ON
AMINO ACIDS IN FOODS AND IN AQUEOUS SOLUTIONS
BY
DARSHAN SINGH BHATIA B.Sc. (Hons.), M.Sc. (Tech.), Universit of Punjab, (1943T University of Punjab, (1945)
SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF
DOCTOR OF SCIENCE at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY (1950)
Signature of Author.
Certified by...
Signature Redacted
... . .. .. ...Department of ood Technology, May, 1950
Signature Redacted
.. ~..-... Thesis Supervisor
Signature Redacted
Chairman, Departmental Committee on Graduate Students India
AU3 11 1950
TABLE OF CONTENTS Page Acknowledgments... Abstract ... 2 Introduction... ... 7 Purpose... 9
Van de Graaff Electrostatic Belt Generator... 11
Dosage
Computation...
20Physical Properties of Ionizing Radiations... 26
Chemical Effects of Ionizing Radiations... 33
Microbiological Assays of Amino Acids... 43
Literature
Survey...
... 54Experimental Methods and Techniques...,... 67
RESULTS Section I. The Effect of Super-voltage Cathode Rays on Amino Acids in Foods... 85
Section II. The Effect of Super-voltage Cathode Rays on Aqueous Solutions of Amino
Acids...
104Section III. Studies on the Mechanism of the~7ction of Cathode Rays- on Amino
Acids...*...--
... 123DISCUSSION Section I. The Effect of Super-voltage Cathode Rays on Amino Acids in Foods... 149
Section II. The Effect of Super-voltage Cth-ode Rays on Aqueous Solutions of Amino
Acids...-..
152Section III. Studies on the Mechanism of the =ction of Cathode Rays on Amino
Acids...-.
173Page
Suggestions for Further Work ... 182a APPENDIX
Biographical Note... 184
Calculation of the Results of Microbiological
A ay
... 186Preparation of Stock Solutions... 193
Standard Curves of Amino Acids,... 199
Colorimetric Determination of Histidine... 212
Chemical Constitution of Amino Acids... 216
Microdiffusion Analysis for Ammonia... 218
Ultraviolet Absorption Spectra... 226
Page Table I - Activities of D and DL Forms of Amino
Acids ... e... 50
Table II Lactic Acid Bacteria Which Have Been Used for Microbiological Assays ...
52
Table III - Organisms Other Than Lactic Acid Used To Determine Amino Acids ...
53
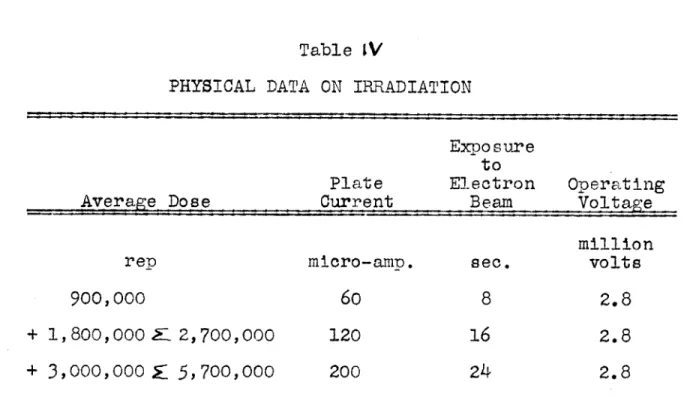
Table IV - Physical Data on Irradiation ...
69
Table V - Composition of Basal Medium ... ,... 74
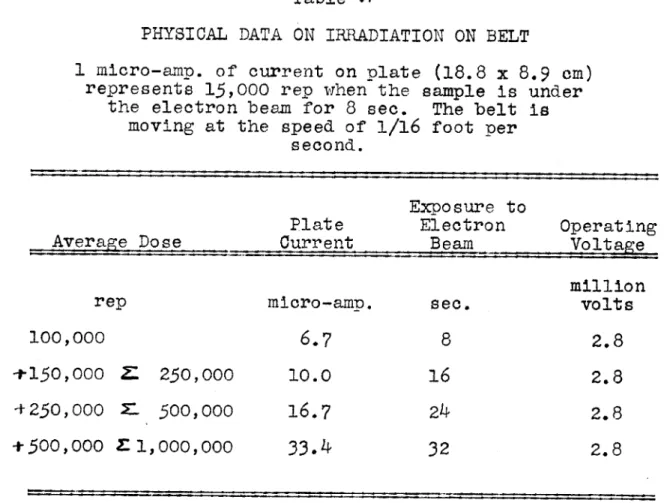
Table VI - Physical Data on Irradiation on Belt ... 78
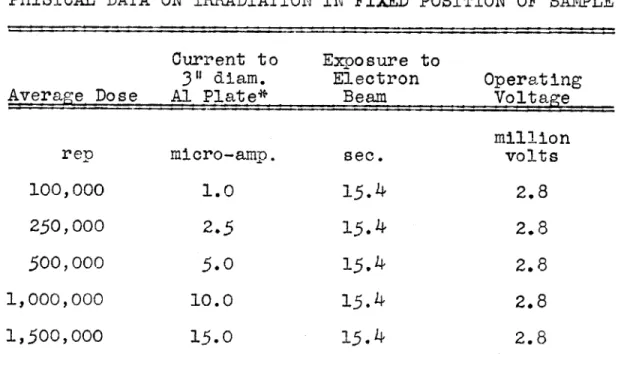
Table VII - Physical Data on Irradiation in Fixed Position of Sample ...
79
Table VIII - Composition of Basal Medium Used for Tyrosine ... 80
Table IX - Effect of Cathode Rays on Physical Condi-tion of Haddock Fillets ...
98
Table X - Effect of Cathode Rays on Amino Acids ...
99
Table XI - Effect of High Voltage Cathode Rays on Aqueous Solutions of dl-Phenylalanine 105 Table XII - Effect of High Voltage Cathode Rays on Aqueous Solutions of L-Histidine HCl.H20 --- *-- -*-4******.*...* 107
Table XIII - Effect of High Voltage Cathode Rays on Aqueous Solutions of L-Tyrosine ... 109
Table XIV - Effect of High Voltage Cathode Rays on Aqueous Solutions of dl-Tryptophan .. 112
Table XV - Effect of High Voltage Cathode Rays on Aqueous Solutions of 1-Leucine ... 114
Table XVI - Effect of High Voltage Cathode Rays on Aqueous Solutions of 1-Leucine ... 115
Table XVII - Effect of High Voltage Cathode Rays on
1-Leucine in Fish Hydrolysate
Table XVIII - Effect of High Voltage Cathode Rays on
Aqueous Solutions of 1-Cystine
Table XIX - Pigmentation of Amino Acid Solutions on Irradiation with Cathode Rays
Table XX - Effect of High Voltage Cathode Rays on Aqueous Solution of 1-Histidine. HC1.H20 (100 ug./ml.) ...
Table XXI Effect of High Voltage Cathode Rays on Aqueous Solution of 1-Histidine. HC1.H20 (100 ug./ml.)
