Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Biochemistry, 26, 25, pp. 8399-8405, 1987-12-15
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=6141d6e8-9443-4020-a147-1cdaf926b5fe
https://publications-cnrc.canada.ca/fra/voir/objet/?id=6141d6e8-9443-4020-a147-1cdaf926b5fe
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1021/bi00399a055
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Conformational differences between linear α(2
➝8)-linked
homosialooligosaccharides and the epitope of the group B
meningococcal polysaccharide
Conformational
Differences
between
Linear
a(2—*8)-Linked
Homosialooligosaccharides
and
the Epitope
of
the Group
B
Meningococcal
Polysaccharide*
Francis Michon, Jean-Robert Brisson, and Harold J. Jennings*
Division
of
BiologicalSciences, NationalResearch Councilof
Canada, Ottawa, CanadaK1A 0R6 ReceivedApril
29, 1987; RevisedManuscriptReceivedJune22, 1987abstract: The a-(2-*-8)-linked sialicacid oligosaccharides
(NeuAc)„
exhibitan unusual degreeof
het-erogeneity in the conformation
of
theirlinkages. Thiswas diagnosedby observation intheir 13CNMR
spectraof
an equivalent andunique heterogeneityinthechemicalshiftsof
theiranomeric carbons and subsequently confirmed bymore comprehensive'H
and 13CNMR
studies.In
these studiesboth one-dimensional and two-dimensional experimentswere carried outon thetrisaccharide (NeuAc)3andcolominicacid. Inaddition tothe unambiguousassignmentof
the signalsin thespectra, theseexperiments demonstratedthatbothlinkagesof
(NeuAc)3 differed in conformation fromeachother andfromtheinner linkagesof
colominicacid. TheNMR
dataindicatethattheseconformationaldifferences extend tobothterminaldisaccharidesof
oligo-saccharideslarger than (NeuAc)5, a resultthathasconsiderable physical and biologicalsignificance.
In
the context
of
the groupBmeningococcalpolysaccharide,it
providesan explanationfortheconformationalepitope
of
the groupBmeningococcal polysaccharide, whichwas proposedon theevidencethat(NeuAc)10,larger than the optimumsize
of
an antibody site, was the smallest oligosaccharide able to bind to groupBpolysaccharidespecificantibodies. Because the two terminal disaccharides
of
(NeuAc)10differ
incon-formation to its inner residues, the immunologically functional part
of
(NeuAc)10residesin its inner six residues. This numberof
residues is now consistentwith
the maximum sizeof
an antibody site.egroup B meningococcal polysaccharideisahomopolymer
of
a-(2—’ 8)-linked sialic acid residues and is structurallyidentical withbothcolominicacid(Bhattacharjeeet al., 1975) and the capsular polysaccharide
of
Escherichia coli K1(Jennings, 1983). It is poorly immunogenic (Wyle et al., 1972), and although groupB meningococcal organisms are able to producelowlevelsofgroup Bpolysaccharidespecific
antibodies inanimals and humans,theseantibodiesare almost
exclusively
IgM
andoflowaffinity
(Mandrell & Zollinger,1982). The poor immunogenicity of the group B
poly-saccharide is probably attributable to tolerance due to
cross-reactive tissue components, and this hypothesis is
strengthenedby theidentificationofa-(2— 8)-linked oligomers of sialic acid common to both the group B meningococcal
polysaccharide(Jennings et al., 1985) and the glycopeptides ofhumanandanimaltissue(Finneet al., 1983a). GroupB
polysaccharide specific antibodies are induced bya confor-mationally controlledepitope. Thiswas unambiguously con-firmed by the fact that an unusually largea-(2—8)-linked
sialic acid oligomer (decasaccharide) was required eitherto
functionas an inhibitor for(Jennings et al., 1985)or tobind
to (Finne & Makela, 1985) these antibodies. The glyco-peptidesofhuman andanimalfetal brainalsocontain thesame
epitopeand inconsequence bindto group Bpolysaccharide
specific antibodies (Finne et al., 1983b; Finne & Makela,
1985). In thispaper theconformational natureofthe epitope associatedwith theformationofgroupBmeningococcal
po-lysaccharide specific antibodieshasbeenconfirmedby high-resolution NMR studies on colominic acid and some ofits
constituent oligosaccharides. These studiesindicate thatat leastfive residuesare requiredbeforealinkagewithasimilar
orientationto thatoftheinternal linkages
of
colominicacidisgenerated.
ThisisNationalResearchCouncilofCanadaPublicationNo.28336.
Experimental Procedures
Materials. Colominic acid (E. coli) was obtained from
Sigma Chemical Co., St. Louis, MO, anda high molecular
weight fractionwas usedin theNMR experiments. Thiswas
obtained by passing colominic acid (Na+ salt) through a Sephadex G-100 columnwithphosphate-buffered saline (PBS) at pH7.0as eluant andisolatingthefraction thatelutedat = 0.1 (20-25 sialicacid residues). Oligosaccharidesof
DP= 2-6were obtained by thepartial
hydrolysisofcolominic acid andfractionationofthe depolymerized fragments in order
of
ascending molecular sizeon an ion-exchange column as describedby Jennings et al. (1985).NMR Methods.
