HAL Id: tel-02074579
https://tel.archives-ouvertes.fr/tel-02074579
Submitted on 20 Mar 2019
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Synergistic interaction earthworm-microbiota : a role in
the tolerance and detoxification of pesticides?
Fatina Jouni
To cite this version:
Fatina Jouni. Synergistic interaction earthworm-microbiota : a role in the tolerance and detoxification of pesticides?. Agricultural sciences. Université d’Avignon, 2018. English. �NNT : 2018AVIG0699�. �tel-02074579�
THESE
Présentée pour obtenir le grade de docteur de
L’Université d’Avignon
2015-2018
Présentée par
Fatina Jouni
Ecole Doctorale (ED 536) : Sciences et Agrosciences
Soutenue le 14 Décembre 2018
Jury :
Claudia Wiegand Professeur, Université de Rennes I Rapporteur Franck Vandenbulcke Professeur, Université de Lille I Rapporteur Camille Dumat Professeur, Université de Toulouse Examinateur Eric Peyretaillade Maitre de Conférence, U Clermont-Ferrand Examinateur Yvan Capowiez Chargé de Recherche, INRA Avignon Examinateur Magali RAULT Maitre de Conférence, Avignon Université Directrice
Synergistic interaction
earthworm-microbiota: A role in the tolerance and
THESE
Présentée pour obtenir le grade de docteur de
L’Université d’Avignon
2015-2018
Présentée par
Fatina Jouni
Ecole Doctorale (ED 536) : Sciences et Agrosciences
Soutenue le 14 Décembre 2018
Jury :
Claudia Wiegand Professeur, Université de Rennes I Rapporteur Franck Vandenbulcke Professeur, Université de Lille I Rapporteur Camille Dumat Professeur, Université de Toulouse Examinateur Eric Peyretaillade Maitre de Conférence, U Clermont-Ferrand Examinateur Yvan Capowiez Chargé de Recherche, INRA Avignon Examinateur Magali RAULT Maitre de Conférence, Avignon Université Directrice
Synergistic interaction e rth
-microbi ta: A role in the tolerance and
Acknowledgements
This journey would not have been possible to achieve without the support and guidance of many people.
Foremost, I would like to express my sincere gratitude to my supervisor Magali Rault, for the continuous guidance and support of my Ph.D. From the initial to the final level of my thesis, her motivation and enthusiasm were determinant for the accomplishement of this work. Thank you for having faith in my abilities, for your constant encouragement, full support and for being a one-of-a-kind supervisor! A very special gratitude goes to Yvan Capowiez, for his immense knowledge and for guiding and supporting me. You have set an example of excellence as a researcher.
Muchas gracias Juan Carlos Sanchez-Hernandez for all insightful comments and encouragement. It was fantastic to have the opportunity to work in your laboratory in Spain. What an enjoyable place to work!
I am deeply grateful to all members of the jury: Claudia Wiegand, Franck Vandenbulcke, Camille Dumat, Eric Peyretaillade and Yvan Capowiez for agreeing to read the manuscript and to participate in the defense of this thesis. I would like to thank my thesis committee members for their guidance, discussion, ideas, and feedback. I am very greatful to Eva Schreck.
Thanks to the team spirit existing between the researchers of my team (BES), for their daily encouragements and supports. I am very grateful to Séverine, Christophe, Alain, Virginie, Joeffrey, Hugues, Corinne and to my kind colleague Adrien.
I also thank Pascal Mirleau for the collaborative work that I undertook in the Station marine d’Endoume (Marseille). I am grateful to Fatma, Marjorie, Caroline, Juliette, Christian, Laurent, Monique, Michel and all members of the team Origine et Evolution de la Biodiversité (OEB).
I would like to thank all my friends and Ph.D colleagues, with whom I have shared moments of big excitement and with I had the best tea breaks in my life: a special thanks to my childhood friend Ghadir, my friends: Hanine, Mariam, Zaynab, Saad, Jean-Baptiste, Karine, Hitomi, Line, Sandrine, Anais, Shiraz, Elodie, Alice, Emilie, Valentin, Christophe, Louise, Milan, Simon, Yves.
I will forever be thankful to my amazing parents and family members, for the love, support, and constant encouragement throughout my years of study and through the process of researching and writing this thesis. I undoubtedly could not have done this without you!!!
Thanks for all your encouragement!
Finally yet importantly, a very special gratitude goes to all unlucky earthworms that passed away under my scissors, in the homogenizer and in the congelator.
I dedicate this thesis
to my parents,
for their constant support and unconditional love.
Thank you for believing in me
Page | 1
Table of Contents
Scientific Productions ... 5 Abreviation ... 6 List of figures ... 7 List of Tables ... 9Summary of the thesis (English) ... 10
Summary of the thesis (French) ... 11
GENERAL INTRODUCTION ... 12
1. The soil inhabitants: biodiversity and functional contributions ... 13
1.1. Soil fauna: diversity and functions ... 13
1.2. Earthworms: the masters of the soil ... 15
1.2.1. Beneficial services of earthworms in soils ... 16
1.2.2. Ecological groups of earthworms ... 17
1.3. Microorganisms screening and functions in soils ... 19
1.4. Factors affecting soil organisms ... 22
1.4.1. Environmental factors and soil properties ... 22
1.4.2. Agricultural practices and xenobiotic compounds ... 23
2. Soil pollution: a global problem ... 25
2.1. Pesticides: benefits and hazards ... 26
2.2. Organophosphates insecticides ... 29
2.2.1. Mechanism of action ... 29
2.2.2. AChE Target of OPs : a potential biomarker ... 30
2.2.3. Vigourous protecting mechanisms ... 31
2.3. Impact of OP on soil organisms ... 33
2.3.1. Impact on earthworms ... 33
2.3.2. Impact on microorganisms... 36
Page | 2
3.1. Earthworms‘ gut microzone: a biological filter ... 38
3.2. Fate of ingested microorganisms in earthworms‘ gut ... 40
4. Aims of the study ... 42
Chapter 1: Interspecific differences in biochemical and behavioral biomarkers in endogeic earthworms exposed to ethyl-parathion ... 46
1. Introduction ... 47
2. Materials and methods ... 49
2.1. Chemicals ... 49
2.2. Soil and earthworms ... 49
2.3. Earthworm behavior ... 50
2.4. Homogenate preparation ... 51
2.5. Esterase activity assays ... 51
2.6. Acetylcholinesterase inhibition kinetics ... 53
2.7. Native polyacrylamide electrophoresis for esterase isoenzymes ... 53
2.8. Statistical analysis ... 53
3. Results ... 54
3.1. Effect of ethyl-parathion on earthworm body weight and behavior ... 54
3.2. Effect of ethyl-parathion on esterase activities ... 56
4. Discussion ... 60
5. Conclusions ... 62
Chapter 2 : In-vitro sensitivity of B-esterases and metabolic responses of two endogeic earthworms‘ species exposed to OP insecticides ... 63
1. Introduction ... 64
2. Materials and methods ... 64
2.1. Chemicals ... 64
2.2. Soil and earthworms ... 65
2.3. Homogenate preparation ... 65
Page | 3
2.5. Enzyme characterization ... 67
2.6. In vitro B-esterase inhibition by pesticides ... 67
2.7. Data analysis ... 68
3. Results ... 69
3.1. Cellular localization of enzymes ... 69
3.2. Characterization of enzymes using specific substrates ... 70
3.3. In vitro inhibition of B-esterases ... 73
3.4. In vivo response of B-esterases and GST after OP exposure ... 76
4. Discussion ... 77
4.1. Characterization of B-esterases and GST activities ... 77
4.2. Sensitivity of B-esterase to OP pesticides ... 79
4.3. In vivo responses ... 80
5. Conclusion ... 81
Chapter 3 : Interaction earthworms-microbiome: a taxonomic study of gut-symbiont in two endogeic species exposed to ethyl-parathion. ... 82
1. Introduction ... 83
2. Materials and methods ... 84
2.1. Soil and earthworms sampling ... 84
2.2. Experimental setup and sample collection ... 85
2.3. DNA Metabarcoding analysis : ... 86
2.3.1. DNA extraction ... 86
2.3.2. Quantitative PCR procedure ... 86
2.3.3. Tagged amplicon sequencing ... 87
2.3.4. Bioinformatic analysis ... 88
2.4. Community analysis ... 88
3. Results ... 88
3.1. Composition of the bacterial community of soil and casts depending on earthworms species and sampling time ... 88
Page | 4
3.2. Microbiome associated to gut wall ... 93
4. Discussion ... 95
5. Conclusion ... 98
Chapter 4: Soil enzyme activities: dynamic and responses in ethyl-parathion-treated soil under the influence of earthworms ... 99
1. Introduction ... 100
2. Material and methods ... 101
2.1. Chemicals ... 101
2.2. Earthworms and soil sampling ... 102
2.3. Collection of soil, cast ... 102
2.4. Soil enzyme activities ... 103
2.5. Data analysis ... 105
3. Results ... 106
3.1. Effect of soil texture and ethyl-parathion on soil enzyme activities ... 106
3.2. Enzymatic indexes of soil quality... 109
4. Discussion ... 111
4.1. Impact of soil texture and Ethyl-parathion on soil enzyme activities ... 111
4.2. Enzyme-based indexes for soil quality ... 114
5. Conclusion ... 115
General discussion and perspectives ... 116
Page | 5
Scientific Productions
Jouni F., Sanchez-Hernandez J.C., Mazzia C., Jobin M., Capowiez Y., Rault M. 2018. Interspecific differences in biochemical and behavioral biomarkers in endogeic earthworms exposed to ethyl-parathion. Chemosphere 202, 85-93.
