HAL Id: hal-00342448
https://hal.archives-ouvertes.fr/hal-00342448
Submitted on 1 Jun 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Distributed under a Creative Commons Attribution| 4.0 International License
Frenkelia parasites in a small mammal community.
Dynamics of infection and effect on the host.
E. Fichet-Calvet, E. B. Kia, P. Giraudoux, J. P. Quéré, P. Delattre, R. W.
Ashford
To cite this version:
E. Fichet-Calvet, E. B. Kia, P. Giraudoux, J. P. Quéré, P. Delattre, et al.. Frenkelia parasites in a
small mammal community. Dynamics of infection and effect on the host.. Parasite, EDP Sciences,
2004, 11 (3), pp.301-10. �10.1051/parasite/2004113301�. �hal-00342448�
FRENKELIA PARASITES IN A SMALL MAMMAL COMMUNITY.
DYNAMICS OF INFECTION AND EFFECT ON THE HOST
FICHET-CALVET E.*
*****, KIA E.B.**
,****, GIRAUDOUX P.***. QUÉRÉ J.P.*, DELATTRE P.* & ASHFORD R.W.**
,Summary:
A community of small mammals, Clethrionomys glareolus, Arvicola
terreslris, Microtus avalis, M. agrestis, M. subterraneus, Apodemus
spp. and Sorex spp., was studied as hosts of Frenkelia glareoli and
F. microti in Franche-Comte (France). They were monitored in spring,
summer and autumn on an area of about 1,350 ha comprising open field, hedgerow network and forest. Among 1,714 small mammals examined between July 1992 and October 1993, 4 7 % (178/376) of C. glareolus, 9.9 % (14/139) of A. terreslris and
1.3 % (4/311) of Apodemus spp. were infected by F. glareoli. The prevalence of infection with F. microti was 9.2 % (66/716) in
M. arvalis and 8.2 % (6/73) in M, agrestis. M. subterraneus and Sorex spp. were not infected. The maintenance of each parasite in
a rural landscape is assured both by a forest and a grassland host. Multiple logistic regression showed that prevalence was highly age-dependent, with an apparent seasonal pattern. Prevalence varied between 30 % in summer and 6 0 % in early spring for F. glareoli in C. glareolus and between 3 % in autumn to 30 % in early spring for F. microti in M. arvalis. The year, habitat, host sex, relative density had no impact on prevalence. In M arvalis only, sexually active voles were preferentially uninfected, indicating a possible impact of this parasitism on fertility.
KEY WORDS : voles, population dynamics, Frenkelia spp., Coccidia,
prevalence, age effect, agroecosystem, mid-mountain.
MOTS CLÉS: campagnol, dynamique de population, Frenkelia spp., coccidie,
prevalence, effet de I'age, agroecosysteme, moyenne montagne.
Résumé :
D E S FRENKELLIA CHEZ UN PEUPLEMENT DE PETITS MAMMIFÈRES : DYNAMIQUE DE L'INFESTATION ET IMPACT SUR L'HÔTEL'infestation par Frenkelia glareoli ef F. microti a éfé étudiée au niveau d'un peuplement de petits mammifères composé de
Clethrionomys glareolus, Arvicola terreslris, Microtus arvalis, M . agrestis, M . subterraneus, Apodemus spp. et Sorex spp. Les
populations ont été suivies au printemps, en été et en automne dans un agroécosystème comprenant des champs ouverts, du bocage et de la forêt. Parmi les 1714 petits mammifères examinés entre juillet 1992 et octobre 1993. 47 % ( 1 7 8 / 3 7 6 ) des C. glareolus, 9,9 % ( 1 4 / 1 3 9 ) des A. terrestris ef 1,3 % ( 4 / 3 1 ) des Apodemus spp. étaient infestés par F. glareoli. La prévalence de F. microti était de 9,2 % 6 6 / 7 1 6 ) chez M . arvalis et 8,2 % ( 6 / 7 3 ) chez M . agrestis. Aucune infestation n'a été observée chez M . subterraneus et Sorex spp. Dans un tel paysage rural, la maintenance de chaque parasite est assurée par deux hôtes, l'un fréquentant les habitats prairiaux, l'autre les habitats forestiers. Une analyse par régression logistique multiple a montré que les prévalences sont étroitement liées à l'âge de l'hôte alors que les fluctuations saisonnières (30-60 % pour F. glareoli chez C. clethrionomys; 3-30 % pour F. microti chez M . arvalis,) de la
prévalence ne sont qu'apparentes et ne dépendent que de la structure en âge de la population hôte. L'année, l'habitat, le sexe de l'hôte et sa densité relative n'ont pas d'influence sur les prévalences. Chez M . arvalis, les individus sexuellement actifs sont préférentiellement ceux qui sont indemnes de F. microti, suggérant ainsi un possible impact de ce parasitisme sur la fertilité de ces rongeurs in natura.
INTRODUCTION
P
arasites have long b e e n c o n s i d e r e d as possible factors in the regulation o f rodent populations (Elton et al., 1 9 3 5 ) . Around 1 9 8 0 . hypothetical * Centre de biologie et de gestion des populations. Campus de Bail-larguet. Montferrier-sur-Lez, France.** Liverpool school of tropical medicine. United Kingdom. *** Biologie environnementale EA 3184 use INRA, Université de Franche-Comté. Besançon. France.
**** School of public health and Institute of public health research. Tehran University of medical sciences. Iran.
***** FRF. CNRS 269S "Origine, structure et évolution de la biodiver-sité". Laboratoire mammifères & oiseaux. Muséum national d'histoire naturelle, Paris. France
Correspondence : Dr Elisabeth Fichel-Calvet. FRE CNRS 269=5 "Ori-gine, structure et évolution de la biodiversité". Laboratoire mammi-fères & oiseaux, Muséum national d'histoire naturelle. î î , rue Buffon. 75005 Paris. France.
Tel.: 33 (6)1 40 79 30 69 - Fax : 33 (0)1 40 79 30 63. E-mail: calvet@mnhn.fr
m o d e l s w e r e produced, considering host-parasite sys-tems as a special c a s e o f predator-prey interaction (Anderson & May, 1978; Holmes, 1982). Paradoxically, very few studies in nature have tested these models. This w a s true long a g o (Wiger, 1 9 7 7 ) , and there have b e e n few relevant studies since that time. Ecological studies on the effects o f parasites o n host populations are particularly appropriate in the applied study o f the m a n a g e m e n t o f irruptive rodent s p e c i e s (Jäkel el a I.. 1999). W e have carried out a long-term study o f agri-cultural pest rodents in mid-mountain z o n e s in France (Delattre etai, 1992, 1996. 1 9 9 9 ) . and have previously linked a study o f the c e s t o d e parasites o f these rodents (Giraudoux. 1 9 9 1 ; Le Pesteur et al.. 1992). Additional results are presented here, o n the protozoan parasites
Frenkelia spp., o f the brains o f these rodents. Fren-kelia microti w a s first discovered (As "M organism",
thought to b e closely related to Toxoplasma) in Wales,
P a r a s i t e . 2 0 0 4 , 11. 3 0 1 - 3 1 0
Mémoire
3 0 1F I C H E T - C A L V E T E , K I A E . B . , G I R A U D O U X P . ET AL.
by Findlay & Middleton ( 1 9 3 4 ) , in Microtus agrestis at the time o f a population crash.