'H
and 13CNMR spectrawere recorded on BrukerAM500andAM200spectrometers at 300K with acetoneas theinternal chemicalshiftreference (2.225 ppmfor
'H
NMR and31.07ppmfor13CNMR).
Internalacetoneisreferenced toexternal tetramethylsilane. Polysaccharides and oligosaccharideswere exchangedtwicewith 99.7%D20 and then run in 0.4 mL of 99.99% D20 (5-mm tubes) at
concentrations
of
50-100mg/mL.Homonuclearshift-correlated 2-DNMR (COSY)
experi-ments and homonuclear /-resolved 2-D
NMR
(JRES) ex-perimentswere carriedout according toAueetal. (1976) andNagayamaet al. (1980). COSY experimentswith two- and three-steprelayed coherencetransferwere doneaccordingto
Wagner (1983) and Bax andDrobny (1985). The
heteronu-clearshift-correlated 2-D NMR experimentwas carriedout
accordingto Bax et al. (1981),andthechortle experiment
was donewiththe pulsesequenceofPearsonetal.(1985). All phasecyclingswere accordingtothestandard software pro-vided byBruker(DISB86),andallexperimentswere donein
the magnitude mode. Nuclear Overhauser enhancements (NOE)were obtained by difference experimentswith multiple irradiationofeachlineofamultiplet(Neuhaus, 1983; Kinns
&Saunders, 1984). TransientNOEvalueswere obtainedwith
8400 BIOCHEMISTRY MICHON ET AL.
Table I: ChemicalShifts"ofSignals inthe 13CNMR Spectrumof
a-(2—*8)-Linked1 [(NeuAc)3] residuesofI* carbon C b a 1 174.19 173.10 175.59 2 101.36 102.87 97.35 3 41.22 41.70 40.06 4 69.27 74.48 68.28 5 52.55 53.19 53.19 6 73.46 68.81 71.50 7 68.99 69.99 68.49 8 72.55 79.05 76.50 9 63.44 62.05 61.89 C=0 175.73 175.73 177.76 ch3 23.07" 23.16" 22.88 “In ppm from internal acetone. Assignments confirmed by 2-D
NMR (C,H)COSY experiments. 6
Contiguousresiduesfromthe
re-ducingresiduea. "Tentativeassignments.
Table II: ChemicalShifts"ofSignalsinthe13C NMR Spectrumof a-(2—*8)-Linked (NeuAc)4 residuesof(NeuAc)/ carbon d c b a 1 174.30 173.89 173.15 175.60 2 101.19 101.90 102.81 97.35 3 41.20 40.89 41.61 40.08 4 69.33 68.97 74.56 68.26 5 52.56 53.20 53.20 53.20 6 73.43 74.01 68.97 71.57 7 68.97 70.24" 69.69" 68.61 8 72.55 78.51 79.16 76.73 9 63.44 62.29" 61.88" 61.88 C=0 175.41 175.71 175.71 177.72 ch3 23.08" 23.08" 23.15" 22.88 "In ppm from internalacetone. 6Contiguousresiduesfromthe
re-ducing residuea. "Tentativeassignments.
figure 1: Structureoftrisaccharide1. Arrows depictNOEspertinent totheconformationof1.
a 180° selectivepulse(Morris & Freeman, 1978). Proton spin
simulation witha linewidthof0.5 Hz was performedwith the Bruker programpanic.
Results
and DiscussionBoth13Cand
'H
NMR spectroscopic studieson colominicacid andsome ofits component oligosaccharidesconfirmthat theinterresidue linkageconformationoftheterminallylocated
sialic acidresiduesofthis a-(2— 8)Tinked sialicacid
homo-polymer differsfrom thoseofits inner residues. Colominic acid was used in thisNMR study becauseit was easily ob-tainableinamolecularsizesmall enough to yield well-resolved
spectra. However,becausetheyare structurally identical with
colominic acid, the capsular polysaccharides of group B
Neisseria meningitidisandE.coliK1 must alsoexhibit this
phenomenon.
Conformationaldifferenceswere first diagnosedby
obser-vationin the 13CNMR spectraofaseriesofa-(2— 8)-linked
sialooligosaccharides,(NeuAc)„,in whichn = 2-6,extensive heterogeneityparticularlyin the chemical shiftsof theirlinkage
carbons (C2andC8). The chemical shiftsofthe carbonsof thesialicresiduesofthe oligosaccharides andcolominicacid are listedinTables
I-IV,
thelatterbeinglistedin TableIII.