Jouni F., Sanchez-Hernandez J.C., Capowiez Y., Rault M. In-vitro sensitivity of B-esterases and metabolic responses of two endogeic earthworms‘ species exposed to OP insecticides. In preparation-1.
Jouni F., Mirleau P., Chappat J, Mazzia C., Sanchez-Hernandez J.C., Capowiez Y., Rault M. Interaction earthworms-microbiome: a taxonomic study of gut-symbiont in two endogeic species exposed to ethyl-parathion. In preparation-2.
Jouni F., Sanchez-Hernandez J.C., Capowiez Y., Rault M. Soil enzyme activities: dynamic and responses in ethyl-parathion-treated soil under the influence of earthworms. In preparation-3.
- Communications in conferences
Jouni F. Role of earthworm-gut microbiota in pesticide tolerance?. SFECOLOGIE 2018, (French Society for Ecology and Evolution (SFE²)), 22-25 octobre, Rennes - France. Oral Communication
Jouni F. Biochemical and behavioural responses in two endogeic earthworm species exposed to parathion». SETAC 2018 (Society of Environmental Toxicology and Chemistry), 13-17 Mai, Rome – Italy. Poster
Jouni F. Rôle de la synergie ver de terre-microbiote intestinal dans la tolérance à un insecticide organophosphoré. SEFA 2018, (Société Française d'Ecotoxicologie Fondamentale et Appliquée), 28-29 juin, Montpellier - France. Oral Communication Jouni F. « Impact d‘un insecticide organophosphoré sur des biomarqueurs biochimiques et comportementaux chez deux espèces de vers de terre » SEFA 2017 (Société Française d'Ecotoxicologie Fondamentale et Appliquée), 2930 juin, Lille -France. Poster
- Additional experiments not included in the manuscript….
Influence of temperatures 12°C and 22°C on biochemical and behavioral biomarkers, in two endogeic earthworms exposed to ethyl-parathion in two differents soils.
Monitoring and analyzing the pesticide behavior and its transformation products in soil, casts and earthworms‘ gut and tegument.
Page | 6
Abreviation
AChE Acetylcholinesterase CbE Carboxylesterase ChE Cholinesterase CYP450 Cytochrome P450 DNA Deoxyribonucleic aciddNTP deoxynucleotide triphosphates GST Glutathione-S-Transferase ITS Internal Transcribed Spacer
LC50 Lethal concentration for 50% of the individuals
MFO Multi Function Oxydase OP Organophosphate
OTU Operational Taxonomic Units PCR Polymerase Chain Reaction rDNA Ribosomal Deoxyribonucleic acid
Page | 7
List of figures
Figure 1: Soil biodiversity ... 13
Figure 2: Taxonomic groups classification of soil organisms according to their body-size. ... 14
Figure 3: Apporectodea caliginosa ... 15
Figure 4: A complex food web including earthworms and other living organism of soil ... 17
Figure 5: Earthworms niche groupings: epigeic earthworm (lumbricus rubellus). Endogeic earthworm (aporrectodea caliginosa). Anecic earthworm (lumbricus terrestris) ... 18
Figure 6: Bacterial community drivers of many ecological services in the rhizosphere, litter/deadwood, and soil compartments of the forest floor... 20
Figure 7: Ecological processes mediated by bacteria (highlighted in bold) and elements transfer (c in orange, n in green and p in blue) within the coupled biogeochemical cycles of carbon, nitrogen and phosphorus in forest ecosystems ... 21
Figure 8: Pollution practices must be abandoned unless human will lose all contact with the world of instinct. ... 26
Figure 9: Main physiochemical and biological processes contributing to pesticide fate and toxicity .... 27
Figure 10: Total sales of pesticides. ... 28
Figure 11: The conversion of the organophosphorus insecticides to their respective oxons ... 29
Figure 12: Esterase inhibition mechanism for the organophosphate parathion. ... 30
Figure 13: Interactions of esterases (cholinesterases and carboxylesterases) with carbamates (CA), the oxon metabolites of organophosphates (OP) and synthetic pyrethroids (PYR).. ... 32
Figure 14: Earthworm digestive system ... 39
Figure 15: Consequent effect of the drilosphere structures (internal and external) along with the surrounding functions on the dynamic of soil organic matter and microbial activity. ... 42
Figure 16. Effect of ethyl-parathion exposure (7 days) on earthworm weight (a), burrow length (b) and cast production (c). ... 55
Figure 17: Response of acetylcholinesterase (ache) activity in earthworms exposed to ethyl-parathion-contaminated soils for 7 days, and in-gel staining activity after native page electrophoresis. ... 57
Figure 18: Response of carboxylesterase (CbE) activity in earthworms exposed to ethylparathion -contaminated soils for 7 days ... 58
Figure 19: Relationship between acetylcholinesterase (AChE) activity and body weight change (a) or cast production (b) after exposure to ethyl-parathion). ... 59
Figure 20: Cellular distribution of proteins, GST and B-esterases activities ... 70
Figure 21: Determination of B-esterase kinetic parameters in both A. Caliginosa and A. Chlorotica species. ... 71
Figure 22: Determination of GSTkinetic parameters in both A. caliginosa and A. chlorotica species.. 73
Figure 23: Effect of the organophosphate insecticides ethyl-paraoxon and chlorpyrifos-oxon on A. caliginosa and A. chlorotica B-esterases.. ... 74
Figure 24: Response of GST and B-esterases activities in earthworms ... 77
Page | 8 Figure 26: Community clusters in soil and cast at the phylum level for bacterial communities (A) and at
the genus level for fungal communities (B)... 90
Figure 27: Community clusters in soil and cast according to the three times of sampling. ... 90
Figure 28: Relative abundances of bacterial (A,B) and fungal (C,D) phyla in soil and casts ... 92
Figure 29: Community clusters in earthworm's gut of both earthworms' species at the phylum level for bacterial communities (A) and at the genus level for fungal communities (B)... 93
Figure 30: The relative abundances of bacterial phyla (A) and bacterial genus (B) in earthworms gut………..94
Figure 31: The relative abundances of fungal phyla in earthworms gut. ... 95
Figure 32: Principal component analysis of enzyme activities for soil G and soil K ... 107
Figure 33: GMean index for soil enzyme activities ... 108
Figure 34: Sunray plots showing distribution of T-SQI scores calculated for each enzyme activity. .. 110
Figure 35 : IBRv2 scores calculated for each enzyme activity. ... 111
Figure 36 : Graphical abstract illustrating ecotoxicological and microbial experimental design ... 117
Page | 9
List of Tables
Table 1: Earthworm taxonomy………...15 Table 2: Neurotoxic insecticides, mode of action and authorized doses(1) used in French apple
orchards ………28 Table 3: Specific esterase activities, expressed as U.mg-1 proteins (mean±SEM) are measured in
homogenates from the whole body of the endogeic earthworms Aporrectodea caliginosa and Allolobophora chlorotica………..56 Table 4: Kinetic parameters for AChE, CbE and GST activities measured in A. caliginosa and A.