Frenkelia spp are h e t e r o x e n o u s coccidia with sexual
reproduction in the intestines o f birds o f prey, e s p e cially the Buzzard Buteo buteo, and asexual multipli cation in the brains o f rodents. T h e full life history was first described, for F. glareoli in the b a n k vole,
Cle-thrionomys glareolus, by R o m m e l & Krampitz ( 1 9 7 5 ) .
Resistant sporocysts are e x c r e t e d in the f a e c e s o f B u z zards from 7-9 days following infection, for a period of 7-57 days. Sporozoites e m e r g e from the sporocysts w h e n these are ingested by a rodent, and migrate to the brain, w h e r e they p r o d u c e cysts that are visible 17 to 18 days following infection (Geisel et al., 1 9 7 8 ; Laarman et al., 1979). Over a period o f w e e k s , the cysts g r o w to 3 5 0 um in diameter, and contain many thou sand b r a d y z o i t e s , w h i c h are infective to B u z z a r d s w h e n the rodent host is eaten. Heavily infected rodents contain n u m e r o u s cysts, w h i c h o c c u p y a considerable proportion o f all parts o f the brain, and infection lasts for the life o f the host (Tadros & Laarman, 1 9 7 6 ; Laar man et al., 1 9 7 9 ) . T h e earliest age at which the rodents can b e infected is u n k n o w n .
T w o species o f Frenkelia are known to occur in Europe,
F. glareoli, mainly in C. glareolus, and F. microti,
mainly in Microtus spp. (Tadros & Laarman, 1982). Vorisek et al. ( 1 9 9 8 ) have s h o w n evidence that infected rodents are m o r e likely to b e predated than uninfected individuals, as h a p p e n s in certain other host-parasite c o m b i n a t i o n s (review by C o m b e s , 1 9 9 5 ) . Transmission of the parasite is thereby facilitated, and the longevity o f infected rodents is reduced. R e d u c e d longevity o f s o m e individuals d o e s not necessarily have any regu latory effect o n populations. In order to assess any regulatory effect o f the parasite on intermediate host populations, information is first required o n the dis tribution o f the parasite in the host c o m m u n i t y at a local scale.
T h e aim o f this study is to test for a n y effect o f extrinsic ( y e a r , s e a s o n , h a b i t a t ) a n d intrinsic ( h o s t age, s e x a n d relative d e n s i t y ) factors o n the infec tion rates in e a c h s p e c i e s o f the rodent c o m m u n i t y . T h e n , the possibility o f an effect o f t h e parasites o n the h o s t s w a s investigated b y c o m p a r i n g the b o d y w e i g h t and s e x u a l activity o f infected a n d u n i n f e c t e d individuals.
MATERIALS A N D METHODS
STUDY SITEThe study a r e a o c c u p i e s a b o u t 1,350 ha, in Franche-Comté, 10 km north-west o f Pontarlier (47.10° N, 6.24° E, 8 5 0 m a b o v e sea l e v e l ) with m e a n annual rainfall o f 1,500 mm. T h e landscape is
Fig. 1. - L a n d s c a p e c o m p o s i t i o n o f t h e s t u d y site l o c a t e d in t h e J u r a p l a t e a u .
c o m p o s e d o f forest and agricultural land. T h e forest is mostly semi-natural, c o m p o s e d o f m i x e d b e e c h
Fagus sylvatica, o a k Quercus robur, and fir Abies alba,
and there are s o m e spruce Picea abies plantations. T h e agricultural land is either improved grassland or per m a n e n t pasture (Delattre et al, 1988; G i r a u d o u x et al.,
1 9 9 7 ) , and is either, o p e n o v e r w i d e areas ( o p e n field), or e n c l o s e d b y h e d g e r o w s in plots o f ca 1 ha (Fig. 1 ) .
T R A P P I N G A N D SAMPLING
Trapping was carried out in forest (deciduous, mixed, coniferous), h e d g e r o w network ( h e d g e , h e d g e e d g e , enclosures) and o p e n field (permanent grassland) habi tats. B e c a u s e this study is part o f a rodent survey for outbreak m a n a g e m e n t , small m a m m a l s w e r e sampled during the reproduction period: in J u l y and O c t o b e r 1992, and April, J u l y and O c t o b e r 1 9 9 3 . INRA ( F r e n c h Agronomic R e s e a r c h Institute) trap lines w e r e used (Spitz et al., 1 9 7 4 ) . Thirty-four traps w e r e placed at 3 m intervals in e a c h line o f about 100 m. T h e n u m b e r s o f lines set o n e a c h o c c a s i o n , and the distribution by habitat o f the 2 1 4 trap lines ( 2 1 , 8 2 8 trap nights) are s h o w n in T a b l e I. Traps w e r e left in p l a c e for three c o n s e c u t i v e nights, and w e r e visited twice daily. Ani mals w e r e killed b y cervical dislocation a c c o r d i n g to Mills et al. ( 1 9 9 5 ) .
3 0 2 - M é m o i r e P a r a s i t e , 2 0 0 4 , 11, 3 0 1 - 3 1 0
FRENKELIA INFECTIONS IN VOLES
T a b l e I. - D i s t r i b u t i o n o f t h e 2 1 4 trap l i n e s b y habitat a n d b y s e a s o n . A r r o w s d e s i g n t h e h a b i t a t s in w h i c h t h e r o d e n t ralative a b u n d a n c e s a r e c a l c u l a t e d . * c o r r e s p o n d s t o a forest c l e a r i n g .
H O S T POPULATION PARAMETERS
Relative a b u n d a n c e was estimated for e a c h s p e c i e s as the n u m b e r caught per trap line. For C. glareolus, c a p
tures in forest and in hedgerow network w e r e analysed separately. For Microtus arvalis, captures in h e d g e r o w network and in o p e n field w e r e analysed separately. For Microtus agrestis, which was less abundant, c a p tures in forest and in h e d g e r o w network w e r e c o m bined. Arvicola terrestris n u m b e r s w e r e not estimated as only juveniles w e r e sampled, adults being too big to enter the traps.
