HAL Id: hal-02628350
https://hal.inrae.fr/hal-02628350
Submitted on 26 May 2020
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Shusheng Zhu, Jean-Benoit Morel
To cite this version:
Shusheng Zhu, Jean-Benoit Morel. Molecular mechanisms underlying microbial disease control in intercropping. Molecular Plant-Microbe Interactions, American Phytopathological Society, 2019, 32 (1), pp.20-24. �10.1094/MPMI-03-18-0058-CR�. �hal-02628350�
Version postprint
Molecular
mechanisms
underlying
microbial
disease
control
in
1
intercropping
2 3
ZHU S1,2 and MOREL JB3*
4 5
1
State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan 6
Agricultural University, Kunming, Yunnan, China 7
2
Key Laboratory of Agro-Biodiversity and Pest Management of Education Ministry of China, 8
Yunnan Agricultural University, Kunming, Yunnan, China 9
3
BGPI, INRA, CIRAD, SupAgro, Univ. Montpellier, Montpellier, France 10
*: corresponding author: jeanbenoit.morel@inra.fr, Phone +33499624838, Fax
11 +33499624822 12 13 Summary 14
Many reports indicate that intercropping, which usually consists in growing two species next 15
to each other, reduces the incidence of microbial diseases. Besides mechanisms operating at 16
the field level like inoculum dilution, there is recent evidence that plant-centered mechanisms 17
with identified plant molecules and pathways are also involved. First, plants may trigger the 18
induction of resistance in neighboring plants by the well-known mechanism of induced 19
resistance. Second, molecules produced by one plant, either above or below ground, can 20
directly inhibit pathogens or indirectly trigger resistance through the induction of the plant 21
immune system in neighboring plants. Third, competition for resources like light or nutrients 22
may indirectly modify the expression of the plant immune system. The conceptual 23
frameworks of non-kin/stranger recognition and competition may be useful to further 24
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
investigate the molecular mechanisms underlying crop protection in interspecific plant 25
mixtures. 26
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
Introduction 27
Economic, societal and environmental concerns are imposing changes in our agriculture 28
models. In particular, there is a global trend towards re-introducing diversity in our agro-29
systems since it has long been shown to increase plant health (Kessing and Ostfeld, 2016; 30
Mundt, 2002). Intercropping is a subset of diverse cropping systems that provide multiple 31
eco-systemic effects including disease control (Gaba et al., 2015). Several definitions of 32
intercropping can be found and here we will consider the cases where two crops from 33
different species are grown in close proximity, at the same time. Intercropping includes strips 34
of different crops as well as complete, intermingled mixtures of several species. The 35
beneficial effects of intercropping systems on insect resistance have been reviewed 36
(Ratnadass et al., 2012) and are not discussed here. Yield in intercropping systems is often 37
more elevated than in the corresponding monoculture (Li et al., 2014) and this is strongly 38
connected to a reduction of microbial disease (Li et al., 2009). 39
Among 206 studies representing more than 240 unique intercrop-disease combinations, 73% 40
showed reduced impact of disease and only 7% showed an increase (Boudreau, 2013). For 41
instance, wheat-faba bean intercropping reduced powdery mildew on wheat by 49% (Chen et 42
al., 2007) and Ascochyta blight was reduced by 82% on faba beans intercropped with triticale
43
(Fernández-Aparicio et al., 2010). These values obtained in field studies highlight the strong 44
potential of intercropping for disease control. 45
As proposed by Boudreau (2013), a “theoretical framework based on a mechanistic 46
understanding [could] allow a more methodical and efficient designer intercrop strategy”. 47
Many factors beyond plant level interactions have been proposed to explain enhanced 48
resistance seen in intercropping systems (Boudreau, 2013; Gaba et al., 2015; Ratnadass et al., 49
2012) and are out of scope of this review. They include inoculum dilution, spore dispersal 50
interference (Figure 1A) and micro-environmental modifications. The scope of this review is 51
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
to examine reports on plant-centered mechanisms that could be responsible for reduced 52
susceptibility to microbial diseases in intercropping systems of annual crops. We propose a 53
simple framework involving plant-derived signals (including light and exudates) and the plant 54
immune system (understood here as the molecular responses normally triggered upon 55
infection) as the key players to explain pathogen suppression in intercropping (Figure 1B). 56
57
Disease reduction through pathogen inhibition by allelochemicals 58
Plant allelochemicals, some of those having antimicrobial properties, result from exudation, 59
decomposition, leaching or volatilization (Weir et al., 2004; Massalah et al., 2017). In 60
intercropping systems, while pathogens adapted to one plant species can overcome host 61
chemical barriers, adapted pathogens cannot. Antimicrobial compounds released by non-62
host plants can help neighboring host plants to suppress disease. Indeed, there is increasing 63
evidence from intercropping systems that root exudates released by non-host plants play an 64
important role in suppression of plant pathogens. Here we illustrate this phenomenon with 65
examples for soil-borne fungi, oomycete, bacteria, and nematodes. 66
For fungal soil-borne pathogens, root exudates of intercropped species can protect 67
neighboring crop plants by directly inhibiting spore germination and mycelial growth, thus 68
reducing pathogen populations in the soil. This mechanism has been documented in many 69
systems with soil-borne fungal pathogens, such as Fusarium spp (Hao et al., 2010), 70
Verticillium dalhiae (Fu et al., 2015), and Cylindrocladium parasiticum (Gao et al., 2014).
