Diabetologia (1997) 40:126%1277
Diabetologia
9 Springer-Verlag 1997Chronic central neuropeptide Y infusion in normal rats:
status of the hypothalamo-pituitary-adrenal axis, and vagal
mediation of hyperinsulinaemia
A . Sainsbury 1 , E R o h n e r - J e a n r e n a u d I , I. Cusin 1 , K. E. Z a k r z e w s k a 1, P. A . H a l b a n 2, R. C. Gaillard 3, B. J e a n r e n a u d 1 1 Laboratoires de Recherches M6taboliques, Faculty of Medicine, University of Geneva, Geneva, Switzerland
2 Laboratoires de Recherche Louis Jeantet, University of Geneva Medical Centre, Geneva, Switzerland
3 Division d'Endocrinologie et du M6tabolisme, Department of Internal Medicine, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland
Summary
Neuropeptide Y in the hypothalamus is a potent physiological stimulator of feeding, and may contribute to the characteristic metabolic defects of obesity when hypothalamic levels remain chronically elevated. Since corticosterone and insulin are impor- tantJJregulators of fuel metabolism, the longitudinal effects of chronic (6 days) intracerebroventricular in- fusion of neuropeptide Y in normal rats on the hypo- thalamo-pituitary-adrenal axis and on insulin secre- tion were studied. Neuropeptide Y-infused rats were either allowed to eat ad libitum, o r were pair-fed with normophagic control rats. Neuropeptide Y in- creased t h e basal plasma concentrations of adreno- corticotropic hormone and corticosterone during the first 2 days of its intracerebroventricular infusion and increased cold stress-induced plasma adrenocor- ticotropic hormone concentrations. After 4-6 days of central neuropeptide Y infusion, however, basal plas- ma adrenocorticotropic hormone and corticosterone concentrations were no different from control values (except in ad libitum-fed rats in which corticosteron- aemia remained elevated), they were unaffected by the stress of cold exposure, and the hypothalamiccontent of corticotropin-releasing factor immuno- reactivity was significantly decreased. A state of hy- perinsulinaemia was present throughout the 6 days of intracerebroventricular neuropeptide Y infusion, being more marked in the ad libitum-fed than in the pair-fed group. The proportions of insulin, proinsulin, and conversion intermediates in plasma and pancreas were unchanged. Hyperinsulinaemia of the pair-fed neuropeptide Y-infused rats was accompanied by muscle insulin resistance and white adipose tissue in- sulin hyperresponsiveness, as assessed by the in vivo uptake of 2-deoxyglucose. Finally, bilateral subdia- phragmatic vagotomy prevented both the basal and the marked glucose-induced hyperinsulinaemia of animals chronically infused with neuropeptide Y, de- monstrating that central neuropeptide Y-induced hy- perinsulinaemia is mediated by the parasympathetic nervous system. [Diabetologia (1997) 40: 1269-1277]
Keywords
Intracerebroventricular, neuropeptide Y, hypothalamo-pituitary adrenal axis, insulin, proinsu- lin, vagus nerve, glucose utilisation.Received: 7 May 1997 and in revised form: 24 July 1997 Corresponding author: Dr. A.Sainsbury, Diabetes Research Group, Garvan Institute of Medical Research, 384 Victoria Street, Darlinghurst, Sydney NSW 2010, Australia
Abbreviations: NPY, Neuropeptide Y; i. c. v., intracerebroven- tricular; HPA, hypothalamo-pituitary-adrenal; ACTH, adre- nocorticotropic hormone; CRE corticotropin-releasing factor; HPLC, high performance liquid chromatography; TFA, tri- fluoroacetic acid; BSA, bovine serum albumin; PVN, paraven- tricular nucleus.
Neuropeptide Y (NPY) is a 36 amino acid peptide of the peripheral and central nervous system [1]. Cen- trally it is found in particularly high concentrations in the hypothalamus [1], where it is involved in the regulation of many neuroendocrine and autonomic functions [2, 3]. NPY is particularly well known for its role as a potent, physiological stimulator of feed- ing acting within discrete hypothalamic sites [4].
Defects in the activity of NPY-ergic neurones in the hypothalamus may contribute to the develop- ment of obesity, at least in rodents [5]. In support of this hypothesis, elevated levels of NPY and/or its
1270 A. Sainsbury et al.: Hormono-metabolic effects of central neuropeptide Y t r a n s c r i p t h a v e b e e n o b s e r v e d in t h e h y p o t h a l a m i o f
g e n e t i c a l l y o b e s e r o d e n t s such as fa/fa a n d cp/cp rats [6, 7] as well as o b / o b a n d d b / d b m i c e [8]. This in- c r e a s e has b e e n d e t e c t e d e a r l y a f t e r w e a n i n g in faJfa rats, c o r r e s p o n d i n g with the t i m e w h e n t h e i r o b e s i t y s y n d r o m e first b e c o m e s a p p a r e n t [6]. F u r t h e r m o r e , c h r o n i c a d m i n i s t r a t i o n o f e x o g e n o u s N P Y to specific h y p o t h a l a m i c nuclei or into t h e c e r e b r a l v e n t r i c l e s o f n o r m a l rats results in m a n y o f t h e d e f e c t s o f obesity, including a m a r k e d i n c r e a s e in f o o d i n t a k e , acceler- a t e d b o d y w e i g h t gain, h y p e r c o r t i c o s t e r o n a e m i a a n d h y p e r i n s u l i n a e m i a , l e a d i n g to m u s c l e insulin resis- t a n c e with r e s p e c t to glucose u p t a k e , a n d i n c r e a s e d t r i g l y c e r i d e s t o r a g e d u e to i n c r e a s e d insulin r e s p o n - siveness in w h i t e a d i p o s e tissue [9-16]. It is o f n o t e t h a t h y p e r c o r t i c o s t e r o n a e m i a a n d hy- p e r i n s u l i n a e m i a are c o m m o n f e a t u r e s o f m a n y states of o b e s i t y a s s o c i a t e d with insulin r e s i s t a n c e in ro- d e n t s a n d in m a n [17-20], a n d t h e s e h o r m o n a l p e r t u r - bations are o f k e y i m p o r t a n c e in t h e N P Y - i n d u c e d o b e s i t y s y n d r o m e . A l t h o u g h p r e v i o u s studies h a v e in- v e s t i g a t e d t h e e f f e c t s o f N P Y o n t h e h y p o t h a l a m o - p i - t u i t a r y - a d r e n a l ( H P A ) axis a n d o n insulin s e c r e t i o n a f t e r a c u t e i n j e c t i o n [3, 21], little is k n o w n a b o u t t h e h o r m o n a l e f f e c t s o f c h r o n i c c e n t r a l N P Y administra- tion. Such k n o w l e d g e w o u l d c o n t r i b u t e to the u n d e r - s t a n d i n g o f t h e a e t i o l o g y o f obesity, c o n s i d e r i n g t h e c h r o n i c a l l y e l e v a t e d h y p o t h a l a m i c N P Y levels associ- a t e d with this p a t h o l o g y [6-8]. This w o r k t h e r e f o r e a i m e d to c h a r a c t e r i s e the t i m e c o u r s e o f c h a n g e s in s e c r e t i o n o f c o r t i c o s t e r o n e a n d insulin d u r i n g 6 days o f i n t r a c e r e b r o v e n t r i c u l a r (i. c.v.) N P Y infusion in n o r m a l rats, as well as t h e r o u t e s b y w h i c h t h e s e two h o r m o n e s are a f f e c t e d b y c e n t r a l NPY.
