HAL Id: hal-02952415
https://hal.archives-ouvertes.fr/hal-02952415
Submitted on 2 Nov 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0
phthalates and bisphenol A mixture reduce
INSL3/RXFP2 signaling
Valentine Suteau, Claire Briet, Maÿlis Lebeault, Louis Gourdin, Daniel
Henrion, Patrice Rodien, Mathilde Munier
To cite this version:
Valentine Suteau, Claire Briet, Maÿlis Lebeault, Louis Gourdin, Daniel Henrion, et al.. Human
amniotic fluid-based exposure levels of phthalates and bisphenol A mixture reduce INSL3/RXFP2
signaling. Environment International, Elsevier, 2020, 138, pp.105585. �10.1016/j.envint.2020.105585�.
�hal-02952415�
Contents lists available atScienceDirect
Environment International
journal homepage:www.elsevier.com/locate/envint
Full length article
Human amniotic fluid-based exposure levels of phthalates and bisphenol A
mixture reduce INSL3/RXFP2 signaling
Valentine Suteau
a,b, Claire Briet
a,b,c, Maÿlis Lebeault
a,b, Louis Gourdin
a,c, Daniel Henrion
a,
Patrice Rodien
a,b,c, Mathilde Munier
a,b,c,⁎aUMR CNRS 6015, INSERM 1083, MITOVASC Institute, 3 rue Roger Amsler, 49000 Angers, France bDepartment of Endocrinology, University Hospital, 4 rue Larrey, 49933 Angers, France
cReference Center for Rare Diseases of Thyroid and Hormone Receptors, University Hospital, 4 rue Larrey, 49933 Angers, France
A R T I C L E I N F O Handling Editor: Shoji Nakayama Keywords:
Endocrine disrupting chemical Mixture
Phthalates bisphenol A INSL3/RXFP2 signaling
A B S T R A C T
Background: The presence of chemical pollutants in the environment can affect human health. Epidemiological
and in vivo experimental studies reveal reprotoxic effects (undescended testis) of phthalates (diethylhexyl phthalate (DEHP), dibutyl phthalate (DBP)) and bisphenol A (BPA), resulting in particular of a decrease in INSL3 (Insulin-Like 3 peptide) production. This hormone is essential for normal testis development and acts on a G protein-coupled receptor: RXFP2.
Objectives: The aim of this study was to evaluate the individual and combined impacts of DEHP, DBP, and BPA
on human RXFP2 (hRXFP2) activity.
Methods: We used HEK293 cells transiently transfected with hRXFP2 and receptor activity was analyzed by
measuring intracellular cAMP production. The mixture was established at concentrations reported in human amniotic fluid, for the three compounds.
Results: Individually, DEHP, DBP and BPA increased the response to INSL3 by 19.3 to 27.5%. This potentiating
effect was specific for RXFP2, because it was absent in the cells which did not express this receptor. On the other hand, and interestingly, the mixture of the three compounds reduced significantly the response to INSL3 by 12%, and the observed effects were opposite to those predicted, suggesting an antagonist effect.
Discussion-Conclusion: Taken together, our results demonstrate for the first time that a mixture of phthalates and
BPA present in human amniotic fluid disturbs the human RXFP2 function. Moreover, we demonstrate that mixture can produce potential antagonistic effects that are not displayed by the compounds, individually.
1. Introduction
Testis descent requires the INSL3/RXFP2 signaling. The deletion of INSL3 (Insulin-Like 3 peptide) or RXFP2 genes in male mice induces a bilateral cryptorchidism with testicles remaining in juxta-renal position (Gorlov et al., 2002; Nef and Parada, 1999; Overbeek et al., 2001; Zimmermann et al., 1999). This is confirmed by cryptorchidism in-duced in mice by treatment with an INSL3 antagonist in utero (Yuan et al., 2010). Moreover, loss of function mutations in the RXFP2 gene are also associated with cryptorchidism in men (Ayers et al., 2019; Ferlin et al., 2008). Cryptorchidism is one of the most common
congenital malformation, affecting up to 1.6% to 9% of male newborns (Sijstermans et al., 2008; Virtanen and Toppari, 2008; Wagner-Mahler et al., 2011) and is considered part of the Testicular Dysgenesis Syn-drome (TDS) (Wohlfahrt-Veje et al., 2009). In some countries, a rising incidence of cryptorchidism is observed, suggesting a link with the exposure of the pregnant women, and the fetus, to endocrine disrupting chemicals (EDC), and more specifically to bisphenol A (BPA) and phthalates (Juul et al., 2014).
BPA is one of the most massively produced EDC with over three million tons manufactured annually worldwide (Jiang et al., 2018). Because of its incomplete polymerization and its degradation at high
https://doi.org/10.1016/j.envint.2020.105585
Received 25 October 2019; Received in revised form 22 January 2020; Accepted 14 February 2020
Abbreviations: INSL3, Insulin-Like 3 peptide; TDS, Testicular Dysgenesis Syndrome; EDC, endocrine disrupting chemicals; BPA, bisphenol A; DEHP, diethylhexyl
phthalate; DBP, dibutyl phthalate; GPCR, G-protein coupled receptor; IBMX, Isobutyl-methylxanthine; FSK, forskolin; DMSO, dimethyl sulfoxide; PDE, phospho-diesterases
⁎Corresponding author at: Laboratoire MITOVASC, UMR CNRS 6015 - INSERM U1083, Bâtiment IRIS2, 3 rue Roger Amsler, 49100 Angers, France.
E-mail addresses:Valentine.Courant@chu-angers.fr(V. Suteau),claire.briet@chu-angers.fr(C. Briet),MaylisLebeault@chu-angers.fr(M. Lebeault),
gourdin.louis@gmail.com(L. Gourdin),daniel.henrion@univ-angers.fr(D. Henrion),PaRodien@chu-angers.fr(P. Rodien),
mathilde.munier@univ-angers.fr(M. Munier).
Available online 29 February 2020
0160-4120/ © 2020 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
temperature from polycarbonate, exposure to BPA is important in food containers (Burstyn et al., 2013; Carwile et al., 2009; Woodruff et al., 2011). Phthalates are classified in two groups: high molecular weight phthalates, such as diethylhexyl phthalate (DEHP), and low molecular weight phthalates, such as dibutyl phthalate (DBP) (Marie et al., 2015). Epidemiological and experimental data have linked exposure to phthalates or BPA and INSL3 expression and secretion. In humans, there is an inverse correlation between urinary or cord blood centrations of phthalates or BPA, respectively, and the plasmatic con-centration of INSL3 (Chang et al., 2017; Chevalier et al., 2015; Pan et al., 2015). Besides, it has been shown that maternal exposure to DEHP induces a reduction of INSL3 production mainly in boys (Araki et al., 2014a). Moreover, rats exposed in utero to phthalates have a testicular descent defect during the INSL3-dependent androgen-in-dependent phase (Mylchreest et al., 1999), associated with a decreased expression of the INSL3 gene (McKinnell et al., 2005; Wilson et al., 2004, 2007). This is confirmed in rat cellular models (Chauvigné et al., 2011; Li et al., 2012; Song et al., 2008). In human fetal testes, BPA decreases INSL3 expression (Ben Maamar et al., 2015; N’Tumba-Byn et al., 2012), whereas in human adult testes, it increases INSL3 pro-duction (Desdoits-Lethimonier et al., 2017). Studies have focused mainly on the effects of BPA and phthalates on the production and/or secretion of INSL3. However, the INSL3 receptor, RXFP2, a G-protein coupled receptor (GPCR), plays obviously a role in INSL3 signaling and consequently in the physiology and pathophysiology of the testis. We have previously shown that BPA decreased the activity of the FSH re-ceptor which is also a GPCR (Munier et al., 2016). In this context, we hypothesized that EDC could disturb RXFP2 function. RXFP2 is coupled to Gαs to activate adenylyl cyclase and increases cAMP production (Bathgate et al., 2013; Halls et al., 2015). It is expressed in the gu-bernaculum with a role in testicular descent but also in the testis and ovary with a role in maintaining gonadal function (Bathgate et al., 2013).
Due to their ubiquity, BPA, DEHP and DBP are present in many biological fluids such as pregnant women's urine, amniotic fluid and umbilical cord blood serum (Huang et al., 2014; Main et al., 2006; Philippat et al., 2013; Woodruff et al., 2011). Therefore, each in-dividual is chronically exposed to mixtures of environmental chemical factors resulting in toxicological interactions that cannot be predicted by reprotoxicological studies of single molecules. It is increasingly ac-knowledged that combinations of compounds that individually have no effect can produce significant effects when tested as mixtures re-presenting human-like exposure (Delfosse et al., 2015; Fini et al., 2017; Kortenkamp, 2014).