T h e weight o f the desiccated e y e lens (ELW) gives the best indication o f a g e for small mammals (Lord, 1959; Martinet. 1966, rev. in Morris, 1971). Eyes were removed and preserved for a minimum o f two w e e k s in 10 % formalin, then the lenses w e r e extracted, dried for two hours at 100° C, and w e i g h e d to a precision o f 0.1 mg. F e m a l e s w e r e classified as sexually active if they w e r e pregnant or lactating, as w e r e males with seminal vesicles over 4 0 m m2 (length x breadth). Litter size was
estimated by the n u m b e r o f e m b r y o s . PARASITES
Carcasses w e r e preserved in 10 % formalin before e x a mination. T h e brain w a s r e m o v e d by dissection o f the skull, and stained for at least 24 h in undiluted Semi-c h o n ' s a Semi-c e t i Semi-c Semi-c a r m i n e . T h e y w e r e then w a s h e d in dis tilled water, transferred to 1 % HC1 in 7 0 % ethanol to differentiate, until the brain material was very pale pink in c o l o u r (usually a few h o u r s ) , and placed in glycerine to clear. T h e stained, cleared brains w e r e then sliced with a scalpel and the slices w e r e e x a mined with a dissecting m i c r o s c o p e ( x 100) to detect a n y p a r a s i t e s . L o b u l a t e d cysts w e r e identified as
F. microti, and large round cysts as F. glareoli (Tadros et al., 1972; Tadros & Laarman, 1978). A few very small
round cysts w e r e regarded as unidentifiable e x c e p t in juvenile A. terrestris in w h i c h 2 / 1 0 2 w e r e Toxoplasma
gondii, and 3 / 1 0 2 w e r e Frenkelia, glareoli (Kia et al.,
in p r e s s ) .
STATISTICAL ANALYSIS
Year, season, habitat, host sex, age and relative density effects on prevalence were analysed with a multiple logistic regression using a binary factor (infected = 1, non infected = 0 ) as the dependent variable and year (two levels: 1992, 1993), season (three levels: spring, summer and autumn), habitat (three levels: open field, hedgerow network and forest), host sex (two levels), host age (continuous ELW) and host relative density (continuous abundance index) as independent variables. T h e strategy o f data treatment was first to enter all the variables in a global model, and to perform a forward stepwise regres sion to select the non redundant variables. T h e second stage was to enter these selected variables with their interactions in a restricted model as recommended by Kleinbaum & Klein ( 2 0 0 2 ) . This analysis was performed with Systat 9. SAS Institute Inc. (1999).
T h e effect o f infection on weight was analysed using ANCOVA with weight as the d e p e n d e n t variable and the host s e x and infection ( t w o levels: infected, unin fected) as the independent variables. Host a g e (conti nuous ELW) was entered as the covariate in the model. T h e effect o f infection on sexual activity was analysed using multiple logistic regression including sexual acti vity (active = 1, inactive = 0 ) as the dependent variable and the infection (two levels: infected, uninfected), year (two levels), season (three levels), habitat (three levels), host sex, host age (continuous ELW) and host relative density (continuous a b u n d a n c e i n d e x ) as the inde pendent variables. As the goal o f this analysis was to obtain a single estimate o f the Frenkelia infection, adjusted for year, s e a s o n , habitat, host sex, host age, and host relative density, the interactions w e r e not included in the model (Kleinbaum & Klein, 2 0 0 2 ) . T h e effect o f infection on fertility in each s e x was analysed using ANCOVA with seminal vesicle size or litter size as the d e p e n d e n t variable and infection ( t w o levels: infected, uninfected), season (three levels) and host age ( c o n t i n u o u s ELW) as independent variables (Legendre & Legendre, 1 9 9 8 ; Sokal & Rohlf. 1998).
P a r a s i t e . 2 0 0 4 . 11. 3 0 1 - 3 1 0
3 0 3
FICHET-CALVET E., KIA E.B., GIRAUDOUX P. ET AL.
RESULTS
H O S T RANGEO
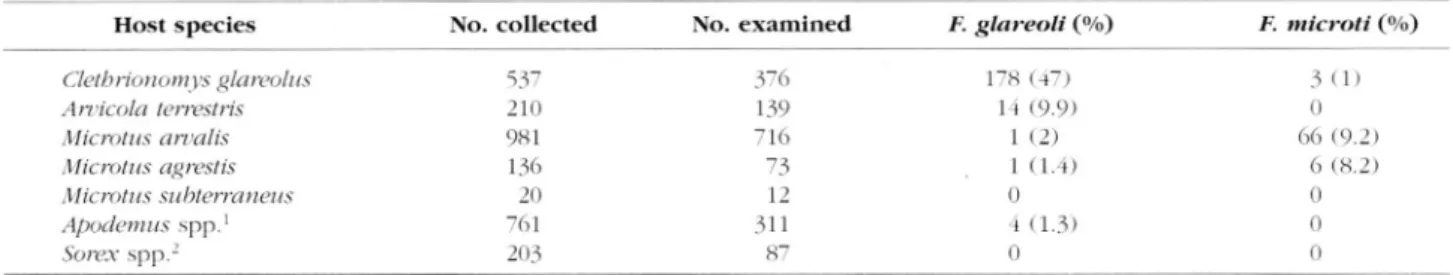
f 2 , 8 4 8 animals c o l l e c t e d , 1,714 w e r e e x a mined for Frenkelia infection (Table II). F.gla-reoli was mainly found in C. glagla-reoli, s e c o n
darily in Arvicola terrestris, and rarely in M. arvalis,
M. agrestis and Apodemus spp. F. microti was most fre
quent in .M. arvalis and M. agrestis and was also found rarely in C. glareolus. Microtus subterraneus and Sorex spp w e r e n e v e r found infected.
Further analysis is restricted to the infection in the four
most important hosts, C. glareolus, A. terrestris, M. arva
lis and M. agrestis.
PREVALENCE
• Frenkelia glareoli in Cletbrionomys glareolus
The influence o f year, season, habitat, host sex, age and relative density on prevalence was analysed in a global model by forward stepwise regression. T h e main effect on prevalence was due to host a g e ( c h i2 = 19.204, p <
0 . 0 0 0 1 ) , w h e r e a s the other factors were not significant. Host age is highly significant with an odds ratio o f 1.043 (p < 0 . 0 0 0 1 ) , indicating an increase o f prevalence with
H o s t s p e c i e s N o . c o l l e c t e d N o . e x a m i n e d F. glareoli (%) F. microti (%)
Cletbrionomys glareolus 5 3 7 3 7 6 1 7 s I R ) 3 ( 1 )
Articola terrestris 2 1 0 1 3 9 l r ( 9 . 9 ) 0
Microtus amalis 9 8 1 7 1 6 1 ( 2 ) 6 6 ( 9 . 2 )
Microtus agrestis 1 3 6 7 3 1 ( 1 . 4 ) 6 ( 8 . 2 )
Microti is su bterra net is 2 0 12 0 0
Apodemus s p p .1 7 6 1 3 1 1 -i ( 1 . 3 ) 0
Sorex s p p .2 2 0 3 8 7 0 0
1 Apodemus flat icollis p r e d o m i n a t e d , but A. sylvaticus a l s o o c c u r r e d : n o a t t e m p t w a s m a d e t o d i s t i n g u i s h j u v e n i l e s p e c i m e n s , s o b o t h s p e
c i e s a r e g r o u p e d t o g e t h e r . 2 Sorex coronatus a n d .V. araneus w e r e n o t d i s t i n g u i s h e d for t h e p r e s e n t s t u d y .