Table XXII - Effect of High Voltage Cathode Rays on Aqueous Solution of 1-Histidine. HC1.H20 (100 ug./ml.) ... Table XXIII Effect of High Voltage Cathode Rays on
Aqueous Solution of 1-Histidine. HC1.H20 (100 ug./ml.) ...
Table XXIV - Standard Curve for Use in the
Colori-metric Determination of Histidine ...
Table XXV - Microdiffusion Analysis for Ammonia
Table XXVI Deamination of Histidine Monohydrochlo-ride (100 ug./ml. in 0.1N H01) by Irradiation with High Voltage Cathode Rays ...
Table XXVII Deamination of Histidine Monohydro-chloride in 0.1M Solution in 0.1N HC1 by Irradiation with High Voltage Cathode Rays ... ,....,.
Table XXVIII - Ammonia Yield from Amino Acids in O.1M Solution in 0.1N H01 on
Irradiation with a Cathode-ray Dose of 250,000 rep ...
IV
Page 117 119 122 124 125 126 127 128 131133
136
ITable XXIX - Effect of Irradiation with High Voltage Cathode Rays on pH of dl-Phenyl-alanine (200 ug./ml.)
Table XXX - Effect of Irradiation with High Voltage Cathode Rays on pH of dl-Tryptophan (50 ug./ml.) .... ,. ...
Table XXXI - Effect of Irradiation with High Voltage Cathode Rays on pH of 1-Tyrosine
(100 ug./ml.)
Table XXXII Effect of Irradiation with High Voltage Cathode Rays on pH of 1-Histidine. HC1.H20 (100 ug./ml.)
Table XXXIII - Decomposition of Amino Acids by High Voltage Cathode Rays ...
Table XXXIV - Ionic Yield of the Decomposition of
dl-Phenylalanine by High Voltage
Cathode Rays ... ,... .. Table XXXV - Ionic Yield of the Decomposition of
1-Tyrosine by High Voltage Cathode Rays ...
Table XXXVI - Ionic Yield of the Decomposition of
dl-Tryptophan by High Voltage Cathode Rays ...
Table XXXVII - Ionic Yield of the Decomposition of 1-Leucine by High Voltage Cathode Rays
.
Table XXXVIII
Table XXXIX
-- Ionic Yield of the Decomposition of 1-Cystine by High Voltage Cathode
Rays ... .*.*0**0***....*0.,.4**
164
Ionic Yield of the Decomposition of1-Histidine Monohydrochloride by
High Voltage Cathode Rays ... ,.
165
Table XL - Amino Acid Content of Haddock .. ... 169137
139
142 144 157 160 161 162363
Table XLI - Relative Radiosensitivities of Some
Amino Acids to Irradiation with
High Voltage Cathode Rays ...
171
Table XLII Relative Radiosensitivities of Some Amino Acids to Irradiation with
High Voltage Cathode Rays .b** * 172
Table XLIII - Unknown Test Series of Tryptophan
(Sample X) ...
191
Table XLIV - Stock Solutions of Amino Acids ... 195 Table XLV - Source of the Synthetic Amino Acids Used 198
Table XLVI - Concentration of Amino Acid Solutions
for Standard Curves ... ,... 200 Table XLVII - Titration Data for the Standard Curve
of 1-Arginine.HC1... 201 Table XLVIII Titration Data for the Standard Curve
of 1-Cystine ... 202 Table XLIX - Titration Data for the Standard Curve
of 1-Histidine.HCI.H20 ...
203
Table L - Titration Data for the Standard Curve of
1-Leucine ...
204
Table LI - Titration Data for the Standard Curve of1-Lysine-Hydroohloride ... 205 Table LII - Titration Data for the Standard Curve
of dl-Methionine ...
206
Table LIII - Titration Data for the Standard Curve
of dl-Phenylalanine ...,... 207 Table LIV - Titration Data for the Standard Curve
of dl-Threonine ...
208
Table LV Titration Data for the Standard Curve of
dl-Tryptophan
... ,....209
Table LVI - Titration Data for the Standard Curve
Table LVII - Titration Data for the Standard Curve
of dl-Valine ... 211 Table LVIII - Suitable Solution Strengths in
Deter-mining Ammonia with the Hydrochloric-Barium Hyd-roxide Procedure ... 224 Table LIX - The Ultraviolet Absorption Spectrum of
Aqueous Solution of dl-Phenylalanine (200 ug./ml.) Irradiated by Cathode
Rays ... 227 Table LX - The Ultraviolet Absorption Spectrum of
Aqueous Solution of dl-Tryptophan (50 ug./ml.) Irradiated by Cathode
Rays ... ... 229 Table LXI - The Ultraviolet Absorption Spectrum of
Aqueous Solution of 1-Tyrosine
(100 ug./ml.) Irradiated by Cathode
Rays ...
230
Table LXII - The Ultraviolet Absorption Spectrum ofAqueous Solution of 1-Histidine. HCl.H 0 (100 ug./ml.) Irradiated by
Catho e Rays ...
231
Table LXIII - The Ultraviolet Absorption Spectrum ofAqueous Solution of dl-Alpha Alanine (0.lM 1N 0.3N NaOH) Irradiated by
Cathode Rays . ... ...
232
Table LXIV - The Ultraviolet Absorption Spectrum ofAqueous Solution of Benzene (37.2 mg./100 ml.) Irradiated by Cathode
TABLE OF FIGURES
Figure I - Van de Graaff Electrostatic Belt
Generator ... Figure Ia - Van de Graaff Generator
Figure II - Cathode Ray Stream from Van de
Generator ...
Figure III - Combined X-ray Target and Cath
Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure IV -V -VI -VII VIII Ix x xi -XII XIII *... Graaff ... ..y ode Ray Window ... ... Standard Curve 1-Arginine
Standard Curve 1-Cystine Standard Curve 1-Histidine
- Standard Curve 1-Leucine .- Standard Curve 1-Lysine
Standard Curve dl-Methionine Standard Curve dl-Phenylalanine
Standard Curve dl-Threonine
- Standard Curve dl-Tryptophane
- Standard Curve 1-Tyrosine ... Figure XIV - Standard Curve dl-Valine ...
Figure XV - Effect of High Voltage Cathode Rays on Aqueous Solutions of dl-Phenylalanine. Figure XVI - Effect of High Voltage Cathode Rays on
Aqueous Solutions of 1-Histidine. HC1.H20.
Figure XVII - Effect of High Voltage Cathode Rays on Aqueous Solutions of 1-Tyrosine Figure XVIII - Effect of High Voltage Cathode Rays
on Aqueous Solutions of dl-Tryptophane ... . 13
14
18 1987
88
89
90
9192
93
94
95
96
97
106
108
110113
Figure XIX - Effect of High Voltage Cathode Rays
on Aqueous Solutions of 1-Leucine.. 116 Figure XX - Effect of High Voltage Cathode Rays
on 1-Leucine in Protein Hydrolysate 118 Figure XXI - Effect of High Voltage Cathode Rays
on Aqueous Solutions of 1-Cystine.. 120 Figure XXII Standard Curve for Histidine
(Diazotization) ... ,. 129 Figure XXIII - Ammonia Yield from Histidine
Mono-hydrochloride in 0.1 Molal Solut ion . . . . ... . . .
