Comparison of the Hemostatic Efficacy of Pathogen-Reduced
Platelets vs Untreated Platelets in Patients With
Thrombocytopenia and Malignant Hematologic Diseases
A Randomized Clinical Trial
Frédéric Garban, MD, PhD; Audrey Guyard, PhD; Helene Labussière, MD; Claude-Eric Bulabois, MD; Tony Marchand, MD; Christiane Mounier, MD; Denis Caillot, MD; Jacques-Olivier Bay, MD, PhD; Valérie Coiteux, MD; Aline Schmidt-Tanguy, MD; Catherine Le Niger, MD; Christine Robin, MD; Patrick Ladaique, MD; Simona Lapusan, MD; Eric Deconinck, MD, PhD; Carole Rolland, MSc; Alison M. Foote, PhD; Anne François, MD; Chantal Jacquot, MD; René Tardivel, MD; Pierre Tiberghien, MD, PhD; Jean-Luc Bosson, MD, PhD; for the Evaluation of the Efficacy of Platelets Treated With Pathogen Reduction Process (EFFIPAP) Study Group
IMPORTANCEPathogen reduction of platelet concentrates may reduce
transfusion-transmitted infections but is associated with qualitative impairment, which could have clinical significance with regard to platelet hemostatic capacity.
OBJECTIVETo compare the effectiveness of platelets in additive solution treated with amotosalen–UV-A vs untreated platelets in plasma or in additive solution in patients with thrombocytopenia and hematologic malignancies.
DESIGN, SETTING, AND PARTICIPANTSThe Evaluation of the Efficacy of Platelets Treated With Pathogen Reduction Process (EFFIPAP) study was a randomized, noninferiority, 3-arm clinical trial performed from May 16, 2013, through January 21, 2016, at 13 French tertiary university hospitals. Clinical signs of bleeding were assessed daily until the end of aplasia, transfer to another department, need for a specific platelet product, or 30 days after enrollment. Consecutive adult patients with bone marrow aplasia, expected hospital stay of more than 10 days, and expected need of platelet transfusions were included.
INTERVENTIONSAt least 1 transfusion of platelets in additive solution with amotosalen–UV-A treatment, in plasma, or in additive solution.
MAIN OUTCOMES AND MEASURESThe proportion of patients with grade 2 or higher bleeding as defined by World Health Organization criteria.
RESULTSAmong 790 evaluable patients (mean [SD] age, 55 [13.4] years; 458 men [58.0%]), the primary end point was observed in 126 receiving pathogen-reduced platelets in additive solution (47.9%; 95% CI, 41.9%-54.0%), 114 receiving platelets in plasma (43.5%; 95% CI, 37.5%-49.5%), and 120 receiving platelets in additive solution (45.3%; 95% CI, 39.3%-51.3%). With a per-protocol population with a prespecified margin of 12.5%, noninferiority was not achieved when pathogen-reduced platelets in additive solution were compared with platelets in plasma (4.4%; 95% CI, −4.1% to 12.9%) but was achieved when the pathogen-reduced platelets were compared with platelets in additive solution (2.6%; 95% CI, −5.9% to 11.1%). The proportion of patients with grade 3 or 4 bleeding was not different among treatment arms. CONCLUSIONS AND RELEVANCE Although the hemostatic efficacy of pathogen-reduced platelets in thrombopenic patients with hematologic malignancies was noninferior to platelets in additive solution, such noninferiority was not achieved when comparing pathogen-reduced platelets with platelets in plasma.
TRIAL REGISTRATIONclinicaltrials.gov Identifier:NCT01789762
JAMA Oncol. 2018;4(4):468-475. doi:10.1001/jamaoncol.2017.5123 Published online February 1, 2018.
Editorialpage 458
Supplemental content
Author Affiliations: Author affiliations are listed at the end of this article.
Group Information: The Evaluation of the Efficacy of Platelets Treated With Pathogen Reduction Process (EFFIPAP) Study Group members are listed at the end of the article. Corresponding Author: Frédéric Garban, MD, PhD, Department of Hematology, CHU de Grenoble Alpes CS10217, 38043 Grenoble CEDEX 9, France (fgarban@chu-grenoble.fr).
P
latelet transfusion to prevent or treat bleeding due to thrombocytopenia is standard therapy worldwide.1 In recent years, the risk of transfusion-transmitted infections (TTIs) has been greatly reduced.2However, infec-tious agent transmission—in particular, bacteria—remains a significant risk associated with platelet transfusion.3-5One solution to further reduce the risk of TTIs is photochemical pathogen reduction (PR),6
a process by which pathogens are significantly depleted in number,7albeit with some exceptions.8,9However, PR technologies, such as amotosalen– UV-A treatment, induce functional platelet alterations.10,11 Previous clinical studies12,13
have raised concerns about the clinical effectiveness of pathogen-reduced platelets com-pared with standard untreated platelets. A 2013 Cochrane meta-analysis14of published clinical trials reported a non-statistically significant relative risk of 1.06 (95% CI, 0.93-1.21; P = .39) with regard to grade 2 or higher bleeding associated with pathogen-reduced platelets and concluded that not enough evidence was available to be sure that such platelets work as effectively as standard platelets to prevent bleeding. A recently updated analysis15reported a 1.10 (95% CI, 0.97-1.25; P = .15) relative risk of grade 2 or higher bleeding associ-ated with pathogen-reduced platelets.
We conducted a randomized clinical trial to test the hypothesis that amotosalen–UV-A–treated platelets in addi-tive solution (P-PR/PAS) are noninferior to untreated plate-lets in plasma (P-P) or to untreated plateplate-lets in additive solu-tion (P-PAS) with regard to hemostatic efficacy in minimally selected patients with thrombocytopenia and malignant hematologic diseases. Introducing both P-P and P-PAS as ref-erence treatments was deemed to be important because treat-ment with platelets in plasma remains the criterion standard with regard to platelet transfusion.
Methods
Study Oversight
The trial was performed according to the Declaration of Helsinki16
and the principles of good clinical practice. The trial protocol (Supplement 1) was approved by the Comité de Protection des Personnes Sud-Est and the Agence Nationale de Sécurité du Médicament. Before enrollment, patients were required to give written informed consent. All patient data were deidentified.