chlorotica homogenate (mean ± SD)………72 Table 5: Molar concentrations of pesticides to yield in vitro 50 % of enzyme inhibition (IC50) of
acetylcholinesterase (AChE) and carboxylesterase (CbE) activities of A. caliginosa and A. chlorotica and correlation coefficient (R2) for nonlinear regressions………...…75 Table 6: Variation in soil enzyme activities (mean ± SD, n=19) in soil after 4 days of exposure to ethyl-
Page | 10
Summary of the thesis (English)
Pesticides used to protect plants from pests, threat grievously non-target organisms such as earthworms. Due to their feeding and burrowing activities, earthworms are in direct contact with soil particles and microorganisms, as well as pollutants including pesticides. This work investigated (1) the effect of an organophosphate ―ethyl-parathion‖ on the sensitivity of two endogeic earthworms‘ species, Aporrectodea caliginosa and Allolobophora chlorotica; and (2) the role of the gut-microbiota, in synergy with the earthworm‘s detoxification pathways, in pesticide tolerance or detoxification. In the first part, biochemical and behavioral responses showed that A. caliginosa is more sensitive to ―ethyl-parathion‖ exposure than A. chlorotica. The endpoints measured ranged from physiological (weight), biochemical (AChE, CbEs, GST) to behavioral biomarkers (cast production and burrowing activity). Our findings showed that the sensitivity of A. caliginosa could be mainly due to the intrinsic sensitivity of its AChE to ―ethyl-parathion‖. The role of the carboxylesterases, acting as bioscavenger of OP, and the role of the detoxifying enzymes GST did not appear to be efficient mechanisms involved in A. chlorotica tolerance. In the second part, we aimed to characterize the microbiome within the ingested soil, the cast and the gut tissue of A. caliginosa and A. chlorotica in control or polluted soils. Our results showed differences in the microbial composition between these compartments. In this line, we suggested that these two earthworms‘ species harbor a species-specific microbiome in their gut. In particular, our findings showed that the earthworm‘s gut acts as a ―biological filter‖ for ingested microbial communities during the gut passage. At the level of the gut, we identified four dominated genus within the gut of A. caliginosa versus two dominated genus in the gut of A. chlorotica. Notably, we identified a Rhodococcus strain, which is highly abundant in the gut of A. chlorotica. Previous studies reported Rhodococcus strains for their ability to degrade some group of pesticides. We suggest that the presence of this strain could contribute to the tolerance of A. chlorotica. Finally, we showed that the effect of ethyl-parathion on soil enzyme activities mainly depend on soil texture rather than the presence and/or the species of earthworms.
According to our findings, it is of considerable importance to include more than one species to assess toxicity from organophosphorus insecticides, due to the interspecific differences that can occur within the same ecological category. Moreover, the identification and the functional analysis of the microorganisms found in the earthworm‘s gut and able to intervene in pesticide detoxification could enhance our knowledge about the fate of the pesticide inside the organism, and could be an important tool for bioremediation program.
Keywords: Aporrectodea caliginosa, Allolobophora chlorotica, organophosphates, biochemical biomarkers, behavior, enzymatic activities, microbiota, biological filter.
Page | 11
Summary of the thesis (French)
Les pesticides utilisés pour protéger les plantes des insectes nuisibles constituent une menace pour les organismes non cibles tels que les vers de terre. En raison de leur activité de bioturbation de sol, les vers de terre sont en contact direct avec les particules et les micro-organismes du sol, ainsi qu'avec les polluants, notamment les pesticides. L‘objectif de ce travail est d‘étudier (1) l‘effet d‘un organophosphoré (OP) «éthyl-parathion» sur la sensibilité de deux espèces de vers de terre endogés, Aporrectodea caliginosa et Allolobophora chlorotica; et (2) le rôle du microbiote intestinal, en synergie avec les voies de détoxification du ver de terre, dans la tolérance ou la détoxification des pesticides. Dans la première partie, les réponses biochimiques et comportementales ont montré que A. caliginosa est plus sensible à l'exposition à «l‘éthyl-parathion» que A. Chlorotica. Les résultats portent sur l‘analyse de biomarqueurs physiologiques (poids), biochimiques (AChE, CbEs, GST) et comportementaux (production de turricules et activité de creusement). Nous avons montré que la sensibilité de A. caliginosa semble liée à la sensibilité intrinsèque de l‘AChE à «l‘éthyl-parathion». De plus, le rôle des carboxylestérases, capables de piéger les insecticides OP, ainsi que le rôle de détoxification des GST notamment, ne semblaient pas être des mécanismes efficaces impliqués dans la tolérance de A. chlorotica. Dans la deuxième partie, nous avons caractérisé, en présence ou non d‘insecticide, le microbiote dans le sol ingéré, les turricules et les intestins des 2 vers de terre. Nos résultats ont montré des différences dans la composition microbienne de ces compartiments. A cet égard, nous avons suggéré que chacune de ces espèces hébergent un microbiote spécifique de l‘espèce dans leur intestin. Nos résultats ont notamment montré que l‘intestin du ver de terre agit comme un «filtre biologique» pour les communautés microbiennes ingérées lors du passage dans l‘intestin. A ce niveau, nous avons identifié, au niveau bactérien, quatre genres dominants dans l'intestin de A. caliginosa et deux genres dominants dans l'intestin de A. chlorotica. Nous avons notamment identifié une souche de Rhodococcus, très abondante dans l'intestin de A. chlorotica. Des études ont montré que des souches de Rhodococcus peuvent dégrader certains groupes de pesticides. Nous suggérons que la présence de cette souche pourrait contribuer à la tolérance de A. chlorotica. Enfin, nous avons montré que l‘effet de l‘éthyl-parathion sur les activités enzymatiques du sol dépend principalement de la texture du sol et non pas de la présence et/ou de l‘espèce de ver de terre.
Selon nos conclusions, il est extrêmement important d'inclure plus d'une espèce pour évaluer la toxicité des insecticides organophosphorés, en raison des différences interspécifiques pouvant se produire au sein d'une même catégorie écologique. De plus, l'identification et l'analyse fonctionnelle des micro-organismes présents au niveau de l‘intestin et susceptibles d'intervenir dans la détoxication des pesticides permettraient d‘améliorer nos connaissances sur le devenir du pesticide dans l'organisme et pourraient constituer un outil important dans les programmes de bioremédiation.
