Review Article
Theme: Intellectual Property Law and the Pharmaceutical SciencesGuest Editors: Marilyn N. Martinez and Srikumaran K. Melethil
The International Patent System and Biomedical Research: Reconciling
Aspiration, Policy and Practice
Antony Taubman1,2,3,4
Received 19 January 2007; accepted 7 July 2008; published online 7 November 2008
Abstract. This article reviews how the international environment shapes international patent law and practice with bearing on biomedical innovation. The cluster of issues is encapsulated in two core paradoxes. Thefirst concerns how public goods, such as new pharmaceuticals, may be produced through the deliberate creation of private rights that exclude material from the public domain. The second paradox concerns how“technological neutrality” and overall policy balance in the application of general patent law principles requires technology-specific interventions by regulators. The article illustrates how centrifugal and centripetal trends influence diverse national approaches to applying patentability criteria for pharmaceutical products.
KEY WORDS: international patent law; pharmaceutical innovation.
LOCATING THE INTERNATIONAL DIMENSION IN LIFE SCIENCES PATENTING
Pathogens show scant respect for national boundaries, human physiology is not shaped by national allegiance, and
theflow of medical science is not neatly confined to discrete
jurisdictions. The struggle to combat human disease and to promote health is inherently international in character and is recognized as an element of maintaining international peace and security.
The drafters of the Constitution of the World Health Organization (WHO), which was adopted in 1946 and entered into force on 7 April 1948, enshrined principles that
they saw as “basic to the happiness, harmonious relations,
and security of all peoples.” These principles included the
tenets that “[t]he health of all peoples is fundamental to
the attainment of peace and security and is dependent upon
the fullest co-operation of individuals and States. The achievement of any State in the promotion and protection of health is of value to all. Unequal development in different countries in the promotion of health and control of disease, especially communicable disease, is a common danger.” Thus, the healthcare policymaker is inevitably and naturally drawn to work with an international, indeed global, perspective, especially concerning communicable diseases.
By contrast, the law, policy, and practice of patents are, by and large, confined to national jurisdictions, and they can differ significantly in different countries. The ultimate scope and reach of patent law are ultimately determined by national statutes, not international mechanisms (apart from a handful of regional systems such as the European Patent Office (the EPO; as established by the European Patent Convention)).
Certainly, no “worldwide patent” exists, and there is no
realistic prospect of a true global patent right in any reasonable time frame: a 2002 World Intellectual Property Organization (WIPO) study (“WIPO Patent Agenda: Options for Development of the International Patent Sys-tem,” document A/37/6, August 19, 2002) noted that “few people see even a basic system of international grant as a realistic goal in the short term”.
Patents are“territorial” in that the rights they provided
under a patent have effect only in the national legal jurisdiction where it is granted. In other words, the granting of a patent in one country does not constrain activity in another country. A patent in one country is also legally “independent” from a patent on the same invention in
another country. This means that a finding that a patent is
valid or invalid in one jurisdiction does not determine its legal status elsewhere—so a patent allowed in one country may be rejected in another country and vice versa. This independence is a basic tenet of the foundational international treaty
1550-7416/08/0400-0526/0 # 2009 American Association of Pharmaceutical Scientists 526
DOI: 10.1208/s12248-008-9049-0
Based on a presentation to the Annual Meeting of the American Association of Pharmaceutical Scientists, San Antonio, October, 2006. This article represents the personal observations of the author only and should not be construed as representing the views of WIPO, its Member States, or its Secretariat. It draws on earlier research undertaken at the Australian Centre for Intellectual Property in Agriculture, College of Law, Australian National University (on leave).
1Global IP Issues Division, WIPO, Geneva, Switzerland.
2Australian Centre for Intellectual Property in Agriculture, College
of Law, Australian National University, Canberra, Australia.
312 chemin des Manons, 1218 Le Grand-Saconnex, Geneva,
Switzerland.
4To whom correspondence should be addressed. (e-mail: antony.
governing patents, the Paris Convention for the Protection of Industrial Property. Thus, if the inventor does not separately obtain a patent in that other country, the invention may fall fully into the public domain.
Patents remain rooted in the soil of the jurisdiction where they are granted and do not transcend its borders. Even so, there is a coherent international dimension to patent law, policy, administration and practice. Therefore it does
make sense to speak of an “international patent system,”
even when there is no international patent. Yet, many intellectual property (IP) policymakers, preeminently those concentrating on life sciences innovation and the
pharmaceu-tical sciences, argue firmly for the maintenance of national
“policy space” and “flexibility” in the international standards. They prefer to sustain a high degree of residual legal and
policy autonomy, of heterogeneity, in the patent system (1) in
contrast to the inherently international character of chal-lenges to public health, the formulation of health policy, and the conduct of biomedical research.
Notwithstanding the drive for national autonomy in IP law and practice, the administrative burdens and strong expect-ations of policymakers mean that some forms of international harmonization will be essential to promote the effective development and dissemination of new medical technologies. Today, a coordinated effort in patent policymaking is integral to our ability to respond to the evolving global health crises. The need for some international coherence and coordination is not a new phenomenon: international cooperation and coordi-nation on patent matters date back to 1883 with the establishment of the foundational multilateral treaty, the Paris Convention, which was elaborated over many years and remains central to IP law and practice today.
Addressing the systemic challenges of IP policy, which are substantial and of critical importance, arguably requires an international approach, now more than ever, even while pressure remains strong for regulatory diversity and a differential approach depending on national economic and social conditions. If the patent system is to be effective in promoting the development and dissemination of new medical technologies, especially pharmaceuticals, its sound operation and the effective functioning of patents as a public policy instrument is unlikely to be achieved by national systems operating in isolation from one another.
THE DYNAMICS OF THE INTERNATIONAL DIMENSION
The emergence and continuing evolution of an interna-tional patent system are not inevitable, despite the long-established international dimension of patent law and the pattern of international cooperation and convergence that have occurred over the past 120 years.
Forces impacting the international patent system can be described as being either centripetal (encouraging legal and administrative convergence) or centrifugal (reasserting the need for national regulatory autonomy and distinctiveness). Centripetal forces include established policy rationales for common standards, for regulatory convergence, for legislative coherence, and for administrative cooperation to enable pooling of resources for gains in efficiency and quality of regulatory processes. Centrifugal influences include objective
differences in national infrastructure and economic and technological development, in differing national priorities and economic and social challenges, and calls for regulatory
diversity and policyflexibility. As innovation and technology
diffusion models are increasingly transjurisdictional, incon-sistencies in national policy and practice are argued to be an injurious constraint on the development and transfer of new technologies in the health domain. However, as convergence takes effect, remaining areas of divergence rise in prominence and critics of existing levels of convergence emphasize that “one size doesn’t fit all.”