Study Design and Outcomes
In this noninferiority trial, patients with malignant hemato-logic disorders who required platelet transfusion were enrolled in 13 tertiary university hospitals and randomized (1:1:1) to 1 of 3 platelet transfusion arms: P-PR/PAS (Intersol, Fresenius Kabi AG, and Intercept Blood System, Cerus Europe BV), P-P, or P-PAS (Intersol). The primary outcome was the proportion of patients with 1 or more moderate to severe bleeding events (World Health Organization [WHO] grade ≥2) up to 30 days after randomization.
The secondary end points included the proportion of pa-tients with grade 3 or 4 bleeding events, the number of days
with grade 2 or higher bleeding events, the 24-hour corrected count increment (CCI) after the first transfusion, the interval between the first and second transfusions, the number of plate-let units and the total number of plateplate-lets transfused per patient, and platelet transfusion refractoriness (treatment failure) defined by a 24-hour CCI inferior to 4.5.12
Eligibility Criteria
The main eligibility criterion was adult patients with bone mar-row aplasia (platelet count <10 × 103/μL [to convert to 109/L, multiply by 1]), an expected hospital stay of more than 10 days, and an expected need of at least 2 platelet transfusions. Patient eligibility criteria included postchemotherapy apla-sia, autologous and allogeneic stem cell transplantation, and other causes of prolonged aplasia (aplastic anemia, myelodys-plastic syndrome requiring chemotherapy). Patients with docu-mented refractoriness to platelet transfusion who required a specific platelet product potentially unavailable in a timely fashion (cytomegalovirus antibody negative, HLA compat-ible, or washed platelets) or anticoagulant treatment at enroll-ment were not included.
Data Collection
Bleeding events were monitored daily until the end of apla-sia, transfer to another hospital department, the need for a spe-cific platelet product (eg, HLA-matched product), or up to 30 days after enrollment. Data collected were recorded in a cen-tralized electronic case report form managed by an indepen-dent clinical research organization (Clininfo).
Randomization
Randomization was centralized with stratification per cen-ter. The computerized randomization list was drawn up by the independent clinical research organization and the patient’s randomization number accessed through a secure site by a member of the site study team. Randomization was per-formed within 48 hours before the first platelet transfusion after enrollment.
Key Points
QuestionAre pathogen-reduced platelets in additive solution noninferior to untreated platelets for the prevention of World Health Organization grade 2 or higher bleeding?
FindingsIn this multicenter, 3-arm randomized clinical trial that analyzed 790 patients with thrombocytopenia and malignant hematologic diseases, bleeding of grade 2 or higher occurred in 47.9% of patients receiving pathogen-reduced platelets in additive solution, 43.5% of patients receiving platelets in plasma, and 45.3% of patients receiving platelets in additive solution. With a prespecified margin of 12.5%, noninferiority of pathogen-reduced platelets was not achieved when compared with untreated platelets in plasma but was achieved when compared with untreated platelets in additive solution.
MeaningPathogen-reduced platelets in additive solution might be associated with reduced hemostatic efficacy compared with untreated platelets in plasma while being noninferior to untreated platelets in additive solution.
Platelet Transfusions
Criteria for prophylactic platelet transfusion were in accor-dance with current French guidelines17
and randomized clinical trials that involved platelet transfusion18-20
: a plate-let count less than 10 × 103/μL or more in case of fever, splenomegaly, anticoagulation, disseminated intravascular coagulation, or invasive medical procedures. Criteria for thera-peutic platelet transfusion included severe hemorrhage and bleeding during surgery. Transfusion criteria were identical in all the study centers.
Patients received apheresis or pooled whole blood–derived platelets. All trial platelet products are authorized for routine use in France and were similarly prepared in accordance with cur-rent regulatory requirements, including prestorage leukodeple-tion (eMethods inSupplement 2). Both P-PAS and P-P were γ-irradiated, whereas P-PR/PAS was not. Platelet dose per trans-fusion followed the current French guidelines of 0.5 to 0.7 × 1010 platelets per kilogram. Trial blood product supply was ensured continuously by the Etablissement Français du Sang. The 24-hour CCI was calculated as previously described.12,21
Bleeding Assessment
Bleeding was assessed daily by research staff using patient interview, physical examination, and any relevant medical re-sults, as previously described,18with the exception of blood in feces, which was assessed using colorimetry and micro-scopic hematuria using dipsticks, with both performed at study center discretion. A daily bleeding grade was assigned cen-trally using WHO criteria by a member of the site study team masked to the study arm. All research staff had received web-based bleeding evaluation training. In addition, each pa-tient’s anonymized bleeding score was adjudicated vis-a-vis the anonymized hospital medical report by an independent committee of clinical hematologists to determine the final maximum bleeding score.
Study Masking
All persons involved in monitoring and evaluating bleeding were masked to the study arm and differed from those pre-paring and administering the study products. Platelet bags were all similarly labeled as being part of the study protocol. The P-P product differed slightly from the 2 other platelets products with regard to product color, and P-PR/PAS bags were stamped with a small transparent identification, with both differences discernable if a deliberate effort was made.
Adverse Events
All adverse events were recorded and reviewed by the Evalu-ation of the Efficacy of Platelets Treated With Pathogen Reduction Process (EFFIPAP) Safety Committee.
Sample Size Calculation
This noninferiority study was designed with a statistical power of 80% to detect an absolute difference of at least 12.5% (Δ for noninferiority) for the primary outcome between the P-PR/PAS arm and each of the 2 control arms, assuming a 60% incidence rate of bleeding grade 2 or higher.18With use of nQuery Advisor, version 7.0 (Statistical Solutions), the required sample size was
810 patients, with 270 per arm. After analyzing initial recruit-ment, the targeted sample size was increased by 30 patients to take into account that some randomized patients might not require transfusion as expected at the time of enrollment.