* . * .r 0000 135
Figure XXIV - U. V. Absorption Spectra of Irrad-iated and Control dl-Phenylalanine
(200 mcg./m1.) ... 138 Figure XXV - U. V. Absorption Spectra of Control
and Cathode Ray Irradiated Solu-tions of dl-Tryptophane (50 mcg./
ml.) ... ... .... ... 140 Figure XXVI - Ultraviolet Absorption Spectra of
1-Tyrosine Solutions Irradiated by Supervoltage Cathode Rays
(100 mcg./ml.) ... ... 1.... 143 Figure XXVII Ultraviolet Absorption Spectrum
of 1-Histidine.HC1 (100 mg./m.).. 145 Figure XXVIII U. V. Absorption Spectra of Control
and Cathode Ray Irradiated Solu-tions of dl- Alanine (0.1 M in
N/10
NaOH) ... . 147 Figure XXIX - U. V. Absorption Spectra of Controland Irradiated Solutions of Benzene in Water (37.2 mg./100 ml.) ... 148 Figure XXX - Decomposition of dl-Phenylalanine by
Figure XXXI - Slope-Ratio Method ... 187
Figure XXXII- Slope-Ratio Method ... 190 Figure XXXIII - Standard Unit (Conway) ... 219
ACKNOWLEDGMENTS
The author wishes to express his appreciation to Professor Bernard E. Proctor who suggested the problem for this thesis. Without his useful and inspiring
sug-gestions, this work would not have been completed.
It is apreciated that members of the Staff of the Department of Food Technology, especially Professor Ernest E. Lockhart, gave valuable and time saving sug-gestions in the course of this study.
The author expresses his gratitude to the Government of Punjab, India who awarded him a scholarship for the first two years of his stay in this country and to the Massachusetts Institute of Technology for appointing him as Research Assistant, thus enabling him to finish his third year of education at the Institute.
Van de Graaff generator was made available to the author through the courtesy of Professor John G. Trump
and the work was facilitated by the help of Mr. Kenneth A. Wright who ran all the irradiations, sometimes at very odd hours of the day. These things are gratefully acknowledged.
2
ABSTRACT
This investigation was undertaken to asoertain the effects of ionizing radiations on proteins and component parts thereof. Supervoltage cathode rays (3-million volts), produced by Van de Graaff electrostatic generator, were used as the ionizing radiation. The experimental work for this thesis was divided into three distinct sections and each is discussed separately.
Section I
The Effects of Supervoltage Cathode R on Amino Acids in Foods
This study was conducted on haddock. Haddock fillets, :sealed in polyethylene bags, were irradiated with cathode
rays at the three levels of 900,000; 2,700,000; and
5,700,000 rep. Ten amino acids, arginine, cystine, his-tidine, leucine, lysine, valine, tryptophan, phenylalanine, threonine and methionine were determined microbiologically in the irradiated and the non-irradiated fillets. Leuconostoc mesenteroides P-60 was used as the test organism. Irradiated
and the control fillets were broiled in the conventional manner and presented to a taste panel of nine judges.
It was observed that irradiation bleaches the surface of the fillet and results in partial coagulation of the tissue at the surface. The results of amino acid
deter-minations have shown that cathode ray irradiation has no adverse effect (within the limits of microbiological assay) on any one of the amino acids studied. The highest level
of irradiation was about 5 times the dose necessary for ster-ilization of fish.
The results of the organoleptic studies suggest that there are some persons to whom the irradiation at levels necessary for sterilization causes inappreciable flavor changes. However, the limiting number of judges on the test panel permits no valid conclusion. In the light of the results obtained, it may be stated that haddock fillets may be sterilized adequately by irradiation with cathode rays, without any adverse effect on the amino kcift
of fish.musole. Section II
The Effects of Supervoltage Cathode Rays on eous Solutions of Amino Acids
This section deals with the effect of cathode rays on six amino acids; namely, histidine, cystine, leucine,
tryptophan, phenylalanine, and tyrosine. Solutions in concentrations of l0 g. to l0- g./ml. were irradiated at levels of 100,000; 250,000; 500,000; and 1,000,000 rep. The retention of amino acid in irradiated solutions was determined microbiologically.
4
Irradiation of solutions of histidine, phenylalanine, tryptophan, and tyrosine produced yellow pigmentation in the solution. The amino acid determinations showed that all these amino acids are adversely affected in dilute solutions and the decomposition of amino acid is related exponentially to the dose of radiation. It was found that with an equal dose of radiation, a dilute solution of an amino acid is relatively more affected than a more con-centrated one (dilution phenomenon). Also an amino acid is more affected when present alone in aqueous solution than when present along with additional substances (protection phenomenon).
Ionic yields (M/N) of the decomposition of amino acids were calculated and it has been found that ionic yields in solutions of concentrations 0.10 - 0.25 mg./ml. are less than
or equal to unity. Under the conditions of the experiments, ionic yields were lower in dilute solutions than in more concentrated solutions. The specific inactivation dose
(D/C) has been determined and it was found that the values (D/C) are almost independent of concentration in cases of histidine, phenylalanine, and tyrosine.
The relative radio-sensitivities of amino acids to cathode rays have been determined and the order of radio-sensitivity was found to be histidine 3 >phenylalaninep
The above observations on the effect of cathode rays on amino acids in aqueous solution give an evidence of the indirect action.
Section III
Studies on the Mechanism of the Action of Cathode y on Amino Acids
Solutions of histidine containing 100 micrograms/ml. were irradiated at 4 doses of 100,000; 250,000; 500,000;
and 1,000,000 rep and the residual amino acid was deter-mined microbiologically and chemically. The chemical method makes use of the red color developed on treating histidine with diazotized sulfanilic acid and thus the method is
spec-ific for the imidazole ring.
The values of residual amino acid, as determined by the colorimetric method, were found to be lower than the microbiological values. The difference in the twr values
is attributed to the concurrent deamination of the alpha
amino group. This means that cathode rays decompose histidine by splitting the imidazole ring and by concurrent
deamina-tion of the alpha amino group in the side chain and that the intact molecule of histidine is essential for its micro-biological activity.
It has been found that cathode rays liberate ammonia from amino acid in aqueous solution. Ammonia determinations were made according to the Microdiffusioh Analysis (Conway).
6
The yields of ammonia in 0.1 M solution of histidine, cystine, phenylalanine, tyrosine, leucine, and tryptophan were 13.63,
7.05, 4.48, 4.03, 3.72, and 1.60 micrograms NH
3/ml.