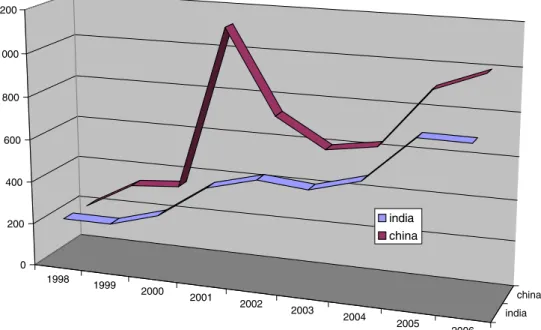
The increasing globalization of the patent system also shows profound changes to geographical patterns of activity. Until very recently, almost all patenting activity was sourced from three major jurisdictions: the USA, the countries within the European patent system (which largely coincide with, but are not coextensive with, the members of the European Union (EU)), and Japan. Recent patenting activity, however, shows a steep rise in domestic patenting activity in China, India, the Republic of Korea, and in several other developing countries. This trend is disproportionately high in the life sciences. The emergence of these economies as major players is likely to be the single greatest influence on the interna-tional patent system over the next few decades.
A review of the international patent system in 2007 reveals seemingly contradictory trends. The system is used far more extensively than was imagined a decade ago, measured either as
a crude count of applicationsfiled, in terms of growing diversity
across jurisdictions (including across perceived“north–south”
divides), acrossfields of technology, and across active users of
the system (including both private and public sectors). The
patent system yields“private” rights (2), in the sense that they
are typically recognized as items of private property. However, this does not imply private ownership. Current innovation policies in many countries promote the use of such private rights by the public sector and public interest entities. This is
leading to the steady accumulation of publicly held patent
portfolios and the emergence of a distinct discipline of public interest IP management (the deployment of private rights directly to serve public policy objectives). An exemplar of this approach has been the formulation of public/private partner-ships as tailored mechanisms for the development of new treatments for neglected diseases. These partnerships may develop and aggressively exploit patent estates for explicit
public health outcomes rather than for commercial goals (3).
More generally, the broader social responsibilities of public sector and not-for-profit patent holders have put the issue of humanitarian licensing and other forms of public interest IP
management on the agenda (4).
The international system is also making publicly avail-able an exponentially increasing amount of information about new technology in a uniform and accessible format. This public disclosure is, in principle, empirically based and focused on the practical teaching of how to implement claimed inventions. It disseminates information that might otherwise remain largely undisclosed and potentially legally constrained by confidentiality. The Patent Cooperation Trea-ty (PCT), a key element of the international patent architec-ture, provides an international application route to facilitate the acquisition of national and regional patents. Its legal operation and practical administration yield a steadily
grow-ing body of technological knowledge. It also stands in contrast to some national patent information systems which are, in principle, accessible to the public but still difficult in practice to access or use in many countries. The PCT system also produces extensive metadata of freely usable public domain information about technology, patterns of technological activity and ownership, directions in technological
develop-ment, activities of individual inventors and firms, and
preliminary assessments of the validity of claims (5).
The very existence of an international patent system promoted and entrenched a near-universal policy of early publication of patent applications. It also helped to promote open access and free-of-charge policies. As a consequence, affordable, accessible information technology delivers this material to users across the world who, only a few years ago, could not afford it and had no technological capacity to access and analyze it.
The international patent system is promoting the transpar-ent socially beneficial dissemination of technology. Neverthe-less, it is currently facing both an internal challenge concerning its operating efficiency and an external challenge concerning the legitimacy of its policy role. The sheer pressure of the growing volume of applications and new technologies strain the ability of national offices to sustain patent quality (i.e., the degree to which patents as actually granted correspond to the public interest as enshrined in the principles of patent law). Commen-tators and policymakers are also voicing concern about the directions taken by patent law and its social and economic impact. This concern arises at the level of principle: is the patent system inherently a legitimate policy tool for public health innovation and, at the level of functionality, is the system capable of ensuring patent quality?
The World Trade Organization (WTO) Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS;
6) has been the most potent and direct influence towards
internationalization of the patent system. The implementation of TRIPS obligations has induced considerable convergence of national legal standards in patent law in many developing countries. By explicit design of its negotiators, it had specific impact in the area of pharmaceutical patents and on the protection of clinical trial data submitted for regulatory approval of new drugs. A major impetus towards including IP law within the trade law system had been the need expressed by the major developed economies for higher and more consistent standards of IP protection, especially for pharma-ceutical product patents and regulatory data. These issues, contested during the Uruguay Round negotiations on TRIPS, remain contentious, forming the subject of a series of trade disputes within the WTO system. To a degree that is arguably now in need of moderation, the discourse and analysis surrounding TRIPS is dominated by coercion, the potential for trade sanctions, and zero-sum calculations of interests. For
one discussion of an alternative approach (7). Furthermore, the
centrifugal influences on patent law have in fact led to the sole amendment made to the wide-ranging package of trade agreements administered by the WTO since they came into force over a decade ago. An amendment to TRIPS has been agreed, with the express goal of safeguarding access to
pharmaceuticals and preserving nationalflexibilities; this was
preceded by a landmark political declaration by the world’s trade ministers, the Doha Declaration on TRIPS and Public
Health (8).
The past decade has seen significant growth in adherence to optional, à la carte, elements of the international system, such as the PCT. It has also seen increasingly diverse usage of the system by inventors in and from developing countries and by public sector institutions. This focus reflects both the increasing capacity of leading developing countries in life sciences innovation and conscious policy initiatives to capture benefits of public sector research. It also reflects the growth of public sector investment in core areas of health, food, and agriculture. In contrast to TRIPS, which is part of a single negotiated package of agreements, individual nations elect whether or not to adhere to the PCT. Recently, the trend in PCT membership has shifted from an early preponderance of developed economies or economies in transition towards developing countries. The latter one now forms the majority of the PCT members. There remains a considerable imbal-ance in the usage of this system, with patent applicants from the developed world predominating. Nonetheless, current trends reveal double-digit growth, sustained over 5 years or more, on the part of certain key developing countries. If sustained, this trend will ultimately lead to a shift in the center of mass of this aspect of the international patent
system (Fig.1).