GmbH, Wehrheim, Germany). An i. m. injection of amoxicillin (50 mg/kg; Smith Kline Beecham, Th6rishaus, Switzerland) was given, and animals were left for 7-10 days to recover from surgery, during which time they were handled daily and habituated to the blood-sampling procedure by thrice weekly rinsing of the jugular catheter with isotonic saline containing 25 IU/ml heparin and 20 000 IU/ml penicillin.
I. c. v. infusion of neuropeptide Y (NPY). Porcine NPY (15 ~g/ day; Bachem, Bubendorf, Switzerland) or its vehicle for con- trol rats (0.04 mol/1 phosphate-buffered saline, pH 7.4, with 0.2 % bovine serum albumin (BSA) and 0.01% ascorbic acid) was i. c. v. infused for 6 days via a subcutaneously placed osmo- tic minipump (model 2001; Alza Corporation, Palo Alto, Ca- lif., USA) as detailed elsewhere [22]. One group of NPY-in- fused rats and their controls were allowed to eat ad libitum. A second group of NPY-infused animals was pair-fed to the amount of food consumed by the vehicle-infused control rats in order to prevent NPY-induced hyperphagia. In this group, pellets were distributed at 07.45, 12.00, 17.30 and 23.30 hours (6, 19, 37.5 and 37.5 % of the daily food allowance of 22.0 g, re- spectively). During i. c. v. infusion, food intake and body weight were measured daily, and a blood sample was collected from the jugular catheter into EDTA-coated tubes between 09.00 and 10.00 hours each day, 1-2 h after removal of food from cages. Food was replaced after blood sampling (ad libitum-fed rats), or at 12.00 hours (pair-fed rats). Plasma was stored at - 20~ until measurement of basal hormone and metabolite concentrations.
Adrenocorticotropic hormone (A CTH) and corticosterone se- cretion induced by cold stress. An additional group of ad libi- turn-fed NPY-infused rats and their controls were exposed for 2 h to a temperature of 5 ~ between 10.30 and 12.30 hours on the second day after the start of i. c.v. infusion, as well as on the fourth day. A blood sample was collected prior to cold ex- posure as described above, and an additional sample was col- lected after the 2-h cold exposure for measurement of cold-in- duced plasma ACTH and corticosterone concentrations. Food was unavailable for 1-2 h before as well as during this experi- ment.
Materials and methods
Animals. Lean heterozygous (Fa/fa) female rats (9-12 weeks old) of the Zucker strain, bred in our laboratories, were housed individually under conditions of controlled temperature (23 ~ and illumination (07.00-19.00 hours) and were provid- ed with standard laboratory chow (Provimi Lacta, Cossonay, Switzerland) and tap water. All experiments were approved by the ethical committee for animal experimentation of the Geneva University Faculty of Medicine, as well as by the Swiss Federal and Geneva Cantonal Veterinarian Offices.
Placement of chronic jugular and intracerebroventricular (i. c.v.) cannulae. Under sodium pentobarbital anaesthesia (55 mg/kg i. p.; Siegfried, Zofingen, Switzerland), a catheter fil- led with 1.5:1 weight/volume polyvinylpyrrolidon (Merck, Darmstadt, Germany) in isotonic saline containing 250 IU/ml heparin (Liquemin; Roche Pharma, Reinach, Switzerland) and 10 000 IU/ml penicillin (Hoechst-Pharma, Zurich, Switzer- land) was placed in the right atrium via the jugular vein. A can- nula was placed in the right lateral cerebral ventricle as pre- viously described [22]. The jugular catheter was subcutaneous- ly tunnelled to be externalised and fixed to the skull next to the i.c.v, cannula using dental cement (Paladur; Heraeus Kulzer
Insulin secretion induced by i. v. glucose injection. On day 4 or 5 after minipump implantation, an i. v. bolus of glucose (300 mg/ kg) was injected via the jugular catheter between 10.00- 11.30 hours. The catheter was rinsed with 0.5 ml of isotonic sa- line, and a 2 ml blood sample was collected between 1 and 3rain post-injection into tubes containing 40~1 EDTA (24 mg/ml in isotonic saline, pH 7.4). Rats were immediately recompensed with 2 ml of donor blood. This large volume of blood was necessary for subsequent high performance liquid chromatography (HPLC) of plasma for the assay of insulin, proinsulin and conversion intermediates.
Measurement of in vivo glucose utilisation during euglycaemic- hyperinsulinaemic clamps. After 6 days of i. c. v. infusion, pair- fed NPY-infused rats and a vehicle-infused control group were fasted for 6-8 h and prepared for euglycaemic-hyperinsu- linaemic clamps (using maximally effective plasma insulin con- centrations) associated with the labelled 2-deoxyglucose tech- nique to measure in vivo insulin-stimulated glucose utilisation index in individual tissues (diaphragm, quadriceps and gas- trocnemius, separated into red and white components, soleus, tibialis, extensor digitorum longus, inguinal white adipose tis- sue) [14]. Uptake and phosphorylation of 2-deoxyglucose by various tissues during the clamp was estimated as described [14] and expressed as a glucose utilisation index (ng/min x mg
A. Sainsbury et al.: Hormono-metabolic effects of central neuropeptide Y 1271 tissue). Since the inguinal white adipose tissue weight of pair-
fed NPY-infused rats was significantly greater than that of con- trol rats, the glucose utilisation index of this tissue was expres- sed in ng/min x ~tg D N A (total D N A content of fat pad was un- altered by NPY infusion).
Measurement of hypothalamic corticotropin-releasing factor
(CRF)-immunoreactivity. After 6 days of i.c.v, infusion, ani-
mals were killed by decapitation between 11.00 and 15.00 hours (3-7 h after removal of food from cages), the brain was removed, frozen on a cold table, and the hypothalamus was dissected and stored at -80 ~ until measurement of total CRF immunoreactivity and protein content as published else- where [23, 24]. Pancreases were also removed, snap frozen in liquid nitrogen, and stored at -80 ~ for later H P L C analysis of the different molecular forms of insulin.
