Original Article
Lipidomic and transcriptomic analyses of Chlamydomonas
reinhardtii under heat stress unveil a direct route for the
conversion of membrane lipids into storage lipids
B. Légeret1,2,3†, M. Schulz-Raffelt1,2,3†, H. M. Nguyen1,2,3†, P. Auroy1,2,3, F. Beisson1,2,3, G. Peltier1,2,3, G. Blanc4& Y. Li-Beisson1,2,3
1Commissariat à l’Energie Atomique et aux Energies Alternatives, Institut de Biologie Environnementale et Biotechnologie, CEA
Cadarache, Saint-Paul-lez-Durance, France,2Centre National de la Recherche Scientifique, UMR7265, Saint-Paul-lez-Durance, France,3Aix-Marseille Université, UMR7265, Marseille, France and4Laboratoire Information Génomique & Structurale, UMR7256 (IMM FR3479) CNRS Aix-Marseille Université, Marseille, France
ABSTRACT
Studying how photosynthetic cells modify membrane lipids in response to heat stress is important to understand how plants and microalgae adapt to daily fluctuations in tempera-ture and to investigate new lipid pathways. Here, we investi-gate changes occurring in lipid molecular species and lipid metabolism genes during early response to heat stress in the model photosynthetic microorganism Chlamydomonas reinhardtii. Lipid molecular species analyses revealed that, after 60 min at 42 °C, a strong decrease in specific polyunsat-urated membrane lipids was observed together with an increase in polyunsaturated triacylglycerols (TAGs) and diac-ylglycerols (DAGs). The fact that decrease in the major chloroplastic monogalactosyldiacylglycerol sn1-18:3/sn2-16:4 was mirrored by an accumulation of DAG sn1-18:3/sn2-16:4 and TAG sn1-18:3/sn2-16:4/sn3-18:3 indicated that newly accumulated TAGs were formed via direct conversion of monogalactosyldiacylglycerols to DAGs then TAGs. Lipidomic analyses showed that the third fatty acid of a TAG likely originated from a phosphatidylethanolamine or a diacylglyceryl-O-4′-(N,N,N,-trimethyl)-homoserine betaine lipid species. Candidate genes for this TAG synthesis pathway were provided through comparative transcriptomic analysis and included a phospholipase A2 homolog and the DAG acyltransferase DGTT1. This study gives insights into the mo-lecular events underlying changes in membrane lipids during heat stress and reveals an alternative route for TAG synthesis. Key-words: Microalgae; lipid remodeling; acyltransferase; high temperature; phospholipase; diacylglycerols; galactolipids; lysolipids; oil accumulation; polyunsaturated triacylglycerols. Abbreviations: DAG, diacylglycerol; DGDG, digalactosyldia-cylglycerol; DGTS, diacylglyceryl-O-4 ′-(N,N,N,-trimethyl)-homoserine; FAMEs, fatty acid methyl esters; FFA, free fatty
acids; GC-FID, gas chromatography–flame ionization detector; MGDG, monogalactosyldiacylglycerol; MM, minimal medium; N, nitrogen; PtdEtn, phosphatidylethanolamine; PtdGro, phos-phatidylglycerol; PtdIns, phosphatidylinositol; SQDG, sulfoqui-novosyldiacylglycerol; TAG, triacylglycerol; TAP, Tris-acetate phosphate; TGDG, trigalactosyldiacylglycerol; TLC, thin layer chromatography; UPLC-MS/MS, ultra performance liquid chromatography–tandem mass spectrometry.
INTRODUCTION
With the continuous rise in global temperatures, the re-sponse and adaptation of photosynthetic cells to heat stress are a critical parameter for crop productivity and ecosystem stability (Barnabás et al. 2008, Iba 2002). Understanding the basic mechanisms underlying cellular response and adapta-tion to heat stress is particularly relevant in the context of industrial cultivation of microalgal species because cells grown in outdoor ponds or photobioreactors are subjected to rapid and wide temperature fluctuations during the day and, due to greenhouse effect, have to cope with tempera-tures as high as 45 °C, even in temperate regions (Ras et al. 2013). Such a temperature largely exceeds the temperature range for optimal growth (15–30 °C for most species), and results in a sharp decrease in productivity (Lobell et al. 2011, Stefan et al. 2014).
Understanding of plants’ response to heat stress at a mo-lecular mechanistic level is a critical first step to provide targets for genetic engineering of thermo-tolerant crop species. Thus, responses of plant/algal species to heat stress have been characterized intensively (Iba 2002, Suzuki et al. 2006, Neilson et al. 2010, Mühlhaus et al. 2011, Liu et al. 2012). It is now known that heat stress response is a highly conserved process and involves multiple pathways and regulatory networks requiring coordination of several subcellular compartments (Mittler et al. 2012, Schroda et al. 2015). Latest system-level study of the heat stress re-sponse in the model green microalga Chlamydomonas reinhardtii has revealed that cells respond to heat stress via arresting cell cycle, accumulating molecular chaperons,
Correspondence: Y. Li-Beisson. Fax: +33442256265; email: yonghua. li@cea.fr and G. Blanc. Fax: +33442256265; email: guillaume.blanc@igs. cnrs-mrs.fr
and degradation of large molecules to generate compounds with roles in stress protection (Hemme et al. 2014).
Increasing evidence also suggests the importance of mem-brane lipids in heat stress biology (Horváth et al. 2012). It has been shown that membrane remodelling and regulation of membrane fluidity play an important role in response to heat stress (Murata & Los 1997, Los & Murata 2004, Chen et al. 2006, Zheng et al. 2011, Yao et al. 2012, Hemme et al. 2014, Schroda et al. 2015). A study of changes occurring in membrane lipids of C. reinhardtii after a few hours to 2 d of heat stress and during a 24 h recovery has shown the existence of several phases that involve degradation of membrane lipids and accumulation of polyunsaturated TAGs (Hemme et al. 2014). However, the potential relationship between the changes in lipids and the pathways involved remains mostly unknown.
In this study, in order to gain insights into the lipid pathways involved in response to heat stress, we focus on the early changes occurring in the lipidome of the model green microalga C. reinhardtii. We provide evidence that within the first hour, heat-stressed cells reduce the content of specific lipid constituents of their plastidial membranes (polyunsaturated MGDGs) by converting them to storage TAGs via DAG inter-mediates. Some candidate genes for this pathway of direct conversion of membrane to storage lipids are proposed based on the analysis of changes induced by heat stress in the tran-scriptome. Both lipidome and transcriptome analyses support the idea that the step of MGDG to DAG conversion is catalysed by a new type of enzyme. This study thus provides new molecular insights into the early phase of the membrane lipid remodelling process triggered by heat stress.
MATERIALS AND METHODS
Strains and culture conditions
Chlamydomonas reinhardtii wild-type strain CC125 (also named 137C, mt nit1nit2) was grown in an Infors (Infors HT, Bottmingen, Switzerland) in liquid MM (no acetate) (i.e. photoautotrophic conditions) under continuous illumina-tion (100μmol photons m 2s 1) at 25 °C, under shaking (120 r.p.m.), and supplied with 2% CO2(i.e. photoautotrophic
condition). For the distribution of TAG molecular species ac-cumulated under mixotrophic conditions, cells were cultivated in TAP medium under air with otherwise similar conditions.
Induction of heat stress
For the heat stress experiments, mid-logarithmic phase grown cells (approximately 5 × 106cells mL 1) cultivated under standard conditions were harvested by centrifugation at 600 g for 3 min, then resuspended into the same amount of pre-heated warm MM medium and incubated in an Infors (Infors HT, Bottmingen, Switzerland) preset at 42 °C. Other parame-ters were kept the same as standard conditions. Cells were kept at 42 °C for 0, 30 or 60 min and then harvested immediately by centrifugation at 4 °C for 2 min at 600 g. Samples taken at time 0 were used as control.
Measurement of cell size and cell concentration
Cell concentration and size were measured using automated cell counter (MultisizerTM3 Coulter Counter, Beckman
Coulter, Brea, CA, USA) before and during heat stress experiments.
Chlorophyll and starch quanti
fication
Cellular chlorophyll and starch amount were measured as previously described (Siaut et al. 2011).
