Publisher’s version / Version de l'éditeur:
Wood Science, 6, 2, pp. 159-166, 1973-10
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
The swelling of wood in polar organic solvents
Ashton, H. E.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=a4973a54-7f52-498b-8c91-1d9920aa4e06
https://publications-cnrc.canada.ca/fra/voir/objet/?id=a4973a54-7f52-498b-8c91-1d9920aa4e06
The Swelling
of
Wood
In Polar Organic Solvents
ABSTRACT. The effectiveness of dimethyl sulfoxide, dimethylformamide, N-methyl pyrrolidone, and pyridine in swelling yellow hirch, beech, white pine, and Douglas-fir was determined. Changes in the radial and tangential dimensions during simple im- mersion were followed until maximum swelling was obtained. The complete swelling behavior in cases of rapid swelling was found to I)(. descrihtd hy the hyperbolic formula:
swelling a t time t = equilibrium swelling b. trnle * h I S the swelling resistance coef-
ficient. For slower swelling, in the initial period the swelling is directly proportional to time, not to its square root. Birch was swoll[%n the most by solvents, and pine the least, in relation t o their densities. Beech and pine swelled the fastest, w h ~ l r Douglas- fir was most resistant. Dimethyl sulfoxide caused the greatest final swelling, but dimethylformamide swelled wood more rapidly The latter solvent could be used with simple immersion, except with fir. To swell wood effectively with dimethyl sulfoxide would require a vacuum impregnation process.
- --
~ H I ' : M I C A L TREATMI':NTS for wood ~ n i g h t im-
C
prove the durability of organic coatings on wood by providing a better surface for coating adhesion. Alternatively, if treatments could in1- prove the dimensional stability of the wood sub- strate, the demands made upon the mechanical properties of films would be reduced, and coating durability should be improved.Swelling of wood
b y
water results from the hydration of hydroxyl groups in the an~orphous regions of cellulose. Chemical treatments, as dis- t i n p i s h e d from those that act by physical bulking, are usually designed to react with these cellulose hydroxyls. One of the problen~s in treating woocl is to react it, under normal conditions of tempera- ture and pressure, with sufficient reagent to change even its surface properties.O n e approach to greater reactivity is to swell the wood so that more functional groups are readily available t o the reagents. Water, of course, is a good swelling agent for wood but cannot be used for reagents that react with hydroxyls.
In recent years organic compounds that are excellent solvents for high polynlers have come
~ n t o use The) dlssolve consldernbl) more c k f
these 111gh-~nolecular w e ~ g h t ~natc-rl~llc t l ~ , ~ n clo convent~onal solvents and for bonlr, such ds dcr) l o n ~ t r ~ l e , are the only p r a d ~ c a l solvents Thci dre also excellent solvents for polysacchar~des In a d d l t ~ o n , these s o l ~ e n t s are reportetl ( ( 1 Ile excellent
nledla for c a r r p ~ n g out chern~cdl r t . ~ ~ t l o n s because both react~on r.~te\ and ~ l e l d s 'lrc ~narkedly
improved
S ~ n c e cellulose I S an extreniel\ III!.~ n~olecular w e ~ g h t polysacchar~de, ~t was c o n s l ~ l e r t ~ l probable that these solvents would Improve c ht 111lta1 treat ment of wood In two ways by swelling wood to a
greater extent thdn prev~ously pctssll~lc and
bv
Improvlng the des~red react~onb u ~ t h 1 1 1 ~ t u ~ ~ c t ~ o n a l
- - -
The author is Research Officer, Building Materials Section, Division of Ruilding Kesearch, National Research Council, Ottawa, Canada. Measure- ments on the wood hlocks were made by R. C. Seeley and J. .I. Wood. This paper I - I rontribu tion from the I ) ~ v ~ s r o n of Building Kesearch. National Research Council of Canada, and is puh- lished with the approval of the Director of the Division. It was received for publication in September 1972.
groups in wood. This paper reports the swelling effect of three such solvents on four different woods.
Procedure
T h e selected solvents were dimethyl sulfoxide ( D M S O ) , dimethylformamide ( D M F ) and
N-
methyl pyrrolidone ( M P ) . Pyridine was used as a control or comvarison solvent. because its swell- ing properties have already been recorded for several woods ( 1 ) . Also, since pyridine causes 20 to 25 percent more swelling than water (2, 3 ) , any swe1l;ng greater than that caused by pyridine would be a considerable advance over water- induced swelling.