Reverse-phase HPLC of plasma and pancreatic extracts. In the
ad libitum-fed group of NPY-infused rats and their controls, plasma (collected after i.v. glucose injection) and pancreases were analysed by H P L C to determine the relative proportions of insulin, proinsulin, and their conversion intermediates. Pan- creases were processed for H P L C as described by Schnetzler and colleagues [25]. Pancreatic extract (700 ~tl) or 1-5 ml of plasma (for controls, plasma from 6 rats was pooled due to low insulin concentrations) were purified on C18 Sep-Pak col- umns (Waters Associates, Milford, Mass., USA) as described [25]. We loaded 40-250 ~tl of sample (containing 360 ng immu- noreactive insulin for pancreatic extracts and 6-12 ng for plas- ma J~amples) onto a Lichrosphere 100-RP-18 column, 4 • 250 mm (Merck, Darmstadt, Germany) connected to a Sys- tem Gold pump module 126 and detector module 166 (Beck- man Instruments, Fullerton, Calif., USA). Elution buffers were: A) 50 mmol/1 phosphoric acid, 20 mmol/1 triethylamine and 50 mmol/1 sodium perchlorate, pH 3.0) and B) 9 : 1 aceto- nitrile/water volume/volume. Insulin-like immunoreactive ma- terial was eluted at 1 ml/min and 38.5 % buffer B from 0- 25 min, followed by a linear increase in the proportion of buf- fer B to 42.5 % between 25 and 95 min. We collected 1 ml frac- tions into glass tubes containing 100 ~tl 0.5 mol/1 borate/1% BSA, pH 9.2. Organic solvents in fractions were evaporated under vacuum, and lyophilised fractions were reconstituted in 1 ml of 0.05 mol/1 phosphate buffer, pH 7.4, containing 1% BSA, and stored at - 2 0 ~ until radioimmunoassay by the method of Herbert [11]. Peaks corresponding to mature insu- lin, proinsulin, and the conversion intermediates des 31,32- and des 64,65-split proinsulin were identified by comparison with previously characterised standards [26].
anaesthetised with sodium pentobarbital (55 mg/kg, i.p.), and the carotid artery was cannulated. After 30 min a bolus of glu- cose (300 mg/kg) was injected via the carotid catheter, which was rinsed with 0.2 ml of isotonic saline. At 0, 5, 10, 20, 30, 40, 60 and 90 min after glucose injection 150 ixl blood samples were collected into heparinised tubes for subsequent determi- nation of plasma insulin and glucose concentrations. Integrat- ed areas under the resultant insulin response curves were cal- culated (after subtracting baseline values) between t = 0 and t = 60 min after glucose injection, when insulinaemia had re- turned to baseline values in all groups of rats. This integral is referred to as glucose-stimulated insulin output.
Plasma hormone measurements. Plasma corticosterone con-
centrations were measured by radioimmunoassay [11], and a kit from CIS Bio International (Gif-Sur-Yvette, France) was used for measurement of plasma ACTH. Insulinaemia was measured by radioimmunoassay [11] using guinea pig anti-rat insulin serum (Linco Research Inc., St. Louis, Mo. USA). Plas- ma glucose concentrations were determined using a kit from Boehringer (Mannheim, Germany).
Statistical analysis. For the daily measurements of food intake,
body weight, change in body weight, basal plasma ACTH, cor- ticosterone, insulin and glucose concentrations, differences in normal and vagotomised rats were assessed by one-way analy- sis of variance (ANOVA) with repeated measures followed by post-hoc Duncan's range tests. When two groups were found to be significantly different from each other, multiple Bonfer- roni comparisons were made to locate differences at each time point. Plasma A C T H and corticosterone concentrations before and after cold exposure (in ad libitum-fed NPY-infused and control rats) were compared by the Kruskal-Wallis non- parametric A N O V A test followed by Dunn's multiple com- parisons tests in order to detect differences betffeen the two groups of rats under basal or stimulated conditions, as well as differences within groups of rats induced by cold stress. Hypo- thalamic levels of CRF immunoreactivity in the three groups of rats were compared by one-way A N O V A followed by a Stu- dent-Newman-Keuls multiple comparisons test. Glucose-sti- mulated insulin output was compared between sham-vagoto- mised and vagotomised rats (i.c.v. infused with vehicle or NPY) by one-way A N O V A and Student-Newman Keuls mul- tiple comparisons. Differences between control and NPY-in- fused rats with respect to glucose utilisation index of various tissues and inguinal white adipose tissue weight were made by one-tailed Student's t-tests. For all analyses, values of p < 0.05 were accepted as being statistically significant.
Experiments involving vagotomiscd rats. Female Sprague-
Dawley rats, bilaterally vagotomised (subdiaphragmatic) or sham-vagotomised at 8 weeks of age, were purchased from IFFA C R E D O (L'Arbresle, France) and equipped with chron- ic jugular and i.c.v, cannulae as described above, using i.m. ketamin/xylazine anaesthesia (45 and 9 mg/kg, respectively, Parke-Davis and Bayer AG, Leverkusen, Switzerland). At 12 weeks of age, rats were designated to one of four experimental groups: 1) sham-vagotomised + i. c.v. vehicle; 2) sham-vagoto- mised + i. c.v. NPY; 3) vagotomised + i. c. v. vehicle; 4) vagoto- mised + i. c.v. NPY. NPY or vehicle were i. c.v. infused as de- scribed above, and the food intake of all four groups was re- stricted to 22.0 g per day, the average amount consumed by sham-vagotomised and vagotomised rats prior to NPY infu- sion. Pellets were distributed as described for the pair-feeding protocol. Body weight, basal insulinaemia and glycaemia were measured daily as described for the other groups of rats. After 6 days of i.c.v, infusion, animals were fasted for 3-4 h,
Results
Effect o f 6-day i. c.v. N P Y infusion on feeding and body weight gain. I . c . v . N P Y i n f u s i o n c a u s e d a m a r k e d i n c r e a s e in a d l i b i t u m f o o d i n t a k e w h i c h w a s s u s t a i n e d f o r t h e 6 d a y s o f its i n f u s i o n . F o o d i n t a k e o n d a y 6 w a s 52 _+ 7 g in N P Y - i n f u s e d r a t s c o m p a r e d t o 19.1 g f o r c o n t r o l s (n = 6 - 1 5 , p < 0.001). T h e f o o d i n t a k e o f p a i r - f e d N P Y i n f u s e d r a t s w a s r e s t r i c t e d t o 22.0 g p e r d a y as d e s c r i b e d u n d e r M a t e r i a l s a n d M e t h o d s . T h e b o d y w e i g h t g a i n o f a d l i b i t u m - f e d N P Y - i n f u s e d r a t s w a s s i g n i f i c a n t l y g r e a t e r t h a n t h a t o f c o n t r o l r a t s (27.4 + 4.3 g/6 d a y s vs 7.9 + 2.0 g/6 days, n = 6 - 1 5 , p < 0.001) w h e r e a s t h e b o d y w e i g h t
1272 A. Sainsbury et al.: Hormono-metabolic effects of central neuropeptide Y
==
v I 0 ,< 5 0 0 . 4 0 0 - 3 0 0 - 2 0 0 - 100 - 0 0 A 6 0 0 ~" 500 =~ 400 o 8 300, u o ~ 2 0 0 , m 1 0 0 ' AAA i w1".
o i :~ ~ ,~ ~ Time (days)Fig.1. Basal plasma ACTH (upper panel) and corticosterone (lower panel) concentrations during i.c.v, infusion of NPY (15 btg/day) in normal rats allowed to eat ad libitum, or pair- fed with vehicle-infused controls ( @, NPY ad libitum-fed; A, NPY pair-fed; O, vehicle-infused controls). Plotted values are means+SEM of 6-15 rats per group. 4,p<0.01 and *** p < 0.001 vs corresponding value of vehicle-infused control rats. Ap < 0.05; AAp < 0.01 and AAAp < 0.001 vs correspond- ing value of pair-fed NPY-infused rats
gain of pair-fed NPY-infused rats was no different from that of the control group.