Lipid extractions
To avoid any potential changes in lipidome due to lipase activ-ities, in this study, cells of C. reinhardtii were harvested by centrifugation at 1000 g for 2 min then immediately quenched by boiling in hot isopropanol containing 0.01% butylated hydroxytoluene (BHT) (w/v) for 10 min at 85 °C (Li-Beisson et al. 2010). Samples were then vortexed for 10 min to break the cells, to which hexane was added to reach a ratio of hexane/isopropanol (3/2, v/v). The mixture was vortexed again, and left for a few minutes on the bench at room temperature. An aqueous Na2SO4solution (6.6%, w/v) was added to allow
phase separation. After being centrifuged briefly, the upper phase was collected and transferred to a new glass tube. A mix-ture of isopropanol/hexane (2/7, v/v) was used to re-extract the lipids. Upper hexane phase was pooled with the previous organic extracts and dried under N2flow. The extracted lipids
were then resuspended in chloroform/methanol (2/1, v/v), and ready for all subsequent analyses.
Quantification of lipid classes
Lipid classes were first separated by TLC. Lipid classes were then quantified based on densitometry method via compar-ing with a standard curve generated from loadcompar-ing known amount of standards to the same plate as described previ-ously in (Siaut et al. 2011). Briefly, a fraction of the total extracted cellular lipids was deposited onto a 20 × 20 cm silica gel 60 F254 TLC plate (Merck KGA, Darmstadt, Germany) using an ATS5 automatic TLC sampler (Camag, Muttenz, Switzerland). The TLC plate was developed either in a mixture of hexane/diethyl ether/acetic acid (17/3/0.2, v/v/v) for neutral lipid separation or developed in a mixture of acetone/toluene/water (91/30/8, v/v/v) for polar lipid separation. After development, the plates were air dried, and lipids were revealed after dipping in a mixture of (20 g CuSO4 and 80 mL H3PO4 dissolved in water in a total
volume of 1 L) and heated at 170 °C for 20 min. The lipid stan-dards used were triheptadecanoin (C17:0 TAG, Sigma-Aldrich, Saint-Louis, USA), monogalactosyl distearoylglyceride (MGDG, Larodan Fine Chemicals AB, Malmö, Sweden), digalactosyl distearoylglyceride (DGDG, Larodan Fine Chemicals AB), 1,2-dipalmitoyl-sn-glycerol-3-phospho-(1′-rac-glycerol) (PtdGro, Avanti Polar Lipids, AL, USA) and 1,2-dipalmitoyl-sn-glycerol-3-phosphoethanolamine (PtdEtn, Avanti Polar LIpids).
Recovery of lipids from TLC plate
Silica-containing lipids were scrapped off a TLC plate, after lipids being revealed by spraying with a 0.01% (v/v) primuline solution dissolved in acetone/water (80/20, v/v) (Li-Beisson et al. 2010). The recovered silica powder was loaded onto a glass Pasteur pipette stuffed with a pinch of glass wool at the bottom. Chloroform/methanol/water (5/5/1, v/v/v) was used to elute the lipids. It was then dried under N2gas and subjected
to mass spectrometry identification by UPLC-MS/MS.
Fatty acid composition analyses
For fatty acid compositional analyses, one part of the extracted lipids were converted to FAMEs by acid-catalysed transmethylation (Li et al. 2006). Triheptadecanoin was added as an internal standard. The FAME mixtures were ex-tracted into hexane and analysed by gas chromatography coupled to flame ionization detector and mass spectrome-try (GC-FID-MS) (Agilent 7890A GC and Agilent 5975C MS, Agilent Technologies, Palo Alto, CA, USA). The Zebron ZB-WAX column (Zebron Corporation, Anaheim, CA, USA) (30 m × 0.32 mm × 0.50μm) was used for separa-tion of individual FAMEs. The GC condisepara-tions were as follows: split ratio of 1:20, injector and flame ionization detector temperature 240 °C and oven temperature pro-gramme 50 °C for 2 min, then increasing at 15 °C min 1 to 150 °C and then increasing again at 6 °C min 1to 240 °C and holding at this temperature for 4 min. The flow rate of the carrier gas (H2) was 1 mL min 1. FAMEs were identified based
on retention time and mass spectrum and quantified based on FID trace (Siaut et al. 2011).
Lipid molecular species analyses by UPLC-MS/MS
For lipid molecular species analyses, the extracted lipids were subjected to analyses by UPLC-MS/MS as previously reported (Nguyen et al. 2013). Briefly, the lipid mixtures were first separated on a KinetexTM (Kinetex, Atlanta, GA, USA) C182.1 × 150 mm 2.6μm column (Phenomenex,
Torrance, CA, USA) connected to an ultimate RS 3000 UPLC system (Thermo Fisher, Waltham, MA, USA). This LC system is connected to a quadrupole-time-of-flight (QTOF) 5600 mass spectrometer (AB Sciex, Framingham, MA, USA) equipped with a duo-spray ion source. Same set of samples were run both at positive and negative mode for quantification of polar membrane lipids and neutral lipids, respectively. Lipid identification was based on reten-tion time, mass accuracy peaks from the MS survey scan compared with theoretical masses and fragment ions from MS/MS scan. Relative quantification was achieved with MULTIQUANTsoftware (AB Sciex) on the basis of intensity values of extracting masses of different lipids previously identified. Lipid molecular species were noted as lipid class (total number of carbon atoms: total number of double bonds) or as lipid class (sn-1 fatty acid/sn-2 fatty acid) when the sn position of fatty acids was known.
Determination of the sn position of fatty acids
For many Chlamydomonas lipid molecular species, the sn-1 or sn-2 position of a fatty acid was attributed based on previ-ous experimental determinations (Giroud et al. 1988). For some other species not reported in Giroud et al. (1988), the sn positions were attributed by comparing the relative inten-sities of the MS/MS fatty acid fragments to those of the char-acterized species of the same class, or to those of a commercial lipid standard of known stereochemistry run under the same LC-MS/MS conditions. In addition, for the DAG34:7 and TAG52:10 species, a lipase-based positional analysis was performed as described next. DAG34:7 and TAG52:10 were collected after UPLC separation of a Chlamydomonas lipid extract (about 15 analytical runs were pooled for each lipid species). Purity of lipids was checked by analysing an aliquot of each lipid species by UPLC-MS/ MS. The remaining part of the sample (about 5μg lipids) was dried under a stream of N2, and 995μL of a Trisbuffer
(40 mM Tris–HCl, 25 mM H3BO4 and pH 7.2) was added.
The mixture was sonicated, and 5μL of a solution of Rhizopus oryzae lipase (Sigma 62305) at 100 U mL 1 was added. In a control experiment, DAG34:1 (sn1-palmitoyl and sn2-oleoyl) (Avanti Polar Lipids) was used to check the lipase sn1,sn3 regiospecificity under the reaction condi-tions used. After 15 min incubation at 22 °C, 1 mL of isopropanol and 3 mL of methyl tert-butyl ether were added. After centrifugation, the upper organic phase was collected, dried under N2and resuspended in the solvent mixture used
for UPLC-MS/MS analysis.
RNA extraction, qRT-PCR and RNAseq experiments
Total RNA was extracted from cells grown to exponential phases and exposed to heat stress (42 °C) for a certain num-ber of minutes (0, 5, 15 and 25 min) in an incubator (Infors). Extracted RNA was resuspended into RNase/DNase free water as detailed in Nguyen et al. (2013). Reverse transcrip-tion reactranscrip-tion was performed on 1μg of total RNA using the superscript Vilo cDNA synthesis kit (Life Technologies, Rockville, MD, USA). The RACK1 gene of C. reinhardtii is used as a housekeeping control for normalization. The gene-specific primers for candidate genes used in this study are shown in Supporting Information Table S1. qRT-PCR reactions were prepared with MESA FAST qPCR MasterMix Plus for SYBR® Assay No ROX (Eurogentec, Seraing, Belgium) and performed in 384-well plates using the LightCycler® 480 instrument (Roche, Basel, Switzer-land) using conditions: 95 °C for 10 min, 45 cycles at 95 °C for 10 s, 60 °C for 15 s and 72 °C for 10 s. The relative transcript ratio of selected genes was calculated based on the 2-ΔΔCT method (Livak & Schmittgen 2001) with aver-age CT obtained based on triplicate measurements. Relative expression ratios were calculated based on three biological and two technical replicates.
An RNA-seq library was constructed from 10μg of total RNA extracted for each time point replicate (eight in total) using the mRNA-seq sample preparation kit (RS-100-0801)
according to the manufacturer’s instructions (Illumina, San Diego, CA, USA). RNA was subjected to poly(A) selec-tion using Sera-Mag magnetic Oligo-dT beads followed by fragmentation and then used for cDNA synthesis with ran-dom hexamers. The cDNA product then underwent end repair, A-tailing, adapter ligation and PCR amplification. Each library was sequenced using an Illumina sequencer, gen-erating 23.9–25.1 million 50-nt single-end reads for each time point replicate. The sequencing reactions were performed at Beijing Genome Institute (BGI)-Hong Kong.
