HAL Id: hal-02723344
https://hal.inrae.fr/hal-02723344
Submitted on 1 Jun 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Copyright
Calmodulin from Neurospora crassa. General properties
and conformational changes
J.A. Cos, C. Ferraz, J.G. Demaille, R. Ortega-Perez, Diederik van Tuinen, D.
Marmé
To cite this version:
J.A. Cos, C. Ferraz, J.G. Demaille, R. Ortega-Perez, Diederik van Tuinen, et al.. Calmodulin from
Neurospora crassa. General properties and conformational changes. Journal of Biological Chemistry,
American Society for Biochemistry and Molecular Biology, 1982, 257 (18), pp.10694-10700.
�hal-02723344�
Vol. 257, No. 18, Issue of September 25, pp. 10694-10700, 1982 Printed in U.SA.
Calmodulin from Neurospora crassa
GENERAL PROPERTIES AND CONFORMATIONAL CHANGES*
(Received for publication, May 3, 1982)
Jos A. Coxt
From the Department of Biochemistry, University of Geneva, Switzerland
Concepcion Ferraz and Jacques G. Demaille§
From the Centre de Recherches de Biochimie Macromoldculaire du Centre National de la Recherche Scientifique and Institut National de la Santd et de la Recherche Midicale U-249, B.P. 5015, Montpellier, France
Ruben Ortega Perezl, Diederik van Tuinen, and Dieter MarmeII
From the Laboratoire de Microbiologie Gendrale, Dpartement de Biologie Vgjtale, University of Geneva, Switzerland
Calmodulin from Neurospora crassa has been
puri-fied to electrophoretic homogeneity. Equilibrium gel
filtration experiments suggest that its Ca-binding
prop-erties are indistinguishable from those of vertebrate
calmodulins. The isoelectric point of 4.04 and
electro-phoretic behavior under nondenaturing conditions
in-dicate that N. crassa calmodulin is slightly less acidic
than its vertebrate counterpart. The amino acid
com-position is typical of plant calmodulins with the
excep-tion that trimethyllysine is absent and that the content
of Ser is unusually high. The tryptic peptide map of N.
crassa calmodulin reveals an important number of
point mutations as compared to vertebrate calmodulin.
Differences in primary structure may explain why N.
crassa calmodulin is less potent in the activation of
myosin light chain kinase than calmodulins from
higher organisms. The far UV circular dichroic spectra
of the Ca-, Mg-, and metal-free forms of N. crassa
cal-modulin are similar to those of vertebrate calcal-modulin;
in contrast, the near UV circular dichroic spectra are
very different, apparently due to the differences in Tyr
content. The single Tyr residue of N. crassa calmodulin,
presumably located in position 138, undergoes an
in-version of optical chirality upon addition of Ca2+, but
not of Mg
2+, to the metal-free protein.
Calmodulin, which modulates a large number of Ca-de-pendent events inside the cell (for review, see Refs. 1-3), has been isolated from several eukaryotes including microorga-nisms such as Physarum polycephalum (4), Chlamydomonas
* The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
f
Supported by Swiss Science Foundation Grant 3.237.77. § Supported by a grant from the Centre National de la Recherche Scientifique (ATP Modulation de l'Action des Hormones au Niveau Cellulaire), Grant CRL 81.3.023 from the Institut National de la Sante et de la Recherche M6dicale, and grants from the Direction Generale' a la Recherche Scientifique et Technique (ACC Relations Dyna-miques des Macromolecules Biologique), the Fondation pour la Recherche Medicale Francaise, and the Muscular Dystrophy Associ-ation of America, Inc.¶
To whom correspondence and reprint requests should be ad-dressed.11 Present address, Institut fiir Biologie III, Albert-Ludwigs-Uni-versitat, Schanzlestrasse 1, 7800 Freiburg-im-Brisgau, Germany.
reinhardii (5, 6), Dictyostelium discoideum (7), Tetrahymena pyriformis (8), Paramecium tetraurelia (9), and Saccharo-myces cerevisiae (10). In general, the amino acid sequence of
the protein has been highly conserved in animals of very different origins (11). Differences, however, exist between vertebrate and protozoan calmodulins, the sequence of
Tet-rahymena calmodulin shows substitutions at 11 residues and
one deletion (12). An important number of sequence differ-ences can also be foreseen for Dictyostelium calmodulin on the basis of the peptide maps (7). These findings suggest that the study of calmodulins from lower organisms may provide insight into the evolution and modification of this class of biologically important proteins.
