Matrix metalloproteinase-9 (gelatinase B) is selectively elevated in CSF during relapses and stable phases of multiple sclerosis
Texte intégral
(2) 2328. D. Leppert et al.. pathogenesis of multiple sclerosis (i) in cellular invasion and disruption of the blood–brain barrier, and (ii) in myelin breakdown. The MMPs are a family of zinc-containing endopeptidases with overlapping substrate affinities against extracellular matrix components (Yong et al., 1998). Earlier studies of MMPs in the CSF of multiple sclerosis patients were restricted to methods that allowed only semiquantitative detection of MMP-9 and -2, while other MMPs and TIMPs could not be studied. Increased levels of MMP-9 were detected in the CSF of 40–64% of multiple sclerosis patients, whereas MMP-2 was expressed constitutively (Gijbels et al., 1992; Paemen et al., 1994). However, the expression of MMPs in CSF has not been associated with any specific underlying form of multiple sclerosis (relapsing–remitting versus primary progressive) or with clinical disease activity. In this report we quantitatively measured four different MMPs (MMP-2, -3, -7 and -9) and two TIMPs (TIMP-1 and -2) in CSF of patients with relapsing–remitting multiple sclerosis and primary progressive multiple sclerosis, and correlated the levels of expression with clinical and laboratory indices of disease activity.. Material and methods Patients Fifty-two patients (33 women, 19 men) with clinically definite or laboratory-supported definite multiple sclerosis (Poser et al., 1983) were included in the study. Seven patients had primary progressive multiple sclerosis and 45 had relapsing– remitting multiple sclerosis. Of the latter group, 22 patients had a spinal tap during a clinical relapse and 23 patients had one during a stable phase of the disease, according to the criteria previously described (Poser et al., 1983; Miller et al., 1991). At the time of lumbar puncture none of the patients with relapsing–remitting multiple sclerosis were in a secondary progressive state. Information on the presence of oligoclonal bands in CSF was available for 49 patients; 88% (43/49) were positive. None of the patients had received corticosteroids or other immunosuppressive agents within 6 weeks prior to the lumbar puncture. For each patient, clinical status was assessed with the expanded disability status scale (EDSS) (Kurtzke et al., 1983); clinical assessments were carried out by observers blinded to the CSF data.. ELISA (enzyme-linked immunosorbent assay) for MMP-9 The murine monoclonal capture antibody 4H3 (Cossins et al., 1997) was raised against recombinant human MMP-9 (expressed in CHO cells) by British Biotech (Oxford, UK). This antibody recognizes both the pro- and the active form of the enzyme and is now commercially available from R&D Systems Europe (Abingdon, UK). Ninety-six-well plates (Maxisorb; Nunc, Roskilde, Denmark) were coated with purified antibody 4H3 (2.5 µg/ml) in 0.05 M carbonate/ bicarbonate buffer (pH 9.6) for 16 h at 4°C. After three washes with PBS (phosphate-buffered saline) (without Mg21 and Ca21), plates were blocked with 1% BSA (bovine serum albumin) in PBS for 1 h at 4°C. The plates were washed three times using PBS containing 0.1% Tween 20. Undiluted CSF samples were added to duplicate wells. A standard curve was derived from recombinant MMP-9 in twofold dilutions from 50 to 0.78 ng/ml in PBS/1% BSA/0.1% Tween 20. Positive controls consisted of purified recombinant MMPs and normal serum diluted 1 : 25. After three washes with PBS/0.1% Tween 20, plates were incubated with peroxidaseconjugated sheep anti-human MMP-9 polyclonal antibody (0.35 µg/ml) for 1 h at room temperature. Plates were washed three times and then incubated with TMBlue substrate (Universal Biologicals, London, UK) for 8 min at room temperature. All incubation and washing steps were performed with 100 µl/well. The reaction was stopped using 50 µl/well of 1.0 N HCl, and the absorbance was measured using a microplate reader (Titertek MS2) at 450 nm with a reference wavelength of 620 nm. The detection limit was 0.03 ng/ml.. ELISA for MMP-7 Materials, volumes and procedures were identical to those used for the ELISA of MMP-9, except for the antibodies used. The monoclonal capture antibody 7E4 against human MMP-7 was used at 40 µg/ml. A peroxidase-conjugated sheep anti-human MMP-7 polyclonal antibody (0.1 µg/ml) was used for detection. The detection limit was 23 ng/ml.. ELISAs for MMP-2 and MMP-3 Controls Twelve CSF samples from patients with the following diseases were used as controls: degenerative retinal diseases (4); cervical myelopathy (1); narcolepsy (1); migraine (3); posttraumatic epilepsy (1); trigeminal neuralgia (1); and aortic valve disease (1).. Pre-launch kits for MMP-2 and MMP-3 (detection limits were 0.51 and 0.055 ng/ml, respectively) were a gift from R&D Systems and were used according to the manufacturer’s recommendations.. ELISAs for TIMP-1 and TIMP-2 Assays After routine analyses, centrifuged CSF samples were frozen and stored at –70°C. All assays were performed with firsttime thawed aliquots and analysed blindly.. ELISAs for TIMP-1 and TIMP-2 (detection limits were 1.25 and 3.0 ng/ml, respectively) were purchased from Amersham (Little Chalfont, UK) and used according to the manufacturer’s recommendations..
