HAL Id: hal-00719816
https://hal.archives-ouvertes.fr/hal-00719816
Submitted on 21 Jul 2012HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
physician
Marie Jauffret-Roustide, Julien Cohen, Isabelle Poisot-Martin, Bruno Spire,
Michael Gossop, Patrizia Carrieri
To cite this version:
Marie Jauffret-Roustide, Julien Cohen, Isabelle Poisot-Martin, Bruno Spire, Michael Gossop, et al.. Distributive sharing among HIV-HCV co-infected injecting drug users: the preventive role of trust in one’s physician. AIDS Care, Taylor & Francis (Routledge), 2011, �10.1080/09540121.2011.596515�. �hal-00719816�
For Peer Review Only
Distributive sharing among HIV-HCV co-infected injecting drug users: the preventive role of trust in one’s physician
Journal: AIDS Care - Psychology, Health & Medicine - Vulnerable Children and Youth Studies
Manuscript ID: AC-2010-11-0621.R2 Journal Selection: AIDS Care
Keywords: drug users, high-risk behaviours, co-infection HIV/HCV, trust in physician
For Peer Review Only
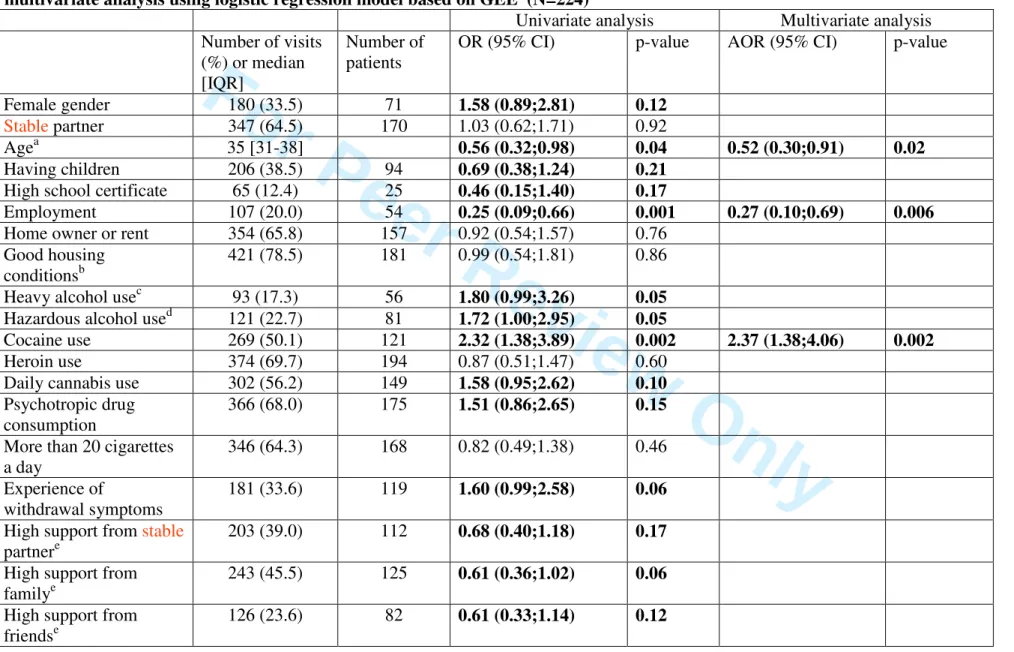
Table I : Factors associated with lending of injection equipment among IDU patients infected with HIV and HCV: univariate and multivariate analysis using logistic regression model based on GEE (N=224)
Univariate analysis Multivariate analysis Number of visits
(%) or median [IQR]
Number of patients
OR (95% CI) p-value AOR (95% CI) p-value
Female gender 180 (33.5) 71 1.58 (0.89;2.81) 0.12
Stable partner 347 (64.5) 170 1.03 (0.62;1.71) 0.92
Agea 35 [31-38] 0.56 (0.32;0.98) 0.04 0.52 (0.30;0.91) 0.02
Having children 206 (38.5) 94 0.69 (0.38;1.24) 0.21
High school certificate 65 (12.4) 25 0.46 (0.15;1.40) 0.17
Employment 107 (20.0) 54 0.25 (0.09;0.66) 0.001 0.27 (0.10;0.69) 0.006
Home owner or rent 354 (65.8) 157 0.92 (0.54;1.57) 0.76 Good housing
conditionsb
421 (78.5) 181 0.99 (0.54;1.81) 0.86
Heavy alcohol usec 93 (17.3) 56 1.80 (0.99;3.26) 0.05
Hazardous alcohol used 121 (22.7) 81 1.72 (1.00;2.95) 0.05
Cocaine use 269 (50.1) 121 2.32 (1.38;3.89) 0.002 2.37 (1.38;4.06) 0.002
Heroin use 374 (69.7) 194 0.87 (0.51;1.47) 0.60 Daily cannabis use 302 (56.2) 149 1.58 (0.95;2.62) 0.10
Psychotropic drug consumption
366 (68.0) 175 1.51 (0.86;2.65) 0.15
More than 20 cigarettes a day
346 (64.3) 168 0.82 (0.49;1.38) 0.46
Experience of
withdrawal symptoms
181 (33.6) 119 1.60 (0.99;2.58) 0.06
High support from stable partnere
203 (39.0) 112 0.68 (0.40;1.18) 0.17
High support from familye
243 (45.5) 125 0.61 (0.36;1.02) 0.06
High support from friendse
126 (23.6) 82 0.61 (0.33;1.14) 0.12
For Peer Review Only
