Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Internal Report (National Research Council of Canada. Division of Building Research), 1959-11-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=8d20c7da-8935-49fe-8d0d-08585944b65b https://publications-cnrc.canada.ca/fra/voir/objet/?id=8d20c7da-8935-49fe-8d0d-08585944b65b
NRC Publications Archive
Archives des publications du CNRC
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20386627
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at Continuing studies on the nature of the Kingston concrete problem Swenson, E. G.; Gillott, J. E.
NATIONAL RESEARCH COUNCIL OF CANADA
DIVISION OF BUILDING RESEARCH
OONTINUING STUDIES ON THE NATURE OF THE
KINGSTON CONCRETE PROBLEM
by
E.G. Swenson and J.E. Gillott
Report No.
190
of the
Division of Building Researoh
Ottawa
PREFACE
The effects of outside factors, external to the
aggregate itself, upon the development of expansion in concrete made with Kingston limestone coarse aggregate have now been amply demonstrated and reported. It is still important, even trom a practical point of view, to pursue studies to determine the cause of the expansion. Such studies will undoubtedly contribute to the knowledge on concrete and in addition may lead to the development of a rapid test which is now urgently required for the identification of limestone aggregates having the particular characteristic involved. Studies concerned with the nature of the mechanism are now reported.
The senior author,
Mr.
Swenson, was for many years the researoh officer in charge of the oement and oonorete work of the Division. The work now reported was oomp1eted just prior to his resignation to take a position in industry. Dr. Gillott, also a researoh offioer with the Division, has been engaged with Mr. Swenson on the minera1ogioal aspeots of the problem and will oontinue this work.Ottawa
December
1959
N. B. Hutoheon Assistant Direotor
•••••••••••••••••••••••••••••••••••••••
A.
EXPERIMENTALTABLE OF CONTENTS
Page
1
1. Expansion of the Carbonate Rocks in Alkaline
Solution ••••••••••••••••••••••••••••••••••••• 1
3. Pore Size •••••••••••••••••••••••••••••••••••••••
4.
Dolomite-calcite Composition •••••••••••••••••••• 2. Influence of Reactive Limestone on IonConcentration of Alkaline Solutions
••••••••••
4
6
6
5. Further Results of ASTM Tests ••••••••••••••••••• 7
6.
Petrographic and X-ray Examination of RocksUsed as Aggregate •••••••••••••••••••••••••••• 10 7. Petrographic and X-ray Examination of Concrete
Aggregate •••••••••••••••••••••••••••••••••••• 13 B. DISCUSSION OF POSSIBLE MECHANISMS ••••••••••••••••••
14
1. Alkali-silica Reaction ••••••••••••••••••••••••••14
2. The Clay Component ••••••••••••••••••••••••••••••16
3. other Foreign Components •••••••••••••セ •••o •••••• 174.
Carbonate Alteration - Dedolomitization ••••••••• 17 C. CONTINUING STUDIES ••••••••••••••••••••••••••••••••• 18REFERENCES •••••••••••••••••••••••••••••••••••••••0 • • • • •
19
APPENDIX A ... RESULTS OF MORTAR BAR TEST, ASTM C227-52T ON KINGSTON JOB AGGREGATES AND REFERENCE MATERIALS
APPENDIX B - RESULTS OF THE CONROW TEST, ASTM c342-55T, ON KINGSTON JOB AGGREGATES AND REFERENCE MATERIALS
CONTINUING STUDIES ON THE NATURE OF THE KINGSTON CONCRETE PROBLEM
by
E.G. Swenson and J.E. Gillott
This is the second detailed report of research carried out on the Kingston concrete problem. The first such report (1) gives in detail experimental and field records that are primarily concerned with the practioal aspects of the problem. An earlier general report (2) and a published paper
(3)
deal with preliminary studies.The present report includes that part of the investigation that was directed mainly セッキ。イ、ウ olarifying the nature of the
reaotion. The mechanism of the reaction has not yet been deter-mined and present indioations suggest that it may be much more
oomplioated than was first believed. This report is therefore in the nature of a progress report and studies are being oontinued. The neoessary baokground material is to be found in the above references.
The similarities and differences between the Kingston reaction and the alkali or cement-aggregate reactions reported extensively in the literature have been detailed (1,2,3). The
points of similarity observed fram the experimental studies indioate that the Kingston reaction is a related phenomenon. The differenoes are such, however, that the theorios developed to explain the
alkali-silica reaotion
(4,5,6,7,8,9)
do not appear to be applicable in this case. Authorities on this type of problem have so farbeen unable to explain the reaotion. Several possible hypotheses have been oonsidered and these will be disoussed in seotions
following the preliminary experimental studies described below.
A. EXPERIMENTAL
1. Expansion of the Carbonate Rooks in Alkaline Solutions
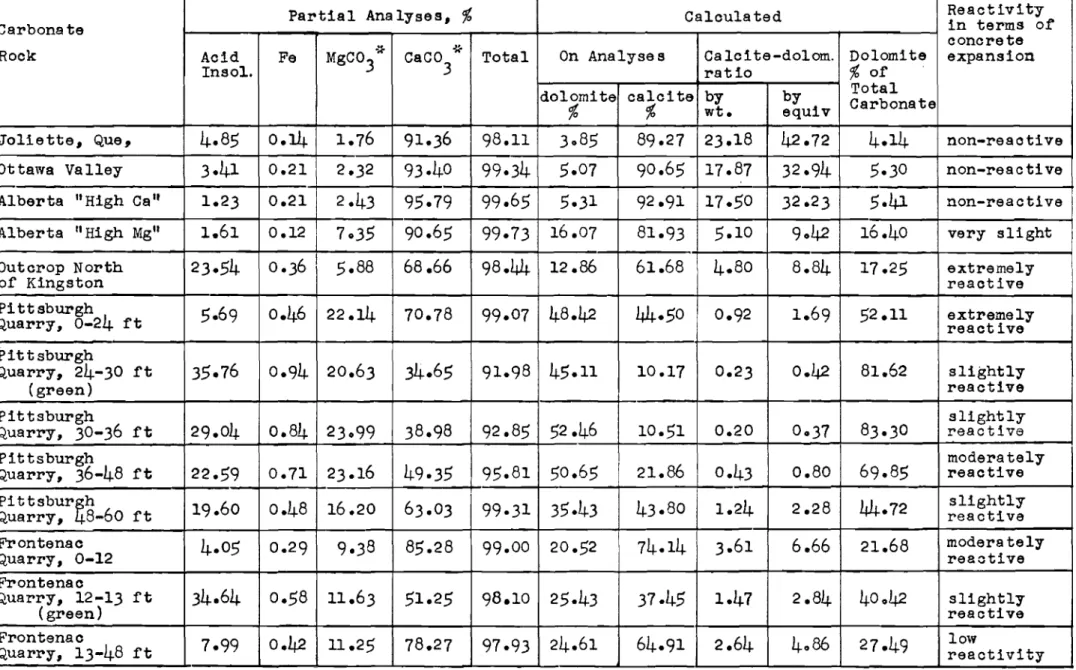
The presenoe of a oonsiderable clay fraotion in the re-active Kingston limestone suggested the possibility that relatively weak clay seams were opened up by strong alkaline solution, and
of the rock itself rather than the result of an inherent expansion of the rock. Volume change measurements were therefore made on limestone rock samples immersed in alkaline solutions made up to simulate the solutions in moist ooncrete.
The primary test solutions used were made up of sodium and potassium hydroxides, lime, and gypsum in three different conoentrations as shown in Table I. These were oonsidered as rough approximations of solutions found in moist concretes made with oements of high, medium, and low alkali oontents. Solution No.4 was sodium hydroxide.
(8) Crushed rock
Five hundred grams of 1/2- to 3/4-in. crushed Pittsburgh O-to 24-ft limestone were plaoed in a 500-ml flask and vacuum.
saturated with solution No.1. The flask was fitted with a
graduated column and the liqUid adjusted to a starting level.