Assignmentsmadeon thetrisaccharide (1) shown in Figure 1 andon colominic acidwere obtainedfrom2-D
(H,H)
andTable III: ChemicalShifts"ofSignalsinthe13CNMR Spectrumof a-(2—8)-Linked(NeuAc)sandColominic Acid6
carbon residuesof (NeuNAc)'s‘ colominicacid e d c b a 1 174.32 173.88 173.88 173.07 175.60 174.00 2 101.18 101.90 101.72 102.92 97.35 101.78 3 41.12 40.78 40.78 41.60 40.02 40.76 4 69.23 68.94 68.94 74.46 68.19 69.13 5 52.53 53.15 53.15 53.15 53.15 53.23 6 73.39 74.04 74.04 68.94 71.40 74.01 7 68.94 70.23* 69.80* 69.71* 68.55 69.86 8 72.55 78.56 78.56 79.06 76.43 78.72 9 63.41 62.28 62.05 61.80 61.80 62.06 C=0 175.70 175.70 175.70 175.70 177.75 175.76 ch3 23.10* 23.24* 23.24* 23.10* 22.89 23.30
"In ppmfrom internalacetone. 6Assignmentsobtainedfromchortle experiment. "Contiguousresiduesfromthereducingresiduea. ^Tentative
assignments.
TableIV: ChemicalShifts" of Signals inthe l3CNMR Spectrumofa-(2—-8)-Linked (NeuAc)6
carbon residuesof(NeuAc)66 f e d c b a 1 174.31 173.94 173.94 173.94 173.15 175.61 2 101.17 101.85 101.74 101.74 102.80 97.35 3 41.21 40.74 40.74 40.74 41.60 40.08 4 69.20 69.07 69.07 69.07 74.55 68.29 5 52.56 53.21 53.21 53.21 53.21 53.21 6 73.41 74.02 74.02 74.02 69.07 71.58 7 69.07 70.27 69.84 69.84 69.33 68.66 8 72.54 78.57 78.65 78.65 79.22 76.75 9 63.44 62.29 62.05 62.05 61.83 61.83 C=0 175.71 175.71 175.71 175.71 175.71 177.71 ch3 23.10" 23.29" 23.29" 23.29" 23.10" 22.87 “In ppm from internalacetone. 6Contiguousresiduesfromthereducingresiduea. "Tentativeassignments.
0
n*6
figure 2: 13Cresonances oftheanomeric carbonsof a-(2-*8)-linked
homosialooligosaccharides (NeuNAc)„wheren = 2-6.
(13C,H)COSYexperiments and the assignments madeon the
other oligosaccharideswere madewitholigosaccharide 1as a model. Whilethe assignments madeon colominicacidare
in substantial agreementwiththose reported byBhattacharjee
etal.(1975), theyconfirmthatthelatterassignments made on the closely spaced C4 and C7 signals should have been reversed.
Thesignalsoftheanomeric carbonsofthe oligosaccharides are shown in Figure2 and revealthe heterogeneity in their chemical shifts. Exceptforthe signalsoftheanomeric carbons ofthereducing sialicacid residues, which remainconstant at 97.35 ppminthe spectraofallthe oligosaccharides, coincident signals donotoccur untilthe hexasaccharideisreached, and these signals at 101.74 ppmalso coincide with that ofthe
anomeric carbon signal
of
colominicacid. A similarpattern ofchemicalshiftheterogeneityisalsoexhibited bytheother linkagecarbons at C8on theaglyconic sialicacid residuesof the oligosaccharides (TablesI-IV).
Fromtheseresultsit
can beinferredthatheterogeneity inlinkage conformation inthe hexasaccharide is presentin both its terminaldisaccharidesandthatby analogy thisisalsotrueforcolominicacid. The conformational dependenceof13C
NMR
chemicalshifts oflinkagecarbonswas firstproposed by Colson et al. (1974) and was laterusedempirically tolocateconformationallycontrolled
determinants in complex polysaccharides (Jennings et al., 1984). However, only recentlyhasthis correlationbetween
glycosidic torsion angle(\p)andchemicalshiftbeenvalidated
withHSEAcalculationson aseriesofoligosaccharideshaving
either a-D-glucopyranosylor a-D-galactopyranosylresidues at the nonreducing end(Bocket al., 1986). Our 1-Dand2-D
’H
NMR studieson trisaccharide1 andcolominicacid alsoprovidefurther evidence forthevalidity ofthis correlation.
Inordertocompare the differences in the linkage
confor-mations between the oligosaccharidesandcolominic acid, a 1-D and 2-D
‘H
NMR studywas carriedout on 1 andcolo-minicacid. Trisaccharide1,shownin Figure 1, was chosen asthe oligosaccharide to studybecauseitapproximatesto the
Table V: ChemicalShifts0ofSignalsinthe*HNMR Spectrumof
a-(2—*-8)-Linked1 [(NeuAc)3]andColominicAcid residuesof1
proton c b a colominicacid
H-3a 1.729 1.639 1.748 1.737 H-3e 2.757 2.691 2.201 2.673 H-4 3.668 3.547 3.983 3.600 H-5 3.821 3.782 3.863 3.819 H-6 3.615 3.555 3.877 3.626 H-7 3.576 3.839 3.760 3.896 H-8 3.897 4.113 3.996 4.102 H-9 3.884 4.132 3.940 4.188 H-9'‘ 3.633 3.666 3.733 3.665 ch3 2.028 2.064° 2.060° 2.083 “Spin simulated parameters. In ppm from internal acetone. ‘Primarygeminal protonsare distinguishedbymeans ofa prime for
the proton withthe largest vicinalcoupling constant. 0
Assignments
maybereversed.