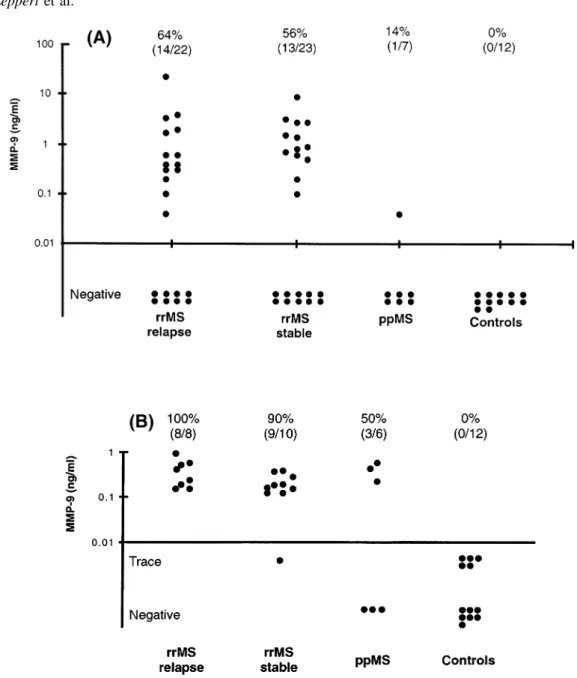
(3) MMP-9 in multiple sclerosis CSF. 2329. Zymography MMP activity in CSF was determined as previously described (Leppert et al., 1996). Briefly, SDS (sodium dodecyl sulphate)–polyacrylamide gels were copolymerized with 1 mg/ml of type A gelatin derived from porcine skin (Sigma; G-8150). Ten microlitres of CSF or standard supernatant (see below) was electrophoresed under non-reducing conditions. After electrophoresis, the gel was incubated in 2% Triton X-100 for 2 3 30 min to remove SDS, and then incubated overnight at 37°C in buffer (150 mM NaCl, 50 mM Tris– HCl, pH 7.6, containing 5 mM CaCl2 and 0.02% NaN3). After staining with 0.5% Coomassie blue G-250 (Sigma), proteolytic activity was identified as a clear band on a blue background. Gels were dried between cellophane sheets with a GelAir dryer (Bio-Rad, Hercules, Calif., USA) and then scanned with a yellow filter using Adobe Photoshop (Adobe Systems, Mountain View, Calif., USA) in grey-scale mode. Densitometric analysis of zymographic lysis zones at 92 kDa was performed using a CS1 image documentation system with Wincam 2.2 software (Cybertech, Berlin, Germany). The standard curve for densitometric quantitation of MMP-9 was derived from serum-free supernatant of shorttime cultured T-cells (Fig. 1B) (Leppert et al., 1995). The stock concentration of supernatants was measured by ELISA. Correlations between zymographic lysis and concentrations of MMP-9 in diluted supernatant were calculated using a sigmoid curve-fitting program (Delta Graph Pro3; Monterey, Calif., USA). Figure 1A shows the zymographic dose– response curve for standard MMP-9 at 92 kDa.. Statistical analysis Data were analysed using non-parametric statistical tests. Spearman rank correlations of levels of MMP-9 with CSF indices and TIMP-1 were calculated. Concentrations of MMP-9 and of EDSS and CSF indices within subgroups of patients were compared using the Mann–Whitney U test. ELISA concentrations of MMPs and TIMPs in the CSF of multiple sclerosis and control patients were compared using the Kruskal–Wallis test. P values , 0.05 were considered significant.. Results MMP-9 is elevated in CSF of relapsing– remitting and primary progressive multiple sclerosis The levels of MMP-9 measured by ELISA are shown in Fig. 2A. 64% (14/22) of CSF samples taken during an acute phase (‘relapse’) of relapsing–remitting multiple sclerosis were positive for MMP-9, compared with 56% (13/23) of samples obtained during a clinically stable period (‘stable’) of relapsing–remitting multiple sclerosis. The levels were only slightly higher in relapses (mean 6 SD, 2.56 6 5.73 ng/ml; median, 0.5 ng/ml) than in stable patients (1.85 6. Fig. 1 Densitometric quantitation of zymographed MMP-9. (A) A sigmoid correlation between the amounts of MMP-9 and zymographic lysis zones, as measured by arbitrary square units on the original zymograph, is shown. It was obtained using defined quantities of MMP-9 (see Material and methods). (B) Zymography used for calculations in (A). The image has been inverted so that lysis zones appear as dark bands on a light background. Bands for 15 and 30 pg/ml of MMP-9 were digitally enhanced to allow visualization on photographic prints.. 