Effect of 6-day
L c. v.NPYjnfusion on the hypothala-
mo-pituitary adrenal (HPA) axis.
In animals allowed to eat ad libitum, i. c. v. NPY infusion caused signifi- cant elevations in plasma A C T H levels compared to control values during the first 3 days of infusion (Fig. 1 upper panel). However, this effect of NPY was not sustained with continued infusion of the peptide; within 4--6 days of NPY infusion in ad libitum-fed rats, plasma A C T H levels were not significantly differ- ent from those of control rats. In the same manner, plasma A C T H levels of pair-fed NPY-infused rats ten- ded to be higher than those of control rats during the first day of N P Y infusion, but were similar to control values during the remaining 5 days of peptide infusion. C o m p a r e d to control values, plasma corticoster- one levels were significantly increased by i. c.v. NPY infusion in b o t h ad libitum- and pair-fed rats (Fig. 1, lower panel). However, whereas Figure 1 shows that the hypercorticosteronaemia of ad libitum-fed NPY- infused rats remained present during the entire 6- day infusion period, that of pair-fed NPY-infusedE
Q . v I F- 0 E 13. r v rs
0 0 0 0 E h--8001
.5
600
400200'
0 J600 ]
400200
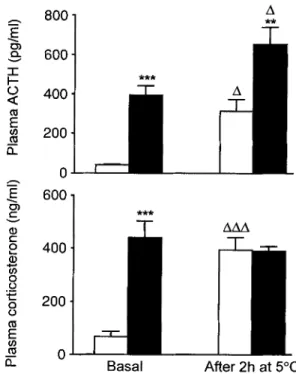
"l- AAA 0 Basal After 2h at 5~Fig.2. Plasma ACTH (upper panel) and corticosterone (lower panel) concentrations under basal conditions and after 2 h at 5 ~ measured after 2 days of i.c.v, infusion of NPY ( II, 15 ~tg/day) or vehicle for control rats ( [] ). All animals were allowed to efftad libitum, but food was unavailable for 1-2 h prior to basal blood sampling and during cold exposure. Plot- ted values are means + SEM of 8-9 rats per group. ** p < 0.01 and ***p < 0.001 vs corresponding value of vehicle-infused control rats. A p < 0.05 and AAA p < 0.001 vs basal value of the corresponding group
rats was normalised from the third day of N P Y infu- sion onwards.
Figure 2 further shows that after 2 days of i.c.v. N P Y infusion with ad libitum feeding, both basal plasma A C T H and corticosterone concentrations were considerably greater than the corresponding va- lues of control rats. Plasma A C T H concentrations were further increased, in both control and NPY-in- fused rats, after 2 h of cold exposure (5 ~ relative
to their respective basal (23~ values. The cold
stress-induced plasma concentrations of A C T H were m u c h greater in NPY-infused rats than in control rats. The cold exposure also caused a m a r k e d increase in plasma corticosterone concentrations of control rats. In NPY-infused rats, the high plasma corticoster- one concentrations measured under basal conditions were not further increased by cold exposure, presum- ably because they had already reached m a x i m u m pla- teau values under basal conditions.
On day 4, the same cold exposure in control rats still caused increases in plasma concentrations of A C T H and corticosterone, while this failed to occur in ad libitum-fed NPY-infused rats (data not shown).
A f t e r 6 days of i. c. v. N P Y infusion, the quantity of corticotropin-releasing factor (CRF)-immunoreac- tivity in the hypothalamus was significantly reduced
A. Sainsbury et al.: Hormono-metabolic effects of central neuropeptide Y 1273
20'
E
o {3. r E l ) t - v ii n~0
5"
T
15.
I
*
10-
0 Fig. 3. H y p o t h a l a m i c c o n t e n t of C R F i m m u n o r e a c t i v i t y after 6 days of i. c.v. infusion of N P Y (15 ~tg/day) in n o r m a l rats al- lowed to eat ad libitum, or pair-fed with vehicle-infused con- trols (11, N P Y ad libitum-fed; [ ] , N P Y pair-fed; rq, vehicle-in- fused controls). Plotted values are m e a n s + S E M of 6-10 rats p e r group. * p < 0.05 a n d ** p < 0.01 vs control rats4" ~ 0 " ~ 3 e - v e - .~ 2 .E E t . . . . -t . . . . ? o c-~ ~ ~._..~...-.o---____~ 0
o
i
2
+
4
5
T i m e ( d a y s )Fig.& Basal plasma insulin concentrations during i.c.v, infu- sion of NPY (15 ~tg/day) in normal rats allowed to eat ad libi- tum, or pair-fed with vehicle-infused controls ( 9 , NPY ad libi- tum-fed; A, NPY pair-fed; O, vehicle-infused controls). Plot- ted values are means + SEM of 6-15 rats per group. * p < 0.05; 9 * p < 0.01 and ***p < 0.001 vs corresponding value of vehi- cle-infused control rats
relative to control values (Fig. 3). This decrease in hy- pothalamic CRF content was observed in both pair- fed and in ad libitum-fed NPY-infused rats, and the difference between the two groups of NPY-infused rats was not significant. There was no difference be- tween the three groups of rats shown in Figure 3 with respect to total protein content per hypothala- mus (data not shown).
Effect o f 6-day i. c. v. N P Y infusion on insulin output.
Chronic i.c.v. N P Y infusion resulted in significant increases in basal insulinaemia in both ad libitum-fed
rats and in rats that were pair-fed with vehicle-infused controls (Fig. 4). This NPY-induced hyperinsulinae- mia was present after 1 day of i.c.v. NPY infusion and was sustained during the entire 6 days of N P Y in- fusion.
In addition to basal hyperinsulinaemia, insulin se- cretion in response to i.v. glucose injection (300 rag/ kg) was also increased in rats infused with N P Y (ad libitum- or pair-fed) compared with vehicle-infused control rats (data not shown). This is despite the fact that plasma glucose concentrations were increased to identical values in all groups of rats by the i.v. glu- cose bolus.