Mots-clés: Aporrectodea caliginosa, Allolobophora chlorotica, organophosphorés, biomarqueurs biochimiques, comportement, activités enzymatiques, microbiote, filtre biologique
Page | 12
Page | 13
1. The soil inhabitants: biodiversity and functional
contributions
1.1. Soil fauna: diversity and functions
Imagine walking through the nature and hearing the leaves crunching underfoot. Underneath those leaves, a complex ecological kingdom colonizes the soil and represents an impressively important reservoir of biodiversity. The soil is an anchor and support for the flora, fauna and microorganisms. In this area, many ecological functions are performed including: nutrient cycles, biogeochemical processes, phytoextraction, drainage, storage of water and many other elements and compounds like carbon, nitrogen, phosphorus, pesticides, heavy metals etc... Forcefully, the soil biota modifies considerably its environment by soil mixing, bioturbation, aeration and aggregate formation. Therefore, the properties of the soil maintain the biodiversity and the interaction between all its components, by providing a biological, chemical and physical habitat (Fig. 1) (Bardgett 2002; Epeide et al. 2008; Menta 2012; Ferris & Tuomisto 2015).
Figure 1: Soil biodiversity
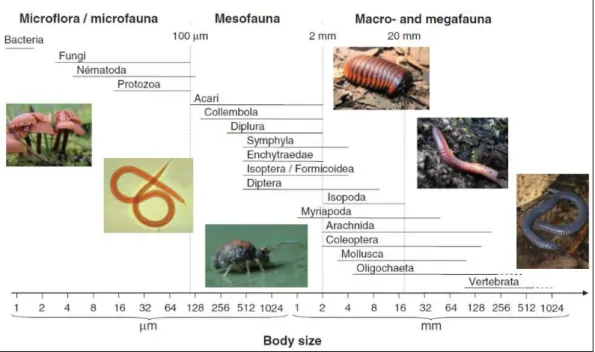
Page | 14 Soil fauna organisms are numerous and diverse. They include microflora and microfauna (bacteria, fungi, nematode…), mesofauna (collembola, enchytraedae, diptera…) and macro-and megafauna (isopoda, mollusca, oligochaete…) (Figure 2) (Decaëns 2010; Swift et al. 1979).
Figure 2: Taxonomic group’s classification of soil organisms according to their body-size. By Swift et al. (1979), illustrated by Decaens (2010)
Natural history and biology of many groups of them are well known, while for others it is still misunderstood. In their habitat, they interact with microorganisms and have significant influences on many processes in agroecosystems. Particularly, they enhance the breakdown and dispersion of soil organic matter, nutrient cycling and dynamics, influence soil structure and porosity thus, they increase soil fertility and primary production (Huhta 2007; Crossley et al. 1989; Coleman & Wall 2015). Potentially, they are used as bio-indicator of soil quality and pollution (Cortet et al. 1999; de Lima et al. 2017). For instance, earthworms belong to the soil macrofauna and are known to be the major component of soil fauna communities and the largest of invertebrates‘ biomass in soils (Fragoso & Lavelle 1992; Shakir & Dindal 1997; Blouin et al. 2013 ; Salehi et al. 2013).
Page | 15
1.2. Earthworms: the masters of the soil
Extensively found in soil all around the world and contributing largely to its biomass (80%), earthworms are commonly the larger members of the oligochaeta class, belonging to the phylum of Annelida because of their segmented body (Fig. 3; Table 1) (Edwards & Bohlen 1996). These
―
old friends of farmers‘‘ (Darwin 1881; Leena et al. 2012) are hermaphrodites and count for more than 1800 species classified under their morphological and phylogenetic characteristics as well as their ecological and behavioral pattern (Lavelle 1988; Kooch et al. 2008; Römbke et al. 2015).
Table 1 : Earthworm taxonomy
Figure 3: Apporectodea caliginosa
In his final book, ―The formation of vegetable mould, through the action of worms” Darwin published his findings and concluded: “The plough is one of the most
ancient and most valuable of man’s inventions; but long before he existed the land was in fact regularly ploughed, and still continues to be thus ploughed by earth-worms. It may be doubted whether there are many other animals which have played so important a part in the history of the world, as have these lowly organized creatures.” - Charles Darwin 1881 - We need to be grateful to Darwin, because of him earthworms have become valuable. He was fascinated and amazed by those invertebrates and spent considerable time studying them and their behavior (Darwin 1881). Kingdom Animalia Phylum Annelida Class Clitellata Order Oligochaeta Family Lumbricidae
Page | 16
1.2.1. Beneficial services of earthworms in soils
Earthworms are considered as major terrestrial ecosystem engineers for their ability to modify physical (aggregate structure, porosity), chemical (nutrient supply and cycling) and biological (soil fauna, microbial and enzymes activities) properties of the soil profile.
Effectively, due to the accumulation of their biogenic structures (casts, galleries…) they modify soil structure, porosity and aeration, and improve soil fertility and plants roots penetration. Hence, they improve water infiltration, drainage and storage in soils. Moreover, they are crucial drivers of soil organic matter dynamics by breaking down residues and debris, and regulation of the mineralization and humification processes by mixing soil layers (Rhea-fournier & González 2017; Chauhan 2014; Bhadauria & Saxena 2010; Grdisa et al. 2013;).
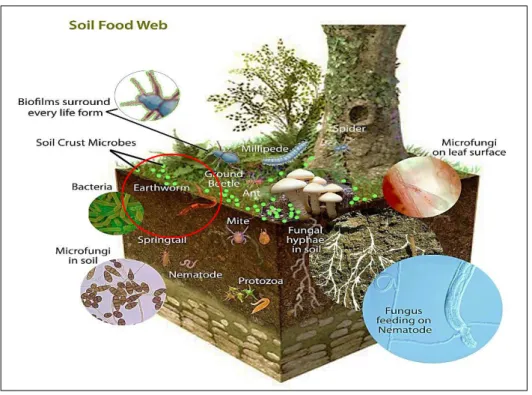
Earthworms interact with microorganisms and other living organisms in the process of soil organic material turnover, and contribute efficiently to the complex soil food web (Fig. 4). They mix debris into the mineral soil by their strong engineering effects, causing a dramatic alteration in soil properties and a redistribution of organic matter in whole profiles, which is beneficial for other organisms. However, little is known about the consequences of these engineering effects on soil food web structure (Frouz et al. 2013). Earthworms‘ feeding and burrowing activities help in the release of soil incorporated with organic matter, which afterwards, return to the soil via their casts. Casts are the excrements rejected behind the worm after digesting the soil, which has already passed through their digestive tract. It is a natural fertilizer rich in nutrients and may be deposit inside the soil or at the soil surface. Studies have documented that casts of earthworms contain higher mineral amounts of nitrogen, phosphate, potassium, sulfur, calcium and zinc, as well as, a higher microbial population and activity than the bulk soil (Haynes et al. 2003; Teng et al. 2012; Chauhan 2014).
Page | 17
Figure 4: A complex food web including earthworms and other living organism of soil. http://endofite.com/
All of these miraculous services toward the soil make earthworms worthy to study. Evidently, earthworms are presumed to be potential biological indicator to evaluate soil fertility and pollution. They can be used in agricultural practices assessing ecotoxicologial risk and monitoring program of different contaminants. This can be done by studying their abundance, species composition and biochemical and behavioral stress-biomarkers (Paoletti 1999; Lionetto et al. 2012; Haeba et al. 2013). Thence, earthworms with their activities could potentially promote ―the second green revolution‖ and spread benefits to the soil and farmers (Sinha et al. 2010).
1.2.2. Ecological groups of earthworms
To make identification easier, three ecological earthworms‘ categories have been described based on morphological, behavioral and ecological characteristics (Fig.5): epigeics, anecics and endogeics (Bouché 1977; Lee 1985; Lavelle & Spain 2001).
Page | 18
Figure 5: Earthworms niche groupings: Epigeic earthworm (Lumbricus rubellus). Endogeic earthworm (Aporrectodea caliginosa). Anecic earthworm (Lumbricus terrestris). Figure adapted from Fraser and Boag, photos of earthworms copyright Ross Gray. (Modified)
Epigeics (above the earth, surface dwellers) are small worms characterized by their highly pigmented skin usually red or brown. This coloration serves as a protection of UV rays and a camouflage from their natural predators. They live mainly on the surface of the soil in the litter horizon, feed on leaf litter and tend not to make burrows (poor burrowing ability). They play crucial role in the decomposition of leaves, other plant detritus and organic matter that falls in the land. Redworms also called manure worms, belong to this group and commonly used in the vermicomposting system. Examples: Dendrobaena octaedra, Eisenia fetida (ideal worm for vermicompost) and Lumbricus rubellus.