Depressive symptomsf 373 (69.5) 181 1.66 (0.91;3.02) 0.10 Opioid substitution treatment - No OST - Buprenorphine treated - Methadone treated 194 (36.3) 184 (34.5) 156 (29.2) 121 91 73 0.99 (0.53;1.84) 1.71 (0.93;3.14) 0.98 0.08 Benzodiazepines use 263 (48.9) 146 1.51 (0.92;2.46) 0.10 Anxiolytics use* 306 (56.9) 143 1.06 (0.63;1.76) 0.83 Hypnotics use 198 (36.8) 112 1.79 (1.08;2.97) 0.02 Neuroleptics use 30 (5.6) 23 3.63 (1.67;7.88) 0.001 Barbiturate use 58 (10.8) 43 0.65 (0.19;1.12) 0.01 Trust in physiciang 418 (78.4) 178 0.51 (0.29;0.92) 0.03 0.51 (0.29;0.92) 0.03Number of years since first drug injectiona
15 [13-18] 0.74 (0.41;1.32) 0.31
Number of years since first positive HIV testa
10 [7-12] 1.16 (0.57;2.38) 0.69
Undetectable viral load 131 (24.8) 63 1.26 (0.73;2.18) 0.42 CD4 cell count per
mm3<500
349 (65.4) 169 0.96 (0.58;1.60) 0.89
HIV clinical stage - A (ref) - B - C 263 (49.0) 245 (45.6) 29 (5.4) 146 97 13 1.08 (0.63;1.85) 0.72 (0.18;2.88) 0.77 0.65 a
For 10 years increase
b
Good housing conditions were defined as the rank 3 and 4 (quite or very comfortable vs. uncomfortable or low comfort) using a four-point Likert scale.
c
Heavy alcohol use was defined as alcohol consumption > 150 units per month for men and > 100 units per month for women.
d
Binge drinking was defined as drinking more than four alcohol units on any one occasion.
e
Support from partner, family and friends was measured on a four-point Likert scale and individuals with high support (rank 4) were compared
For Peer Review Only
to those with lower ranks (no, limited or moderate support from the partner).
f
Patients were defined with depressive symptoms if CES-D>17 for men and >23 for women. * other than benzodiazepines
For Peer Review Only
1
Distributive sharing among HIV-HCV co-infected injecting drug users: the
preventive role of trust in one’s physician
Abstract
This study, based on data from the MANIF 2000 cohort study, investigates the relationship between the lending of injecting equipment, drug use and experience with HIV care. The sample comprised 224 HIV-HCV-coinfected patients who reported having injected drugs in the previous 6 months and their 538 visits to clinical services. Longitudinal data were collected for medical status, and self-reported risk behaviors. A logistic regression GEE model was used to identify correlates of distributive sharing. After multiple adjustment, patients who reported trust in physicians were significantly less likely to report lending injection equipment while cocaine users were at increased risk. Promoting dialogue between physicians and IDUs may play an important role in HIV-HCV positive prevention.
