Advance Access publication October 6, 2005.
421
Tumour necrosis factor-
␣ up-regulates macrophage
migration inhibitory factor expression in endometrial
stromal cells via the nuclear transcription factor NF-
B
W.G.Cao
1, M.Morin
1, V.Sengers
1, C.Metz
2, T.Roger
3, R.Maheux
1and A.Akoum
1,41Unité d’endocrinologie de la reproduction, Centre de Recherche, Hôpital Saint-François d’Assise, Centre Hospitalier Universitaire de Québec, Faculté de Médecine, Université Laval, Québec, Canada, 2Institute for Medical Research at North Shore-LIJ, NY, USA and 3Service des Maladies Infectieuses, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland
4To whom correspondence should be addressed at: Laboratoire d’Endocrinologie de la Reproduction, Centre de Recherche, Hôpital Saint-François d’Assise, 10 rue de l’Espinay, Local D0-711, Québec, Québec, Canada G1L 3L5. E-mail: ali.akoum@crsfa.ulaval.ca
BACKGROUND: A series of controlled changes including proliferation, secretion and menstrual shedding occur in
the human endometrium during every normal menstrual cycle. Macrophage migration inhibitory factor (MIF), a
multifunctional cytokine with numerous proinflammatory, immunomodulatory and angiogenic properties, appears
to be expressed in the human endometrium and to follow a regulated cycle phase-dependent expression, but the
mechanisms underlying endometrial MIF expression remain to be fully elucidated. METHODS AND RESULTS:
Results from enzyme-linked immunosorbent assay (ELISA) demonstrated a significant dose- and time-dependent
increase in MIF secretion by human endometrial cells in response to tumour necrosis factor-alpha (TNF-
␣) (0.1–100
ng/ml). This increase was also observed at the mRNA level as shown by reverse transcription (RT)–PCR. Curcumin
(10
ⴚ8mol/l), a known nuclear factor (NF)-
B inhibitor, inhibited the TNF-␣-induced pIB phosphorylation as shown
by western blotting, NF-
B translocation into the nucleus as shown by electrophoretic mobility shift assay, and MIF
synthesis and secretion as measured by ELISA and RT–PCR. The expression of a dominant-negative NF-
B
inhibi-tor (I
B) significantly decreased the TNF-␣-induced MIF promoter activity as analysed by transient cell
transfec-tion. CONCLUSIONS: These results indicate clearly that TNF-
␣ up-regulates the expression of MIF in endometrial
stromal cells. This took place possibly through NF-
B activation, and may play an important role in the physiology of
the human endometrium.
Key words: endometrium/MIF/NF-κB/TNF-α
Introduction
Human endometrium undergoes a series of controlled changes including proliferation, secretion and menstrual shedding during every normal menstrual cycle. These dynamic processes require a network of hormonal, growth and immunoinflammatory fac-tors in which cytokines locally produced within the endometrial tissue play a crucial role (Tabibzadeh, 1996; 1998; Lebovic
et al., 2000; von Wolff et al., 2000; Bigonnesse et al., 2001;
Boucher et al., 2001; Critchley et al., 2001). Of particular inter-est is the role that appears to be played by tumour necrosis factor-alpha (TNF-α) in human endometrial physiology.
TNF-α was first identified as a cytokine secreted by endo-toxin-activated macrophages that induced the necrosis of tumours (Carswell et al., 1975). TNF-α is now known as a pluripotent cell mediator and angiogenic cytokine that promotes the production of other cytokines in various cells (Feldmann and Maini, 1999). The human endometrium is characterized by a variety of cell types, including fibroblasts, immune cells, vascular cells and
epithelial cells, all of which express TNF-α (Hunt et al., 1992; Philippeaux and Piguet, 1993; Tabibzadeh et al., 1995; Chegini
et al., 1999; von Wolff et al., 1999; Bergqvist et al., 2000; 2001).
Despite discrepancies in the cycle expression patterns, these stud-ies suggest a local role for TNF-α in a variety of normal endome-trial functions. Increased expression of this cytokine was shown to cause pathophysiological effects reflected by its involvement in implantation failure (Hazout, 1995), abortion (Giacomucci
et al., 1994) and endometriosis (Zhang et al., 1993).
Our previous studies identified macrophage migration inhib-itory factor (MIF) as a potent mitogenic factor for human endothelial cells released by ectopic endometrial cells (Yang
et al., 2000) in women with endometriosis (Kats et al., 2002b).