71
Allelochemicals, especially phenolic acids (such as p-coumaric acid and cinnamic acid), have 72
been described as major antifungal chemicals in root exudates (Hao et al., 2010; Gao et al., 73
2014; Ren et al., 2008). 74
Plant-parasitic nematodes can also be inhibited by root exudates from non-host plants. For 75
instance, some Asteraceae species have been shown to suppress plant-parasitic nematodes 76
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
when intercropped with susceptible crops (for reviews Tsay et al., 2004). Various nematicidal 77
compounds produced by Asteraceae plants, such as thiophenes and thiarubrines, have been 78
shown to negatively affect plant-parasitic nematodes and thus help neighbor plants in 79
intercropping system by reducing nematode population sizes (Tsay et al., 2004). In addition to 80
direct allelochemical effects on the nematode life cycle, some root exudate compounds 81
produced by non-host plants can reduce nematode damage by modifying the behavior of the 82
nematode. For example, lauric acid in crown daisy (Chrysanthemum coronarium) root 83
exudates can attract Meloidogyne incognita and induce nematode death at low concentration, 84
while it repels the nematode from roots at higher concentration. This pattern results from the 85
interference of lauric acid with the expression of a nematode gene encoding a neuromodulator 86
peptide involved in chemotaxis (Dong et al., 2014). Solanum sisymbriifolium, produces high 87
levels of hatching agents, and is a resistant trap crop for potato cyst nematodes (Globodera 88
spp.) (Timmermans et al., 2010; Dias et al., 2012). This plant is an excellent candidate to 89
intercrop with a nematode susceptible host for potato cyst nematode population suppression. 90
For soil-borne Oomycete pathogens, some non-host plant roots can attract zoospores that later 91
are killed by antimicrobial secreted substances against which these zoospores are not adapted. 92
This mechanism has been well studied for Phytophthora disease control in the maize-pepper 93
intercropping system. Non-host maize plants could form a “root wall” below the ground that 94
restricts the spread of zoospores in the field and thus indirectly protect host pepper plants. The 95
maize roots attract zoospores of P. capsici into the “root wall” and simultaneously secrete 96
antimicrobial compounds, such as 2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H) 97
(DIMBOA), that kill zoospores (Yang et al., 2014); this strategy has been termed “attract and 98
kill”. It differs slightly from the push-pull mechanism for insect control in intercropping 99
where one plant repels the insect towards another attractive one (where it remains) (Ratnadass 100
et al., 2012). This “attract and kill” phenomenon has been found for several plant species
101
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
including rapeseed (Fang et al., 2016), garlic, Chinese chive and fennel (Zhu S, unpublished 102
data). Many plants could be selected as potential intercropping species for the management of 103
soil-borne Oomycete pathogens. The existence of this strategy has not been reported in 104
intercropping systems for bacterial disease suppression. 105
There are a limited number of volatile organic compounds (VOCs) that can directly inhibit 106
pathogen growth (references in Heil and Karban, 2010). For instance, several volatile 107
molecules produced by Chinese chives could inhibit Fusarium oxysporum f. sp cubense, a 108
soil-borne pathogen of banana (Zhang et al., 2013). Whether VOCs can directly inhibit the 109
growth of foliar pathogens in intercropping remains unexplored. 110
111
Modification of the plant immune system by neighbors 112
In contrast to the direct inhibition of pathogens by allelochemicals described above, some 113
mechanisms involved in intercropping require the participation of plant immune responses. 114
Two major mechanisms, further illustrated below, could lead to the induction of resistance 115
towards microbes in intercropping systems. First, a pathogen adapted to one plant may land 116
on a non-host, neighboring plant, and activate the plant immune system. Second, a molecule 117
(e.g. root exudate) and/or a perturbation of the environment (e.g. shade or competition for 118
nutrients) by one plant could trigger the activation of an immune response in a neighboring 119
plant. We provide below several examples illustrating these mechanisms. 120
Induction of the plant immune system by non-adapted pathogens produced by neighbors 121
Induced resistance has been documented in varietal mixtures where different genotypes of the 122
same species are grown in the same field (Mundt, 2002). The proposed mechanism is that the 123
virulent, adapted pathogen will multiply on the susceptible hosts, and disseminate spores to 124
genetically resistant neighbors where they will induce the plant immune system that will be 125
effective against another pathogen yet adapted to this plant. In the case of intercropping two 126
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
plant species (rather than distinct varieties of the same crop), resistance could be induced by 127
pathogen propagules produced by the susceptible species landing on the neighbor non-host 128
species. Non-host induced resistance has been shown for different pathogens in the context of 129
intercropping (Ding et al., 2015). When this mechanism is at play, the resistance of one crop 130
relies on the susceptibility of the neighboring one. This contrasts with the simultaneous 131
reduction of disease on two intercropped species; as exemplified by the reduction of both leaf 132
blight on potato and northern leaf blight on maize when these two plants were intercropped 133
(Li et al., 2009). However, this mechanism could operate belowground; for instance one may 134
speculate that a plant recruiting a specific microbial community could trigger in the 135
neighboring plant an enhancement of the plant immune system. 136
Modification of the expression of the plant immune system in intercropping situations 137
Recent studies provide evidence that (i) growing two plants from different species next to 138
each other affects the expression of markers related to the plant immune system, and that (ii) 139
crop protection is observed when adapted pathogens challenge such plants. When watermelon 140
was intercropped with wheat, Phenylalanine Ammonia Lyase (PAL; involved in the 141
biosynthesis of secondary metabolites) activity was higher and the induction of several 142
defense-related genes was enhanced upon infection compared to watermelon grown alone (Xu 143
et al., 2015). This correlated with a strong reduction of disease incidence associated with the
144
soil-borne pathogen Fusarium oxysporum on watermelon. When intercropped with onion, 145
tomato showed an enhanced induction of genes involved in secondary metabolites synthesis 146
and responses to biotic stress (as measured by RNA-Seq). This molecular response correlated 147
with a decrease of wilt on tomato caused by the soil-borne pathogen Verticillium dalhiae (Fu 148
et al., 2015).