Recently, we described the isolation of calmodulin from the mycelia of the higher fungus (Ascomycetes) Neurospora
crassa (13). With respect to the electrophoretic mobility on
polyacrylamide gels, the activation of bovine heart phospho-diesterase, and the absorption spectrum, N. crassa calmodulin differs from animal calmodulin, but resembles that from plants. In this study, a more thorough and complete charac-terization of N. crassa calmodulin is carried out with emphasis on the Ca-dependent conformational changes in the environ-ment of the single Tyr residue.
EXPERIMENTAL PROCEDURES
Materials-N. crassa calmodulin was purified as described by
Ortega Perez et al. (13) with one additonal chromatography. The lyophilized preparation was dissolved in 20 mM Tris-HCI, pH 7.5, 80 mM KCI, 25 or 90 M CaCI2and passed through a Sephacryl S-200
column (150 x 1 cm) equilibrated in the same buffer to which 45CaC12
was added (50,000 cpm/ml). Calmodulin was monitored by atomic absorption and by liquid scintillation counting of 45Ca. Metal-depleted calmodulin (less than 0.05 mol of Ca2+/mol of protein) was obtained by passage of 5 mg of protein through a column containing 1 ml of Chelex (Bio-Rad) equilibrated in 60 mM N- {tris[hydroxymethyl] methyl-2-amino} ethanesulfonic acid, pH 7.0, 150 mM KCl. Bovine brain calmodulin was prepared according to the method of Watterson
et al. (14). Ram testis calmodulin was prepared as described by Autric et al. (15).
Electrophoresis-Polyacrylamide (15%) disc gel electrophoresis in
the presence of 0.1% sodium dodecyl sulfate, 1 mM EDTA, or 1 mM CaCl2was carried out as described previously (16).
Isoelectric Point Determination-Isoelectric focusing was carried
out on agarose IEF (Pharmacia Fine Chemicals, Uppsala, Sweden) according to the method recommended by Pharmacia. The polyam-pholyte used was Servalyt 3-4 (Serva, Heidelberg, West Germany).
Amino Acid Analysis-The amino acid composition of N. crassa
calmodulin was determined on a Beckman Multichrom Analyser Model 4255 (17) after 24, 48, and 72 h of hydrolysis in 5.6 N HCI at
10694
at INRA Institut National de la Recherche Agronomique, on June 19, 2012
www.jbc.org
Neurospora crassa Calmodulin
110 C under vacuum. A single column technique (6 x 250 mm) was adopted, using a 1 M Na+ buffer, pH 7.0, as the last buffer. Mono-, di-, and trimethyl derivatives of lysine were separated as described by Molla et al. (18).
Peptide Mapping-High pressure liquid chromatography peptide
maps of ram testis calmodulin and of N. crassa calmodulin were obtained as described by Autric et al. (15). For those peaks of the N.
crassa calmodulin digest that were not found in ram testis calmodulin
and appeared to be well separated from adjacent peaks, individual fractions (0.5 min, 0.75 ml) were dried and hydrolyzed by 5.6 N HC for 2 h at 145 C under vacuum, and amino acid analysis was carried out as described above.
Enzyme Activity-Myosin light chain kinase activity was assayed
according to the method of Molla et al. (18) using calmodulin solutions
with concentrations determined by amino acid analysis.
Circular Dichroism-Spectra were recorded at room temperature
on a Jasco J-20A spectropolarimeter with a 1-nm slit as described by Cox and Stein (16). The concentration of metal-depleted N. crassa
calmodulin in 60 mM
N-{tris[hydroxymethyl]methyl-2-amino}eth-anesulfonic acid, pH 7.0, 150 mM KC1 was 0.5 mg/ml in a 0.05-cm cell used below 250 nm and 3 mg/ml in a 0.5-cm cell used above 250 nm.
The mean residue molecular weight of N. crassa calmodulin, calcu-lated from the amino acid composition, was found to be 113.4. The circular dichroic data were expressed in terms of ellipticity/decimol
of amino acid residue, [0], below 250 nm and in terms of difference in molar absorptivity, Ae, above 250 nm.