Transcriptome analysis
Reads were aligned onto the C. reinhardtii genome assembly version V5 using BOWTIE2 (Langmead & Salzberg 2012), and TOPHAT2 (Kim et al. 2013a) for aligning reads spanning exon junctions. Only alignments that had not more than two mismatches with the reference sequence were retained. When a read produced more than one valid alignment, the genomic region producing the best alignment score was considered as its point of origin. Reads producing more than one alignment with identical best scores were considered as originating inside a repeated sequence and were discarded in subsequent per-gene read count analyses. For each time point, we mapped 18.4–19.2 million reads to single copy sequences on the C. reinhardtii genome.
Of 17 672 C. reinhardtii predicted genes, 5286 had raw read counts<50 in all time points and were discarded from subse-quent analysis. The remaining 12 386 genes had their read counts normalized for library size using the variance stabilizing transformation function implemented in the DESeq2 package (Love et al. 2014).
RESULTS
Response to heat stress involves a strong decrease
in some membrane lipids and an accumulation of
TAGs
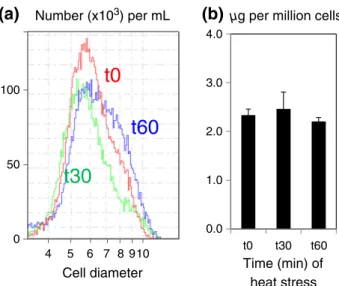
To characterize the initial response of C. reinhardtii to heat stress, cells were cultivated at 25 °C and then subjected dur-ing 30 or 60 min to a heat stress at 42 °C (Supportdur-ing Infor-mation Fig. S1). During this short-period heat treatment, it was observed that cell size and chlorophyll content remained constant between control and heat-stressed cells (Fig. 1A & B). At the end of 1 h treatment, one part of the cell population seemed to increase slightly in cell size (Fig. 1A). Under all three conditions (time 0, 30 min or 60 min of heat stress), total fatty acid content per cell did not change significantly albeit showed a slight tendency to increase (Fig. 2A). There are also no changes in fatty acid composition; therefore, the ratio of polyunsaturated to satu-rated fatty acids at a whole cell level remains constant (Fig. 2B).
To determine whether major quantitative changes occur dur-ing the early phase of response to heat stress, absolute quanti-fication of membrane lipid classes and TAGs was performed based on TLC and densitometry. We observed reductions in
MGDG, PtdGro, DAG DGTS and PtdEtn, while DGDG remained unaltered (Fig. 3A). Analysis of neutral lipids showed a threefold increase in total TAG content after 1 h heat stress (Fig. 3B). The absolute TAG level increased from 0.1 to 0.35μg per million cells (as quantified by TLC) within 1 h. The changes in polar membrane lipids and TAG amount are depen-dent on the length of heat treatment, that is, the differences are more dramatic as treatment time prolongs (as drawn from data for 0, 30 and 60 min of heat stress). However, starch level remained constant during this period under the cultivation con-ditions used in this study, that is, photoautotrophic growth (Fig. 3C).
Some polyunsaturated plastidial and extraplastidial
membrane lipid species are selectively removed in
heat-stressed cells
The observation that specific membrane lipids decreased and TAG increased while total cellular fatty acid content and fatty acid composition remained constant suggested that degradation of membrane fatty acids was compensated by de novo synthesis of storage fatty acids, or that more simply, there was conversion of membrane lipids to storage lipids. To gain detailed insights into the changes occurring in membrane lipid composition, a survey of all membrane lipid molecular species was conducted using UPLC-MS/MS. Because stronger re-sponse were observed for 60 min heat stress as compared with the 30 min heat stress (Fig. 3), for UPLC-MS/MS experiment, we focused our analyses on the 60 min heat stress. The sn1 or sn2 position of fatty acid on the glycerol backbone was attrib-uted based on previous literature (Giroud et al. 1988) and/or using MS and MS2 analysis and comparison with lipid stan-dards of known stereochemistry.
4 5 6 7 8910 100 50 0 0.0 1.0 2.0 3.0 4.0 t0 t30 t60
(a)
(b)
t0
t30
t60
µg per million cells
Time (min) of heat stress Cell diameter
Number (x103) per mL
Figure 1. Impact of short-term heat stress on cell size and chlorophyll content in Chlamydomonas reinhardtii. (a) Monitoring of cell size distribution in control (0 min), 30 and 60 min heat-stressed cells (noted as t0, t30 and t60, respectively). (b) Chlorophyll content in response to heat stress. Values are means of three biological replicates, and error bars represent 95% confidence intervals.
Among all lipid classes, MGDG, PtdGro, DGTS and PtdEtn were reduced, whereas DGDG and PtdIns remained largely constant; SQDG seems to increase slightly under higher tem-perature (Supporting Information Fig. S2). This analysis also showed that relative comparisons in lipid classes based on UPLC-MS/MS are consistent with quantitative analyses as determined by TLC (Fig. 3A).
Examination of lipid molecular species showed that within each lipid class, only certain molecular species were reduced under heat stress (Fig. 4). The most dramatic reductions (be-tween 40–80% reduction) were observed for MGDG18:3/ 16:4, DGTS16:0/18:3 and PtdEtn18:0/18:3. It is worth noting that around 30–50% reductions were also observed for most PtdGro species. PtdGro, as the single phospholipid of chloroplast, is known to play essential roles in plant development, and PtdGro has also been known to be in-volved in phase transitions in response to freezing tempera-tures in higher plants (Wada & Murata 2007). Its reduction observed here in response to temperature increases
demonstrated the important and conserved role of PthGro metabolite in the adaptation of membranes to temperature stress.
Besides the usual lipids, we detected the formation of consid-erable amount of 2-lyso-DGTS16:0 and 2-lyso-PtdEtn18:0, accompanied by reduction in their respective metabolic precur-sors DGTS16:0/18:3 and PtdEtn18:0/18:3. The identification and relative quantification of DGTS16:0/18:3 and lyso-DGTS16:0 are shown in Supporting Information Fig. S3, and the over-accumulation of lyso-PtdEtn in heat-stressed cells is shown in Supporting Information Fig. S4.
The decrease in the major MGDG18:3/16:4 is
mirrored by an accumulation of DAG18:3/16:4 and
TAG18:3/16:4/18:3 species
During TAG quantification by TLC, we noticed a consider-able shift in the migration distance (Rf: retention factor) be-tween TAGs of control cells (at 25 °C) as compared with heat-stressed cells (Supporting Information Fig. S5). Such an
0 5 10 15 20 25 30 mol% µ
g FAs per million
cells
(a)
(b)
Fatty acids
[number of carbons: number of unsaturations (position of unsaturations)] 0.0 2.0 4.0 6.0
t0
t30
t60
Figure 2. Cellular fatty acid content (a) and molar composition (b) remain constant during 0, 30 and 60 min heat stress at 42 °C. Control (0 min), 30 and 60 min heat-stressed cells are noted as t0, t30 and t60, respectively. Values are means ± 95% confidence interval of three biological replicates. FA, fatty acid.
Figure 3. Quantitative alterations in polar lipid classes, TAG and starch content in response to 30 and 60 min heat stress. (a) Polar lipids content, (b) TAG content, (c) Starch content. Control (0 min), 30 and 60 min heat-stressed cells are noted as t0, t30 and t60, respectively. Values are means ± 95% confidence interval of three biological replicates. *Asterisk indicates significant difference from the control cells (P< 0.01).
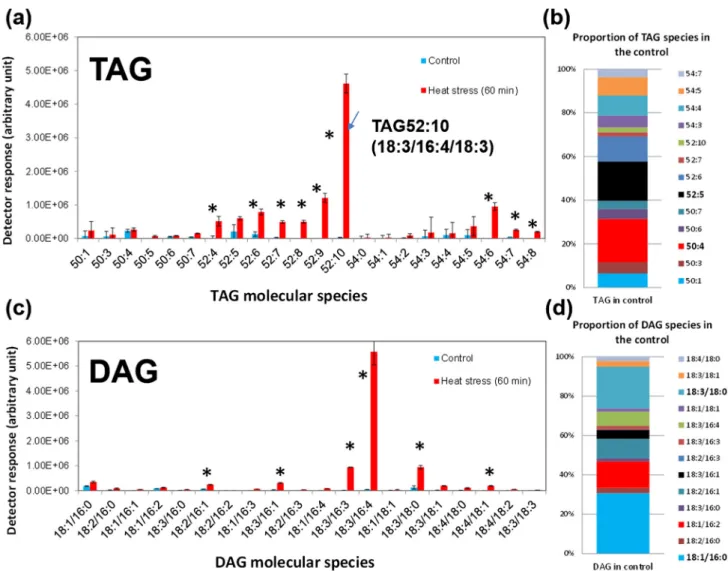
effect is usually due to differences in the degree of unsaturations of the fatty acyl chains esterified to the glycerol backbone. We thus extracted the lipids from the correspond-ing area (as circled in the red box) of the TLC silica plate (in Fig. 5), and the recovered lipids were identified as only TAGs, and the majority of which are TAG52:10 (total num-ber of carbons: total numnum-ber of double bonds) and TAG52:11, whereas the major TAG molecular species present in normal grown cells is TAG52:5; this dramatic shift from sat-urated to polyunsatsat-urated TAG species explains the observed difference in the migration distance on the TLC plate.