TableVI: VicinalandGeminal Coupling Constants(Hz) of a-(2-*8)-Linked1 [(NeuAc)3]°andColominicAcid‘
residuesof1 /h,h c b a colominicacid 3a,3e -12.2 -12.2 -12.9 -12 3a,4 12.0 12.1 11.5 12 3e,4 4.4 4.4 5.0 5 4,5 10.0 10.2 10.3 10 5,6 10.3 10.3 10.3 10 6,7 1.9 1.0 0.5 <3 7,8 9.1 2.3 6.9 <3 8,9 2.6 4.7 2.8 5 8,9' 6.4 6.9 3.9 6 9,9' -12.1 -12.3 -12.3 -12
"Spin simulated parameters. ‘Taken from resolution-enhanced
spectrum.
smallest structure incorporating both terminaldisaccharides ofcolominic acid. Strictlyspeaking, the tetrasaccharide would have been a more precisemodel but the analysisof its *H
NMR spectrum would havebeen prohibitive.
The
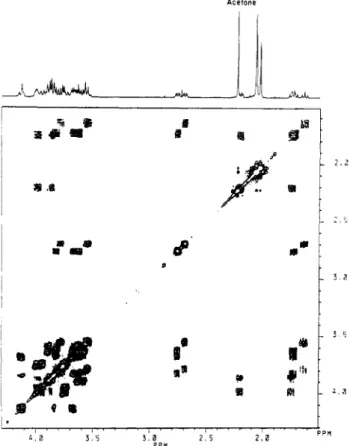
’H
NMR
spectrumof
thetrisaccharide (1) isshownin Figure 3 and was
difficult
to assign directly due to itscomplexity; therefore,aproton homonuclear correlated 2-D NMR
[(H,H)
COSY] experimentwithtwo- andthree-steprelaycoherencetransferwas performed,and thespectrumis
alsoshownin Figure3. Asaresultofthisexperiment,it was possible to assignallthe protons ofeach sialic acid residue
in 1. Improved resolutionofthe
'H
NMR spectrumof1 was alsoobtainedwhen signalswere further separated along the / axis by two-dimensional /-resolved spectroscopy. Mea-surement ofchemical shiftsandcouplingconstants fromthe contour plot of the two-dimensional /-resolved spectrum permitted rapidand unambiguousassignmentof individualprotons within the unresolved multiplets observed in the one-dimensional spectrum. The chemicalshifts andcoupling constants oftheprotons in 1 are listedin Tables Vand
VI,
respectively. Additionalconfirmation ofthe assignmentswas also obtained by a proton simulation experiment. In this
experiment the coupling constants and chemical shifts obtained from the /-resolved spectrum were fed into a program, a nine-spin systemforeachsialicacid residueof1. The signals associatedwitheachresidueare shownin Figure4,andthe
spectrum obtained by the addition of all these signals is
identicalwiththatoftheresolution-enhanced one-dimensional
'H
NMR spectrumof 1.Having unambiguouslyassignedalltheprotonresonances, NOEexperimentscouldthen becarriedout. SelectedNOE difference spectrafor 1are showninFigure5,andthe relative
8402 BIOCHEMISTRY MICHON ET AL.
TableVII: Nuclear Overhauser Enhancementsfor a-(2—8)-Linked1 [(NeuAc)3]
observedNOE(negative)
saturatedsignal0 H3a H3a h4 h5 h6 H, Hg H, H<y
H3e(a) (a) 12 (a)4
H3e(c) (c) 11 (c) 6 (c)2
H3e(b) (b) 13 (b)9
H3a(c) (c) 14 (c)7 (b) 1
H3a(a) (a) 10 (a)2
H3a(b) (b) 17 (b)4 (b) 10
Hg(b) (c) 1 (b) 4 (b) 5 (b) 17 (b) +
IHs(b) (c) 2 (b) 5 (b) 5
»H,(b) (a)4 (b) 6
“aisthereducingresidue, bisthemiddleresidue, andcisthe nonreducingresidueof1.
Acetone
,A>L_
withthree-steprelay,of1 in D20(310K)withthe 1-D spectrum
above. Theinitial(r3,t2)matrix of256 X2048pointswas zero-filled to1024X 2048 points andprocessedwithunshiftedsinebell window functions, a magnitude calculation,andsymmetrization about the diagonal. Thefinal resolution in bothdimensionswas 1 Hz/point.
NOEsobtainedfromthesespectraare listedin TableVII. An
examinationofthecouplingconstantsandNOEsofthe pro-tons associatedwiththetwo differentlinkagesin 1indicates
astriking difference in conformationbetween these linkages. Thatconformational differencesare confinedto thelinkage regionsof1isconfirmed by thefact thatthe coupling constants
andNOEs ofthering protonsof1 indicatethatthe
confor-mationsoftheindividual sialic acid rings remain essentially inthesame conformation(5C2) asdescribedfortheirrespective monomericunits (Joachims et al., 1967). Thisisalsotruefor the exocyclic chain oftheterminal residue(c)of1,the
pre-ferredconformationofwhichissimilarto thatproposed by others(Brownet al., 1975; Sabesanet al., 1983;Lindonet al., 1984).