THE CORE ISSUES
The interplay between biomedical innovation and IP raises complex questions, with roots deeply embedded in many areas of public policy. Examples include the policy formation and regulation concerning pharmaceuticals, food, and agriculture; the ethics of reproductive technologies, stem cell research, use of genetic resources, and the equitable sharing of the ensuing benefits; and the rights and interests of indigenous and other traditional communities. It is inevitable that such issues will ultimately be considered and resolved in diverse ways across jurisdictions and cultural settings.
IP protection in thisfield is not a recent consequence of
the emergence of modern biotechnology. Inventions in the life sciences, such as Pasteur’s improved beer-making yeast
(9), were patented in the nineteenth century. In 1883, the
Paris Convention explicitly extended IP protection to
agri-cultural science and natural products (10). Yet, the recent
rapid surge in patents across the life sciences has led to a renewed interest in several fundamental questions such as:
& Can a synthesized or extracted chemical compound
be considered patentably “novel” if it is chemically
identical to a compound that already exists in nature?
& When is a therapeutic compound or an isolated
nucleotide sequence a“mere discovery” versus a true
invention?
& When should the claimed invention in a patent
application be considered truly inventive, and when is it merely obvious, a predictable or routine labora-tory development, in the case of:
○New polymorphs or salts?
○The identification of new therapeutic properties?
○The development of a new dosage forms of known
pharmaceuticals?
& How do we resolve moral and bioethical issues
concern the technology itself, such as concerns over stem cell research, and when do they concern the grant or ownership of exclusive patent rights over isolated chemical structures such as nucleotide sequences? How should these exclusive rights be exercised and to what ends?
& What is different about the new pharmaceutical from a legal, regulatory, and ethical point of view? When considering the chemistry of life, such as genes, DNA, and the protein, they code for—are these to
be considered“just” a chemical compound or should
they be considered something more?
Established patent jurisdictions such as the USA and the EPO/European Union continue to develop answers to these
questions (11). However, as use of the patent system
internationalizes and broadens in scope (geographically, culturally, and across levels of economic development) and as more diverse actors (such as public institutions or public– private partnerships) develop an active stake, answers to these questions may likewise evolve in more diverse ways, posing challenges for the international patent system. TWO PARADOXES
These issues can be reduced to two fundamental
para-doxes. Thefirst and central paradox of IP law is that it aims to
promote the production of public goods by creating exclusive private rights, purposefully creating public benefit by exclud-ing material from the public domain. Patents legally entitle their owners to exclude third parties from the use of the claimed invention to create an incentive structure for the development and implementation of a valuable invention and to encourage disclosure to the public on how to carry out the invention. The challenge is to set the right balance between
legitimate rights for the inventor and beneficial access to technology for the public.
Consider, for example, an invention of borderline “inventiveness”. Would the public interest be better served by refusing the patent and thereby consigning this innovation to the public domain, free for others to use, leaving open pathways for research and development using this
innova-tion? As expressed by the US Supreme Court:“It was never
the object of [the patent] laws to grant a monopoly for every trifling device, every shadow of a shade of an idea, which would naturally and spontaneously occur to any skilled mechanic or operator in the ordinary progress of manufac-tures. Such an indiscriminate creation of exclusive privileges tends rather to obstruct than to stimulate invention. It creates a class of speculative schemers who make it their business to watch the advancing wave of improvement, and gather its foam in the form of patented monopolies, which enable them to lay a heavy tax upon the industry of the country, without
contributing anything to the real advancement of the art” (12,
13,14). Or would the public interest be better served by the
granting of property rights for that invention, creating an incentive for investment in the development and actual implementation of the technology? The US Supreme Court again recalled that the economic philosophy behind patent
law is that“encouragement of individual effort by personal
gain is the best way to advance public welfare through the
talents of authors and inventors in ‘Science and useful
Arts.’”(15). Such borderline questions may need to be
resolved differently in diverse national jurisdictions. Never-theless, there may also be broader policy interest in ensuring a reasonable degree of consistency and convergence in outcomes from different national systems.
The second paradox is that the patent system is, in
principle, neutral as to thefield of technology that is covered.
Yet, to maintain technology neutrality patent law applies a 1998 1999 2000 2001 2002 2003 2004 2005 2006 india china 0 200 400 600 800 1000 1200 india china
Fig. 1. Overall participation in the international patent system. PCT publications by nationality of applicant. IPC Class A61K: preparations for medical purposes
range of technology-specific interventions. A basic premise of
patent law is that allfields of technology should be treated
consistently or without discrimination (16). However,
main-taining the optimal balance between public and private interests in relation to biomedical patents has necessitated the institution of specific measures that recognize the
particular characteristics of thisfield of technology. In other
words, paradoxically, a technology-neutral approach requires technology-specific interventions such as:
& Explicit rules on whether patents should be available for specific areas of technology, such as newly identified secondary therapeutic uses of known com-pounds, or for bare nucleotide sequences without clearly disclosed utility
& The morality and ordre public exceptions to patent-able subject matter that potentially exclude inven-tions such as genetically modified mammals
& The exceptions to the reach of patent rights (such as the scope of research exceptions and the reach of patents on the use of research tools such as DNA sequences)
& The interaction of the patent system with the
regulation of pharmaceuticals for safety and efficacy, for instance in linking entry of generic drugs to patent enforcement
& Rules governing IP management in the public
interest for public-funded biomedical innovation
& Compulsory licensing and government use provisions in the public health domain, especially the tailored system for pharmaceutical patents established
through the Doha process (8)
& Forms of disclosure of technological information
uniquely required in the life sciences, notably the deposit of microorganisms for the purposes of patent procedure and specific mechanisms for disclosure of DNA sequences. It is in this regard that a distinct international system is established under the Buda-pest Treaty for the mutual recognition of deposit of microorganisms for patent purposes
& Proposals to require inventors who make use of genetic resources and traditional knowledge to disclose source, origin, and aspects of legal provenance, including evidence of benefit-sharing. This is argued to clarify and strengthen the relationship between the TRIPS agreement and the Convention on Biological Diversity (CBD) and the protection of traditional knowledge— increasingly present in national laws, and urged inter-nationally by India, Brazil and other developing nations
(17).