To gain a comprehensive picture of the TAG molecular spe-cies present in heat-stressed cells as compared with control cells, the storage lipids were analysed by UPLC-MS/MS
(Fig. 5A). This analysis showed that in photoautotrophically healthy grown C. reinhardtii, basal level of TAGs was very low and is composed of only a few molecular species (around 13, their relative composition shown in Fig. 5B). By contrast, in the heat-treated cells, 22 species (including nine new ones) could be detected. The major increase was observed for TAG52:10. Lipidomic analysis also showed that in addition to TAGs, heat-stressed cells accumulated DAGs (Fig. 5C). The mass spectrometry identification of TAG52:10 as TAG18:3/ 16:4/18:3 is shown in Fig. 6A, whereas the major DAG formed under heat-stressed cells was found to be DAG18:3/16:4 (Fig. 6B), which is also more unsaturated than the basal level of DAG species (DAG18:1/16:0) found in control cells. In or-der to confirm the sn position of the fatty acids in DAG34:7 Figure 4. Relative alterations in lipid molecular species in response to 60 min heat stress. Values are means ± 95% confidence interval of three biological replicates. *Asterisk indicates significant difference from the control cells as determined by the Student’s t-test P < 0.01.
and TAG52:10, these species were purified by UPLC, and a stereospecific positional analysis was performed using R. oryzae lipase, which specifically removes the acyl groups pres-ent in the sn1 and sn3 positions of an acylglycerol. Analysis of reaction products by UPLC-MS/MS showed that only MAG16:4 was formed, which demonstrated that sn2 position was only esterified by 16:4 fatty acid in both lipids and hence that the DAG34:7 and TAG52:10 accumulated in the heat-stressed cells were indeed DAG sn1-18:3/sn2-16:4 and TAG sn1-18:3/sn2-16:4/sn3-18:3. This is the same backbone as that of the major plastidial galactolipid MGDG 34:7 (sn1-18:3/sn2-16:4).
To provide further evidence that DAGs and TAGs formed under heat stress in the wild-type (WT) cells are indeed due to the remodelling of the plastidial galactolipid MGDG, we ex-amined the lipidomic responses of the crfad7 mutant to heat stress. Crfad7 mutant has been shown to be affected in the only plastidial-locatedω-3 fatty acid desaturase of Chlamydomonas (Nguyen et al. 2013) (Pflaster et al. 2014). This mutant offers
unique opportunity to address the question of MGDG re-modelling because the molecular makeup of the MGDG species in the mutant is considerably different from that of WT, that is,>80% reduction in MGDG18:3/16:4 and simul-taneous increase in MGDG18:2/16:3. When subjected to 1 h heat stress at 42 °C, we observed a decrease in MGDG18:3/16:4 (similar to what occurs in the WT), but also, a more significant decrease in the newly formed and more abundant MGDG18:2/16:3 in the mutant. This is again mirrored by a shift in the molecular species of DAGs and TAGs accumulated under heat stress, that is, from DAG18:3/16:4 in the WT to DAG18:2/16:3 in the crfad7, and from TAG52:10 in the WT to TAG52:8 in the mutant (Supporting Information Fig. S6). Taken together, the re-duction in the major molecular species of MGDG18:2/16:3 in the crfad7 mutant together with the accumulation of DAG18:2/16:3 and TAG52:8 supports our argument that un-der heat stress, MGDG can be remodelled to provide the DAG backbone for TAG synthesis.
Figure 5. TAGs and DAGs formed under heat stress are mainly of polyunsaturated species. Total lipid extracts were subjected to UPLC-MS/MS analyses under negative mode. (a) Relative amount of TAG molecular species present in control and in heat-stressed cells. (b) TAG molecular species composition for control cells only. (c) Relative amount of DAG molecular species present in control and in heat-stressed cells. (d) DAG molecular species composition for control cells only. Values are means of three biological replicates with 95% confidence interval shown. *Asterisk indicates significant difference from the control cells (P < 0.01).
Transcriptome analysis provides candidates for the
heat stress-induced TAG synthesis route
To identify candidate proteins of the TAG formation pathway in heat-stressed Chlamydomonas cells, we determined the global transcriptional responses that are associated with heat stress using the Illumina RNAseq approach. More than 196 million sequence reads, 50 bp in length, were generated from four time points (two biological replicates for each time point) during a heat stress at 42 °C, that is, 0 (T = 25 C), 5, 15 and 25 min. Under the current experimental conditions, heat-stress responses were observed. The sequence reads were aligned onto the C. reinhardtii genome and analysed to mea-sure gene expression levels. Of 17 672 predicted genes, 5286 had raw read numbers below 50 at any point and were desig-nated as weakly expressed. Of the remaining 12 386 genes, 10 051 had significant expression variability during heat stress as determined by the likelihood ratio test (LRT– adjusted for multiple tests;α = 0.01) implemented in the DESeq2 package (Love et al. 2014). Among the variable genes supported by statistical test, 3376 had≥1.5-fold change in transcription ac-tivity relative to 25 °C at any point during the heat stress, with approximately one-third showing up-regulation and two-thirds showing regulation. Up-regulated and down-regulated genes (≥1.5-fold change) are distributed evenly in most Kyoto Encyclopedia of Genes and Genomes (KEGG) biological pathways, except for four pathways described in the succeeding texts. Altogether, the results of the RNAseq
study indicate that heat stress impacts massively genome tran-scription and that many metabolic pathways are affected.
As shown in Fig. 7, the KEGG pathway‘protein processing in endoplasmic reticulum’, containing heat shock proteins, chaperone functions and proteins involved in the proteasome targeting pathway, is characterized by a significant excess of up-regulated genes relative to random distribution, indicating this pathway is heavily solicited to cope with heat stress-induced denaturation of proteins. This is consistent with previ-ous studies (Hemme et al. 2014) and also confirmed heat-shock response of our treated cells in this experiment. In contrast, functional categories involved in ribosome biogenesis, N-glycan biosynthesis and lysosome-like functions contain an ex-cess of down-regulated genes. Thus, C. reinhardtii appears to inhibit ribosome formation and consequently protein synthesis as part of the response to heat stress. This observation is in line with previous reports of a ~17% decrease in cytosolic ribosome content after 3 h of heat stress (Mühlhaus et al. 2011) that persisted during a 24 h heat stress period (Hemme et al. 2014). Thus, our data indicate that this specific response of the cytosolic ribosomal apparatus to heat stress is transcription-ally regulated and rapidly set up following a temperature shift to 42 °C. Down-regulation of N-glycan biosynthesis genes sug-gests that cell wall synthesis and maintenance functions are globally decreased during heat stress.
We focused our analyses on the changes in transcription ac-tivity of a compiled list of 273 C. reinhardtii genes involved in Figure 6. Molecular identification of TAG(18:3/16:4/18:3) and DAG(18:3/16:4) via MS scan after UPLC. M, molecular weight.
lipid metabolism (Supporting Information Table S2). This list is based on a homology search to known lipid metabolism genes in the higher model plant Arabidopsis thaliana reported in Aralip website (http://aralip.plantbiology.msu.edu/pathways/ pathways) and described in Li-Beisson et al. (2010). Of the 273 genes, 46 were considered as weakly expressed, and 196 showed significant expression variability (LRT), of which 64 had≥1.5-fold change at any point in the time series relative to 25 °C, with 24 showing up-regulation and 40 showing down-regulation (Table 1). The profiles of transcription for genes in-volved in fatty acid synthesis are relatively constant compared with other functional categories, suggesting that this pathway maintains a steady activity during heat stress (Fig. 7). Other
up-regulated genes include two plastid-lipid-associated pro-teins (PLP2 and PLP6) and two membrane-lipid synthesis genes (DGD1 and SQD3). DGD1 catalyses the formation of DGDG from MGDG, thus consistent with the relative con-stant level of DGDG amount under heat stress albeit with sig-nificant reduction in its substrate MGDG. SQD3 catalyses the formation of sulfolipid SQDG, which showed slight increase in heat-stressed cells (Fig. 4).