Subsequent studies from our laboratory showed a cycle phase-dependent expression of MIF during the menstrual cycle, with a particular increase occurring in the late secretory phase, sug-gesting a tight regulation and perhaps different roles for this factor in the reparative, reproductive and inflammatory-like processes that occur in human endometrium during the normal
menstrual cycle (Kats et al., 2005). MIF, one of the first described cytokines, was identified for its ability to inhibit macrophage migration within the inflammatory sites (Bloom and Bennett, 1970; David, 1966). More recent studies have shown, however, that MIF is rather a multifunctional cytokine/ hormone endowed with a multitude of proinflammatory, immunomodulatory, angiogenic and tissue remodelling effects (Metz and Bucala, 1997; Nishihira, 1998; Calandra and Roger, 2003; Nishihira et al., 2003). Accordingly, we believe that MIF may in different ways influence human endometrial tissue functions and be involved in its normal cyclic changes.
The mechanisms underlying MIF expression in the human endometrial tissue remain unknown. Herein we report that TNF-α had a direct stimulatory effect on MIF expression by human endometrial stromal cells. Cell treatment with TNF-α caused a significant increase in MIF protein secretion and mRNA synthesis, which appeared to be likely mediated by the activation of the nuclear transcription factor NF-κB. Interac-tion between TNF-α and MIF, owing to the properties of these two major and multifunctional cytokines, may play a consider-able role in the physiology of the human endemetrium.
Materials and methods
Subjects and tissue handling
Endometrial biopsies were obtained during laparoscopy for tubal liga-tion from eight normal fertile women who had not received hormonal or anti-inflammatory therapy for at least 3 months before surgery
(mean age ± SD, 30.71 ± 4.03 years). Four women were in the
prolif-erative phase of the menstrual and four in the secretory phase. Written informed consent was obtained from these women under a study pro-tocol approved by the Ethical Committee on Human Research at Laval University, Quebec, Canada. Biopsies were obtained by aspira-tion with the use of a Pipelle (Unimar Inc., Prodimed,
Neuilly-En-Tchelle, France), immediately placed at 4°C in sterile Hank’s
bal-anced salt solution containing 100 U/ml penicillin, 100 μg/ml
streptamycin and 0.25 μg/ml amphotericin B (Invitrogen Life
Tech-nologies, Burlington, ON, Canada) and transported to the laboratory.
Cell culture and stimulation
Endometrial stromal cells were obtained and characterized according to our previously described procedure (Boucher et al., 2000). Cells were subcultured to eliminate contamination by macrophages or other leuko-cytes and used between passages 3 and 5. Extensive characterization of cell cultures prepared using this protocol previously confirmed >95% purity with cells retaining cytoskeletal markers of their endometrial stro-mal origin. For enzyme-linked immunosorbent assay (ELISA) and reverse transcription (RT)–PCR studies, endometrial cells were plated in 12-well plates in Dulbecco’s minimal essential medium F-12 medium,
supplied with 10% fetal bovine serum (FBS), 5 μg/ml insulin, 5 μg/ml
transferrin and 1% antibiotics–antimycotics. Medium was changed every 48 h. When cells proliferated to confluence, the culture medium was replaced overnight with serum-free medium, then with serum-free and
phenol red-free medium containing various concentrations of TNF-α (0–
100 ng/ml) (R & D systems, Minneapolis, MN, USA) for different time
periods (0–24 h). In some cultures, curcumin (10−8 mol/l) (Sigma
Chemi-cal Co., St Louis, MO, USA), an extract from plant known for inhibiting NF-κB (D’Acquisto et al., 2002; Ghosh and Karin, 2002) was added 1 h
before TNF-α. The culture supernatants were collected for MIF ELISA,
whereas cells were collected for mRNA or nuclear protein extraction.
Immunocytofluorescence
Endometrial stromal cells were plated on chamber slides (Nalge Nunc International, Naperville, IL, USA). At confluence, cells were
incu-bated overnight with serum-free medium, and then with TNF-α at
1 ng/ml for 6 h. Cells were then fixed in formaldehyde [3.5% v/v in phosphate buffered saline (PBS)] for 15 min at room temperature, rinsed in PBS–0.01% Tween 20, incubated with a goat anti-human MIF antibody [0.66 mg/ml in PBS/0.2% bovine serum albumin (BSA)/ 0.01% Tween 20] (R & D Systems) for 90 min at room temperature, rinsed in PBS–0.01% Tween 20, incubated with fluoresceine isothio-cyanate (FITC)-conjugated anti-goat antibody (1/50 dilution in PBS-BSA-Tween) (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), rinsed in PBS–0.01% Tween 20 and mounted in Mowiol containing 10% para-phenylenediamine (Sigma). Cells incubated with-out the primary antibody or with goat immunoglobulins (Sigma) at the same concentration as the primary antibody were included as negative controls. Slides were observed under a microscope equipped for fluo-rescence (Leica mikroskopie und systeme GmbH, Model DMRB; Postfach, Wetzlar, Germany) and photographed.