T a b l e II. - P r e v a l e n c e s a n d host r a n g e in a small m a m m a l c o m m u n i t y i n f e c t e d b y Frenkelia glareoli a n d F. microti.
Fig. 2. - P r e v a l e n c e o f Frenkelia glareoli a n d a b u n d a n c e o f its host Cletbrionomys glareolus ( n u m b e r o f c a p t u r e s p e r 1 0 0 m o f trap l i n e ) in t h e h e d g e r o w n e t w o r k a n d in t h e forest. N u m b e r s u n d e r e a c h b a r c o r r e s p o n d t o t h e r o d e n t s e x a m i n e d for i n f e c t i o n .
Frenkelia glareoli in Clethrionomys glareolus
H e d q e r o w n e t w o r k & F o r e s t
Fig. 3. - D i s t r i b u t i o n o f Frenkelia glareoli in Clethrionomys glareolus b y e y e l e n s w e i g h t (F.LW in m g ) a n d s e x o f h o s t .
age. T h e host age effect is illustrated in Figure 3 w h e r e the age structure is presented for e a c h session. Infected individuals w e r e present at each session and in e a c h ELW class over 3 mg. Prevalence remained high throu ghout the year (Fig. 2 ) with the lowest prevalence in July 1993 (32 % ) and the highest in April 1993 (62 % ) . T h e s e seasonal variations w e r e not significant w h e n host age was taken into account. T h e model explains only 4 % ( 2 5 0 . 4 7 5 - 2 4 0 . 5 7 7 / 2 5 0 . 4 7 5 ) o f the total varia tion, suggesting that m a n y o t h e r factors than host a g e explain 9 6 % o f the variation o f prevalence o f F.
gla-reoli in C. glareolus.
• Frenkelia glareoli in Arvicola terrestris
B e c a u s e A. terrestris were captured almost exclusively in enclosed grassland, the variable "habitat" was excluded from this analysis. In addition, relative density o f this spe cies was not evaluated since only juvenile specimens were caught. Among the four remaining variables, year, season, host s e x and host age (1.6 < ELW < 11.1), the multiple logistic regression shows that only the two last had an effect on prevalence (Chi2 = 3-983, p = 0.046 and
Chi2 = 21.200. p < 0.0001 respectively). Infection was
twice as c o m m o n in females ( 8 / 6 l ) than in males ( 4 / 5 8 ) .
• Frenkelia microti in Microtus arvalis
First, the forward stepwise regression showed host age, relative density, and year to b e significant variables having an effect on the Frenkelia infection. T h e other variables, season, host s e x and habitat, were not corre lated with prevalence. Host age is highly significant with an odds ratio o f 1.069 (p O.OOOl), indicating increasing infection with age. This effect is illustrated in Figure 5 where the age structure is presented for each session. Infected individuals were present at each session, with very young ones in summer with ELW between 2 and 3 mg. In autumn, the youngest infected vole had ELW over 3-5 mg. Host relative density is significant with an odds ratio o f 0.807 (p < 0.001) indicating that the pre valence o f F. microti is negatively correlated with the abundance o f its host. T h e year effect is described by an OR o f 0.635 indicating a lower prevalence in 1993 than 1992. Figure 4 shows prevalence to b e highest in spring ( 2 9 % in April 1993), when the vole population was at its lowest; prevalence declined in the breeding season (17 % in July 1992; 3 % in July 1993). reaching its lowest in autumn (7 % in O c t o b e r 1992; 3 % in O c t o b e r 1993), when the host population was at its greatest. T h e s e sea sonal variations were not significant when host age was taken into account. In the restricted model containing the main factors, host age, host relative density and year, and their interactions, the 2-way interactions, i.e., "year x host relative density" and the 3-way interaction "year x host age x host density" were significant, whereas the main factor turned to non-significant (Table III). This means
V a r i a b l e s C h i2
R e s i d u a l d e v i a n c e P
Null 2 0 8 . 8 8 2
Host a g e ( 1 . 2 < e l w < "".5) 6 0 . 0 8 4 181.277 < 0.0001 Host relative density ( 2 . 8 < ai < 1 4 . 4 ) 2 0 . 6 1 0 169.946 < 0.0001 Host a g e x host relative density 8 . 0 8 3 165.854 0.004 Y e a r x host relative density 4 . 4 6 2 1 6 3 . 8 1 5 0.035 Y e a r x host a g e x host relative density 3 . 9 6 9 1 6 1 . 9 2 2 0 . 0 4 6 Y e a r x host a g e 0 . 0 9 3 161.922 0 . - 6 1 Y e a r 0 . 0 2 8 161.922 0 . 8 6 8 T a b l e III. - Logistic r e g r e s s i o n results for Frenkelia microti i n f e c t i o n in Microtus arvalis in a h e d g e r o w n e t w o r k ( I I N W ) a n d o p e n field m o d e l . F l w = e y e l e n s w e i g h t in mg, ai = a b u n d a n c e i n d e x in n u m b e r o f v o l e s t r a p p e d p e r 1 0 0 m. n = 6 7 0 .
that these variables were not additive, and also that the combined effect o f host age and host density o n preva lence has to b e considered year by year. This restricted model explains 22 % ( 2 0 8 . 8 8 2 - 1 6 1 . 9 2 2 / 2 0 8 . 8 8 2 ) o f the total variation, suggesting that other factors are involved in Frenkelia infection in M. arvalis.
• Frenkelia microti in Microtus agrestis
As the M. agrestis sample was not large enough to segre gate captures b e t w e e n hedgerow network and forest, the data w e r e pooled, and habitat was excluded from the model. Here, the main effect on infection is ckte to host age only (Chi2 = 7.851, p = 0 . 0 0 5 ) whereas year,
season, host s e x and relative density are not significant. EFFECT OF PARASITES ON WEIGHT
AND SEXUAL ACTIVITY OF THE HOST
T o assess the possible impact o f parasitism on weight and sexLtal activity in rodents, infection in the two n u m e r o u s and well sampled hosts, C7. glareolus and