Most of the other up-regulated genes belong to lipid-derived signalling pathways, for example, linoleate 9S-lipoxygenase (LOX), phospholipase A2 (PLA2), 12-oxophytodienoic acid reductase (OPDA reductase) and so on, or are coding proteins required for lipid degradation and remobilization, for example, Figure 7. Transcriptional changes of genes involved in selected Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways during thefirst 25 min of heat stress. Aside pathway IDs and descriptions are indicated under the format (X/Y/Z), X = total number of genes in the KEGG pathway, Y = number of gene that are significantly up-regulated and Z = number of genes that are significantly down-regulated; asterisks show pathways containing a significant excess of up-regulated genes or down-regulated genes according the unidirectional binomial test (α = 0.05 after Bonferroni correction for multiple tests).
Table 1. A list of lipid genes showing significant response to heat stress in Chlamydomonas reihardtii as analysed by RNAseq. Expression
JGI v5.5
(Augustus u111.6) ID Name
abbreviations Putative function
Log2 (42 °C/25 °C) Up-regulated in response to
heat stress
Cre16.g689150 SQD3 Sulfoquinovosyltransferase 2.15
Cre01.g053000 GPD2 Glycerol-3-phosphate dehydrogenase (NAD+) 1.75
Cre16.g684043 Aldehyde dehydrogenase (NAD+) 1.52
Cre05.g232002 ACX2 Acyl-CoA oxidase 1.28
Cre12.g557750 DGTT1 DAG acyltransferase. DGAT Type 2 1.21
Cre12.g512300 LOX Linoleate 9S-lipoxygenase (LB associated) 1.18
Cre12.g514000 CS1 Ceramide synthetase 1.04
Cre09.g405500 MLDP Major lipid droplet protein (LB associated) 1.00
Cre03.g188650 PLP2 Plastid-lipid-associated protein PAP/fibrillin family protein
0.99
Cre12.g505400 PLA2 Phospholipase A2 (LB associated) 0.92
Cre03.g210513 OPR1 12-Oxophytodienoic acid reductase (OPDA reductase) 0.92
Cre10.g425100 Lipase Patatin lipid acylhydrolase 0.87
Cre08.g381250 PLA2 Phospholipase A2 0.79
Cre11.g476550 ADH11 Mitochondrial trans-2-enoyl-CoA reductase; alchol dehydrogenase
0.77 Cre03.g188700 PLP6 Plastid-lipid associated protein PAP/fibrillin family
protein
0.75
Cre03.g209393 OPR2 12-Oxophytodienoic acid reductase (OPDA reductase) 0.71
Cre06.g299800 LCL1 Long-chain acyl-CoA synthetase 0.69
Cre02.g106050 Esterase/lipase/thioesterase (α/β hydrolase fold) 0.68
Cre14.g630883 FAB2 Acyl-[acyl-carrier-protein] desaturase 0.66
Cre13.g583600 DGD1 DGDG synthase 0.63
Cre11.g468050 VIPP1 Vesicle-inducing protein in plastids 1-like 0.61
Cre10.g431350 IPLA2 Esterase/lipase/thioesterase (oxylipin related) 0.6
Cre09.g388750 TEF21 Phosphoinositide phosphatase (LB associated) 0.59
Cre17.g718600 LIP3 Acylglycerol lipase 5 (lysophospholipase); (LB associated)
0.58 Down-regulated in response
to heat stress
Cre01.g026250 AGAL1 Alpha-galactosidase 0.51
Cre03.g162600 PGP3/
PGPS3
Phosphatidylglycerolphosphate synthase 0.61
Cre07.g312400 KDG1 DAG kinase (ATP dependent) 0.61
Cre03.g167924 Very-long-chain (3R)-3-hydroxyacyl-[acyl-carrier
protein] dehydratase 0.61 Cre03.g162601 PGP3 CDP-DAG-glycerol-phosphate 3-phosphatidyltransferase 0.61 Cre13.g567650 Esterase/lipase/thioesterase 0.63
Cre09.g387763 Glycerol-3-phosphate dehydrogenase (NAD(P)+) 0.65
Cre16.g678213 Palmitoyl-protein thioesterase 0.65
Cre12.g514300 CS2 Ceramide synthetase 0.71
Cre05.g248150 LPEAT1 Lysophospholipid acyltransferase 0.74
Cre12.g561550 CDS1 Mitochondrial half-size ABC transporter, membrane
protein (PG synthesis)
0.76
Cre05.g234801 TAG lipase 0.78
Cre12.g498750 LIP2/LIPG2 TAG lipase 0.8
Cre17.g702750 3-Dehydrosphinganine reductase (short-chain
dehydrogenase)-sphingolipid
0.8
Cre01.g005200 TGL2 Lipase class 3 0.81
Cre06.g272400 PAP2 superfamily 0.82
Cre09.g401145 Sphingomyelin phosphodiesterase 2 0.83
Cre17.g711150 FAD2 Omega-6 fatty acid desaturase (delta-12 desaturase) 0.84
Cre08.g379450 Beta-galactosidase 0.86
Cre12.g538450 EPT1 Ethanolaminephosphotransferase (PE synthesis) 0.89
Cre06.g299050 DGTT3 DAG acyltransferase, DGAT Type 2 0.92
Cre07.g350000 TAG lipase/lipid acylhydrolase (oxylipin pathway) 0.93
Cre12.g521650 Esterase/lipase/thioesterase 0.96
Cre12.g530550 SPHK1 Sphingosine kinase (DAG kinase) 1.17
Cre16.g669150 Acetaldehyde dehydrogenase/alcohol dehydrogenase 1.17
Cre17.g726400 CS3 Ceramide synthetase 1.21
Cre17.g707300 LPEAT2 Lysophospholipid acyltransferase 1.42
acyl-CoA oxidase (ACX2) and esterase/lipase/thioesterase (α/β hydrolase fold). Down-regulated genes of lipid metabo-lism include PtdGro synthase, DAG kinase, lysophospholipid acyltransferase (LPEAT), DAG acyltransferase type II (DGTT3/DGTT4), desaturases and several lipid hydrolyzing enzymes (Table 1). We further verified this transcriptomic response by quantitative RT-PCR on a few genes encoding enzymes of alternative pathways to TAG formation, that is, five DAG acyltransferases (DGAT1, DGTT1, DGTT2, DGTT3 and DGTT4), phospholipid:DAG acyltransferase (PDAT) and also the major lipid droplet protein (MLDP). Among all candidate genes, DGTT1 and MLDP were up-regulated; DGTT2/3/4 and PDAT were slightly down-regulated under heat stress, thus verifying our transcriptomic data (Supporting Information Fig. S7). The up-regulation of DGTT1 and MLDP is consistent with the formation of TAGs under heat stress and has previously been observed in other TAG-inducing conditions (Miller et al. 2010, Schmollinger et al. 2014, Urzica et al. 2013).
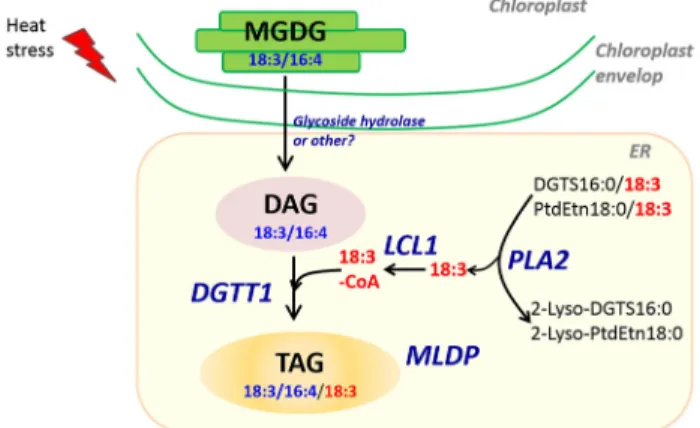
Based on these data, a likely scenario for the production of DAGs and TAGs from MGDG is proposed in Fig. 8. We are still not certain about the identity of the enzyme(s) removing the head group of MGDG to produce DAG. Nevertheless, this transcriptomic analysis showed that DGTT1 can contribute to TAG formation utilizing acyl-CoAs provided by DGTS and PtdEtn through the action of phospholipase A2 (PLA2) and acyl-CoA activating enzymes (LCL1) under heat stress. Over-all, transcriptional response of Chlamydomonas to heat stress is consistent with lipidomic changes, thus indicating that main regulations occur at the transcriptional level.