149
Partial control of the pathogenic fungus Cylindrocladium parasiticum can be achieved on 150
soybean by intercropping with maize (Gao et al., 2014). In this situation, soybean 151
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
pathogenesis-related (PR) genes were induced to higher levels after infection when maize and 152
soybean root interacted with each other. No significant change in expression of these defense-153
related genes was observed when root contact was prevented. This suggests that a diffusible 154
signal from maize roots induced the expression of most PR genes tested as well as the PAL 155
gene in soybean roots. Quite interestingly, exudates from maize (but not from soybean) were 156
shown to contain salicylic acid, a potent inducer of defense-genes and systemic acquired 157
resistance (SAR; Fu and Dong, 2013), making this compound a good candidate for being part 158
of the signal. 159
There are, however, few studies identifying molecules responsible for the modification of 160
expression of the plant immune system in intercropping. Intercropping maize and pepper can 161
reduce blight on pepper roots caused by Phytophthora capsici (Yang et al., 2014) as well as 162
foliar disease on maize caused by the necrotrophic fungal pathogen Bipolaris maydis (Ding et 163
al., 2015). More specifically, root exudates from healthy pepper could reduce lesions caused
164
by B. maydis on maize plants (Ding et al., 2015). The expression of marker genes from 165
several defense pathways related to the plant immune system was measured in roots and 166
shoots of healthy maize plants treated with exudates from healthy pepper. An induction of the 167
AOS (Allene Oxide Synthetase) and AOC (Allene Oxide Cyclase) genes involved in the
168
biosynthesis of jasmonic acid was detected in maize roots. Although jasmonic acid was not 169
directly measured, these results are consistent with the role of this molecule in defense against 170
necrotrophic pathogens (Campos et al., 2014). A slight induction of the genes involved in 171
DIMBOA biosynthesis was also observed in maize plants pre-treated with pepper exudates. 172
This correlated with the accumulation of this secondary metabolite in the roots and shoots of 173
maize plants. DIMBOA and its major derivative were later shown to have an antimicrobial 174
activity on B. maydis in vitro. Thus, an unknown element of root exudates from pepper could 175
trigger some sort of systemic acquired resistance in maize. Interestingly, besides its 176
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
antimicrobial function, DIMBOA and some of its derivatives were shown to affect histone 177
deacetylases and subsequently the transcription of several hundreds of plant genes in 178
Arabidopsis, among which a large set are known to be involved in response to biotic stress
179
(Venturelli et al., 2015). Therefore, one can speculate that some molecule(s) from pepper 180
exudates activate defense-related genes in maize roots and the production of molecules, 181
including DIMBOA, which would exert their effects through direct antimicrobial activity (as 182
in Yang et al., 2014) and indirectly through the further activation of defense-related genes. 183
This example suggests that molecules known for their allelopathic effects on microbes (see 184
above) could also affect the plant immune system. More recently, it was shown that p-185
coumaric acid secreted by rice roots could inhibit the germination of fungal spores and 186
constitutively induce PR gene expression in watermelon, conferring protection against F. 187
oxysporum when directly applied to watermelon (Ren et al., 2008; 2016). Likewise, a large
188
set of potent inducers of the plant immune system like salicylic acid (Khorassni et al., 2011) 189
and jasmonic acid (Strehmel et al., 2014) have been found in root exudates. 190
Finally, VOCs emitted by neighboring plants could also play a role in intercropping by 191
modifying plant resistance (reviewed in Heil and Karban, 2010). However, the majority of the 192
reports on VOCs in intercropping deal with the induction of defense against insects and there 193
are only few reports on the role of VOCs in intercropping against microbial diseases (e.g. 194
Gomez-Rodrıguez et al., 2003). 195
Stranger recognition may change the expression of the plant immune system 196
Several studies investigating plant adaptation to non-kin/stranger recognition also provide 197
evidence that expression of plant immune system can be influenced by neighboring plants. 198
Stranger recognition, which is well-known in animals, is defined as the ability to differentiate 199
related individuals from non-related ones either within species (conspecific) or between 200
species (heterospecific) (Biedrzycki and Bais, 2010). The latter situation best represents what 201
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