2.29 ng/ml; median, 0.8 ng/ml); this difference was not significant (P 5 0.35). Only one out of seven CSF samples from primary progressive multiple sclerosis patients showed detectable amounts of MMP-9, and controls (n 5 12) were all negative by ELISA. All CSF samples scoring negative in the ELISA were subsequently analysed by gelatin zymography (Fig. 2B). This method is more sensitive than ELISA (Kolb et al., 1998) for the detection of MMP-9 (detection threshold for biological samples, 10 pg/ml versus 30 pg/ml in the ELISA). This is probably due to the electrophoretic separation, which reveals low concentrations of enzymes that are undetectable in solution. The narrow dynamic range of densitometry (Fig. 1A) and the fact that complexed and degraded forms of MMP-9 cannot be quantitated make zymography only a semiquantitative technique, and therefore results cannot be compared directly with those from ELISA. Nonetheless, we have established that the two techniques are roughly.
(4) 2330. D. Leppert et al.. Fig. 2 Quantitation of MMP-9 in CSF by ELISA and zymography. (A) CSF samples were first analysed by ELISA. The detection limit of the ELISA, defined as 2 SDs above the zero dose optical density, was determined as .0.03 ng/ml. (B) CSF samples that were negative for MMP-9 by ELISA were subjected to zymography. Lysis zones at 92 kDa were quantitated by densitometry and indexed with the values from standard MMP-9 as described in Material and methods (Fig. 1). Lysis zones between 10 and 125 pg/ml could be identified readily by visual inspection, but fell below the dynamic measuring range in the densitometry (Fig. 1B) and were therefore labelled as ‘trace amounts’. Percentage values indicate the number of CSF samples with quantifiable amounts of MMP-9.. comparable at MMP-9 concentrations that are above the threshold for detection by ELISA (data not shown). In lower ranges (15–125 pg/ml) MMP-9 could readily be identified by visual inspection (Fig. 1B) but could not be quantitated by densitometry (classified as ‘trace’ amounts); 5 out of 12 (42%) control CSFs fell into this category. Figure 2B shows that MMP-9 was detectable by zymography in all CSF samples from relapsing–remitting multiple sclerosis. When the ELISA and zymography data were combined, quantifiable MMP-9 was present in 98% (44 out of 45) of relapsing– remitting multiple sclerosis CSF samples (from both acute. and stable patients) and 57% (4 out of 7) of samples from primary progressive multiple sclerosis. In contrast, controls were negative for quantifiable MMP-9.. Correlation of MMP concentrations as measured by ELISA with clinical and CSF parameters in relapsing–remitting multiple sclerosis Patients were divided into two groups according to the detectability of MMP-9 by ELISA. Patients who scored.