DISCUSSION
Due to global warming and increased frequencies of heat waves across our planet, understanding heat stress response is becoming of critical importance for agriculture productivity. Heat stress response has previously been intensively studied, but mostly at the protein level (Barnabás et al. 2008, Schroda et al. 2015). A study has more lately shown that heat stress response involves remodelling of lipid membranes and
accumulation of polyunsaturated storage lipids (Hemme et al. 2014). Whether pathways involved were similar to the ones involved in other stresses or specific to heat stress was un-known however. Here, we studied the early changes occurring upon heat stress in the lipidome and the transcriptome of C. reinhardtii, which is so far the best developed algal model for the study of both lipid metabolism and heat stress biology (Liu & Benning 2013, Merchant et al. 2012, Merchant et al. 2007, Nordhues et al. 2010, Schroda et al. 2015). Evidence is provided that the selective and rapid reduction in some membrane lipids induced by heat stress occurs via their direct conversion to DAGs and TAGs by a distinctive pathway we discuss in the succeeding texts.
Table 1. (Continued)
Expression
JGI v5.5
(Augustus u111.6) ID Name
abbreviations Putative function
Log2 (42 °C/25 °C)
Cre06.g265850 TCP2 Lipase, active site (Peptidase S41) 1.47
Cre14.g618050 PLP3 Plastid-lipid-associated protein PAP/fibrillin family protein
1.76
Cre17.g735600 TAG lipase 1.8
Cre08.g381100 Phospholipase A2 1.93
Cre03.g205050 DGTT4 DAG acyltransferase, DGAT Type 2 1.97
Cre17.g704150 GDP5 Glycerophosphoryl diester phosphodiesterase 1.99
Cre03.g183650 GDP4 Glycerophosphoryl diester phosphodiesterase 2.2
Cre01.g011300 SCPL50 Esterase/lipase/thioesterase (Peptidase S10, serine carboxypeptidase)
2.49
Cre09.g397216 Cytochrome P450, family 3, subfamily A 2.95
Total RNA was extracted from three biological replicates. Time points were taken for 0 (T1), 5 (T3), 10 (T5) and 25 (T7) min after heat stress, and only an example (T7) is shown here. Similar responses were observed for each time point in a kinetic order. padj(LTR)***< < 0.05 is shown.
Figure 8. A possible route for the conversion of MGDG to TAGs in heat-stressed cells. This model is based on integration of transcriptomic responses to lipidomic changes. It should be noted that we could not exclude the contribution of proteins/enzymes that are under post-transcriptional regulation. The subcellular location of this pathway is drawn partly based on the observation under Transmission Electron Microscope (TEM) that lipid droplet formed under heat stress is mostly present in the cytosol but in close proximity to plastid (Hemme et al., 2014), and also based on the known location of galactolipids and phospholipids in the cells of Chlamydomonas. LCL1, long-chain acyl-CoA synthetase; DGTT1, DAG acyltransferase type II, isoform 1; MLDP, major lipid droplet protein; PLA2, phospholipase A2; PLP, plastid-lipid-associated protein PAP.
Heat stress and remodelling of membrane lipids
We observed in this study that selective degradation of mem-brane lipids (mostly MGDG) occurs as part of memmem-brane re-sponse to heat stress. As a result, the ratio of DGDG to MGDG increased from 0.17 to 0.26 (calculated from Fig. 3A), conforming with membrane adaptations in higher plants where the ratio of non-bi-layer forming lipids (i.e. DGDG) to bi-layer forming lipids (i.e. MGDG) increases in response to tempera-ture rises (Zheng et al. 2011). Thus, head group exchanges of glycerolipids are also one of the mechanisms that C. reinhardtii employ to adapt to temperature rises.
Moreover, this selective degradation of polyunsaturated membrane lipid species resulted in an increase in the composi-tion of saturated fatty acids of the membrane lipids under heat stress (although without overall changes in fatty acid composi-tions at a whole cell level). Inversely, in response to cold stress, the ratio of polyunsaturated fatty acids to saturated fatty acids in the membranes increases (Valledor et al. 2013). The compo-sitional adaptation of membrane lipids serves to maintain the correct membrane fluidity at the new environmental condi-tions, a phenomenon known as the‘homeoviscous adaptation’, and we show in this study that it is also a conserved mechanism in the primitive alga C. reinhardtii.
Besides physical–chemical adaptions of membranes via modulating its membrane lipid composition, selective removal of polyunsaturated fatty acyl groups might confer also survival advantage in an increasingly oxidative cellular environment as the generation of reactive oxygen species (ROS) is known to increase under heat stress (Sgobba et al. 2015). Polyunsatu-rated fatty acids contained in the membrane lipids are easily accessible and preferred substrates for lipid oxidative enzymes (Andreou & Feussner 2009). Containing these fatty acids from membrane lipids to protein-bound lipid droplet in the form of TAGs (as discussed in the succeeding texts) could be a strategy C. reinhardtii developed to avoid lipid peroxidation. This strategy is quicker and cost less energy to convert them to TAGs (Fig. 8) than to degrade lipids andβ-oxidize fatty acids (Graham 2008), and the intact polyunsaturated fatty acyl-chains now contained in the lipid droplet is also readily accessible to make the membrane lipids when such a need arises, for example, during the recovery phase (Hemme et al. 2014).
Heat stress and accumulation of TAGs
TAG accumulation is either developmentally programmed like in the case of oilseeds or occurs in response to a stress condition (Goold et al. 2014). The latter usually serves as a buffer zone for sequestration of otherwise toxic FFAs. TAG accumulation in Chlamydomonas has been intensively investigated, and latest progress has been summarized in three recent reviews (Li-Beisson et al. 2015, Liu & Benning 2013, Merchant et al. 2012). Nutrient starvation (N, sulfur, phosphate, zinc and iron), high salinity and some chemicals are known to trigger TAG ac-cumulation in C. reinhardtii (Kato et al. 2013, Kim et al. 2015, Kim et al. 2013b, Siaut et al. 2011, Urzica et al. 2013, Wang et al. 2009). Among different‘triggers’, N starvation-induced TAG accumulation is the most efficient and has been the focus
of many studies in C. reinhardtii (Boyle et al. 2012, Fan et al. 2012, Fan et al. 2011, Goodson et al. 2011, Siaut et al. 2011, Urzica et al. 2013, Wang et al. 2009, Work et al. 2010). By contrast, little is known about TAG accumulation under other environmental stresses such as heat stress. A recent study has shown that accumulation of TAG species occurs in Chlamydomonas in response to heat stress from 1 h to several days at 42 °C (Hemme et al. 2014). Our data show that synthesis of TAGs is a very early response to a heat stress (42 °C) be-cause after 30 min of heat stress, TAG amounts have already increased more than twofold (Fig. 3B).
Although heat stress involves TAG synthesis, important dif-ferences were observed with the TAGs accumulated under N starvation. The first difference is that TAGs formed under heat stress are mainly made of polyunsaturated fatty acids, for ex-ample, the two major TAG species are TAG52:10(18:3/16:4/ 18:3) and TAG52:11(18:4/16:4/18:3), whereas under N starva-tion, saturated and monounsaturated fatty acids are the major acyl chains present in TAG fraction (Fan et al. 2011, Liu et al. 2013, Nguyen et al. 2013, Siaut et al. 2011) (TAG52:3, TAG52:4, TAG52:5 and TAG52:6; Supporting Information Fig. 8). However, because our work was performed under pho-toautotrophic conditions (no acetate supply, CO2is the only
carbon source) and most previous reports of TAG accumula-tion under N starvaaccumula-tion were carried out under mixotrophic condition (i.e. in acetate-containing medium) (Liu et al. 2013), it could be argued that the difference in TAG species distribu-tion between heat stress and N starvadistribu-tion could be due to the presence or absence of acetate. However, this is not likely because we found that under N starvation, TAGs present in photoautotrophically grown cells are similar to those grown mixotrophically (Supporting Information Fig. 8). The major reason for the difference in fatty acid composition of TAGs in N-starved cells and heat–stressed cells is that the former are mostly formed using de novo synthesized fatty acids (Fan et al. 2011), while the latter are synthesized by conversion of polyunsaturated membrane lipids and recycling of their poly-unsaturated acyl chains as discussed previously.