Statistical Analysis
Analyses are reported for the per-protocol population. All ran-domized patients who received at least 1 platelet concentrate that conformed to the assigned treatment arm were included in the per-protocol analysis. These were patients who had re-ceived products as planned in the protocol, with a few rare exceptions in which 1 or more off-protocol platelet concen-trates were transfused in addition to the assigned study prod-uct. Statistical analyses were performed using Stata, version 13.1 (StataCorp). The detailed statistical analysis plan is avail-able in Appendix F inSupplement 1. Nominal data are ex-pressed as number (percentage) and continuous data as mean (SD) except when the distribution was not normal, in which case median (interquartile range [IQR]) is used. For the primary outcome, a 1-sided test22,23with a prespecified noninferiority margin of 12.5% was used in accordance with previous12,24
and ongoing studies.25
Because of multiple com-parisons, a first-order risk Bonferroni correction was applied, resulting in a .025 significance threshold, with Wald 95% CIs calculated using binomial distribution. For the primary out-come, center effects were tested using Logit regression.
For secondary end points, superiority comparisons, an analysis of variance, or Kruskal-Wallis test, depending on nor-mality, was used for continuous data and a χ2test for nominal data. A 2-sided P < .025 significance level was used. When a difference was significant, a post hoc Scheffe, Wilcoxon, or χ2 test was used. The 95% CIs are given for proportions of bleed-ing events usbleed-ing binomial distribution. When daily bleedbleed-ing assessments were missing, a multiple imputation method using the mean of data before and after the missing data26was applied as predefined in the protocol.
Results
Study Population and Platelet Products
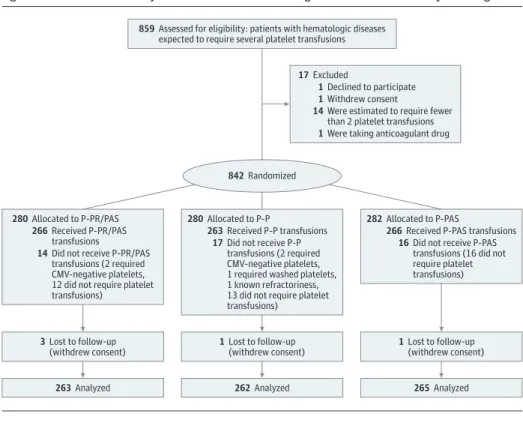
From May 16, 2013, through December 24, 2015, a total of 859 consecutive patients were screened and 842 enrolled and ran-domized (Figure). Of these, 790 patients were evaluable (mean [SD] age, 55 [13.4] years; 458 men [58.0%]). The number of pa-tients included per center varied (median, 56; range, 17-126). However, patients were evenly distributed among the 3 study arms in all 13 centers (eFigure 1 inSupplement 2). A total of 4983 platelet products were transfused. Of these, 90 transfusions (1.8%) were not in accordance with the study arm (off-protocol platelet transfusions), mainly because of nonavailability of the assigned product. All 790 evaluable patients received at least one on-protocol platelet transfusion. Of them, 734 (92.9%) re-ceived exclusively on-protocol platelets, whereas only 7 (0.9%) received less than 50% of on-protocol platelets. The distribu-tion among the study arms of off-protocol platelet transfu-sions is given in eTable 1 inSupplement 2. In view of the small proportion of off-protocol transfusions, the per-protocol
popu-lation and intention-to-treat popupopu-lations were considered to be identical. No substantial differences were found in patient char-acteristics among the study arms (Table 1). Test results for cen-ter effect were negative. The median follow-up duration was 18 days (IQR, 12-25 days) in all 3 arms. Three patients died during the study period of causes unrelated to study treatment.
Mean platelet dose per transfusion was 4.5 × 1011
(0.6 × 1011 ) but differed among the treatment arms (Table 2). Platelet characteristics (ratio between apheresis platelets and pooled whole blood–derived platelets, mean platelet storage age, and
ABO incompatibility) exhibited moderate to no imbalance among the treatment arms (eTable2 inSupplement 2).
Primary End Point
Grade 2 or higher bleeding occurred in 126 patients in the P-PR/ PAS group (47.9%; 95% CI, 41.9%-54.0%), 114 patients in the P-P group (43.5%; 95% CI, 37.5%-49.5%), and 120 patients in the P-PAS group (45.3%; 95% CI, 39.3%-51.3%) (Table 2). Thus, considering a prespecified 12.5% margin, noninferiority was not achieved when comparing P-PR/PAS and P-P, whereas Figure. Evaluation of the Efficacy of Platelets Treated With Pathogen Reduction Process Study Flow Diagram
859 Assessed for eligibility: patients with hematologic diseases expected to require several platelet transfusions
280 Allocated to P-PR/PAS 266 Received P-PR/PAS
transfusions
14 Did not receive P-PR/PAS transfusions (2 required CMV-negative platelets, 12 did not require platelet transfusions)
3 Lost to follow-up
(withdrew consent) 1 Lost to follow-up(withdrew consent) 1 Lost to follow-up(withdrew consent)
263 Analyzed 262 Analyzed 265 Analyzed
282 Allocated to P-PAS
266 Received P-PAS transfusions 16 Did not receive P-PAS
transfusions (16 did not require platelet transfusions) 280 Allocated to P-P
263 Received P-P transfusions 17 Did not receive P-P
transfusions (2 required CMV-negative platelets, 1 required washed platelets, 1 known refractoriness, 13 did not require platelet transfusions)
17 Excluded
1 Declined to participate 1 Withdrew consent
1 Were taking anticoagulant drug 14 Were estimated to require fewer
than 2 platelet transfusions
842 Randomized
CMV indicates cytomegalovirus; P-P, untreated platelets in plasma; P-PAS, untreated platelets in additive solution; and
P-PR/PAS, amotosalen–UV-A–treated platelets in additive solution. Table 1. Baseline Characteristics of the Study Population Randomized to Receive Platelets
in P-PR/PAS, P-P, or P-PAS Characteristic P-PR/PAS (n = 263) P-P (n = 262) P-PAS (n = 265) Total (N = 790) Men, No. (%) 145 (55.1) 160 (61.1) 153 (57.7) 458 (58.0) Age, mean (SD), y 55 (13.2) 55 (13.6) 54 (13.5) 55 (13.4) Weight, mean (SD), kg 75 (16.8) 74 (15.1) 74 (15.3) 74 (15.8) BMI, mean (SD)a 26 (5.1) 25 (4.6) 26 (4.8) 26 (4.9)
Diagnosis (treatment), No. (%)
Acute leukemia (induction or second line) 133 (50.6) 130 (49.6) 132 (49.8) 395 (50.0) Acute leukemia (consolidation) 38 (14.5) 22 (8.4) 38 (14.3) 98 (12.4) Other hematologic malignant tumor
with thrombocytopenia
7 (2.7) 8 (3.1) 6 (2.3) 21 (2.7)
Autologous hematopoetic stem cell transplantation
45 (17.1) 50 (19.1) 40 (15.1) 135 (17.1) Allogeneic hematopoetic stem cell
transplantation
40 (15.2) 51 (19.5) 48 (18.1) 139 (17.6)
Aplastic anemia 0 (0) 1 (0.4) 1 (0.4) 2 (0.3)
Follow-up duration, median (IQR), d 17 (12-24) 18 (11-25) 18 (12-26) 18 (12-25) Hemoglobin at enrollment, median (IQR), g/La 88 (81-97) 89 (78-99) 89 (79-98) 89 (79-98)
Platelet count at enrollment, median (IQR), ×103/μLb
25 (17-40) 25 (17-39) 25 (16-41) 25 (16-40) Death during the study period, No. (%) 1 (0.4) 0 (0) 2 (0.8) 3 (0.4)
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range;
P-P, untreated platelets in plasma; P-PAS, untreated platelets in additive solution;
P-PR/PAS, amotosalen–UV-A–treated platelets in additive solution. SI conversion factor: To convert platelets to ×109/L, multiply by 1. aMissing data restricted the sample
to 789 patients (263 in the P-PR/PAS group, 261 in the P-P group, and 265 in the P-PAS group).