Fluorescence Measurements-Following excitation of the sample
(2.2 mg/ml of metal-free calmodulin in 60 mM N- {tris[hydroxymethyl] methyl-2-amino} ethanesulfonic acid, pH 7.0, 150 mm KCI) at 282 nm, which ensures maximal emission, fluorescence spectra were recorded
at room temperature on a Baird Atomic spectrofluorometer FC 100 with both slits at 2 nm. Saturating amounts of CaC12or MgCl2were
added to obtain the spectra of the Ca- and Mg-saturated protein, respectively.
RESULTS
Final Purification of N. crassa Calmodulin-Fig.
shows the elution profile of the Sephacryl S-200 chromatography of partially purifiedN. crassa
calmodulin. The pure proteineluted with a Kay value of 0.38 (fraction 36), compared to 0.45
for bovine brain calmodulin; hence,
N. crassa
possesses a higher Stokes radius than brain calmodulin. In most prepa-rations, a UV absorbing peak was observed which eluted between calmodulin and the free salts (fraction 48); this peakfraction no
FIG. 1. Sephacryl S-200 chromatography of a crude calmod-ulin preparation (see "Materials"). Fractions of 2 m. Elution was
carried out at a flow rate of 8 ml/h. The void volume and the total volume of the mobile phase correspond to fractions 20 and 63,
respectively. Absorbance at 258 nm (- - -). Total Ca2+ concentration as monitored by atomic absorption (O--O) or by Ca scintillation
counting ( --- ). The inset represents a scale expansion of the
Ca2+ concentration in the elution profile from fractions 44 to 51.
strongly binds Ca2 + but does not contain proteins, as judged from the negative Coomassie test (19). In some preparations of N. crassa calmodulin, the UV fluorescence spectrum sug-gested the presence of a Trp-containing contaminant. The latter was removed by DE52-cellulose chromatography as described by Cox et al. (20).
Specific Extinction Coefficient and Calcium Content-The
specific extinction coefficient of N. crassa calmodulin exhaus-tively dialyzed against 60 mM N- {tris[hydroxymethyl]methyl-2-amino}ethanesulfonic acid, pH 7.0, 160 mM KCI, 50 M CaC12at 258 and 280 Im was determined at two wavelengths using a protein concentration determined in triplicate accord-ing to the method of Lowry et al. (21) or that of Bradford (19) with bovine brain calmodulin as a standard. Both methods gave the same results. The A25r of 1.36 and
the
A28~ 0of
1.08 agree with the fact that N. crassa calmodulin contains only Tyr residue/mol.
The Ca content of N. crassa calmodulin was determined according to the method of Hummel and Dryer (22) under experimental conditions identical with those described in Fig. 1. Control experiments were run as described in the case of insect calmodulin (20). Protein-bound and free [Ca2 +] were determined by liquid scintillation counting and by atomic absorption spectrophotometry. As seen in Fig. 1, similar re-sults were obtained by both methods. The protein concentra-tion was determined spectrophotometrically or according to the method of Bradford (19) with brain calmodulin as a standard. Assuming Mr = 16,500 (see below), N. crassa cal-modulin binds 3.0 mol of Ca2
+/mol
of protein at a free [Ca2"]
of 90 pM and 2.2 mol of Ca2+ at a free [Ca2+] of 24.5 pM. Under similar conditions, identical values were found for bovine brain calmodulin, suggesting that the Ca-binding properties of calmodulin of both organisms are very similar. The same conclusion was reached previously in the case of insect cal-modulin (Cox et al. (23). It should be noted that, at high ionic strength, we (23) and Haiech et al. (24) could saturate only the three high affinity Ca-binding sites of bovine brain cal-modulin.
Electrophoretic Properties-Fig. 2A depicts the
electro-phoretic mobility of N. crassa calmodulin under nondenatur-ing conditions. RF = 0.54 in the presence of Ca2+, compared to
0.69 in the presence of 1 mim EGTA.1 This important Ca-dependent shift in mobility is consistent with the prediction that the metal-depleted form is more acidic than the Ca-saturated protein, as was also found in the case of brain calmodulin (16). Both the metal-free and Ca-saturated forms of N. crassa calmodulin migrate slightly more slowly than those of brain calmodulin. This is in agreement with the findings that calmodulin of N. crassa is somewhat less acidic and that its Stokes radius is distinctly higher than that of brain.