Key words : drug users, high-risk behaviors, co-infection HIV/HCV, trust in physician
For Peer Review Only
2
Background
Even in countries that have adopted needle exchange programs (NEP) and expanded access to opioid substitution treatment (OST), the prevalence of HCV in injecting drug users (IDUs) remains extremely high (60-70%) and even higher in those HIV-infected through drug use (Jauffret-Roustide et al., 2009). Among IDUs, HCV, like HIV, is mainly transmitted through needle sharing. However, HCV is more easily transmitted than HIV because its prevalence is greater, it is more resistant to desiccation (Centers for Disease Control and Prevention", 1998) and it is also linked to the sharing of drug paraphernalia (water, filter, spoon) (Hagan et al., 2001; McCoy, Metsch, Chitwood, Shapshak, & Comerford, 1998). HCV infection can consequently occur after substantially less injection and fewer sharing episodes (Hahn et al., 2002; Miller et al., 2002). All around the world, the current harm reduction (HR) policy seems to have been less effective in controlling HCV than HIV and consequently HR packages for controlling HCV are under evaluation. Targeting HIV-HCV co-infected patients’ risk behaviors is important, as interventions on this population may have a major role in controlling the spread of both HIV and HCV. Risks involved in injection practices greatly contribute to the spread of blood-borne transmitted diseases among IDUs (Alter, 2006) .
A range of interventions has been implemented to reduce the spread of blood-borne diseases among IDUs.
Even though HR interventions have been found to have an impact upon HIV prevalence and incidence among IDUs (Cazein et al., 2008; Des Jarlais et al., 2005; Van Den Berg, Smit, Van Brussel, Coutinho, & Prins, 2007), some HIV positive IDUs continue to engage in high-risk behaviors. There is an urgent need to develop and strengthen effective interventions for HIV-positive IDUs who are still engaged in high-risk behaviors.
The French HR policy has provided expanded and free access to OST (70% coverage), NEP (Emmanuelli & Desenclos, 2005) but HCV prevalence among IDUs is still high (Jauffret-Roustide et al., 2006).
While several studies document incidence rates of HCV and the pattern of risk factors, little is known about HIV-HCV risk practices in co-infected IDUs. Also, few studies take account of the serological status of IDUs involved in high-risk behaviors, and fewer studies have focused on distributive sharing among HIV/HCV coinfected IDUs (Metsch et al., 2007).
For Peer Review Only
3
The present study used data from the MANIF 2000 cohort of HIV-HCV co-infected patients to identify whether experience with HIV care and access to ART, social demographic characteristics and specific use of drugs could influence the practice of distributive sharing among active IDUs. The study also investigated the role of the physician, and specifically of the trust in physician among HIV-HCV co-infected IDUs in relation to high-risk behaviors.
Method
Sample and setting
The sample comprised 224 HIV-HCV co-infected patients who reported having injected drugs at least once in the 6 months prior to interview. Only visits where patients reported having injected were included in the data analysis (538 visits).
The sample was drawn from the MANIF 2000 cohort which enrolled 467 patients who were HIV-infected through injection drug use between July 1995 and May 1998. Patients were enrolled in hospital-based outpatient HIV clinics and followed-up until 2007. Inclusion criteria for enrolment in the cohort included only patients with a CD4+ cell count >300/ul in the last visit prior to enrolment and with clinical stages A or B. This cohort was designed to focus on social and behavioral aspects of HIV-positive IDUs and particularly on their access and adherence to ART.
All individuals who agreed to be interviewed signed an informed consent form, approved with the study protocol by the French committee responsible for the evaluation of work involving human subjects.