INTERNATIONAL LEGAL FRAMEWORK IN CONTEXT
Getting the balance of interests right is of course most critical in the public health area. This is why many countries and TRIPS itself have special forms of intervention in this area. TRIPS requires patents to be available under national law for any legitimate inventions, without discrimination as to
place of invention, field of technology, and whether the
invention is imported or locally produced (18). However, it is
permissible to exclude patents on inventions if their commer-cial exploitation would jeopardize human, animal, or plant
life or health (19). TRIPS also allows (but does not require)
countries to exclude diagnostic, therapeutic, and surgical methods for the treatment of humans from the scope of
inventions eligible for patenting (20) since some countries
assess the balance of interests differently for such inventions than for other areas of technology.
International rules, in particular TRIPS, are most often viewed as a constraint on national policymaking, as a reduction of legislative choice, and as an enforced conver-gence of national laws. For instance, as a result of TRIPS, it is now mandatory to make patents available for pharmaceutical products and for patent terms to run for at least 20 years. This contrasts with the past autonomous policy choices of many countries. The kind of earlier regulatory diversity that the TRIPS negotiations reversed was documented at the time in “Uruguay Round—Group of Negotiations on Goods—Nego-tiating Group on Trade-Related Aspects of Intellectual Property Rights, including Trade in Counterfeit Goods— Existence, Scope and Form of Generally Internationally Accepted and Applied Standards/Norms for the Protection
of Intellectual Property” (21). International rules are also
viewed as a means of safeguarding regulatory diversity. Once compliant with the international standards, countries have, in
principle, a free hand to exercise flexibility within the policy
space defined by these general international rules. The
pressure for flexibility has been most acutely felt within the
area of pharmaceutical patents and the life sciences because of the high levels of public interest.
This pressure came conspicuously to a head in 2001 when concern about access to key antiretroviral drugs led WTO Members to issue the Doha Declaration. This statement
captured the essence of thefirst patent paradox, the contrast
between exclusive rights and access to technology: “IP
protection is important for the development of new medi-cines…we recognize the concerns about its effects on prices.” Equally, Doha formalized the concept of national policy flexibility to promote public health: “TRIPS does not and should not prevent…taking measures to protect public health. Accordingly, while reiterating our commitment to [TRIPS]… [TRIPS] can and should be interpreted and implemented in a manner supportive of…right to protect public health and, in particular, to promote access to medicines for all. In this connection, we reaffirm the right…to use, to the full, the
provisions in [TRIPS], which provide flexibility for this
purpose.” While the Doha process has concentrated on policy options for compulsory licensing of pharmaceutical patents,
the domains of national legislative and policyflexibility range
across a broader range of measures and policy players
(TableI; Fig.2).
Despite the continuing political emphasis on centrifugal dynamics and interests, there remains a strong policy and public interest rationale for the international convergence of national patent systems. In particular, greater administrative and procedural cooperation on the international level will be
essential to enhance “patent quality” (i.e., the correlation
between patents granted and public interest). A vigorous debate continues as to whether, and if so how, the interna-tional harmonization of the substantive law of patentable inventions would advance the attainment of patent quality. A
centrifugal view on patent quality maintains that patentability criteria and their practical application must be defined and exercised by sovereign nations in a fully independent manner, consistent with the economic needs and policy priorities of each country. Conversely, a centripetal view maintains that sufficient resources will never be available to allow the practical realization of full practical independence and that there are sufficient shared interests to justify a common legal basis for closer cooperation and worksharing.
Recalling that patents are independent and territorially confined to individual jurisdictions, the practical reality is that
most patents arefiled for essentially the same subject matter
in multiple jurisdictions. Thus, patent granting authorities are, in parallel, undertaking a great deal of duplicated work. This is occurring at a time when offices around the globe are confronted with near-unmanageable workloads. The sheer logic of this situation may encourage work sharing, mutual recognition of search and examination, and pooling of search and examination resources. Harmonization is inevitable, be it promoted by legal platforms such as the draft Substantive Patent Law Treaty or conducted through an ad hoc arrange-ment between cooperating offices. In practice, offices pay
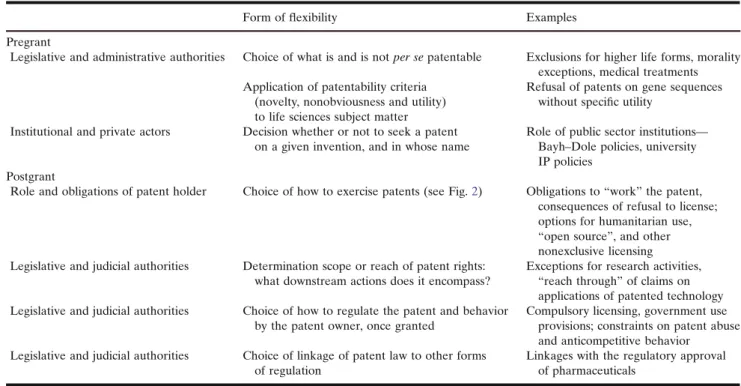
Table I. Pre- and Postgrant Fields of Flexibility in Patent Law and Practice
Form offlexibility Examples
Pregrant
Legislative and administrative authorities Choice of what is and is not per se patentable Exclusions for higher life forms, morality exceptions, medical treatments Application of patentability criteria
(novelty, nonobviousness and utility) to life sciences subject matter
Refusal of patents on gene sequences without specific utility
Institutional and private actors Decision whether or not to seek a patent on a given invention, and in whose name
Role of public sector institutions— Bayh–Dole policies, university IP policies
Postgrant
Role and obligations of patent holder Choice of how to exercise patents (see Fig.2) Obligations to“work” the patent, consequences of refusal to license; options for humanitarian use, “open source”, and other nonexclusive licensing Legislative and judicial authorities Determination scope or reach of patent rights:
what downstream actions does it encompass?
Exceptions for research activities, “reach through” of claims on applications of patented technology Legislative and judicial authorities Choice of how to regulate the patent and behavior
by the patent owner, once granted
Compulsory licensing, government use provisions; constraints on patent abuse and anticompetitive behavior
Legislative and judicial authorities Choice of linkage of patent law to other forms of regulation
Linkages with the regulatory approval of pharmaceuticals
18
Market orientation
Exclusivity/leverage over technology
“Conventional” private sector pipeline – tight vertical integration,
close exclusivity within one firm and affiliates
Public-private partnership for neglected disease burdens with cross-subsidization from market
product: diverse approaches to leveraging exclusive rights for health access, not commercial
return Conventional commercial collaboration – cross-licensing, technology partnerships, joint ventures, firms as technology integrators, etc. ‘Open source’ or public health patent pool
models with private sector downstream development pipeline: facilitated technology access upstream, strong commercial involvement in downstream
development and dissemination
“traditional” public sector research: noncommercial orientation, public domain,
no downstream leverage
?
commercial patent pools: based on pre-competitive technology platforms non-exclusive push or pull
incentive mechanisms: prize funds, advance purchase
commitments
attention to one another’s work, with some requiring the submission of search and examination outcomes in other jurisdictions.