A
control, using Ottawa Valley unreactive limestone, was set up in exactly the same way and the two systems placed side by side in a conditioned room at 50 per oent relative humidity and 73°F. To the top of each graduated tUbe was attached a soda-lime tube to prevent carbonation. Volume change readings were taken periodi-cally over a period of
9
months. The samples under test are shown in Fig. 1.At the end of the test, an estimated one-sixth of the reaotive rock particles were broken up, the first ones having begun to break up after only about 3 weeks. The remainder of
the rooks appeared to be unaffected. None of the particles in the oontro1 samples was cracked.
Fluctuations in volume readings caused by small temperature variations were accounted for by subtracting the control readings from the test sample readings to obtain the net volume change of the latter. The resulting values showed a gradual inorease in total volume of the test limestone. This is shown graphioally in Fig. 2.
The net inorease in volume of the reactive rock over the unreactive material indicates that an inherent expansion has
ocourred in the reactive rock. The cracking of some of the particles should not a.ffect the liquid volume in this test. The order of
this expansion is observed to be that found for ooncretes made with the same rock and a high alkali cement.
(b) Rock Erisms
Special samples of limestone rock were obtained at the following levels in the Pittsburgh quarry: 6 to 7 ft, 10.5 to 12 ft, 20 to 21 ft, 24 to 30 ft (green and grey-green), and 30 to 36 ft.
3
-These beds were selected because it was possible to obtain relatively large pieces from which prisms could be out by
diamond saw. Prisms 1 1/2 by 1 1/2 by
5 3/4
in. were cut from each bed (exoept the 20- to 2l-ft bed, which tended to break up). Some prisms were out so that bedding planes were parallel to the length (V) and others so that bedding planes were perpen-dioular to the length (H). The ends were planed and polished to permit fitting of speoial plates whioh were olamped on for periodic measurements of length ohange. These prisms, and similar oontrol prisms of the ottawa Valley limestone, were immersed in the solutions shown in Table I and plaoed in sealed luoite containers. Control samples were immersed in water. Periodioally the prisms were removed and measured for length, the operation being done quickly to minimize carbonation. Figure3
shows the specimen in a oontainer and also one of the measuring plates in position on a sample.Table II gives a reoord of length ohanges over 10 months and also indioates the cracking behaviour. Lengths at one-day immersion were taken as zero length for subsequent oalculations. The small but variable length inoreases, which occurred from dry to 1 day immersed, are recorded but are attributed to normal moisture movement.
The reference ottawa Valley prisms, after a small initial volume ohange that was probably due to further moisture movement, remain essentially stable in solutions 1 and 2 as well as in water. The prisms from the
6-
to 7-ft bed immersed in water and solutions 2 and3
remain stable but the two prisms immersed in solution 1 show a gradual abnormal expansion of approximately the same rate and degree that was observed for oonoretes made with reactive rock and a high alkali oement (Fig.4).
The prism with the bedding planes parallel to the length (V) showed a lower expansion than the one with the bedding planes perpendicular tothe length (H), indioating that the expansion is probably faoilitated by weakness oaused by stratification. Both samples remained
un-craoked, however, indicating that an abnormal inherent expansion in the rock had taken place. A slioe was taken off the H sample at
6
months, along its length, and this process apparently did not affect the progress of growth nor did it reveal any inoipient oraoking.The prisms from the 10 1/2- to 12-ft bed were much more unstable in solution 1 than those from the
6-
to 7-ft bed. The prism with bedding planes parallel to the length showed very rapid and high expansion and appeared intaot except for two short fine oracks at one end and parallel with the length. These cracks were not oonsidered capable of produoing any appreoiable change in4
-length oracked very rapidly, with major cracks along bedding planes and some perpendicular to them. The prism immersed in solution No. 2 showed an abnormal but slower expansion and
remained free from cracking. A very slight showing of abnormal expansion is observed for the prism in solution N0.3. The prism immersed in sodium hydroxide (No.4) expanded in a manner ウゥュゥャ。セ
to those in solution No.1, but cracking was much more extensive, both parallel and perpendicular to bedding planes. The edges on each side of the cracks protruded considerably. Some of these results are plotted in Fig. 5. The rate and degree of expansion of this rock appears to depend on the alkali concentrations.
Of the two prisms from the 24- to 30-ft green bed, one was cut from a grey part and another from the green part, both with bedding planes parallel to the length. The "grey" sample
cracked very early. The "green" sample began to crack at a later age, with large cracks along major bedding planes, but with a "checkered" cracldng pattern developing later. The checkered
seotions measure about 1/2 to 1 in. across, the cracks having a white edging similar to the rim-like appearanoe of the green rook in concrete at later ages. The size of these "checker" sections is roughly 1 in.
The prism from the 30- to 36-ft bed cracked very early in solution No.1, with cracking along the bedding plane.
It would appear from these experiments that expansion of ooncretes made with "reactive" limestones and containing a rela-tively high alkali content may expand abnormally as a result of two separate phenomena: the actual expansion of some of the lime-stone, and the cracking of some particles at bedding planes or joints. Both appear to depend on alkali concentration. The crushing of the limestone into sizes used in concrete may cause
fracturing of the weaker" joints" and thus account for the decrease in rate and degree of expansion with a reduction in particle size. The latter behaviour is also found in alkali-reactive siliceous aggregate but is aocounted for theoretically on quite a different basis(4) •
2. Influence of Reactive Limestone on Ion Concentration of Alkaline Solutions
-
- -.-It was previously shown (3) that in the quick chemical test (ASTM C289-54T), the reactive limestone yielded an abnor-mally high value for reduction in alkalinity. This was not unexpected for dolomitic limestones. Other investigators have reported that alkali-reactive aggregates reduce, not only the hydroxyl ion concentration, but to some extent also the potassium
5
-and sodium ion concentrations
{8}.
Consideration of the several possible mechanisms for the reaction indicated that it would be useful to know whether each of the major ions present in the free water in concrete is to some degree removed during the reaotion.Samples of the Pittsburgh 0- to 24-ft reactive rock and the 24- to 30-ft green, unreactive rock were crushed to the six sand sizes and recombined in equal proportions, including the pan material. One hundred and fifty grams of each of these samples was placed in a polyethylene bottle. Two hundred ml of solution No. 1 were added to each bottle and the mixture was oontinually agitated by a mechanical shaker. After certain
intervals the shaking was stopped, the solids allowed to settle, and l-ml portions of the clear supernatant solution were pipet ted off, aliquotted to 200 ml, and analyzed.
Control samples, using Ottawa Valley and Joliette lime-stones, were digested in the same manner in solution No.1. Similar tests were made with other solutions; the results of analyses are shown in Table III.
It is observed that the hydroxyl ion concentration decreased with time for all carbonate rocks and for all
solutions. Reference to Table IV shows that this decrease may be related to one or both of two factors: the clay-ailica fraotion and the dolomite content. It would appear, however, that it bears no relation to the degree of reactivity of the limestone in concrete since the only rock in this series that produced exoessive expansion in concrete is the Pittsburgh 0" to 24-ft series.
The sodium ion concentration remained essentially unohanged for solutions Nos. 1 and 2. It would therefore appear that the
sodium ion either takes no part in any react·ion or, if it is a reactant, it is regenerated and remains a soluble product. In
solution No.4 an increase in sodium ion concentration was obtained for all samples. No explanation is immediately evident for these anomalous values.
An inorease in potassium ion conoentration was obtained for solution No. 1 containing the reactive 0- to 24-ft rock and the inactive Ottawa Valley limestone, but no appreciable change was observed for the other two samples. No explanation can be
offered for these anomalous results.
The sulphate ion ooncentration increased in all samples and this increase is attributed to oxidation of sulphides present in these rocks. The initial low oalcium ion concentration due to the high alkali ooncentration present (10) remained essentially unchanged in all samples.