In the presence of sodium dodecyl sulfate, the two proteins behave differently (Fig. 2B); N. crassa calmodulin migrates faster in the presence of EGTA than in the presence of Ca2+, whereas the opposite prevails for brain calmodulin. For in-stance, brain calmodulin is the faster migratory component in the presence of 1 mM CaCl, whereas N. crassa calmodulin migrates ahead in the presence of 1 mM EDTA.
The isoelectric point of N. crassa calmodulin was deter-mined as described under "Experimental Procedures" and equals 4.04. Under similar conditions, bovine brain calmodulin displays an isoelectric point of 3.97. This small, but significant, difference was confirmed by the fact that, during electrofo-cusing of a mixture of both calmodulins in a medium contain-'The abbreviations used are: EGTA, ethylene glycol bis(,f-ami-noethyl ether)-N,N,N',N'-tetraacetic acid; Lys(Mea), e-N-trimethyl-lysine.
10695
at INRA Institut National de la Recherche Agronomique, on June 19, 2012
www.jbc.org
at INRA Institut National de la Recherche Agronomique, on June 19, 2012
Neurospora crassa Calmodulin
y
40 80 time Imin)
FIG. 3. Determination of mono-, di-, and trimethyllysine in
a N. crassa calmodulin hydrolyzate. The elution time of
mono-methyllysine (MML) and Lys(Mea) (TML) is shown by the arrows. Dimethyllysine elutes between them. I (a.u.) stands for the fluores-cence intensity of the o-phthaldialdehyde derivates of amino acids in arbitrary units. E time (min) 2 a ,. ,, _
This, as well as the marked differences in the retention times of most peptides, suggests a number of point mutations be-tween N. crassa and vertebrate calmodulins, explaining why it is difficult to locate the N. crassa peptides in the available sequence of vertebrate calmodulin (28).
Far UV Circular Dichroism-The circular dichroic spectra
below 250 nm of the Ca- and Mg-saturated protein, as well as of the metal-free form, depicted in Fig. 5, are very similar to those of bovine brain calmodulin (29-31). Hence, a similar secondary structure can be predicted: 30-35% a-helix, 50% random coil, and 15-20% fl-pleated sheet for the metal-de-pleted form. In the Ca-saturated form, the a-helix increases 5-8%, with a corresponding decrease of random coil.
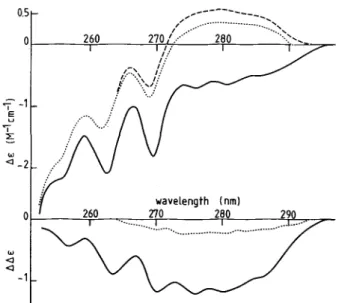
Near UV Circular Dichroism-Fig. 6 (top) shows the
cir-cular dichroic spectra between 250 and 300 nm for the Ca- or Mg-saturated or metal-free calmodulin of N. crassa. The sharp negative peaks at 262.5 and 269.5, as well as the shoulder at 255 nm, correspond to the Ltb transitions of Phe. The AE6, nm
value for the Ca-saturated protein is -0.23 M-1Phe cm-'. Hence, according to the reasoning of Wnuk et al. (32), 6 to 7
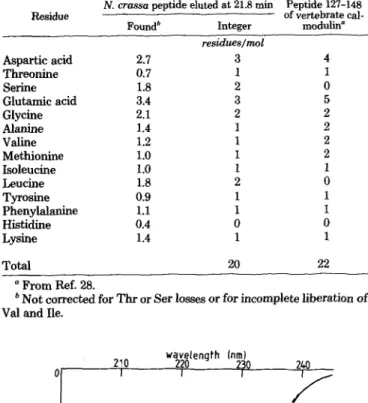
TABLE II
Amino acid composition of the tyrosine-containingpeptide of N. crassa calmodulin
N. crassa peptide eluted at 21.8 min Peptide 127-148 Residue of vertebrate
cal-Foundb Integer modulin
residues/mol Aspartic acid 2.7 3 4 Threonine 0.7 1 1 Serine 1.8 2 0 Glutamic acid 3.4 3 5 Glycine 2.1 2 2 Alanine 1.4 1 2 Valine 1.2 1 2 Methionine 1.0 1 2 Isoleucine 1.0 1 1 Leucine 1.8 2 0 Tyrosine 0.9 1 1 Phenylalanine 1.1 1 1 Histidine 0.4 0 0 Lysine 1.4 1 1 Total 20 22 a From Ref. 28.
b Not corrected for Thr or Ser losses or for incomplete liberation of Val and Ile.
time (min)
FIG. 4. High pressure liquid chromatography tryptic
pep-tide maps of ram testis (A) and N. crassa (B) calmodulins.