M. arvalis was analysed.
• Frenkelia glareoli in C glareolus
T a b l e IV s h o w s the effect o f F. glareoli infection on the weight o f C. glareolus with season, host s e x and
C. glareolus M. arvalls m o d e l m o d e l S o u r c e o f v a r i a t i o n F P F P Host i n f e c t i o n 0 . 0 1 9 0 . 8 8 9 0 . 5 8 3 0 . 4 4 5 H o s t s e x 9 . 8 5 7 0 . 0 0 2 1 3 . 9 9 1 0 . 0 0 0 2 S e a s o n 3 6 . 4 7 4 < 0 . 0 0 0 1 1 3 1 . 2 7 7 < 0 . 0 0 0 1 I n f e c t i o n x s e x 0 . 8 8 2 0 . 3 4 8 4 . 7 4 9 0 . 2 9 "7 I n f e c t i o n x s e a s o n 4 . 6 4 9 0 . 0 1 0 1.421 0 . 2 4 2 S e x x s e a s o n 2 6 . 0 2 4 < 0 . 0 0 0 1 4 . 6 7 2 0 . 0 0 9 I n f e c t i o n x s e x x s e a s o n 0 . 6 6 8 0 . 5 1 3 0 . 8 5 6 0 . 4 2 5 Host a g e ( e l w ) 1 8 4 . 2 1 9 < 0 . 0 0 0 1 5 4 6 . 8 0 5 < 0 . 0 0 0 1
T a b l e IV. - Intrinsic a n d e x t r i n s i c s o u r c e s o f variation in t h e b o d y w e i g h t in Clelhrlonomys glareolus i n f e c t e d with Frenkelia glared! a n d in Microlus arratis i n f e c t e d with Frenkelia microti t h r o u g h A N C O V A . H o s t a g e . e s t i m a t e d b y t h e e y e l e n s w e i g h t ( e l w ) . is e n t e r e d a s a c o v a r i a t e in e a c h m o d e l .
FRENKKUA INFECTIONS IN VOLES
P a r a s i t e . 2 0 0 4 . 7 7 . 3 0 1 - 3 1 0
FICHET-CALVET E., KIA E.B., GIRAUDOUX P. ETAL.
Fig. 4. - Prevalence of Frenkelia microti and abundance of its host Microtus arvalis (number of captures per 100 m of trap line) in the hedgerow network and in the open field habitats. Numbers under each bar cor respond to the rodents examined for infection.
Frenkelia microti in Microtus arvalis
H e d g e r o w n e t w o r k & O p e n field
Fig. 5. - D i s t r i b u t i o n o f Frenkelia microti in Microtus amalis b y e y e l e n s w e i g h t ( E L W in m g ) a n d s e x o f h o s t .
a g e taken into a c c o u n t through ANCOVA. Weight is significantly affected b y age, s e x and s e a s o n but not by Frenkelia infection. T h e two w a y interaction, infec tion x season, is significant. This is illustrated in Figure 6
Fig. 6. - M e a n b o d y w e i g h t (in g with s t a n d a r d e r r o r b a r s ) in infected a n d u n i n f e c t e d C/ethrionomys glareolus, b y s e a s o n . N u m b e r c l o s e t o e a c h s y m b o l i n d i c a t e s t h e s a m p l e s i z e .
s h o w i n g that, in summer, the infected voles are h e a vier than the uninfected o n e s .
Using multiple logistic regression, sexual activity in both s e x e s is significantly correlated with year, season, host s e x and age, but not with habitat, relative den sity or Frenkelia infection ( T a b l e V ) . For males, the length x breadth o f the seminal vesicles is highly cor related with host a g e ( F1 2 0 0 = 5 8 . 9 8 0 , p < 0 . 0 0 0 1 ) and
C. glareolus M. arvalis m o d e l m o d e l V a r i a b l e s C h i2 P C h i2 P I n f e c t i o n 0 . 2 0 0 0 . 6 5 5 6 . 2 0 5 0 . 0 1 3 Y e a r 3 . 9 8 0 0 . 0 4 6 3 2 . 9 4 0 < 0 . 0 0 0 1 S e a s o n 1 8 . 8 6 7 < 0 . 0 0 0 1 9 7 . 8 8 8 < 0 . 0 0 0 1 H a b i t a t 0 . 1 5 1 0 . 6 9 7 1 7 . 1 0 5 < 0 . 0 0 0 1 I lc >st s e x 1 6 . 0 4 4 < 0 . 0 0 0 1 0 . 4 7 0 0 . 4 9 3 H o s t a g e 1 6 . 5 7 8 < 0 . 0 0 0 1 4 2 . 7 9 1 < 0 . 0 0 0 1 H o s t r e l a t i v e d e n s i t y 0 . 1 4 5 0 . 7 0 3 1 1 . 8 0 2 0 . 0 0 0 6
T a b l e V. - L o g i s t i c r e g r e s s i o n results for s e x u a l activity in
Clethio-nomys glareolus i n f e c t e d w i t h Frenkelia glareoli a n d in Microtus arvalis i n f e c t e d with Frenkelia microti.
FRENKELIA INFECTIONS IN VOLES
s e a s o n ( F2 2 0 0 = 53-222, p < 0 . 0 0 0 1 ) but not with Fren-kelia infection ( F , 2 0 0 = 2.506, p = 0 . 1 1 5 ) . T h e r e w e r e
insufficient pregnant females in the sample to test for differences in litter size.
• Frenkelia microti in M. atvalis
T h e s a m e analysis as a b o v e s h o w s that the variation in weight o f M. ari'alis was mainly due to host a g e and s e x , and to season, but not to Frenkelia infection. T h e significant two w a y interaction, season x host sex, is due to the increased weight o f females in autumn ( T a b l e IV).
T h e sexual activity in M. atvalis also s h o w e d a multi factorial d e p e n d e n c y pattern, significantly correlated with year, season, host age, relative density, habitat and infection ( T a b l e V ) . T h e most interesting correlations are those c o n c e r n i n g habitat and Frenkelia infection. Their partial coefficients s h o w that o p e n field and infection are negatively correlated with sexual activity (r = - 0 . 1 3 1 . p < 0.0001 and r = - 0.069, p = 0 . 0 1 4 respectively). This suggests a l o w e r sexual activity in o p e n field than in h e d g e r o w network, and in infected voles than in uninfected o n e s . Seminal vesicle size was not influenced b y Frenkelia infection ( F , 3 5 9 = 0.949,
p = 0.330) but only by age ( F1 3 5 9 = 102.341, p < 0 . 0 0 0 1 )
and s e a s o n ( F ,3,9 = 22.567, p < 0 . 0 0 0 1 ) . Litter size was
related to the s e a s o n only ( F ,9 ] = 1 0 . 4 7 8 , p < 0 . 0 0 0 1 )
but not to Frenkelia infection ( F , 9 1 = 0.022, p = 0 . 8 7 1 ) .
DISCUSSION
OCCURRENCE OF FRENKELIA SPP. IN INTERMEDIATE HOSTS
T h e main host for F. glareoli is clearly C. glareolus, with almost 5 0 % p r e v a l e n c e overall. T h e other important host for this s p e c i e s is A. terrestris, w h i c h appears to b e a n e w host record. Bearing in mind the fact that only juvenile animals o f this species w e r e sampled, the
10 % prevalence is p r o b a b l y an underestimate o f the real prevalence.