Pathways of conversion of membrane lipids to
storage lipids
Reduction in MGDG18:3/16:4, simultaneous to occurrence of DAG18:3/16:4 and TAG18:3/16:4/18:3 with the same stereo-chemistry, strongly suggests a conversion of MGDG to DAG and TAG. Acyl-chains (18:3) used to esterify the sn3 position of DAG is most likely supplied by DGTS16:0/18:3 or PtdEtn18:0/18:3 because reduction in both lipids was observed together with the over-accumulation of 2-lyso-DGTS16:0 and 2-lyso-PtdEtn18:0 (Figs 3 & 4). A pathway consisting of MGDG to DAG conversion and acyl recycling for the sn3 po-sition of TAGs is consistent with the observation that there are no changes in total fatty acid composition and fatty acid content between control and heat-stressed cells (Fig. 2). The acyl traf-ficking from DGTS and/or PtdEtn to TAGs may involve a phospholipase A2 (PLA2), an acyl activating enzyme (LCL1) and a diacylglycerolacyltransferase (DGTT1) (Fig. 8). It should
be noted that this proposed pathway is based on lipidomic and transcriptomic datasets and we cannot at this stage rule out the contributions of those enzymes that are regulated at the post-transcriptional level.
Accumulation of TAGs following stress-induced lipid re-modelling has been reported in several plant species (Benning & Ohta 2005, Heemskerk et al. 1988, Moellering & Benning 2011), and a pathway has been characterized in freezing toler-ance in A. thaliana (Moellering et al. 2010). Although freezing tolerance in Arabidopsis also involves the conversion of MGDG to DAGs and TAGs, it is likely that the initial step of MGDG to DAG conversion is catalysed by a different type of enzyme. Indeed, during freezing tolerance, conversion of MGDG to DAG is achieved by a galactolipid:galactolipid galactosyltransferase/sensitive-to-freezing 2 (GGGT/SFR2) that transfers MGDG galactosyl groups to other galactolipid species to form oligogalactolipids (particularly TGDG) (Moellering et al. 2010). Despite repeated efforts, we could not detect the formation of TGDGs or other oligogalactolipids in Chlamydomonas under heat stress. This is consistent with a previous study showing that isolated plastidial envelops of Chlamydomonas do not synthesize oligogalactolipids including TGDG (Mendiola-Morgenthaler et al. 1985). Furthermore, phylogenetic analysis has indicated that SFR2 orthologs are re-stricted to land plant species and not found in bacteria or sim-ple algae (Fourrier et al. 2008). In addition, the expression of the Chlamydomonas gene encoding the protein with the highest sequence similarity to SFR2 was not found to be up-regulated upon heat stress in our transcriptomic study. Taken together, these data strongly support the view that in Chlamydomonas response to heat stress, MGDG to DAG con-version is catalysed by a type of enzyme different from the Arabidopsis galactosyltransferase SFR2 acting in freezing tol-erance. Identification of the Chlamydomonas enzyme converting MGDG to DAG would provide a definitive proof to the pathway proposed here as well as an interesting tool for the genetic engineering of lipid metabolism in photosyn-thetic organisms.
ACKNOWLEDGMENTS
We thank thefinancial support by the Direction Générale de l’Aviation (DGA-CAER). This work has been carried out thanks to the support of the A*MIDEX project (no. ANR-11-IDEX-0001-02) funded by the « Investissements d’Avenir » French Government programme, managed by the French National Research Agency (ANR). Support was also provided by the HélioBiotec platform (funded by the European Re-gional Development Fund, the Région Provence Alpes Côte d’Azur, the French Ministry of Research and the Commissariat à l’Energie Atomique et aux Energies Alternatives). The IGS laboratory is partially supported by CNRS and Aix-Marseille University. We acknowledge the use of the PACA-Bioinfo Platform, supported by IBISA and France-Génomique (ANR-10-INBS-0009). We also thank Stéphan Cuiné and Emmanuelle Billon (CEA Cadarache) for technical help.
There are no conflicts of interest.
REFERENCES
Andreou A. & Feussner I. (2009) Lipoxygenases– structure and reaction mech-anism. Phytochemistry 70, 1504–1510.
Barnabás B., Jäger K. & Fehér A. (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell & Environment 31, 11–38. Benning C. & Ohta H. (2005) Three enzyme systems for galactoglycerolipid
bio-synthesis are coordinately regulated in plants. Journal of Biological Chemistry 280, 2397–2400.
Boyle N.R., Page M.D., Liu B., Blaby I.K., Casero D., Kropat J.,… Merchant S.S. (2012) Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. Journal of Biological Chemistry 287, 15811–15825. Chen J.P., Burke J.J., Xin Z.G., Xu C.C. & Velten J. (2006) Characterization of
the Arabidopsis thermosensitive mutant atts02 reveals an important role for ga-lactolipids in thermotolerance. Plant, Cell & Environment 29, 1437–1448. Fan J., Yan C., Andre C., Shanklin J., Schwender J. & Xu C. (2012) Oil
accumu-lation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii. Plant and Cell Physiology 53, 1380–1390. Fan J.L., Andre C. & Xu C.C. (2011) A chloroplast pathway for the de novo
biosynthesis of triacylglycerol in Chlamydomonas reinhardtii. Febs Letters 585, 1985–1991.
Fourrier N., Bédard J., Lopez-Juez E., Barbrook A., Bowyer J., Jarvis P.,… Thorlby G. (2008) A role for sensitive to freezing2 in protecting chloroplasts against freeze-induced damage in Arabidopsis. The Plant Journal 55, 734–745. Giroud C., Gerber A. & Eichenberger W. (1988) Lipids of Chlamydomonas reinhardtii– analysis of molecular-species and intracellular site(s) of biosynthe-sis. Plant and Cell Physiology 29, 587–595.
Goodson C., Roth R., Wang Z.T. & Goodenough U. (2011) Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost. Eukaryotic Cell 10, 1592–1606.
Goold H., Beisson F., Peltier G. & Li-Beisson Y. (2014) Microalgal lipid droplets: composition, diversity, biogenesis and functions. Plant Cell Reports 1–11. Graham I.A. (2008) Seed storage oil mobilization. Annual Review of Plant
Biology 59, 115–142.
Harris E. (2001) Chlamydomonas as a model organism. Annual Reviews in Plant Physiology and Plant Molecular Biology 52, 363–406.
Heemskerk J.W.M., Bögemann G., Helsper J.P.F.G. & Wintermans J.F.G.M. (1988) Synthesis of mono- and digalactosyldiacylglycerol in isolated spinach chloroplasts. Plant Physiology 86, 971–977.
Hemme D., Veyel D., Mühlhaus T., Sommer F., Jüppner J., Unger A.-K.,… Schroda M. (2014) Systems-wide analysis of acclimation responses to long-term heat stress and recovery in the photosynthetic model organism Chlamydomonas reinhardtii. The Plant Cell 26, 4270–4297.
Horváth I., Glatz A., Nakamoto H., Mishkind M.L., Munnik T., Saidi Y.,… Vigh L. (2012) Heat shock response in photosynthetic organisms: membrane and lipid connections. Progress in Lipid Research 51, 208–220.
Iba K. (2002) Acclimative response to temperature stress in higher plants: ap-proaches of gene engineering for temperature tolerance. Annual Review of Plant Biology 53, 225–245.
Kato N., Dong T., Bailey M., Lum T. & Ingram D. (2013) Triacylglycerol mobili-zation is suppressed by brefeldin A in Chlamydomonas reinhardtii. Plant and Cell Physiology 54, 1585–1599.
Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R. & Salzberg S. (2013a) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology 14, R36.
Kim H., Jang S., Kim S., Yamaoka Y., Hong D., Song W.,… Lee Y. (2015) The small molecule fenpropimorph rapidly converts chloroplast membrane lipids to triacylglycerols in Chlamydomonas reinhardtii. Frontiers in Microbiol-ogy 6, 13.
Kim S., Kim H., Ko D., Yamaoka Y., Otsuru M., Kawai-Yamada M.,… Lee Y. (2013b) Rapid induction of lipid droplets in Chlamydomonas reinhardtii and Chlorella vulgaris by Brefeldin A. PLoS ONE 8, e81978.
Langmead B. & Salzberg S.L. (2012) Fast gapped-read alignment with Bowtie 2. Nature Methods 9, 357–359.
Li-Beisson Y., Beisson F. & Riekhof W. (2015) Metabolism of acyl-lipids in Chlamydomonas reinhardtii. Plant Journal 82, 504–522.