Calmodulin (0.5 mg) was digested and the peptides separated as described (18). Only the elution profile as monitored at 220 nm is shown for the sake of clarity. The eluate was also monitored at 275 nm for the aromatic residues. The two tyrosine-containing peptides of ram testis calmodulin correspond to the peaks at 16.3 and 21 min. The only tyrosine-containing peptide of N. crassa calmodulin corre-sponds to the peak at 21.8 min.
belongs to the COOH-terminal portion of the molecule even though it is somewhat shorter than peptide 127-148 of verte-brate calmodulin. The peptide shows significant differences in
composition, e.g. the presence of 2 Leu and 2 Ser residues.
FIG. 5. Far WUV circular dichroic spectra ofN. crassa calmod-ulin. -, 0.1 m CaCI2; --- , 2 mM MgC12+ 1 mM EGTA; -- -, 1
mM EDTA.
10697
co o 2. 1 Fat INRA Institut National de la Recherche Agronomique, on June 19, 2012
www.jbc.org
of the 8 Phe residues seem to be incorporated in helical segments. The broad negative peaks above 273 nm in the Ca-saturated protein can be attributed to the single Tyr residue present in N. crassa calmodulin. The AE2so0m value of -0.66
M-' Tyr cm-' is higher than the AE value reported for nonhel-ical Tyr-containing compounds; this suggests that the Tyr conformation is stabilized by strong interaction between the phenolic groups and polar groups in a hydrophobic environ-ment (33). A remarkable feature of N. crassa calmodulin is shown in Fig. 6 (top). Upon metal depletion, the sign of the intensities of the Tyr transitions is inversed; for instance,
AE280 m changes from -0.66 to 0.53 M- ' Tyr. This suggests that, in the metal-free form of N. crassa calmodulin, the Tyr conformation is stabilized in a dissymmetric environment of opposite type to that of the Ca form.
Fig. 6 (bottom) shows that, in the absence of Ca2+, 2 mM MgC12 changes the spectrum of the metal-depleted protein in the region of the Tyr bands, but not in the Phe region. In the presence of 100 M CaC12, MgC12has no effect on the spectrum (not shown). The difference in spectrum between the Ca- and metal-free forms confirms the important change in the envi-ronment of the Tyr residue. Furthermore, it suggests that, upon saturation by Ca2 +, 3 Phe residues become incorporated in helical segments.
Tyrosine Fluorescence-Fig. 7 shows the fluorescence spec-tra of the Ca-, Mg-, and metal-free forms of N. crassa cal-modulin. The maximum of emission corresponds to 310 nm. If the relative fluorescence intensity at 310 nm of the metal-free protein is taken as 1.0, Ca-saturated calmodulin shows a fluorescence intensity of 1.99, and the Mg form, a value of 1.26. Hence, the divalent cation dependency is less pronounced in the case of N. crassa calmodulin than in that of Octopus calmodulin, where the relative values are 1.0, 3.0, and 2.19 for the metal-free, Ca, and Mg form, respectively (34). This discrepancy between the two proteins suggests different de-grees of quenching (34) which, in turn, may result from the differences observed in the primary structure of the Tyr-containing peptide.
Myosin Light Chain Kinase Activation-N. crassa
cal-modulin activates bovine heart or brain phosphodiesterase to the same extent as vertebrate brain or testis calmodulin, but at concentrations 5- to 8-fold higher (13). To ascertain this
E
FIG. 6. Near UV circular dichroic spectra of N. crassa cal-modulin. Top, - , 0.1 mM CaC2; --- , 2 mM MgC12 + 1 mM EGTA;
- - -, 1 mM EDTA. Bottom, difference spectra: , Ca form/metal-free form; --- , Mg form/metal-form/metal-free form.
wavelength (nm)
FIG. 7. Tyrosine fluorescence spectra of N. crassa calmodu-lin. -, 0.1 mM CaC12; --- , 2 mM MgC12 + 1 mM EGTA; - - -, 1 mM
EDTA. The fluorescence intensity of the metal-free form at 308 nm is taken as 1.0. Excitation wavelength was 282 nm.