The PCT already functions to reduce the administrative burden of patent authorities in multiple jurisdictions. Legally, the PCT forms a union of sovereign states, the International
Patent Cooperation Union, “for cooperation in the filing,
searching, and examination, of applications for the protection of inventions, and for rendering special technical services
(22).” It also provides a common international search and
examination process that yields a written opinion on the patentability of the claimed invention before an application
enters national patenting procedures (23). The PCT provides
for a degree of cooperation and practical convergence,
without prejudice to substantive national law (24). The
increasing practical availability of these international search and examination opinions and information about the entry of international applications into the national phase provides an unprecedented degree of transparency in the patenting
process (25).
TRIPS nominally harmonized patent law around the three core patentability criteria: novelty, nonobviousness/
inventive step, and utility/industrial applicability (19). In so
doing, it documented the convergence of views on the kind of exclusions from the public domain that would best advance the public interest. In the Anglo-American legal tradition, this utilitarian framework can be traced back to at least the 1623 Statue of Monopolies passed by the English Parliament
(26). However, TRIPS provided no guidance on substantive
legal questions raised by these general principles, such as the range of background knowledge (“prior art”) considered relevant in assessing novelty, how to assess when a claimed invention is obvious to the relevant person skilled in the art, and what test for utility is required. Thus, in setting an agreed general standard that leaves substantive questions open,
TRIPS effectively permitted policy flexibility. TRIPS also
explicitly provided certain areas of flexibility on patentable
subject matter and a priori exclusions from patentability (20,21).
INTERNATIONAL LAW AND BIOPIRACY
Developments in the life sciences, notably the perception that developed countries have vastly increased their capacity to derive value and benefit from genetic materials originating in developing countries, have triggered an intense debate over the equities of the patent system as currently configured. This has led to calls to recalibrate the equitable balance within the patent system and has reshaped the dynamics of patent law and policy in the international arena. Most strikingly at present, this has led to proposals to rewrite international patent law standards making it mandatory to disclose genetic resources and traditional knowledge used in the course of developing an invention. In some formulations, the obligation is extended to require evidence of the legal circumstances of source or origin, with the requirement for prior informed consent for its use of the indigenous materials. National laws have also been amended along these lines in a number of economies, creating a distinctive new requirement for those seeking patent protection for life sciences inventions. A divisive debate continues internation-ally over the appropriate scope, if any, and effectiveness of
such disclosure requirements. Nevertheless, such practices are emerging as a matter of national practice in a number of jurisdictions. This issue exemplifies the centrifugal trend in national responses to the two paradoxes of life sciences patent policymaking, especially the question of how to reconcile differential treatment of specific technological areas with the broader goal of nondiscriminatory implementation of the basic principles of patent law.
NATURAL MATERIALS: DEFINING INNOVATION One consistent concern with life science patenting has
been the legitimacy of patents on “naturally occurring”
materials: in other words, on newly identified or character-ized, isolated, purified, or synthesized chemical forms that correspond to those already existing in nature. Contemporary concerns over patenting of gene sequences are but a new chapter in a long-running debate. In the early years of last century, beriberi was a major cause of death in Asia. Suzuki discovered that a substance in rice bran (aberic acid, later called thiamin or vitamin B1) prevented the disease. This was
the first vitamin to be isolated, and in 1911, it received
Japanese patent 20785. Similarly, within the USA, a landmark
case concerned adrenalin, which wasfirst isolated for possible
therapeutic use by Takamine. A patent on isolated adrenalin was upheld because its isolation was considered to be novel and of therapeutic relevance. Judge Learned Hand concluded
that “even if it were merely an extracted product without
change, there is no rule that such products are not patentable.
Takamine was thefirst to make [adrenaline] available for any
use by removing it from the other gland-tissue in which it was found, and, while it is of course possible logically to call this a purification of the principle, it became for every practical purpose a new thing commercially and therapeutically. That
was a good ground for a patent” (27). Thus, a distinction
emerged between a patent on a naturally occurring com-pound versus its artificially extracted and isolated form, for which specific utility has been identified. Plainly, a patent claim for adrenalin in its natural state, within the human suprarenal gland, would be invalid.
The same general issue confronts the patent examiner today: when a naturally occurring nucleotide sequence legitimately be viewed as an invention versus a simple discovery of the workings of nature? Despite the centrifugal trend in debate, there is a degree of practical convergence on this question between different patent offices, pivoting on the very understanding highlighted by Justice Hand: i.e., that the isolation of a gene sequence must deliver a new state of affairs of practical utility to humanity, not simply the discovery that a certain nucleotide sequence exists in nature
(28). For instance, European law provides that“[a]n element
isolated from the human body or otherwise produced by means of a technical process, including the sequence or partial sequence of a gene, may constitute a patentable invention, even if the structure of that element is identical
to that of a natural element” (29). Yet a patent is awarded for
an invention, not for a bare chemical structure per se, even if it is newly disclosed. Therefore, there must be clear, practical utility associated with the newly identified or isolated gene sequence. For this reason, within the EU, a bare nucleic acid sequence is not patentable without its function being
indicat-ed. If a gene sequence is used to produce a protein, the protein and its function must be identified. If the nucleotide sequence has a different function, such as transcription promoter activity, that should likewise be indicated. Similarly, in 2001, the US Patent Office issued Utility Examination Guidelines that requires a patent on an isolated gene to
demonstrate“specific, substantial, and credible” utility (30).
Particularly in developing countries such as Brazil, China, and India, there is an emerging global practice of allowing patents on gene sequences, provided that the disclosed sequences meet the criteria of being truly inventive and practically useful for a defined purpose, rather than being simply an observation. This idea is built into the very concept of “invention” in many jurisdictions, where it is conceived as a specified solution to a technical problem, and not as a
scientific insight (31).
One continuing point of diversity in national legislative approaches is for a priori exclusions of certain subject matter. Debate continues over new chemical structures, derivatives,
or “follow on” innovations in the pharmaceutical sector.