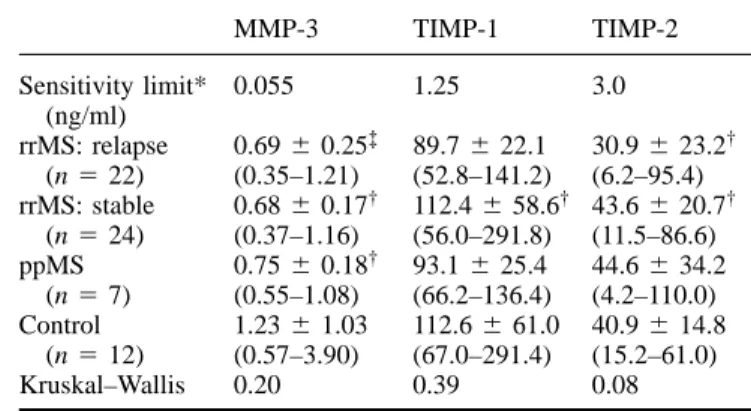
(5) MMP-9 in multiple sclerosis CSF. 2331. Table 1 Concentrations of MMP-3, TIMP-1 and TIMP-2 in CSF of multiple sclerosis patients. Sensitivity limit* (ng/ml) rrMS: relapse (n 5 22) rrMS: stable (n 5 24) ppMS (n 5 7) Control (n 5 12) Kruskal–Wallis. MMP-3. TIMP-1. TIMP-2. 0.055. 1.25. 3.0. 0.69 6 0.25‡ (0.35–1.21) 0.68 6 0.17† (0.37–1.16) 0.75 6 0.18† (0.55–1.08) 1.23 6 1.03 (0.57–3.90) 0.20. 89.7 6 22.1 (52.8–141.2) 112.4 6 58.6† (56.0–291.8) 93.1 6 25.4 (66.2–136.4) 112.6 6 61.0 (67.0–291.4) 0.39. 30.9 6 23.2† (6.2–95.4) 43.6 6 20.7† (11.5–86.6) 44.6 6 34.2 (4.2–110.0) 40.9 6 14.8 (15.2–61.0) 0.08. *For MMP-3, sensitivity was defined as 2 SDs above the mean optical density of 6 zero standard replicates. The corresponding concentration was calculated and averaged from three standard curves. For TIMP-1 and TIMP-2 the sensitivity values were calculated by the manufacturer by the same method. Samples were analysed in the following dilutions: undiluted for MMP-3, 1 : 5 for TIMP-1 and 1 : 3 for TIMP-2. Values are mean 6 SD (range). Due to the lack of CSF material one sample† and three samples‡ could not be analysed. ppMS 5 primary progressive multiple sclerosis; rrMS 5 relapsing–remitting multiple sclerosis.. subgroup. Half (5 out of 10) of these patients were found to have an increased CSF/serum albumin ratio (.5.5 3 10–3), indicative of damage to the blood–brain barrier. Fig. 3 Correlation of MMP-9 levels (measured by ELISA) with IgG index (A) and pleocytosis (B) in the CSF of patients with acute (closed circles) and stable (open circles) relapsing–remitting multiple sclerosis. Rho values of the Spearman rank correlation were 0.658 (A) and 0.659 (B).. positive for MMP-9 by ELISA had higher CSF cell numbers compared with those in whom MMP-9 was only detectable by zymography (mean 6 SD, 8.9 6 8.6 versus 4.8 6 5.9/µl; P 5 0.039). A similar dichotomy was observed for the IgG (immunoglobulin G) index (12.4 6 6.2 versus 8.4 6 3.6; P 5 0.045). However, higher levels of MMP-9 did not correlate with current or subsequent clinical disability, as in both groups the EDSS score was similar at the time of lumbar puncture and 1–1.5 years later (data not shown). ELISA values of MMP-9 correlated strongly with the IgG index in patients with relapsing–remitting multiple sclerosis (Pn 5 27 , 0.01) (Fig. 3A) and with CSF pleocytosis (P , 0.01) (Fig. 3B), but not with the CSF/serum albumin ratio (P 5 0.62). The correlation with CSF pleocytosis was still significant when acute and stable relapsing–remitting multiple sclerosis were analysed separately (P 5 0.043 for acute relapsing–remitting multiple sclerosis, P 5 0.023 for stable relapsing–remitting multiple sclerosis). However, in 10 patients with relapsing–remitting multiple sclerosis (37%) quantifiable amounts of MMP-9 were present, although CSF cell counts were normal (,4/µl) (Fig. 3B); this did not occur in any control. Furthermore, there was no correlation of MMP-9 and absolute CSF cell count (P 5 0.58) in this. Levels of MMP-2, MMP-3, MMP-7, TIMP-1 and TIMP-2 are not altered in multiple sclerosis CSF Table 1 shows that CSF levels of MMP-3, TIMP-1 and TIMP-2 were similar in multiple sclerosis patients and controls. MMP-2 could be detected in all CSF samples by ELISA both in multiple sclerosis patients and in controls, but levels ranged in all samples between the detection limit (0.51 ng/ml) and the lowest standard value (1.95 ng/ml), and were therefore not evaluated quantitatively; this finding was supported by results from zymography (data not shown). MMP-7 was not detectable by ELISA in either multiple sclerosis or control samples. TIMP-1 and MMP-9 are known to be secreted as complex heterodimers, as are TIMP-2 and MMP-2; TIMP-1 and TIMP-2 function to inhibit the proteolytic activity of the MMPs (Yong et al., 1998). No significant correlation between the concentrations of MMP-9 and TIMP-1 (ELISA values) in relapsing–remitting multiple sclerosis was found (P 5 0.64).. Discussion There is accumulating evidence that matrix metalloproteinases play a key role in the pathogenesis of neuroimmunological diseases. Several matrix metalloproteinases, such as MMP-2 and MMP-9 (gelatinase A and B), MMP-7 (matrilysin) and MMP-3 (stromelysin-1) are expressed in and.