DMSO, D M F , and methyl pyrrolidone were all conlmercial grades. They were. first dried over anhydrous sodium sulfate, then over anhydrous calcium sulfate, and finally filtered ( 4 ) . Because these materials are good solvents for some inor- ganic salts, determinations of residue after evapo- ration were made on the D M S O and D M F to ensure that the drying agents had not been dis- solved. T h e solids content of D M S O was 0.016 percent and appeared to be organic sulfur com- pounds. T h e DMI: did not contain any non- volatile material. Later analysis by gas-liquid chronlatography showed the D M S O content to be 91 percent and the M P 100 percent. Reagent grade pyridine was refluxed over K O H and dis- tilled at 114-1 15°C ( 5 ) .
Samples of four woods were provided by the Forest Products Research Laboratory, Canada De- partment of the Environnlent. Because of their similarity to European woods that had been studied previously ( 6 ) , yellow birch (Ge~rlld crlleghat~;e~~- J ~ J ) , beech (1;agzc~ g~.a~~rl;fol;a), and white pine
( P ~ I I ~ J J ~ I . U ~ I I J ) were selected. Douglas-fir ( P ~ e ~ c d o ~ ~ z c g d ~ne~zzies;i) was also chosen, because of its great commercial importance. T w o pieces of each wood were supplied in I - by 1-inch (25- by 2 5 - n i n ~ ) strips. They were cut into 2-inch lengths so that their dimensions corresponded to the first sample size used by Kumar ( 6 ) . T h e individual pieces were sanded gently and the edges slightly rounded with 400 silicon-carbide paper to remove any loose fibers.
T h e blocks were ovendried at 105'C for 24 hours and then kept in a desiccator over phosphor- ous pentoxide for 3 weeks. Weights and dimen- sions were recorded several times during this period. T h e dimensions remained fairly constant, but the weights gradually increased by about 314 of 1 percent although the PzOs was fresh. T h e
Figure 1 .
-
Apparatus for immersion of wood blocks in solvent.blocks were weighed on an analytical balance that is sensitive to 0.1 nlg and is maintained in a con- stant temperature and humidity room ( 2 3 2 2°C; 50?2 percent R . H . ) . Dimensions in both direc- tions across the grain were measured to 0.001 inch
( 1 mil) with a hand nlicronleter equipped with a ratchet stop. Longitudinal measurements were not made, because little swelling occurs in this clirec- tion. Initially a jig was set up to insure that measurenients were taken at the same location each time. During the experiment, howevet, the blocks soon became to2 swollen to fit into the jig. Subsequent n~easurenlents were made as close to the center of the faces as could visually be estimated.
Because the three test solvents are very hygro- scopic, the swelling experiments were carried out in vacuum distillation units with the distillation tubes replaced by drying tubes containing anhy- drous calcium sulfate (Fig. 1 ) . During the course
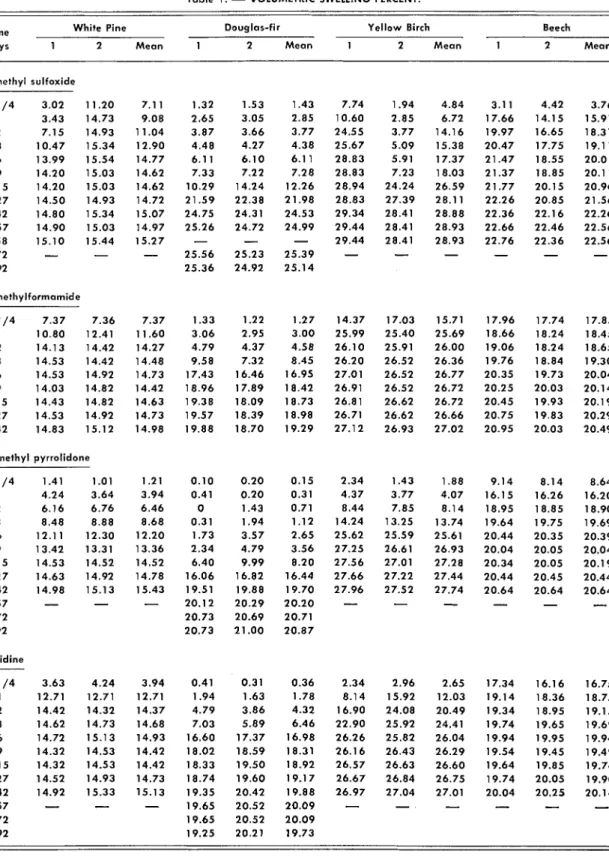
Table 1 .