Ultraviolet absorption spectroscopic studies were done
on histidine, tyrosine, tryptophan, and phenylalanine. Histidine does not have any fine structure in the spectrum but an increase in the general absorption on irradiation
indicated that the molecule of histidine was affected by cathode rays. The aromatic amino acids have characteristic maxima and minima in the spectra. Absorption spectra of the irradiated solutions of tyrosine, phenylalanine, and trypto-phan showed that the ring structure was broken in all the
cases, signifying that high voltage cathode rays are cap-able of splitting the benzene and the indole rings. The typical U. V. absorption spectrum of benzene disappeared on irradiation of benzene solution.
These investigations show that the decomposition of
aromatic amino acids by cathode rays takes place both through deamination and fission of the ring structure.
INTRODUCTION
A number of investigators have shown that ionizing
radiations have a lethal effect on microorganisms (46,'& 5 q,
jos, II' ). The possibility of utilizing X-ray
irrad-iation as a means of sterilizing foods was demonstrated by Proctor et al in 1943 (70). Brasch and Huber showed later that they were able to sterilize a wide variety of medicines, therapeutics, and food stuffs with penetrating electrons of ultrashort duration as emitted from the capacitron (7, 8 ). Dunn et al (21 ) found that super-voltage X-rays and cathode rays destroyed bacteria, yeasts, and molds in massive
con-centrations in pure cultures and when associated with liquid or solid substances such as milk, water, apple juice, ground spices, soil and catgut sutures. They indicated the possi-bility of sterilizing fluids by a continuous process.
These investigations demonstrated that supervoltage cathode rays and X-rays have potentialities as a means of processing foods. Extensive studies on the effect of X-rays and cathode rays on vitamins and enzymes have been carried out in the Food Technology Laboratories at the Massachusetts Institute of Technology. Proctor and Goldblith (71,-72,38) have studied the effects of ionizing radiations on niacin, riboflavin, ascorbic acid, and carotene. All these vitamins
are adversely affected in dilute solutions. Other unpub-lished work in these laboratories (73 ) has shown that vita-mins are relatively more radio-resistant in foods than in
8
pure solutions of equivalent concentrations. Investigations on the effects of high energy X-rays and cathode rays on enzymes in foods have indicated that the enzymes are only slightly affected at the dosages necessary for steriliza-tion ( 2- ).In addition to vitamins and enzymes, amino acids form another group of compounds of great importance. The author is not aware of any published reports on the effects of
cathode rays on amino acids in foods. In view of the possi-bility of sterilization of foods by irradiation with super-voltage cathode rays, it became necessary to find out as to how the amino acids would be affected on irradiation.
PURPOSE
The work for this thesis was undertaken to investigate the effects of super-voltage cathode rays on amino acids in foods and in aqueous solutions. This thesis was divided into three sections:
SECTION I: The Effects of Super-voltage Cathode Rays on Amino Acids in Foods.
SECTION II: The Effects of Super-voltage Cathode Rays on Aqueous Solutions of Amino Acids.
SECTION III: Studies on the Mechanism of the Action of Cathode Rays on Amino Acids.
SECTION I: Since the food technologist is interested in the nutritional and organoleptic properties of irradiated protein foods, the present investigation is started on this basis. Haddock muscle is selected as the typical protein because it is rich in all the "essential" amino acids. This section deals with the effect of irradiation by cathode rays on the nutritional and organoleptic properties of haddock. Amino acids are determined microbiologically.
SECTION II: This section deals with the effects of super-voltage cathode rays on amino acids in aqueous solutions.
This investigation is carried out on six amino acids; namely, histidine, leucine, tryptophan, cystine, tyrosine, and
10
SECTION III: This section comprises of investigations on the mechanism of the action of cathode rays on amino acids. This study is conducted on six amino acids described above. The choice of the amino acids was a deliberate one and was guided by an attempt to include amino acids of varied chemi-cal constitution. Leucine represents an amino acid with a straight aliphatic chain and histidine is an amino acid con-taining one amiro group in the side chain and one imino
(--NH-) group in the ring. Cystine represents one of the group of sulfur containing amino acids. The other three
amino acids contain an aromatic mucleus, tyrosine and phenyl-alanine containing the benzene nucleus and tryptophan having the indole grouping. These investigations include the follow-ing studies:
(a) microbiological determination of amino acids. (b) chemical determination of amino acid histidine. (c) ultraviolet absorption spectroscopic studies on
irradiated amino acids.
The chemical formulae and the molecular weights of the amino acids studied are shown in the appendix, page 2/6 .
VAN DE GRAAFF
12
The source of high-energy electrons was a pressure in-sulated Van de Graaff electrostatic generator of the belt type at the Massachusetts Institute of Technology (al) rated at 3-million volts constant potential. This generator, shown in Figure ILa is completely contained in a steel pressure
tank 4-1/2 feet in inside diameter and about 12 feet high, containing a mixture of N2 and SF6 at 13 atmospheres pressure.
It is beyond the scope of this thesis to describe in details the principles of electrostatic belt generators of this type. This subject has been thoroughly reviewed by Trump (1*s) and Van de Graaff et al Qo$. However, to acquaint the reader with the salient features, a brief description
(abstracted from the above mentioned papers) of the operating principles of Van de Graaff generator is given here. As
shown in Figure I, the generator (io9) consists of a well rounded high voltage terminal supported from ground on an insulating column and of a charge-conveying system consisting of a rapidly moving insulating belt which continuously trans-fers charge between ground and terminal. Electric charge of one polarity is sprayed by corona on the belt at its grounded end and is transported by the belt into the hollow terminal, where it is removed.
Within the terminal, charge of the opposite polarity is deposited on the belt and transferred to ground. The maximum voltage which can be attained by the terminal depends only on its ability to insulate. As charge is brought to the terminal, its voltage rises at the rate
HIGH VOLTAGE TERMINAL
UPPER SPRAY
POINTS--COLLECTOR -
-UPPER PULLEY(INSULA
TED FROM TERMINAL) INSULATING MOTOR -DRIVEN BELT - PULLEY-- PULLEY--PULLEY-- PULLEY--PULLEY--w -4
LOWER SPRAY
POINTS-CONTROLLABLE SPRAY VOLTAGE
----VAN DE GRAAFF ELECTROSTATI
C
I
I
I-I
k
1-
:1!'
I
it
9I r, 4I
~j}
Em
I
F
-_I
IT
71T
A
P11
/
t-1 V* i 2 ----4 I ii
I I I , . I A I I I .where i is the net current to the terminal and C is its
capacitance to ground. This rate is usually about 1 million volts per second. At any instant the voltage of the genera-tor is V = where Q is the stored charge. The terminal will
maintain that equilibrium potential at which the charging current delivered by the belt is just balanced by the load current; this equilibrium voltage may be varied over a wide range by controlling either the belt or load currents.