Anecics (out of the earth, subsoil dwellers) are among the largest varieties of earthworms that can grow up to several meters, and noted for their dark antero-dorsal pigmentation. They feed on decaying leaves and are known to emerge on the surface of the soil at night to search for food. Known as the powerful deep-dwelling class of earthworms, they migrate within the various soil strata by making vertical burrows, where they pull organic matter and decaying leaves in order to feed on them. Examples: Lumbricus terrestris, Apporectodea longa and Dendrobaena platyura
Page | 19 Endogeics (Within the earth, topsoil dwellers) are medium-sized and appear pale in color (pink, blue or grey). They lake pigmentation as they spend their entire lives in the dark beneath the ground and out of sun. Scientists know the least about their life cycle and behavior and are least recognizable to most people. In contrast to anecic earthworms, endogeic earthworms make horizontal burrows by swallowing large quantity of soil and organic matter. Crucially, they allow better plant root penetration and aeration of the soil. Examples include Allolobophora chlorotica, Apporectodea caliginosaand Pontoscolex corethrurus.
Over and above, earthworms are widely considered as precious candidates in microcosm experiments (Fründ et al. 2009). However, the epigean segmented Red wiggler species Eisenia fetida, is the mostly used as model soil invertebrates. Accordingly, it thrives widely in many habitats all around the world and it is adapted to variable climates (Wang et al. 2015; Plytycz et al. 2018). Used in ecotoxicological tests, it is also the favorite amongst earthworms in vermicomposting (Sparks 2014. Römbke et al. 2015; Hyunseong 2016). Despite the use of this species as a model, however, it is not found in mineral soil, and very uncommon in cultivated fields. Then, endogeic and anecic species such as Lumbricus terrestris and Aporrectodea caliginosa, which are more commonly found in cultivated fields, are ecologically important in terrestrial ecosystems of many temperate regions (Bouché, 1977; Bauer & Römbke 1997). This statement emphasis the need to develop analysis using ecologically relevant earthworms‘ species, as terrestrial models (Pelosi et al. 2013).
1.3. Microorganisms screening and functions in soils
Microbes can be found in almost any habitat, in every nook and cranny we could think of. They live in water, soil, digestive system of human and animals and sometimes in extreme environments. Inside our bodies, at microscopic level, a vast number of microbes colonized us. This microbiome consists of billions of microorganisms including bacteria, fungi, parasites, virus and other microbial and eukaryotic species (Schulz et al. 2013; Mora et al. 2016).
Further, a microscope is usually needed to see microorganisms, but their functions in soils are strikingly noticeable. Microorganisms play a critical role in maintaining good
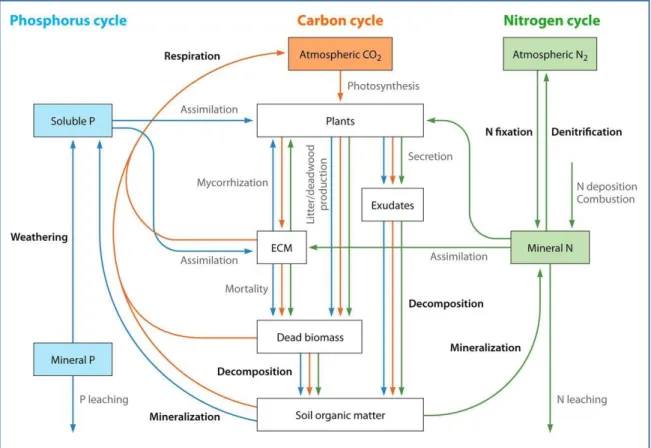
Page | 20 soil health, structure and fertility. Collectively, they intervene in the breaking down of decaying plant biomass and fungal mycelia, humification, nutrients recycling (nitrogen, phosphorus, potassium, carbon…) (Fig. 6) (Llado et al. 2017).
Figure 6: Bacterial community drivers of many ecological services in the rhizosphere, litter/deadwood, and soil compartments of the forest floor.
Connectedly, their crucial role in returning nutrients to their mineral forms helps the
plants to take up again cycling nutrients, which favor their growth. In addition to supplying nutrients, microorganisms benefit plants also by producing a variety of substances that promote plant growth, including auxins, gibberellins and antibiotic (Gyaneshwar et al. 2002; Jacoby et al. 2017). Biochemical properties of soils associated to the cycles of elements such as carbon, nitrogen, phosphorus and sulfur, are used to point out soil quality. These properties include both general and specific biochemical parameters. In effect, general parameters involve microbial biomass, dehydrogenase activity, nitrogen mineralization potential and soil respiration; specific parameters are related to the activity of hydrolytic enzymes such
Page | 21 as phosphatase, urease and β−glucosidase. Most of these processes (Fig. 7) (Llado et al. 2017) are mediated by soil enzymes, which are produced by soil microorganisms, roots and to some extend by soil animals.
Figure 7: Ecological processes mediated by bacteria (highlighted in bold) and elements transfer (C in orange, N in green and P in blue) within the coupled biogeochemical cycles of carbon, nitrogen and phosphorus in forest ecosystems.
Therefore the composition of soil biota determines the potential of the community for enzymes synthesis. Soil enzyme activities directly influence energy and nutrients transformation and cycling. This feature is associated with the presence of extracellular and intracellular enzymes, which are essential in the process of decomposition and mineralization of organic material (Dilly‘ & Nannipieri 1998; Gil-Sotres et al. 2005; Friedlova 2010).
The soil matrix is considered as a favorable niche (Lavelle & Spain 2001). Added, in a typical healthy soil, in a single gram, live and reproduce a wealth of millions of microorganisms. Identification, characterization and classification of microorganisms were generally based on their morphological, physiological and cultural characteristics. This was and still performed using conventional culture-based
Page | 22 methods. These methods do not provide prompt results, because only a small fraction (99% of microorganisms in the external environment are uncultivable) of the various microbial community can be cultured on synthetic media (Janssen et al. 2002; Stewart 2012; Ling et al. 2015). Recent molecular methods, which are more sophisticated, offer better solutions in identifying and characterizing ―uncultivable‖ microorganisms. These emerged methods continue to replace conventional culture techniques. Hence, molecular methods are increasingly incorporated in laboratories, despite being expensive, however, they are faster and offer reliable specificity in detection and identification (Rappé & Giovannoni 2003; Nichols et al. 2010; Fakruddin et al. 2013; Desai & Armstrong 2003).
It is of current concerns to understand interactions between microbes and soil components such as bacteria, fungi, roots and animals (Barea et al. 2002a). As specified above, these interactions could act either in nutrient cycling and plant growth, but also in the biological control of plant pathogens, improving soil quality (Johansson et al. 2004). Microorganism‘s biomass and activity are greatly influenced by higher trophic levels of the soil food web (Fig. 5). Despite the fact that there is evidence that soil microbial communities are linked to ecosystem functioning, the understanding of the functional importance of different groups of the soil biota and the connections between them (the member soil food web) is limited (de Vries et al. 2013). One of the interests of understanding such interactions lies in the modification of microbial enzyme activities, which could be used as a potential tool for measuring soil quality.
1.4. Factors affecting soil organisms
1.4.1. Environmental factors and soil properties
Biotic factors and climatic conditions that influence the environment and soil, can also affect the biology and life processes of living organisms including, microorganisms and earthworms.