E
S
E C
- log [CaM]
FIG. 8. Activation of calcium-dependent skeletal myosin
light chain kinase as a function of increasing concentrations of calmodulin from N. crassa (0) or ram testis (x). The
concen-tration of Ca2 +was 0.1 mM.
difference in another calmodulin-activated enzyme, the activ-ities of myosin light chain kinase were assayed with vertebrate and N. crassa calmodulin. Fig. 8 confirms that about 8-fold higher concentrations of N. crassa are required for half-max-imal activation than in the case of vertebrate calmodulin (2.5 nM for ram testis versus 20 nM for N. crassa calmodulin).
DISCUSSION
The results detailed here suggest that, although N. crassa calmodulin shares the general characteristics of all other calmodulins known to date, it has features distinct from those of animal and even plant calmodulins. For instance, small, but significant, physicochemical differences, such as isoelectric point and Stokes radius, exist between N. crassa and brain calmodulin, which is apparently not the case between those from plant and animal species (35). Another example is related to one of the most salient features of the amino acid compo-sition of calmodulins: the content of methylated Lys. All vertebrate, invertebrate, and plant calmodulins possess meth-ylated Lys in position 115, whereas N. crassa calmodulin possesses none. The absence of these particular amino acids has been reported in the case of calmodulins from the basidi-omycete (higher fungus) Russula (27), Dictyostelium (7), and the green algae Chlamydomonas (5). The absence of Lys(Me3), however, is not a characteristic of all calmodulins from microorganisms since the presence of one Lys(Me3) was reported in calmodulin from the ciliated protozoa
Tetrahy-10698
w
at INRA Institut National de la Recherche Agronomique, on June 19, 2012
www.jbc.org
Neurospora crassa Calmodulin
mena (12) and Paramecium (9). It should be noted that the
enzymatic modification of Lys-115 occurs post-translationally (36) and does not necessarily reflect differences in the calmod-ulin gene in different organisms; lack of Lys(Me3) could mean that the methylating enzyme is not present or is inactive in the organism.
The tryptic peptide pattern of N. crassa calmodulin sug-gests that important differences exist in the primary structure between vertebrate and fungi calmodulins. In a general way, the reported peptide maps of plant and invertebrate calmod-ulins strongly resemble those of vertebrate calmodulin (26, 37), whereas those of Chlamydomonas (5) and Dictyostelium (7) are markedly different from the peptide map of bovine brain calmodulin. This suggests that, in microorganisms such as N. crassa, the calmodulin gene was the subject of many more point mutations than in animals and plants. The phe-nomenon of multiple gene mutations seems to be illustrated in the amino acid composition of the Tyr-containing peptide of N. crassa calmodulin. On the basis of conformational analyses (see below) and of amino acid composition, it is proposed that this peptide is the COOH-terminal peptide. The alignment of the amino acids of this peptide with those of the COOH-terminal peptide of vertebrate calmodulin indi-cates that the corresponding gene fragment of N. crassa underwent two deletions and three substitutions.
These modifications did not change dramatically the func-tion of the protein since 1) the Ca-binding properties are remarkably similar to those of vertebrate calmodulin, and 2)
N. crassa calmodulin can fully activate heart
phosphodiester-ase (13) and smooth muscle myosin light chain kinphosphodiester-ase. It is not clear why 5- to 8-fold higher concentrations of N. crassa calmodulin are required as compared to vertebrate calmodu-lin. A similar finding has been reported in the case of calmod-ulin from barley and fungi (27), Dictyostelium (7) and
Tetra-hymena (8), but cannot yet be related to particularities in the
primary structure; the absence of Lys(Me3) N. crassa calmod-ulin is certainly not involved (18).