(6) 2332. D. Leppert et al.. around multiple sclerosis plaques (Cuzner et al., 1996; Maeda and Sobel et al., 1996; Cossins et al., 1997). Gelatinases have been shown to mediate T-cell migration across subendothelial basement membrane in an in vitro model of the blood–brain barrier (Leppert et al., 1995, 1996), and the degree of tumour necrosis factor (TNF)-induced blood–brain barrier leakage correlates with levels of MMP-9 in brain tissue (Rosenberg et al., 1995). In addition, MMPs may contribute to myelin breakdown due to their proteolytic activity against myelin basic protein (Proost et al., 1993; Chandler et al., 1995). The release of degradation products of myelin basic protein could enhance the autoimmune response and epitope spreading (Proost et al., 1993) in multiple sclerosis. In an earlier report, using semiquantitative zymography, increased levels of MMP-9 were present in 40–64% of multiple sclerosis CSF samples and 35% of optic neuritis samples, but only in 0–20% of control samples, whereas levels of MMP-2 were not increased (Gijbels et al., 1992; Paemen et al., 1994), a finding that was corroborated in this study. MMPs other than MMP-2, MMP-9 and TIMPs have not been measured previously in multiple sclerosis CSF. Here we show that MMP-9 levels in CSF were elevated in 100% of relapsing–remitting multiple sclerosis and 57% of primary progressive multiple sclerosis CSF samples when the results of a quantitative (ELISA) and a semiquantitative but more sensitive method (zymography) were combined. MMP-9 was equally increased during both relapses and clinically stable phases of relapsing–remitting multiple sclerosis. Control samples did not contain quantifiable amounts of MMP-9 either by ELISA or zymography, confirming results of a previous report using the same set of detection methods (Kolb et al., 1998). Likewise, upregulation of MMP-9 and MMP-7 was observed in actively induced and adoptive experimental autoimmune encephalomyelitis and experimental autoimmune neuritis at peak clinical disease severity (Clements et al., 1997; Redford et al., 1997; Hughes et al., 1998; Kieseier et al., 1998), but expression declined rapidly in later phases. A similar transient induction of MMP expression could account for the failure to detect MMP-9 in some patients with viral meningitis (Kolb et al., 1998). In multiple sclerosis, upregulation of MMP-9 appears to be sustained, as levels were increased in all patients with acute or chronic states of relapsing–remitting multiple sclerosis and in most patients with primary progressive multiple sclerosis. Of interest, levels of MMP-9 in relapsing–remitting multiple sclerosis show a correlation with the IgG index, a parameter of chronic intrathecal immune response, confirming the result of an earlier study (Paemen et al., 1994). We conclude that CSF MMP-9 is a sensitive marker for relapsing– remitting multiple sclerosis. However, levels of MMP-9 do not seem to correlate with clinical disease activity or subsequent disability. Furthermore, this finding is not specific to multiple sclerosis, as increased levels of MMP-9 are also present in other neuroinflammatory diseases, such as meningitis and encephalitis (Gijbels et al., 1992; Paemen et al., 1994; Kieseier et al., 1998; Kolb et al., 1998).. MMP-2 and -3 and TIMP-1 and -2 were constitutively expressed and not increased in multiple sclerosis CSF. Increased levels of TIMP-1 have been found during viral meningitis, where MMP-9 levels 10–100 times higher than in multiple sclerosis occur (Kolb et al., 1998). The lack of a parallel increase of TIMP-1 and MMP-9 in multiple sclerosis could reflect an inability of the assay to detect subtle changes of TIMP-1. Alternatively, the failure to induce compensatory upregulation of inhibitors against excess proteolytic activity could be a specific feature of multiple sclerosis. Hypothetically, this could result in a continuous process of neural damage that might promote secondary chronic progression. The selective increase of MMP-9, but not MMP-2, in CSF and the correlation of MMP-9 concentrations with CSF pleocytosis suggests that mononuclear leukocytes are the major source of MMPs in multiple sclerosis. MMP-9 is the predominant metalloprotease in mononuclear cells, whereas MMP-2 is produced only in small amounts (Welgus et al., 1990; Xie et al., 1994; Leppert et al., 1995; Johnatty et al., 1997). A correlation of CSF cell count and levels of MMP9 in multiple sclerosis has been described by Gijbels et al. (1992) but was not confirmed in a follow-up study from the same group (Paemen et