Earthworms devour and rely on their house comprising soil, organic debris, microorganisms and other materials. Soil temperature, moisture, pH and organic matter are factors of primary importance in the regulation of earthworm‘s population, growth, reproduction and health. Earthworms do not have specialized breathing
Page | 23 system, they breathe through their skin. Adequate moisture is needed to keep their skin moist in order to facilitate their cutaneous respiration. Effectively, they take water from the ingested soil that must be moist, but not overly, unless they die suffocated. They survive in relatively low oxygen environment even when submerged in water if it contains dissolved oxygen. During global warming or drought or frost, earthworms ‗‘the coldblooded organisms‘‘, may die because of the extreme conditions. High levels of rainfall cause the drowning of earthworms, while low levels of rainfall can dry out them. In both cases, heat or cold can affect the metabolism and reproduction of earthworms. Hence, the ideal environment for earthworms to thrive is a ventilated and drained soil, optimum temperatures (range from 10 to 20°C for cool temperate species and 20 to 30°C for tropical and subtropical species) and a neutral pH range 5.0 to 8 ( Edwards & Bohen 1996; Lavelle 1988; Curry 2004; Wood 2018).
Otherwise, soil microorganisms control soil organic matter decomposition and nutrient availability, by exerting a prevailing influence on the net carbon balance of ecosystems. A growing body of literature pointed out that climate, soil features, vegetation, substrate quantity and quality and land uses are relevant determinants of the abundance, structure and activity of soil microbial community. In effect, climate change has a direct effect on soil environments and can particularly alter temperature and moisture. These variations may have profound impacts on physiology and growth of some specific groups of microbes within communities. Therefore, it is anticipated that environmental parameters and management practices may influence
the microbial community and activities in soil, but, the relationships between microbes
and these alterations have been poorly understood, and convincing data are still scarce (Zhang et al. 2005; Nielsen et al. 2010; Tsiknia et al. 2014; Wu et al. 2018).
1.4.2. Agricultural practices and xenobiotic compounds
Intensive soil management, amendment and farming practices, tillage and conventional plough affect physical, chemical, and biological characteristics of soil. These practices have considerable impact on soil biota, productivity and sustainability. While organic management tend to favor microbial and earthworms biomass and activity, conventional practices lead to nutrients leaching, soil degradation and damage severely earthworm populations and microbial communities (Sheibani & Ahangar 2013; Smeaton et al. 2003; Mathew et al. 2012 ; Wang et al. 2012). By way of illustration, many studies have documented the negative effect of
Page | 24 tillage practices and soil disturbances on earthworms, by establishing a strong relationship between earthworm‘s abundance and no-tillage system. The decrease of earthworms abundance is due to loss of nutrient as soil organic matter, but also mechanical damage, burrows destruction, changes in soil physical properties and predation (van Capelle et al. 2012; Brown 2003).
Additionally, microbial population varies between soils, and depends on soil structure, pH, moisture, redox potential and other parameters. Organic management practices are recognized to affect positively the microbial biomass, however, microbial biomass, dynamic and respiration as well as enzyme activities have been noted to be negatively affected by tillage and conventional regimes. This is due to changes in carbon inputs and availability as well as soil organic matter concentrations (Wander et al. 1995; Shannon et al. 2002; Stark et al. 2007; Kallenbach & Grandy 2011). Further, depending on their origin and fate in the water, air or soil, xenobiotic compounds represent a serious hazard to all living organisms. For instance, heavy metals in soil affect earthworms‘ population density and species depending on their ecological group. Thusly, the sensitivity of earthworms to heavy metals depended on species. Based on studies, Lumbricus rubellus, Lurnbricus castaneus and Lumbricus terrestris, were more tolerant to heavy metals than Aporrectodea rosea, Aporrectodea caliginosa and Allolobophora chlorotica, that showed a high sensitivity and were absent in sites near to smelter. This sensitivity was explained by a lower calcium secretion in their gut, due the capability of calcium to sequestrate and eliminate various metals through the chlorogogenous tissue (Spurgeon & Hopkin 1996; Nahmani et al. 2003). Concerning pesticides, many studies have investigated the effect of pesticides on earthworms (Pelosi et al. 2014). Those studies have been conducted mainly in laboratory, and shown that earthworms are impacted at all organization levels. For instance, pesticides disrupt the activity level of enzymes involved in oxidative stress (Schreck et al. 2008, Schreck et al. 2012), they cause acute toxicity to earthworms by inhibiting acetylcholinesterase and carboxylesterase activities, (Rault et al. 2008, Collange et al. 2010) leading to behavioral and physiological disturbances (Sanchez-Hernandez 2009). Field studies have shown that earthworms‘ density in orchards sprayed with organophosphates, was very low in comparison to adjacent uncultivated fields (Reinecke & Reinecke 2007). In addition, significant decreases in cholinesterase activity were found in earthworms
Page | 25 coming from the IPM (Integrated Pest Management) and conventional orchards (Denoyelle et al. 2007).
Withal, pesticides applications and other pollutants also affect soil microorganisms by decreasing their number and activity (Gianfreda & Rao 2008). Toxicants may interfere with vital function such as respiration, division and other processes. This may have undesirable impacts on non-target microorganisms. However, it was documented that it is difficult to predict the effect of pesticides on microorganisms because, some pesticides stimulate the growth of microorganisms but other decline it. Hence, in order to apprehend the effect of pesticides on soil microflora, it is necessary to inquire the concentration of the spread pollutants and the capability of the microorganism to adapt to existent pollutant or to degrade it (Lo 2010; Kalia & Gosal 2011).
Fauna and flora are not the unique inhabitants of the soil Pollutants exist also
2. Soil pollution: a global problem
Nowadays, the soil becomes a reservoir for industrial and domestic pollutants. The soil pollution is generally due to the deposition of a variety of contaminants in the atmosphere, in addition to human activities especially farming system. These pollutants are in high concentrations to be of risks to plants, animals, and humans (Fig. 8). They include metals, toxic chemicals compounds, organic and inorganic pollutants, pesticides… Once inside the soil, they can enter the food chain, threat food safety and alter significantly the soil composition, diversity and many critical functions of soils (Zalidis et al. 2002; Fabietti et al. 2010).
Page | 26
Figure 8: Pollution practices must be abandoned unless human will lose all contact with the world of instinct. https://fractalenlightenment.com/24754/issues/aggrandized-ego-alienated-soul-contesting-the-atrophy-of-instinct-in-an-age-of-anxiety
2.1. Pesticides: benefits and hazards
Pesticides represent a group of human-made chemicals covering a wide range of compounds including insecticides, fungicides, herbicides, rodenticides, molluscicides, nematicides and others. They are applying as well in residences, gardens, agricultural fields and greenhouses, in order to harm devastating pests and vector borne diseases. Hence, they contribute to the protection of crop and improve yields and productivity. For these reasons, their role has become crucially important in increasing food production, to satisfy the high food demand of the growing population density (Aktar et al. 2009; Popp et al. 2013).
However, are they harming only pests at which they are target?
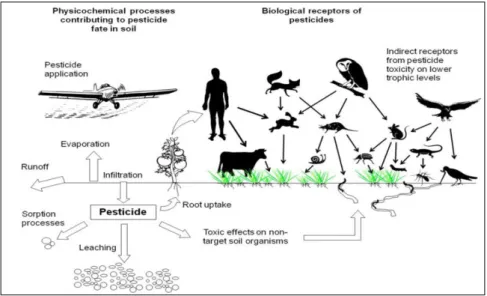
Pesticides can be applied by manual or hydraulic spraying in addition to aerial or truck based spraying techniques. They exert toxic effect to production workers, loaders, mixers, sprayers and farmers during manufacturing and formulation. When moving from the application site, pesticides can reach other ecosystems like soil and water, even downward through the soil to the groundwater (Fig. 9). Leaching, persistence and degradation of pesticides depend on their chemical and physical
Page | 27 characteristics and the transport processes. At this level, they threat target and non-target organisms including humans, mammals, fish, birds, plants, microorganisms and valuable species of soils (arthropods, earthworms…) (Kazemi et al. 2012; Carvalho 2017).