- 6
セDespite some anomalous values, and allowing for some margin of experimental error, it appears that a reaction oocurs whioh removes hydroxide ions but does not remove alkali ions. The reaction under consideration, therefore, appears to be different from the alkali-silica type
(8).
A possible reaction whioh could account for the above results is dedolomitization in a strongly alkaline solution, and may be represented as:
cac0
3• MgC03 + 2Na+ + 20H-
セmァHohIR
+ caco3 + 2Na+ + C03--This reaotion would occur if the solubility of dolomite is of the same order as that of MgC03• Information on the solubility of dolomite is not available, bセ、 solubility products of MgC03 in highly alkaline solutions are also not known. Preliminary x-ras studies showed evidence, however, of the formation of some Mg(OH)2 in the reaction. In the suggested equation, the hydroxide ion oonoentration is reduced but the sodium (or potassium) ion is regenerated.
3. Pore Size
Studies of certain Iowa limestones (11) suggested a oorrelation between field performance of concrete and pore-size distribution do\Yn to 0.1 micron. Using the sarne technique, with mercury pressure absorption to 2000 lb, it was found in
this laboratory that none of the carbonate rocks studied contained more than a very small proportion of pores greater than 0.1 micron. The mercury absorption varied framO.2 per cent for a reactive
and a non-reactive rock to 1.3 per cent for a non-reaotive rook. The intermediate values appeared to bear no relation to expansion of concrete where these materials were used as ooarse aggregate, and appeared to bear no relation to durability. It was apparent that the determination of pore-size distribution of suoh rooks requires apparatus oapable of pressures much higher than 2000 lb.
4.
Dolomite-calcite CompositionPrevious results had indicated that unreactive carbonate rooks were either highly oalcitio or highly dolomitic, and that the reactive rocks were of some intermediate composition. A partial analysis was made on the samples studied to determine the relative proportions of dolomite and oalcite present and to deter-mine if these oould be oorrelated with reaotivity. The analytioal results and oaloulated values are given in Table IV. A soale of reactivity was assigned to the carbonate rooks studied whioh was
7
-based on the expansion of concrete prisms obtained in previous studies (1). On this basis the reactivity was plotted against per cent dolomite as shown in Fig.
6.
It is observed that the Pittsburgh beds give a definite peak of reactivity at about
52
per cent dolomite, the reactivity falling off on either side of this composition. The Frontenac beds, however, show increasing reactivity towards a much lower dolomite content, apparently corresponding to the reactive sample obtained from an outcropping near the junction of Highways15
and 401. It is clear, however, that the highly calcitic and thehighly dolomitic carbonate rocks show little or no reactiVity. These results, although not conclusive, are interesting since they support previous indications that the reaotive carbonate rocks have some intermediate dolomite-calcite composition. It is emphasized, however, that the samples tested are themselves
variable in composition and that preoise differentiation would require oareful sampling of each of a very much larger number of distinct strata in each quarry.
5.
Further Results of ASTM Tests(a) The Quick Chemical Method (ASTM C289-54T)
Previously reported results of the Quick Chemical test were negative for the reactive 0- to 24-ft Pittsburgh series and also for the green 24- to 30-ft bed (3). These results are re-produced in Table V along with additional values for repeat tests. The failure of the test to yield positive results for the alkali" reactive Kingston material was not unexpected since the dolomite present would tend to give a spuriously high reduction in alkalinity through a reaction between mァcoセ and NaOH to produce Mg(OH)2 (8,12). It is believed that this would セャウッ tend to lower the value for
silica obtained in this test (8).
At the suggestion of Dr. R.C. Mielenz, the test was
repeated on the acid insoluble residue of the reactive 0- to 24-ft material and the values obtained, recalculated in terms of the original limestone, are shown in Table V. The adverse influence of the MgCO was eliminated, and the results, when applying the
accepted
」イセエ・イゥ。
of Mielenz et al (8) and Halstead and Chaiken (12), would indicate a potentially alkali-reactive aggregate, presumably susceptible to the alkali-silica type of reaction. Although the reliability of this modification of the Quick Chemical test is open to some question, the results indicate that there probably is some active silica present. This test was not carried out on the other materials, reactive and non-reactive, and hence must be considered inconclusive.8
-(b) The Mortar .Bar Test J.ASTM cRRQMURセャ
In the first publication on studies of the Kingston lime-stone problem
(3),
some results of mortar bar tests were given in bar graph form. These showed that the Kingston limestone which produced excessive expansion in concrete showed only a moderate expansion in this test and, on the basts of accepted criteria, the material would be classified as a safe material for aggregate in concrete. The test was considered, therefore, as having failed to detect the alkali-aggregate reaction in the Kingston case. It was concluded that (1) the test method was inadequate, (2) thatthe deleterious component was not within the so-called "pessimum" range, or
(3)
that the Kingston reaction involved a mechanism different from the usual alkali-silica type.The detailed results of all mortar bar tests are given
in Appendix A. In addition to the above discussion, some additional points are worthy of mention in connection with Appendix A. Criteria for reactive aggregate are: (1) expansions to exceed 0.05 per cent at
3
months and 0.10 per cent at6
months for the high water-cement ratio, and (2) expansions to exceed 0.05 per cent at6
months and 0.10 per cent at 12 months for the low water-cement ratio.The Montreal cement, although its total alkali is a little lass than for the Belleville cement, curiously enough produced higher expansions with Kingston 0- to 24-ft material as well aa with the alkali-reactive Republican River reference sand. The low alkali Exshaw cement satisfied the criteria of the test but produced abnormal expansions at later periods, a behaviour also observed
for concretes made with the reactive Kingston limestones and the same cement.
The pozzolan, California oalcined shale, was very offective in inhibiting alkali-aggregate reaction with the Republican River sand, but was less effective in reducing expansion of bars made with the reactive 0- to 24-ft rock. It will be recalled that in
concrete made with this limestone it was effective in the early stages but at later ages proved ineffective. Increase in water-cement ratio increased the rate of expansion for the Republican River reference sand, but appeared to have no significant effect
for the Kingston material. The blends of inactive green with the reactive 0- to 24-ft material reduced the expansion as was found for concretes.
The land-mined sand was found to be free of alkali-aggregate reaolJion by this test. The tests using the fine grading, to
correspond with natural grading of セQゥウ sand, were carried out to make certain that the pan fraction contained no deleteriously reactive materials.
materials Mix
were
- 9 ...
The possibility was considered that the "partial" failure of the mortar bar test to detect the alkali-aggregate reactivity of the Kingston limestone was due to the proportions of deleterious component and inactive material not being within the "pessimwn"
range; however expansion records of concrete prisms made with blends of reaotive 0- to 24-ft rock and inaotive green rock, described in the previous report (1), show essentially a dilution effect only.
The same holds for the blends used in the mortar bar test (Appendix A). The possibility was also considered that the "inactive" green material was actually so highly reactive that no expansion would
occur. To oheck this, three blends of inaotive Ottawa Valley ャゥュ・セ
stone and the "green" Kingston limestone were used in conorete to get a combination within the "pessimum" range. Blends were: 90 and 10 per oent, 80 and 20 per oent, and 70 and 30 per oent. The
oorresponding per oent expansions were: at
6
months: 0.010, 0.009, and 0.009; at 12 months: 0.016, 0.016, and 0.017; and at 15 months: 0.018, 0.015, and 0.017. These results would appear to indioate that the "green" Pittsburgh 24- to 30-ft dolomite is actually in... active to the alkali reaotion.(c) The Conrow Test, ASTM cST_MUセt
In the previous publication on the Kingston oonorete problem (3) no values of this test were detailed but it was
indioated that results were negative. It was pointed out, however, that the land-mined sand showed some response. to the test and that it was, therefore, to be oonsidered suspeot. Results of the oon-crete prism tests had sho\vn that the contribution of this sand to the excessive expansion of Kingston conoretes must be of a small order.
Details of the Conrow Test results on Kingston job and selected reference materials are given in Appendix B. combinations similar to those used for the mortar bar test tested. Certain points of interest may be noted.