al., 1994), although in the latter paper cell counts were significantly higher in patients with detectable MMP-9 in CSF when compared with controls by the Mann–Whitney U test. These partially discrepant results may indicate that MMP-9 originates predominantly from immune cells infiltrating the brain parenchyma, which may be reflected inaccurately by the CSF cell count depending on the spatial relation of multiple sclerosis lesions to the CSF space. This could explain why more than a third of relapsing–remitting multiple sclerosis patients with increased levels of MMP-9 had a normal CSF cell count. Alternatively, astrocytes and microglial cells in multiple sclerosis plaques could be a source of MMP-9 and MMP-2, but their quantitative contribution is thought to be minor compared with those of infiltrating immune cells (Cuzner et al., 1996; Maeda and Sobel et al., 1996; Cossins et al., 1997). As half of the patients had an increased CSF/serum albumin ratio, it is conceivable that leakage of MMP-9 across the defective blood–brain barrier contributes substantially to levels of MMP-9 in the CSF space. Our failure to detect MMP-7 in multiple sclerosis CSF could be explained by limited diffusion into the CSF or by transient production or a short half-life of this metalloproteinase; also, the ELISA might be insensitive to small increases of MMP-7 in multiple sclerosis. The results of the present study support the lack of strict correlation between levels of MMP-9 and clinical disease activity in multiple sclerosis, a not unexpected finding given the frequent bursts of subclinical disease activity detected by MRI (Willoughby et al., 1989; Kermode et al., 1990). In a small longitudinal study of multiple sclerosis patients in acute relapse, high expression of MMP-9 in CSF, as measured by zymography, coincided with gadolinium-enhancing lesions on MRI (Rosenberg et al., 1996). In a cross-sectional study.
(7) MMP-9 in multiple sclerosis CSF of 40 patients no correlation between the number of active multiple sclerosis lesions and MMP-9 concentration could be established (K. Gijbels, personal communication). Similarly, the numbers of hyperintense multiple sclerosis lesions, as found in T2- and proton-weighted MRI scans, did not correlate with MMP-9 in CSF (Paemen et al., 1994). The beneficial effects of both steroids and IFN-β (interferon-β) in multiple sclerosis might result from their suppressive action on MMP production (Leppert et al., 1996; Rosenberg et al., 1996; Stu¨ve et al., 1996), but their longterm effect on the clinical outcome is modest (Goodkin et al., 1994; Hughes et al., 1996). Enzyme inhibitors of the hydroxamic acid class are novel compounds that inactivate the proteolytic capacity of metalloproteinases, resulting in decreased leukocyte migration across subendothelial basement membrane models in vitro (Leppert et al., 1995). Furthermore, they have been shown to ameliorate the clinical course and reduce inflammatory cell infiltration in experimental autoimmune encephalomyelitis (Gijbels et al., 1994; Hewson et al., 1995; Clements et al., 1997) and experimental autoimmune neuritis (Redford et al., 1997). Inactivation of destructive proteolytic enzymes could be a specific and efficient approach for the treatment of multiple sclerosis. As non-protein molecules of low molecular weight, they readily appear in the CSF of inflamed brain tissue (Gijbels et al., 1994) and would not be expected to induce neutralizing antibodies that represent a significant problem with IFN-β (IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group, 1996; Abdul-Ahad et al., 1997). The sustained overexpression of MMP-9 and possibly other proteases in the parenchyma and CSF in multiple sclerosis suggests that chronic therapy with these enzyme inhibitors would be desirable. Of the four MMPs we tested, only MMP-9 is quantitatively upregulated in multiple sclerosis, and it could therefore be a specific target for future therapies. However, the role of most other members of the MMP family (e.g. MMP-1, -10, -11, -12, -13 and -15) and of TNF-α converting enzyme, a related protease that releases membrane-bound TNF-α into its soluble form (Black et al., 1997; Moss et al., 1997), remains uncertain. This precludes the definition of the ideal target profile of an enzyme inhibitor for multiple sclerosis therapy at this time. Further studies to define the longitudinal course of expression and the transcriptional regulation of MMP-9 and other metalloproteinases in the CSF and plaque tissue from multiple sclerosis patients are needed.. Acknowledgements