To understand the impact of BPA, DEHP and DBP, individually and in combination, on RXFP2 activity, we investigated theirs effects in HEK293 cells transiently transfected with human RXFP2 and responsive to INSL3. This cell-based system offers the possibility to specifically identify disruption of RXFP2 function, independently of other pathways that may be affected by BPA, DEHP and DBP. The mixtures used are based on concentrations of BPA, DEHP and DBP measured in human amniotic fluid as describedTable 1. The design of an environmentally-relevant mixture provides new data for in vitro testing of the combined effects. We showed that DEHP, DBP and BPA individually potentiate the cAMP response to INSL3, whereas the combination of these three che-micals decreases the response to the hormone.
2. Materials and methods
2.1. Reagents
Isobutyl-methylxanthine (IBMX) was provided by Sigma-Aldrich (St Louis, US), Forskoline by VWR (Radnor, US) and both were dissolved in DMSO at 1 M. INSL3 was purchased from Phoenix Pharmaceuticals (Burlingame, US) and dissolved in H20 at 10−4M concentration and
stored at −20 °C.
Plasmid: human RXFP2 cloned into a pcDNA3.1Zeo expression vector (pcDNA3.1Zeo-hRXFP2), was kindly provided by Ross A. D. Bathgate (University of Melbourne, Australia).
2.2. Chemicals mixture
Diethylhexylphthalate (DEHP) and bisphenol A (BPA) were pur-chased from Sigma-Aldrich (St Louis, US), dibutylphthalate (DBP) from Chem Service (West Chester, US). All were dissolved at 1 M in dimethyl sulfoxide (DMSO) (PAN Biotech, Aidenbach, Germany) and stored at −20 °C in glass vials.
For individual effects assessment, DEHP and DBP were tested be-tween 10−9 and 10−5 M and BPA was tested between 10−7 and
10−11M. For combined effects assessment, we reviewed in literature
the mean concentration reported in human amniotic fluid of each in-dividual chemical (Table 1). In binary and ternary mixtures, mixture 1× represented the mean concentration in human amniotic fluid and used concentrations ratios reflecting human exposure levels (i.e. 10−7 M for DEHP and DBP, 10−9 M for BPA), mixture 0.1×
re-presented the compounds concentration 10 times less concentrated than concentrations in human amniotic fluid, and 10× represented compounds concentration 10 times more concentrated than con-centrations in human amniotic fluid. Mixture solution stocks were prepared at 100,000× human amniotic fluid concentrations in DMSO and stored at −20 °C, in glass vials. For exposure studies in the cell model, mixtures were prepared at 10× in DMEM/F12. Then, serial dilutions in DMEM/F12 were realized to obtain mixtures 1× and 0.1×. Final DMSO concentration in each cultured well was 0.01% and was kept constant in all dilutions tested.
2.3. Cell culture and transient transfection
HEK 293 cell line was maintained in Dulbecco's modified Eagle's medium–F12 medium (DMEM/F12) (PAN Biotech, Aidenbach, Germany) containing 10% heat-inactivated fetal calf serum (FCS Biowest Nuaille, France), 2 mM L-glutamine (Lonza, Verviers, Belgium), 100 UI/ml penicillin and 100 μg/ml streptomycin (Lonza, Verviers, Belgium). Cells were routinely cultured in 75 cm2tissue
cul-ture flasks (Greiner Bio-one) at 37 °C with 5%CO2 and 95% humidity. HEK 293 cells were transiently transfected in 96-well plates (56,000 cells/well) with the 83.5 ng pcDNA3.1Zeo-hRXFP2 and 83.5 ng pGloSensorTM-22F cAMP plasmid (Promega) encoding an engineered cAMP-sensitive luciferase (Binkowski et al., 2011), using poly-ethylenimine (PEI) (Polyscience) (500 ng). The medium was changed 18 h after transfection with fresh medium containing 10% heat-in-activated charcoal-stripped fetal calf serum (CS FCS) (Life Technolo-gies, Carlsbad, US). The transfection efficiency was determined by FACS analysis and the mean was 30 ± 3% cells transfected.
2.4. cAMP assay
cAMP production was determined using the Promega GloSensor cAMP assay (Promega) as previously described (Munier et al., 2016). Briefly, transfected cells (pGloSensorTM-22F cAMP and pcDNA3.1Zeo-hRXFP2), seeded in white-walled, clear flat-bottomed 96 well plates (Greiner, Bio-One), were incubated for 2 h at room temperature with a substrate-containing medium (GloSensor™ cAMP assay, Promega) di-luted at 2% in DMEM/F12 containing 10% CS FCS. The luminescence was recorded immediately after injection of the drugs for 1 h on Flex-Station® 3 Multimode Plate Reader (Molecular Device) (1000 msec integration). Receptor activity was quantified by Area Under the Curve (AUC) with GraphPad Prism 6 (GraphPad Software, Inc). Concentration dose-response data were fitted using a three-parameter equation. For each analysis, at least three independent experiments were performed in triplicate.
2.5. Mixture effect prediction
Predictions for combined exposures were calculated by assuming additive effects (Groten et al., 2001). We used the following equation for the binary mixture: FCmix = (FC1 + FC2) − 1, or
FCmix= (FC1+ FC2+ FC3) − 2 for the ternary mixture, where FC1
and FC2and FC3are the fold changes for molecule 1 and 2 and 3 alone.
Observed values lower or higher than these predictions are determined as antagonistic or synergistic, respectively. A Holm-Sidak’s multiple comparison test was used to determine if the observed effects were significantly non-additive. If the observed values are not significantly above or below the predicted values, an additive effect is recorded. If the observed values are significantly above the predicted values, a sy-nergistic effect is recorded. If the observed values are significantly below the predicted values, an antagonistic effect is recorded. 2.6. Statistical analysis
Data were analyzed using GraphPad PRISM 6 (GraphPad Software, Inc., San Diego, CA) and represented mean ± SEM of at least 3 in-dependent experiments performed in triplicate for each condition. Data are expressed as a percentage of the solvent or positive controls, where applicable. Differences between cells with and without hRXFP2 were analyzed using non-parametric Mann-Whitney test. Differences were considered significant at p < 0.05(*), p < 0.01(**), p < 0.001(***) and p < 0.0001(****).
3. Results
3.1. Individual effects of DEHP, DBP and BPA on INSL3-dependent cAMP production
To characterize the mechanisms of action of DEHP, DBP and BPA on RXFP2 activity, we used HEK293 cells transiently transfected with plasmid encoding for human RXFP2 (hRXFP2), and named HEK293-RXFP2. RXFP2 activity was evaluated by measuring intracellular cAMP production using a live cell bioluminescence approach (Glosensor technology). This technology is the fusion of firefly luciferase (Photinus pyralis) protein with cAMP-binding domain of protein kinase A, which, upon cAMP binding emits light in the presence of substrate (D-luci-ferin). TheFig. S1shows the validation of the cellular model. We first verified that compounds did not impact the cell viability using MTT assay (Figure supplementary S2A-C). According to the dose-response curve for INSL3 on hRXFP2, we examined the effects of molecules alone on the response to two concentrations of INSL3: 10−9and 10−6 M,
corresponding to the EC50and the Cmax, respectively (Fig. S1). Each
compound was tested at five concentrations, including the one reported in amniotic fluid, i.e.: 10−7M for DEHP and DBP, and 10−9M for BPA
(Chen et al., 2011; Edlow et al., 2012; Fini et al., 2017; Huang et al., 2009; Jensen et al., 2012; Philippat et al., 2013; Shekhar et al., 2017). At concentrations ranging from 10−9to 10−7M DEHP enhanced the
cAMP response to INSL3 Cmax, up to 128 ± 14% at 10−8M, without
impact on the response to EC50(Fig. 1A and B). While DBP had no effect
on the response to INSL3 Cmax, it increased the response to EC50, in a
dose-dependent manner, up to 137 ± 16% at 10−6M (Fig. 1D and E).
BPA induced a minimal decrease (4 ± 2%) of the maximal response to INSL3 (Fig. 1G), but a 122 ± 9% increase of the response to EC50, at
10−10M (Fig. 1H). No significant effect on RXFP2 basal activity was
observed (Fig. 1C, F and I). In summary, the three compounds tested individually increase the cAMP response to INSL3.