Procedure
Data collection (every 6 months) was based on medical records, a face-to-face interview and a self-administered questionnaire. Clinical data, including CD4 cell count, HIV viral load and HIV clinical stage were derived from clinical records. The face-to-face interview was administered by trained nurses who gathered psychosocial information (social support and patient-physician relationship) as well as information about patients’ personal experience with HIV infection and care. The questionnaire collected socio-demographic data, year of first drug injection, incarceration, several items about substance use and at-risk behaviors. All the data collected referred to the 6 months prior to the given visit.
Injecting drug use at any given visit thereafter was defined as the injection of heroin or morphine, psychotropic drugs, cocaine, buprenorphine or any other drugs in the 6 months before that visit.
For Peer Review Only
4
Depressive symptoms were detected using the French version of the Center for Epidemiological Studies Depression Scale (CES-D) (Fuhrer & Rouillon, 1989). Although CES-D cannot be considered as a clinical tool for diagnosing depression, patients were considered to present depressive symptoms if their CESD score was greater than 17 (for men) or greater than 23 (for women) (Radloff, 1977).
A measure of the trust between physicians and their patient was calculated using a single item using a 5-point Likert scale to score patient agreement with different suggested levels of trust in their physician. In the data analysis, the variable was dichotomized to contrast individuals reporting very high and high agreement vs those reporting moderate, low or very low agreement.
In order to minimize the risk of under-reporting, the definition of the outcome variable of “distributive sharing at a given visit” was based on several questions:
1. Having lent syringe/needle or other paraphernalia at least once in the 6 previous months before the visit
2. Having lent needle/syringe to someone else during their last injection if it occurred in the 6 months prior to the visit
3. Having lent other paraphernalia (filter, spoon) to someone else during their last injection if it occurred in the 6 months prior to the visit.
Statistical methods
To determine factors associated with distributive sharing, only visits of those individuals reporting active injection during follow-up were included. A logistic regression GEE model was used to assess the independent effect of patient-physician relationship and individual characteristics on distributive sharing.
Variables were considered eligible for entering the final models if in their p value was <0.25 in the univariate analysis. The final model was built using a backward procedure based on the log likelihood ratio test with p<0.05.
All the analyses were performed using Stata version 10.1.
Results
Among the 224 co-infected patients who also reported having injecting drugs at least once during the whole follow-up (4 years on average, 993.5 person-years), 166 reported injection
For Peer Review Only
5
cessation for at least one year during follow-up. The 243 individuals who were excluded because they never reported injection over the whole observation period, had longer duration of follow-up (6 years on average, 1638.5 person-years).
The 224 patients in the study sample were mainly men (68.3%, 153 patients), median [IQR] patient age was 35 [32–38] years. Thirty eight were employed at enrolment (17.1%) and 25 (11.5%) had a high school certificate but the majority (30%) had 9 years of education and 25% did not have a primary school certificate.. At enrollment in the cohort, 142 (63.4%) were living in a stable relationship and 138 (61.6%) reported being the owner or tenant of their house. At enrollment, median [IQR] time since first injection was 14 [12–17] years, and 168 (75.3%) presented depressive symptoms. Sixty-nine patients (30.8%) started ART during follow-up.
Among the 224 patients accounting for 538 visits where injection 6 months prior the visit was reported, 157 (71%) reported having shared injecting equipment in 282 visits (54%) ; distributive sharing was reported by 57 patients (25%) in 82 visits (15%) and borrowing injection equipment was reported by 119 patients (53%) at 173 visits (32%).
The distribution of the main characteristics and behaviors observed at the selected visits and the associations between such characteristics and distributive sharing are presented in Table I. When focusing on substance use behaviors, individuals reporting injection at these visits also reported, respectively, heroin or cocaine use in 70% and 50% of the selected visits while benzodiazepine in 49% of the visits. Experience of withdrawal symptoms was reported in 34% of visits.After adjustment for socio-demographic factors (age and employment), individuals reporting cocaine use were at increased risk of distributive sharing. Conversely, individuals reporting trust in physicians were significantly less likely to report lending injection equipment (Table I). However the adjustment for IMR (i.e. for inequity in ART access) did not affect the relationship between trust in physicians and distributive sharing.