The High Voltage Terminal. The ideal terminal would be an isolated conducting sphere. The necessity, however, of physically supporting the high voltage terminal from ground,
as well as of terminating an electrically charged belt and an acceleration tube inside the terminal, makes it desirable to modify its spherical shape in the region of the column. The column is constructed of a series of conducting members sep-arated by insulating supports. Resistors or corona are used to divide the terminal voltage uniformly between these equi-potential planes. The charge-conveyor belt and acceleration tube pass within this column and benefit by the uniform dis-tribution of potential. A typical terminal and column arrange-ment for an electrostatic generator is illustrated in Figure
1.0-The charge-conveyor system consists of multiple rubber fabric belt which travels between the ground plane and the interior of the high voltage terminal at a constant speed
varying between 4,000 and 6,000 feet per minute. As indicated in Figure I, electric charge is sprayed on the belt at the grounded end from a row of corona points extending across the
16
width of the belt and directed at the pulley. The charge is physically carried by the belt to the terminal and the gen-erated electric power is equal to the mechanical work done in moving the electric charge to terminal potential. The current capacity of an electrostatic generator depends upon the width and speed of the belt and upon the dielectric strength of the gaseous medium in which it operates. Air, nitrogen, Freon (CC12 F2) and sulphur hexafluoride (S F6) have been found to be suitable as insulating media.An increase in the dielectric strength of the gaseous medium favorably affects all of the physical dimensions of the apparatus, including the terminal diameter, the terminal-to-tank spacing, the length of the column, and the required belt width and speed for a given generator current. In the limit, the volume of electrostatic apparatus of given voltage rating varies inversely as the cube of the dielectric strength.
The Acceleration Tube. The purpose of the accelerating tube is to provide a region in which charged particles of the desired type can be produced and accelerated to emerge from the tube in a beam. To accomplish this, the tube must be highly evacuated so that the charges being accelerated will not suffer collisions in their passage down the tube.
The acceleration tube is mounted within the generator column and in a position parallel to the charge conveyor belt. The source of electrons is a hot tungsten filament
contained within the high voltage terminal and driven by the upper pulley. The insulating column of the tube is a rigid self-supporting structure made of alternate glass rings and flat stainless steel disks sealed together by a thermoplastic technique developed in the laboratory of Department of Elec-trical Engineering, Massachusetts Institute of Technology. Each disk has an axial 3-inch diameter hole and is electri-cally connected to the equipotential plane of the generator column at that level. The electrons are thus progressively accelerated as they pass from the filament axially downward through the planes of the diaphragms. X-rays are produced when the electrons impinge on the water-cooled gold disk, which is 0.25 inch thick. By withdrawal of the gold disk from the path of the stream the electrons may be allowed to pass through the 0.002-inch aluminum window into the atmosphere as a high energy cathode ray stream as shown in Figure II. The ground potential end of the acceleration
tube terminates in the target arrangement shown in Figure III. The acceleration tube is continuously evacuated by a mercury diffusion pumping system.
18
-J
-S Utc.AA%
Mad
-ELECTRON BEAM
GROUNDED BEAM-DEFINING GROUNDED STEEL BLOCK ACCELERATION
TUBE FLANGE
VACUUM GUARD RING,
COPPER BELLOWS
RETRACTING MECHANISM
'WATER LINES
0.25" GOLD DISK INSULATED STEEL DISK
RETRACTABLE X-RAY SAMPLE HOLDER / RETRACTABLE WATER-COOLED GOLD TARGET 0.002" ALUMINUM WINDOW 5 cc SAMPLE HOLDER IONIZATION CHAMBER
COMBINED X-RAY TARGET and CATHODE RAY WINDOW
LADORATORY FoR NUCLEAR SCENCE ANo ENGEER MASSACHUSETTS INS11ulf :, TEC.%CL O'
20
In the study of the physical and biological effects of high-energy rays, it is desirable to know the actual
distribu-tion of the ionizadistribu-tion energy in the absorber material.
Trump et al (1*4) reported measurements on the distribution of ionization in depth of water, aluminum, copper and lead pro-duced by mono-energetic electron beams over the energy range from 0.3 to 1.5 million volts. In a more recent study,
Trump et a. (jo) have reported measurements on the distribu-tion of ionizadistribu-tion in depth of aluminum produced by steady beams of 2 and 3 million electron volts. "A thin parallel-plate ionization chamber was used to measure the distribu-tion of ionizadistribu-tion in depth caused by the high energy elec-trons. The distribution of current density in a plane trans-verse to the beam was also measured. It was observed that the ionization has a broad maximum at about one-third the greatest range (1o0, this distribution arising from the high
scatter-ing tendency of the primary electrons within the absorber. The penetrating power of electrons in aluminum is close to 1 gram/sq. cm. per 2 million volts. The range in centimeters can be obtained by dividing the range in grams/sq. cm. by the density of the absorber material. In addition to its primary dependence on electron energy and absorber density,
the distribution of ionization energy in an absorber irrad-iated by essentially parallel and mono-energetic electrons is affected by electron scattering. Although the ionization density is greatest near the end of the path of a single
electron, the effect of scattering on a beam of such
parti-cles is to shift the region of highest ionization and energy absorption to a depth of about one-third the maximum penetra-tion for that energy. Having known the distribution of ion-ization, it becomes a simple matter to determine the dosage of irradiation.
In principle, the cathode ray dosage rate is determined by the numbers of impinging electrons per second and their accelerating voltage. The total cathode ray power falling on a disk of diameter D is EI where E is the accelerating potential and I the total beam current to the disk. The average power P absorbed per gram of material distributed uniformly over this disk to a depth R expressed in grams/sq. cm. is
P = EI Ki K2 watts
w
(D/2)z R gramwhere K1 is the fraction of the total power absorbed in range R and K2 is a factor close to unity which takes account of
the possible change in electron backscatter from the disk area after a sample of the same diameter has been placed upon
it.
To illustrate the calculations for dosage rate, the
following example is taken from the paper by Trump et al (io1):
"Consider the specific case in which it is desired to irradiate a stationary sample 3 inches in diameter (7.62 cm.) with 3-million cathode rays. The current to the 3-inch
collecting disk is taken as 10 microamperes. R is found to be 1.03 grams/aq. cm. and K is 0.84. K2 is taken as unity. Then
P = 3 x 106 x 10x 10- 6 x 0.84 x 1 0.54 watts
7.62 2
1.03 gramand the time required to deliver an average dose of 106 rep (8.3 joules per gram) is
t = 8 = 15.4 sec.
Maximum and minimum doses can be calculated from the average
by applying the appropriate dose ratios both for the
distribu-tion in depth and the transverse distribudistribu-tion."
In the present study, irradiation of samples was done both in the fixed position and on a moving belt. The deter-mination of the dosages of cathode irradiation in the fixed position has been described above.
The moving belt was calibrated by biochemical means; that is, by comparing the destruction of the riboflavin when irradiated on the belt with that obtained in the fixed posi-tion.* Riboflavin in a concentration of 100 micrograms per ml. was used as the material irradiated.
The retention of riboflavin in a sample irradiated on the belt is calculated from the fluorescence data. The dosage
*Calibration studies were done by Dr. S. A. Goldblith of the Department of Food Technology, Massachusetts Institute of Technology.
that samples received in traversing the electron beam is
calculated by reading off the graph of dosage in fixed target position vs.