Li-Beisson Y. Shorrosh B., Beisson F., Andersson M., Arondel V., Bates P.,… Ohlrogge J. (2010) Acyl lipid metabolism In The Arabidopsis Book (ed R. Last). American Society of Plant Biologists, Rockville, MD.
Li Y.H., Beisson F., Pollard M. & Ohlrogge J. (2006) Oil content of Arabidopsis seeds: the influence of seed anatomy, light and plant-to-plant variation. Phyto-chemistry 67, 904–915.
Liu B. & Benning C. (2013) Lipid metabolism in microalgae distinguishes itself. Current Opinion in Biotechnology 24, 300–309.
Liu B., Vieler A., Li C., Daniel J.A. & Benning C. (2013) Triacylglycerol profiling of microalgae Chlamydomonas reinhardtii and Nannochloropsis oceanica. Bioresource Technology 146, 310–316.
Liu G.-T., Wang J.-F., Cramer G., Dai Z.-W., Duan W., Xu H.-G.,… Li S.-H. (2012) Transcriptomic analysis of grape (Vitis vinifera L.) leaves during and af-ter recovery from heat stress. BMC Plant Biology 12, 174.
Livak K.J. & Schmittgen T.D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 ΔΔCT method. Methods 25, 402–408. Lobell D.B., Schlenker W. & Costa-Roberts J. (2011) Climate trends and global
crop production since 1980. Science 333, 616–620.
Los D.A. & Murata N. (2004) Membranefluidity and its roles in the perception of environmental signals. Biochimica et Biophysica Acta (BBA) - Biomembranes 1666, 142–157.
Love M., Huber W. & Anders S. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15, 550. Mendiola-Morgenthaler L., Eichenberger W. & Boschetti A. (1985) Isolation of
chloroplast envelopes from Chlamydomonas. Lipid and polypeptide composi-tion. Plant Science 41, 97–104.
Merchant S.S., Kropat J., Liu B., Shaw J. & Warakanont J. (2012) TAG, you’re it! Chlamydomonas as a reference organism for understanding algal triacylglyc-erol accumulation. Current Opinion in Biotechnology 23, 352–363.
Merchant S.S., Prochnik S.E., Vallon O., Harris E.H., Karpowicz S.J., Witman G. B.,… Brokstein P. (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245–250.
Miller R., Wu G.X., Deshpande R.R., Vieler A., Gartner K., Li X.B.,… Benning C. (2010) Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiology 154, 1737–1752.
Mittler R., Finka A. & Goloubinoff P. (2012) How do plants feel the heat? Trends in Biochemical Sciences 37, 118–125.
Moellering E.R. & Benning C. (2011) Galactoglycerolipid metabolism under stress: a time for remodeling. Trends in Plant Science 16, 98–107.
Moellering E.R., Muthan B. & Benning C. (2010) Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330, 226–228.
Mühlhaus T., Weiss J., Hemme D., Sommer F. & Schroda M. (2011) Quantitative shotgun proteomics using a uniform 15 N-labeled standard to monitor proteome dynamics in time course experiments reveals new insights into the heat stress re-sponse of Chlamydomonas reinhardtii. Molecular & Cellular Proteomics 10. Murata N. & Los D.A. (1997) Membranefluidity and temperature perception.
Plant Physiology 115, 875–879.
Neilson K.A., Gammulla C.G., Mirzaei M., Imin N. & Haynes P.A. (2010) Proteomic analysis of temperature stress in plants. Proteomics 10, 828–845. Nguyen H.M., Cuiné S., Beyly-Adriano A., Légeret B., Billon E., Auroy P.,…
Li-Beisson Y. (2013) The green microalga Chlamydomonas reinhardtii has a single ω-3 fatty acid desaturase that localizes to the chloroplast and impacts both plastidic and extraplastidic membrane lipids. Plant Physiology 163, 914–928. Nordhues A., Miller S.M., Mühlhaus T. & Schroda M. (2010) Chapter two–
new insights into the roles of molecular chaperones in Chlamydomonas and Volvox. In International Review of Cell and Molecular Biology (ed Kwang W.J.), pp. 75–113. Academic Press.
Pflaster E.L., Schwabe M.J., Becker J., Wilkinson M.S., Parmer A., Clemente T. E., … Riekhof W.R. (2014) A high-throughput fatty acid profiling screen reveals novel variations in fatty acid biosynthesis in Chlamydomonas reinhardtii and related algae. Eukaryotic Cell 13, 1431–1438.
Ras M., Steyer J.P. & Bernard O. (2013) Temperature effect on microalgae: a crucial factor for outdoor production. Reviews in Environmental Science and Bio-Technology 12, 153–164.
Schmollinger S., Muhlhaus T., Boyle N.R., Blaby I.K., Casero D., Mettler T.,… Merchant S.S. (2014) Nitrogen-sparing mechanisms in Chlamydomonas affect the transcriptome, the proteome, and photosynthetic metabolism. Plant Cell 26, 1410–1435.
Schroda M., Hemme D. & Muhlhaus T. (2015) The Chlamydomonas heat stress response. Plant Journal 82, 466–480.
Sgobba A., Paradiso A., Dipierro S., De Gara L. & de Pinto M.C. (2015) Changes in antioxidants are critical in determining cell responses to short- and long-term heat stress. Physiologia Plantarum 153, 68–78.
Siaut M., Cuine S., Cagnon C., Fessler B., Nguyen M., Carrier P.,… Peltier G. (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relation-ship with starch reserves. BMC Biotechnology 11, 7.
Stefan S., Frank E., Ehsan E.R., Henning K. & Rikard G. (2014) Impact of heat stress on crop yield– on the importance of considering canopy temperature. Environmental Research Letters 9, 044012.
Suzuki I., Simon W.J. & Slabas A.R. (2006) The heat shock response of Synechocystis sp PCC 6803 analysed by transcriptomics and proteomics. Journal of Experimental Botany 57, 1573–1578.
Urzica E.I., Vieler A., Hong-Hermesdorf A., Page M.D., Casero D., Gallaher S. D.,… Merchant S.S. (2013) Remodeling of membrane lipids in iron-starved Chlamydomonas. Journal of Biological Chemistry 288, 30246–30258. Valledor L., Furuhashi T., Hanak A.-M. & Weckwerth W. (2013) Systemic cold
stress adaptation of Chlamydomonas reinhardtii. Molecular & Cellular Proteomics 12, 2032–2047.
Wada H. & Murata N. (2007) The essential role of phosphatidylglycerol in photosynthesis. Photosynthesis Research 92, 205–215.
Wang Z.T., Ullrich N., Joo S., Waffenschmidt S. & Goodenough U. (2009) Algal lipid bodies: stress induction, purification, and biochemical characteriza-tion in wild-type and starchless Chlamydomonas reinhardtii. Eukaryotic Cell 8, 1856–1868.
Work V.H., Radakovits R., Jinkerson R.E., Meuser J.E., Elliott L.G., Vinyard D. J., … Posewitz M.C. (2010) Increased lipid accumulation in the Chlamydomonas reinhardtii sta7-10 starchless isoamylase mutant and increased carbohydrate synthesis in complemented strains. Eukaryotic Cell 9, 1251–1261. Yao S., Brandt A., Egsgaard H. & Gjermansen C. (2012) Neutral lipid accumula-tion at elevated temperature in condiaccumula-tional mutants of two microalgae species. Plant Physiology and Biochemistry 61, 71–79.
Zheng G., Tian B.O., Zhang F., Tao F. & Li W. (2011) Plant adaptation to frequent alterations between high and low temperatures: remodelling of membrane lipids and maintenance of unsaturation levels. Plant, Cell & Environment 34, 1431–1442.
Received 31 August 2015; received in revised form 1 October 2015; accepted for publication 6 October 2015
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
Figure S1. Experimental setup.
Figure S2. Major alterations in membrane lipids were com-pared using UPLC-MS/MS on total lipid extracts before and af-ter 1 hour heat stress.
Figure S3. Identification of 2-lyso-DGTS in control and heat-stressed cells.
Figure S4. Over-accumulation of 2-lyso-PtdEtn in control and heat-stressed cells.
Figure S5. TAG accumulation in heat stressed cells of Chlamydomonas as revealed by TLC.
Figure S6. Lipidomic analysis of the crfad7 mutant under heat stress.
Figure S7. qRT-PCR analyses of transcriptional response of se-lected genes to heat stress.
Figure S8. Comparison of TAG molecular species distribution in photoautrophically (MM)- or mixotrophically (TAP)- grown cells when subjected to nitrogen starvation.
Table S1. List of primers used in this study for quantitative RT-PCR.
Table S2. Transcriptional changes of genes involved in lipid metabolism during heat stress.