is found in intercropping situations. In Arabidopsis, transcriptome analysis of roots in 202
conspecific competition experiments involving different ecotypes (a situation in between 203
conspecific and heterospecific competition) identified four categories of genes: transporters, 204
auxin-related, secondary metabolite and pathogen response genes that differed among 205
treatments (Biedrzycki et al., 2011). In particular, it was shown that PR genes were more 206
highly expressed in the case of plants grown next to non-kin. However, this over-induction of 207
defense was not correlated with reduced susceptibility to the bacterium Pseudomonas tomato. 208
When examining analysis of heterospecific competition, a global trend emerges with respect 209
to its effects on the expression of the plant immune system. A pioneer study by Schmidt and 210
Baldwin (2006) showed that several defense genes were expressed to higher levels in 211
Solanum nigrum plants cultivated with various interspecific competitors compared to those
212
grown alone. Broz et al (2010) showed that growing strangers (Festuca idahoensis) in the 213
neighborhood of Centaurea maculosa constitutively modified the production of secondary 214
metabolites associated with defense against pests compared to the situation where only C. 215
maculosa plants were grown. However, it was hard to conclude from this study whether
216
intercropping had a direct impact on herbivore resistance in both species. Arabidopsis plants 217
grown together with the weak plant competitor Hieracium pilosella were shown to express 218
higher defense-related genes like PRs in leaves than when grown with congeners, and the 219
overall expression pattern remarkably resembled that observed upon oomycete infection 220
(although no oomycete was detected in these experiments; Schmid et al., 2013). Jasmonic 221
acid-dependent defense seems to be affected by heterospecific competition: in soybean/canola 222
competition experiments, a negative regulator of JA was down-regulated by competition 223
(Horvath et al., 2015) while JA biosynthesis genes were induced in maize/canola competition 224
experiments (Moriles et al., 2012). This somehow mirrors the observation made in the case of 225
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
maize/pepper intercropping presented above where root exudates from pepper could mimic 226
the effects of intercropping (Ding et al., 2015). 227
The mechanisms behind non-kin/stranger recognition in plants have been little studied but 228
unidentified root exudates seem to play a role in Arabidopsis (Biedrzycki and Bais, 2010). It 229
is likely that some of the allelopathic compounds produced by roots and known to participate 230
in competition may also be responsible for the modification of defense in intercropping. For 231
instance, the volatile alpha-pinene, produced by the roots of many plant species when 232
competing with other species, was shown to increase H2O2 production and the activity of
233
several enzymes related to the oxidative burst (Singh et al., 2006), which is also involved in 234
the response to pathogens. Since alpha-pinene was initially described as a potent inhibitor of 235
root growth required for plant-plant competition, this example suggests that the detrimental 236
effects of plant-plant competition may also indirectly contribute to an enhancement of the 237
plant immune system and therefore to an ecologically positive effect (by protecting their 238
neighbors). 239
Relationships between nutrition, light and disease in intercropping 240
Intercropping is well-known to improve yield and this effect is in part due to improved 241
nutrition. For instance, the uptake of P and micro-nutrients like Fe, Zn and Mn is improved in 242
several intercropping systems (Li et al., 2014). On the other hand, mineral nutrition is 243
paramount for plant health and competition for nutrients may strongly impact plant 244
physiology (Dordas, 2009). One may thus expect indirect effects on plant health in 245
intercropping through plant nutrition. For instance, increasing P, Fe, Zn or Mn reduces in 246
most cases disease susceptibility (Dordas, 2009), consistent with disease suppression in 247
intercropping systems. Yet, in the case of nitrogen, there is a paradox between its improved 248
uptake in intercropping and the reduction of disease (Chen et al., 2007). Indeed, for most 249
obligate pathogens, nitrogen increases plant susceptibility (Dordas, 2009). For instance, the 250
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
increase of N in wheat leaves intercropped with faba bean was associated with a reduction 251
(and not an increase) of disease caused by powdery mildew on wheat (Chen et al., 2007). The 252
mechanisms that allow increased N content without increasing disease thus merit further 253
investigation. 254
Other mechanisms linking plant defense to competition-related pathways may also occur in 255