b
Missing data restricted the sample to 785 patients (262 in the P-PR/PAS group, 261 in the P-P group, and 262 in the P-PAS group).
noninferiority was achieved when comparing P-PR/PAS and P-PAS (eFigure 2 inSupplement 2). The absolute risk differ-ence for grade 2 or higher bleeding was 4.4% (95% CI, −4.1% to 12.9%) when comparing P-PR/PAS and P-P (relative in-crease of 10.1%; 95% CI, −8.7% to 32.7%) and 2.6% (95% CI, −5.9% to 11.1%) when comparing P-PR/PAS and P-PAS (rela-tive increase of 5.7%; 95% CI, −11.9% to 27.0%).
Secondary End Points
All secondary end point results are given in Table 2. Severe bleeding events of WHO grade 3 or 4 occurred in 82 patients (10.4%; 95% CI, 8.2%-12.5%), with no significant difference be-tween treatment arms (P = .68). The number of days with grade 2 or higher bleeding was not significantly different among the treatment arms when considering all patients or patients ex-periencing at least 1 day with grade 2 or higher bleeding.
The median number of platelet transfusions per patient was 5 (IQR, 3-8). Patients in the P-PR/PAS group received sig-nificantly more transfusions (median, 6; IQR, 4-9) compared with patients in the P-P group (median, 5; IQR, 2-7; P < .001) but not compared with the patients in the P-PAS group (median, 5; IQR, 3-8; P = .17). Patients in the P-PR/PAS group were significantly more likely to receive a second platelet transfusion less than 2 days after the first transfusion (73 [31.6%]) compared with patients in the other treatment arms (P-P: 29 [13.2%]; P-PAS: 35 [15.2%]; P < .001 for both tests). The median total amount of platelets received per patient was
similar in the 3 arms (P-PR/PAS: median, 22.6; IQR, 14.5-36; P-P: median, 22.2; IQR, 11.5-34.3; P-PAS: median, 21.9; IQR, 13.2-37.8; P = .67). Red blood cell transfusions did not differ either (P-PR/PAS: median, 4; IQR, 2-7; P-P: median, 4; IQR, 2-8; P-PAS: median, 4; IQR, 2-6; P = .89). The mean (SD) 24-hour CCI after the first transfusion was 7.9 (6.3) overall and signifi-cantly lower in the P-PR/PAS arm compared with the other 2 arms (P-PR/PAS: 5.0 [5.2]; P-P, 10.2 [6.4]; P-PAS: 8.2 [6.0]; P < .001 for both tests). Treatment failure occurred more frequently in the P-PR/PAS arm. In relation to such treatment failures, 8 patients in the P-PR/PAS arm and 2 patients in the P-P arm received alternative nonstudy platelet products (ie, P-PAS) on request of the clinical team.
Adverse Events
Adverse events considered as probably or certainly linked to the platelet transfusion were scarce (Table 3). The frequency of allergic reactions differed between treatment groups, with a higher frequency in the P-P group. The frequency of pulmo-nary events did not differ among the treatment arms, and none were considered to be linked to platelet transfusion.
Discussion
In our study, noninferiority was not achieved when compar-ing P-PR/PAS and P-P, whereas noninferior was achieved when Table 2. Primary and Secondary End Points in Patients Receiving P-PR/PAS, P-P, or P-PAS
End Point P-PR/PAS (n = 263) P-P (n = 262) P-PAS (n = 265) P Value Primary end point
Patients with ≥1 episode of bleeding of grade 2 or higher,
No. (%) [95% CI]a 126 (47.9)[41.9-54.0] 114 (43.5)[37.5-49.5] 120 (45.3)[39.3-51.3] NA
Secondary end points
Patients with ≥1 episode of bleeding of grade 3 or 4, No. (%) [95% CI] 26 (9.9) [6.3-13.5] 25 (9.5) [6.0-13.1] 31 (11.7) [7.8-15.6] .68 No. of days with bleeding grade 2 or higher, median (IQR), d
All patientsb 0 (0-2) 0 (0-1) 0 (0-2) .27
Patients with at least 1 d of bleeding of grade 2 or higherc 2 (1-8) 3 (1-7) 3 (1-7) .84
No. of platelet transfusions per patient, median (IQR), unit 6 (4-9) 5 (2-7)d 5 (3-8)e .001
Platelet dose per transfusion, mean (SD), ×1011 4.1 (0.4) 4.9 (0.6)d 4.4 (0.5)d <.001
Total dose of platelets transfused per patient, median (IQR), ×1011 22.6 (14-36) 22.2 (12 to 34) 21.9 (13 to 38) .67
No. of RBC transfusions per patient, median (IQR), units 4 (2-7) 4 (2-8) 4 (2-6) .89
24-h CCI after the first platelet transfusion, mean (SD)f 5.0 (5.2) 10.2 (6.4)d 8.2 (6.0)d <.001
<2-d Interval between the first and second platelet transfusions, No. (%)g 73 (31.6) 29 (13.2)d 35 (15.2)d <.001
Transfusion failure
No. of evaluable 24-h CCIs 1367 1186 1313
24-h CCI<4.5, No. (%) [95% CI] 743 (54.2)
[51.6-56.8] 372 (31.3) [28.6-33.9]d 523 (39.9) [37.2-42.5]d <.001 Abbreviations: CCI, corrected count increment; IQR, interquartile range;
NA, not applicable; P-P, untreated platelets in plasma; P-PAS, untreated platelets in additive solution; P-PR/PAS, amotosalen–UV-A–treated platelets in additive solution; RBC, red blood cell.