Questions include the conditions under which the following are legitimate inventions as opposed to a routine adaptation of known technology, including:
& Purified or newly isolated substances
& New formulations and combinations of known compounds
& New therapeutic applications of “selection” patents within a known range, or species of compounds within a known genus
& New dosage forms or regimens
& New delivery routes, new salts and esters,
poly-morphs, and metabolites
Typically, in established patent systems, such claimed inventions have been assessed case by case, in the light of the technological background. A relatively recent trend has been to specify a priori rules in patent legislation, ruling out certain classes of pharmaceuticals. The Andean Community Decision 486 rules out new patents for new uses of products or
processes that are already patented (32). India’s patent law
was amended in 2005 to rule out“the mere discovery of a
new form of a known substance which does not result in the enhancement of the known efficacy of that substance or the mere discovery of any new property or new use for a known substance or of the mere use of a known process, machine or apparatus unless such known process results in a new product
or employs at least one new reactant” (33). An explanatory
note clarifies that “For the purposes of this clause, salts, esters, ethers, polymorphs, metabolites, pure form, particle size, isomers, mixtures of isomers, complexes, combinations and other derivatives of known substance shall be considered to be the same substance, unless they differ significantly in properties with regard to efficacy.” A technology-specific intervention was made in the USA, when the patent code was amended in 1995 to create a presumption that any biotech-nological process which yields a nonobvious product is itself
nonobvious (34).
Much commentary in international debate casts skepti-cism over the legitimacy of patents on follow-on innovations such as new forms of known compounds. Some studies have cast doubt on the therapeutic value of such new drugs. Yet, a
WHO-commissioned report points out that: “[i]ncremental
innovation can play an important part in the development of improved products that address public health needs. For instance, improving safety, simplifying the delivery of a drug or vaccine, or improving the efficiency with which it can be manufactured, can have an important impact on clinical outcomes or affordability and acceptability. Many of the modifications needed to align existing interventions more closely with the needs of poorer populations are likely to be
of the incremental variety” (35). To resolve competing
objectives—promoting valuable incremental innovation, while not burdening the public with exclusive rights over trivial adaptations—policymakers confront a dilemma: wheth-er to legislate technology-specific a priori rules on patentabil-ity or to rely on the continuous practical review and refreshment of broad patent principles as patent offices and courts assess claimed inventions against the evolving
techno-logical background, as in the life sciences what is“obvious”
today may have been highly inventive 12 months ago. In addition, questions of public expectation, moral issues, and public well-being result in regional differences in patentability decisions. Some jurisdictions categorize certain technologies as being inherently contrary to morality, such as the European ban on patents for inventions involving the modification of human genetic identity, human cloning, commercial exploitation of embryos, or the modification of animals’ genetic identity to cause them suffering while
yielding no substantial medical benefit (36). The public
interest is typically considered on a case-by-case basis in assessing morality and public well-being. For example, in China, a patent may still be available if an invention has positive therapeutic properties, despite being potentially detrimental due to undesirable side effects or the potential for abuse of the technology.
The EPO Howard Florey/Relaxin case (37) provides an
exemplary overview of the points raised in this “morality”
debate and highlights how established general principles are applied to valuable, but potentially controversial, new
tech-nologies. It concerned a European patent (38)filed in 1983,
granted in 1991, and opposed by a coalition of green members of the European Parliament. The case was appealed
and only finally decided in 2002. The patent was for a
synthesized DNA sequence that coded for a human H2-preprorelaxin, functionally equivalent to naturally occurring H2-relaxin. It was derived from human tissue obtained, with consent, during childbirth. It enabled the production, through novel nonhuman biological processes, of clinical quantities of relaxin for therapeutic use. In its natural form, it was difficult to obtain in sufficient quantities for therapeutic use or even systematic investigation. In effect it had to be harvested (with the prior informed consent of human subjects) from the minute quantities of relaxin naturally produced within the human body. It was practically undesirable and technically not feasible to continue to obtain human relaxin produced by human physiology, creating a technical problem for research-ers hoping to explore its therapeutic potential. Accordingly, the availability of recombinant, synthetic relaxin was, in Justice Hand’s phrase from the Adrenaline case, “for every practical purpose a new thing commercially and
therapeuti-cally (30).”
This hormone is structurally related to insulin, which led to certain false assumptions about its structure. This meant
that the eventual derivation of its sequence with a defined function contradicted general expectations about its charac-teristics, reinforcing the claim that this invention was not
obvious in the terms of patent law. It wasfirst investigated for
its role in loosening the pelvic ligaments and ripening the cervix prior to childbirth, with potential application in treating difficult births. This line of investigation failed when
problems arose in finding a stable and effective means of
delivery. Relaxin was subsequently tested as a potential
treatment forfibrosis, and scleroderma in particular (39).
Even though relaxin existed in nature, the availability of greater quantities of recombinant relaxin produced instead by bacteria meant the isolated sequence was potentially a patentable invention, illustrated how in the life sciences what
“occurs in nature” can yet be a genuine invention (27). The
very point of the invention was to create a nonhuman means of producing the hormone relaxin—despite earlier misleading scientific analyses, based on apparent similarities with insulin. It transpired that human relaxin (or more strictly two distinct forms of the hormone) were significantly different than that derived from other mammalian species. For example, porcine relaxin was therapeutically useless for humans. The essence of the invention was creating the capability of producing cloned human relaxin through genetically modified bacteria. The objective of the invention was to avoid harvesting relaxin from the human body but rather to produce it through the cloning of unicellular hosts. Accordingly, humans were not the source of the recombinant relaxin. Thus, the patent case turned, legally and technologically, on the transfer of a distinctively human gene sequence from consenting human subjects to single-cell bacteria—violating, in a literal sense at
least, the“integrity” of the human genome and conferring a
distinctively human trait on a markedly distinct organism. Given the positive ethical aspect of the search for treatments for human ailments, decision makers have been reluctant to rule out such inventions on the basis of moral objections. Considering the opposition, the EPO observed
that“to find a substance freely occurring in nature is a mere
discovery and therefore unpatentable. However, if a
sub-stance found in nature has first to be isolated from its
surroundings and a process for obtaining it is developed, that process is patentable. Moreover, if this substance can properly be characterised by its structure and it is new in the absolute sense of having no previously recognised
existence, then the substance per se may be patentable” (40).