Despite the factthat thelinkageregions between the
res-iduesof1are complex, bothare composedoffourbondswith
potential for unrestricted rotational freedom, the coupling
constants(Table
VI)
andNOEdata(TableVII)
indicatethatthelinkagecarbons adopt aratherpreciseconformation. For
.jaIUwxJi—ll_,11
JiLii
a)a. I 4.0 5.9 58 3.’ 3.C
figure 4: Comparisonofthesimulated and experimental spectrum
of1. Simulationforeachindividualresidue (a,b,and c)isshown
in (a), (b),and(c)andtheirsum in (d). The resolution-enhanced
experimentalspectrumisshownin (e).
the exocyclic chainofresidue bof1,astrongNOEbetween
HjjandHjj (Figure5) and the smallvicinalcoupling constants (Table
VI)
associatedwith these protons indicatethat bothsets ofrotamers about theC8-C7 bond and the C7-C6 bond
are confined toa preferredrotamer with<t>(H8,C8, C7, H7)
= +60° forthe C8-C7 bond and
<j>(H7, C7, C6, H6) = -60°
forthe C7-C6 bond. In addition the orientationofthe
non-reducing terminal sialicacid residue (c)in relation toresidue b can be determined from the NOE between H|a and H8,
whichputs these two protons inclose proximity.
Theconformationofthelinkage between residuesbanda of1 (Figure 1) iscompletely differentto thelinkagebetween residuescandb. Residuea,whichisinits preferred/3-dform
as determinedby
'H
and 13C chemicalshift data (Tables I andV) (Brownet al., 1975;Jennings&Bhattacharjee, 1977),is bent back toward the middle residue (b). This can be
deduced by the enhancement of the H7 signal when H9 is
irradiated, indicatingtheproximityofthesetwo protons. The couplingconstantsare alsoinagreementwitha large change
in thelinkage conformationbetween residues b anda. Inthis case thecouplingconstant betweenHjjand H7isalsosmall, indicating that they are in gauche orientation, while the
couplingconstant betweenH7CandH|cislarge, showingthat
these two protons are trans to eachother.
The chemical shiftsandcouplingconstants associatedwith
the protonsofthesialicacid repeatingunitofcolominicacid were derivedfromits resolution-enhanced
'H
NMR spectrum andare alsolistedinTablesVandVI. Similarvaluesfortheseparameterswere alsoreportedforsome oftheseprotonsby
TableVIII: Nuclear Overhauser EnhancementsforColominicAcid
saturated signal
observedNOE(negative)0
H3a H3e h4 h6 Hy h5 h7 h8 h9 H3a 32 9 7 1 4 H3e 32 15 3 1 2 h5 5 2 8 1 h7 <1 8 8 8 3 h8 3 1 9 6 9 14 h9, 4 17 23 0InterresidueNOE
intensityvaluesare underlined.
2LA
(iv)figure 5: NOEdifference spectraof1. Resonancesofresidueaare notunderlined,thoseofresidue bare underlinedonce,and thoseof
residuec are underlined twice. The spectra represent theirradiation
of(i)H3aofb, (ii) H3aofaandH3aofc,(iii) H3eofb,(iv)H3eof c, (v)H8and H9ofb,and(vi)H8 (mostly) ofb. The spectrumof 1 isshownin (vii).
directlyon colominicacid. SelectedNOEdifferencespectra
are shownin Figure6, andtherelativeNOEs obtained from
thesespectraare listedin Table
VIII.
Fromthisinformationit
can be determined that the conformation of the linkage betweentwoconsecutivesialic acidresidues (a and bof2 as shown in Figure 7) located internally in colominic acid isdifferent from thatofboth linkagesin1. Althoughthe linkage
conformation
of
2ismore similartothatbetween residuesc and bof
1than thatbetween residues banda of1,thedif-ferencesin the formerare stillstrikingand show, despite the
possibilityofaveraging,aconsiderabledifference in the ori-entationofthesialic acid residues. That theorientationof residuesa and bof2 isdifferenttothatofresiduescand b of1can bedeterminedfromtheNOEdataof2(Table
VIII)
wherean enhancementon Hgwas not onlygeneratedbyir-radiatingH3a,as was thecase withthe equivalent signals (Hb,
H3a) in 1(Table
VII),
butalsobyirradiatingH3e. Thereforefor 2, H8 must be situated midway between, and in close proximityto, both geminalH3 protonsofresiduea. Onthe
basisofcoupling constants(Table
VI)
andNOEs (TableVIII)
assigned to Hb, Hb, and Hg, the remainder of the linkage regionof2 appears to besimilarto thatbetween residuesc
7 CH3
' 1 ' 1 1 1 1 1 1
1 1 1 ! 1 1 1 1 1 ' 1 1 1 1 1 '
4.2 4.0 3.8 3.6 3.4 3.2 3.0 2.8 2.6 2.4 2,2 2.0 1.8 l.S PPM
figure 6: TransientNOEdifference spectraofcolominic acidwith
50-ms delayafterselectiveinversionof (i)theH3aresonance and(ii)
theH3e resonance. The reference spectrum isshownin (iii),and
interresidueenhancementsare underlined.