%
retention. The dosage thus calculated isdivided by the plate current reading and the dosage recorded as dosage (rep) per microampere of plate current reading.
Units of Dosage
The subject of units and standards for the measurement of dosages of radiations has been reviewed by Evans (a4).
Cathode ray dose is best expressed in ergs and joules for
physical studies, but may be expressed in Roentgen-equivalent-physical (rep) for biological tudies. If the energy lost by ionization in the tissues is the same as the energy loss for one roentgen of gamma radiation absorbed in air, the dose is spoken of as one roentgen-equivalent-physical. For example, if one gram of tissue absorbs 108 beta rays having an average energy of 0.52 Mev. the total energy absorbed would be 52 x 106 Mev. per gram or 83 ergs per gram or 1 rep. Then
1 rep = 83 ergs/gm. tissue
It is to be noted that 1 r of photons, which produces about 83.8 ergs per gm. of air, will produce a somewhat different energy dissipation in various tissues, because of the differ-ence in chemical composition. The somewhat conventional state-ment that 1 r of photons = 1 rep is therefore an approximation
The conversion factors for these units of radiation energy are
1 joule = io 7 ergs
1 roentgen-equivalent-physical (rep) = 83 ergs per gm. of tissue 106 rep = 8.3 joules per gram of tissue
The irradiation time is found by dividing the desired dose expressed in joules by the average dosage rate P.
26
PHYSICAL PROPERTIES OF
Since this thesis deals with the chemical and physical effects of cathode rays, it is quite appropriate to include a little discussion on the physical properties of differ--ent radiations which are available for studies on biological
materials. The radiations with which we are concerned are the so-called ionizing radiations which may be classified as
follows: A. Electromagnetic radiations X-rays Y-rays B. Negative particles #-rays cathode rays C. Positive particles o<-particles protons deuterons positrons D. Neutral particles neutrons E. Miscellaneous cosmic rays
We also deal with ultraviolet light which, of course, is a non-ionizing radiation.
Ionization
An atom consists of a positively charged nucleus surrounded by a constellation of negative electrons. The atom, as a whole,
28
is electrically neutral. When an ionizing radiation passes through matter, it results in the ejection of electrons from atoms. An atom so ionized is left positively charged and is referred to as an ion.The electron which is ejected from an atom in the proc-ess of ionization eventually becomes attached to another atom and makes it a negative ion; and one usually speaks of the production of an ion-pair. However, it may be mentioned that it is the positive ions and not the negative ions which are of biological importance.
Practically all the energy dissipated by radiation in tissue ultimately becomes degraded to heat energy.
Excitation
In addition to ionization, a second method by which radia-tions dissipate energy in tissue is by excitation. When an electron in an atom or molecule is raiged to a state of higher energy, the atom or the molecule is said to be in a state of excitation.
Ultraviolet Light
Ultraviolet light for biological experiments is usually obtained from a mercury lamp. In quantitative experiments it is necessary to use monochromatic ultraviolet light, since the biological effectiveness per unit of energy varies very much with wavelength. The energy of a single quantum of ultraviolet light is connected with the wavelength (A) in Angstroms by the relation:
Energy in electron volts = 12,400
X-radiation like ultraviolet light is an electromagnetic radiation emitted in quanta and on account of their greater penetrating power, it is not usually convenient to measure the total energy incident on- a surface, but to measure the
energy absorbed in a given volume. The unit of dose employed is roentgen-defined as the quantity of X- or Y-radiation such that the associated corpuscular emission per 0.001293 g. of air (1 cm.3 of air at 00 and 760 mm. pressure) produces, in air, ions carrying 1 electrostatic unit of quantity of electricity of either sign.
The -rays used in biological work are those of radium and its products sealed in a closed container with sufficient thickness of wall to absorb X- and fi-rays (at least 0.5 mm. platinum or equivalent). &(-rays, like X-rays, are measured
in terms of roentgens. The radiations, which are natural X-rays of short wavelength, are emitted in a number of
dis-crete wavelengths ranging, with this filtration, in quantum energy from about 0.2 to about 2.2 Mev.
-Rays and Cathode y
-rays are fast electrons emitted from radioactive mate-rials and cathode rays are artificially accelerated electrons. The most convenient source of 3-rays is a thin-walled vessel
containing radon gas; a wall thickness of 0.05 mm. of glass or aluminum suffices to remove the *C-rays. The dose of
30
cathode rays is specified in terms of roentgen-equivalent-physical (rep) which is defined on page 24 .
,OC-Rays
o(-rays are the nuclei of helium atoms emitted sponta-neously by radioactive materials and also obtainable artifi-cially with the cyclotron. Their range in tissue is very short and produce many more ionizations per micron path in tissue than do electrons. By an obvious extension of the definition of the roentgen, we may define 1 r offC-rays as that dose which liberates in 0.001293 g. of air ions
carry-ing 1 e.s.u. of charge of either sign. Protons and Neutrons
Protons are hydrogen nuclei moving at high speed and are not emitted by radioactive materials but are obtainable as a beam from a cyclotron. Protons give a number of ioniza-tions per micron path intermediate between electrons and
oC -rays. Hydrogen nuclei already in the tissue are pro-jected at high speed as protons when the tissue is irradiated by fast neutrons. The "n" unit for fast electrons has been described by Evans (zQ.
Some useful units and their interconversion are given below:
Electron volt - 1 ev = 1.602 x 10-12 erg
= 3.82 x 10-20 gram-calories 1 Mev = 106 ev
1 r = 1 esu/cc. std. air
= 2.083 x 109 ion pairs/cc. std. air = 1.61 x 1012 ion pairs/gm. air
= 5.24 x 107 Mev./gm. air
= 83.8 erg/gm. air
1 ion pair in air produced by electrons, X- or Y-rays = 32.5 ev
1 ion pair in air produced byaC-rays, protons or neutrons = 35 ev
Primary Ionization and Energy Dissipation
Electrons, protons and o(-particles pass through tissue in paths which may be taken as straight for distances of a few microns. As the particle traverses the tissue it loses energy as a result of collisions with atoms. As the ioniz-ing particle slows down, the number of ionizations per micron path -- ion-density or specific ionization -- increases.
The ionization caused by a collision of the primary particle (electron, proton or a(-particle) is called primary ioniza-tion.