The EPO decision reviewed a wide range of grounds of opposition at the level of basic principle in a manner that provides a comprehensive insight into the interplay between
law and policy in thisfield. The opponents charged that the
patent was not novel, since as the gene encoding relaxin had always been present in human body. Nevertheless, it was held
that even naturally occurring substances isolated for thefirst
time with no previously recognized existence were patent-able. Against the claim that the patent had no inventive step or was obvious, since conventional techniques had been used to isolate the gene sequence, it was held that the very existence of the substance in the form disclosed was a surprise and confounded expectations about the nature of relaxin. The method alone used to achieve the invention was not enough to determine obviousness. The opponents likened
the claimed invention to a“mere discovery” such as patenting
the moon or a new animal found in a remote area. The Opposition Division agreed that it was a substance freely occurring in nature, and would not be patentable, but took the view that it was not a mere discovery to newly isolate and characterize a substance. A further distinction was that the invention was a solution to an established technical problem (creating therapeutic supplies of relaxin). On the question of whether the invention was contrary to morality and whether it was indeed an offense to human dignity (by isolating a gene from tissue and by using pregnancy for a technical, profit-oriented process), the decision held that the general public would not view the invention as too abhorrent for a patent to be granted. However, it was required that the tissue had been donated with consent within framework of gynecological operations. Bioethics standards have previously been consis-tent with the approval of using human body parts, removed during an intervention, for medical purposes, and any life-saving substances had been isolated in this manner.
Against the claims that patenting human genes amounted
to a form of “modern slavery” (entailing dismemberment of
women and their piecemeal sale to commercial enterprises) and that patenting human genes amounted to the intrinsically
immoral patenting of human life, the final decision took the
view that there was no slavery: a patent on DNA encoding H2 relaxin does not create rights over individual human beings, and the invention achieved the opposite of dismemberment. To the contrary, the whole point—the technical problem solved— was to avoid harvesting relaxin from the human body and instead to produce it through cloning the unicellular hosts.
Patenting a“human gene has nothing to do with the patenting
of human life,” and there was no moral distinction discerned between patenting a gene and other substances found in the human body, such as adrenaline. The reasoning in the decision may or may not attract the approval of all commentators, but it certainly set out the kind of key distinctions that need to be weighed in assessing the patentability of such inventions. A further review of these issues in can be found in the Australian Law Reform Commission, Issues Paper on Gene Patenting and
Human Health, 2003 (41), which cites the examples of Claes
(42), Schrecker et al. (43), and Keays (44).
The following table sets out the key points of opposition and the responses made by the EPO in its decision, to provide an accessible overview of how patent law in one leading
jurisdiction works through such issues (TableII).
CONCLUSIONS
TRIPS has induced considerable convergence in patent law, removing key exceptions to patentable subject matter (notably mandating patents on pharmaceutical products) and precluding discrimination between technologies in patent law. Yet, as illustrated by the range of technology-specific measures
in patent law, life sciences innovation is not“just another” field
of technology. Ensuring that the widely accepted general principles of the patent system are effectively applied in the life sciences necessitated specific regulatory interventions. On
the other hand, human engineered “natural” products have
been developed and patented for well over a century. Modern biotechnology and pharmaceutical science do not necessarily bring wholly new and distinct challenges for the patent system. However, at a time when life sciences policymakers must
respond both to hope for new technologies for human health and to concern about equitable access to the benefits of new technologies, continuing specific attention will be made to the life sciences. Considerations will be based upon distinctive social and economic factors associated with these technologies, including:
& The ethical concerns associated with biotechnology as such and separate ethical questions about extending patents to biotechnology subject matter (such as gene sequences and higher life forms)
& The importance of the pharmaceutical technologies for meeting the fundamental human needs
& The strong north–south dimension both of research
priorities and access to and transfer of technology
& The relatively high proportion of public funds
invested in life sciences research, with concomitant public expectations of enhanced welfare outcomes
& Questions of ownership, control, and consent of
genetic resources when used in biomedical innova-tion, be they derived from human agricultural or other biological sources
Such considerations lead to distinct regulatory interven-tions with bearing on patents in the life sciences, including:
& Specific restrictions on patentable subject matter, such as methods of medical treatment or essentially
biological processes (16)
& Amendments to TRIPS to facilitate compulsory
licensing for pharmaceutical products, for those countries with limited manufacturing capacity
& The Budapest system for deposit of microorganisms for patent purposes
& Mechanisms for disclosure of origin or consent for genetic resources and traditional knowledge
& Specific morality-based exclusions from patentability The broad principles of patent law represent a steady
accumulation of understanding how to resolve the first
paradox, by ensuring that patents are available only for genuine inventions that are of practical utility and that are fully disclosed to the public. These principles have been repeatedly tested in the context of controversial life sciences technologies and have been shown to be surprisingly resilient and appropriate.
Patenting activities in the life sciences will continue to be subject to particular scrutiny and public concern because they represent the tip of the iceberg of technological development
infields that are of enormous public importance. Concerns
about setting the appropriate bounds of exclusive rights over life sciences technologies will continue to arise as new technologies work their way through the patent system. Specific legislative interventions may be required to ensure that the patent system remains true to its core principles. Paradoxically, the characteristics of life sciences innovation mean that differential treatment of patent law principles may be necessary to ensure nondiscriminatory impact in practice. Sustaining the patent system as a public policy tool for socially beneficial impact is a continuing challenge but one with profound importance for developed and developing countries alike.
It is not a minor task to adapt and apply these general principles to specific cases in the face of overlapping concerns impacted by international law, scientific and technological developments, and social and economic issues. This challenge is further magnified by an increasingly diverse array of countries that take an active role in the use of patent mechanisms to capture the benefits of life sciences innovation. Above all else, the development of an overarching, international standard for the application of patent law principles poses a dynamic
Table II. Core Patentability Issues in the Relaxin Case
Opponents argument EPO decision
Not novel, as the gene-encoding relaxin had always been present in human body
Novel: natural substances isolated for thefirst time with no previously recognized existence were patentable No inventive step (i.e., obvious)—
a conventional method had been used to isolate the DNA
Inventive. The very existence of the substance in the form disclosed was a surprise, confounding expectations. Method used to obtain it not significant in assessing obviousness Mere discoveries are not patentable.