The conformational changes induced in calmodulins by Ca2 + are of considerable importance since the Ca-dependent exposure of a hydrophobic plate on the surface of the protein is critical for the interaction with the target enzymes (38, 39) and subsequent activation. The CD data on the Phe bands of
N. crassa calmodulin suggest that most of the Phe residues
are incorporated in helical segments in the Ca-saturated pro-tein and may contribute to the stability of the hydrophobic plate. Upon Ca removal, 3 Phe residues lose their rigid con-formation. Interestingly, Mg2 + has no effect whatsoever on the chirality of these Phe residues, which confirms that Mg2 + cannot induce the formation of the hydrophobic plate.
N. crassa calmodulin possesses 1 single Tyr residue, which
facilitates the interpretation of conformational studies such as circular dichroism and UV fluorescence. Circular dichroism suggests that the Tyr residue belongs to one of the Ca-binding loops and remains in a highly organized environment even upon metal depletion. This behavior is reminiscent of the Tyr-138 in mammalian calmodulin; indeed, spectrophotometric titration (29), UV difference spectrophotometry (40), and pro-ton nuclear magnetic resonance (41, 42) indicate that, inde-pendently of the presence of Ca2+ in the fourth site, the Tyr-138 residue is buried in a particular environment. The confor-mational studies thus confirm our statement that the unique Tyr-containing peptide is homologous to the COOH-terminal tryptic peptide in vertebrate calmodulin.
In the absence of Ca2 +, the orientation of the aromatic side chain of Tyr-138 is inversed; the residue is engaged in a nonpolar environment of opposite chirality. This description is apparently also valid for the Tyr-138 residue in vertebrate
calmodulins for the following reasons. In native brain calmod-ulin, Tyr-138 apparently does not contribute much to the spectrum as compared to Tyr-99; however, upon nitration of Tyr-138, its contribution to the CD spectrum in the region of 270 and 290 nm is strongly amplified (approximately 10-fold) and becomes predominant. Interestingly, bovine brain nitrosyl calmodulin shows an inversion of the chirality of the Tyr CD bands upon removal of Ca2+ (30). It can be concluded that inversion of chirality of the Tyr-138 residue upon metal re-moval is common to calmodulins from widely different origins. It should be noted that Mg2 + does not change the chirality of Tyr-138 in metal-free calmodulin, suggesting that the latter ion is not capable of orienting the Tyr side chain towards the hydrophobic region of the fourth Ca-binding domain. This might explain why Mg2" is not an activating ion.
Acknowledgments-We wish to thank Prof. E. A. Stein
(Biochem-istry) and Prof. G. Turian (Microbiology) for their interest and financial support and M. Comte for skillful technical assistance.
REFERENCES
1. Cheung, W. Y. (1980) Science (Wash. D. C) 207, 19-27
2. Means, A. R., and Dedman, J. R. (1980) Nature (Lond.) 285, 73-77
3. Klee, C. B., Crouch, T. H., and Richman, P. G. (1980) Annu. Rev.
Biochem. 49, 489-515
4. Kuznicki, J., Kuznicki, L., and Drabikowski, W. (1979) Cell Biol.
Int. Rep. 3, 17-23
5. Van Eldik, L. J., Piperno, G., and Watterson, D. M. (1980) Proc.
Natl. Acad. Sci. U. S. A. 77, 4779-4783
6. Gitelman, S. E., and Witman, G. B. (1980) J. Cell Biol. 87, 764-770
7. Bazari, W. L., and Clarke, M. (1981) J. Biol. Chem. 256, 3598-3603 8. Kakiuchi, S., Sobue, K., Yamasaki, R., Nagao, S., Umeki, S.,
Nozawa, Y., Yazawa, M., and Yagi, K. (1981) J. Biol. Chem. 256, 19-22
9. Rauh, J. J., and Nelson, D. L. (1981) J. Cell Biol. 91, 860-865 10. Hubbard, M., Bardley, M., Sullivan, P., Shepherd, M., and
Forres-ter, I. (1982) FEBS Lett. 137, 85-88
11. Takagi, T., Nemoto, T., Konishi, K., Yazawa, M., and Yagi, K. (1980) Biochem. Biophys. Res. Commun. 96, 377-381
12. Yazawa, M., Yagi, K., Toda, H., Kondo, K., Narita, K., Yamazaki, R., Sobue, K., Kakiuchi, S., Nagao, S., and Nozawa, Y. (1981)
Biochem. Biophys. Res. Commun. 99, 1051-1057
13. Ortega Perez, R., van Tuinen, D., Marme, D., Cox, J. A., and Turian, G. (1981) FEBS Lett. 133, 205-208
14. Watterson, D. M., Harrelson, W. G., Jr., Keller, P. M., Sharief, F., and Vanaman, T. C. (1976) J. Biol. Chem. 251, 4501-4513 15. Autric, F., Ferraz, C., Kilhoffer, M.-C., Cavadore, J. C., and
Demaille, J. G. (1980) Biochim. Biophys. Acta 631, 139-147 16. Cox, J. A., and Stein, E. A. (1981) Biochemistry 20, 5430-5436 17. Moore, S., and Stein, W. H. (1963) Methods Enzymol. 6, 819-831 18. Molla, A., Kilhoffer, M.-C., Ferraz, C., Audemard, E., Walsh, M.