All limestone samples reaoted negatively but with slightly greater expansions for the Kingston limestones compared with the reference Ottawa Valley limestone when the high alkali cements were used. No significant differenoes were observed for the two gradings and the two water-oement ratios. Decrease in cement content, with and without the pozzolan, decreased the expansion.
The reactive referenoe Kaw River sand responded as expected. The land-mined Kingston sand showed varying reaotion with the
different cements, and approached the oriticc.l limit of 0.2 per cent for this test, when Montreal cement was used. The fine
10
-fine material may have been the main source of reaction. The pozzolan, although it showed the expeoted effectiveness with the Kaw River referenoe sand, appeared to have no signifioant effeot with the Kingston sand.
Although the results of these tests were negative, the results suggest that sources of sand in this area be tested for the type of unsoundness revealed by the Conrow method.
6.
PetrograEhio and X-ray Examination of Rocks Used as Assregate (a) ottawa Vallez limestoneThe rook is a dark grey fossiliferous limestone with oooasional impressions of brachiopods. Blaok graphitic material gives a shiny lustre to parts of irregular fraoture surfaces and there are veins and pockets of reorystallised caloite.
In thin section the rock is seen to consist of a finely crystalline limestone sprinkled with organic material. There are also scattered irregular grains of quartz about 0.05 mm in diameter and Widely distributed euhedral rhombs of dolomite of about 0.04 mm diameter. The slice cuts irregularly through abundant fossil fragments which occasionally have a border of fibrous calcite. The fossils sometimes have a dusky peripheral rim which gives way rapidly to a oore of interlocking anhedral calcite crystals which are of larger size and more free of inclusions than those in the matrix. This is made up of bright polarizing-carbonate and brown
dusky grains of organic and argillaceous material. Graphite occurs as opaque six-sided platelets.
An examination of the residue, heavy in liquid of specific gravity of
2.89,
shows irregular grains of limonite and haematite to be common together with some pyrite. Irregular to rectangular crystals of a carbonate belonging to the aragonite group oocurwhich are about 0.1 mm in length. These are colourless in ordinary light but contain minute dark inclusions. They show very pale
polarization oolours and extinguish in a diagonal relationship to the cross wires. There are occasional irregular or elongated
crystals of tourmaline showing pleochroism from colourless to green and straight extinction. One colourless elongated grain of zircon was observed showing high relief and straight extinction. There are occasional brown, irregular crystals of moderate to high relief showing strain (sphene?). There is one grain which shows a pale green colour in reflected light and which is opaque in ordinary light.
No mineralogical difference could be detected by microscopic examination of a thin section taken from the rock soaked in alkaline
11
-Debye-Scherrer x-ray powder films of the ottawa Valley limestone and the rock soaked in alkaline solution do not differ. They show calcite to be the dominant phase with weak dolomite lines. There are also weak lines due to chlorite and illite on the pattern of the untreated rock.
(b) f1ttsburgh QuarrZt Kingston, idNUセ to 12-ft bed
The rock is a grey, fine grained limestone shoWing thin bedding and small veins and pockets of recrystal1ised calcite.
In thin section the rock is seen to be a dolomitic lime-stone or calcareous dolomite. There are euhedral dolomite rhombs (approximately 0.05 mm) sometime s containing tiny incluslons,
enclosed in a bright polarizing calcareous イイセエイゥク containing dusky organic material. This is distributed throughout the matrix and also concentrated in ill-defined rounded patches or grains. There are distorted rhombic or rectangular pseudomorphs (1 to
1.5
mm cross-section) occupied by interlocking calcite crystals. Thesection is crossed by a narrow contorted vein of lfulonite and there are small crystals of pyrite, rather unequally distributed and
related to the bedding. The groundmass contains irregular grains of quartz and a very occasional crystal of plagioclase felspar showing lamellar twinning.
The residue, heavy in liquid of specific gravity of 2.89 contains limonite, pyrite, and some haematite. There is much
black isotropic material, which in reflected light is silver grey, and which often displays cubic or rectangular form. Rhombs of carbonate are common and green tourmaline, sometimes pleochroic, is present.
The rock treated with alkaline solution is similar
mioro-scopically to the untreated rock and there is no apparent mineralogical difference. The hand specimen is cracked parallel, perpendicular,
and oblique to the bedding planes.
Debye-Scherrer x-ray powder patterns of the rock and the rock SUbjected to attack by alkaline solution, show calcite and dolomite as the dominant phases but the dolomite lines are weaker on the diffraction pattern of the sample soaked in alkali.
(c) セエウ「オイ・ィ セ。イイケL kゥョヲAウセッョL
6-
to 7-ft bedThe rock is of similar appearance to the
10.5-
to 12-ft bed though black layers are finer and less persistent forming12
-appearance of the rock but there is probably less of the dark rectangular mineral. Grains of colourless cryptocrystalline material and dark organic material may form a larger proportion
of the rock than in the 10.5- to 12-ft bed.
The rook subjected to attack by alkaline solution appears microscopically identical to the untreated rock. The hand specimen is apparently uncracked though small-scale cracking is visible
microscopically.
The intensity of the x-ray.reflections from dolomite and calcite are approximately equal suggesting that the rock contains about equal proportions of the two minerals. The diffraction pattern of the rock soaked in alkali does not apparently differ from that of the untreated rock.
(d) Pittsbursh Quarrx, kゥョァウエッョセ RPセ to 2l-ft be£
A thin section cut from the 20- to 2l-ft bed is of similar appearance to the 10.5- to l2-ft and
6-
to 7-ft beds.(e) Pittsbursh Quarry, Kingston, 0- to 24-ft series oomposite
A sample of the composite 0- to 24-ft series in the Kingston quarry was subjeoted to x-ray analysis. The diffraction pattern
shows strong reflections from both calcite and dolomite but the dolomite reflections are less intense than the caloite refleotions. Weak quartz refleotions also occur.
A sample of the same rook, crushed to a size comparable to the aggregate used in concrete beams was soaked for several months in alkaline solution. An x-ray film of this material was compared with the film obtained from the original rook. These films differ in that the dolomite reflections have vanished (with the exception of a very faint line at the position of the most intense dolomite line). The caloite refleotions have become enhanoed and three additional lines have appeared on the diffraction pattern. These lines appear to oorrespond with the strongest lines of bruoite. An attempt was made to confirm this finding by differential thermal analysis.
The thermogram shows an endothermio peak rather broader and at a temperature somewhat lower than is normally given by brucite. However, the correspondence is suffioiently close in the absence of further data to make it likely that brucite 1s present in the sample.
13
-The lack of dolomite is curious as rhombic crystals, apparently dolomite, are present in microscope sections cut from rock prisms known to have expanded (see description of 10.5- to l2-ft and
6-
to 7-ft beds).(f) Pittsburgh Quarr:y:, Kingston, "green bed", 24- to 30-ft
The rock is a greenish pelitic dolomite containing scattered detrital grains of quartz of the order of 0.5 to 1 mm diameter.
Microscopic examination shows a large concentration of detrital quartz generally about 0.1 rom diameter but sometimes reaching a size of 1 to 1.5 mm. Dolomite rhombs (0.1 mm) are the other major constituents. The dusky matrix is composed of crypto-crystalline pelitic and organic material speckled with black grains of iron in the form of pyrite and leucoxene. Small crystals of plagioclase felspar, showing lamellar twinning, also occur.
Heavy liquids and magnetic means were employed to concen-trate accessory minerals. Tourmaline, apatite, and zircon are present and rarely well formed octahedra of ceylonite. There are grains, generally concentrated in the more magnetic fractions, of cryptocrystalline material patchily coloured green, possibly of glauconite.
The rock SUbjected to attack by an alkaline solution is similar. The ィ。ョ、jセー・」ゥュSョ displays numerous irregular cracks. X-ray patterns of the rock and the rock soaked in alkali do not apparently differ. Dolomite is the_dominant mineral
present with Ie sser amounts of calcite, illite, quartz, and chlorite.