a
P = .03 for the noninferiority test between P-PR/PAS and P-P; P = .01 for the noninferiority test between P-PR/PAS and P-PAS. See eFigure 2 in Supplement 2.
b
The sample was restricted to 694 patients (232 in the P-PR/PAS group, 226 in the P-P group, and 236 in the P-PAS group) with a minimum daily hemorrhagic grade of 2 or higher.
c
The sample was restricted to 294: patients (105 in the P-PR/PAS group, 86 in
the P-P group, and 103 in the P-PAS group).
dP < .001 for results from post hoc analyses comparing P-PR/PAS with each of
the control treatments.
eP = .17 for results from post hoc analyses comparing P-PR/PAS with each of the
control treatments.
f
The sample was restricted to 695 patients (222 in the P-PR/PAS group, 242 in the P-P group, and 231 in the P-PAS group).
gThe sample was restricted to 719 patients (247 in the P-PR/PAS group, 227 in
comparing P-PR/PAS and P-PAS with regard to grade 2 or higher bleeding in minimally selected patients with thrombocyto-penia and hematologic diseases.
These findings were observed with a predefined 12.5% noninferiority margin considered as appropriate from a clini-cal perspective. Furthermore, an identiclini-cal margin has been used in several prior or ongoing studies that assessed the hemostatic efficacy of platelets administered at different doses18
or having undergone PR.12,24,25
Platelet additive solu-tion was introduced in Europe in the early 2000s and subse-quently in North America to increase plasma for fraction-ation and to reduce plasma-related adverse reactions.27,28 Although reporting no significant differences regarding bleed-ing complications and transfusion interval, to our knowl-edge, the only published clinical trial29
that prospectively assessed PAS reported a significant reduction of 1- and 24-hour count increments and CCIs with P-PAS compared with P-P. To assess a potential independent effect of PAS (in treated or untreated platelets) and a “creeping inferiority” risk with regard to platelet quality,30we thought it appropriate to in-troduce both P-PAS and P-P as reference treatments.
Our finding that noninferiority was not achieved between P-PR/PAS and P-P differs from that of the sole sufficiently powered study of hemostatic efficacy: the SPRINT trial.24,31
That study, which had a similar 12.5% margin, reported noninferi-ority for grade 2 or higher bleeding frequencies when compar-ing P-PR/PAS and P-P. However, the mean number of days with grade 2 bleeding was greater in the P-PR/PAS arm.
We observed noninferiority between P-PR/PAS and P-PAS. Thisfindingagreeswithseveralsmall,Europeanrandomizedclini-cal trials32,33that examined bleeding occurrence as a secondary objective. In contrast, the Dutch-Belgium Hemato-Oncology Co-operative Group (HOVON) study12
reported significant differences in grade 2 or higher bleeding between P-PR/PAS and P-PAS (and between P-PR/PAS and P-P). More recently, the Italian Platelet Technology Assessment Study (IPTAS)13reported an absolute grade 2 or more bleeding risk difference of 6.1% when compar-ing P-PR/PAS and P-PAS (22% vs 15.9%, a 37% relative increase). However, the reported differences did not reach significance, and conclusions on noninferiority could not be made because of pre-mature termination and low statistical power.
In agreement with a review of prior studies,15 the fre-quency of severe bleeding (grade 3 and 4) was not different among the treatment arms. While reassuring, all reported stud-ies, including ours, are insufficiently powered to adequately assess differences regarding such bleeding events.
The overall frequency of grade 2 or higher bleeding in our study was intermediate between that reported in several pre-vious studies12,18,24but similar to the frequencies observed in
2 large randomized clinical trials of platelet transfusion.19,20The reported incidence of bleeding depends on factors that may dif-fer among studies, such as assessment method and frequency, criteria used to grade bleeding, and patient population.
In accordance with previous studies,12,13,24,32
P-PR/PAS was also associated with a decreased interval between 2 transfu-sions and a lower 24-hour CCI. Treatment failure frequency was also increased. These later factors suggest impaired in vivo plate-let viability and/or reduced circulation capacity. Reduced num-bers of platelets in P-PR/PAS may have contributed to the reduced interval while not contributing to the reduced CCI.18
Of note, for grade 2 or higher bleeding incidence and for 24-hour CCI and frequency of treatment failures, results with P-PAS were intermediate between P-PR/PAS and P-P. Such findings suggest that PAS might affect platelet quality in accordance with a recent in vitro study.34Consequently, using P-PAS as sole control when evaluating test platelets may in-deed not be appropriate. Untreated platelets in plasma, their natural environment, should remain the criterion standard for studies that evaluate in vivo efficacy of platelets undergoing treatment that may impair their function.
Transfusion-associated adverse events were infrequent, mainly low-grade allergies and fever, and in line with current French hemovigilance data.35
The higher frequency of aller-gies associated with P-P was expected.27Evidence that sup-ports increased pulmonary adverse events with P-PR/PAS, as possibly suggested in the SPRINT trial,24,31,36was not found.