The above-mentioned levels of basal and stimulat- ed insulinaemia represent measures of total insulin- like immunoreactivity in plasma, which includes not only m a t u r e insulin but also proinsulin, and the con- version intermediates des 64,65- and des 31,32-split proinsulin [27]. These intermediates are f o r m e d by endoproteolytic cleavage at the A-chain/C-peptide and the B-chain/C-peptide junctions of proinsulin, re- spectively, followed by enzymatic trimming of resi- dual C-terminal basic amino acids [27]. To determine w h e t h e r the composition of total insulin-like immu- noreactivity in plasma after i.v. glucose stimulation was altered by i. c. v. NPY infusion, a plasma sample collected 1-3 min after a bolus glucose injection was fractionated by H P L C prior to radioimmunoassay. In ad libitum-fed NPY-infused rats as in control rats, mature insulin contributed to over 90 % ef the total plasma insulin-like immunoreactivity, the remaining being m a d e up of proinsulin and conversion inter- mediates (data not shown). Six days of i. c.v. N P Y in- fusion in ad libitum-fed rats had no effect on either the wet weight of the pancreas, or on the total quanti- ty of insulin-like immunoreactivity in the pancreas (Table 1). The composition of total pancreatic insu- lin-like immunoreactivity was no different in NPY- treated rats c o m p a r e d to control rats, with over 94 % of the pancreatic insulin-like immunoreactivity being due to m a t u r e insulin in both cases, intact proinsulin, des 64,65- and des 31,32-split proinsulin making up the remaining 6 % (Table 1).
Metabolic consequences o f 6-day i. c. v. N P Y infusion with pair-feeding regime. As shown in Figure 5, the in-
sulin-stimulated glucose utilisation index of muscles m e a s u r e d during euglycaemic-hyperinsulinaemic clamps was decreased after 6 days of i. c.v. N P Y infu- sion with pair-feeding to control rats, indicating mus- cle insulin resistance. N P Y infusion t e n d e d to also cause a decrease in insulin-stimulated glucose utilisa- tion index in other muscles studied and not shown in the figure, although the intergroup difference was not statistically significant. In contrast, the glucose utilisation index of inguinal white adipose tissue was significantly increased after 6 days of central N P Y administration with pair-feeding (Fig.5). Inguinal
1274 A. Sainsbury et al.: Hormono-metabolic effects of central neuropeptide Y Table 1. Effect of 6-day i.c.v. NPY infusion (15 ~tg/day) on
pancreas weight, total insulin content, and relative proportions of insulin biosynthetic products in the pancreas
Control i.c.zNPY
0.68• 0.74•
Wet weight of pancreas (g) Total insulin-like immunoreactivity per pancreas (~tg)
% Mature insulin
% des 31,32-split proinsulin % des 64,65-split proinsulin % Proinsulin 24.7• 32.1• 95.2• 94.1• 2.5• 3.1• 0.7• 0.8• 1.6• 2.0•
All animals were allowed to eat ad libitum during the i. c. v. in- fusion. Values are means _+ SEM of 3-7 rats per group. There was no significant difference between control and NPY-infused rats
w h i t e a d i p o s e tissue w e i g h t was significantly g r e a t e r in p a i r - f e d N P Y - i n f u s e d rats t h a n in c o n t r o l rats (1.4 + 0.2 g vs 1.0 + 0.1 g, n = 5-6, p < 0.05). W h e n ad l i b i t u m - f e d d u r i n g 6-day i. c. v. N P Y infusion, this in- c r e a s e in inguinal w h i t e a d i p o s e tissue weight was m o r e p r o n o u n c e d (2.4 + 0.2 g vs 1.1 + 0.2 g in vehi- cle-infused controls, n = 5-7, p < 0.005).
Fig.5. Glucose utilisation index of various muscles (Diaph, diaphragm; RQ, red quadriceps; RG, red gastrocnemius; Sol, soleus; EDL, extensor digitorum longus) and of inguinal white adipose tissue (WATi), measured during euglycaemic-hyperin- sulinaemic clamps after 6 days of i. c. v. infusion of NPY (15 ~tg/ day) in normal rats that were pair-fed with vehicle-infused con- trols ([], NPY pair-fed; I-1, vehicle-infused controls). Plotted values are means _+ SEM of 5-6 rats per group. * p < 0.05 vs corresponding value of control rats
Impact o f vagotomy on i.c.v. NPY-induced insulin
output.
F o r t h e s e e x p e r i m e n t s , t h e f o o d i n t a k e o f all f o u r g r o u p s o f rats ( s h a m - v a g o t o m i s e d a n d v a g o t o - mised, i.c.v, i n f u s e d with vehicle o r N P Y ) was re- stricted to t h a t o f v e h i c l e - i n f u s e d rats (22.0 g p e r day). Such p a i r f e e d i n g was i m p o r t a n t b e c a u s e sub- strate- a n d v a g u s n e r v e - m e d i a t e d insulin o u t p u t are i n f l u e n c e d b y s u b s t r a t e availability [28]. T h e r e was n o significant d i f f e r e n c e b e t w e e n t h e f o u r g r o u p s o f rats with r e s p e c t to a b s o l u t e b o d y weight, b o d y weight gain, o r basal g l y c a e m i a d u r i n g t h e 6 - d a y infu- sion p e r i o d .T h e m e a n basal i n s u l i n a e m i a m e a s u r e d d u r i n g 6 days o f i. c. v. infusion o f vehicle o r N P Y in sham-va- g o t o m i s e d rats o r rats with b i l a t e r a l s u b d i a p h r a g m a t - ic v a g o t o m y a r e s h o w n in F i g u r e 6. I. c.v. N P Y infu- sion p r o d u c e d a significant i n c r e a s e in t h e basal insu- l i n a e m i a o f s h a m - v a g o t o m i s e d rats, b u t h a d n o e f f e c t o n the basal i n s u l i n a e m i a o f v a g o t o m i s e d rats. A s fur- t h e r s h o w n b y F i g u r e 6, i. v. g l u c o s e - s t i m u l a t e d insulin o u t p u t was c o n s i d e r a b l y i n c r e a s e d b y i. c. v. N P Y infu- sion in s h a m - v a g o t o m i s e d rats, b u t was u n a f f e c t e d by i. c. v. N P Y in v a g o t o m i s e d animals. N o t e t h a t the gly- c a e m i c r e s p o n s e in the 90 m i n a f t e r i. v. glucose injec- tion was i d e n t i c a l in t h e f o u r g r o u p s o f rats s h o w n in F i g u r e 6.