Figure 9: Main physiochemical and biological processes contributing to pesticide fate and toxicity (conceptual scheme elaborated from Köhne et al 2009, modified from Sanchez-Hernandez 2011).
According to the Pesticide Action Network (https://www.pan-europe.info) and despite the European strategies attempts to reduce pesticide inputs (Plan Ecophyto II), chemical control is still intensively used. In spite of much opinion about the sustainability of agriculture and the entering on the market of new product that can be used at low dose, the average of pesticide use did not decrease in recent years, as reported by the Eurostat on pesticide sales (Fig. 10). After a decrease observed in 2013, it can be implied that pesticide use has grievously expanded due to the increasing resistance of organisms and plants. Whilst, the initiation of organic methods and practices remained at a low level. In 2016, almost 400,000 tonnes of pesticides sold in Europe were used in the agricultural field.
Page | 28
Figure 10: Total sales of pesticides
Data are collected from Eurostat last updated on 08/08/2018. (http://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do)
Among pesticides, insecticides represent a group of highly toxic compounds and there effects on wildlife were extensively studies (Köhler & Triebskorn 2013). Their application rates are generally lower than other classes of pesticides such as fungicides or herbicides, however, they exert strong impact on non-target organisms. A particular progression of interest in the effects of neurotoxic insecticides (Table 2) such as organophosphates, pyrethroids, and neonicotinoids was observed (Köhler & Triebskorn 2013).
Table 2: Neurotoxic insecticides, mode of action and authorized doses(1) used in French apple
orchards
Chemical classes Commercial formulation
Active ingredient Mode of action Authorized dose
(1)
Pyrethroïds Decis Expert® Deltamethrin Sodium channel
modulation 0.75 g hL
-1
Carbamates Pirimor G Pirimicarb Acetylcholinesterase
inhibition 37.5g.hL
-1
Organophosphates Oleobladan®
Pyrinex® Ethyl-Parathion Chlorpyrifos Acetylcholinesterase inhibition 250g.hL
-1
50 g hL-1
Spinosyns Success4® Spinosad Acetylcholine receptor stimulation
9.6 g hL-1
Neonicotinoids Supreme® acétamipride Acetylcholine receptor
stimulation 5 g hL
Page | 29
2.2. Organophosphates insecticides
2.2.1. Mechanism of action
Organophosphate insecticides (OP) display a variety of structures and are still the most widely used including chlorpyrifos-ethyl, parathion-ethyl, malathion, diazinon... Once inside the organism, OPs are bioactivated through the cytochrome P450 mixed function oxidase enzymes. CYP450 constitute a family of enzymes mostly involved in the transformation and conversion of the P=S compound to its toxic oxon metabolite (P=O) (Fig. 11).
Figure 11: The conversion of the organophosphorus insecticides to their respective oxons
The inhibitory effect of oxon metabolites lies in a phosphorylation reaction, which creates a covalent bond with the catalytic serine group of esterase. Among this group, cholinergic enzymes called ―Cholinesterases‖ are the target of OP compounds. Hence, due to the high affinity of the active site of serine esterase, the phosphorylation induces the destruction of the OP and then, the persistent inhibition of the serine esterase lasting hours to days. Whereas, this inhibition is usually considered irreversible if a process of ―aging‖ (non enzymatically mediated dealkylation) takes place, some reactivation can occur if the aging process is not complete (Fig. 12).
Both target esterases ―acetylcholinesterase‖ and non-target (butyrylcholinesterase and carboxylesterases) are affected by oxons (Satoh & Gupta 2010).
Page | 30
Figure 12: Esterase inhibition mechanism for the organophosphate parathion. The organophosphate (parathion) is first activated via mixed-function oxidases (MFO) to the “active” oxon-form, which is the inhibitory structure of the compound. Paraoxon then binds to the esterase and is hydrolyzed in the process by the addition of water, releasing p-nitrophenol. The phosphorylated esterase can then either
release the phosphate group and regain catalytic activity, or become “aged” where the phosphate
remains permanently bound and the enzyme loses catalytic activity (from Wheelock et al 2005).
2.2.2. AChE Target of OPs : a potential biomarker
The inhibition of Cholinesterases is the most used biomarker in ecotoxicological risk assessment and pesticide exposure. Cholinesterases (ChEs) constitute a group of hydrolases that are highly sensitive to organophosphate and carbamate pesticides. Found in plasma, mammalian erythrocytes, neuromuscular junction and other organs, CHEs include two types based on their substrate specificity acetylcholinesterase (AChE), and butyrylcholinesterase (BuChE). AChE is considered critical to a normal control of nerve impulse transmission, because it catalyzes the hydrolysis of the neurotransmitter acetylcholine in the nervous system. Its inhibition leads to an accumulation of the neurotransmitter acetylcholine in the nerve endings thus, a continued stimulation of acetylcholine receptors. BuChE is found mainly in the plasma with no physiological functions assigned. In vertebrates, it appears in liver, lung, heart, at cholinergic synapses, in developing embryonic tissues and a functional role of BuChE can be found in regulation of cell proliferation (Mack & Robitzki 2000).
In addition, the activity of AChE is easy to measure and its sensitivity is dose-dependent (Lionetto et al. 2013). The classical use of AChE activity in ecotoxicology,
Page | 31 displays a wide range of methodological approaches that involve both laboratory toxicity bioassays and field monitoring surveys (Denoyelle et al. 2007; Yaqin et al. 2008; Yaquin & Hansen 2010; Otero and Kristoff, 2016). Withal, it is critical to apprehend the eventual consequences of AChE inhibition on the organism, especially at behavioral and physiological levels (Scott & Sloman 2004). For instance, several studies have shown that a direct relationship could be established between AChE inhibition and several functions including behavioral, biochemical and physiological disturbance (Carlock et al. 1999; Rickwood & Galloway 2004; Nunes 2011; Ghorab & Khalil 2015).
2.2.3. Vigourous protecting mechanisms
Generally, there are diverse detoxifying enzymatic systems that provide protection against synthetic and natural pollutants (Li et al., 2007). The biotransformation of xenobiotic occurs in two stages. An initial phase of functionalization (Phase I), consists in the addition of a functional group to the exogenous molecule to make it hydrophilic, therefore more easily excretable. In the second phase of conjugation (Phase II), a polar group is added to the functionalized or original molecule. The first phase, primarily involves CYP450 and esterases such as Carboxylesterases (CbEs), whereas the conjugation phase involves the glutathione-S-transferases (GST), which is the most important enzyme group (Li et al., 2007). Both phases increase the polarity of the xenobiotic, further processing can take place for example the excretion (phase III).
2.2.3.1. Carboxylesterases
Carboxylesterases belong to the serine-esterase family of enzymes that are widely distributed in all living organisms (animals, plants, and microorganisms). As their name suggest, these enzymes are involved in the hydrolysis of carboxyl esters into the corresponding alcohol and carboxylic acid via the addition of water (Junge 1975). Alike cholinesterases, they belong to the so-called B-esterases family. CbEs have a wide distribution and are expressed in tissues known to maintain a barrier function, and which are exposed to xenobiotics. They are found in greatest amounts in liver, epithelia of the lung and intestine, skin, kidney and brain (Wheelock et al. 2008;
Page | 32 Hatfield et al. 2017). Effectively, CbEs consist of multiple isozymes with a variable levels and activities. Their expression and activity are tissue and organism-dependent, (Hosokawa 2008; Imai 2006; Wheelock et al. 2008).
In addition, CbEs physiological function remains unclear since no real endogenous substrates have been identified (Hatfield et al 2016), whereas, these enzymes play a substantial role in the metabolism and detoxification of many xenobiotic containing ester group including, agrochemicals and pharmaceuticals (Fig. 13). In particular, carboxylesterases hydrolyze pyrethroids (Abernathy & Casida 1973; Stok et al. 2004; Wheelock et al. 2005) and bind stoichiometrically to carbamates (Gupta & Dettbarn 1993; Sogorb & Vilanova 2002) and organophosphates (Casida & Quistad 2004).