7. p・エイッァイ。eィセョ、 X-ray Examination of Concrete Aggregate
Thin sections were prepared from concrete in which the aggregate conststed of ottawa Valley limestone, the "green" bed in the Pittst'lrgh quarry, and a composite of the 0- to 24-ft series in bhe lame quar-r-y ,
The aggregate is in each case similar to the appropriate rocks described previously though at least one fragment of the
ottawa Valley aggregate is more dolomitic than the sample described suggesting some variation from bed to bed within the quarry from which this rock is obtained. There is also considerable variation among the aggregate from the 0- to 24-ft series in respect to
grain size, dolomite content, amount of quartz and transparency to transmitted light.
-lJ+-A similar variation can be seen among the oセ to 24-ft series aggregate on polished surfaces of the concrete. Some
。ァセイ・ァ。エ・ is surrounded by narrow dark rims (approximately 0.3
mm) and these are generally associated with light grey aggregate. Thin sections made to include rims do not reveal any apparent mineralogical difference between rim and core but the rim
some-times seems clearer and more transparent to transmitted light though it is often more apparent in reflected light. Thin bands of discoloration occasionally cross the aggregate and there are cracks that cut through both matrix and aggregate. The darker blUish-grey aggregate is generally free of rim formation.
A sample of concrete made with low-alkali cement and Kingston ッセ to 24-ft series aggregate when polished shows rims affecting both dark and light grey aggregate. The rims are
sllnllar In appearance to those seen surrounding aggregate in the high-alkali concrete but broader and reach 2 to 3 mm in width.
Similar rims do not surround aggregate composed of ottawa Valley limestone, the Kingston "green" (24- to 30-ft) bed nor the 30- to 36-ft bed. Rims are also lacking on concrete aggregate . composed of limestone from Montreal and Joliette, Quebeo, and on two samples of limestone from Alberta. Cracks within the aggregate fragments are present in all samples but seem fewest in aggregate composed of the Kingston "green" and 30- to 36-ft bed.
Debye-Scherrer x-ray powder patterns were obtained from
Kingston 0- to 24-ft series aggregate separated into three fractions composed of rim material surrounding aggregate, rim centres, and non-rimmed aggregate. The throe patterns are apparently identical and correspond closely with the x-ray powder pattern obtained from the composite sample of the 0- to 24-ft series in the Kingston quarry. Dolomite and caloite are the dominant minerals present with the caloite reflections being soolewhat stronger than the
dolomite lines. An attempt to concentrate brucite (if present) by flotation of powdered aggregate proved unsuccessful.
B. DISCUSSION OF POSSIBLE MECHANISMS
.
...._-
...
1. Alkali-silica r・。」エゥッセThat the Kingston reaction is related to the alkali-silica type of alkali-3ggregate reaction cannot be disregarded in any consideration of mechanism. The points of similarity are strong:
(1) abnormal expansion of ooncrete, followed by map-cracking where moisture conditions vary at two surfaces; (2) the degree and rate of expansion; (3) the influence of the alkali content, whether this derives from the cement or is added; (4) the more aggressive
15
-influenoe of sodium than potassiQTI hydroxide; (5) the influenoe of temperature, with evidenoe of an optimum;
(6)
the direct influence of moisture; and (7) the apparently satisfactoryproperties of the aggregate when subjected to conventional tests. Additional factors which point to similarity, but whioh oontain elements of difference, are:
(1) Deorease in expansion with deorease in particle size of aggregate. It is possible that this property is related to the break-Up at planes of weakness during reduction of particle size, rather than a decrease in the effeotiveness of a fixed amount of alkali with inoreasing surfaoe area (4). The rather low expansion obtained with the mortar bar test is to be recalled.
(2) Pronounoed rim formation around affeoted aggregate partioles, but with an appearanoe different from
that obtained in aDroli-silica reactions.
(3) Microscopic evidence of fracturing of aggregate and paste, but with a difference in the general appearance of the affected concreto.
The evidence pointing to a different type of reaction is equally atrong. Some of these points require amplification.
(1) E!!1ur:.e_ to 、セセ・N」エ the
.:e..
ᆪNセセョセセ⦅AAャ エィᆪMセァァイセセセヲ any known alkali-reaotive mineral or rock typo. - Although the partly deoayed micaceous material--or'the-qu5riZ-material present may be thereaotive oomponent, the total amount present is small, and is
present in much larger amount in the non-reaotive "green" bed than in the very reaotive 0- to 24-ft series of the Pittsburgh quarry. The results of the Quiok Chamioal test on the acid insoluble
residue, and the considerable expansions obtained in the mortar bar test suggest, however, that an alkali-silica reaotion may contribute to some degroe to the observed expansion of ooncrete.
(2) The セセNセQGAYNelNᆪヲ 。ァqᆪセッゥ⦅セャ・ L。NeQセエウ of alkali-silica ァセ
oompat.:!E.:l;,e キゥセRMjウNAスNRュセセNャ_ZLLZZN_ゥャャゥLセイP。アセセャLセN - It may be that the gel, if formed, is distributed tihr-ougnout the very fine pores of the limestone partioles and is therefore difficult to detect. Since the total silioa content is small in the reaotive rock, and since only a part of it oould oonceivably reaot, it is difficult to conceive of sufficient gel being formed to produce the very high rates and degrees of expansion of ooncrete observed. If the curront theories pertaining to alkali-silic3 reaction are valid, the Kingston reaction would appear to belong to a different category of reaction.
.. 16
-(3) Inhibitors of the alkali-silica t e of alkali-a
reaction were not effective in the K1n ston reaction • - The failure of siliceous pozzolans to maintain a low degree of
expansion in concretes would appear to be an anomalous situation, since these materials are supposedly effective in reacting with
the alkalies thus making them less available for any other reaction. On this assumption :i.t follows that the alkali-silica reaction
involving a siliceous pozzolan is not capable of competing with some reaction that demands alkali as a reactant or as a catalyst.
(4) Inorease in water-cement ratio a ears effect on the rate and 、・セ・・ of t e react
(5) Areas of ooncrete surrounded by cracks aPEear to イ・ュ。セセ
relatively intact. - ThIs is true of many of the field cases, some
of
which are over 20 years old (1). In cases of 。ャォ。ャゥセsilica reaotions, there is a general disintegration throughout. From the evidence, summarized above, the presence of
alkali-silica reaction in the Kingston case appears most unlikely as the major meohanism. It remains, however, an alkali-aggregate reaction with certain major points of similarity to the 。ャォ。ャゥセ
silica type.
2. セcャ。ケ ComEonent
Several independent laboratories as well as the DBR laboratory have identified the clay in the Kingston limestones
as illite. In addition, some chlorite was found by this laboratory. Both of these clays are of the non-swelling type. That either of them might be transformed into a swelling type in the presence of a strongly alkaline solution was not found to be the case in x-ray analyses of original rock and rims of rock from affected concrete, and also rock long ゥセョ・イウ・、 1n alkaline solutions (13). X-ray analyses by this laboratory failed to detect the presence of any form of montmorillonite, or any product indicating a transformation of the illite. The clay-silica fractions used in these studies were separated from the carbonates by ion-exchange techniques to avoid any transformation owing to the method of separation.
The clay fraction in the reference Ottawa Valley limestone is also illite. The 24- to 36-ft green rock (non-expansive)
contains a much larger amount of the same olay than the reactive 0- to 24-ft series.
The studies to date have revealed no certain evidence that the clay fraction in the reactive rock is responsible for the
... 17 ....
the disintegration of some of the rock particles may be caused by high clay concentration. Investigations of the clay fraction are continuing (13).
3.