intercropping. For instance, shade avoidance, one of plant’s responses to competition for light, 256
is in part regulated by the ratio of red and far-red light perceived by plants, a ratio that can be 257
affected in intercropping situations (Zhu et al., 2014). Shade-avoidance has been shown to 258
affect the expression of many classical defense-related genes and this was associated with 259
modifications of the susceptibility of plants to various pathogens (reviewed in Ballaré et al., 260 2012). 261 262 Future challenges 263
Intercropping thus seems to directly and indirectly affect the plant immune system. Although 264
only few molecules in root exudates have been shown to induce the plant immune system, 265
there is yet no proof that they are responsible for the increased protection observed in 266
intercropping. Mutants for the production of these molecules will be required to test this 267
hypothesis. It remains difficult to assess whether intercropping is priming the plant immune 268
system, enabling plants to over-react only upon pathogen attack or constitutively activating 269
strong defenses. This question is critical to answer, as both strategies are likely to have 270
different effects of plant fitness and thus yield. 271
Identifying the molecular mechanisms underlying disease reduction in intercropping 272
represents a major technical challenge. Indeed, as shown above, competition effects are at 273
play in most experimental systems and are thus difficult to exclude from the equation. 274
Researchers should always keep in mind that the plant immune system and competition are 275
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
intermingled by several means; measuring traits associated with these two biological 276
processes is needed in experimental systems designed to study intercropping. Some technical 277
solutions have also been developed in the field of allelopathy (He et al., 2012) and may be 278
useful to establish the respective roles of molecular communication and competition. 279
Many studies cited in this review were conducted under controlled laboratory conditions. 280
There is thus a need to demonstrate the relevance of these mechanisms in the field. There are 281
indeed only a limited number of examples indicating that patterns observed in controlled 282
conditions translate into the field. For instance, Fu et al (2015) used the manual inoculation 283
with Verticillium dahlia of tomato intercropped with onion to alleviate possible effects from 284
“root wall” or inhibition of the pathogen. An enhanced induction of defense could be shown 285
in the field and it explained as much as 36% of the disease reduction. More field experiments 286
with controlled inoculation should be performed to test whether plant-centered mechanisms 287
(Figure 1B) significantly contribute to disease reduction in intercropping and differentiate 288
these from the other effects operating at the field level in such agronomical systems (Figure 289
1A). 290
Communication in plants has received increased attention in recent decades (Heil and Karban, 291
2010). However, several communication channels and molecules are still unexplored, and 292
their roles in disease reduction in intercropping have been poorly investigated. Volatile 293
organic compounds released by plants can induce and prime plant defense against pathogens 294
(Pierick et al., 2014). Beyond pest control, the participation of such molecules in disease 295
control in intercropping against microbes remains to be evaluated. 296
Different crops may select and attract specific microbes by secreting specific root exudates 297
and, therefore, alter the composition and diversity of microbial communities in the 298
rhizosphere. For instance, the exudates of maize, one of the most popular crops used in 299
intercropping, have been shown to attract some plant-beneficial bacteria (Neal et al., 2012). In 300
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
the watermelon/rice intercropping system, strong changes of microbial communities were 301
observed, with bacterial populations increasing by more than two-fold in watermelon 302
rhizosphere intercropped with rice (Ren et al., 2008). However, the link between these 303
changes in microbial community and disease protection in intercropping remains to be 304
established. 305
As it is clear that some species mixtures work better for protecting their neighbors than others 306
(see for instance Fernandez-Aparicio et al., 2010; Boudreau, 2013), it will be important to 307
investigate the potential of a large combination of plant species to provide additional solutions 308
for crop protection. Understanding why disease severity can sometimes increase in 309
intercropping will also be important to improve this agronomic practice. 310
311
Acknowledgements 312
We are thankful to Dr HUANG H, Dr LIU Y and Dr FREVILLE H for critical reading, Pr K. 313