Apodemus spp, Microtus arvalis and Al. agrestris are
incidental hosts, with low prevalence o f infection. A similar result was found in the Czech Republic by Vorisek et al. ( 1 9 9 8 ) . It is not clear w h e t h e r these low prevalences are due to innate resistance in most indi viduals, l o w e r e x p o s u r e to infection (unlikely for
Microtus spp, as these are infected with F. microti
w h i c h has the s a m e transmission m e c h a n i s m ) , or high mortality o f i n f e c t e d a n i m a l s . T h e main hosts o f
F. microti are confirmed to b e M. aivalis ( 9 % preva
l e n c e ) and M. agrestis ( 8 % p r e v a l e n c e ) . C. glareolus is clearly an incidental host for this parasite.
This study s h o w s that e a c h o f the Frenkelia species is maintained by two main intermediate hosts, which inhabit w o o d e d habitats such as forest or hedges in h e d g e r o w network, and grassland such as o p e n field or fields in h e d g e r o w network.
VARIATIONS IN PREVALENCE
Host a g e is the main factor influencing the prevalence o f Frenkelia in voles ( T a b l e V I ) . This positive relation has b e e n pointed out in rodents infected with many parasites such as c e s t o d e s ( B e h n k e et al., 1 9 9 3 , 1999), trematodes (Duplantier & S e n e , 2 0 0 0 ) , protozoa (Tur ner, 1 9 8 6 ) , bacteria ( G o d e l u c k el al., 1994; Fichet-Calvet et al, 2 0 0 0 ) and viruses (Mills et al, 1992). T h e s e results suggest that as the rodents age, the pro bability o f infection increases. In M. arvalis, the infec tion can o c c u r very early in its life, around 20-30 days in summer. This age was extrapolated from the FLW m e a s u r e s o f captive-bred animals (Martinet. 1 9 6 6 ) . In
C. glareolus, host a g e is the only factor correlated with
the p r e v a l e n c e o f F. glareoli w h e r e a s year and host relative density also s h o w e d a distinct influence on the prevalence o f F. microti in M. arvalis. F. microti was more prevalent in 1992 than 1 9 9 3 and during this time, the density o f M. arvalis was stable. As the buzzard population declined in 1993 (pens, obs.), it is suggested
S o u r c e P r e v a l e n c e S e x u a l a c t i v i t y S o u r c e o f v a r i a t i o n Fg in Cg Fg in At Fin in Ma Fm in Mg Cg Ma Y e a r 0 0 - ( 1 9 9 3 ) 0 - ( 1 9 9 3 ) - ( 1 9 9 3 ) S e a s o n 0 0 0 0 + ( s p r i n g & s u m m e r ) + ( s p r i n g & s u m m e r ) Habitat 0 N1 0 N1 0 - ( O p e n F i e l d ) H o s t s e x 0 + ( f e m a l e ) (1 0 + ( m a l e ) 0 H o s t a g e + + + + + + H o s t relative d e n s i t y 0 N1 - 0 0 + I n f e c t i o n 0 - ( i n f e c t e d )
T a b l e VI. - S u m m a r i z e d e f f e c t s v a r i a b l e s o n t h e p r e v a l e n c e o f Frenkelia i n f e c t i o n a n d o n s e x u a l activity. 0 = not significant, + p o s i t i v e , - n e g a t i v e . I n f o r m a t i o n b e t w e e n b r a c k e t s i n d i c a t e s w h i c h l e v e l is s o u r c e o f variation for n o m i n a l factors. Fg = Frenkelia glareoli. Fin =
Frenkelia microti. Cg = Clethrionomys glareolus, At = Articola terrestris. Ma = Microtus aivalis. Mg = Microtus agrestis. X I = n o n i n c l u d e d .
P a r a s i t e . 2 0 0 4 . 11. 3 0 1 - 3 1 0
FICHET-CALVET F... KIA E.B.. GIRAUDOUX P. ET AL
that the reduction in prevalence may b e related to the d e c r e a s e in density o f buzzards. T h e negative corre lation b e t w e e n p r e v a l e n c e and host relative density indicates that w h e n the voles are most numerous, the infection rate is lowest. T h e buzzard is a c o m m o n pre dator o f the two voles, but the density effect is not dis cernible in C. glareolus, p r o b a b l y b e c a u s e o f the rela tive stability o f their population.
Even though p r e v a l e n c e fluctuates seasonally, s e a s o n has n o impact o n p r e v a l e n c e w h e n host a g e is taken into account. In spring, w h e n the population o f C. gla
reolus and M. arvalis was at its minimum, consisting
only o f old adults that had survived the winter, pre v a l e n c e o f both Frenkelia spp. was maximal (F.
gla-reoli: 62 %, F. microti: 2 9 % ) . Infected animals w e r e
then diluted by newly born individuals b e t w e e n spring and autumn and, as the older individuals died off, ove rall p r e v a l e n c e declined (F. glareoli: 3 2 %, F. microti: 3 % ) . T h e s e findings agree with those o f Laarman et al. ( 1 9 7 9 ) in the Netherlands w h e r e , in winter and early spring, most o f b a n k voles w e r e infected w h e r e a s o n l y 2 5 - 3 0 % w e r e infected in summer. In the Czech Republic, Vorisek et al. ( 1 9 9 8 ) found a mean preva l e n c e o f 16 % in spring w h i c h is similar to that obser ved here in the C. glareolus living in forest. T h e decli ning prevalence o f F. microti b e t w e e n July and O c t o b e r is e x p l i c a b l e partly by the e x t e n s i o n o f the breeding s e a s o n into the autumn, and continuing dilution with y o u n g individuals.
T h e data suggest that F. microti and F. glareoli are equally transmitted all through the year. T h e high pre v a l e n c e o f F. glareoli in y o u n g o f b o t h C. glareolus in J u l y and A. terrestris in April, indicating a high rate o f transmission in spring and early summer, supports this hypothesis.
In M. agrestis, the prevalence o f F. microti in April 1 9 9 3 ( 2 2 % ) was greater than that observed in Finland (6 % ) in the s a m e s e a s o n (Soveri et al., 2 0 0 0 ) . In the winter of 1 9 9 2 - 1 9 9 3 , buzzards w e r e unusually abundant o n the Jura plateau, w h i c h may explain this difference. Habitat had n o impact on the prevalence o f Frenkelia spp. T h e bank voles were equally infected in hedgerow network as in the forest. T h e irregularity o f the forest boundaries and clearings m a k e the forest a m o s a i c w h e r e the permeability o f the parasite is equal to that in the h e d g e r o w network. A comparative study in a landscape with larger areas o f u n b r o k e n forest would b e necessary to s h o w any impact o f habitat o n pre valence. Infection in Microtus spp. is equally preva lent in e n c l o s e d and o p e n grassland and, here again, a larger o p e n field would b e necessary to s h o w any impact o f the habitat in relation to the behaviour o f the buzzard, w h i c h s p e n d s m o r e time on o p e n than closed habitats.
