or high
CD274 and low P2RX7 have a better overall survival than patients
with high CD274 and high P2RX7. This result suggests that
immunotherapies may be efficient in double positive patients and
questions the ability of P2RX7 to represent a valuable biomarker
for
αPD-1/PD-L1 therapies. In this context, we showed in another
study
26that the expression of P2RX7B splice variant in tumor
immune cells is associated with less infiltrated tumors in lung
adenocarcinoma. Mechanistically, we observed that the
differ-ential expression of the P2RX7B splice variant in immune cells
within tumor area correlates with the expression of a less
func-tional P2RX7 and lower leukocytes recruitment into LUAD.
We demonstrated that a small-molecule activator of P2RX7
boosts immune surveillance by unleashing the effector functions
of adaptive immune T cells and improving the efficacy of αPD-1
treatment. This therapeutic strategy holds new hopes for cancer
patients; by increasing tumor immunogenicity, it could
first
increase the number of patients eligible to immunotherapies and
second, it could also be used as a neoadjuvant or adjuvant
therapies of locally advanced lung tumors.
Methods
Mice. Mice were housed under standardized light–dark cycles in a temperature-controlled air-conditioned environment under specific pathogen-free conditions at IRCAN, Nice, France, with free access to food and water. All mouse studies were approved by the committee for Research and Ethics of the local authorities (CIEPAL #28, protocol numbers MESRI 23707, 13656) and followed the European directive 2010/63/UE, in agreement with the ARRIVE guidelines. Experiments were performed in accord with animal protection representative at IRCAN. P2rx7−/−(B6.129P2-P2rx7tm1Gab/J) and il18−/−mice were from the Jackson Laboratory. LSL-KRasG12Dare from the Jackson Laboratories (ref 008179). P2rx7-flox mice were engineered as follow: ES clones (C57/BL/6) containing a construct for the conditional elimination or re-expression of P2RX7 (purchased from The European Conditional Mouse Mutagenesis Program) were injected into blastocytes of C57Bl6/N, chimeric mice were selected and crossed with deleter mice that are transgenic for the Flip-recombinase under the control of the ubiquitous Actin promoter to produce p2rx7loxP/loxPmice. Our p2rx7loxP/loxPmice, with loxP sequencefloxing the second exon of p2rx7, were crossed with (C57BL/6NTacGt (ROSA)26Sor<tm1(ACTB-Cre,-EGFP)) transgenic mice which express the Cre recombinase under the control of the b-actin promoter to produce p2rx7exon2−/− mice or with LysM-Cre mice (B6.129P2-Lys2tm1(cre)lfo from the Jackson Laboratories (obtained from Dr B. Chazaud, France) to generate myeloid cell conditional p2rx7 knockout and WT control (p2rx7loxp/loxp) mice. Control C57BL/ 6J OlaHsD female (WT mouse) was supplied from Envigo (Gannat, France).
In vivo treatments. Five 105tumor cells were injected s.c. into the leftflank of WT mice. Pharmacokinetic analysis (Fig.1g), to characterize the clearance of HEI3090 showed that after a period of 18-h HEI3090 concentration is <10 nM. Therefore, we have decided to inject HEI3090 daily. Mice were treated i.p. with vehicle (PBS, 10% DMSO) or with HEI3090 (1.5 mg/kg in PBS, 10% DMSO), which corresponds to the highest soluble dose. For therapeutic settings, treatment started at day 3, when tumor reached ~10–15 mm2, for a maximum of 20 days and mice received vehicle or HEI3090 (3 mg/kg in PBS, 10% DMSO) daily. Depleting and neutralizing antibodies from BioXCell were given i.p. in the rightflank at days −1, 3, 7, and 10. αPD-1 antibody was given i.p. at days 4, 7, 10, and 13 (or as stated in the legend of thefigure) post-tumor cell inoculation. Antibodies are listed in Supplementary Table 1.αPD-1 and HEI3090 were injected separately, with at least 30 min delay between the two injections. Two hundred microliters liposome clo-dronate (Liposoma) were injected i.p. 3 days before LLC tumor cell inoculation in WT mice and then every 3 days, at least 1 h before HEI3090 treatment after the treatment started. CD8+T cells were sorted from peripheral lymph nodes of cured or naïve WT mice with Dynabeads®Untouched™ Mouse CD8 Cells (Invitrogen) according to the supplier’s instructions. 5.105CD8+T cells were adoptively transferred into 8-week-old naïve WT mice (i.v.) 1 day before tumor inoculation. 5.105LLC cells were injected s.c. into the leftflank of these mice and given no further treatment.
Intratracheal delivery of adenoCre induces oncogenic KRAS in lung airway cells, leading to multifocal adenocarcinomas and a median survival of about 6 months49. Starting with tumors established for 3 months in adult LSL-KrasG12D mice of either gender, treatment with vehicle or 1.5 mg/kg HEI3090 daily by i.p. injection was performed for 21 additional days.
Adoptive transfer inp2rx7-deficient mice. Spleens from WT C57BL/6J female mice were collected and digested in RPMI 1640 medium containing 5% FCS, 1-mg/ ml collagenase IV (Sigma-Aldrich), and 50 U/ml DNase I (Roche) for 7 min at 37 °C. Single-cell suspensions of spleens were prepared by passage through 100 µm cell strainers (BD Biosciences) and counted. For WT DCs isolation, spleens were digested with the spleen dissociation kit (Miltenyi Biotech) and isolated with the CD11c Microbeads UltraPure (Miltenyi biotech) according to the supplier’s instructions. 5.106splenocytes or 1.2.106DCs were injected i.v. in p2rx7−/−mice 1 day before subcutaneous injection of 5.105LLC cells into the leftflank. Mice were treated i.p. every day for 12 days with vehicle (PBS, 10% DMSO) or with HEI3090 (1.5 mg/kg in PBS, 10% DMSO). At day 12, tumors were collected, weighted, and digested, whenflow cytometry analyses were done.