3.2. Individual effects of DEHP, DBP and BPA on the downstream effectors of RXFP2
HEK293 do not endogenously express RXFP2 (Atwood et al., 2011). To examine the putative impact of compounds on the downstream
Table 1 Composition of the mixtures containing DEHP, DBP and BPA based on human amniotic fluid levels. Molecules Plasma concentration in pregnant women (µg/l) Urinary Concentration in pregnant women (µg/l) Concentration in cord blood (µg/l) Concentration in amniotic fluid (µg/l) Mean concentration (µg/ l) (M) a Final concentration in mixtures (M) b 0.1× 1× 10× Dibutylphthalate (DBP) Primary metabolite Monobutylphthalate (MBP) MBP: 0.94 ( Minatoya et al., 2018 ) DBP: 18.83 ( Woodruff et al., 2011 ); MBP: 11 ( Swan 2008 ); 58.1 ( Philippat et al., 2013 ); 78.8 ( Huang et al., 2009 ) DBP: 68,14 ( Huang et al., 2014 );; 6 ( Zhang et al., 2009 )47,6 (DBP) – 2.9 (MBP) ( Brucker-Davis et al., 2008 ) MBP: 83.5 ( Huang et al., 2009 ); 4.23 ( Li and Sun, 2018 ) DBP: 29.71 (1.07 10 −7 ) MBP: 24.25 (1.09 10 −7) 10 −8 10 −7 10 −6 Diethylphthalate (DEHP) Primary metabolite Monoethylphthalate (MEHP) MEHP: 10.4 ( Araki et al., 2014a ), 0.34 ( Caserta et al., 2018 ), 3.24 ( Li and Sun, 2018 ); 3.07 ( Minatoya et al., 2018 ) DEHP: 10,8 ( Philippat et al., 2013 ); 226,53 ( Woodruff et al., 2011 ); MEHP: 2 ( Swan 2008 ); 13 ( Chevrier et al., 2012 ); 19 ( Lin et al., 2011 ); 26 ( Huang et al., 2009 ); 5 ( Suzuki et al., 2012 ); 21.2 ( Li and Sun, 2018 ) DEHP: 187,16( Huang et al., 2014 )); 119 (DEHP) – 520 (MEHP) ( Latini, 2003 ); MEHP: 0.36 ( Caserta et al., 2018 ), 12.9 ( Li and Sun, 2018 ) MEHP: 0.86 ( Jensen et al 2012 ); 23 ( Huang et al., 2009 ); 1.7–2.6; 0.83 ( Li and Sun, 2018 ); 9.62 ( Jensen et al., 2015 ); 2.4 ( Wittassek et al., 2009 ) DEHP: 96.78 (2.48 10 −7 ) MEHP: 39.45 (1.42 10 −7) 10 −8 10 −7 10 −6 Bisphenol A (BPA) 0,49 ( Burstyn et al., 2013 ); 7.43 ( Shekhar et al., 2017 ) 1.63 ( Woodruff et al., 2011 ); 1,36 ( Aris 2014 ); 0.4 ( Jiménez-Díaz et al., 2016 ) 1,26 ( Fénichel et al., 2012 ); 0,9 ( Brucker-Davis et al., 2011 ); 1,23 ( Aris 2014 ); 16 ( Gerona et al., 2013 ); 0.47 ( Chen et al., 2011 ); 7.75 ( Shekhar et al., 2017 ); placenta: 3,4 ( Fernández et al., 2016 ) 1,70 (7.43 10 −9 ) 10 −10 10 −9 10 −8 aMean human amniotic fluid concentrations of DEHP, DBP and BPA based on a literature review, and converted to M in brackets. bConcentrations of the various compounds in the different mixtures.
effectors of the RXFP2, we first measured the cAMP production in na-tive HEK293 after incubation with DEHP, DBP and BPA and we did not observe any effect (Fig. 2A-C). Next, we tested the effect of the most potent concentration of DEHP (10−8 M), DBP (10−6 M) and BPA
(10−10M) on forskolin-induced cAMP-accumulation in HEK293-RXFP2
and HEK293 cells (Fig. 2D-F). Forskolin is a specific activator of ade-nylate cyclase, the enzyme responsible for the synthesis of cAMP. In presence of DEHP, there was an increase of the response to forskolin in HEK293-RXFP2 cells that reached 126 ± 5% of the control value. This effect was not observed in native HEK293 cells (Fig. 2D). Similarly, BPA potentiated up to 115 ± 5% the cAMP production stimulated by for-skolin in HEK293-RXFP2, but not in native cells (Fig. 2F). DBP did not modify the response to forskolin (Fig. 2E). Finally, the effects of the three compounds on phosphodiesterases (PDE) were analyzed. The PDE are the enzymes responsible for the degradation of cAMP in the cells. There was a further increase of the INSL3-stimulated cAMP production by DEHP, DBP or BPA in the presence of IBMX, a non-selective PDE
inhibitor (Fig. 2G-I). Interestingly, in presence of IBMX, the RXFP2 basal activity was also increased by DEHP, DBP and BPA up to 117 ± 4%, 121 ± 3%, 121 ± 5%, respectively (Fig. 2J-K), revealing an agonistic effect. Altogether, these findings indicate that the effects on downstream effectors are mediated by RXFP2.
3.3. Combined effects of DEHP, DBP and BPA on INSL3-dependent cAMP production
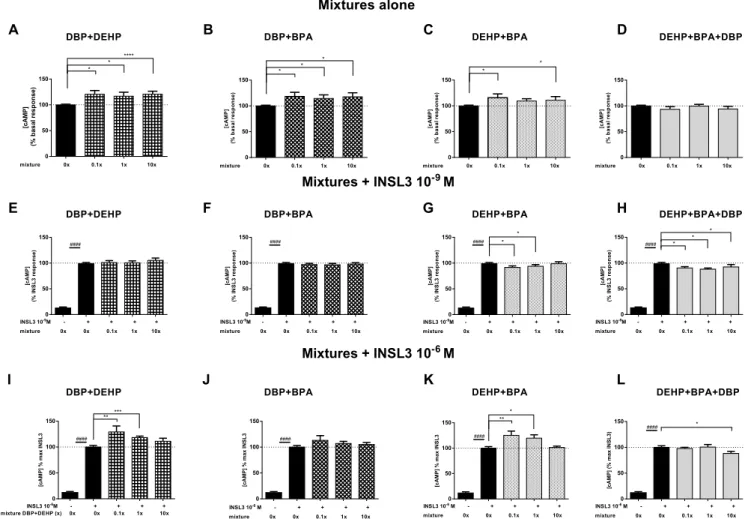
We realized binary and a ternary mixtures of the three compounds. We tested the mixture at 0.1×, 1×, 10× concentrations, where 1× represents the concentrations of individual chemicals reported in human amniotic fluid (see Material and Methods section 2.2). The dif-ferent mixtures did not impact the cell viability (Figure supplementary S2 D-F). For DBP + DEHP mixture, an increase in RXFP2 basal activity up to 120.7 ± 5.7% for 1× (Fig. 3A) and in response to CmaxINSL3 up
to 129.2 ± 11.5% for 0.1× was observed (Fig. 3I). No effect was
Fig. 1. Effects of individual chemicals on INSL3-stimulated cAMP production. HEK 293 were transiently transfected with hRXFP2 and then were incubated with
increasing concentrations of DEHP (A-C), DBP (D-F) or BPA (G-I) and stimulated with INSL3 10−6M (A, D, G) or 10−9M (B, E, H), or without INSL3 (C, F, I). The
cAMP concentration measured in the absence of compounds was arbitrarily set at 100%. Data represented means ± SEM of at least four independent experiments performed in triplicate. Triangles indicate average level of chemicals found in human amniotic fluid. The difference between exposed and unexposed cells was evaluated using non-parametric Mann Whitney test (*, p < 0.05, **, p < 0.01, ***, p < 0,001) as well as the difference between medium with or without INSL3 (####, p < 0,0001).
observed on response to INSL3 EC50(Fig. 3E). DBP + BPA mixture
enhanced to 118.6 ± 7.8% the basal activity with no effect on re-sponse to INSL3 (Fig. 3B, F and J). The DEHP + BPA mixture increased the RXFP2 basal activity and the response to Cmax INSL3 to
115.8 ± 7.4% and 125.3 ± 8.4%, respectively (Fig. 3C and K).
Interestingly, this mixture decreased slightly the response to INSL3 EC50
(Fig. 3G). When the three compounds were mixed, no effect was ob-served on basal activity (Fig. 3D). However, a 12% decrease of the response to EC50and CmaxINSL3 was observed with the 1× and 10×
mixtures, respectively (Fig. 3H and L). All results are summarized in the
heat map,Fig. 4.
Depending on their mode of action, the toxicity of the compounds within the mixture is classified into two groups: (1) mixtures that have no interaction between molecules and (2) mixtures that show interac-tion between molecules (Hernández et al., 2017). Effects of constituents of group 1 mixtures are additive, and the impact of the mixture can be predicted from the individual effects of the compounds. In group 2, the experimentally observed effects diverge from the simple addition of individual effects: if higher than predicted by calculation the
compounds have synergistic effects; if lower than predicted, they have antagonistic effects. The expected response amplitude was calculated, according to putative additive effects of individual compounds, and compared to observed responses using the model described in Materials and Methods section 2.5.Fig. 5shows that within the ternary mixture, there was an antagonistic interaction of BPA, DEHP, DBP molecules between them (the experimentally observed effects were lower than predicted ones).
