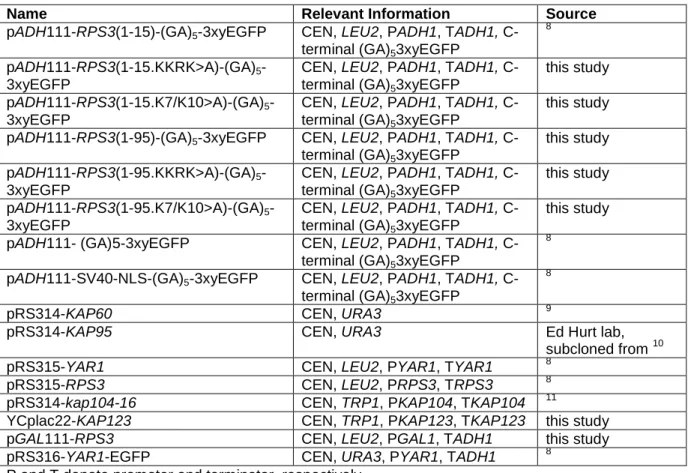
Nuclear import of dimerized ribosomal protein Rps3 in complex with its chaperone Yar1
Texte intégral
Figure
Documents relatifs
tested, with the exception of CHX (Table1). Analysis of other fluorescent and non-fluorescent colonies revealed similar phenotypes. To confirm further that the activity of the
Mean Hydrophobicity Has Strongly Decreased in Those Transmembrane Regions of Cr-ATP6 That Are Not Critical for Function—The transfer of the atp6 gene from the mtDNA to the nucleus
The x-ray structure of the catalytic domain of the Lactobacillus casei enzyme (12) shows that it forms a hexamer with a subunit fold unrelated to that of eukaryotic Ser兾Thr
[r]
[r]
This study summarizes the refit of the 8.2 m LOA Government of Nunavut owned research vessel RV Papiruq to make it suitable for fisheries research in coastal (i.e.,
Taken together, in vivo and in vitro analyses demonstrated that Syo1 can simultaneously bind Rpl5 and Rpl11 to form a heterotrimeric Syo1-Rpl5-Rpl11 complex.. To gain further
HeLa cells were permeabilized by digitonin and incubated with purified, Alexa488-labelled Syo1 or GST-SV40NLS-mRFP in transport buffer (C, upper panel), transport buffer