Secondary Ionization
At each primary ionization an electron is ejected by collision of the primary particle with an atom of the tissue. Often this electron is ejected with insufficient energy to make any ionizing collision on its own account, but if the electron is ejected with rather more energy it may produce a few ionizations. Occasionally, the electron ejected at a primary ionization has an energy of several hundred or even thousand electron volts and is able to produce a large number
32
of ionizations. The secondary electron thus forms a separate track branching off the main track and is called a &-ray. Such -6-rays play a rather important part in certain biologi-cal actions of radiation.CHEMICAL EFFECTS oF
34
The Ionic Yield
The yield of radiochemical reactions is expressed as the number of molecules of a specified substance formed or destroyed per ion-pair produced, a ratio represented as M/N. In experiments on dilute aqueous solutions the yield is
sometimes expressed in terms of micromoles reacting per 1000 r per litre of solution irradiated. To convert these units into molecules reacting per ion pair, it is necessary to know the mean energy dissipation per ionization. Conver-sion factors for expressing yields in radiochemical reactions as M/N values have been tabulated by Lea.* Energy dissipa-tions are 35 ev per ion-pair for aC-rays and 32.5 ev per ion-pair for electrons.
In the present discussion, only the radiochemical actions in liquids and solutions will be discussed. Kailan (so) has determined the ionic yield for a number of organic liquids exposed to 8- and X-rays and found values ranging from 0.1 to 8.
Indirect Action in Aqueous Solutions
A number of authors ( 2-1,$o, ' ' ' /
)
have studied the chemical changes produced by X-rays and radioactive radi-ations in dilute solutions. All such reactions are indirect reactions; that is to say, most of the molecules of solute*Action of Radiations on Living Cells by D. E. Lea, 1947, p. 34.
the radiation, but their reaction follows excitation or ion-ization of the solvent molecules.
Ionization or excitation of a water molecule by an ioniz-ing particle causes the production of an intermediary body which is usually referred to in the literature as "activated water." Providing that there is no reverse reaction, and that the products of the reaction do not compete for the activated water, the quantity of the solute remaining unchanged
dimin-ished linearly with the dose. If, however, there is a pro-tective agent present which secures most of the activated water, or if the products of the reaction themselves compete for activated water, then the gradient of the curve becomes less steep Cs the percentage of unchanged solute diminishes, due to the solute obtaining a continually smaller proportion of the total amount of activated water produced in the irra-diated solution.
Lea* has tabulated ionic yields for a number of reactions investigated in dilute aqueous solutions. Almost all the
ionic yields lie between 0.1 and 2.0. Lea suggests that acti-vated water is formed at the rate of about 1 molecule per
ion-pair.
Chemical Mechanisms off the Indirect Action in Aqueous Solutions According to Weiss (9) the ionization of water results in the formation of free hydroxyl radicals and hydrogen toms which
36
then undergo reactions with cell constituents or solutes in the solutions irradiated. The mainfeatures, according to Weiss are as follows:
The passage of an ionizing particle ionizes water H20 radiation H20+ + e~
The positive water ion dissociates as follows:
H20+ H+ + OH
e- + H20 H2O~
H20~ OH + H
The electron may alternatively, but probably rarely, combine with a hydrogen ion to form a free hydrogen atom.
H + e- H
In addition to the above-mentioned reactions, OH radicals may be formed by ejection of electrons from hydroxyl ions nor-mally present in water.
OH~ radiation > OH + e
If the hydroxyl radicals and the hydrogen atoms are close enough together, they may recombine (111)
H + OH H20
and no net change occurs. Lea has pointed out (5i1) that the distance between H and OH is usually so large on a molecular scale that other things are likely to happen before recom-bination occurs.
formed according to the reaction H + 02 HO2 2 HO2 )H2O2 + 02
The OH radicals which accumulate combine to give oxygen according to the reaction
OH + OH > H20 + 0
0 + 0
4
0The oxidation of inorganic ions by OH radicals and their reduction by H atoms take place as follows:
Fe++ + OH , Fe+++ + OH~ Ce++++ + H Ce+++ + H+
If we accept the hypothesis that the action of ioniz-ing radiation in aqueous solutions is indirect, we would expect ( I) the following features:
(A) that with an equal dose of radiation a dilute solu-tion would be relatively more affected than a more concen-trated one (dilution phenomenon) and
(B) that any additional substance in the solution, capable of reacting with the intermediate product, would divert some of this from its partner to itself (protection phenomenon).
38
The protection phenomenon has been confirmed through extensive studies by Fricke, Dale and Forssberg (.7, 6,2-),
Dale (16 ) lists the concentration (02) of a number of solutes which suffice to mduce to about 50% the number of molecules of alloxazin-adenine-dinucleotide reacting with a given dose, the dinucleotide itself being present in a concentration of C = 6.1 x 10-7 gram-molecule per liter.Reduced Yield in Dilute Solutions
Providing that the solute concentration is sufficiently high for nearly all the active radicals to be eliminated by collisions with solute molecules rather than by collision with each other, the ionic yield will be independent of the solute concentration. Lea points out that if the solute con-centration is so low that an appreciable proportion of the total number of active radicals combine with one another rather than react with solute molecules, then the ionic
yields will fall.
Mode of Chemical Action of X-Rays on a Ndn-Aaueous Solution Broda (it ) has recently published figures for the decom-position of ammonium persulphate in glycerine, the persulphate concentration varying from 0.04 to 0.13 g./cm.3. The 37%
dose appears to be independent of concentration over this range and it is inferred that there is no indirect action in glycerine solutions such as occurs in aqueous solutions. Lea, however, points out* that it is unwise to make this
deduction on the basis of observations which do not extend to low concentrations of the solute. Obviously more work needs to be done to elucidate the mode of chemical action of radiations in non-aqueous solutions.
The Target Theory
According to this theory, one speaks of the molecule or structure in which ionization has to be produced as the
"target," and the production of ionization in it as a "hit." The biological effect observed is due to the production of ionization in some particular molecules.
The production of gene mutation by ionization of the
gene molecule, or of chromosome breakage following the passage of an ionizing particle through the chromosome are actions of radiations to which the target theory is applicable.
Accord-ing to Lea, three investigations should form a part of any attempt to determine whether a given biological action of radiation is of the target-theory type.
1. Determination of the manner in which the numbers of organisms or cells affected increased with the dose of radia-tion.
2. Determination of the manner in which the effect of a given dose depends upon the intensity at which it is admin-istered.
3. Investigation of the relative effectiveness of differ-ent types or wavelengths of radiation,
40
The biological effect may be due to change produced in a single molecule by the single-ionization of that molecule or the ionizing particle may produce a number of ionizations in the cell. For a single-ionization type of action, the following results are to be expected:
(a) The survival curve is exponential.
(b) The effect of a given dose is independent of the intensity at which it is given, or of the manner in which it is fractionated.
(c) For the same degree of effect, the dose required with different radiations increases in the order X-rays, hard X-rays, soft X-rays, neutrons, oC-rays. It is probable also that the effect of a given dose will be independent of the temperature (Lea, 1947).