-
VOLUMETRIC SWELLING PERCENT.Time White Pine Douglas-fir Yellow Birch Beech days 1 2 Mean 1 2 Mean 1 2 Mean 1 2 Mean Dimethyl sulfoxide 1/4 3.02 1 3.43 2 7.15 3 10.47 6 13.99 9 14.20 15 14.20 2 7 14.50 42 14.80 5 7 14.90 58 15.10 72
-
92 Dimethylforrnarnide 1/4 7.37 7.36 1 10.80 12.41 2 14.13 14.42 3 14.53 14.42 6 14.53 14.92 9 14.03 14.82 15 14.43 14.82 2 7 14.53 14.92 42 14.83 15.12 N-methyl pyrrolidone Pyridine W O U D S C I E N C E Vo1.6. N o . 2 161of the experiment the blocks were remo\ced from the solvent only to measure their dimensions. They were not weighed, because they would have ab- sorbed water vapor, and the swelling properties of the solvents alone were wanted.
T w o blocks of each wood were taken at ran- dom and placed in each of the four solvents. Porcelain desiccator plates were used to keep the blocks immersed at least 1 inch below the liquid level. Vacuum or pressure impregnation was not used, because the rate o f swelling under normal soaking was of as much interest as the final per- cent swelling. Maximum swelling is attained rapidly by the other procedures, but no informa- tion on the rate is provided. Dimensions were measured frequently at the beginning but at longer intervals as the test progressed. T h e sol- vents were replaced with fresh material when they became markedly discolored by the wood extractives.
Results
T h e blocks were measured in both directions across the grain. Only the beech had growth rings sufficiently parallel to the surfaces to classify the swelling as solely radial or tangential. T h e fir was quarter sawn, while the birch and vine were intermediate between quarter and flat sawn. Be- cause the grain directions were not separable, it was necessary to consider them together-in analyz- ing the results. O n e such method is to use the parameter percent volun~etric change. This has been defined ( 7 ) as the sum of the radial and tangential changes calculated as a percentage of the original values.
T h e percent volumetric swellings obtained in these studies are given in Table - 1. W i t h most of the duplicate samples the results are in good agreement: W i t h some, however, there was a marked difference, especially during the period of rapid swelling. In these cases the individual as well as the mean values must be considered in analyzing the swelling results.
Examination of the values in Table 1 shows that one wood may swell more rapidly in one sol- - . vent but attain greater v o l u n ~ e in another. For example, after 6 days Douglas-fir had swelled 6
percent in D M S O and 1 7 percent in D M F while the final swelling was 2 5 percent in D M S O but only 19 percent in D M F . W i t h regard to the dif- ferent woods, birch swelled the most and pine the least. Birch was also the fastest swelling wood in DMSO and D M F , but beech swelled more rapidly in pyridine and methyl pyrrolidone. Douglas-fir
T I I L D A Y S
Figure 2 .
-
Representative swelling curves.was the slowest swelling wood in all four solvents. Representative swelling curves are plotted in Figure 2. It can be seen that the fastest swelling combination is birch in DM17, while the slowest is Douglas-fir in methyl pyrrolidone. These two cases were selected for analysis of the swelling rates.
Discussion
'The extent of swelling can readily be deter- mined from the final percent volumetric increase. For convenience the mean values are given in Table 2 together with Kumnr's results. T h e maximum swelling results for the individual samples were subjected to analysis of variance. It was found that the differences between woods and between solvents were significant at the 99.9
percent confidence level. There was also a significant interaction between the two factors. This can be seen in the table where fir swelled more than beech in DMSO, less in DMF, and about the sanle in the other two solvents. Similarly, D M S O swelled birch more than methyl pyrroli- done, but white pine about the same. It is, there- fore, necessary to consider each combination of solvent and wood when examining the experi- mental results. Also, because of the variability, it does not appear possible to relate swelling effect O C T O B E R 1 9 7 3
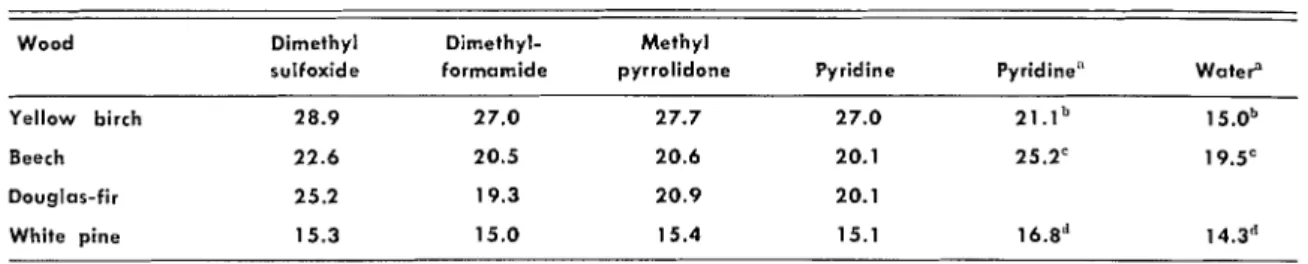
Table 2.