Other Foreign cッュeッョ・eセThe possibility that an internal sulphate reaction might produce excessive expansion in the concrete has already been
shown to be improbable on the basis of experiments described in a previous report (1). The rock itself expanded in an alkaline medium with no tricalcium aluminate present with which to form calcium sulphoaluminate. A large amount of free or easily freed sulphide, as revealed by the high hydrogen sulphide concentration obtained by vacuum saturating the reactive rock, is a peculiar characteristic of this limestone, however, which demands further study.
A considerable quantity of strontium was found in one sample of reactive rock by one laboratory. This has not yet been verified.
It is known that an unstable form of calcium carbonate, aragonite, is transformed, under certain conditions, to calcite with an accompanying increase in volume. No evidence for the presence of aragonite in the reactive limestone has yet been found.
4.
Carbonate Alteration - d・、ッャセュゥエゥコ。エゥッョSteidtmann (14) and others have shown that carbonate rock analyses cluster touards the end-members of the dolomite-limestone
series. Rocks in which dolomite comprises either 0 to 10 per oent or over
90
per oent of the total r-ock are much more common than rocks of intermediate oomposition. As dolomite is frequently of replacement origin(15)
this suggests that intermediate oompositions may be unstable.In the Pittsburgh Quarry the most reactive 0.... to 24-ft series contains an average of about
45
per cent calcite and48
per cent dolomite. Samples from the next most reactive 36.... to 48-ft level contain proportions of 22 and51
per cent. The un-reactive "green" bed contains approximately 10 per cent calcite and45
per cent dolomite and in the unreactive 30'" to 36-ft bed the proportions are 11 and 52 per cent. Thus analyses show that the Kingston 0- to 24-ft series falls within the intermediate composition range and this may be the unde r-LyLng cause for the reactivity.18
-The possibility that dedolomitization occurs in highly alkaline solution has already been considered in this report. This would require that the solubility of dolomite is greater than that of Mg(OH)2 in alkaline solution. This is probable in view of the re la ti ve solubilit les of MgC0
3
and Mg(OH)2 in water butun-fortunately, such ゥョヲッイョセエゥッョ is not only unavailable, but would appear to be difficult to obtain experimentally. X-ray evidence is that some Mg(OH)2 is formedo
If hexagonal axes of reference are used for all solid reactants, the previously suggested chemical equation may be
written to give a minimum of one unit cell of each of the probable solid products:
6
CaMg(c0 3) 2 + 12 n。ohセ 2 cells dolomite6
CaC0 3 1 c0l1 calcite +6
Mg(OH)26
cells bruciteDiffering values for the lattice constants of these minerals appear in the literature but in all casen, provided sodium carbonate goes into solution completely, the above reaction should lead to a decrease in volume. Crystallization of the sodium carbonate as a hydrate, on any considerable scale, however, would probably reverse this and result in expansion.
Dolomite ヲッイョセエゥッョ at the expense of calcite should lead to a reduction in volume in the ratio of 100 to
88.
In fact dolomites are often no more porous than limestones(15)
so that replacement has apparently been volume-for-volume rather than the more generally expected molecule-for-molecule type of replacement.An ion-by-ion replacement of Mg++ by Ca++ in the dedolo-mitization reaction would lead to an increase in volume in the
above ratio.
That dedolomitization is involved in the mechanism responsible for excessive expansion of concrete containing Kingston limestones is a possibility in view of evidenoe from studies of the different limestone beds outlined in a previous section. It is possible, however, that seoondary reactions may oocur and this question is under continuing study.
C. CONTINUING STUDIE£
Sinoe this report surrmarir.es progress in the study of the nature of the Kingston aggregate problem, and since the mechanism of the reaction has not yet been discovered, no
general conclusions are made at this time. Continuing studies include carbonate r-oclra from other areas that have experienoed aggregate problems. Evidenoe now being accumUlated indi'cates
19
-that at least two U.S. limestones react in the same manner as the Kingston rock. A more exhaustive analysis of these rocks has been started and it is hoped that these studies will not only bring light to boar on the Kingston reaction, but will also add to our knowledge of the behaviour of carbonate rock in
cement. REFERENCES... ... 1.
6.
7.
8.
9.
10. 11.Swenson, E.G. Cament-aggregate roaction in Kingston, Ontario. National Resoarch CotUlci1. Division of Building Research,
Intornal Report 182, October 1959.
Legget, R.F. and EeG. sセPョッッョN A concrete problenl at Kingston, Ontario. National Rosoarch Council, Division of Building Research, Internal Report 115, April 1959.
sセ・ョウッョL E.G. A Canadian roactive aggregate undetected by ASTM
tests. ASTM Bull. No. 226, Decembor 1957.
Powers, T.C. and H.H. Stoinour. An interpretation of published researches on the alkali-aggregate reaction. Proc. ACI, Vol. 51, p.497 and 785, Feb. and April 1955.
Hansen, W.C. Studies relating to the mechanism by which the alkali-aggregate roaction produces expansion in concrete. Journal, ACI, January QYTQセL Pr-oc , ACI, Vol. 40, p.213-27.
Verbeck, G.J. and C. Gramlich. Osmotic studies and hypothesis concerning alkali-aggregate reaction. Proc. ASTM, Vol. 55, p.lllO,
1955-Broun, L.S. Some observations on the mechanics of alkali-aggregate roaction. ASTM Bull. No. 205, p.40, April 1955. Mie1enz, R.C., K.T. Green and E.J. Benton. Chemical test for
reactiVity of aGGregates vTith c emerrt alkalis; chemical
pr-oce sae s in comerrb-caggr-egab e reaction. Journal, ACI,
Nov. 1947; Proc. Vol.
44,
p.193-224.Pike, rNgセL D. Hubbard and H. Insley. Mechanisms of alkali-aggrogate reaction. Journal, ACI, Vol. 27, No.1, Sept. 1955; Proc. Vol. 52.
Kalouook, G.L. Studies of portions of the Quaternary System
sッ、。Mャゥュ・Mウゥャゥ」ョMセ。エッイ at 25°C. Journal of Research, NBS,
VoL. 32, ーセRXUL QYlQャセN
Lendsh, J., F.E. Ruch and C.Lo Hiltr op , RoLationship of physical proportion of 80LlO IO'JC\ car-bonabo otjgl"eeate to tho durability of concroto \) Pro aorrt c d at Annua L l.Iooting of Highufly Resoarch BOaI'd, VlnshingtoJ:1, Jnn .. 1958 ..
12.
20
-Chaiken, B. and W.J. Halstead. Correlation between chemical and mortar bar tests for potential alkali reactivity of concrete aggregates. Presented at Annual Meeting of Highway Research Board, Washington, Jan.
1959.
Gillott, J.E. and R. Masson. Clay minerals in concrete aggregates of Kingston dolomitic limestone. (To be published as DBR Internal Report
191).
Steidtmann, E. Origin of dolomite as disclosed by stains and other methods. Bull. Geol. Soc.
Am.,
Vol. 28,p.437,
1917.
15.
Pettijohn, F.J. Sedimentary rocks. 2nd Ed., Harper Bros., New York,1957.
TABLE I
ION CONCENTRATION OF ALKALINE SOLUTIONS USED IN TESTS ON LIMESTONES
--Sol'n Compounds Original Concentrat Lons , gm.ions per liter
No. Present Na+ K+ Ca++ OH'" SOh--I NaOH 2.002 KOH 2.002 1.252 1.018 0.005 0.152 Ca(OH)2 cas04 2
"
0.1V+3 0.239 0.197 0.008 0.128 3"
0.062 0.030 0.024 0.014 0.014 4 NaOH 2.604 2·391..
0.005..
onlyTABLE II
EXPANSION AND CRACKING BEHAVIOUR OF LIMESTONE PRISMS IN ALKALINE SOLUTIONS AND WATER
V
=
bedding planes parallel to lengthH
= "
n perpendicular to lengthA L at Linear Expansion, per cent
Rock SolIn Bed I-day (based on zero at I-day immersion)
No. Plane Immersion 6-7 days\14 daysll mO.\2 mo.14 mo.16 mo.18 mo.110 mo. State
Ottawa Valley 1
-
.010 .005-
Nooセ .008 .007 .004 .• 004 .004 ) No cracking Reference 2...