.oe of Survival Curve
We are now studying a single ionization type of action. The number of hits is simply proportional to the dose of
radiation given. If the dose given is such that only a small proportion of the targets are hit, no distinction need be made between the total number of hits and the number of targets hit. A straight line is obtained by plotting the yield of the reaction against the dose. If the dose used is larger so that the number of targets hit is a considerable propor-tion of the whole number, several hits may be obtained in a single target. Although the total numbers of hits increase in strict proportionability to the dose, the number of targets
hit increases more slowly, so that the yield plotted against the dose gives a curve which is convex upwards. If one is following the killing of bacteria, the numbers killed by successive increments of dose are not equal but each incre-ment of
dose
kills the same proportion of the number of organisms which have survived until then.*Effect off Radiations on Covalent Bonds
"In covalent compounds ( I ) the atoms are grouped to-gether into molecules by strong exchange forces. The mole-cules in turn are held together by relatively very weak forces of the Van der Waals type. The energy required to ionize a molecule is much greater than the energy required to break a bond within the molecule. When a molecule is ionized by radiation and subsequently neutralized, it is given more
than enough energy to break the bonds and will break up into fragments. The main types of actions occurring in organic comoounds are classified as follows by Allen ( I ): (a) con-densation to large molecules, (b) degradation to smaller molecules, (c) dehydrogenation, (d) hydrogenation.
Goldblith et al (34 ) have recently shown that when niacin in a concentration of 100 X per ml. was irradiated, decarboxylation to the extent of 28% occurred with as little as 0.17 x 106 rep of cathode rays, whereas splitting of the pyridine ring did not begin to occur until o.66 x 106 rep of cathode rays had been supplied.
4L2
Zirkle (115) has recently published a review article in which he has elaborated upon the relationship between
MICROBIOLOGICAL ASSAYS OF AMINO ACIDS
Since all the assays for amino acids were done by micro-biological methods, it seems justified to include a section on microbiological determination of amino acids. It is not intended to exhaustively review the literature on this sub-ject but an attempt is made to give the main principles and features of a microbiological assay. The experimental tech-niques of the assay, as employed in this study, are described in the section on experimental methods.
Principle of Microbiological Determinations
The ability of certain microorganisms to grow on
syn-thetic media permits the development of methods for the quanti-tative determination of each separate nutrilite in the medium. So for the development of a microbiological method, the first task that the experimentalist comes up to, is to select a suitable strain of the organism which may be a bacterium, yeast or a mold. The organism to be selected should fulfil the following requisites:
1. It should be specific for the nutrilite under investi-gation.
2. The organism should be reliable; that is to say, it should give consistent growth response under given conditions.
3. The organism should give a linear response over a wide range of concentrations of the nutrilite.
4. Nutritional requirements of the test organism should be known.
Methods of Measuring Growth iepnse
The extent of bacterial or yeast growth is measured by acidimetric titration or by turbidimetric observation in a photoelectric colorimeter. Acid-base titrations may be per-formed either by using a suitable internal indicator or
electrometrically. The extent of mold growth is determined either by weighing the mycelia or by linear measurement of their surface.
Assay Procedures
Hydrolysis of Proteins
Before a protein sample can be analyzed microbiologically by common lactic acid organisms, the protein must be hydrolyzed to amino acids. For hydrolysis we can make use of any one of the three catalytic agents; namely, enzymes, strongly disso-ciated acids and alkalies.
Proteolytic enzymes suffer from several disadvantages: (a) The hydrolysis seldom goes to completion.
(b) Due to the heat lability of the proteolytic enzymes, the temperature of the reaction usually has to be maintained at 8000. or less.
(c) The enzymes are proteins and undergo partial auto-lysis, thus increasing the amino acid content of the sample under test.
Strongly dissociated acids and alkalies do not suffer from the above disadvantages and hence are usually employed. The concentration of the reagent to be used in any specific
46
case is somewhat dependent on the protein to be hydrolyzed but is inversely proportional to the time and temperature of the reaction. It is usual to employ a volume of reagent
equal to 5 to 20 times the weight of the protein to be hydrolyzed. When the hydrolysis is carried out at atmos-pheric pressure, the following concentrations are usually employed*:
H0l 3 - 12 N, commonly 6 N H2so 4 4 - 8 N, commonly 8 N
Hl commonly 57 per cent
NaOH commonly 5 N
Ba (OH)2 commonly 14 per cent
Hydrolysis is considered complete when a maximum number of -COOH or -NH2 groups have been liberated. The carboxylic groups can be readily estimated by one of the modifications of the Schiff-Sorensen formal titration methods (9 ) while the amino nitrogen can be determined by the Van Slyke method
(qz).
Hydrolytic Losses of Amino Acids
Destruction of amino acids can and in some cases does occur during hydrolysis. The amount of destruction, however, is dependent upon the conditions; namely, the strength of acid or alkali, time and termperature of hydrolysis, presence or absence of oxygen or oxygen carriers, etc. For greatest
accuracy several methods of hydrolysis and the addition of
*Block and Bolling: "The Amino Acid Composition of Proteins and Foods," 1945, P. 283.
and after hydrolysis are recommended.
Alkaline hydrolysates have been used only for the deter-mination of tryptophan and phenylalanine. Lately workers have determined all amino acids except tryptophan in acid hydrolysates. Complete racemization of the amino acid during alkaline hydrolysis is assumed normally ( 41' 6S~, 48 / 17
86 ).
Krehl et a (48) investigated the possibilities of race-mization during alkaline hydrolysis and came to the conclu-sion that racemization of pure 1 (-) tryptophan does not take place when treated with 5 N NaOH or 14% Ba(OH)2-8H20. Their results substantiated the previous observations (93 ) that racemization of pure amino acids with alkali is induced with difficulty. Because of the fact that racemization does occur in the hydrolysis of proteins, it appears that racemization precedes the hydrolytic cleavage of the peptide bond (4'8). Microbiological Assay
Basal medium containing all the nutrilites (except the one to be estimated) known to be essential for the test
organism is prepared. The sample is run at a series of con-centration levels in parallel with the pure substance to be determined. The concentration of this substance present in the sample is then determined by comparing the effect of
sample and standard on growth at the various concentration levels.
48
Recovery Experiments
When a known amount of the amino acid being determined is added to the sample, it must be quantitatively recovered. High or low recoveries indicate stimulation or suppression of response to the amino acid by other substances present in the sample.
Agreement Between Different Assay Organisms
If two different organisms having different nutritional requirements yield the same assay value on a sample, chances are that the assay walue is correct.
Historical Development off Microbiological Assays
This subject has been reviewed very exhaustively by Dunn (-io)and Snell (79, So, 81 ).
It was reported in September 1943 by Kuiken et al (47 ) of the Texas Agricultural Experiment Station that nine amino acids (cystine, glutamic acid, isoleucine, leucine, lysine, phenylalanine, threonine, tryptophan and valine) were
essen-tial for the growth of Lactobacillus arabinosus 17-5 and that three amino acids (isoleucine, leucine and valine) could be determined by microbiological techniques similar to those
utilized for vitamin assays. The basal medium employed was that developed by Snell and Wright (82-) in 1941 for the
determination of nicotinic acid modified to contain p-amino-benzoic acid, a tomato juice eluate and 17 amino acids in place of acid-hydrolysed casein. Green and Black in 1943
(35) reported on the determination of tryptophan with L. arabinosus and Shankman et al developed basal media for the