Limitations
Our study has some weaknesses. It was not sufficiently pow-ered to allow for a third comparison between P-P and P-PAS, an issue that deserves further investigation. Furthermore, all efforts were made to maximize masking of study investiga-tors, the medical team, and patients to treatment arm. How-ever, with a deliberate effort, both P-P (as in all studies com-paring P-P and P-PAS) and P-PR/PAS bags could be recognized as such. The low occurrence of TTI4
prevented the inclusion of a safety end point with regard to TTI risk reduction. Lastly, our results regarding prophylactic platelet transfusion in pa-tients with thrombocytopenia and hematologic diseases can-not substitute for careful clinical assessment of similarly treated platelets in other clinical contexts, such as acute bleeding in the context of posttraumatic coagulopathy.37-39
Our results highlight the difficultly in maintaining an optimal functional quality of blood products while ensuring maximum TTI prevention. We suggest that differing geo-graphic and temporal infectious epidemiologic contexts, as well as the increased cost of providing platelet support, are additional issues that should also be considered.
Table 3. Transfusion-Related Adverse Events
Adverse Event
No. (%) of Adverse Events
P Value P-PR/PAS (n = 1822) P-P (n = 1422) P-PAS (n = 1739) Allergic reactions 9 (0.5) 17 (1.2)a 5 (0.3)b .006 Severe 2 (0.1) 1 (0.1) 2 (0.1) NA Fever 10 (0.6) 3 (0.2) 1 (0.1) .06
Other (not severe) 2 (0.1) 0 (0.0) 1 (0.2) NA
Abbreviations: See Table 2.
aP = .20 for results from post hoc
analyses comparing P-PR/PAS with P-P.
bP = .10 for results from post hoc
analyses comparing P-PR/PAS with P-PAS.
Conclusions
In our study, P-PR/PAS was not inferior to P-PAS with regard to clinical hemostatic efficacy in a hematologic context. However, such noninferiority was not achieved when
com-paring P-PR/PAS and P-P. Furthermore, our CCI results sug-gest reduced in vivo platelet recirculation resulting from the use of PAS and PR. Pathogen reduction technology, in addi-tion to platelet storage in PAS, could be associated with reduced hemostatic efficacy compared with untreated platelets stored in plasma.
ARTICLE INFORMATION
Accepted for Publication: November 9, 2017. Published Online: February 1, 2018. doi:10.1001/jamaoncol.2017.5123
Author Affiliations: University Grenoble Alpes, Centre National de Recherche Scientifique, Techniques de l'Ingénierie Médicale et de la Complexité–Institut Mathématiques Appliquées de Grenoble 38000, Grenoble, France (Garban, Bulabois, Rolland, Bosson); Service d’Hématologie, Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France (Garban, Bulabois); Etablissement Français du Sang, Grenoble, France (Garban); Centre d’Investigation Clinique 1406–Innovation Technologique, Institut national de la santé et de la recherche médicale, Grenoble, France (Guyard, Bosson); Service de Biostatistiques, Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France (Guyard, Bosson); Service d’Hématologie, Hôpital Lyon Sud, Hospices Civils de Lyon, Lyon, France (Labussière); Service d’Hématologie, Centre Hospitalier Universitaire de Rennes, Rennes, France (Marchand); Service d’Hématologie, Institut de Cancérologie Lucien Neuwirth, Saint-Priest-en-Jarez, France (Mounier); Service d’Hématologie, Centre Hospitalier Universitaire de Dijon, Dijon, France (Caillot); Service d’Hématologie, Centre Hospitalier Universitaire de Ferrand, Clermont-Ferrand, France (Bay); Service d’Hématologie, Centre Hospitalier Universitaire de Lille, Lille, France (Coiteux); Service d’Hématologie, Centre Hospitalier Universitaire d’Angers, Angers, France (Schmidt-Tanguy); Service d’Hématologie, Centre Hospitalier Universitaire de Brest, Brest, France (Le Niger); Service d’Hématologie, Hôpital Henri Mondor, Assistance Publique Hôpitaux de Paris, Créteil, France (Robin); Service d’Hématologie, Institut Paoli Calmettes, Marseille, France (Ladaique); Service d’Hématologie, Hôpital Saint-Antoine, Assistance Publique Hôpitaux de Paris, Paris, France (Lapusan); Service d’Hématologie, Centre Hospitalier Universitaire de Besançon, Besançon, France (Deconinck); Cellule Publication, Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France (Foote); Etablissement Français du Sang, La Plaine Saint-Denis, France (François, Jacquot, Tardivel, Tiberghien); Etablissement Français du Sang, Rennes, France (Tardivel); Unité mixte de recherche 1098, Institut national de la santé et de la recherche médicale, Université de Franche-Comté, Etablissement Français du Sang, Besançon, France (Tiberghien).
Author Contributions: Drs Garban and Bosson had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Garban, Caillot, Bay, Ladaique, Rolland, François, Jacquot, Tiberghien, Bosson.
Acquisition, analysis, or interpretation of data: Garban, Guyard, Labussière, Bulabois, Marchand,
Mounier, Caillot, Bay, Coiteux, Schmidt-Tanguy, Le Niger, Robin, Lapusan, Deconinck, Rolland, Foote, Tardivel, Bosson.
Drafting of the manuscript: Garban, Guyard, Caillot, Rolland, Foote, Tiberghien, Bosson.
Critical revision of the manuscript for important intellectual content: Garban, Guyard, Labussière, Bulabois, Marchand, Mounier, Caillot, Bay, Coiteux, Schmidt-Tanguy, Le Niger, Robin, Ladaique, Lapusan, Deconinck, François, Jacquot, Tardivel, Tiberghien, Bosson.
Statistical analysis: Guyard, Bosson. Obtained funding: Tiberghien.
Administrative, technical, or material support: Garban, Guyard, Marchand, Schmidt-Tanguy, Deconinck, Rolland, Foote, François, Jacquot, Tardivel, Tiberghien.
Study supervision: Garban, Labussière, Le Niger, Lapusan, Jacquot, Tiberghien, Bosson. Conflict of Interest Disclosures: None reported. Funding/Support: The study was sponsored and funded by the Etablissement Français du Sang (the French public transfusion service created by and under the supervision of the French Ministry of Health) independently of any pathogen reduction or additive solution manufacturer. Data collection and analysis were performed by the Grenoble Clinical Investigation Center independently of the study sponsor.