We wish to thank Dr M. Spycher for her help with the preparation of patient data. We also wish to thank the Experimental Biology Unit at the University of Surrey, and R&D Systems Europe for assistance in the production and conjugation of sheep polyclonal antibodies against human MMPs and for prelaunch kits for MMP-2 and -3. This work was supported by grants (32–41655.94 and 31–43043) from. 2333. the Swiss National Foundation, the Schering Foundation, Swiss Life Insurance and the Swiss Multiple Sclerosis Society.. References Abdul-Ahad AK, Galazka AR, Revel M, Biffoni M, Borden EC. Incidence of antibodies to interferon-β in patients treated with recombinant human interferon-b1a from mammalian cells. Cytokines Cell Mol Ther 1997; 3: 27–32. Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour necrosis factor-α from cells. Nature 1997; 385: 729–33. Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett 1995; 201: 223–6. Clements JM, Cossins JA, Wells GM, Corkill DJ, Helfrich K, Wood LM, et al. Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-α inhibitor. J Neuroimmunol 1997; 74: 85–94. Cossins JA, Clements JM, Ford J, Miller KM, Pigott R, Vos W, et al. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol 1997; 94: 590–8. Cuzner ML, Gveric D, Strand C, Loughlin AJ, Paemen L, Opdenakker G, et al. The expression of tissue-type plasminogenactivator, matrix metalloproteases and endogenous inhibitors in the central nervous system in multiple sclerosis: comparison of stages in lesion evolution. J Neuropathol Exp Neurol 1996; 55: 1194–204. Gijbels K, Masure S, Carton H, Opdenakker G. Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol 1992; 41: 29–34. Gijbels K, Galardy RE, Steinman L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J Clin Invest 1994; 94: 2177–82. Goodkin DE. Role of steroids and immunosuppression and effects of interferon beta-1b in multiple sclerosis. [Review]. West J Med 1994; 161: 292–8. Hartung HP. Pathogenesis of multiple sclerosis. In: Abramsky O, Ovadia H, editors. Frontiers in multiple sclerosis. London: Martin Dunitz; 1997. p. 45–60. Hewson AK, Smith T, Leonard JP, Cuzner ML. Suppression of experimental allergic encephalomyelitis in the Lewis rat by the matrix metalloproteinase inhibitor Ro31–9790. Inflamm Res 1995; 44: 345–9. Hughes RA, Sharrack B. More immunotherapy for multiple sclerosis [editorial; comment]. [Review]. J Neurol Neurosurg Psychiatry 1996; 61: 239–41. Comment on: J Neurol Neurosurg Psychiatry 1996; 61: 251–8. Hughes PM, Wells GMA, Clements JM, Gearing AJH, Redford EJ, Davies M, et al. Matrix metalloproteinase expression during experimental autoimmune neuritis. Brain 1998; 121: 481–94. IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Neutralizing antibodies during.
(8) 2334. D. Leppert et al.. treatment of multiple sclerosis with interferon beta-1b: experience during the first three years [see comments]. Neurology 1996; 47: 889–94. Comment in: Neurology 1996; 47: 865–6, Comment in: Neurology 1997; 49: 641–2. Johnatty RN, Taub DD, Reeder SP, Turcovski-Corrales SM, Cottam DW, Stephenson TJ, et al. Cytokine and chemokine regulation of proMMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol 1997; 158: 2327–33. Kermode AG, Thompson AJ, Tofts P, MacManus DG, Kendall BE, Kingsley DP, et al. Breakdown of the blood–brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Brain 1990;113: 1477–89. Kieseier BC, Kiefer R, Clements JM, Miller K, Wells GMA, Schweitzer T, et al. Matrix metalloproteinase-9 and -7 are regulated in experimental autoimmune encephalomyelitis. Brain 1998; 121:159–66. Kolb SA, Lahrtz F, Paul R, Leppert D, Nadal D, Pfister HW, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in viral meningitis: upregulation of MMP-9 and TIMP-1 in cerebrospinal fluid. J Neuroimmunol 1998; 84: 143–50. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–52. Kwon EE, Prineas JW. Blood–brain barrier abnormalities in longstanding multiple sclerosis lesions. An immunohistochemical study. J Neuropathol Exp Neurol 1994; 53: 625–36. Leppert D, Waubant E, Galardy R, Bunnett NW, Hauser SL. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol 1995; 154: 4379–89. Leppert D, Waubant E, Bu¨rk M, Oksenberg JR, Hauser SL. Interferon beta-1b inhibits gelatinase secretion and in vitro migration of human T cells: a possible mechanism for treatment efficacy in multiple sclerosis. Ann Neurol 1996; 40: 846–52. Maeda A, Sobel RA. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol 1996; 55: 300–9. Miller DH, Barkhof F, Berry I, Kappos L, Scotti G, Thompson AJ. Magnetic resonance imaging in monitoring the treatment of multiple sclerosis: concerted action guidelines [see comments]. J Neurol Neurosurg Psychiatry 1991; 54: 683–8. Comment in: J Neurol Neurosurg Psychiatry 1992; 55: 978. Montgomery AM, Sabzevari H, Reisfeld RA. Production and regulation of gelatinase B by human T-cells. Biochim Biophys Acta 1993; 1176: 256–68. Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α [published erratum appears in Nature 1997; 386: 738]. Nature 1997; 385: 733–6. Paemen L, Olsson T, So¨derstro¨m M, Van Damme J, Opdenakker G. Evaluation of gelatinases and IL-6 in the cerebrospinal fluid of patients with optic neuritis, multiple sclerosis and other inflammatory neurological diseases. Eur J Neurol 1994; 1: 55–63 Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers. GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983; 13: 227–31. Proost P, Van Damme J, Opdenakker G. Leukocyte gelatinase B cleavage releases encephalitogens from human myelin basic protein. Biochem Biophys Res Commun 1993; 192: 1175–81. Redford EJ, Smith KJ, Gregson NA, Davies M, Hughes P, Gearing AJ, et al. A combined inhibitor of matrix metalloproteinase activity and tumour necrosis factor-α processing attenuates experimental autoimmune neuritis. Brain 1997; 120: 1895–905. Rosenberg GA, Kornfeld M, Estrada E, Kelley RO, Liotta LA, Stetler-Stevenson WG. TIMP-2 reduces proteolytic opening of blood–brain barrier by type IV collagenase. Brain Res 1992; 576: 203–7. Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG. Tumor necrosis factor-α induced gelatinase B causes delayed opening of the blood–brain barrier: an expanded therapeutic window. Brain Res 1995; 703: 151–5. Rosenberg GA, Dencoff JE, Correa N Jr, Reiners M, Ford CC. Effect of steroids on CSF matrix metalloproteinases in multiple sclerosis: relation to blood–brain barrier injury. Neurology 1996; 46: 1626–32. Stu¨ve O, Dooley NP, Uhm JH, Antel JP, Francis GS, Williams G, et al. Interferon β-1b decreases the migration of T lymphocytes in vitro: effects on matrix metalloproteinase-9. Ann Neurol 1996; 40: 853–63. Tuohy VK, Fritz RB, Ben-Nun A. Self-determinants in autoimmune demyelinating disease: changes in T-cell response specificity. [Review]. Curr Opin Immunol 1994; 6: 887–91. Welgus H, Campbell EJ, Cury JD, Eisen AZ, Senior RM, Wilhelm SM, et al. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest 1990; 86: 1496–502. Wells GM, Catlin G, Cossins JA, Mangan M, Ward GA, Miller KM, et al. Quantitation of matrix metalloproteinases in cultured rat astrocytes using the polymerase chain reaction with a multicompetitor cDNA standard. Glia 1996; 18: 332–40. Willoughby EW, Grochowski E, Li DK, Oger J, Kastrukoff LF, Paty DW. Serial magnetic resonance scanning in multiple sclerosis: a second prospective study in relapsing patients. Ann Neurol 1989; 25: 43–9. Xia M, Leppert D, Hauser SL, Sreedharan SP, Nelson PJ, Krensky AM, et al. Stimulus specificity of matrix metalloproteinase dependence of human T cell migration through a model basement membrane. J Immunol 1996; 156: 160–7. Xie B, Dong Z, Fidler IJ. Regulatory mechanisms for the expression of type IV collagenases/gelatinases in murine macrophages. J Immunol 1994; 152: 3637–44. Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci 1998; 21: 75–80.. Received May 13, 1998. Revised July 17, 1998. Accepted August 10, 1998.
(9)
Figure

Documents relatifs
Si après la première lecture vous pouvez mettre quelques mots (au brouillon), c'est tant mieux. Maintenant vous pouvez lire les phrases séparément pour les remplir sans
Relie chaque évènement à la carte par une flèche selon le lieu de son déroulement.. Les Alliés débarquent en Normandie en
The originality of a non-persuasive conception is to shake the following idea that, in either an implicit or explicit way, subtends the majority of other theories: the
Cognitive and cortical plasticity deficits correlate with altered amyloid-β CSF levels in Multiple Sclerosis.. Francesco Mori, Silvia Rossi, Giulia Sancesario, Claudia Codecà,
Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer
Namely, the signal decay rate which should re flect redox processes in the selected organ depends on, beside the reduction of nitroxides, also on several kinetic factors such as
parodontopathies (pathologies des tissus de soutien de la dent) observées chez la femme enceinte en consultation prénatale dans le service de Gynécologie
There are many persons, for example, who, while they hold no allegiance to any particular body of religious beliefs, would hold that their world-view is a