Modeling of the Degradation of Poly(ethylene
glycol)-co-(lactic acid)-dimethacrylate Hydrogels Accounting for
Diffusion Limitations
Vincent E.G. Diedericha, Thomas Villigera, Marco Lattuadab*, Giuseppe Stortia*
a
ETH Zurich, Department of Chemistry and Applied Biosciences, Institute for Chemical and Bioengineering, Vladimir-Prelog-Weg 1, CH-8093 Zurich, Switzerland
b
Adolphe Merkle Institute, University Fribourg, Route de l’ancienne papeterie CP 209, CH-1723 Marly, Switzerland
* Corresponding authors.
Email: giuseppe.storti@chem.ethz.ch Email: marco.lattuada@unifr.ch
Time dependence of the sol fraction
We will rewrite Equations (17) of the main text:( )
(
)
(
)
` 1 1 1 1 1 2 1 sol s f f f g w s g s s f s s ξ ξ ξ α ξ α ξ α = − − ∂ = ∂ = ⋅ − ⋅ ⋅ − − − ⋅ ⋅ = − (1)The third of Equations (1) at s=1 can have either one or two roots. The first one is the trivial solution ξ = 1, which leads to a sol fraction of 1 (no gel). On the other hand, for specific values of the exponent f, there might be a smaller root, which leads to the correct fraction of gel, as long as the conversion of functional groups α is large enough. Clearly, this only happens if the exponent f is also sufficiently larger than 2. This root must be found numerically. The expression of the sol fraction now becomes: ( ) ( ) ( ( )) ( ) ( ( )) ( ) ( ) ( ) ( ) ( ( )) ( ) 1 1 2 1 1 2 1 1 1 2 2 ` 1 1 , 1 1 1 1 1 1 2 2 2 1 1 1 1 1 1 1 1 2 2 2 1 1 A A A A A A A A A A A A sol A s A g s s g g A s A A A s A s s s s s s A g w A A A A s A ξ ξ ξ ξ ξ α ξ ξ ξ α ξ ξ ξ ξ α ξ α ξ α ξ ξ ξ ξ α ξ ξ ξ α α ξ ξ α ξ − − − − − − − − − − − = − ∂ =∂ +∂ ⋅∂ = − ⋅ − − + ⋅ ⋅ − ⋅ − ⋅ ⋅ − − − ⋅ ∂ ∂ ∂ ∂ ∂ ∂ ∂ ⋅ = ∂ − ⋅ ⋅ − ∂ ⋅ = = − ⋅ − − + ⋅ − ⋅ − ⋅ − − ∂ − ⋅ − − ⋅ − −(1 α)=0 (2)
In Equations (2) the inverse function theorem has been used to compute the partial derivative of ξ, which is a function of s, from the last equation (1).
The solution of the equations is carried out as follows. Equation (15) in the main body of the manuscript is used both in its algebraic and in its differentiated forms to eliminate the volume and the time derivative of the volume. This leads to the
(
)
(
)
(
)
1 2 1 2 2 1 1 2 1 2 BG BG BG C BG C C C C F BG C F B F F F C BG C BG G C F BG C F V V V V d d x m k dt dt x d d m k dt V dt d d x x m k dt V dt x xφ
φ
φ
φ
φ
φ
φ
φ
φ
φ
φ
φ
φ
φ
φ
φ
φ
φ
φ
φ
+ = ⋅ ⋅ ⋅ − ⋅ − + = − ⋅ ⋅ − − − ⋅ − ⋅ + = ⋅ ⋅ ⋅ − − − + ⋅ − (3) where: ( )(
) (
)
(
)
( )(
)(
)
1 2 3 3 , , , 0 , , 1 2 2 3 3 3 , 0 1 , , , , , , , , 0 , 3 1 1 2 2 1 3 1 1 2 2 BG geBG gel C gel F gel
C b C gel C gel C gel b C l C gel F gel BG g gel b C gel C ge el C gel F gel l d d d x V V d dt a dt dt dt d V V V a b V a V φ φ φ χ φ φ φ φ φ φ φ φ φ φ φ φ − + + − − = − − + + + + − + ⋅ − + ⋅ ⋅ − + ⋅