EFFECTS OF PARASITES ON HOST WEIGHT AND SEXUAL ACTIVITY
In the overall samples, w h e n animals o f all a g e s w e r e represented, and before correcting for age, infected voles o f both s p e c i e s w e r e systematically heavier than uninfected individuals (C. glareolus: 1 9 . 3 ± 3 - 1 g, n = 1 7 2 vs 1 8 . 3 ± 3 . 5 g, n = 1 9 0 ; M. arvalis: 2 1 . 6 ± 6 . 3 g, n = 6 3 vs 1 8 . 2 ± 6 . 2 g. n == 6 0 7 ) . For C7. glareoli infected by F. glareoli, this trend was particularly true in sum mer w h e n voles were reproducing. H o o g e n b o o m & Dijkstra ( 1 9 8 7 ) found a similar effect in another hete-r o x e n o u s coccidian in the m u s c l e s o f M. ahete-rvalis, Sahete-r-
Sar-cocystis cernae, in w h i c h infection was associated with
increased weight, but this study was not fully adjusted for age, and older, heavier animals are m o r e likely to be infected. W h e n the sample is restricted to similar season and age cohort, as for Psammomys obesus infec ted with Bartonella spp. or Babesia spp. in Tunisia, the weight is equal in. infected and uninfected animals (Fichet-Calvet et al, 2 0 0 0 ) . In our study, infection with
Frenkelia spp. had n o impact on b o d y weight w h e n
season, s e x and age w e r e taken into account. T h e s e last three factors are normally the main determinants of b o d y weight. More interesting are the results c o n c e r ning sexual activity, which also depends o n season and a g e ( T a b l e V I ) . Sexual activity was also d e p e n d e n t on year, with a higher probability o f inactivity in 1 9 9 3 . This lack o f sexual activity could explain w h y the
M. arvalis population crashed in 1 9 9 4 following a
period o f high density lasting three years (unpublished data). M. arvalis was less sexually active in o p e n field than in h e d g e r o w network, indicating that the crash b e g a n in o p e n field before continuing in h e d g e r o w network. Sexual activity was positively related with density, reflecting continuation o f reproduction into the autumn and an accumulation o f several cohorts b o r n in the previous spring and s u m m e r w h e n .M. arvalis was abundant. In males, the infection was not corre lated with the size o f the seminal vesicles. In pregnant females, the infection did not affect the litter size. H o w e v e r , infected individuals o f M. arvalis w e r e less sexually active than uninfected o n e s . This suggests that
F. microti m a y delay female sexual maturity. M e c h a
nisms such as a delay in the first p r e g n a n e ) or an increasing time b e t w e e n litters have b e e n s h o w n in
C. glareolus infected with c o w p o x virus in UK ( F e o r e et al, 1 9 9 7 ) . Our result is consistent with a possible
regulation o f host population by parasitism. An addi tional regulatory effect o n the intermediate host p o p u lations could operate through an increased risk o f predation leading to reduced longevity and a reduced n u m b e r o f litters produced by predated individuals. Against this, there is n o e v i d e n c e o f reduced preva l e n c e in the oldest animals, indicating that longevity is not reduced in infected individuals.
FRENKELIA INFECTIONS IN VOIES
ACKNOWLEDGEMENTS
F
inancial support o f the Franche-Comté regional council is gratefully a c k n o w l e d g e d . E. F - C b e n e fited from a grant from t h e Société d e Secours des Amis d e s S c i e n c e s (Paris). Many thanks to the Réseau d'Observation Prédateurs-Rongeurs-Environne ment for providing data o n buzzard dynamics. T h e authors are grateful t o J-M. Duplantier for his useful c o m m e n t s on the earlier version o f the manuscript.REFERENCES
ANDERSON R.M. & MAY R.M. Regulation and stability of host-parasite population interactions. I. Regulatory processes.
Journal of Animal Ecology. 1978, 47, 219-247.
BEHNKE J . M . , BARNARD C , H I R S T J . L . . MCGREGOR P . K . . GIL
BERT F. & LEWIS J.W. The prevalence and intensity of infec tion with helminth parasites in Mus spivtus from the Setubal Peninsula of Portugal. Journal of Helminthology,
1993. 67, 115-122.
BEHNKE J . M . . LEWIS J A W . MOHD ZAIN S.N. & GILBERT F . S . Hel
minth infections in Apodemus sylvaticus in southern England: interactive effects of host age, sex and year on the prevalence and abundance of infections. Journal of
Helminthology. 1999. 73, 31-44.
COMBES C. Interactions durables. Écologie et évolution du parasitisme. Masson, Paris, 1995.
DELATTRE P., PASCAL M . , LE PESTEUR M.H., GIRAUDOUX P. &
DAMANGE J . P . Caractéristiques écologiques et épidémiologi-ques de l'Echinococcus multilocularis au cours d'un cycle complet des populations d'un hôte intermédiaire (Microtus
airalis). Canadian Journal of Zoology, 1988, 66. 2740-2750. DELATTRE P., GIRAUDOUX P., BAUDRY J . , MUSARD P., TOUSSAINT M., TRUCHETET D . , STAHL P . , LAZARINE-POULE M., ARTOIS M.,
DAMANGE J . P . & QUÉRÉ J . P . Land use patterns and types of common vole (Microtus airalis) population kinetics. Agri
culture, Ecosystem and Environ ment. 1992, 39, 153-169.
DELATTRE P.. GIRAUDOUX P.. BAUDRY J . . QUÉRÉ J . P , & FICHET E.
Effect of landscape structure on Common Vole (Microtus
airalis) distribution and abundance at several space scales. Landscape Ecology, 1996, 11. 279-288.
DELATTRE P.. D E SOUSA B . , FICHET-CALVET F . , QUÉRÉ J . P . &
GIRAUDOUX P. Vole outbreaks in a landscape context: evi dence from a six year study of Microtus arvalis. Landscape
Ecology. 1999. 14. 401-4 12.
DUPLANTIER J.M. & SENT. M. Rodents as reservoir hosts in the transmission of Schistosoma mansoni in Richard-Toll. Senegal. West Africa. Journal of Helminthology, 2000. 74, 129-135.
FEORE S.M.. BENNETT M.. CHANTREY J . , JONES T.. BAXBY D . &
BEGON M. The effect of cowpox virus infection on fecun dity in bank voles and wood mice. Proceedings of the
Royal Society of London. Series B. Biological Sciences, 1997, 264, 1457-1461.
FICHET-CAIAFT E.. JO.MAA I.. BEN ISMAIL R. & ASHFORD RAW Pat
terns of infection of haemoparasites in the fat sand rat.
L'sammomys ohesus. in Tunisia, and effect on the host. Annals of Tropical Medicine and Parasitology, 2000, 94, 5 5 - 6 8 .
FINDLAY G.M. & MIDDLETON A.D. Epidemic disease among voles (Microtus) with special reference to Toxoplasma.
Journal (J'Animal Ecology. 1934, 3, 150-160.