Fig. 2. Effects of individual chemicals on cAMP production in HEK 293, on forskolin (FSK)-stimulated cAMP production and on inhibition of phosphodiesterase
(PDE) by IBMX. (A-C) Natives HEK 293 were incubated for 60 min with increasing concentrations of DEHP, DBP and BPA. Triangles indicate average level of chemicals found in human amniotic fluid. The basal cAMP level in the absence of compounds was arbitrarily set at 100% (means ± SEM of three independent experiments performed in triplicate) and differences were evaluated using the non-parametric Mann–Whitney test. (D-F) HEK293 and HEK293-RXFP2 cells were stimulated with 10–5M forskolin (an adenylate cyclase (AC) agonist) and DEHP (from 10−9to 10−7M), DBP (from 10−9to 10−5M) and BPA (from 10−10to 0−7M)
(means ± SEM of three to five independent experiments performed in triplicate). The cAMP production in the presence of forskolin alone was arbitrarily set at 100% for each cell type. Triangle indicate average level of chemicals found in human amniotic fluid. The differences between exposed and unexposed cells were evaluated using non-parametric Mann–Whitney test (*, p < 0.05). (G-I) HEK293-RXFP2 cells were incubated with 10−3M IBMX (a non-specific PDE inhibitor) for 2 hs and
then stimulated with INSL3 10−6M (G) or 10−9M (H, I) and with or without increasing concentrations of DEHP, DBP and BPA. The cAMP production in the presence
of INSL3 with IBMX alone was arbitrarily set at 100%. Data represented means ± SEM of three independent experiments performed in triplicate. Triangle indicate average level of chemicals found in human amniotic fluid. Difference between exposed and unexposed cells were analyzed with non-parametric Mann-Whitney test (*, p < 0.05, **, p < 0.01, ***, p < 0,001) as well as difference between medium alone and INSL3 alone and medium with or without IBMX (###, p < 0, 001, ####, p < 0,0001). (J-L) After incubated for 2 hs with 10−3M IBMX, HEK 293-RXFP2 cells were exposed to increasing concentrations of DHEP, DBP or BPA. Data
represented mean ± SEM of at least three independent experiments performed in triplicate. Triangle indicate average level of chemicals found in human amniotic fluid. Difference between exposed and unexposed cells was analyzed with non-parametric Mann- Whitney test (*, p < 0.05, **, p < 0.01, ***, p < 0,001) and between medium with or without 10−3M IBMX (####, p < 0,0001).
Fig. 3. Effects of chemicals mixture on INSL3-stimulated cAMP production. HEK 293 were transiently transfected with hRXFP2 and then were incubated with
increasing concentrations (0,1×, 1×, 10×, where 1× represented the mean concentration in human amniotic fluid) of DBP + DEHP (A, E, I), DBP + BPA (B, F, J), DEHP + BPA (C, G, K) or DEHP + BPA + DBP (D, H, L) and stimulated without (A-D) or with INSL3 10−9M (E-H) or 10−6M (I-L). The cAMP concentration
measured in the absence of compounds was arbitrarily set at 100%. Data represented means ± SEM of at least three independent experiments performed in triplicate. The difference between exposed and unexposed cells was evaluated using non-parametric Mann Whitney test (*, p < 0.05, **, p < 0.01, ***, p < 0,001) as well as the difference between medium with or without INSL3 (####, p < 0,0001).
4. Discussion
Phthalates and BPA are ubiquitous in the environment and humans, including pregnant women (Woodruff et al., 2011), are largely exposed to them. While epidemiological or experimental studies have focused on the impact of these endocrine disruptors on INSL3 production (Bay and Anand-Ivell, 2014), we here studied, for the first time, their impacts on the INSL3 human receptor, hRXFP2 both individually and in com-bination. Noteworthy, our experiments were performed with con-centrations of disruptors reported in vivo in amniotic fluid (Chen et al., 2011; Edlow et al., 2012; Fini et al., 2017; Huang et al., 2009; Jensen et al., 2012; Philippat et al., 2013; Shekhar et al., 2017).
To unambiguously study the effect on RXFP2, independently of other cellular targets, the receptor was isolated from its native en-vironment and analyzed in a heterologous expression system (HEK293 cells transfected with the human RXFP2). We found that DEHP, DBP and BPA potentiate the production of cAMP stimulated by INSL3. Several facts argue for the specific impact of those compounds on RXFP2. First, their effects required the presence of the receptor because there was no increase in cAMP production in untransfected cells. Second, the response to INSL3 was also increased when PDEs were inhibited by IBMX, while the forskolin-induced cAMP production was increased only in the presence of RXFP2, ruling out a direct effect on PDE or adenylyl cyclase, respectively. This effect on the response to forskolin suggests an INSL3-independent effect on RXFP2. Indeed, DEHP, DBP and BPA rose the basal cAMP accumulation in HEK293-RXFP2 cells, in presence of IBMX, which appears to be able to unmask a mild agonistic activity. Third, the traditional targets of DEHP, DBP and BPA, the oestrogen receptor α (ERα) (Lee et al., 2012; Legler et al., 2002) or GPER (Thomas and Dong, 2006) are not expressed by HEK293 cells neither is the ERβ (Thomas et al., 2005). Fourth, the reported positive or negative effects of DEHP, DBP and BPA on cell viability (Chen et al., 2013; Meruvu et al., 2016) even at lower concentrations (≤10−6M) (Kim et al., 2017; Sauer et al., 2017) were not observed
here. However, MTT assay was performed earlier in our study (1 h, as cAMP assay, vs 24–72 h), evaluating cytotoxicity rather than cell
growth.
Among the compounds studied here the minimal effective con-centration (ECmin) varied from 10−11 M for BPA (8.8% increase), to
10−9M for DEHP (25.7% increase) or 10−8M for DBP (15.3% increase)
(Fig. 1). BPA is the most potent compound in the modulation of the response to INSL3 and noteworthy, the minimal effective concentra-tions found to be active in the present work, remain below the reported concentrations in human amniotic fluids (nanomolar range). In term of the potency to induce cAMP production independently of INSL3, DBP and BPA (ECmin = 10−9 M) were more potent than DEHP
(ECmin= 10−8M) (Fig. 2J-L). For comparison, the ECminof INSL3, to
produce cAMP, was 10−10M (Fig. S1). Although, 10 to 100 times less
potent than INSL3, we can therefore conclude that BPA and phthalates are hormonally active compounds. The effects of phthalates and BPA were tested on two distinct concentrations of INSL3: 10−9and 10−6M
corresponding to EC50and Cmax, respectively (Fig. S1). This choice
al-lows us to evaluate the consequence of each combination of molecules tested on the potency (EC50) and efficacy (Cmax) of INSL3. Thus, the
conditions DBP or BPA alone increase the potency of INSL3, whereas DEHP + BPA and DEHP + DBP + BPA reduce it. Moreover, DEHP alone, DEHP + DBP and DEHP + BPA potentiate the efficacy of INSL3, while BPA alone and DEHP + DBP + BPA decrease it. Our results il-lustrate that the allosteric modulations of INSL3 by the different mix-tures (1, 2 or 3 compounds) tested are complex. DEHP, DBP and BPA seem to act as RXFP2 allosteric modulators. Currently, there are no data on allosteric sites in RXFP2. However, the structural similarity between RXFP1 and RXFP2 (80% sequence similarity in transmembrane do-main) suggests that small hydrophobic molecules (like phthalates and BPA) could use the same binding sites as ML290 in RXFP1. ML290 is a synthetic non-peptide low molecular weight agonist of RXFP1 (Xiao et al., 2013). The structural model of RXFP2 and molecular docking of ML290 showed that the small molecule interacts with key hydrophobic residues in helix 3 and helix 7 in transmembrane domain (Hu et al., 2016). DEHP, DBP or BPA are hydrophobic molecules and could bind in the same putative binding site in RXFP2. When one or two compounds may bind in this site, this could lead to a stabilization of the active state
Fig. 4. Heat map of basal activity and INSL3 response after mixture exposure of HEK293-hRXFP2. Each value was colored according to its percentage value. The
difference between exposed and unexposed cells was evaluated using non-parametric Mann Whitney test (*, p < 0.05, **, p < 0.01, ***, p < 0,001). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
of the receptor driving an increase of agonist activity (Thal et al., 2018). But, the binding of three compounds (ternary mixture) likely leads to a steric hindrance that may prevent the conformational changes (rotation and/or translation of helix) necessary for the activation of RXFP2 and probably stabilize an inactive state (Thal et al., 2018). Besides, one of the structural particularities of RXFP2 is the presence of an N-terminal LDLa module that is essential for signalling. This module interacts with the transmembrane domain in a manner analogous to a tethered ligand (Bruell et al., 2013; Kong et al., 2014). The binding of the ternary mixture may again prevent the interaction of the LDLa module with the transmembrane domain and thus the activation of the receptor due to steric hindrance.