Figure 13: Interactions of esterases (cholinesterases and carboxylesterases) with carbamates (CA), the oxon metabolites of organophosphates (OP) and synthetic pyrethroids (PYR). Inhibition of esterases by CAs yields a carbamylated complex which is unstable and the esterase activity is rapidly recovered in the presence of water. Organophosphates inhibit irreversibly the hydrolysis activity of ChEs and CbEs by the formation of a stable phosphorylated complex. Under this condition, restoration of the esterase activity requires the synthesis of new enzyme. Synthetic pyrethroids interact only with CbEs, and these esterases hydrolyze them to yield the corresponding alcohol and carboxylic acid. Scheme elaborated from Sogorb & Vilanova (2002) and Thompson & Richardson (2004).
The inhibition of CbEs by OPs induces the formation of a stable enzyme-inhibitor complex, which is considered a stoichiometric mechanism for decreasing the OP concentration at the target site ―AChE‖ (Maxwell 1992; Chanda et al 1997). Then, CbEs act as bioscavenger protecting organisms from organophosphates in several organisms (Maxwell 1992; Maxwell & Brecht 2001).
PYR
Page | 33
2.2.3.2. Cytochrome P450 (CYP450)
Phase I of metabolisation is catalyzed by the CYP450 group of enzymes. As specified above, these enzymes are involved in the bioactivation of organophophates insecticides. However, The CYP gene superfamily catalyzes mono-oxygenation reactions of a wide range of xenobiotic and endogenous substrates, which highly contributes to the detoxication mechanisms in all living organisms (Ortiz de Montellano 2015). CYP450 are found across diverse range of organisms including bacteria, plants, fungi, insects and mammals. They are involved in many cases of resistance of insect to insecticides (Després et al. 2007).
2.2.3.3. Glutathion-S-Transferase (GST)
Glutathione-S-transferases (GSTs; E.C.2.5.1.18) are members of multifunctional isoenzymes. They display various activities and participate in several types of reaction. Ubiquitously distributed in nature, they are found in organisms as diverse as animals, plants, microbes, and insects. These transferases are major phase II drug metabolizing enzymes. Added, their main biological roles consist of the detoxification of harmful electrophilic endogenous and exogenous compounds as xenobiotic, thus, they protect cells from oxidative stress (Armstrong 1991). These enzymes catalyze the addition of reduced glutathione (GSH≡γ-L-glutamyl-L-cysteinyl-glycine) to xenobiotic substrates. This reaction facilitates the elimination of the molecule that becomes generally less reactive and more soluble.
Further, GSTs could be either cytosolic or membrane-bound (Jakobsson et al. 1999) and their activities have been associated to insecticides resistance (Carvalho et al. 2013; Pavlidi et al. 2018).
2.3. Impact of OP on soil organisms
2.3.1. Impact on earthworms
Pesticides that are extensively used, threat target as well as non-target organisms amongst are earthworms. Besides, earthworms that are always in direct contact with their home that includes the soil and its component, they are at a high risk to absorb
Page | 34 such agrochemicals through the skin or to ingest them with food. Many literatures have documented the negative effects of pesticides on earthworms at different levels, because of their vulnerability to such applied chemicals and their crucial role as bioindicator. Pesticides act by hundred mechanisms thus, many studies have evidenced the impact of pesticides on earthworms each one at different organization level. They induced morphological, behavioral, physiological, reproductive, nervous and osmoregulatory disorders in earthworms. They decrease biomass and density by increasing mortality of individuals and inhibiting feeding behavior, reproduction and growth. Along with, they disturb the activity of several enzymes including those immersed in oxidative stress (Dureja et al. 1999; Pelosi et al. 2014).
The first ecotoxicological studies, had mainly focused on mortality (Edwards 2004; Capowiez et al 2005), then a growing interest in the development of sublethal biomarkers pointed out the importance to study the effect on both reproduction and growth (Bauer and Römbke 1997). For example, triazine herbicide and the organophosphosphate insecticide Galition were found toxic to Eisenia fetida, due to their significant impact in the growth inhibition of this species (Milanovic et al. 2014). Moreover, two organophosphates pesticides diazinon and chlorpyrifos were reported to cause toxic effect on growth, maturation and fecundity of Aporrectodea caliginosa (Booth et al. 2000), while the OP parathion and a carbamate propoxur, impact the reproduction of Aporrectodea caliginosa,Aporrectodea longa and Eisenia fetida (Kula & Kokta 1992). In order to investigate different organization levels, changes in behavior are of growing interest to link the toxic effects at the ecosystem level (Hellou 2011). In this context, impact on weight loss and burrowing activity of a neonicotinoid insecticide such as imidacloprid, has been observed on two earthworms species: Aporrectodea nocturna (anecic) and Allolobophora icterica (endogeic) (Capowiez et al. 2005, Capowiez et al. 2006). However, according to the concept of a hierarchical cascade of biological responses to pollutants, sub-individual biomarkers should be linked to behavioral responses. OP and carbamate pesticides are a good model to test this hypothesis as their primary mechanism of acute toxicity is the inhibition of AChE activity involved in the functioning of the nervous system. In chlorpyrifos-spiked polluted soils, the avoidance response of the earthworms Lumbricus terrestris was not significant and no correlation could be established either with the dose of OP pesticide used or with AChE inhibition (Martinez-Morcillo et al. 2013). Other studies
Page | 35 have shown that the outcome of avoidance behavior response test is pesticide concentration-dependent, using the carbamate insecticide methomyl (Pereira et al. 2010). However, most of the studies concerning the impact of pesticides were conducted under laboratory at the molecular level. Eisenia fetida treated with the organophosphate insecticide malathion, recorded a genotoxicity by the alteration of the DNA structure of spermatogonia, the disruption of sperms disposition and viability accompanied by a significant decrease in the body weight (Espinoza-navarro & Bustos-obregón 2005).
Under laboratory conditions, AChE inhibition in response to OP or Carbamates pesticides has been validated in earthworms (Booth et al. 2000, Venkateswara Rao et al. 2003, Caselli et al., 2006). Some inhibitions were observed in orchards after one spraying event (Reinecke & Reinecke, 2007) or repeated treatments (Denoyelle et al., 2007) but sometimes, no effect could be obtained under field conditions (O‘Halloran et al. 1999, Booth and O‘ Halloran, 2001). Over-and-above, studies suggest that the toxicity of a same pesticide is depended on the ecological group of the worm, its behavior and feeding manners (Kammenga et al. 2000). The impact of the pesticide methyl-parathion on the growth of three different earthworms was species-specific. The substantial impact of this pesticide was observed in the anecic species Lampito mauritii and the endogeic Metaphire posthuma, in comparison with the epigeic Allolobophora parva (Suthar 2014). Finally yet importantly, organophosphates pesticides methyl-parathion, chlorpyrifos and phorate showed devastating effects on the nervous system of earthworms (Reinecke & Reinecke, 2007; Mhamane & Reddy 2014).
More recently, some laboratory studies have suggested the inclusion of CbE activity in the assessment of pesticide exposure and toxicity in these soil organisms (Collange et al. 2010; Sanchez-Hernandez & Wheelock, 2009). Many authors postulate that the sensitivity of CbE activity to both OP and Carbamates insecticides modulates the acute toxicity of these agrochemicals. Thus, CbE activity measurements in some terrestrial invertebrate such as earthworms exposed to chlorpyrifos-spiked soils showed a higher percentage of CbE inhibition than AChE activity, irrespective of the tissue used for esterase measurements (Collange et al. 2010; González Vejares et al. 2010). Changes in enzymes involved in oxidative stress have also been observed under pesticide exposure on Aporrectodea