Cannot patent the moon or a new animal found in a remote area
Mere discovery tofind a substance freely occurring in nature: not patentable. But it is not a mere discovery to newly isolate and characterize a substance. The moon and animals are not novel; not solutions to technical problems
Invention was contrary to morality or ordre public
General public would not view the invention as too abhorrent for a patent to be granted
Isolating a gene from tissue taken from a pregnant woman an offense to human dignity: pregnancy is used for a technical profit-oriented process
Tissue was donated with consent within framework of gynecological operations; many life-saving substances isolated in this way, patented, and welcomed by the public; bioethics norms approve use for other purposes of parts of the human body removed during an intervention
Patenting human genes amounts to a form of modern slavery: it involves
dismemberment of women and their piecemeal sale to commercial enterprises
No slavery—a patent covering DNA encoding H2 relaxin does not confer any rights to individual human beings. No dismemberment— the whole point is to avoid harvesting relaxin from the human body, producing it through cloning unicellular hosts. Humans are not the source Patenting human genes means that human
life is patented. This is intrinsically immoral
Patenting a“human gene has nothing to do with the patenting of human life.” No moral distinction can be seen between the patenting of genes and other important human substances (e.g., adrenaline)
challenge for policymakers as they balance the necessary regulatory diversity with a continuing core conception of the public interest. Rapid technological advances create mean that
technical criteria for patent eligibility, such as what is“obvious”
to a skilled expert outpace the regular tempo of legislative reform and responses. The fundamental logic and the essential principles of patent law retain broad acceptance, but wrangling will inevitably continue over how these principles should be adapted for new technologies. Historically, much of the practical content of patent law has been shaped through adversarial court cases, and national and international law is generally restricted to expressing broadly adaptable principles. But a significant recent development has seen increasing desire on the part of legislators to react to technologically-specific concerns, especially in the life sciences, through technologically specific interventions, in the hope of ensuring the patent system remains an effective, balanced instrument of public policy. Yet much of the actual economic impact of the patent system arises from choices about how patent rights, once granted, are
actually exercised in thefield—with the growing trend towards
public institutions and innovators in the developing world to take out patents in the life sciences—much of the economic and social impact of the system will result from their choices, as much as from formal legislative structures.
REFERENCES
1. Abbott, F.M. 2005. Toward a new era of objective assessment in thefield of TRIPS and variable geometry for the preservation of multilateralism (March 2005). Journal of International Economic Law 8(1): 77–100. Available at SSRN: http://ssrn.com/ab stract=915556.
2. Preamble of TRIPS, “[r]ecognizing that intellectual property rights are private rights”.
3. Taubman, Antony. 2004. Public–private management of intellec-tual property for public health outcomes in the developing world the lessons of access conditions in research and development agreements. Geneva: The Initiative on Public–Private Partner-ships for Health (IPPPH).
4. Chokshi, D.A. 2006. Improving access to medicines in poor countries: The role of universities. PLoS Med 3(6): 136. doi:10.1371/journal.pmed.0030136.
5. WIPO patent report 2006, at http://www.wipo.int/freepublica tions/en/patents/931/wipo_pub_931.pdf.
6. Agreement on trade-related aspects of intellectual property rights (1994) art. 8.
7. Taubman, A. 2003. Collective Management of Trips: APEC, New Regionalism and Intellectual Property. http://dspace.anu. edu.au/handle/1885/43004.
8. Declaration on the TRIPS agreement and public health (14 November, 2001) No. WT/MIN(01)/DEC/2.
9. Improvement in brewing beer and ale pasteurization (US Patent 135,245).
10. Paris Convention, art. 1.
11. Directive 98/44/EC of the European Parliament and of the Council of 6 July 1998 on the legal protection of biotechnological inventions, 1998 O.J. (L 213) 13 (‘EU Biotech Directive’) art. 5.2.
12. U.S. Supreme Court. Atlantic Works v. Brady, 107 U.S. 192 (1883).http://supreme.justia.com/us/107/192/case.html.
13. U.S. Supreme Court. Slawson v. Grand Street R. Co., 107 U.S. 649 (1883).http://supreme.justia.com/us/107/649/case.html. 14. U.S. Supreme Court. Great Atlantic & Pacific Tea Co. v.
Supermarket Equipment Corp., 340 U.S. 147 (1950). http:// supreme.justia.com/us/340/147/.
15. U.S. Supreme Court. Mazer v. Stein, 347 U.S. 201 (1954).http:// supreme.justia.com/us/347/201/case.html..
16. TRIPS art 27 http://www.wto.org/english/docs_e/legal_e/27-trips_04c_e.htm.
17. IP/C/W/442 (18 March 2005). 18. TRIPS art 27.1.
19. TRIPS art 27.2. 20. TRIPS art 27.3.
21. Note prepared by the International Bureau of WIPO, MTN. GNG/NG11/W/24 (5 May 1988).
22. PCT, art. 1. 23. PCT, art. 16.
24. Patent Cooperation Treaty, art. 27.3.
25. WIPO’s Gateway to Patent Services and Activities.www.wipo. int/patentscope.
26. Statute of Monopolies 1623 (c.3 21_Ja_1), An Act concerning Monopolies and Dispensations with penall Lawes and the Forfeyture thereof. http://www.statutelaw.gov.uk/content.aspx? activeTextDocId=1518308.
27. Parke-Davis & Co. v. H. K. Mulford & Co., 189 F. 95, 103 (S.D. N.Y. 1911).
28. Amgen, Inc. v. Chugai Pharmaceutical Co. 927 F.2d 1200, 1206 (Fed. Cir. 1991).
29. EU Biotech Directive, art. 5.2.
30. Utility Examination Guidelines, 66 Fed. Reg. 1092, 1098 (5 Jan 2001).
31. Indian Patent Law, s.3j.
32. e.g. Andean Community Decision 486: Article 21.
33. The Patents (Amendment) Act, 2005, No. 15 of 2005, para 3. 34. USC § 103(b).
35. CIPIH p 147.
36. Biotech Directive art 4.
37. Howard Florey/Relaxin (1995) EPOR 541. 38. Patent EP 0101309, Howard Florey Institute.
39. Seibold, JR. 2000. Recombinant human relaxin in the treatment of scleroderma. Annals of Internal Medicine, 132: 871–879. 40. Howard Florey/Relaxin (1995) EPOR 541, Ibid, 548.
41. Australia Law Reform Commission (2003). Issue Paper 27: Gene Patenting and Human Health. http://www.WHO.Int/Genomics/ Elsi/Regulatory_Data/Region/Wpro/126/En/Index.Html. 42. Claes, T. 2000. Cultural background of the ethical and social
debate about biotechnology. In Biotechnology, patents and morality, ed. S. Sterckx, 179–182. Aldershot: Ashgate.
43. Schrecker, T. et al. 1997. Ethical issues associated with the patenting of higher life forms. Montreal: Westminster Institute for Ethics and Human Values, McGill Centre for Medicine Ethics and Law. www.strategis.ic.gc.ca/pics/ip/ schrecef.pdf.
44. Keays, D. 1999. Patenting DNA and amino acid sequences: an Australian perspective. Health Law Journal 7: 69–76.