In our experiments, BPA potentiated the response to INSL3 response at concentrations in the same range (10−10M) of those (10−9M)
re-ported for BPA to increase the production of INSL3 in human adult testicular explants (Desdoits-Lethimonier et al., 2017). Thus, the global INSL3/RXFP2 signaling can be potentiated by BPA. Conversely, the positive effect of phthalates on the response to INSL3 we observe here, is opposite to the negative correlation reported between exposure to them and INSL3 concentrations in the cord blood (Araki et al., 2014a; Chang et al., 2015, 2017; Pan et al., 2015). In addition, 10−5 and
10−4M DEHP had no effect on the production of INSL3 by human adult
testicular explants (Desdoits-Lethimonier et al., 2012). It therefore ap-pears that phthalates act differently on INSL3 production and INSL3
Fig. 5. Predicted and observed fold change (FC) values for basal activity and INSL3 responses after mixture exposure of HEK293-RXFP2. The predicted values were
calculated assuming additive effects. The values were plotted on Log2-scale such that Log2(FC) = 0 corresponds to no effect. The difference between predicted and
effects.
A key issue when addressing the impact of compound cocktails is chemical interactions, which could modify both the amplitude and the direction of the action of the different compounds (Hernández et al., 2017). The effect additivity model was used to evaluate the type of interaction between the molecules within the mixture (additive, sy-nergism, antagonism). We assume the basic hypothesis of additivity and we analyze statistically if the observed effects (FC observed) deviate from the additive prediction (FC prediction). The combination of DEHP + DBP resulted in an increase in basal receptor activity and in response to INSL3 Cmax(Fig. 3). Although not significant, the effect of
DEHP + DBP does not appear to follow an effect additivity model on basal RXFP2 activity (p = 0.059 for 0.1×; p = 0.059 for 1×; p = 0.055 for 10×), since FC tended to be higher than FC observed (Fig. 5). In contrast, the effect of DEHP + DBP on INSL3 Cmaxresponse
followed an additive manner (FC predicted = FC observed) (Fig. 5). This corroborates previous studies showing that a binary DEHP + DBP or more complex phthalates mixtures acted in a cumulative additive fashion to induce reproductive malformations (including undescended testis and Insl3 expression) in male rats (Howdeshell et al., 2007, 2015). The mixture DBP + BPA caused only an increase in basal receptor activity (Fig. 3). Although the DBP + BPA mixture significantly in-creases basal receptor activity, the combination of effects does not appear to follow an additive model, as the FC observed tended to be less than FC predicted (p = 0.086 for all dilutions) (Fig. 5). While experi-mentally, DBP + BPA mixture has no effect on the response to INSL3 (EC50and Cmax) (Fig. 3), according to the predictive effect additivity
model, DBP + BPA mixture should have potentiated the response to INSL3 (Fig. 5). Each compound, BPA and DBP, appears to antagonize the potentiating effects of the other compound. DEHP + BPA rises the basal RXFP2 activity and potentiates the INSL3 Cmaxresponse (Fig. 3).
The comparison between FC observed and FC predicted shows that there is no difference between the two values (Fig. 5). Therefore, BPA and DEHP follow an effect additivity model regarding the basal and Cmaxresponse. In contrast, DEHP + BPA mixture decrease the INSL3
EC50 response (Fig. 3). Although not significant, the effect of
DEHP + BPA does not look to follow an effect additivity model on INSL3 EC50 response (p = 0.087 for 0.1×; p = 0.095 for 1×;
p = 0.095 for 10×), since FC predicted tended to be higher than FC observed (Fig. 5). Finally, the ternary mixture has no effect experi-mentally on basal activity and reduced the response to INSL3 (EC50and
Cmax) (Fig. 3). The comparison of FC observed and FC predicted shows
that the combination of the effects of DBP, DEHP and BPA is not ad-ditive on basal RXFP2 activity and INSL3 EC50 response (p < 0.05) (Fig. 5). With regard to INSL3 Cmaxresponse, although not significant
(p = 0.070 for 0.1×; p = 0.082 for 1×; p = 0.082 for 10×), the effect additivity model seems to be not applicable (Fig. 5). Thus, a significant interaction between the disrupting effect of the compounds results in an inhibition of the INSL3 action and a decrease in RXFP2 basal activity. The effects observed with compounds tested individually may be re-levant in vivo since they were studied at concentrations close to those reported for biological liquids. However, humans are exposed to mix-tures of multiple chemical molecules. Analyses focusing on a single compound may underestimate the real risk of exposure to multiple molecules (Kortenkamp, 2014). Our study supports this hypothesis since we demonstrated that while BPA, DEHP and DBP alone po-tentiated the response to INSL3, the cocktail of the three compounds reduced it. The fact that the association of compounds with partial agonistic effects could result in a weak inhibiting mixture has also been reported by others (Howard and Webster, 2009; Yu et al., 2019).
During the first trimester of pregnancy, INSL3/RXFP2 signaling plays a key role in the development and growth of the male re-productive tract, especially by regulating the first phase of testicular descent (Mäkelä et al., 2019). Exposure to phthalates or BPA has been associated with the development of cryptorchidism in animal models and humans (Fernández et al., 2016; Fisher et al., 2003). Our results
show that ternary mixture reduced the basal activity of RXFP2 sug-gesting that this receptor may have a constitutive activity. Whether the disruption of this basal activity could be deleterious in vivo is unknown. The effect of the ternary mixture was stronger on the response to EC50
(10−9M) than to C
max(10−6M) INSL3. Indeed, the cord blood INSL3
concentration ranges between 50 and 100 pg/ml (9 to 18 10−12M)
(Ivell et al., 2017). A lower response of RXFP2 to low concentrations of INSL3, may contribute to the cryptorchidism observed in male new-borns exposed in utero to BPA and/or phthalates, along with the known anti-androgenic effects of the compounds.
5. Conclusion
In conclusion, we identify the INSL3 receptor as a target of endo-crine disruptors which could participate in the occurrence of cryp-torchidism. The study elucidates a new biological pathway which may be used to determine a new mechanism of action for potential EDC. In addition, the mixture of several disruptors has effects that differ from the ones of each disruptor alone. This further stresses the mandatory assessment of disruptors in cocktails further to their individual eva-luation.
CRediT authorship contribution statement
Valentine Suteau: Methodology, Formal analysis, Investigation,
Writing - original draft. Claire Briet: Visualization. Maÿlis Lebeault: Investigation. Louis Gourdin: Investigation. Daniel Henrion: Resources, Writing - review & editing. Patrice Rodien: Conceptualization, Resources, Writing - review & editing. Mathilde
Munier: Conceptualization, Formal analysis, Writing - original draft,
Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influ-ence the work reported in this paper.
Acknowledgements
V.S. was supported by funding from La Société Française d’Endocrinologie.
We thank Ross A.D. Bathgate (University of Melbourne, Australia) for generously giving us the human RXFP2 plasmid.
Appendix A. Supplementary material
Supplementary data to this article can be found online athttps:// doi.org/10.1016/j.envint.2020.105585.
References
Araki, A., Mitsui, T., Miyashita, C., Nakajima, T., Naito, H., Ito, S., et al., 2014a. Association between maternal exposure to di(2-ethylhexyl) phthalate and re-productive hormone levels in fetal blood: the Hokkaido study on environment and
children’s health. PLoS ONE 9, e109039.https://doi.org/10.1371/journal.pone.
0109039.
Aris, A., 2014. Estimation of bisphenol A (BPA) concentrations in pregnant women, fe-tuses and nonpregnant women in Eastern Townships of Canada. Reprod. Toxicol. 45,
8–13.https://doi.org/10.1016/j.reprotox.2013.12.006.
Atwood, B.K., Lopez, J., Wager-Miller, J., Mackie, K., Straiker, A., 2011. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell
lines as revealed by microarray analysis. BMC Genomics 12, 14.https://doi.org/10.
1186/1471-2164-12-14.
Ayers, K., Kumar, R., Robevska, G., Bruell, S., Bell, K., Malik, M.A., et al., 2019. Familial bilateral cryptorchidism is caused by recessive variants in RXFP2. J. Med. Genet.
https://doi.org/10.1136/jmedgenet-2019-106203.
Bathgate, R.A.D., Halls, M.L., van der Westhuizen, E.T., Callander, G.E., Kocan, M., Summers, R.J., 2013. Relaxin Family Peptides and Their Receptors. Physiol. Rev. 93,
Bay, K., Anand-Ivell, R., 2014. Human testicular insulin-like factor 3 and endocrine
dis-rupters. Vitam. Horm. 94, 327–348.https://doi.org/10.1016/B978-0-12-800095-3.