Flow cytometry and antibodies. Tumors were mechanically dissociated and digested with 1-mg mL−1collagenase A and 0.1-mg mL−1DNase I for 20 min at 37 °C. Then single-cell suspensions of tumors were prepared by passage through 100 µM cell strainers (BD Biosciences). Surface staining was performed by incu-bating cells on ice, for 20 min, with saturating concentrations of labeled Abs in PBS, 5% FCS, and 0.5% EDTA. After blocking Fc receptors using CD16/32 anti-bodies, cells were stained with the appropriate combination of antibodies (see Supplementary Table 1). The transcription factor staining Buffer Set (eBioscience) was used for the FoxP3 staining. For intracellular cytokines, staining was per-formed after stimulation of single-cell suspensions with Phorbol 12-myristate 13-acetate (PMA at 50 ng mL−1, Sigma), ionomycin (0.5μg mL−1, Sigma) and 1μL mL−1Golgi Plug™ (BD Biosciences) for 4 h at 37 °C 5% CO2. Cells were incubated
with Live/Dead Near-IR stain (Invitrogen), according to the manufacturer’s pro-tocol prior to Ab surface staining. Then, intracellular staining was performed using Cytofix/Cytoperm™ kit (BD biosciences) following the manufacturer’s instructions. The production of IFN-γ and IL-10 was simultaneously analyzed in CD45+, NK, CD4+T, or CD8+T cells. Datafiles were acquired and analyzed on Aria III using Diva software (BD Biosciences) or on the CytoFlex LX (Beckman Coulter) and analyzed using FlowJo software (LLC). Gating strategies are shown in Supple-mentary Fig. 12.
Immunohistological analysis of tumors. Collected tumors or lungs werefixed in 3% formamide for 16 h prior inclusion in paraffin. We used the following anti-bodies: anti-CD3, anti-CD8, anti-IL-18,αPD-L1, and anti-Ki67 (see Supplementary Table 1). After staining, slides were captured and analyzed using NDP view2-software. For the analyses,five zones per tumor were randomly selected and cells were counted using ImageJ software. Results are expressed as number of positive cells per total cell number.
Characterization of lung lesions in the LSL-KRasG12Dmouse model. At the end of the treatment mice were sacrificed, exsanguinated and lungs processed for histologic and immunological analyses. After deparaffinization, HE stains were performed and slides were captured and analyzed using NDP view2 software. Tumor burden was calculated by determining the mean of total tumor area per lung using the NDP view2 software. To count the cells and determine the per-centage of Ki67-positive cells within lesions, ten lesions per lung, from grade 2 to 5 according to the Sutherland scoring50, were randomly selected, their perimeter was
determined, and positive and negative nuclei were counted using ImageJ software. Results are expressed as number of cells per mm2and the percentage of Ki67-positive cells.
Ex vivo macrophages and BMDC stimulation. Peritoneal lavage was done with RPMI 1640 medium on WT or p2rx7−/−mice. 4.105macrophages were seeded in a 96-well plate overnight in RPMI 1640 containing 10% FBS, 2% sodium pyruvate, 1% penicillin/streptomycin, and 50-µMβ-mercaptoethanol. After two washes with the complete medium, cells were primed for 4 h with 100 ng/ml LPS (Sigma-Aldrich) at 37 °C and then stimulated for 30 min at 37 °C with ATP (Sigma-Aldrich) with or without 50 µM of HEI3090 or with 10 µM nigericin. When indicated, NLRP3 inflammasome was inhibited with 1 µM of MCC950 (Invivogen) for 1 h at 37 °C before cell stimulation. To prepare BMDC, leg bones were removed from C57Bl/6 mice, cut with scissors, andflushed with sterile PBS pH 7.4 via syringe. Bone-marrow-derived dendritic cells (BMDCs) were obtained from bone-marrow cells seeded in Petri dishes and cultured in RPMI medium containing 10% fetal calf serum, 2 mM L-Glutamine, 50-U/mL Penicillin, 50 µg/mL Streptomycin and 20% conditioned medium from GM-CSF-producing J558L cells. Medium was refreshed every 3 days. On the 7th day of the differentiation protocol, semi-adherent BMDCs were collected using PBS containing 10 mM EDTA and re-plated in new Petri dishes with fresh medium. Mature and semi-adherent BMDCs were used for experiments on the 14th day of culture. Supernatants were collected and stored at−80 °C before cytokine detection by ELISA using mouse IL-1 beta/IL-1F2 (R&D) and IL-18 (MBL) according to the supplier’s instructions.
Cells were lysed with Laemmli buffer (10% glycerol, 3% SDS, 10 mM Na2HPO4)
with protease inhibitor cocktail (Roche). Proteins were separated on a 12% SDS-PAGE gel and electro transferred onto PVDF membranes, which were blocked for 30 min at RT with 3% bovine serum albumin. Membranes were incubated with primary antibodies diluted 1/1000 at 4 °C overnight. The following antibodies were