2
figure 7: Structureofdisaccharide2locatedinternally incolominic acid. ArrowsdepictNOEs pertinenttotheconformation of2.
and bof1,although minorchangeswouldstillbecompatible
withthe data. TheNOEbetween Hband Hg, isalso present
in 2,andthe couplingconstants between H^b,
H?
andH7\
H|bare ofthesame orderofmagnitudeasthosefoundforthe equivalent protonsin1(Table
VI).
Aminor difference intheNOEpatternbetween 1and2was that irradiationofH3aand H3a also causeda smallenhancement on H7b; however, this may not be important to the linkage conformation. CPK modelsof2showthatthecloseproximity ofbothgeminalH3 protonsof2toH7bisunlikelyandthatthereforetheseNOEs couldbe attributedto threespin effects.
The aboveNMR analyses demonstratethatthe linkagesin
1 are conformationally differenttoeachother and to theinner linkagesofcolominicacid. Theextent ofthisdifferencecan
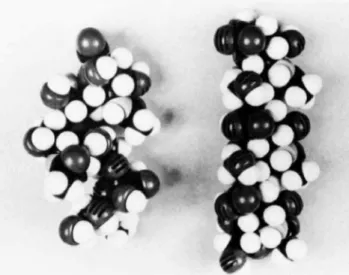
be seen by examining CPK modelsof 1 and an equivalent
internallylocated trisaccharidesequenceofcolominicacid. The modelsare shownin Figure8,and two dominantfeatures
8404 BIOCHEMISTRY
figure 8: Space-fillingmodels(CPK) oftrisaccharide1(left)and
thesame trisaccharidelocatedinternallyincolominicacid(right).
readily visualized. First, due tothe folding backofthe re-ducing sialic acid residueof
I,
trisaccharide 1ismuchshorterthantheinternal trisaccharide. Second,the carboxylate groups of1 appear to berandomly arranged, whereas those
of
theinternal trisaccharideare essentially linedupon onesideof the molecule. Thisisexemplifiedbycomparingthecoupling constantsofthe H9 protonsof1 and colominic acid (Table
VI).
For I thevicinal couplingconstants ofthe H9protonsofeachresidueare all different,whereas thoseofcolominic
acid are all thesame.
Alltheseobservationshaveimportantbiological implications inthat the unusual length requirementof«-(2—*-8)-linked sialic
acidoligosaccharides (dccamer) to bind to groupB menin-gococcalpolysaccharide specific antibodies (Jenningset al., 1985; Finne
&
Makela, 1985)can be rationalized. The fact thatadecasaccharide istheminimumsize requirediscom-pletely consistent with our
NMR
studies because we have shown that the two terminal disaccharides of the deca-saccharidewoulddifferinconformationfromits innerresidues.Thus, theimmunologically functional moietyofthe decamer
istheinnersix residues. This numberisnow consistentwith the inhibition studies of Kabat and Mayer (1961) using a
dextran-antidextransystemin whichtheinhibition power
of
dextran-derived oligosaccharides maximized at the hexa-saccharide. Also consistentwiththelackofaconformationally
controlled determinantinthe dextransisthefactthat linear
a- and/9-D-(l-*4)-linkedanda- and /9-d-(1—6)-linked
oli-gosaccharidesrelated to dextranexhibitno conformational heterogeneity intheirlinkagesasdeterminedby theabsence
ofanomericandlinkage l3Cchemicalshiftheterogeneity in
their13C NMR spectra (Bocketal., 1984). It isinteresting
to notethatthe dependenceoftheconformationofthe group
Bmeningococcal polysaccharideon chain length probablyalso
explainsa similar unusuallylargeoligomer (eight sialicacid
residues) requirement
of
abacteriophage endosialidase,whichcleavescolominic acid (Finne & Makela, 1985).
ThatthegroupB polysaccharide containsapreferred
con-formationnot foundinthe smaller oligosaccharidescould be
dueto cooperativestabilization, whichisdependenton chain length. Theforcesinvolved in thisstabilizationare not known with certainty but wouldbea very important factor inany theoretical calculationsoftheconformationofthedeterminant. Obviously anypotential energycalculationswould requirean oligosaccharideofatleastsix residuestoadequately describe
the preferred conformation
of
colominic acid. Charge isMICHON ET AL.
probably involved, butdue to the factthat the isomeric
a-(2—»9)-linkedgroupC meningococcalpolysaccharide forms
aconventionaldeterminant(Jennings et
al„
1985),additional environmental factors mustbeimportant. Perhaps the uniquedispositionofthecarboxylateandacetamidogroupsin
colo-minic acid, each being lined up on opposite sides of the molecule (Figure 8), could be a factor in the stabilization
energy. This alignmentofthe carboxylate groups leavesthem
all incloseproximity to9-(hydroxymethyl)groups,which is consistentwiththereported(Lifelyetal., 1981)easeoflactone
formationbetweenthesegroups in thegroup B polysaccharide.
