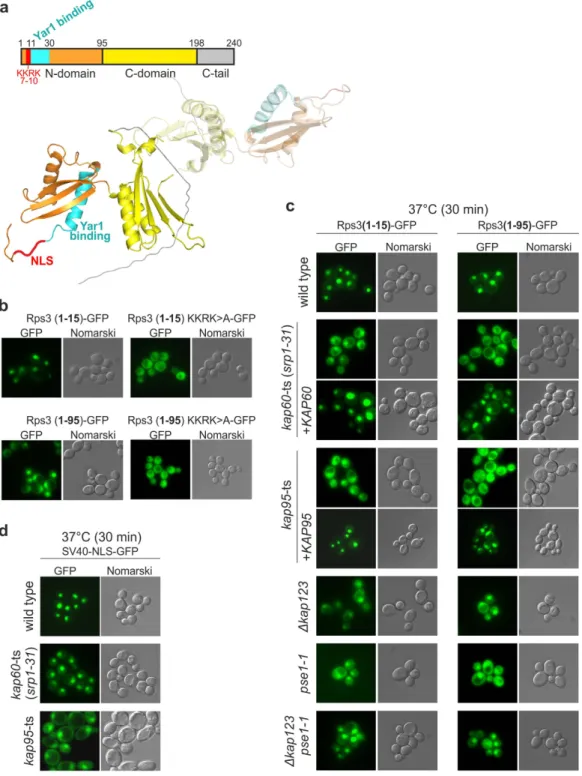
Nuclear import of dimerized ribosomal protein Rps3 in complex with its chaperone Yar1
Texte intégral
Figure

Documents relatifs
[r]
It can be seen that the dummy node in the center is the root node that connectes with all receptors/ligands.. The inverted triangles depict receptor
The inverted triangles depict receptor molecules, circles depict signaling intermediates and squares depict transcription factors.. The experimentally validated signaling pathways
The ballast pump is the device responsible for changing the volume of the glider, causing the net buoyancy to be positive or negative and subsequently causing
Drawing together a diverse range of artistic practices including film, video, photography, sculpture, text installation, performance, and mixed media, the works presented in Among
Taken together, in vivo and in vitro analyses demonstrated that Syo1 can simultaneously bind Rpl5 and Rpl11 to form a heterotrimeric Syo1-Rpl5-Rpl11 complex.. To gain further
HeLa cells were permeabilized by digitonin and incubated with purified, Alexa488-labelled Syo1 or GST-SV40NLS-mRFP in transport buffer (C, upper panel), transport buffer
Here, we report that the ankyrin repeat protein Yar1 directly interacts with the small ribosomal subunit protein Rps3 and accompanies newly synthesized Rps3 from the cytoplasm into