Fig.6. Basal plasma insulin concentrations (upper panel) and insulin output after an i.v. bolus injection of 300 mg/kg glucose (lower panel) during i.c.v, infusion of NPY (15 vg/day) in sham-vagotomised or vagotomised rats compared to vehicle- infused sham or vagotomised rats (I3, vehicle-infused sham va- gotomised; [ ] NPY sham-vagotomised; vehicle-infused va- gotomised; NPY vagotomised). Food intake of all animals was restricted to 22.0 g/day (pair-feeding). Plotted values are means + SEM of 4-6 rats per group. * p < 0.05 vs vehicle-in- fused sham-vagotomised rats
Discussion
T h e aim o f this s t u d y was to c h a r a c t e r i s e the longitu- dinal c h a n g e s in activity o f t h e h y p o t h a l a m o - p i t u i - t a r y - a d r e n a l ( H P A ) axis a n d in insulin s e c r e t i o n
d u r i n g 6 days o f i n t r a c e r e b r o v e n t r i c u l a r (i. c.v.) infu- sion o f n e u r o p e p t i d e Y ( N P Y ) in n o r m a l rats, a n d to d e t e r m i n e the i m p a c t o f s u b d i a p h r a g m a t i c v a g o t o m y o n N P Y - i n d u c e d insulin s e c r e t i o n .
A. Sainsbury et al.: Hormono-metabolic effects of central neuropeptide Y 1275 Past research has shown that acute injection of
NPY into the cerebral ventricles or into the hypotha- lamic paraventricular nucleus (PVN) stimulates the H P A axis of several species [3]. Such NPY-induced changes include increases in corticotropin-releasing factor (CRF) m R N A levels and immunoreactivity in the PVN [29, 30], and increases in plasma adrenocor- ticotropic hormone (ACTH) and corticosterone con- centrations [3]. In vitro, NPY stimulates C R F secre- tion from hypothalamic fragments [3], and it may also increase the sensitivity of cultured pituitary cells to CRF-induced secretion of A C T H [3], although this has not been a unanimous finding [31, 32]. There is some evidence that endogenous NPY may influ- ence the activity of the HPA axis in vivo, since NPY- ergic nerve terminals have been shown to make con- tact with CRF-synthesising perikaryon of the PVN [3], and since i. c. v. infusion of anti-NPY serum elimi- nated the stress-induced increase in plasma A C T H concentrations in dogs [3].
In the present study it was shown that NPY stimu- lated the basal and stress-induced activity of the H P A axis during the first 2 days of its i. c.v. infusion. Although feeding is known to increase plasma A C T H and corticosterone concentrations [33], this effeet of i. c.v. NPY infusion was not simply due to the associated increase in food intake (hyperphagia), since it was observed, albeit to a lesser extent, even when NPY-induced hyperphagia was prevented by a pair-feeding regime. However, after 4 to 6 days of continuous i.c.v. NPY administration, basal plasma concentrations of A C T H and corticosterone were normalised or tending towards normal control values, there was no change in their plasma concentrations in response to stress, and the hypothalamic content of CRF-immunoreactivity was reduced relative to con- trol values. These findings suggest that chronic cen- tral NPY administration ultimately inhibits the activ- ity of the HPA axis. One possible explanation for this is that the NPY Y1 receptor, which is thought to be responsible for the effects of N P u on the HPA axis [34], may be down-regulated in the face of 6-day exposure to its agonist, while such down- regulation would not occur for the NPY Y5 receptor, responsible for the effect of NPY on food intake [35]. A n addi- tional possibility is that the elevated plasma concen- trations of corticosterone during the first few days of i. c.v. NPY infusion could inhibit the activity of the HPA axis by feedback inhibition of CRF and A C T H secretion [36, 37]. The latter scenario is presently fa- voured since it would explain not only the normalisa- tion of basal plasma A C T H and corticosterone levels observed after several days of central NPY adminis- tration, but also the total lack of stress-induced secre- tion of these hormones at this time, as well as the de- crease in hypothalamic CRF-immunoreactivity [37]. These data suggest that the hypercorticism of geneti- cally obese rodents [19] may not be caused by the
associated increase in hypothalamic NPY-ergic activ- ity, and other routes, such as increases in arginine va- sopressin regulation of the HPA axis [38], need to be investigated.
Although the effects of acute central NPY injec- tion on insulin secretion are small or absent, as stu- died and reviewed [21], a sustained increase in basal insulinaemia was apparent during chronic (6 days) central NPY infusion in normal rats. This effect of NPY, which is in agreement with reports of others [10, 11], was manifest after just 1 day of its i. c. v. infu- sion. It was present both in animals that were ad libi- turn-fed during central NPY infusion, as well as in those that were pair-fed along with vehicle-infused control rats, demonstrating that this hyperinsulinae- mia was not simply a consequence of NPY-induced hyperphagia. The basal hyperinsulinaemia of rats in- fused with NPY was accompanied by a hypersecre- tion of insulin in response to stimuli such as i.v. injec- tion of glucose as presently shown, or ingestion of a meal [12].
In patients with non-insulin-dependent diabetes mellitus (NIDDM), increases in the absolute or rela- tive amounts of proinsulin in the plasma (compared to plasma concentrations of mature insulin) are en- countered [39-41]. It is hypothesised that the chronic stimulation of insulin secretion in N I D D M patients leads to an increased output of immature insulin se- cretory granules in which the ratio of proinsulin and conversion intermediates to insulin is higher than that of mature granules [27, 40, 41]. It was therefore of interest to study proinsulin secretion in an animal model presenting sustained hyperstimulation of insu- lin secretion. In the present study, rats i. c.v. infused with NPY for 6 days, although hyperinsulinaemic, show no evidence of perturbations in the molecular forms of secreted insulin and related peptides, or in the relative proportions of these molecules in the pancreas. It must therefore be concluded that in- creased insulin turnover in the beta cell per se, as in the present model, does not necessarily lead to any perturbation in proinsulin conversion.
This work shows for the first time, using pair-feed- ing conditions to completely abolish N P Y - i n d u c e d hyperphagia, that 6 days of central NPY infusion re- sulted in a state of insulin resistance in muscles (as in- dicated by reduced 2-deoxyglucose uptake and phos- phorylation relative to control rats) and a hyperre- sponsiveness of such insulin-induced glucose uptake and phosphorylation in white adipose tissue. Al- though such changes in peripheral glucose metabo- lism have been observed in freely feeding, hyperpha- gic i. c.v. NPY-infused rats [12-14], the present data show that even in the absence of an increase in sub- strate supply, central NPY infusion placed the ani- mals in a "thrifty" phenotype channelling glucose car- bon away from utilisation and into lipid accretion pathways. This is reflected by the increases in inguinal
1276 A. Sainsbury et al.: Hormono-metabolic effects of central neuropeptide Y white adipose tissue weight of b o t h pair-fed a n d ad li-
b i t u m - f e d NPY-infused rats c o m p a r e d to controls. These m e t a b o l i c effects of chronic i. c.v. N P Y infu- sion m a y be a c o n s e q u e n c e of the t r a n s i e n t hypercor- t i c o s t e r o n a e m i a [15] a n d especially t h e sustained hy- p e r i n s u l i n a e m i a [16] presently r e p o r t e d u n d e r b o t h ad libitum- a n d pair-feeding conditions.