The closeproximity ofthese groups could alsoresult in hy-drogen bonding, possibly with the participation of water
molecules, which could also be a factor in stabilizing the
conformation
of
thegroup Bpolysaccharide.Inconclusion,itisinterestingto speculateastowhether the groupBpolysaccharideconformationaldeterminantisunique. Certainlythereissome preliminaryevidence that would in-dicatethatitisnot. For instance, thereare reports(Mehmet
etal., 1986)ofasimilarsizedependenceforoligosaccharides
obtained from keratan sulfateinbindingto keratan-specific
monoclonal antibodies. Also,the largemolecularsize(12-14 residues)ofdermatan sulfate oligosaccharides required to bind
to heparin cofactor II seems unusually large forthe entire
oligosaccharide tobeincludedin abindingsite(Tollefsenet
al., 1986).
Registry No. (NeuAc)3, 76152-09-5; (NeuAc)4, 96425-83-1;
(NeuAc)j, 110935-75-6; (NeuAc)6, 96425-82-0; colominic acid, 9013-15-4.
References
Aue, W. P„ Bartholdi, E. & Ernst, R. R. (1976) J. Chem. Phys. 64,2229-2246.
Bax,A., & Morris,G. (1981)J.Magn. Reson.42,501-505.
Bax,A.,& Drobny,G. (1985)J. Magn.Reson.61, 306-320. Bhattacharjee, A. K.,Jennings, H.J.,Kenny,C.P., Martin,
A.,& Smith,I.C.P.(1975) J.Biol.Chem. 250, 1926-1932. Bock, K., & Pedersen, C. (1984) Adv. Carbohydr. Chem.
Biochem. 42, 193-225.
Bock,K., Brignole,A.,& Sigurskjold,B.W. (1986) J. Chem.
Soc.,Perkin Trans. 2, 1711-1713.
Brown, E. B., Brey, W.S.,Jr., & Weltner, W., Jr. (1975) Biochim. Biophys. Acta 399, 124-130.
Colson,P., Jennings,H.J., & Smith, I. C.P. (1974) J. Am.
Chem. Soc. 96, 8081-8087.
Finne, J., & Makela, P. H. (1985) J. Biol. Chem. 260,
1265-1270,
Finne, J., Finne, V., Deagostini-Bazin, H., & Goridis, C.
(1983a)Biochem.Biophys. Res.Commun. 112,482-487. Finne, J.,Leinonen, M., & Makela, P.H. (1983b)Lancet2
(No. 8346), 355-357.
Flippen, J. L. (1973) Acta Cryslallogr., Sect. B. Struct.
Crystallog. Cryst.Chem. 29, 1881-1886.
Jennings, H.J.(1983) Adv. Carbohydr.Chem.Biochem. 41,
155-208.
Jennings, H.J., & Bhattacharjee,A. K. (1977) Carbohydr.
Res. 55, 105-112.
Jennings,H.J.,Katzenellenbogen, E.,Lugowski,
C„
Michon,F., Roy, R., & Kasper, D. L. (1984) PureAppl. Chem. 56,
893-905.
Jennings, H.J., Roy,R., & Michon, F.(1985)J. Immunol.
134, 2651-2657.
Joachims,J.,Taigel,G., Seeliger,A.,Lutz,P., & Driesen,H. (1967) Tetrahedron Lett., 4363-4369.
Kabat,E. A.,& Mayer, M. M. (1961) in Experimental
Kinns,M.,
&
Saunders,J.K.M. (1984)J.Magn.Reson. 56,518-520.
Lifely,M.R.,Gilbert, A.S.,
&
Moreno, C.0981) Carbohydr. Res. 94, 193-203.Lindon, J. C., Vinter, J. C., Lifely, M. R.,
&
Moreno, C.(1984) Carbohydr. Res. 133, 59-74.
Mandrell,R. E., &Zollinger, W.D. (1982) J.Immunol. 129,
2172-2178.
Mehmet,H.,Scudder, P.,Tang,P.W., Hounsell,E. F., Ca-terson, B., & Feizi, T. (1986) Eur. J. Biochem. 157,
385-391.
Morris, G. A., & Freeman, R. (1978) J.Magn. Reson. 29,
433-436.
Nagayama, K., Kumar, A., Wiithrich, K., & Ernst, R. R.
(1980)J. Magn. Reson. 40, 321-334.
Neuhaus, D. (1983) J. Magn. Reson. 53, 109-114.
Pearson, G. A. (1985) J. Magn. Reson. 64, 487-500.
Sabesan, S., Bock, K., & Lemieux, R. U. (1984) Can. J. Chem. 62, 1034-1045.
Tollefsen,D.M., Peacock, M. E., & Monafo, W. J. (1986)
J. Biol. Chem. 261, 8854-8858.
Wagner, G. (1983) J. Magn.Reson. 55, 151-156.
Wyle, F. A.,Artenstein, M. S.,Brandt, B. L., Tramont, D.
L.,Kasper, D.L.,Altieri,P., Berman,S.L., &Lowenthal,
J. P. (1972)J. Infect. Dis. 126, 514-522.
Biosynthesis
of
a
Specifically Deuteriated Diunsaturated
Fatty Acid
(18:2A6,9)
for
2H
NMR
Membrane
Studies'^
John E. Baenziger,*’*’******8 Ian C. P.