(
)
(
(
) (
) (
)
)
3 2 2 0 , , , , , 1 log 1 1 2 CS gel S gel S gel
b C gel C gel dt x V V a φ φ χ φ φ φ = − + − + − + ⋅ (4)
The unfortunate characteristics of these equations is that the gel volume fractions depend on the sol fraction, which is a function of time. Therefore, the time derivative of the sol fraction needs to be explicitly evaluated. This is rather cumbersome, because the time dependence is included in the parameter A, which is linearly dependent on r. First, we have:
(
)
(
)
(
)
(
)
(
)
(
)
, , , , , , 1 1 1 1 1 1 C gel C solC gel C sol sol C
F gel F sol
F gel F sol sol F
BG gel BG sol
BG gel BG sol sol BG
w w w t t t w w w t t t w w w t t t φ φ φ φ φ φ φ φ φ φ φ φ φ φ φ ∂ ∂ ∂ = ⋅ − ⇒ = ⋅ − − ⋅ ∂ ∂ ∂ ∂ ∂ ∂ = ⋅ − ⇒ = ⋅ − − ⋅ ∂ ∂ ∂ ∂ ∂ ∂ = ⋅ − ⇒ = ⋅ − − ⋅ ∂ ∂ ∂ (5)
( )
(
( ))
( )(
( ))
( ) ( )(
( ))
( ) ( )(
( ))
( ) ( )(
( ))
1 1 1 1 1 2 2 2 1 2 1 1 log 1 log 1 2 2 1 1 1 1 log 2 1 1 1 log 1 2 2 2 2 1 1 l 1 1 1 1 2 2sol sol sol
A A A sol A A A A A A A A w w w A t A A t w A A A A A A A A A A ξ ξ ξ ξ ξ ξ α ξ ξ ξ α α ξ ξ ξ ξ ξ ξ α ξ ξ ξ α α ξ α ξ ξ ξ α − − − − − − − − − − ∂ ∂ ∂ ∂ ∂ = + ⋅ ∂ ∂ ∂ ∂ ∂ ∂ = − − − − ⋅ − − + ∂ ⋅ + ⋅ − − ⋅ − − − ⋅ − ⋅ − − + − ⋅ − ⋅ ⋅ − ⋅ − ⋅ − − ( ) ( ) ( ) ( )
(
)
( )(
)
( )(
( ))
( ) ( ) ( )(
( ))
( ) ( ) ( ) 1 2 2 1 2 2 2 1 2 1 1 2 3 2 2 1 2 1 log og 1 1 1 1 1 1 1 1 2 2 1 1 1 1 1 2 1 1 2 2 2 1 1 1 1 1 1 2 2 A A A A A A A A A sol A A A A A A A A A A w A A A A A A A A A A A A A A A α ξ α ξ α ξ ξ ξ ξ α ξ α ξ ξ ξ ξ α ξ ξ α ξ ξ ξ ξ α ξ α ξ ξ ξ ξ − − − − − − − − − − − − − − − − ⋅ ⋅ + ⋅ − + − ⋅ − − ⋅ − ∂ = ⋅ − ⋅ − − − − ⋅ + ∂ ⋅ ⋅ − − ⋅ − ⋅ − ⋅ − − − ⋅ − ⋅ + − ⋅ − ⋅ − ⋅ − ⋅(
−( ))
( ) ( )(
(( ) ())
) ( ) ( ) ( ) ( ) 2 2 1 3 2 2 2 1 2 2 1 1 2 1 1 1 1 log 1 1 2 2 A A A A A A A C C F C BG C C F BG C C C C C F BG C F BG F BG F BG A A A A A A A x x t x t x t A r t f f x x t t x x x x x x α ξ α ξ ξ α α ξ α ξ α ξ ξ ξ α ξ φ φ φ φ φ φ φ φ φ φ φ − − − − − − − ⋅ − ⋅ ⋅ − ⋅ − − + − ⋅ − − ⋅ − ⋅ ∂ = ∂ − ⋅ − ∂ ∂ ∂ ∂ + + ∂ ∂ ∂ ∂ = − ∂ = − ∂ − ∂ ∂ + + + + ( ) 1 1 0 A ξ α ξ − α − ⋅ − − = (6)The volume change equation can be written as:
( )
(
) (
)
(
)
( ) ( )(
) (
)
, , , , , , , , , , , , 1 2 3 3 0 , , 1 2 3 3 0 , , 3 1 1 2 1 2 1 3 1 1 2 2 1BG gel C gel F gel BG gel C gel F gel
BG gel C gel F BG gel C gel C BG C F F sol b C gel C gel C b C gel Cgel g le x V V d w dt t t t x V a V a V φ φ φ φ φ φ φ φ χ φ φ χ φ φ φ φ φ φ φ φ φ ∂ ∂ ∂ = − − + + ⋅ + + ⋅ − − − + + ∂ ∂ + ∂ − + ⋅ + − + + − + ⋅ − −
(
+)
( ) ( ) ( )(
)(
)
2 1 2 2 3 3 0 1 , 0 , , 2 3 1 2 sol sol BG C F C C F C BG C C F BG C C C C C F BG C F BG F BG F BG b C gel b C ge gel C gel l w w A A x x t x t x t t f x x x x x x x x V V V a b V a ξ φ φ φ ξ φ φ φ φ φ φ φ φ φ φ φ φ φ φ − ∂ ∂ ∂ + + ⋅ + ⋅ ∂ ∂ ∂ ∂ ∂ ∂ ∂ + + ∂ ∂ ∂ ∂ ⋅ − − + + + + − + ⋅ ⋅ + ⋅ ( ) ( )(