It is interesting to note that trust in physicians was also associated with high adherence (p=0.001) and better virological outcomes (undetectable viral load p=0.05), but not with “systematic condom use in the 6 months prior to the visit with one’s stable or occasional partner” (p=0.47).
For Peer Review Only
6
Discussion
This study conducted in HIV-HCV coinfected IDUs shows that the continued high level of sharing of injecting equipment remains a public health concern because of continuing concerns about the transmission of blood borne diseases, especially HIV and HCV.
The proportion of IDUs who reported distributive sharing is lower than that of IDUs who reported borrowing equipment. From a public health perspective and a coinfected prevention viewpoint, it is important to differentiate between borrowing and lending among HIV-infected patients. This result suggests that HIV-HCV coinfected IDUs are to take more personal risks in terms of potential exposure to HCV re-infection or super-infections (Currie et al., 2008; van de Laar & Likatavicius, 2009) than they are to expose others to HCV risk by lending their injecting equipment. This behavior emphasizes the strategies used by IDUs to protect their community from HIV transmission by “informed altruism” (Des Jarlais et al., 2004) and the responsibility of IDUs (Rhodes et al., 2007) toward others. Interestingly, during the cohort follow-up, more than half of the IDUs studied had stopped injecting drugs (Bouhnik et al., 2004).
In our study, younger age, unemployment and recent cocaine use were risk factors associated with distributive sharing at least once in the previous six months. In most international literature, cocaine use is an important indicator of risky injecting (Compton, Lamb, & Fletcher, 1995; Reynaud-Maurupt et al., 2000; Santibanez et al., 2005)as it requires more frequent injections than other drugs, such as heroin (Patrick et al., 2001), and particularly exposes IDUs to the risk of HIV and hepatitis transmission (Guadagnino et al., 1995). Increasingly frequent cocaine injection can also lead to other infectious risks, like abscesses or other bacterial infections (Guadagnino et al., 1995; V. Hope, Kimber, Vickerman, Hickman, & Ncube, 2008; V. D. Hope, Marongiu, Parry, & Ncube, 2010). HIV-HCV coinfected IDUs are weakened and more susceptible to exposure to infectious complications due to sharing (Mertz, Viktorin, Wolbers, Fluckiger, & Battegay, 2009). This result also emphasizes the urgent need to associate HR measures with the development of new pharmacological treatment for stimulant dependence.
Age is also an important factor. Previous studies have shown that younger IDUs are more likely to engage in high-risk behaviors (Deren, Kang, Colon, Andia, & Robles, 2004). Young people may tend to take more risks due to lack of information and shorter history and
For Peer Review Only
7
experience of drug injection. It is possible that there the “generation gap” factor also plays a role. Older IDUs lived during a time when AIDS decimated their circle of relationships and generated within them the feeling that they are “survivors" (Nord, 1997). There is a certain sense of guilt for staying alive and they pay particular attention to protecting themselves and others by minimizing risk-taking.
Trust in one’s physician was also found to be associated with a reduced likelihood of distributive sharing.
Since the onset of the AIDS epidemic, published studies have shown the difficult interaction between patients and physicians, particularly when the patient is an IDU (Hindler et al., 1996) and/or HIV-positive.
Although a delay in access to ART was observed for IDUs when ART first became available to HIV-infected persons (Carrieri et al., 1999), today in France, access to ART for IDUs has considerably increased, largely due to the rapid scale-up of OST mainly through primary care, which improves patients' behavioral stabilization and rehabilitation (Spire, Lucas, & Carrieri, 2007). Even if recent studies have shown that HIV-positive IDUs often report having suffered discrimination from health professionals and have poorer adherence to ART (Peretti-Watel, Spire, Lert, & Obadia, 2005), the implementation of OST as a HR tool in primary care is likely to have fostered the dialogue between doctors and patients regarding injection and at-risk practices. This has probably contributed to improve the overall quality of the relationship between HIV-infected IDUs and their physicians.
International literature has also shown that engaging in HIV care has a positive impact on the decrease of injecting drug use among HI-HCV co-infected IDUs (Bouhnik et al., 2004; Smit et al., 2006).