GEISFL O.. KAISER E.. KRAMPITZ H.E. & ROMMEL M. Beitrage zum
Lebenszyklus tier Frenkelien. IV. Pathomorphologische Befunde an den organen experimentell infizierter Rotel-mâuse. Veterinarian Pathology. 1978. 15, 621-630. GIRAUDOUX P. Utilisation de l'espace par les hôtes du ténia
multiloculaire ( Echiuococcus multilocularis) : conséquen ces épidémiologiques. Ph.D. Thesis, University of Dijon, France, 1991, 106 p.
GIRAUDOUX P.. DELATTRE P.. HABERE M.. Qi i HI I.I'.. DEBLAY S.. DÉFAIT R.. DUHAMEL R.. MOISSENET M.F.. SALVI D. & TRU
CHETET D. Population dynamics of fossorial water vole
(Arrico/a terrestris scherman): a land use and landscape
perspective. Agriculture. Ecosystem and Environment, 1997.
66, 47-60.
GODELUCK B., DUPLANTIER J.M., BA K . LN TRAPE J . F . A longitu
dinal survey of Borrelia crocidurae prevalence in rodents and insectivores in Senegal. American Journal of Tropical
Medicine and Hygiene. 199a. 50, 165-168.
HOLMES J.C. Impact of infectious disease agents on the popu lation growth and geographical distribution of animals, IN: Population biology of infectious diseases. Anderson R.M. & May R.M. (eds). Springer-Yerlag. New York. 1982. 37
-51.
HOOGENBOOM I. & DIIKSTRA C. Sarcocystis cemae: a parasite increasing the risk of prédation of its intermediate host,
Microtus airalis. Oecologia, 19S7. 74. 86-92.
JAKEL. T . . KHOPRASERT Y.. ENDEPOLS S.. ARCHER-BAUMANN C . SUASA-ARD K . . PROMKERD P.. KLIFMT D . . BOONSONG P. & HON-GNARK S. Biological control of rodents using Sarcocystis sin-gaporensis. International Journal for Parasitology, 1999. 29. 1321-1330.
KIA E . B . , DELATTRE P., GIRAUDOUX P., QUÉRÉ J.P. & ASHFORD
R.W. Natural infection of water vole Airicola terrestris with
Toxoplasma gondii in Jura plateau, eastern France. Annals of Tropical Medicine and Parasitology (in press).
KLEINBAUM D.G. (N; KLEIN M. Logistic regression. A self-lear ning text. 2 Edn. Springer-Verlag, New York, 2002.
LAARMAN J.J.. TADROS \ W & MARKS J . Studies on frenkeliosis
and Frenkelia-induced coccidiosis in the Netherlands. Tro
pical and Geographical Medicine, 1979, 31, 167-168.
LFGENDRE P. & LEGENDRE L. Numerical Ecology. Elsevier, Ams terdam. 1998.
LE PESTFI R M.H.. GIRAUDOUX P.. DELATTRE P.. DAMANGE J.P. ix
QUÉRÉ J.P. Spatiotemporal distribution of four species of cestodes in a landscape of mid-altitude mountains (Jura. France). Annales de Parasitologic Humaine et Comparée, 1992. 67. 155-160.
L O R D R.D. The lens as an indicator of age in cottontail rab
bits. Journal of Wildlife Management, 1959, 23, 359-360. MARTINET L. Détermination de l'âge chez le Campagnol des champs (Microtus arvalis (Pallas)) par la pesée du cristal lin. Mammalia. 1966. 30. 425-430.
P a r a s i t e . 2 0 0 4 , 11 3 0 1 - 3 1 0
309
F I C H E T - C A L V E T E . , K I A E . B . . G I R A U D O U X P. ET AL.
MILLS J.N., ELLIS B.A., MCKEE K.T.. CALDERON G . F . . MAIZTEGUI J.I., NELSON G . O . , KSIAZEK T . G . , PETERS C.J. & CHILDS J . A
lon-gituninal study of Junin virus activity in the rodent reser-voir of Argentine hemorrhagic fever. American Journal of
Tropical Medicine and Hygiene. 1992. 47, 749-763. MILLS J.N.. CHILDS J . , KSIAZEK T.G.. PETERS C.J. & VELLECA W.M.
Methods for trapping and sampling small mammals for virologie testing. Centers for Disease Control and Preven-tion. Atlanta. 1995.
MORRIS P. A review of mammalian age determination methods.
Mammal Review, 1971, 2, 69-104.
ROMMEL M. & KRAMPITZ H.E. Beiträge zum Lebenszyklus der Frenkelien. I. Die Identität von Isospora buteonis aus dem Mäusebussard mit einer Frenkelienart (F.
clethrionomyo-bnteonis spec, n.) aus der Rötelmaus. Berliner und Mün-chener Tierärztliche Wochenschrift. 1975, 88, 338-340. SOKAL R. R. & ROHLF F./. Biometry. W.H. Freeman & Co,
New-York. 1998.
SO\ERI T., HENTTONEN FL, RUDBÄCK E., SCHILDT R., TANSKANEN R., HUSU-KALLIO J . , HAUKISALMI V . , SUKURA A. & LAAKKONEN J .
Disease patterns in field and bank vole populations during a cyclic decline in central Finland. Comparative
Immu-nology Microbiology and Infectious Diseases, 2000, 23.
73-89.
SPITZ F., LE LOUARN H., POULET A. & DASSONVILLE B .
Standardi-sation des piégeages en ligne pour quelques espèces de rongeurs. Revue d'Écologie (Terre et Vie). 1974, 24. 564-578.
TADROS W . , BIRD R.G. & ELLIS D.S. The fine structure of cysts of Frenkelia (the M-organism). Folia Parasitológica, Praha. 1972. 19, 203-209.
TADROS W . & LAARMAN J.J. Sarcocystis and related coccidian parasites: a brief general review, together with a discus-sion on some biological aspects of their life cycles and a new proposal for their classification. Acta Leidensia. 1 976 . 44. 1-107.
TADROS W. & LAARMAN J.J. Apparent congenital transmission of Frenkelia (Coccidiai: Eimeriidae): first recorded inci-dence. Zeitschrift für Parasitenkunde, 1978, 58, 41-46. TADROS W . & LAARMAN J.J. Current concepts on the biology,
evolution and taxonomy of tissue cyst-forming eimeriid coccidia. Advances in Parasitology, 1982. 20, 293-468.
TURNER C.M.R. Seasonal and age distribution of Babesia,
Hepatozoon, Tiypanosoma and Grahamella species in Clethrionomys glareolus and Apodemus sylvaticus
popu-lations. Parasitology. 1986. 93. 279-289.
VORÍSEK P.. VOTYPKA J . . ZVÁRA K . & SYOBODOVÁ M.
Heteroxe-nous coccidia increase the prédation risk of parasitized rodents. Parasitology, 1998, 117, 521-524.
WIGER R. Some pathological effects of endoparasites on rodents with special reference to the population ecology of microtines. Oikos. 1977. 29. 598-606.
Reçu le 11 décembre 2003 Accepté le 16 juin 2004