.007 .009 .012 .00 .005 .004 .005 .007 ) water-
.003 .007 .007 .010 .009 .007 .007 .010 ) 1 V .002 .012 .016 .023 .056 .090 .120.144
.155 ) No cracking 1 H .003 .005 .016 .030 .096 .143 NQWセ .2i4** .233 ) 6-7 ft 2 V .007 .000 .000 .000 .000 .000 .000 .001 .001 ) 3 V .007 .005 .007 .008 .007 .004 .009 .008 ) No cracking water V .009 .009 .009 .014 .011-
.010 .009 ) 1 V .009 .026 .049 .108 .256 .390 .451 .470 .4861 H .023
-
.150 Large cracks along bedding planes(2 days)
10 1/2- 1 H
..
-
.070 .289 Large cracks along bedding plane12 ft Small n perpendicular to bedding plane
1 H .017 .063 .235 Cracks along bedding Klanes
2 V .007 .002 .012· .Oll .028 .054 .12 .126 .122 No cracks
4
V .007 .01 .014 .01 .016 .014 .017 .020 .022 No cracksV .017 .051 .115 .470 Large cracks along bedding planes
Later, finer cracks perpendicular to bedding plane • 004 "Lip" protrusion at oracks •
water V .020 .005
...
.008 .005 .007 .007-
No cracks24-30 ft 1 V .016 .063 .369 Cracking perpendicular and parallel to bedding plane s
grey
24-30 ft 1 V .003 .000 .002 .004 .023 .090 Large cracks 。ャッョセ length
green Fine cracks forming checker" pattern
30-36 ft 1 V .031 -.002 .004 .033 .266
.435
Cracking along lengthwater V .017
-
.007 .019 .013 •004 .007 .014 .. No crackingTABLE III
CHANGE IN ION CONCENTRATIONS
DURING DIGESTION OF LIMESTONES
IN
ALKALINE SOLUTIONS (Using Sand-Size Grading)Solution Limestone Ions Concentration, セ
Present 100
Original 1 wk. 3 wk. 9 V/k. 6 mo.
" ' - - - - ..•
No. 1 Pittsburgh Na+ 288 276 292 278 290
r
398 416 410 416 4200-24 rt ca++ 1.8 £2 セR L2 ..:::2
series SO - ...4 146 148 172 218 180
OH- 340 335 328 330 323
lNo. 1 Pittsburgh Na++ 288
RXセ
278 284 286K+ 398 41 394 404 402 24-30 rt Ca++ 1.8 セR セR L.2 .c..2 green S04-- 146 160 190 212 220 ッセ 340 327 319 315 309 セッN 1 Ottawa Na+ 288 278
RXセ
RXセ
290 Valley K+ 398 426 39 40 420 Ca++ 1.8 2. <:2 2. L2 Reference S04-- 146 ャAセX 164 166 182 OH- 340 339 335 331 328No. 1 Joliette Na+ 288 282 284 280 290
r
398 422 400 402 396Reference Ca++ 1.8 ':::2 2. "'.2 L..2
8°4-'" 146 144 152 220 154
OH- 340 338 340
---
336No. 2 Pittsburgh Na+ 55 53
セャ
55 58r
77 77 75 73 0-24 ft ca++ 3. 0 セQ L.l 1 1 series S04-- 123 119 129 133 133', OH- 75 64 64 62 59-No.4 Pittsburgh Na+ 550 556 582 566 572
0..
24
ft C3++ 1.8 2 セR .c..2 2series S04--
---
BGMイセ 12 48 72OR- 443
l.W2
438 436 427No.4 Pittsburgh Na+ 550 566 574 576 578
24-30 ft Ca++ 1.8 2 2 ..c2 2 green S04-'"
-_
...
20 1.12 72 60 OR- 443 429 421---
424 セッN 4 Ottawa Na+ 550 580 564 568 578 Valley Ca++ 1.8 2 0::::.2 ""2 2 Reference S04-----
-_
...
14 50 24 011 443 AセQ 438 432 428----
-TABLE IV
DOLOMITE-CALCITE COMPOSITIONS FROM PARTIAL ANALYSES OF CARBONATE ROCKS
Partial Analyses,
%
Caloulated ReactivityCarbonate in terms ッセ
Nセ
*
concreteRook Acid Fe MgC0
3 cac03 Total On Analyses Ca loite-dolom. Dolomite expansion
Insol. ratio
%
ofdolomite oalcite by by Total
%
%
wt. equiv CarbonateJoliette, Que, 4.85 0.14 1.76 91.36 98.11 3085 89.27 23.18 42.72 4.14 non-reaotive Ottawa Valley 3.41 0.21 2.32 93.40 99.34 5.07 90.65 17.87 32.94 5.30 non-reactive Alberta "High Ca" 1.23 0.21 2.43 95.79 99.65 5.31 92.91 17.50 32.23 5.43- non-reactive
I
Alberta "High Mg" 1.61 0.12 7035 90.65 99.73 16.07 81.93 5.10 9042 16.40 very slight Outorop North 23.54 0.36 5.88 68.66 98.44 12.86 61.68 4.80 8.84 17.25 extremelyof Kingston reaotive Pittsburgh 5.69 0.46 22.14 70.78 99.07 48.42 44.50 0.92 1.69 52.11 extremely Ruarry, 0-24 ft reaotive セゥエエウ「オイァィ 35.76 0.94 20.63 34.65 45.11 0.42 81.62 iQuarry, 24-30 ft 91.98 10.17 0.23 slightly (green) reactive Pittsburgh 29.04 0.84 38.98 92.85 52.46 83.30 slightly Quarry, 30-36 ft 23099 10.51 0.20 0037 reactive 'Pittsburgh 23·16 49.35 50.65 21.86 0.43 0.80 69.85 moderately Quarry, 36-48 ft 22.59 0.71 95.81 reaotive pゥエエウ「オイセィ 19.60 0.48 16.20 63. 03 99·31 35.43 43.80 1.24 2.28 44.72 slightly Quarry, 8-60 ft reactive Frontenac 4.05 0.29 9.38 85.28 99.00 20.52 74.14 3.61 6.66 21.68 moderately Quarry, 0-12 reactive Frontenao 34.64 11.63 25.43 37.45 1.47 2.84 40 0
42
Quarry, 12-13 ft 0.58 51.25 98.10 slightly (green) reaotive Frontenao 7.99 0.42 11.25 78.27 97.93 24.61 64.91 2.64 4086 27.49 low セオ。イイケL 13-48 ft reactivityTABLE V
RESULTS OF QUICK CHEMICAL TEST FOR POTENTIAL ALKALI REACTIVITY, ASTM, c289-54T
Si1ioa release, Reduotion in
Aggregate millimole s per Alka1ini ty,
liter millimoles per liter Pittsburgh 0-24 ft, No. 1 19.1
*
160.3*
"
0...24 ft , No. 2 17.7 127.7 tt 24-30 ft, No. 1 8.3*
323.3*
"
24-30 ft, No. 2 19·5 338.7Ottawa Valley (Referenoe) 6.5
*
48.6 .::..Land-mined sand 10.4
*
53.6*
Arnprior sand (Referenoe) 9.7
*
70.3*
Repub1ioan River sand 98.3 oJ!- 63.1
*
(Referenoe)Republioan River sand 114.2 74.2
(Referenoe)
-Pittsburgh, 0-24 ft , on aoid insoluble residue
HVセ of total). Cal- 49.1 23.6
culated to original stone.
Figure 1. Measurement of volume change of reaotive limestone in alkaline solution.