PERRY for proofreading this manuscript and the anonymous reviewers. This work was 314
partially funded by a CASDAR project (BURRITOS), the CONTACT project supported by 315
the French ANR program “Investissement d’Avenir” ANR-10-LABX-0001-01, a CAFEA 316
exchange grant (GDT20165300009) to J-B M and the Natural Science Foundation of China 317
(31760535). We apologize for the many studies that could not be cited in this review because 318
of size limitation. 319
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
References 320
- Badri DV, De-la-Peña C, Lei Z, Manter DK, Chaparro JM, Guimarães RL, Sumner LW, 321
Vivanco JM (2012). Root secreted metabolites and proteins are involved in the early events of 322
plant-plant recognition prior to competition. PLoS ONE 7, e46640. 323
324
- Ballaré CL, Mazza CA, Austin AT, Pierik R (2012). Canopy light and plant health. Plant 325
Physiol. 160(1):145-55. 326
327
- Biedrzycki ML, Bais HP (2010). Kin recognition in plants: a mysterious behavior unsolved. 328
J Exp. Bot. 61(15):4123-8. 329
330
- Biedrzycki ML, Venkatachalam L and Bais HP (2011). Transcriptome analysis of 331
Arabidopsis thaliana plants in response to kin and stranger recognition. Plant Signal Behav 332
6(10):1515-24. 333
334
- Boudreau MA (2013). Diseases in intercropping systems. Annu Rev Phytopathol. 51(1):499-335
519. 336
337
- Broz AK, Broeckling CD, De-la-Peña C, Lewis MR, Greene E, Callaway RM, Sumner LW, 338
Vivanco JM (2010). Plant neighbor identity influences plant biochemistry and physiology 339
related to defense. BMC Plant Biol. 10(1):115. 340
341
- Campos ML, Kang JH, Howe GA (2014). Jasmonate-triggered plant immunity. J Chem Ecol. 342
40(7):657-75. 343
344
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
345
- Chen Y, Zhang F, Tang L, Zheng Y, Li Y, Christie P, Li L (2007). Wheat powdery mildew 346
and foliar N concentrations as influenced by N fertilization and below ground interactions 347
with intercropped faba bean. Plant Soil 291(1-2):1–13. 348
349
- Dias MC, Conceição IL, Abrantes I, Cunha MJ (2012). Solanum sisymbriifolium, - a new 350
approach for the management of plant-parasitic nematodes. Eur. J. Plant Pathol. 133(1): 171-9. 351
352
- Ding X, Yang M, Huang H, Chuan Y, He X, Li C, Zhu Y, Zhu S (2015). Priming maize 353
resistance by its neighbors: activating 1,4-benzoxazine-3-ones synthesis and defense gene 354
expression to alleviate leaf disease. Front Plant Sci. 6:830. 355
356
- Dong L, Li X, Huang L, Gao Y, Zhong L, Zheng Y, Zuo Y (2014). Lauric acid in crown 357
daisy root exudate potently regulates root-knot nematode chemotaxis and disrupts Mi-flp-18 358
expression to block infection. J Exp Bot. 65(1):131-41. 359
360
- Dordas C (2009). Role of nutrients in controlling plant diseases in sustainable agriculture. A 361
review. Agron. Sustain. Dev. 28(1):33–46 362
363
- Fang Y, Zhang L, Jiao Y, Liao J, Luo L, Ji S, Li J, Dai K, Zhu S and Yang M (2016). 364
Tobacco rotated with rapeseed for soil-borne Phytophthora pathogen biocontrol: mediated by 365
rapeseed root exudates. Front. Microbiol. 7:894. 366
367
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
- Fernandez-Aparicio M, Amri M, Kharrat M, Rubiales D (2010). Intercropping reduces 368
Mycosphaerella pinodes severity and delays upward progress on the pea plant. Crop Prot. 369
29(7):744–50. 370
371
- Fu ZQ, Dong X (2013). Systemic acquired resistance: turning local infection into global 372
defense. Annu. Rev. Plant Biol. 64(1):839-63. 373
374
- Fu X, Wu X, Zhou X, Liu S, Shen Y, Wu F (2015). Companion cropping with potato onion 375
enhances the disease resistance of tomato against Verticillium dahlia. Front. Plant Sci. 6: 726. 376
377
- Gao X, Wu M, Xu R, Wang X, Pan R, Kim H, Liao H (2014). Root interactions in a maize/ 378
soybean intercropping system control soybean soil-borne disease, red crown rot. PLoS ONE 379
9(5): e95031. 380
381
- Gaba S, Lescourret F, Boudsocq S, Enjalbert J, Hinsinger P, Journet EP, Navas ML, Wery J, 382
Louarn G, Malézieux E, Pelzer E, Prudent M, Ozier-Lafontaine H (2015). Multiple cropping 383
systems as drivers for providing multiple ecosystem services: from concepts to design. Agron. 384
Sustain. Dev. 35(2):607-23. 385
386
- Gomez-Rodrıguez O, Zavaleta-Mejıa E, Gonzalez-Hernandez VA, Livera-Munoz M, 387
Cardenas-Soriano E (2003). Allelopathy and microclimatic modification of intercropping with 388
marigold on tomato early blight disease development. Field Crops Research 83: 27–34. 389
390
- Hao W, Ren L, Ran W, Shen Q (2010). Allelopathic effects of root exudates from 391
watermelon and rice plants on Fusarium oxysporum f.sp. niveum. Plant Soil 336(1-2):485–97. 392
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
393
- He HB, Wang HB, Fang CX, Lin ZH, Yu ZM, Lin WX (2012). Separation of allelopathy 394
from resource competition using rice/barnyardgrass mixed-cultures. PLoS One. 7(5):e37201. 395
396
- Heil M, Karban R (2010). Explaining evolution of plant communication by airborne signals. 397