Finally, the p r e s e n t investigation d e m o n s t r a t e s that i. c.v. N P Y infusion increases basal a n d stimulat- ed insulinaemia via activation o f t h e vagus nerve, since bilateral s u b d i a p h r a g m a t i c v a g o t o m y preven- ted such N P Y - i n d u c e d increases in insulinaemia. Si- milarly, o t h e r effects of central N P Y injection on the gastrointestinal tract (such as increases i n gastric acid, bile a n d pepsin secretion) are m e d i a t e d by vagal cholinergic p a t h w a y s [42]. R e c e n t w o r k f r o m this la- b o r a t o r y [14] has s h o w n t h a t a d r e n a l e c t o m y is able to p r e v e n t h y p e r i n s u l i n a e m i a during chronic central N P Y infusion in rats. O t h e r investigators h a v e shown t h a t the insulin h y p e r s e c r e t i o n of genetically obese
fa/fa
rats, which p r e s e n t s p o n t a n e o u s l y e l e v a t e d hy- p o t h a l a m i c N P Y expression [6], can be abolished by adrenalectomy, r e s t o r e d by i. c.v. or p e r i p h e r a l gluco- corticoid r e p l a c e m e n t , a n d s u b s e q u e n t l y b l o c k e d by the cholinergic a n t a g o n i s t atropine [43, 44]. It was c o n c l u d e d t h a t glucocorticoids play a permissive role in the p a r a s y m p a t h e t i c a l l y m e d i a t e d hyperinsulinae- mia offa/fa
rats. These d a t a collectively suggest t h a t high N P Y levels in the h y p o t h a l a m i of genetically obese r o d e n t s stimulate insulin secretion by activat- ing p a r a s y m p a t h e t i c efferents to t h e pancreas, the preganglionic n e u r o n e s of which are m a i n l y located in the dorsal m o t o r nucleus of t h e vagus [45], a n d t h a t this effect o f N P Y is d e p e n d e n t o n circulating glucocorticoids, p r o b a b l y acting within the central nervous system.In summary, chronic central N P Y infusion resulted in transient a c t i v a t i o n of the H P A axis f o l l o w e d by an e v e n t u a l inhibition of this axis, a n d a sustained, vagal- ly m e d i a t e d increase in basal a n d s u b s t r a t e - i n d u c e d insulinaemia. C o n s i d e r i n g the k e y role o f insulin in m e t a b o l i c h o m e o s t a s i s [16], f u r t h e r w o r k a i m e d at u n d e r s t a n d i n g h o w N P Y activates vagal efferents to the pancreas, a n d the role of glucocorticoids in this process, could c o n t r i b u t e to o u r k n o w l e d g e of the me- tabolic defects associated with obesity s y n d r o m e s in which N P Y expression in the h y p o t h a l a m u s is chroni- cally elevated.
Acknowledgements.
This study was supported by grants 32- 40806.94 (B.J.), 31-40839.94 (P.A.H.), and 31-039749.93/1 (R. C.G.) of the Swiss National Science Foundation (Bern, Switzerland) and a grant-in-aid of E.Lilly and Company (B.J., Indianapolis, Indiana, USA). We wish to thank Ms. P.Arboit and Ms. I.Antoni for expert technical assistance, and Ms. E Touabi for secretarial help. Mr. P. Germann, Mr. A.Volery, and Mr. D. Chgttelain are also acknowledged for their technical expertise necessary for the realisation of this work.References
1. Hendry SHC (1993) Organization of neuropeptide Y neu- rons in the mammalian central nervous system. In: Colmers WF, Wahlestedt C (eds) The biology of neuropeptide Y and related peptides. Humana Press Inc, New Jersey, pp 65-156 2. Ergene E, Dunbar JC, Barraco RA (1993) Visceroendo-
crine responses elicited by neuropeptide Y in the nucleus tractus solitarius. Brain Res Bull 32:461-465
3. McDonald JK, Koenig JI (1993) Neuropeptide Y actions on reproductive and endocrine functions. In: Colmers WF, Wahlestadt C (eds) The biology of neuropeptide Y and related peptides. Humana Press Inc, New Jersey, pp 419- 456
4. Stanley BG (1993) Neuropeptide Y in multiple hypothala- mic sites controls eating behavior, endocrine, and auto- nomic systems for body energy balance. In: Colmers WF, Wahlestedt C (eds) The biology of neuropeptide Y and re- lated peptides. Humana Press Inc, New Jersey, pp 457-509 5. Dryden S, Frankish H, Wang Q, Williams G (1994) Neuro-
peptide Y and energy balance: one way ahead for the treat- ment of obesity? Eur J Clin Invest 24:293-308
6. Bchini-Hooft van Huijsduijnen O, Rohner-Jeanrenaud F, Jeanrenaud B (1993) Hypothalamic neuropeptide Y mes- senger ribonucleic acid levels in pre-obese and genetically obese (fa/fa) rats; potential regulation thereof by cortico- tropin-releasing factor. J Neuroendocrinol 5:381-386 7. Williams (3, Shellard L, Lewis DE et al. (1992) Hypothala-
mic neuropeptide Y disturbances in the obese (cp/cp) JCR : LA corpulent rat. Peptides 13:537-540
8. Stephens TW, Basinski M, Bristow PK et al. (1995) The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377:530-532
9. Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF (1986) Neuropeptide Y chronically injected into the hypothala- mus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides 7:1189-1192
10. Billington CJ, Briggs JE, Harker S, Grace M, Levine AS (1994) Neuropeptide Y in hypothalamic paraventricular nucleus: a center coordinating energy metabolism. Am J Physiol 266:R1765-R1770
11. Zarj evski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, Jean- renaud B (1993) Chronic intracerebroventricular neuro- peptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology 133: 1753-1758
12. Zarjevski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, Jean- renaud B (1994) Intracerebroventricular administration of neuropeptide Y to normal rats has divergent effects on glu- cose utilization by adipose tissue and skeletal muscle. Dia- betes 43:764-769
13. Vettor R, Zarjevski N, Cusin I, Rohner-Jeanrenaud F, Jean- renaud B (1994) Induction and reversibility of an obesity syndrome by intracerebroventricular neuropeptide Y ad- ministration to normal rats. Diabetologia 37:1202-1208 14. Sainsbury A, Cusin I, Rohner-Jeanrenaud F, Jeanrenaud B
(1997) Adrenalectomy prevents the obesity syndrome pro- duced by chronic central neuropeptide Y infusion in nor- mal rats. Diabetes 46:209-214