00012-2.
Ben Maamar, M., Lesné, L., Desdoits-Lethimonier, C., Coiffec, I., Lassurguère, J., Lavoué, V., et al., 2015. An investigation of the endocrine-disruptive effects of bisphenol a in
human and rat fetal testes. PLoS ONE 10, e0117226.https://doi.org/10.1371/
journal.pone.0117226.
Binkowski, B.F., Fan, F., Wood, K.V., 2011. Luminescent biosensors for real-time
mon-itoring of intracellular cAMP. Methods Mol. Biol. Clifton NJ 756, 263–271.https://
doi.org/10.1007/978-1-61779-160-4_14.
Brucker-Davis, F., Ferrari, P., Boda-Buccino, M., Wagner-Mahler, K., Pacini, P., Gal, J., et al., 2011. Cord Blood Thyroid Tests in Boys Born With and Without
Cryptorchidism: Correlations with Birth Parameters and In Utero Xenobiotics
Exposure. Thyroid 21, 1133–1141.https://doi.org/10.1089/thy.2010.0459.
Brucker-Davis, F., Wagner-Mahler, K., Delattre, I., Ducot, B., Ferrari, P., Bongain, A., et al., 2008. Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Hum.
Reprod. 23, 1708–1718.https://doi.org/10.1093/humrep/den186.
Bruell, S., Kong, R.C.K., Petrie, E.J., Hoare, B., Wade, J.D., Scott, D.J., et al., 2013. Chimeric RXFP1 and RXFP2 Receptors Highlight the Similar Mechanism of Activation Utilizing Their N-Terminal Low-Density Lipoprotein Class A Modules. Front.
Endocrinol. 4, 171.https://doi.org/10.3389/fendo.2013.00171.
Burstyn, I., Martin, J.W., Beesoon, S., Bamforth, F., Li, Q., Yasui, Y., et al., 2013. Maternal Exposure to Bisphenol-A and Fetal Growth Restriction: A Case-Referent Study. Int. J.
Environ. Res. Public Health 10, 7001.https://doi.org/10.3390/ijerph10127001.
Carwile, J.L., Luu, H.T., Bassett, L.S., Driscoll, D.A., Yuan, C., Chang, J.Y., et al., 2009. Polycarbonate Bottle Use and Urinary Bisphenol A Concentrations. Environ. Health
Perspect. 117, 1368–1372.https://doi.org/10.1289/ehp.0900604.
Caserta, D., Pegoraro, S., Mallozzi, M., Benedetto, L.D., Colicino, E., Lionetto, L., et al., 2018. Maternal exposure to endocrine disruptors and placental transmission: a pilot
study. Gynecol. Endocrinol. 34, 1001–1004.https://doi.org/10.1080/09513590.
2018.1473362.
Chang, W.-H., Li, S.-S., Wu, M.-H., Pan, H.-A., Lee, C.-C., 2015. Phthalates might interfere with testicular function by reducing testosterone and insulin-like factor 3 levels.
Hum. Reprod. Oxf. Engl. 30, 2658–2670.https://doi.org/10.1093/humrep/dev225.
Chang, W.-H., Wu, M.-H., Pan, H.-A., Guo, P.-L., Lee, C.-C., 2017. Semen quality and insulin-like factor 3: Associations with urinary and seminal levels of phthalate
me-tabolites in adult males. Chemosphere 173, 594–602.https://doi.org/10.1016/j.
chemosphere.2017.01.056.
Chauvigné, F., Plummer, S., Lesné, L., Cravedi, J.-P., Dejucq-Rainsford, N., Fostier, A., et al., 2011. Mono-(2-ethylhexyl) phthalate directly alters the expression of Leydig cell genes and CYP17 lyase activity in cultured rat fetal testis. PLoS ONE 6, e27172.
https://doi.org/10.1371/journal.pone.0027172.
Chen, M., Edlow, A.G., Lin, T., Smith, N.A., McElrath, T.F., Lu, C., 2011. Determination of bisphenol-A levels in human amniotic fluid samples by liquid chromatography
cou-pled with mass spectrometry. J. Sep. Sci. 34, 1648.https://doi.org/10.1002/jssc.
201100152.
Chen, X., Zhou, Q., Leng, L., Chen, X., Sun, Z., Tang, N., 2013. Effects of di(n-butyl) and monobutyl phthalate on steroidogenesis pathways in the murine Leydig tumor cell
line MLTC-1. Environ. Toxicol. Pharmacol. 36, 332–338.https://doi.org/10.1016/j.
etap.2013.04.013.
Chevalier, N., Brucker-Davis, F., Lahlou, N., Coquillard, P., Pugeat, M., Pacini, P., et al., 2015. A negative correlation between insulin-like peptide 3 and bisphenol A in human cord blood suggests an effect of endocrine disruptors on testicular descent
during fetal development. Hum. Reprod. Oxf. Engl. 30, 447–453.https://doi.org/10.
1093/humrep/deu340.
Chevrier, C., Petit, C., Philippat, C., Mortamais, M., Slama, R., Rouget, F., et al., 2012. Maternal Urinary Phthalates and Phenols and Male Genital Anomalies. Epidemiol.
Camb. Mass 23, 353–356.https://doi.org/10.1097/EDE.0b013e318246073e.
Delfosse, V., Dendele, B., Huet, T., Grimaldi, M., Boulahtouf, A., Gerbal-Chaloin, S., et al., 2015. Synergistic activation of human pregnane X receptor by binary cocktails of
pharmaceutical and environmental compounds. Nat. Commun. 6, 8089.https://doi.
org/10.1038/ncomms9089.
Desdoits-Lethimonier, C., Albert, O., Le Bizec, B., Perdu, E., Zalko, D., Courant, F., et al., 2012. Human testis steroidogenesis is inhibited by phthalates. Hum. Reprod. Oxf.
Engl. 27, 1451–1459.https://doi.org/10.1093/humrep/des069.
Desdoits-Lethimonier, C., Lesné, L., Gaudriault, P., Zalko, D., Antignac, J.P., Deceuninck, Y., et al., 2017. Parallel assessment of the effects of bisphenol A and several of its
analogs on the adult human testis. Hum. Reprod. Oxf. Engl. 32, 1465–1473.https://
doi.org/10.1093/humrep/dex093.
Edlow, A.G., Chen, M., Smith, N.A., Lu, C., McElrath, T.F., 2012. Fetal bisphenol A ex-posure: concentration of conjugated and unconjugated bisphenol A in amniotic fluid
in the second and third trimesters. Reprod. Toxicol. Elmsford N 34, 1–7.https://doi.
org/10.1016/j.reprotox.2012.03.009.
Fénichel, P., Déchaux, H., Harthe, C., Gal, J., Ferrari, P., Pacini, P., et al., 2012. Unconjugated bisphenol A cord blood levels in boys with descended or undescended
testes. Hum. Reprod. 27, 983–990.https://doi.org/10.1093/humrep/der451.
Ferlin, A., Pepe, A., Gianesello, L., Garolla, A., Feng, S., Giannini, S., et al., 2008. Mutations in the Insulin-Like Factor 3 Receptor Are Associated With Osteoporosis. J.
Bone Miner. Res. 23, 683–693.https://doi.org/10.1359/jbmr.080204.
Fernández, M.F., Arrebola, J.P., Jiménez-Díaz, I., Sáenz, J.M., Molina-Molina, J.M., Ballesteros, O., et al., 2016. Bisphenol A and other phenols in human placenta from children with cryptorchidism or hypospadias. Reprod. Toxicol. Elmsford N 59, 89–95.
https://doi.org/10.1016/j.reprotox.2015.11.002.
Fini, J.-B., Mughal, B.B., Le Mével, S., Leemans, M., Lettmann, M., Spirhanzlova, P., et al., 2017. Human amniotic fluid contaminants alter thyroid hormone signalling and early
brain development in Xenopus embryos. Sci. Rep. 7, 43786.https://doi.org/10.
1038/srep43786.
Fisher, J.S., Macpherson, S., Marchetti, N., Sharpe, R.M., 2003. Human ‘testicular dys-genesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl
phthalate. Hum. Reprod. 18, 1383–1394.https://doi.org/10.1093/humrep/deg273.
Gerona, R.R., Woodruff, T.J., Dickenson, C.A., Pan, J., Schwartz, J.M., Sen, S., et al., 2013. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in mid-gestation umbi-lical cord serum in a Northern and Central California population. Environ. Sci.
Technol. 47.https://doi.org/10.1021/es402764d.