Trends Ecol Evol. 25(3):137-44. 398
399
- Horvath D, Hansen SA, Moriles-Miller JP, Pierik P, Yan C, Clay DE, Scheffler B and Clay 400
SA (2015) RNAseq reveals weed-induced PIF3-like as a candidate target to manipulate weed 401
stress response in soybean. New Phytol. 207(1): 196–210. 402
403
- Khorassani R, Hettwer U, Ratzinger A, Steingrobe B, Karlovsky P, Claassen N (2011). 388 404
Citramalic acid and salicylic acid in sugar beet root exudates solubilize soil phosphorus. BMC 405
Plant Biol. 11, 121. 406
407
- Keesing F and Ostfeld R (2016). Is biodiversity good for your health? Science 349 (6245): 408
235-236. 409
410
- Li C, He X, Zhu S, Zhou H, Wang Y, Li Y, Yang J, Fan J, Yang J, Wang G, Long Y, Xu J, 411
Tang Y, Zhao G, Yang J, Liu L, Sun Y, Xie Y, Wang H, Zhu Y (2009). Crop diversity for 412
yield increase. PLoS One. 4(11):e8049. 413
414
- Li L, Tilman D, Lambers H, Zhang FS (2014). Plant diversity and overyielding: insights 415
from belowground facilitation of intercropping in agriculture. New Phytol. 203(1):63-9. 416
417
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
- Massalha H, Korenblum E, Tholl 2, Aharoni A (2017). Small molecules below-ground: the 418
role of specialized metabolites in the rhizosphere. Plant J. 90(4): 788-807. 419
420
- Moriles J, Hansen S, Horvath DP, Reicks G, Clay DE and Clay SA (2012) Microarray and 421
growth analyses identify differences and similarities of early corn response to weeds, shade, 422
and nitrogen stress. Weed Science 60(2): 158–66. 423
424
- Mundt CC (2002). Use of multiline cultivars and cultivar mixtures for disease management. 425
Annu Rev Phytopathol. 40(4):381-410. 426
427
- Neal AL, Ahmad S, Gordon-Weeks R, Ton J (2012). Benzoxazinoids in root exudates of 428
maize attract Pseudomonas putida to the rhizosphere. PLoS ONE 7(4): e35498. 429
430
- Pierik R, Ballaré CL, Dicke M (2014). Ecology of plant volatiles: taking a plant community 431
perspective. Plant Cell Environ. 37(8):1845-53. 432
433
- Ratnadass A, Fernandes P, Avelino J, Habib R (2012). Plant species diversity for sustainable 434
management of crop pests and diseases in agroecosystems: a review. Agron. Sustain. Dev. 435
32(1):273–303. 436
437
- Ren L, Shiming S, Xingming Y, Yangchun X, Qiwei H, Qirong S (2008). Intercropping 438
with aerobic rice suppressed Fusarium wilt in watermelon. Soil Biol. Biochem. 40(3):834–44. 439
440
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
- Ren L, Huo H, Zhang F, Hao W, Xiao L, Dong C, Xu G (2016). The components of rice and 441
watermelon root exudates and their effects on pathogenic fungus and watermelon defense. 442
Plant Signal Behav. 11(6):e1187357. 443
444
- Schmid C, Bauer S, Müller B, Bartelheimer M (2013). Belowground neighbor perception in 445
Arabidopsis thaliana studied by transcriptome analysis: roots of Hieracium pilosella cause 446
biotic stress. Front Plant Sci. 4:296. 447
448
- Schmidt DD and Baldwin IT (2006) Transcriptional responses of Solanum nigrum to methyl 449
jasmonate and competition: A glasshouse and field study. Functional Ecology 20(3): 500–8. 450
451
- Singh HP, Batish DR, Kaur S, Arora K, Kohli RK (2006). Alpha-Pinene inhibits growth and 452
induces oxidative stress in roots. Ann Bot. 98(6):1261-9. 453
454
- Strehmel N, Böttcher C, Schmidt S, Scheel D (2014). Profiling of secondary metabolites in 455
root exudates of Arabidopsis thaliana. Phytochemistry 108: 35–46. 456
457
- Timmermans BGH, Vos J, Van Nieuwburg J, Stomph TJ,Van der PEL, Molendijk PG 458
(2010). Field performance of Solanum sisymbriifolium, a trap crop for potato cyst nematodes. 459
ii. Root characteristics. Ann. Appl. Biol. 150(1):99-106. 460
461
- Tsay TT, Wu ST, Lin YY (2004). Evaluation of Asteraceae plants for control of 462
Meloidogyne incognita. J. Nematol. 36(1):36–41. 463
464
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
- Venturelli S, Belz RG, Kämper A, Berger A, von Horn K, Wegner A, Böcker A, Zabulon G, 465
Langenecker T, Kohlbacher O, Barneche F, Weigel D, Lauer UM, Bitzer M, Becker C (2015). 466
Plants release precursors of histone deacetylase inhibitors to suppress growth of competitors. 467
Plant Cell. 27(11):3175-89. 468
469
- Weir TL, Park SW, Vivanco JM (2004). Biochemical and physiological mechanisms 470
mediated by allelochemicals. Curr Opin Plant Biol. 7(4):472-9. 471
472
- Xu W, Wang Z, Wu F (2015). Companion cropping with wheat increases resistance to 473
Fusarium wilt in watermelon and the roles of root exudates in watermelon root growth. Physio 474
Mol. Plant Pathol. 90:12-20. 475
476
- Yang M, Zhang Y, Qi L, Mei X, Liao J, Ding X, Deng W, Fan L, He X, Vivanco JM, Li C, 477
Zhu Y, Zhu S (2014). Plant-plant-microbe mechanisms involved in soil-borne disease 478
suppression on a maize and pepper intercropping system. PLoS One. 9(12):e115052. 479
480
- Zhang H, Mallik A, Zeng RS (2013). Control of Panama disease of banana by rotating and 481