Role of the Funder/Sponsor: Five of the authors are employees of the Etablissement Français du Sang, a state agency. They were involved in the design of the study as well as interpretation of the data and preparation, review, and approval of the manuscript. However, the funding source had no role in the conduct of the study, data collection, management, or analysis.
Group Members: Jacques-Olivier Bay, MD, PhD (Centre Hospitalier Universitaire de Clermont-Ferrand, Clermont-Clermont-Ferrand, France); Philippe Bierling, MD, PhD (Etablissement Français du Sang, La Plaine Saint-Denis, France); Jean-Luc Bosson, MD, PhD (Centre Hospitalier Universitaire de Grenoble Alpes and University Grenoble Alpes, Grenoble, France); Claude-Eric Bulabois, MD (Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France); Denis Caillot, MD (Centre Hospitalier Universitaire de Dijon, Dijon, France); Martin Carre, MD (Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France); Valérie Coiteux, MD (Centre Hospitalier Universitaire de Lille, Lille, France); Catherine Cordonnier, MD, PhD (Centre Hospitalier Universitaire Henri Mondor, Créteil, France); Eric Deconinck, MD, PhD (Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France); Alison M. Foote, PhD (Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France); Anne François, MD (Etablissement Français du Sang, La Plaine Saint-Denis, France); Frédéric Garban, MD, PhD (Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France); Linda Gimeno, BSc
(Etablissement Français du Sang, La Plaine
Saint-Denis, France); Gaelle Guillerm, MD (Centre Hospitalier Universitaire de Brest, Brest, France); Audrey Guyard, PhD (Centre Hospitalier Universitaire de Grenoble Alpes and University Grenoble Alpes, Grenoble, France); Mathilde Hunault, MD, PhD (Centre Hospitalier Universitaire d’Angers, Angers, France); Chantal Jacquot, MD (Etablissement Français du Sang, La Plaine Saint-Denis, France); Helene Labussière, MD (Centre Hospitalier Lyon Sud, Pierre Benite, France); Patrick Ladaique, MD (Institut Paoli-Calmettes, Marseille, France); Thierry Lamy, MD, PhD (Centre Hospitalier Universitaire de Rennes, Rennes, France); Simona Lapusan, MD (Hôpital Saint Antoine Paris, Paris, France); Catherine Le Niger, MD (Centre Hospitalier Universitaire de Brest, Brest, France); Dominique Legrand, MD (Etablissement Français du Sang, Auvergne Rhône-Alpes, Decines, France); Tony Marchand, MD (Centre Hospitalier Universitaire de Rennes, Rennes, France); Mauricette Michallet, MD, PhD (Centre Hospitalier Lyon Sud, Pierre Benite, France); Christiane Mounier, MD (Institut de Cancérologie Lucien Neuwirth, Saint-Priest-en-Jarez, France); Christine Robin, MD (Centre Hospitalier Universitaire Henri Mondor, Créteil, France); Carole Rolland, MSc (University Grenoble Alpes, Grenoble, France); Aline Schmidt-Tanguy, MD (Centre Hospitalier Universitaire d’Anger, Anger, France); René Tardivel, MD (Etablissement Français du Sang, Rennes, France); Emmanuelle Tavernier, MD (Institut de Cancerologie, Saint-Priest-en-Jarez, France); Pierre Tiberghien, MD, PhD (Etablissement Français du Sang, La Plaine Saint-Denis, France); Anne Verkhoff, MD (Hôpital Saint Antoine, Paris, France); and Ibrahim Yakoub Agua, MD, PhD (Centre Hospitalier Universitaire de Lille, Lille, France). Monitoring and quality control: Fanny Doroszewski, MSc (Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France). Independent clinical research assistants from the OPTIMARC association: pharmacovigilance: Marie Laure Gavard, DPharm (Centre Hospitalier Universitaire de Grenoble, Grenoble, France); study clinical coordination and data management: Jean-Luc Bosson, MD, PhD, Audrey Guyard, PhD, and Carole Rolland, MSc (Centre Hospitalier Universitaire de Grenoble Alpes and University Grenoble Alpes, Grenoble, France); medical writer: Alison M. Foote, PhD (Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France).
Additional Contributions: We thank the patients, site investigators, research coordinators, and blood transfusion personnel who participated in this trial. More specifically, we thank the following persons for their support: Brigitte Bonneaudeau, MSc; Armelle Degeorges, PhD; Eric Jacquot, MD; Remi Courbil, MD; Rachid Djoudi, MD; Isabelle Desbois, MD; Bertrand Pelletier, MD; Gerard Tobelem, MD, PhD; and François Toujas, MSc, MBA, from the Etablissement Français du Sang. We also thank the mermbers of the Evaluation of the Efficacy of Platelets Treated With Pathogen Reduction Process Safety Committee: Michel Mallaret, MD, PhD
(Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France); Jean-Pierre Jouet, MD, PhD (Centre Hospitalier Universitaire de Lille, Lille, France); and Philippe Renaudier, MD, PhD (Agence Regionale de Santé de Nancy, Nancy, France). None of these people received supplementary compensation for study participation.
REFERENCES
1. Stroncek DF, Rebulla P. Platelet transfusions.
Lancet. 2007;370(9585):427-438.
2. Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress.Transfusion. 2010;50(10):
2080-2099.
3. Brecher ME, Hay SN. Bacterial contamination of blood components.Clin Microbiol Rev. 2005;18(1):
195-204.
4. Lafeuillade B, Eb F, Ounnoughene N, et al. Residual risk and retrospective analysis of transfusion-transmitted bacterial infection reported by the French National Hemovigilance Network from 2000 to 2008.Transfusion. 2015;55
(3):636-646.
5. Pietersz RNI, Reesink HW, Panzer S, et al. Bacterial contamination in platelet concentrates.
Vox Sang. 2014;106(3):256-283.
6. Prowse CV. Component pathogen inactivation.
Vox Sang. 2013;104(3):183-199.