Gorlov, I.P., Kamat, A., Bogatcheva, N.V., Jones, E., Lamb, D.J., Truong, A., et al., 2002. Mutations of the GREAT gene cause cryptorchidism. Hum. Mol. Genet. 11,
2309–2318.https://doi.org/10.1093/hmg/11.19.2309.
Groten, J.P., Feron, V.J., Sühnel, J., 2001. Toxicology of simple and complex mixtures.
Trends Pharmacol. Sci. 22, 316–322.
Halls, M.L., Bathgate, R.A.D., Sutton, S.W., Dschietzig, T.B., Summers, R.J., 2015. International Union of Basic and Clinical Pharmacology. XCV. Recent Advances in the Understanding of the Pharmacology and Biological Roles of Relaxin Family Peptide Receptors 1–4, the Receptors for Relaxin Family Peptides. Pharmacol. Rev. 67,
389–440.https://doi.org/10.1124/pr.114.009472.
Hernández, A.F., Gil, F., Lacasaña, M., 2017. Toxicological interactions of pesticide
mixtures: an update. Arch. Toxicol. 91, 3211–3223.https://doi.org/10.1007/
s00204-017-2043-5.
Howard, G.J., Webster, T.F., 2009. Generalized concentration addition: a method for examining mixtures containing partial agonists. J. Theor. Biol. 259, 469–477.
https://doi.org/10.1016/j.jtbi.2009.03.030.
Howdeshell, K.L., Furr, J., Lambright, C.R., Rider, C.V., Wilson, V.S., Gray, L.E., 2007. Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat re-productive tract development: altered fetal steroid hormones and genes. Toxicol. Sci.
Off. J. Soc. Toxicol. 99, 190–202.https://doi.org/10.1093/toxsci/kfm069.
Howdeshell, K.L., Rider, C.V., Wilson, V.S., Furr, J.R., Lambright, C.R., Gray, L.E., 2015. Dose Addition Models Based on Biologically Relevant Reductions in Fetal Testosterone Accurately Predict Postnatal Reproductive Tract Alterations by a
Phthalate Mixture in Rats. Toxicol. Sci. Off. J. Soc. Toxicol. 148, 488–502.https://
doi.org/10.1093/toxsci/kfv196.
Hu, X., Myhr, C., Huang, Z., Xiao, J., Barnaeva, E., Ho, B.A., et al., 2016. Structural Insights into the Activation of Human Relaxin Family Peptide Receptor 1 by
Small-Molecule Agonists. Biochemistry (Mosc) 55, 1772–1783.https://doi.org/10.1021/
acs.biochem.5b01195.
Huang, P.-C., Kuo, P.-L., Chou, Y.-Y., Lin, S.-J., Lee, C.-C., 2009. Association between prenatal exposure to phthalates and the health of newborns. Environ. Int. 35, 14–20.
https://doi.org/10.1016/j.envint.2008.05.012.
Huang, Y., Li, J., Garcia, J.M., Lin, H., Wang, Y., Yan, P., et al., 2014. Phthalate Levels in Cord Blood Are Associated with Preterm Delivery and Fetal Growth Parameters in
Chinese Women. PLoS ONE 9.https://doi.org/10.1371/journal.pone.0087430.
Ivell, R., Agoulnik, A.I., Anand-Ivell, R., 2017. Relaxin-like peptides in male reproduction
- a human perspective. Br. J. Pharmacol. 174, 990–1001.https://doi.org/10.1111/
bph.13689.
Jensen, M.S., Anand-Ivell, R., Nørgaard-Pedersen, B., Jönsson, B.A.G., Bonde, J.P., Hougaard, D.M., et al., 2015. Amniotic Fluid Phthalate Levels and Male Fetal Gonad
Function. Epidemiology 26, 91.https://doi.org/10.1097/EDE.0000000000000198.
Jensen, M.S., Nørgaard-Pedersen, B., Toft, G., Hougaard, D.M., Bonde, J.P., Cohen, A., et al., 2012. Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environ. Health Perspect.
120, 897–903.https://doi.org/10.1289/ehp.1104522.
Jiang, D., Chen, W.-Q., Zeng, X., Tang, L., 2018. Dynamic Stocks and Flows Analysis of Bisphenol A (BPA) in China: 2000–2014. Environ. Sci. Technol. 52, 3706–3715.
https://doi.org/10.1021/acs.est.7b05709.
Jiménez-Díaz, I., Artacho-Cordón, F., Vela-Soria, F., Belhassen, H., Arrebola, J.P., Fernández, M.F., et al., 2016. Urinary levels of bisphenol A, benzophenones and
parabens in Tunisian women: A pilot study. Sci. Total Environ. 562, 81–88.https://
doi.org/10.1016/j.scitotenv.2016.03.203.
Juul, A., Almstrup, K., Andersson, A.-M., Jensen, T.K., Jørgensen, N., Main, K.M., et al., 2014. Possible fetal determinants of male infertility. Nat. Rev. Endocrinol. 10,
553–562.https://doi.org/10.1038/nrendo.2014.97.
Kim, J.H., Kim, S.H., Oh, Y.S., Ihm, H.J., Chae, H.D., Kim, C.-H., et al., 2017. In vitro effects of phthalate esters in human myometrial and leiomyoma cells and increased urinary level of phthalate metabolite in women with uterine leiomyoma. Fertil. Steril.
107, 1061–1069.e1.https://doi.org/10.1016/j.fertnstert.2017.01.015.
Kong, R.C.K., Bathgate, R.A.D., Bruell, S., Wade, J.D., Gooley, P.R., Petrie, E.J., 2014. Mapping key regions of the RXFP2 low-density lipoprotein class-A module that are
involved in signal activation. Biochemistry (Mosc) 53, 4537–4548.https://doi.org/
10.1021/bi500797d.
Kortenkamp, A., 2014. Low dose mixture effects of endocrine disrupters and their im-plications for regulatory thresholds in chemical risk assessment. Curr. Opin.
Pharmacol. 19, 105–111.https://doi.org/10.1016/j.coph.2014.08.006.
Latini, 2003. Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy. A
preliminary report. Biol. Neonate.https://doi.org/10.1159/000067012.
Lee, H.K., Kim, T.S., Kim, C.Y., Kang, I.H., Kim, M.G., Jung, K.K., et al., 2012. Evaluation of in vitro screening system for estrogenicity: comparison of stably transfected human estrogen receptor-α transcriptional activation (OECD TG455) assay and estrogen
receptor (ER) binding assay. J. Toxicol. Sci. 37, 431–437.
Legler, J., Zeinstra, L.M., Schuitemaker, F., Lanser, P.H., Bogerd, J., Brouwer, A., et al., 2002. Comparison of in vivo and in vitro reporter gene assays for short-term
screening of estrogenic activity. Environ. Sci. Technol. 36, 4410–4415.https://doi.
org/10.1021/es010323a.
di-(2-ethylhexyl) phthalate on Leydig cell regeneration in the adult rat testis. Toxicol.
Lett. 215, 84–91.https://doi.org/10.1016/j.toxlet.2012.10.001.
Li, Sun, et al., 2018. Distribution of phthalate metabolites between paired maternal-fetal
samples. Environ. Sci. Technol. 6626–6635.https://doi.org/10.1021/acs.est.
8b00838.
Lin, S., Ku, H.-Y., Su, P.-H., Chen, J.-W., Huang, P.-C., Angerer, J., et al., 2011. Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere 82,
947–955.https://doi.org/10.1016/j.chemosphere.2010.10.073.
Main, K.M., Mortensen, G.K., Kaleva, M.M., Boisen, K.A., Damgaard, I.N., Chellakooty, M., et al., 2006. Human Breast Milk Contamination with Phthalates and Alterations of Endogenous Reproductive Hormones in Infants Three Months of Age. Environ. Health
Perspect. 114, 270.https://doi.org/10.1289/ehp.8075.
Mäkelä, J.-A., Koskenniemi, J.J., Virtanen, H.E., Toppari, J., 2019. Testis Development.
Endocr. Rev. 40, 857–905.https://doi.org/10.1210/er.2018-00140.
Marie, C., Vendittelli, F., Sauvant-Rochat, M.-P., 2015. Obstetrical outcomes and bio-markers to assess exposure to phthalates: A review. Environ. Int. 83, 116–136.
https://doi.org/10.1016/j.envint.2015.06.003.
McKinnell, C., Sharpe, R.M., Mahood, K., Hallmark, N., Scott, H., Ivell, R., et al., 2005. Expression of insulin-like factor 3 protein in the rat testis during fetal and postnatal development and in relation to cryptorchidism induced by in utero exposure to di
(n-Butyl) phthalate. Endocrinology 146, 4536–4544.
https://doi.org/10.1210/en.2005-0676.