It is possible that access to HIV or HCV care, through a more regular and trustful relationship with their HIV physicians can be considered by some patients as a unique opportunity to switch from a “drug user” status to that of an individual followed-up for his/her HIV. This new identity makes some IDUs pay particular attention to their general health and also provides them the opportunity to reduce or renounce injection practices, or to inject only in safe contexts, thus minimizing the risk of transmission of blood borne diseases (Bouhnik et al. 2004).
For Peer Review Only
8
It is important to note that HIV-HCV co-infected IDUs in France can be followed-up by multiple care providers (primary care physicians, HIV physicians, psychiatrists, hepatologists). However, the quality of the relationship may be different depending on the specialty of the physician, probably due to fear of judgment or stigma. For instance, it has been shown that HIV-HCV co-infected patients tend to underreport alcohol use to their hepatologist(Roux et al., 2011). HIV and primary care physicians in France were the first who provided care and prevention counselling for IDUs and were therefore the less judgmental. It is not surpriing that HIV physicians may have a better relationship with their patients, and that such patients are more sensitive to their counselling and adopt harm reduction practices.
At any rate, this result demonstrates the importance of strengthening the dialogue and the therapeutic alliance between physicians (HIV or primary care physician) and their IDU patients. Specific training and collaborations with specialists in addiction is sometimes required (Ireland & McLeod, 1995). Such collaborations may modify the perception that physicians may have regarding their IDU patients, often perceived as “liars and manipulators” (George & Martin, 1992; McGillion, Wanigaratne, Feinmann, Godden, & Byrne, 2000).
This result has also important implications in terms of clinical management but also policy repercussions in terms of HR in the IDU community. In a meta-analysis, (Martin, Garske, & Davis, 2000) a consistent, positive and moderately sized relationship between therapeutic alliance and treatment outcomes was found, and a review of recent studies (Meier, Donmall, Barrowclough, McElduff, & Heller, 2005; Meier, Donmall, McElduff, Barrowclough, & Heller, 2006) suggested that the trust in one’s physician plays an important role in predicting drug treatment outcomes.
In interpreting these findings, certain limitations should be acknowledged. Firstly, in studies concerning self-reported at-risk practices, it is important to consider possible social desirability bias. For an HIV-positive person, distributive sharing is a very sensitive subject because there is a tendency to stigmatize lending behaviors. Though the validity and reliability of self-reports about drug use and risk practices have been established in studies using similar methods for data collection and processing (Darke, 1998; Latkin & Vlahov, 1998), the risk of under-reporting for social desirability bias exists. This risk could result in a unidirectional misclassification of individuals lending their injecting equipment and therefore in a possible underestimation of the associations found. The study was also conducted with a
For Peer Review Only
9
very specific population, i.e. HIV-HCV co-infected IDUs receiving hospital-based outpatient clinical care for their HIV disease. Because access to care in France is free, we assume that the study group may be representative of the wider HIV-infected population of IDUs.
Conclusions
Our study emphasized that high-risk behaviors are not only related to characteristics of patients but imply a strong relation with the social environment of IDUs (Rhodes, 2009). Promoting the dialogue between doctors and their opioid dependent/HIV/HCV patients can improve HIV and HCV prevention among IDUs.
For Peer Review Only
10
References
Alter, M. J. (2006). Epidemiology of viral hepatitis and HIV co-infection. J Hepatol, 44(1 Suppl), S6-9.
Bouhnik, A. D., Carrieri, M. P., Rey, D., Spire, B., Gastaut, J. A., Gallais, H., et al. (2004). Drug injection cessation among HIV-infected injecting drug users. Addict Behav,
29(6), 1189-1197.
Carrieri, M. P., Moatti, J. P., Vlahov, D., Obadia, Y., Reynaud-Maurupt, C., & Chesney, M. (1999). Access to antiretroviral treatment among French HIV infected injection drug users: the influence of continued drug use. MANIF 2000 Study Group. J Epidemiol
Community Health, 53(1), 4-8.