15. Guillaume-Gentil C, Assimacopoulos-Jeannet F, Jeanre- naud B (1993) Involvement of non-esterified fatty acid oxi- dation in glucocorticoid-induced peripheral insulin resis- tance in vivo in rats. Diabetologia 36:899-906
16. Cusin I, Terrettaz J, Rohner-Jeanrenaud F, Zarjevski N, As- simacopoulos-Jeannet F, Jeanrenaud B (1990) Hyperinsu- linemia increases the amount of GLUT4 mRNA in white adipose tissue and decreases that of muscles: a clue for
A. Sainsbury et al.: Hormono-metabolic effects of central neuropeptide Y 1277 increased fat depot and insulin resistance. Endocrinology
127:3246-3248
17. Coleman DL (1988) Classical diabetes models: past les- sions and potential new therapies. In: Shafrir E, Renold AE (eds) Frontiers in diabetes research. Lessons from ani- mal diabetes II. John Libbey & Co Ltd, London, pp 253- 256
18. Nosadini R, Del Prato S, Tiengo A et al. (1983) Insulin re- sistance in Cushing's syndrome. J Clin Endocrinol Metab 57:529-536
19. Guillaume-Gentil C, Rohner-Jeanrenaud F, Abramo F, Bestetti GE, Rossi GL, Jeanrenaud B (1990) Abnormal regulation of the hypothalamo-pituitary-adrenal axis in the genetically obese fa/fa rat. Endocrinology 126: 1873- 1879
20. Jeanrenaud B (1991) Neuroendocrinology and evolution- ary aspects of experimental obesity. In: Oomura Y, Tarui S, Inoue S, Shimazu T (eds) Progress in obesity research 1990. John Libbey, Cornwall, pp 409-421
21. Sainsbury A, Rohner-Jeanrenaud F, Grouzmann E, Jeanre- naud B (1996) Acute intracerebroventricular administra- tion of neuropeptide Y stimulates corticosterone output and feeding but not insulin output in normal rats. Neuroen- docrinology 63:318-326
22. Rohner-Jeanrenaud F, Walker CD, Greco-Perotto R, Jean- renaud B (1989) Central corticotropin-releasing factor ad- ministration prevents the excessive body weight gain of ge- netically obese (fa/fa) rats. Endocrinology 124:733-739 23. Hadid R, Spinedi E, Daneva T, Grau G, Gaillard RC (1995)
Repeated endotoxin treatment decreases immune and hy- pothalamo-pituitary-adrenal axis responses: effects of 9 orchidectomy and testosterone therapy. Neuroendocrinol-
ogy 62:348-355
24. Spinedi E, Giacomini M, Jacquier MC, Gaillard RC (1991) Changes in the hypothalamo-corticotrope axis after bilater- al adrenalectomy: evidence for a median eminence site of glucocorticoid action. Neuroendocrinology 53:160-170 25. Schnetzler B, Murakawa G, Abalos D, Halban P, Selden R
(1993) Adapatation to supraphysiologic levels of insulin gene expression in transgenic mice: evidence for the impor- tance of posttranscriptional regulation. J Clin Invest 92: 272-280
26. Sizonenko S, Halban PA (1991) Differential rates of con- version of rat proinsulin I and II: evidence for slow clea- vage at the B-chain/C-peptide junction of proinsulin II. Biochem J 278:621-625
27. Halban PA (1991) Structural domains and molecular life- styles of insulin and its precursors in the pancreatic beta cell. Diabetologia 34:767-778
28. Woods SC, Porte D Jr (1974) Neural control of the endo- crine pancreas. Physiol Rev 54:5"96-619
29. Suda T, Tozawa E Iwai I e t al. (1993) Neuropeptide-Y in- creases the corticotropin-releasing factor messenger ribo- nucleic acid level in the rat hypothalamus. Mol Brain Res 18:311-315
30. Haas DA, George SR (1987) Neuropeptide Y administra- tion acutely increases hypothalamic corticotropin-releasing
factor immunoreactivity: lack of effect in other rat brain re- gions. Life Sci 41:2725-2731
31. Brooks AN, Howe DC, Porter DWF, Naylor AM (1994) Neuropeptide-Y stimulates pituitary-adrenal activity in fe- tal and adult sheep. J Neuroendocrinol 6:161-166
32. Liu JP, Clarke IJ, Funder JW, Engler D (1994) Studies of the secretion of corticotropin-releasing factor and arginine vasopressin into the hypophysial-portal circulation of the conscious sheep II. The central noradrenergic and neuro- peptide Y pathways cause immediate and prolonged hypo- thalamic-pituitary-adrenal activation. Potential involve- ment in the pseudo-Cushing's syndrome of endogenous de- pression and anorexia nervosa. J Clin Invest 93:1439-1450 33. Dallman MF, Strack AM, Akana SF et al. (1993) Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol 14:303-347
34. Miura M, Inui A, Teranishi A e t al. (1992) Structural re- quirements for the effects of neuropeptide Y on the hypo- thalamic-pituitary-adrenal axis in the dog. Neuropeptides 23:15-18
35. Gerald C, Walker MW, Criscione L e t al. (1996) A receptor subtype involved in neuropeptide-Y-induced food intake. Nature 382:168-171
36. Briick K (1983) Functions of the endocrine system. In: Schmidt RF, Thews G (eds) Human Physiology. Springer- Verlag, Heidelberg, pp 658-687
37. Dallman MF, Akana SF, Scribner KA et al. (1992) Mortyn Jones memorial lecture. Stress, feedback and facilitation in the hypothalamo-pituitary adrenal axis. J Neuroendocrinol 4:517-526
38. KaiYala KJ, Woods SC, Schwartz MW (1995) New model for the regulation of energy balance and adiposity by the central nervous system. Am J Clin Nutr 62:1123S-1134S 39. Yudkin JS (1993) Circulating proinsulin-like molecules. J
Diabetic Complications 7:113-123
40. Rhodes CJ, Alarcon C (1994) What beta-cell defect could lead to hyperproinsulinemia in NIDDM - some clues from recent advances made in understanding the proinsulin-pro- cessing mechanism. Diabetes 43:511-517
41. Porte DJr (1,991) Banting lecture 1990. Beta cells in type II diabetes. Diabetes 40:166-i80
42. Yoneda M, Tamasawa N, Takebe K et al. (1995) Central neuropeptide Y enhances bile secretion through vagal and muscarinic but not nitric oxide pathways in rats. Peptides 16:727-732
43. Stubbs M, York DA (1991) Central glucocorticoid regula- tion of parasympathetic drive to pancreatic B-cells in the obese fa/fa rat. Int J Obesity 15:547-553
44. Fletcher JM, McKenzie N (1988) The parasympathetic ner- vous system and glucocorticoid-mediated hyperinsulinae- mia in the genetically obese (fa/fa) Zucker rat. J Endocri- nol 118:87-92
45. Siaud P, Puech R, Assenmacher I, Alonso G (1990) Adre- nergic innervation of the dorsal vagal motor nucleus: possi- ble involvement in inhibitory control of gastric acid and pancreatic insulin secretion. Cell Tissue Res 259:535-542