P., and Demaille, J. G. (1981) J. Biol. Chem. 256, 15-18 19. Bradford, M. M. (1976) Anal. Biochem. 72, 248-254
20. Cox, J. A., Kretsinger, R. H., and Stein, E. A. (1981) Biochim.
Biophys. Acta 670, 441-444
21. Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) J. Biol. Chem. 193, 265-275
22. Hummel, J.-P., and Dreyer, W. J. (1962) Biochim. Biophys. Acta
63, 530-532
23. Cox, J. A., Malnoei, A., and Stein, E. A. (1981) J. Biol. Chem. 256, 3218-3222
24. Haiech, J., Klee, C. B., and Demaille, J. G. (1981) Biochemistry
20, 3890-3897
25. Cartaud, A., Ozon, R., Walsh, M. P., Haiech, J., and Demaille, J. G. (1980) J. Biol. Chem. 255, 9404-9408
26. Head, J. F., Mader, S., and Kaminer, B. (1979) J. Cell Biol. 80, 211-218
27. Grand, R. J. A., Nairn, A. C., and Perry, S. V. (1980) Biochem. J.
185, 755-760
28. Watterson, D. M., Sharief, F., and Vanaman, T. C. (1980) J. Biol.
Chem. 255, 962-975
29. Klee, C. B. (1977) Biochemistry 16, 1017-1024
10699
at INRA Institut National de la Recherche Agronomique, on June 19, 2012
www.jbc.org
10700
Neurospora crassa Calmodulin
30. Walsh, M., Stevens, F. C., Orkawa, K., and Kay, C. M. (1979) 37. Watterson, D. M., Iverson, D. B., and Van Eldik, L. J. (1980)
Can. J. Biochem. 57, 267-278 Biochemistry 19, 5762-5768
31. Wolff, D. J., Poirier, P. G., Brostrom, C. 0., and Brostrom, M. A. 38. LaPorte, D. C., Wierman, B. M., and Storm, D. R. (1980) Bio-(1977) J. Biol. Chem. 252, 4108-4117 chemistry 19, 3814-3819
32. Wnuk, W., Cox, J. A., and Stein, E. A. (1981) J. Biol. Chem. 256, 39. Tanaka, T., and Hidaka, H. (1980) J. Biol. Chem. 255,
11538-11544 11078-11080
33. Strickland, E. H. (1974) Crit. Rev. Biochem. 2, 113-175 40. Richman, P. G., and Klee, C. B. (1979) J. Biol. Chem. 254, 34. Kilhoffer, M.-C., Demaille, J. G., and G6rard, D. (1981) Biochem- 5372-5376
istry 20, 4407-4414 41. Seamon, K. B. (1980) Biochemistry 19, 207-215
35. Anderson, J. M., Charbonneau, H., Jones, H. P., McCann, R. 0O., 42. Hincke, M. T., Sykes, B. D., and Kay, C. M. (1981) Biochemistry and Cormier, M. J. (1980) Biochemistry 19, 3113-3120 20, 4185-4193
36. Van Eldik, L. J., Grossman, A. R., Iverson, D. B., and Watterson, 43. Takagi, T., Nemoto, T., and Konishi, K. (1980) Biochem. Biophys. D. M. (1980) Proc. Natl. Acad. Sci. U. S. A. 77, 1912-1916 Res. Commun. 96, 377-381
at INRA Institut National de la Recherche Agronomique, on June 19, 2012
www.jbc.org