)(
)
( ) 3 1 2 2 3 3 3 0 1 , 0 , , 1 1 2 3 1 1 2 2 2 C sol b sol sol C gel C b C gel C gel C C F C BG C C F BG C C C C C F BG C F F BG F BG w t V V V w w a b V A A a x x t x t x t t f x x x x x x x x φ ξ φ φ ξ φ φ φ φ φ φ φ φ φ φ φ φ φ − ∂ − ⋅ − ∂ ∂ ∂ ∂ + + ⋅ ⋅ − ⋅ ⋅ + ⋅ ∂ ∂ ∂ + ⋅ ∂ ∂ ∂ ∂ + + ∂ ∂ ∂ ∂ ⋅ − − + + + + 2 BG (7)Supplementary Figures
3500 4000 4500 5000 0,0 0,2 0,4 0,6 0,8 1,0 70 80 90 100 110 120 0 1 2 3 4 5 6 c b S ig n a l in te n s it y ( a .u .) Mass (m/z) Measured 0 MA group 1 MA group 2 MA groups a R e la ti v e A m o u n t (% )Number of EG repeating units (-)
0 MA group 1 MA group 2 MA groups 0 1 2 0 50 100 R e la ti v e A m o u n t (% ) MA groups (-) Average: 1.95 MA groups per chain
Figure S1. a) MALDI-TOF signal of the MA-PEG-MA macromonomer obtained from the dimethacrylation reaction. In black is the measured spectrum, while the colored spectra correspond to the PEG, PEG-MA and MA-PEG-MA macromonomers; b) Relative amounts of the individual species found in the dimethacrylation product. These results were obtained by fitting the MALDI-TOF spectrum; c) Distribution of the number of MA units incorporated into each PEG chain. The average of this distribution is 1.95 MA units per PEG chain.
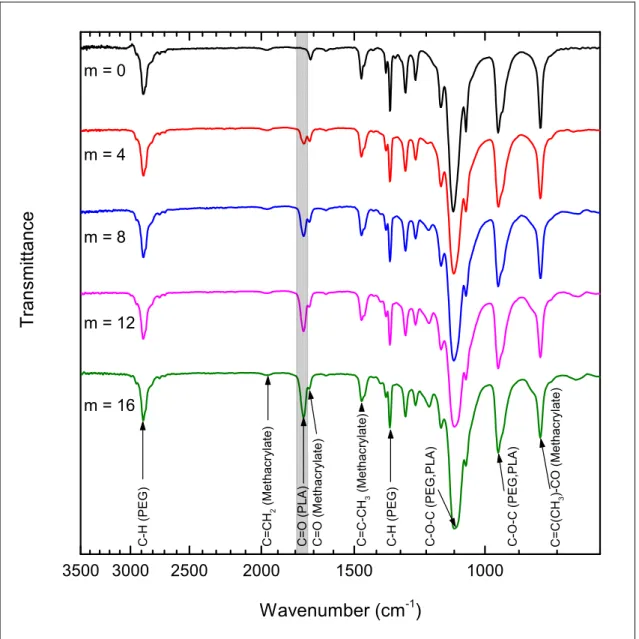
3500 3000 2500 2000 1500 1000 C -O -C ( P E G ,P L A ) C = O ( M e th a c ry la te ) C = C (C H3 )-C O ( M e th a c ry la te ) C -O -C ( P E G ,P L A ) C -H ( P E G ) C = C -C H3 ( M e th a c ry la te ) C = O ( P L A ) C = C H2 ( M e th a c ry la te ) m = 16 m = 12 m = 8 m = 4
T
ra
n
s
m
it
ta
n
c
e
Wavenumber (cm
-1)
m = 0 C -H ( P E G )Figure S2. Infrared spectra of MA-PLA-b-PEG-b-PLA-MA macromonomers with different contents of LA units per PEG chains (increasing from top to bottom, as reported in the figure)
0 4 8 12 16 20 24 0 2 4 6 8 10 12 14 b A tt a c h e d L A U n it s p e r P E G C h a in
Charged LA Units per PEG Chain
a 0 4 8 12 14.7 0,0 0,5 1,0 1,5 2,0 M A U n it s p e r P E G C h a in
Attached LA Units per PEG Chain
Figure S3. a) Quantification by 1H NMR spectroscopy (full symbols) and by MALDI-TOF (open
symbol, preliminary experiment) of the number of LA units per PEG chain. It can be seen that except for the macromonomer with the highest LA content, the number of LA units attached to each PEG chain increases linearly with the charged amount of LA units per PEG chain. The slope of this line
corresponds to the LA conversion, which is around 72%. b) Quantification by 1H NMR spectroscopy of
the number of MA groups per PEG chain as a function of the copolymer composition. It can be seen that the target of 2 MA units per PEG chain is reached for all compositions.