intercropping with Chinese chive (Allium tuberosum Rottler): role of plant volatiles. J Chem 482
Ecol. 39(2):243-52. 483
484
- Zhu J, Vos J, van der Werf W, van der Putten PE, Evers JB (2014). Early competition 485
shapes maize whole-plant development in mixed stands. J Exp Bot. 65(2):641-53. 486
487 488 489
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
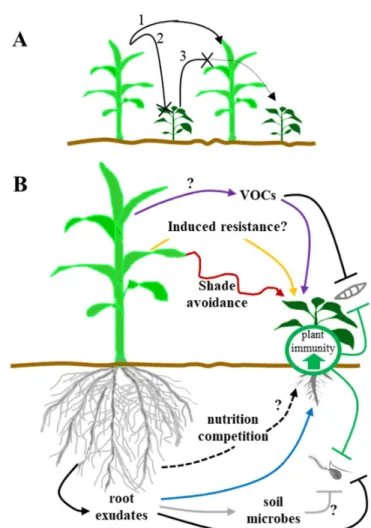
490 491 492 493 494 495 496 497Figure 1. Field and plant-centered mechanisms underlying microbial disease 498
suppression in intercropping systems. 499
A maize and pepper plant system was chosen to illustrate field (A) and plant-centered (B) 500
mechanisms responsible for the reduction of microbial diseases in intercropping. (A) At the 501
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
field level, a pathogen on a first host species (maize) disseminates to a healthy, neighboring 502
host plant (1) with lesser efficiency because of inoculum dilution (2) caused by the 503
intercropped non-host plant (pepper) and also microclimatic changes. On the other hand, a 504
non-host plant species (maize) may create a physical barrier reducing the dispersal of 505
pathogen from host to host (3). (B) Three types of plant-centered mechanisms can contribute 506
to the modification of the plant immune system in intercropping systems: induced resistance, 507
indirect changes in the expression of the plant immune system through competition for 508
resources (e.g. shade avoidance), and direct and indirect effects of the production of 509
molecules (including allelopathic compounds). Above ground mechanisms include induced 510
resistance by non-adapated pathogen (yellow arrow), production of volatile organic 511
compounds (VOCs) that could activate the plant immune system (purple arrows) and/or 512
inhibit the growth of airborne pathogens (black line) and effects like shade-avoidance (red 513
arrow). Below ground mechanisms can be direct with inhibition of pathogens (black line) by 514
root exudates from non-host plant; root exudates can also directly affect the plant immune 515
system of host plants (blue arrow) or affect soil microbial community that may have 516
detrimental effects on pathogens (grey arrows). Nutrition/competition effects (e.g. 517
growth/defense trade-off) can also indirectly affect plant the plant immune system (black 518
dashed arrows). Only mechanisms triggered by a non-host plant (maize) and increasing 519
resistance on host plant (pepper) are shown but reciprocal effects exist. Question marks point 520
to poorly documented mechanisms. Examples are provided in main text. 521
522
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
Figure 1. Field and plant-centered mechanisms underlying microbial disease suppression in intercropping systems.
A maize and pepper plant system was chosen to illustrate field (A) and plant-centered (B) mechanisms responsible for the reduction of microbial diseases in intercropping. (A) At the field level, a pathogen on a
first host species (maize) disseminates to a healthy, neighboring host plant (1) with lesser efficiency because of inoculum dilution (2) caused by the intercropped non-host plant (pepper) and also microclimatic
changes. On the other hand, a non-host plant species (maize) may create a physical barrier reducing the dispersal of pathogen from host to host (3). (B) Three types of plant-centered mechanisms can contribute to the modification of the plant immune system in intercropping systems: induced resistance, indirect changes in the expression of the plant immune system through competition for resources (e.g. shade avoidance),
and direct and indirect effects of the production of molecules (including allelopathic compounds). Above ground mechanisms include induced resistance by non-adapated pathogen (yellow arrow), production of volatile organic compounds (VOCs) that could activate the plant immune system (purple arrows) and/or
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018
Version postprint
inhibit the growth of airborne pathogens (black line) and effects like shade-avoidance (red arrow). Below ground mechanisms can be direct with inhibition of pathogens (black line) by root exudates from non-host
plant; root exudates can also directly affect the plant immune system of host plants (blue arrow) or affect soil microbial community that may have detrimental effects on pathogens (grey arrows).
Nutrition/competition effects (e.g. growth/defense trade-off) can also indirectly affect plant the plant immune system (black dashed arrows). Only mechanisms triggered by a non-host plant (maize) and increasing resistance on host plant (pepper) are shown but reciprocal effects exist. Question marks point to
poorly documented mechanisms. Examples are provided in main text. 93x133mm (150 x 150 DPI)
Molecular Plant-Microbe Interactions "First Look" paper • http://dx.doi.org/10.1094/MPMI-03-18-0058-CR • posted 07/11/2018