Meruvu, S., Zhang, J., Bedi, Y.S., Choudhury, M., 2016. Mono-(2-ethylhexyl) phthalate induces apoptosis through miR-16 in human first trimester placental cell line HTR-8/
SVneo. Toxicol. In Vitro 31, 35–42.https://doi.org/10.1016/j.tiv.2015.11.010.
Minatoya, M., Itoh, S., Yamazaki, K., Araki, A., Miyashita, C., Tamura, N., et al., 2018. Prenatal exposure to bisphenol A and phthalates and behavioral problems in children at preschool age: the Hokkaido Study on Environment and Children’s Health.
Environ. Health Prev. Med. 23.https://doi.org/10.1186/s12199-018-0732-1.
Munier, M., Grouleff, J., Gourdin, L., Fauchard, M., Chantreau, V., Henrion, D., et al., 2016. In Vitro Effects of the Endocrine Disruptor p, p’DDT on Human Follitropin
Receptor. Environ. Health Perspect.https://doi.org/10.1289/ehp.1510006.
Mylchreest, E., Sar, M., Cattley, R.C., Foster, P.M., 1999. Disruption of androgen-regu-lated male reproductive development by di(n-butyl) phthalate during late gestation
in rats is different from flutamide. Toxicol. Appl. Pharmacol. 156, 81–95.https://doi.
org/10.1006/taap.1999.8643.
Nef, S., Parada, L.F., 1999. Cryptorchidism in mice mutant for Insl3. Nat. Genet. 22,
295–299.https://doi.org/10.1038/10364.
N’Tumba-Byn, T., Moison, D., Lacroix, M., Lecureuil, C., Lesage, L., Prud’homme, S.M., et al., 2012. Differential effects of bisphenol A and diethylstilbestrol on human, rat
and mouse fetal leydig cell function. PLoS ONE 7, e51579.https://doi.org/10.1371/
journal.pone.0051579.
Overbeek, P.A., Gorlov, I.P., Sutherland, R.W., Houston, J.B., Harrison, W.R., Boettger-Tong, H.L., et al., 2001. A transgenic insertion causing cryptorchidism in mice. Genes
N Y N 2000 (30), 26–35.
Pan, Y., Jing, J., Dong, F., Yao, Q., Zhang, W., Zhang, H., et al., 2015. Association between phthalate metabolites and biomarkers of reproductive function in 1066 Chinese men
of reproductive age. J. Hazard. Mater. 300, 729–736.https://doi.org/10.1016/j.
jhazmat.2015.08.011.
Philippat, C., Wolff, M.S., Calafat, A.M., Ye, X., Bausell, R., Meadows, M., et al., 2013. Prenatal Exposure to Environmental Phenols: Concentrations in Amniotic Fluid and Variability in Urinary Concentrations during Pregnancy. Environ. Health Perspect.
121, 1225.https://doi.org/10.1289/ehp.1206335.
Sauer, S.J., Tarpley, M., Shah, I., Save, A.V., Lyerly, H.K., Patierno, S.R., et al., 2017. Bisphenol A activates EGFR and ERK promoting proliferation, tumor spheroid for-mation and resistance to EGFR pathway inhibition in estrogen receptor-negative
inflammatory breast cancer cells. Carcinogenesis 38, 252–260.https://doi.org/10.
1093/carcin/bgx003.
Shekhar, S., Sood, S., Showkat, S., Lite, C., Chandrasekhar, A., Vairamani, M., et al., 2017. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen. Comp. Endocrinol. 241,
100–107.https://doi.org/10.1016/j.ygcen.2016.05.025.
Sijstermans, K., Hack, W.W.M., Meijer, R.W., van der Voort-Doedens, L.M., 2008. The
frequency of undescended testis from birth to adulthood: a review. Int. J. Androl. 31,
1–11.https://doi.org/10.1111/j.1365-2605.2007.00770.x.
Song, X.F., Wei, G.H., Liu, X., Zhang, D.Y., Chen, X., Deng, Y.J., 2008. Effects of die-thylhexyl phthalate (DEHP) on INSL3 mRNA expression by Leydig cells derived from
mouse embryos and in newborn mice. J. Int. Med. Res. 36, 512–521.https://doi.org/
10.1177/147323000803600316.
Suzuki, Y., Yoshinaga, J., Mizumoto, Y., Serizawa, S., Shiraishi, H., 2012. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int. J. Androl. 35,
236–244.https://doi.org/10.1111/j.1365-2605.2011.01190.x.
Swan, S.H., 2008. Environmental phthalate exposure in relation to reproductive outcomes
and other health endpoints in humans. Environ. Res. 108, 177.https://doi.org/10.
1016/j.envres.2008.08.007.
Thal, D.M., Glukhova, A., Sexton, P.M., Christopoulos, A., 2018. Structural insights into
G-protein-coupled receptor allostery. Nature 559, 45–53.https://doi.org/10.1038/
s41586-018-0259-z.
Thomas, P., Dong, J., 2006. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of
endo-crine disruption. J. Steroid Biochem. Mol. Biol. 102, 175–179.https://doi.org/10.
1016/j.jsbmb.2006.09.017.
Thomas, P., Pang, Y., Filardo, E.J., Dong, J., 2005. Identity of an Estrogen Membrane Receptor Coupled to a G Protein in Human Breast Cancer Cells. Endocrinology 146,
624–632.https://doi.org/10.1210/en.2004-1064.
Virtanen, H.E., Toppari, J., 2008. Epidemiology and pathogenesis of cryptorchidism.
Hum. Reprod. Update 14, 49–58.https://doi.org/10.1093/humupd/dmm027.
Wagner-Mahler, K., Kurzenne, J.-Y., Delattre, I., Bérard, E., Mas, J.-C., Bornebush, L., et al., 2011. Prospective study on the prevalence and associated risk factors of cryptorchidism in 6246 newborn boys from Nice area, France. Int. J. Androl. 34,
e499–e510.https://doi.org/10.1111/j.1365-2605.2011.01211.x.
Wilson, V.S., Howdeshell, K.L., Lambright, C.S., Furr, J., Earl, Gray L., 2007. Differential expression of the phthalate syndrome in male Sprague-Dawley and Wistar rats after
in utero DEHP exposure. Toxicol. Lett. 170, 177–184.https://doi.org/10.1016/j.
toxlet.2007.03.004.
Wilson, V.S., Lambright, C., Furr, J., Ostby, J., Wood, C., Held, G., et al., 2004. Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression
in the fetal rat testis. Toxicol. Lett. 146, 207–215.
Wittassek, M., Angerer, J., Kolossa-Gehring, M., Schäfer, S.D., Klockenbusch, W., Dobler, L., et al., 2009. Fetal exposure to phthalates – a pilot study. Int. J. Hyg. Environ.
Health 212, 492–498.https://doi.org/10.1016/j.ijheh.2009.04.001.
Wohlfahrt-Veje, C., Main, K.M., Skakkebæk, N.E., 2009. Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clin. Endocrinol. (Oxf.) 71, 459–465.
https://doi.org/10.1111/j.1365-2265.2009.03545.x.
Woodruff, T.J., Zota, A.R., Schwartz, J.M., 2011. Environmental Chemicals in Pregnant Women in the United States: NHANES 2003–2004. Environ. Health Perspect. 119,
878.https://doi.org/10.1289/ehp.1002727.
Xiao, J., Huang, Z., Chen, C.Z., Agoulnik, I.U., Southall, N., Hu, X., et al., 2013. Identification and optimization of small-molecule agonists of the human relaxin
hormone receptor RXFP1. Nat. Commun. 4, 1953.https://doi.org/10.1038/
ncomms2953.
Yu, H., Caldwell, D.J., Suri, R.P., 2019. In vitro estrogenic activity of representative en-docrine disrupting chemicals mixtures at environmentally relevant concentrations.
Chemosphere 215, 396–403.https://doi.org/10.1016/j.chemosphere.2018.10.067.
Yuan, F.P., Li, X., Lin, J., Schwabe, C., Büllesbach, E.E., Rao, C.V., et al., 2010. The role of RXFP2 in mediating androgen-induced inguinoscrotal testis descent in LH receptor
knockout mice. Reprod. Camb. Engl. 139, 759–769.
https://doi.org/10.1530/REP-09-0518.
Zhang, Y., Lin, L., Cao, Y., Chen, B., Zheng, L., Ge, R.-S., 2009. Phthalate Levels and Low Birth Weight: A Nested Case-Control Study of Chinese Newborns. J. Pediatr. 155,
500–504.https://doi.org/10.1016/j.jpeds.2009.04.007.
Zimmermann, S., Steding, G., Emmen, J.M.A., Brinkmann, A.O., Nayernia, K., Holstein, A.F., et al., 1999. Targeted Disruption of the Insl3 Gene Causes Bilateral
Cryptorchidism. Mol. Endocrinol. 13, 681–691.https://doi.org/10.1210/mend.13.5.