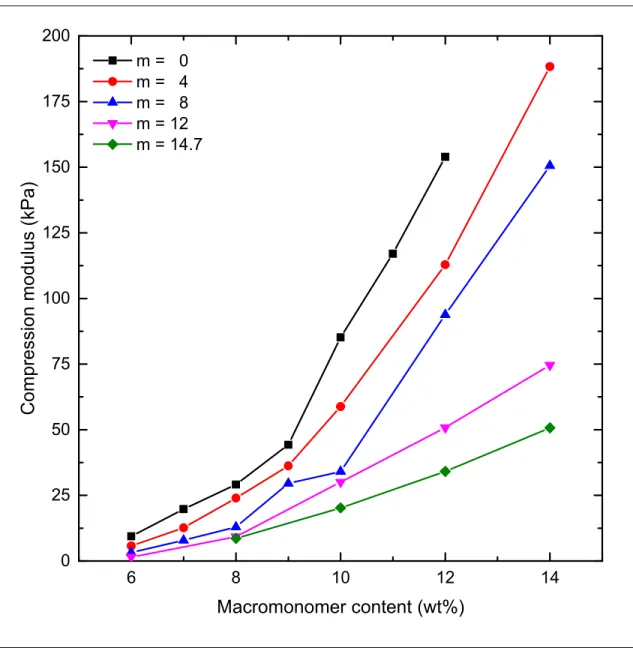
6 8 10 12 14 0 25 50 75 100 125 150 175 200
C
o
m
p
re
s
s
io
n
m
o
d
u
lu
s
(
k
P
a
)
Macromonomer content (wt%)
m = 0 m = 4 m = 8 m = 12 m = 14.7Figure S4. Compression modulus of MA-PLA-b-PEG-b-PLA-MA hydrogels having different
Figure S5. Empirical dependence of the kinetic constant of macromonomer degradation as a function of the macromonomer content.
6 8 10 12 14 1.0x10-7 1.5x10-7 2.0x10-7 2.5x10-7 3.0x10-7 K in e ti c c o n s ta n t (s -1 ) Macromonomer content (wt%) k = - 4.672x10-10 w3m+ 1.728x10-8 w2m- 2.17x10-7 wm + 1.032x10-6
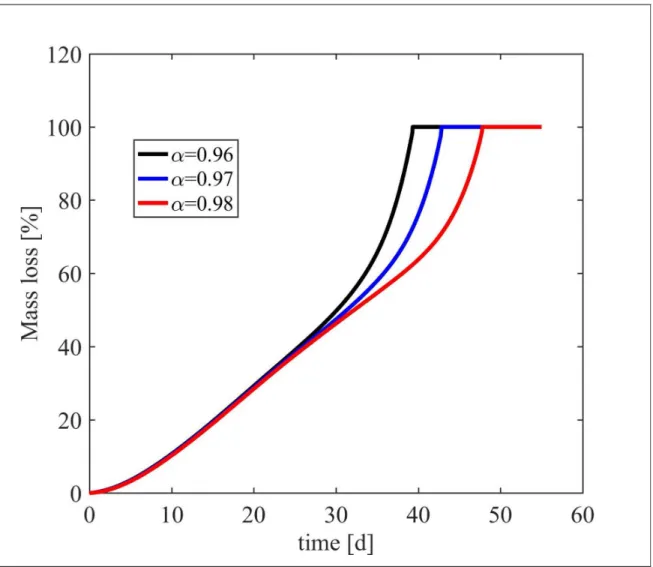
Figure S8. Sensitivity of the mass loss on the value of the initial fraction of reacted crosslinks, α, for m=8 and w=10%.