Cazein, F., Pillonel, J., Le Strat, Y., Lot, F., Pinget, R., David, D., et al. (2008). Surveillance de l’infection à VIH-sida en France, 2007. Bull Epidemiol Hebd, 45-46, 434-442. Compton, W. M., Lamb, R. J., & Fletcher, B. W. (1995). Results of the NIDA treatment
demonstration grants' cocaine workgroup: characteristics of cocaine users and HIV risk behaviors. Drug Alcohol Depend, 37(1), 1-6.
Currie, S. L., Ryan, J. C., Tracy, D., Wright, T. L., George, S., McQuaid, R., et al. (2008). A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus. Drug Alcohol Depend, 93(1-2), 148-154.
Darke, S. (1998). Self-report among injecting drug users: a review. Drug Alcohol Depend,
51(3), 253-263; discussion 267-258.
Deren, S., Kang, S. Y., Colon, H. M., Andia, J. F., & Robles, R. R. (2004). HIV incidence among high-risk Puerto Rican drug users: a comparison of East Harlem, New York, and Bayamon, Puerto Rico. J Acquir Immune Defic Syndr, 36(5), 1067-1074.
Des Jarlais, D. C., Perlis, T., Arasteh, K., Torian, L. V., Hagan, H., Beatrice, S., et al. (2005). Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990-2001. Aids, 19 Suppl 3, S20-25.
Emmanuelli, J., & Desenclos, J. C. (2005). Harm reduction interventions, behaviours and associated health outcomes in France, 1996-2003. Addiction, 100(11), 1690-1700. Fuhrer, R., & Rouillon, F. (1989). La version française de l'échelle CES-D. Description and
translation of the autoevaluation scale (in French). Psychiatrie et Psychobiologie, 4, 163-166.
George, M., & Martin, E. (1992). GP's attitudes towards drug users. Br J Gen Pract, 42(360), 302.
Guadagnino, V., Zimatore, G., Izzi, A., Caroleo, B., Rocca, A., Montesano, F., et al. (1995). Relevance of intravenous cocaine use in relation to prevalence of HIV, hepatitis B and C virus markers among intravenous drug abusers in southern Italy. J Clin Lab
Immunol, 47(1), 1-9.
Hagan, H., Thiede, H., Weiss, N. S., Hopkins, S. G., Duchin, J. S., & Alexander, E. R. (2001). Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public
Health, 91(1), 42-46.
Hope, V., Kimber, J., Vickerman, P., Hickman, M., & Ncube, F. (2008). Frequency, factors and costs associated with injection site infections: findings from a national multi-site survey of injecting drug users in England. BMC Infect Dis, 8, 120.
Hope, V. D., Marongiu, A., Parry, J. V., & Ncube, F. (2010). The extent of injection site infection in injecting drug users: findings from a national surveillance study.
Epidemiol Infect, 1-9.
Ireland, R., & McLeod, I. (1995). Drug users' views on general practitioners. Bmj, 310(6991), 1407-1408.
For Peer Review Only
11
Jauffret-Roustide, M., Emmanuelli, J., Quaglia, M., Barin, F., Arduin, P., Laporte, A., et al.
(2006). Impact of a harm-reduction policy on HIV and hepatitis C virus transmission among drug users: recent French data--the ANRS-Coquelicot Study. Subst Use
Misuse, 41(10-12), 1603-1621.
Jauffret-Roustide, M., Le Strat, Y., Couturier, E., Thierry, D., Rondy, M., Quaglia, M., et al. (2009). A national cross-sectional study among drug-users in France: epidemiology of HCV and highlight on practical and statistical aspects of the design. BMC Infect Dis,
9, 113.
Latkin, C. A., & Vlahov, D. (1998). Socially desirable response tendency as a correlate of accuracy of self-reported HIV serostatus for HIV seropositive injection drug users.
Addiction, 93(8), 1191-1197.
Martin, D. J., Garske, J. P., & Davis, M. K. (2000). Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. J Consult Clin Psychol, 68(3), 438-450.
McCoy, C. B., Metsch, L. R., Chitwood, D. D., Shapshak, P., & Comerford, S. T. (1998). Parenteral transmission of HIV among injection drug users: assessing the frequency of multiperson use of needles, syringes, cookers, cotton, and water. J Acquir Immune
Defic Syndr Hum Retrovirol, 18 Suppl 1, S25-29.