Smith,*’8 and Robin J. Hill8
Department
of
Biochemistry, Universityof
Ottawa, andDivisionof
BiologicalSciences, NationalResearch Councilof
Canada,Ottawa, Ontario, CanadaK1A0R6
ReceivedMay 13, 1987; RevisedManuscriptReceivedAugust 14, 1987
abstract:
A
unique procedurefor
the biosynthesis and subsequent isolationof
a seriesof
specificallydeuteriated c/y,m-octadeca-6,9-dienoicacidshas beendeveloped.
An
auxotrophof
Tetrahymena, whichlacks A9 and A12 desaturaseactivity,issupplementedwith specificallydeuteriated oleic acid and converts
it
to the corresponding deuteriated dy,c/y-octadeca-6,9-dienoic acid, 18:2A6,9. Thedeuteriatedfatty
acidis subsequently isolated by argentationchromatography and
HPLC.
Todemonstrate theutility
of
the procedure, we describe herethe biosynthesisof
cw,c/j-octadeca-6,9-dienoicaciddeuteriated at positions9 and 10. Gas and thin-layer chromatography
of
the isolatedfatty
acid showed thatit
was greater than 99% purewhile 13CNMR
and mass spectrometryof
theG-(trimethylsilyl)
derivativeconfirmedthatthe 18-carbonfatty
acid contains two doublebondslocated at positions6and 9. The yield,froman 11-L culture, wastypically
100 mgof
which 35% was found to be deuteriated at both the 9- and 10-positions. The deuteriatedfatty
acidwas esterified to l-hexadecanoyl-Jn-glycero-3-phosphocholine, and aqueous,multi-lamellardispersions
of
thelipidwere studied by2HNMR.
Each spectrumconsistsof
two overlapping powder patterns andthereforeyields twoquadrupolar splittings. Over atemperature rangefrom0to 40 °C,one splitting decreasesfrom6.6to 1.8kHz
whiletheotherincreases from4.5 to 5.3 kHz. The magnitudesof
the two splittings are equivalent between 10 and 15 °C. The values
of
thesplittings, and their responseto temperature,
differ
significantly from thoseof
the corresponding deuteriated oleic acid in microbialmembranes [Ranee,
M.,
Jeffrey, K. R.,Tulloch, A.P.,Butler, K. W.,&
Smith, I. C. P. (1980) Biochim. Biophys. Acta 600, 245-262] and in bilayersof
l-hexadecanoyl-2-m-octadec-9-enoyl-j«-glycero-3-phosphocholine (POPC) [Seelig, J.,
&
Waespe-Sarcevic,N.
(1978) Biochemistry 17,3310-3315], Theresults suggest
that
afatty
acyl chaincontaining two double bonds has physicochemical properties verydifferent
from thoseof
the corresponding acylchainwith
a single double bond.In
recent years considerable evidencehasaccumulatedsug-gestingaUniquerolefor polyunsaturated lipids in eukaryotic membranes. Theselipids modulateavarietyof
membrane-associated processes [for a review see Spector and Yorek (1985)] and are thought to playan essential rolein neural
tissue(Lamptey &Walker, 1976;Crawfordet al., 1984) and
in theretina (Neuringer et al., 1984).
The effectofpolyunsaturatedlipidsis generally attributed
to their ability to “fluidize” membranes. A high degreeof fThisworkwas supportedby theNaturalSciencesandEngineering ResearchCouncilandtheNationalResearchCouncilofCanada. J.E.B.
isarecipientofan Ontario graduatescholarship.
’Addresscorrespondence to thisauthor atthe NationalResearch
CouncilofCanada.
*
UniversityofOttawa.
8NationalResearchCouncilofCanada.
0006-2960/87/0426-8405$01.50/0
unsaturation inamembraneisthoughttocorrelatewithalow gel toliquidcrystal transition temperatureandahighdegree of mobilityand disorder
of
thelipids. Although the doublebonditselfis arelativelyordered,immobilestructure (Seelig
& Waespe-Sarcevic, 1978;Ranee et al., 1980;Dufourcet al., 1984), thiscorrelation appears to hold formembranes
con-tainingsaturated andmonounsaturated acyl chains(Davis & Keough, 1983; Stubbset al., 1981; Seelig & Seelig, 1977).
The extension tohighly unsaturatedsystems,however,hasno
rigorous physicochemical basis. For example, differential scanningcalorimetryhasshownthatliposomescontaininga
variety ofpolyunsaturated lecithins havesimilar transition temperatures(Coolbearet al., 1983), and fluorescence
depo-larizationofdiphenylhexatriene, in similarbilayers,suggests
thatthe order and ratesofmotionofthelipidacyl chains are
very similar(Stubbset al., 1981). Both techniques suggest

![Table V: Chemical Shifts0 of Signals in the *H NMR Spectrum of a-(2—*-8)-Linked 1 [(NeuAc)3] and Colominic Acid](https://thumb-eu.123doks.com/thumbv2/123doknet/14281540.491668/4.923.479.838.94.275/table-chemical-shifts-signals-spectrum-linked-neuac-colominic.webp)