-
FINAL PERCENT VOLUMETRIC SWELLING.Wood Dimethyl Dirnethyl- Methyl
sulfoxide formarnide pyrrolidone Pyridine Pyridine" WateP
Yellow birch 28.9 27.0 27.7 27.0 21.1" 1 5.0b Beech 22.6 20.5 20.6 20.1 25.2" 19.5= Douglas-fir 25.2 19.3 20.9 20.1 White pine 15.3 15.0 15.4 15.1 16.8" 14.3" "Results of Kumar (1). " ~ e t u l a verrucosa. 'Fagus silvatica. "pinus silvestris.
to solvent propert~es such as solubility parameter, hydrogen bonding, or dipole moment.
It can be concluded that dimethyl sulfoxide swells wood more than does pyritline. Because the latter is more effective than water in causing swelling, D M S O should have a much greater effect than water. Methyl pyrrolidone also swells wood more than the control solvent while dimethylfor- mamide acts about the same. Hence, all four solvents should be more active than water. This is confirmed by the values of 15.0, 19.5, and 14.3
obtained by Kumar ( 6 ) for the same size speci-
mens of European birch, beech, and pine in water. These conclusions, of course, apply only to
the e q u i l ~ b r ~ u m swelling. They would be import-
ant if vacuum impregnation, which rapidly pro- duces saturation, were to be used. If, however, the alnl of treatment is to react mainly the wood surface, the rate of swelling is more important.
In experiments involving process rates it is customary to find the mathematical forn~ula that best fits the results. 1:ormula coefficients that vary with the individual cases can then be compared more easily to determine the effect of experimental
variables. Rate laws usually encountered are
linear, power, exponential, and parabolic. By
taking the logarithm, the square root, or the inverse of time, it is frequently possible to con- vert a nonlinear into a linear relationship. Stamm
(8) reported that the square root of time is
linearly related to weight gain up to about 20
percent weight increase.
W h e n the power, exponential, and parabolic rate laws were applied to the volumetric swelling results, none was found to fit the two cases selected for study. For the slowest swelling com-
bination - fir in methyl pyrrolidone shown in
Figure 3 - the square root function required
additional terms to bring the theoretical curve closer to the actual results. T h e results for the sample with the most rapid swelling are plotted in Figure 4. Of the usual mathematical treatments, the logarithmic curve most closely approximated the results.
After examining several other formulas, it
was found that the hyperbolic function ( 9 ) fitted
the swelling of yellow birch in dimethylforma- mide very well. With this function the percent
volumetric swelling, 11, at any time, /, is given by
the equation :
where
y=
is the swelling at infinite time. T h eequation can be applied either to calculate
y=
fromthe data or merely to find the constant, b, taking
the final swelling value as the equilibrium sweli- ing. It can be seen in Figures 3 and 4 that the latter curve approaches the experimental curve
more closely. It is also evident from Figure 2 and
Table 1 that nearly all the samples exhibited this
type of swelling. The hyperbolic function can apparently be used to describe the complete swell-
ing history. Other mathematical forn~ulas tend
to emphasize one part of the process at the expense of the other.
T h e equilibrium value obtained in the swell- ing studies shows how much the wood swells
while the factor b shows how quickly swelling
takes place. W i t h an increase in b, the time re-
quired to approach the equilibrium value increases, and the change from rapid to slow swelling be- comes more gradual. Hence, fast swelling rates
are associated with low values of b, so that b can
be called the coefficient of swelling resistance.