McGillion, J., Wanigaratne, S., Feinmann, C., Godden, T., & Byrne, A. (2000). GPs' attitudes towards the treatment of drug misusers. Br J Gen Pract, 50(454), 385-386.
Meier, P. S., Donmall, M. C., Barrowclough, C., McElduff, P., & Heller, R. F. (2005). Predicting the early therapeutic alliance in the treatment of drug misuse. Addiction,
100(4), 500-511.
Meier, P. S., Donmall, M. C., McElduff, P., Barrowclough, C., & Heller, R. F. (2006). The role of the early therapeutic alliance in predicting drug treatment dropout. Drug
Alcohol Depend, 83(1), 57-64.
Mertz, D., Viktorin, N., Wolbers, M., Fluckiger, U., & Battegay, M. (2009). Impact of HIV status on outcome of infectious complications in intravenous drug users. J Acquir
Immune Defic Syndr, 51(4), 506-507; author reply 507-508.
Metsch, L. R., Pereyra, M., Purcell, D. W., Latkin, C. A., Malow, R., Gomez, C. A., et al. (2007). Correlates of lending needles/syringes among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr, 46 Suppl 2, S72-79.
Nord, D. (1997). Threats to identity in survivors of multiple AIDS-related losses. Am J
Psychother, 51(3), 387-402.
Patrick, D. M., Tyndall, M. W., Cornelisse, P. G., Li, K., Sherlock, C. H., Rekart, M. L., et al. (2001). Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. Cmaj, 165(7), 889-895.
Peretti-Watel, P., Spire, B., Lert, F., & Obadia, Y. (2005). [Seropositive people infected through intravenous drug use: a persistent vulnerability]. Rev Prat, 55(19), 2093-2100. Radloff, L. (1977). The CES-D scale: a self-report depression scale for research in the general
population. Appl. Psychol. Measure, 3, 385–491.
Reynaud-Maurupt, C., Carrieri, M. P., Gastaud, J. A., Pradier, C., Obadia, Y., & Moatti, J. P. (2000). Impact of drug maintenance treatment on injection practices among French HIV-infected IDUs. The MANIF 2000 Study Group. AIDS Care, 12(4), 461-470. Rhodes, T. (2009). Risk environments and drug harms: a social science for harm reduction
approach. Int J Drug Policy, 20(3), 193-201.
Rhodes, T., Watts, L., Davies, S., Martin, A., Smith, J., Clark, D., et al. (2007). Risk, shame and the public injector: a qualitative study of drug injecting in South Wales. Soc Sci
Med, 65(3), 572-585.
For Peer Review Only
12
Roux, P., Cohen, J., Lascoux-Combe, C., Sogni, P., Winnock, M., Salmon-Ceron, D., et al.
(2011). Determinants of the underreporting of alcohol consumption by HIV/HCV co-infected patients during face-to-face medical interviews: The role of the physician.
Drug Alcohol Depend, 116(1-3), 228-232.
Santibanez, S. S., Garfein, R. S., Swartzendruber, A., Kerndt, P. R., Morse, E., Ompad, D., et al. (2005). Prevalence and correlates of crack-cocaine injection among young injection drug users in the United States, 1997-1999. Drug Alcohol Depend, 77(3), 227-233. Smit, C., Lindenburg, K., Geskus, R. B., Brinkman, K., Coutinho, R. A., & Prins, M. (2006).
Highly active antiretroviral therapy (HAART) among HIV-infected drug users: a prospective cohort study of sexual risk and injecting behaviour. Addiction, 101(3), 433-440.
Spire, B., Lucas, G. M., & Carrieri, M. P. (2007). Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST). Int J Drug Policy, 18(4), 262-270. van de Laar, M. J., & Likatavicius, G. (2009). HIV and AIDS in the European Union, 2008.
Euro Surveill, 14(47).
Van Den Berg, C., Smit, C., Van Brussel, G., Coutinho, R., & Prins, M. (2007). Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction, 102(9), 1454-1462.