0 セ
V
C7
0セ
. /
V
.,/./
i>
/
V
/
1/
-28 00 2 : 3 4 5 6 7 8Time, Month s (I mo
=
28 days)PER CENT VOLUME EXPANSION WITH TIME OF セ -
%
1\ CRUSHEDREACTIVE LIMESTONE IN SOLUTION I. (CORRECTED FOR VOLUME CHANGE OF INACTIVE REFERENCE STONE)
FIGURE 2 ·24
--
c= CD ·20 o セ CD a.-
c: ·16 0 en c: c a. )( 1JJ -12 CD E j 0 > ·08-
CD z ·04 5ll,'20'24--!Figure
3.
Limestone prisms in alkaline solution; end plates for length ohange measurement.·24
イMMMMセMM⦅N⦅⦅MM⦅⦅⦅イ⦅MM⦅イMM⦅イMM⦅⦅⦅⦅ケMMMイ⦅⦅MMN⦅⦅⦅MM⦅⦅N⦅⦅⦅MMセ10
length
i
II9
n
8
2
H.
solution I
0---0prism,
lr--i::J.V
II,
III
o---a
V
II II2
...
•
V
II,
II3
Sample cut from
•
• V
II,
water
prism
See note
oセセ]セ]セセZ[ZZZD]]K]]]MKMMKMMKMセL
o
- 12
QMMMMMMKMMMMMKMMMィGZZZMMMMKMMMMMKMMMMMMZZcセMMK⦅MM⦅⦅⦅⦅エ⦅MM⦅⦅⦅⦅⦅エMM⦅⦅⦅⦅⦅Q-16
3
4
5
6
7
Time, Months (I mo
=
28 days)
EXPANSION OF LIMESTONE
PRISMS IN
ALKALINE
SOLUTIONS, 6 -7
1BED,
PITTSBURGH
QUARRY
FIGURE
4
co
fA C C C. )( W _0 8
1 - - - t - - - Y - - - _ b 1 \ " " ' - - - t - -Not e:
H
=
Bedding planes perpendicular to
V=
II"parallel
IILegend:
·20
·0 4
QMMMMMMMMMャMKセセMMKMMMiMMMMMKMMMMMKMMMM⦅⦅⦅iMMMK⦅MM⦅⦅⦅⦅エ⦅MM⦅⦅⦅⦅⦅エMMN⦅⦅⦅⦅Q...
o Q) c:: ...J...
Q) c.--
-
c: Q) (J10
98
2
セcォゥョァ
I
I
Legend: I 0 - - 0 Solution I セI
A--b, II 2 0 - - 0 II3
. - - . WaterI
セMMN Solution 4 C)) c:. - - e
Control sample --oXAll samples with bedding planes
(J
セッ
/
parol led to lengthセ (J CD
I
I
:>0-
\ "-CI) セ セ c: .. IJV
<,No cracking CD-/
>< UJ/
/4
V
t
/0セM
..
MMセ
v
] I,
[ I L セ•
..
•
,
.---i I-- ,...o
o
-04·24
·28
3
4
5
6
7
Time, Months (I mo
=
28
day s)EXPANSION OF LIMESTONE PRISMS IN ALKALINE SOLUTIONS, 10
セ
-12
1 BED,PITTSBURGH QUARRY FIGURE 5
-
-
c:·20
CD (J セ CD C.-
·16
c: 0 fh c: 0'12
c. )( W セ 0 CD·08
c: ...J Bp, 2024 -3t
セ-
3
.->-
0 c CD セ セ5
0 CD-
C 0 en7
C' c en c CD9
セ 0 cII
Legend:
..
o Pittsburgh
•
• Frontenac
\
• Outcrop samples
\\
I
1\
0Alberta "High Mg
It•
Joliette
\
l:J.Alberta "High Ca"
-
-\
I
\
r-.
セOttawa Valley
"'<.
-
1
<,
'\
;... ... ...0,.'
....
1,." -IW('o
10
20
30
40
50
60
70
Dolomite, percent of total carbonate
80
90
100
RELATION
BETWEEN PERCENT DOLOMITE AND REACTIVITY FOR SOME
CARBONATE
ROCKS STUDIED
FIGURE
6
APPENDIX A
RESULTS OF MORTAR BAR TEST, ASTM C227-52T ON KINGSTON JOB AGGREGATES AND
APPENDIX A
RESULTS OF MORTAR BAR TEST, ASTM C227-$2T, ON KINGSTON JOB AGGREGATES AND REFERENCE MATERIALS
Legend
Cement:
B M E
=Belleville, high alkali
=
Montreal, high alkali=
Exshaw, low alkaliSand Grading:
ASTM
=
as required by test'Fine =as for original Kingston job sample.
Limestones: Pozzolan:
ov =ottawa Valley, rererence pO-24
=
Pittsburgh reactive P24-30=
Pittsburgh "green"Calirornia oalcined shale; 25% replaoe-ment or cereplaoe-ment by weight.
Flow and w/c: Arn Pit RR H
=
Arnprior, reference L=
Kingston land-mined=
Republican River, reactive roference=
required by present ASTM test=
former ASTM requirementAverage of two 1xlx5 inch bars
Aggregate Cement wlc Grading Caloined Expansion, peroent, at
Shale
3 mo. 6 mo. 9 mo. 12 mo.
"":
Limestones PO-24 B H-0.60 ASTM----
.016 .027 .036 ·°35 .060 ov B"
ASTM----
.000 -.003 -.002 -.003 .008 pO-24 M " ASTM ---- NPSセ .045 .048 .050 .073 ov M"
ASTM----
.00 .009 .009 Nooセ .019 pO-24 E n ASTM----
.016 .024 .022 .01 NPSセ i ov E n ASTM----
.007 .013 .008---
.01I
pO-24 B L-O.50 ASTM
----.
.020 .035 .037 .032 .063I
ov B II ASTIIi
---
... ...002 .007 .009---
.015I
pO-24 B, less 25% H-0.60 ASTM
----
.030 .042 .040 .043 .066OV
"
"
ASTM----
.005 .010 .005 .005 .020 PO-24"
"
ASTM + 25% .014
.016 .017 .013 .021 ov"
II ASTM"
.00 .004 .004---
.000 50% pO-24 ) B"
ASTM ---",. .002 .014 .017 .007 .042 50% P24-30) P24-3° B"
ASTM----
-.005 .006 .008--
..
.018 50% pO-24 ) B, less 25%"
ASTM + RUセ .016..
_-
.017 .015 .014 Uセ P24-30) P2 -30 II"
ASTM"
-.011 -.012---
-.008 ...004 セPit B H-O.51 ASTM
----
-.004 -.007 -.007 -.006 -.004Arn B " ASTM
----
-.004 ...004 .000 -.003 .002 RR B " ASTM----
.062 Nャャセ .107..
_-
.116 Pit M"
ASTM ""--- .0 .01 .017 .012 NPRセ Arn M"
ASTM ---. .009 .012 .009 .007 .01 RR M"
ASTM ---- .113 .146 .207 .221 .245 Pit E " ASTM----
.005 .012 .012---
.007 Arn E " AST14 ---- .005 .016 .012-
....
.012 RR E " ASTM----
.017 .062 .148 .186 .212 Pit B lMoNjjセ ASTM----
-.006 .003 .005---
-.001 Arn B " ASTM----
-.007 .001 .002---
.002 RR B"
ASTM----
.070 .084 .094 .083 .096Pit B H..O.51 Fine
----
.002 .006 .006 .002 .012Arn B
I'
Fine----
...ッッセ -.004 ...002---
.001RR B
"
Fine ---- .05 NPYセ .096 .097 .124Pit B, less 25%
"
ASTM ---- .003 .00 .005..
_-
.004Arn II
"
ASTM----
.000 .004.ool
---
.000RR
"
II ASTM----
.091 .121 .1) NQAセP .152Pit II II, ASTM
+ 25% .001 .002 .00) .001 .001
!l.rn
"
II' ASTM"
Nooセ .002 .003--
..
.010RR
"
"
ASTM"
.00 -.002---
-.001 .000-APPENDIX B
RESULTS OF THE CONROW TEST, ASTM c342-55T, ON KINGSTON JOB AGGREGATES AND