0 - 0 A C T U A L R [ S U L I I --- y .27.b ... . a . O ' ---. y
.
a i b l ~ - < I 24 2 20 l a r- $ I 6 zc I 4c
" 1 2 . 1 I : l u i e,
164 O C T O B E R 1 9 7 3 I I 1 1 1 1 1 1 I - - - _ _ _ _ - - * - -----
- , Y - - - ~.
_.-
,,, , . ... . . . ~ - / 1 ,...'. 1 .... -1:I.:'.
/:,*,"'
- .-I - I I , - I ' ,',
- : I , , ''!//
-+;
1 1 I.ti
I) l kThe hyperbolic equation can be applied to the experimental results to calculate the equilib- rium swelling value. The difference between the calculated and observed equilibrium values indi- cates how closely the swelling follows the hyper- bolic rate law. These differences are shown in Table 3. In about half the cases the difference is less than 0.3 percent volunletric swelling. Larger differences are connected with slower swelling rates, which will be discussed later.
Table 4 lists the values of b calculated with
y. taken from the experimental results. It can be seen that the fastest swelling did not in fact occur with birch in DMI:, but with beech in pyridine and DMF, and with one sample of pine in
DMSO. These faster swelling rates were ob-
scured by the lower final swelling volunle of beech and pine compared with that of birch. Except for beech and one sample of pine, dimeth- ylformamide caused the most rapid swelling. It would, therefore, be the most effective solvent
I" 20 l a ;D D O a.J la so uu l o o for use without vacuum impregnation. ,,'.'I O W " $
Slower swelling generally occurred with
Figure 3 .
-
Douglas-fir in methyl pyrrolidone. Douglas-fir although one sample of birch inDMSO resulted in the highest swelling resistance
23 24 22 20 .- z : 1s z I6
-
'" I d ; I 2 I D 8 6 P 2 0 .tI!.Ii. D A Y S experimental curves more closely than the square
Figure 4.
-
Yellow birch In dirnefhylforrnarnide. root equation did. This is shown in Figure 5o - I I i i I , I I I _ _ _ _ _ _ - - - - _ _ _ _
--__
- --- -.. - -.__.
, -,7..-.-,-... -.-.-.-.-.-*.7--.-.-.-:.k.-.-.--.-._
2 6 - . ' 0 . . ,,' - ; - -- ; - .-; - , 1 : - .F - .- - - - - - - - - - - - - -,b
,b
,
Jocoefficient. However, these conclusions are based on the hyperbolic function, and there is some question as to whether it is applicable to cases of
slow swelling. In Table 3 the differences between
calculated and observed results are large, and in
Figure 3 the hyperbolic curve rises too sharply
during the initial swelling, although it approxi- mates the later stages more closely than the other functions. Consequently, the first stages of swell-
ing were examined for all cases where b exceeded
10.
Based on Stamm's work ( 8 ) the square root
law,
y=a+b~"~
was applied. It was first necessaryto determine which of the later results should be excluded. This was done by including, in the cal- culation, observations up to the first observation
past the break in the curve. T h e number of
observations, 72, was then decreased stepwise until
a reasonable approximation of the initial swelling curve was obtained. The slower the swelling, the more observations could be included, because a longer time elapsed before equilibriulll was approached.
When tz had been established for each of
the slow swelling combinations, it was found that
Table 3.
-
DIFFERENCE BETWEEN OBSERVED AND CALCULATED FINAL SWELLING RESULTS." Wood Dimethyl Dimethyl- Methyl Pyridinesulfoxide formamide pyrrolidone Yellow birch 0.34 0.21 1.28 0.74 2.49 0.43 Beech 0.57 0.24 0.29 0.23 Douglas-fir 2.36 1.08 2.40 0.63 White pine 0.49 0.26 1.10 0.33 0.24
"Mean value except where two results are given.
Table 4.
-
SWELLING RESISTANCE COEFFICIENT, b". Wood Dimethyl Dimethyl Methyl Pyridinesulfoxide formamide pyrrolidone
Yellow 8.23 2.46 13.93 9.63
Birch 23.73 6.70
Beech 6.54 1.46 3.32 1.25
Douglas- 23.04 10.26 21.68 11.37
fir 12.35
where three different swelling behaviors are illus- trated. For comparison, the hyperbolic curve ob- tained using all the results from the fastest swell- ing wood in the group is included. With woods
that take 6 days to approach maximum swelling,
the function distorts the initial swelling rate. T h e square root law is not applicable to either slow or fast rates of swelling. Hence, more than diffusion is involved in the swelling of wood.