7. Irsch J, Lin L. Pathogen inactivation of platelet and plasma blood components for transfusion using the INTERCEPT Blood System™.Transfus Med Hemother. 2011;38(1):19-31.
8. Hauser L, Roque-Afonso AM, Beylouné A, et al. Hepatitis E transmission by transfusion of Intercept blood system–treated plasma.Blood. 2014;123(5):
796-797.
9. Schmidt M, Hourfar MK, Sireis W, et al. Evaluation of the effectiveness of a pathogen inactivation technology against clinically relevant transfusion-transmitted bacterial strains.Transfusion.
2015;55(9):2104-2112.
10. Kaiser-Guignard J, Canellini G, Lion N, Abonnenc M, Osselaer JC, Tissot JD. The clinical and biological impact of new pathogen inactivation technologies on platelet concentrates.Blood Rev.
2014;28(6):235-241.
11. Osman A, Hitzler WE, Meyer CU, et al. Effects of pathogen reduction systems on platelet microRNAs, mRNAs, activation, and function.
Platelets. 2015;26(2):154-163.
12. Kerkhoffs JL, van Putten WL, Novotny VM, et al; Dutch-Belgian HOVON Cooperative Group. Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction.Br J Haematol. 2010;150(2):209-217. 13. Rebulla P, Vaglio S, Beccaria F, et al. Clinical effectiveness of platelets in additive solution
treated with two commercial pathogen-reduction technologies.Transfusion. 2017;57(5):1171-1183. 14. Butler C, Doree C, Estcourt LJ, et al. Pathogen-reduced platelets for the prevention of bleeding.Cochrane Database Syst Rev. 2013;3(3):
CD009072.
15. Estcourt LJ, Malouf R, Hopewell S, et al. Pathogen-reduced platelets for the prevention of bleeding.Cochrane Database Syst Rev. 2017;7:
CD009072.
16. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi:10.1001/jama.2013.281053
17. Transfusion of platelets in hematology-oncology medicine.http://www.has-sante.fr/portail /upload/docs/application/pdf/2015-11/fiche_de _synthese_-_transfusion_de_plaquettes_en _medecine_hematologie-oncologie.pdf. Accessed September 30, 2017.
18. Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage.N Engl J Med. 2010;362
(7):600-613.
19. Stanworth SJ, Estcourt LJ, Powter G, et al; TOPPS Investigators. A no-prophylaxis platelet-transfusion strategy for hematologic cancers.N Engl J Med. 2013;368(19):1771-1780. 20. Wandt H, Schaefer-Eckart K, Wendelin K, et al; Study Alliance Leukemia. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies.Lancet.
2012;380(9850):1309-1316.
21. DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med (Chic). 1916;17:863-871. 22. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing.J Gen Intern Med. 2011;26(2):192-196.
23. Dinno A. tostpr: One- and two-sample z tests of proportion-equivalence. Stata software package 2016.https://www.alexisdinno.com/stata/tostpr .txt. Accessed March 29, 2017.
24. McCullough J, Vesole DH, Benjamin RJ, et al. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation.Blood. 2004;104(5):1534-1541. 25. Ypma PF, van der Meer PF, Heddle NM, et al; PREPAReS Study Group. A study protocol for a randomised controlled trial evaluating clinical effects of platelet transfusion products.BMJ Open.
2016;6(1):e010156.
26. Engels JM, Diehr P. Imputation of missing longitudinal data: a comparison of methods.J Clin Epidemiol. 2003;56(10):968-976.
27. Heddle NM, Blajchman MA, Meyer RM, et al. A randomized controlled trial comparing the
frequency of acute reactions to plasma-removed platelets and prestorage WBC-reduced platelets.
Transfusion. 2002;42(5):556-566.
28. Ringwald J, Zimmermann R, Eckstein R. The new generation of platelet additive solution for storage at 22 degrees C.Transfus Med Rev. 2006;
20(2):158-164.
29. Kerkhoffs JL, Eikenboom JC, Schipperus MS, et al. A multicenter randomized study of the efficacy of transfusions with platelets stored in platelet additive solution II versus plasma.Blood.
2006;108(9):3210-3215.
30. Murphy S. Radiolabeling of PLTs to assess viability.Transfusion. 2004;44(1):131-133. 31. Snyder E, McCullough J, Slichter SJ, et al; SPRINT Study Group. Clinical safety of platelets photochemically treated with amotosalen HCl and ultraviolet A light for pathogen inactivation.
Transfusion. 2005;45(12):1864-1875.
32. van Rhenen D, Gulliksson H, Cazenave JP, et al; euroSPRITE trial. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment.Blood. 2003;101
(6):2426-2433.
33. Lozano M, Knutson F, Tardivel R, et al. A multi-centre study of therapeutic efficacy and safety of platelet components treated with amotosalen and ultraviolet A pathogen inactivation stored for 6 or 7 d prior to transfusion.Br J Haematol.
2011;153(3):393-401.
34. van Hout FMA, Bontekoe IJ, de Laleijne LAE, et al. Comparison of haemostatic function of PAS-C-platelets vs. plasma-platelets in reconstituted whole blood using impedance aggregometry and thromboelastography.
Vox Sang. 2017;112(6):549-556.
35. Daurat A, Roger C, Gris J, et al. Apheresis platelets are more frequently associated with adverse reactions than pooled platelets both in recipients and in donors.Transfusion. 2016;56(6):
1295-1303.
36. Corash L, Lin JS, Sherman CD, Eiden J. Determination of acute lung injury after repeated platelet transfusions.Blood. 2011;117(3):1014-1020. 37. Hess JR, Pagano MB, Barbeau JD, Johannson PI. Will pathogen reduction of blood components harm more people than it helps in developed countries?Transfusion. 2016;56(5):1236-1241. 38. Arbaeen AF, Schubert P, Serrano K, Carter CJ, Culibrk B, Devine DV. Pathogen inactivation treatment of plasma and platelet concentrates and their predicted functionality in massive transfusion protocols.Transfusion. 2017;57(5):1208-1217. 39. Nussbaumer W, Amato M, Schennach H, et al. Patient outcomes and amotosalen/UVA-treated platelet utilization in massively transfused patients.