T h e slope of the linear regression line gives the initial swelling rate. These values are listed
in T,able 5 for the cases studied. It is evident that
the slowest swelling occurred with fir in MP, although the swelling resistance coefficient would have placed it third slowest. Similarly, birch in M P had the fastest initial swelling in ,the group, while ,the hyperbolic equation rated it slower than fir in either D M F or pyridine. These results, however, are mainly of academic interest, because the swelling would be too slow to be of practical
use. Such combinations of wood and solvent
require more than simple immersion for effective swelling to take place within a reasonable time.
Conclusions
White 6.43 2.21 7.70 2.66
pine 1.16 Of the three polar solvents studied, two swell
wood more than pyridine, and one about the same
"Mean value except where two results are given. as pyridine. Dimethyl sulfoxide caused the
0
0 5 1 0 I5 20 25 30 35 4 0 45 50 55 6 0 65 70 75
T l h l i . D A Y S
Figure 5.
-
Linear vs. square root regression lines.I I I I I I I I I I I I - D F
.
O M S 0 - D F 0 -0 lll P-
-
---- y - a t b l-
y-
a + b t j y.
y--p
- 0-0 Y E L L O W B I R C H I N M - P Y R O L.-.
D O U G L A S F I R I N O M S 0 0-0 D O U G L A S F I R I N M-
P Y R O L-
- I I I I I I I I I I I 1 I W O O D S C I E N C E V o ' . 6 , N o . 2Table 5.
-
RATE OF INITIAL SWELLING.--
Wood DMSO DMF -- M-pyrol Pyridine
Slope" nb Slope" n" Slope" n" Slope" n"
Yellow birch 1.06 9
-
4.25 6-
Douglas-fir 0.75 9 2.78 6 0.60 9 2.82 6
-. - -.
"Slope
-
Percent volumetric swelling per day. "n number of observations including origin.greatest final volun~etric swelling in three of the four woods.
Yellow birch was swollen the most by the solvents and white pine the least, as expected by their densities. Beech and Douglas-f~r were In- termediate, and bheir relative order varied with the solvent.
Equilibrium swelling is of interest only when swelling takes place very rapidly upon s ~ m p l e in]- mersion or when vacuum or pressure impregnation is used. In most other cases the rate of swelling is of more practical ~niportance.
A
hyperbolic function expresses mathematical- ly the complete swelling behavior of woods that swell rapidly. T h e difference between the cal- culated and the observed final percent volun~etric s w e l l ~ n g shows how closely the actual swelling obeys the hyperbolic law. When this difference is small and the swelling resistance coefficient is less than 10, the use of the equation to determine swelling rates appears valid.W h e n the swelling resistance coefficient is about 10 or higher, a linear equation can be ap- plied to those initial swelling results which yield a representative regression line if slow swelling rates are of interest.
Dimethylformamide generally caused the most rapid swelling of wood, although pyridine was faster in one case. Simple immersion in D M F
would be practical except with Dougls-fir. Swell- ing rates were slower with methyl pyrrolidone and variable with diniethyl sulfoxicle.
Beech was the fastest swelling wood except in D M S O in which white pine swelled most rapidly. 1)ouglas-fir exhibited the greatest re- sistance to swelling. Hence regardless o f the solvent used, vacuum or pressure impregnation would be required to swell fir before chemical treatment
Literature Cited
1. KUMAR, V. B. 1958. Swelling studies in wood. Norsk Skogindustri 12(9) :337.
2. Rrsr, J., and D. F. ARSENAU. 1957. Dimensional stabilization of wood. Forest Prod. J. 7 ( 6 ) :210. 3. NAYER, A. N. 1948. Ph. D. thesis. University of
Minnesota. Cited in Wood Chemistry Vol. 2, 1952. L. E. Wise and E. C. Jahn (eds.). Rein- hold, New York, N.Y. p. 735.
4. VOGEL, A. I. 1956. Practical Organic Chemistry. Longmans, Green and Co., London. p. 140. 5. IBID., p. 175.
6. KUMAR, V. B. 1957. Swelling studies in wood. Norsk Skogindustri 11 ( 7 ) :259.
7. STAMM, A. J. 1956. Dimensional stabilization of wood with carbowaxes. Forest Prod. J. 6 ( 5 ) : 201.
8. STAMM, A. J. 1955. Swelling of wood and fiber- boards in liquid ammonia. Forest Prod. J. 5 ( 6 ) : 413.
9. MARK, H. 1967. American Scientist Vol. 55. p.
265.