ELISA
MIF ELISA was performed using a mouse monoclonal anti-human-MIF antibody (R & D Systems) as a capture antibody, a rabbit polyclonal anti-human-MIF antibody for detection with an alkaline phosphatase-conjugated goat anti-rabbit antibody and para-nitrophenyl phosphate (Sigma) as substrate (Calandra et al., 1995). The optical density was measured at 405 nm and MIF concentrations were extrapolated from a standard curve using recombinant human MIF. The sensitivity limit of the assay was 300 pg/ml, with intra- and inter-assay coefficients of variation <4%.
RT–PCR
Briefly, following appropriate treatment with TNF-α, cells were
washed with ice-cold PBS and extraction of total RNA was performed with Trizol reagent according to the manufacturer’s instructions (Invitrogen). RNA was reverse transcribed in the presence of random primers, and the resulting cDNA was amplified with oligonucleotide primers specific to human MIF (amplimer size 255 bp) and to human glyceraldehyde phosphate dehydrogenase (GAPDH) used as internal control (amplimer size 240 bp). The PCR reaction products were then separated on 1.2% agarose gel by electrophoresis for qualitative ana-lysis of mRNA expression as described previously (Kats et al., 2002b). Quantitative real-time PCRs were carried out in an ABI 7000 Thermal Cycler (Applied Biosystems). Each standard PCR reaction contains 2 ml of RT product, 0.5 ml of each primer (final concentration, 5 pmol/l), 12.5 ml SYBR Green PCR Master Mix consisting of Taq
DNA polymerase reaction buffer, dNTP mix, SYBR green I, MgCl2
and Taq DNA polymerase. Following a 10 min denaturation at 95°C,
the reactions were cycled 40 times with a 15 s denaturation at 95°C
and a 60 s annealing at 59 or 60°C for MIF and GAPDH, respectively.
MIF primers (forward, 5′-GCCCGGACAGGGTCTACA-3′; reverse,
5′-CTTAGGCGAAGGTGGAGTTGTT-3′; amplimer size 125 bp)
(Boeuf et al., 2005), and GAPDH primers (forward, 5
′-CAGGGCT-GCTTTTAACTCTGG-3′; reverse,
5′-TGGGTGGAATCATATT-GGAACA-3′; amplimer size 102 bp) designed with Primer Express™,
version 2.0 (Applied Biosystems), span intron–exon boundaries to avoid amplification of genomic DNA, and were selected so as to have
compatible Tm values (59–61°C). Quantitation of MIF mRNA was
performed using a relative quantitation method. For each experimen-tal sample, MIF mRNA levels were normalized to GAPDH mRNA
levels. After each run, melting curve analysis (55–95°C) was
duplicate, and each run included no-template and no-reverse tran-scription controls.
Western blotting
Briefly, 80 μl lysis buffer (50 mmol/l Tris–HCl, 125 mmol/l NaCl,
0.1% Nonidet-P40, 5 mmol/l ethylenediamine tetraacetic acid, 50 mmol/l NaF, 0.1% phenylmethylsulfonylfluoride and protease inhibitors) was used to extract cytoproteins after collecting cells with
cold PBS supplied with phosphorylation inhibitors. Proteins (20 μg)
were electrophoretically separated using 10% SDS–PAGE, then trans-ferred onto polyvinylidene difluoride membranes (Millipore Corp.,
Bedford, MA, USA) overnight at 4°C. After incubation with anti-human
p-IκB (Active Motif, Carlsbad, CA, USA) in 0.1% Tween 20–PBS for 1.5 h at room temperature, membranes were incubated with horserad-ish peroxidase (HRP)-labelled anti-mouse IgGs for 1 h, and a chemi-luminescence kit was used for detection following the manufacturer’s instructions (Roche, IN, USA). Membranes were stripped and reblot-ted with a monoclonal antibody specific to tubulin (Sigma) (1:50 000 dilution in Tween 20–PBS) used as an internal control for protein loading and transfer.
Extraction of nuclear proteins and EMSA
Nuclear proteins were extracted using a nuclear extract kit according to the manufacturer’s instructions (Active Motif) and protein concen-tration was determined using the Bio-Rad DC Protein assay kit (Bio-Rad Laboratories Ltd, Mississauga, ON, Canada). Nuclear extracts
were stored at –80°C until use.
DNA binding of NF-κB was examined using the consensus
oligonu-cleotide of NF-κB (5′-AGT TGA GGG GAC TTT CCC AGG C-3′).
The oligonucleotide was end-labelled with (32P)γ-ATP (Amersham
Pharmacia Biotech) using T4 polynucleotide kinase (Promega, Madison, WI, USA). The labelled probe was purified using a Probe-Quant G-50 micro-column (Amersham Biosciences, Baie d’Urfe, QC, Canada) (3000 r.p.m., 1 min) and recovered in Tris–EDTA buffer pH
8.0. Binding reactions included 5 μg of nuclear proteins in incubation
buffer containing 50 mmol/l Tris–HCl pH 7.5, 250 mmol/l NaCl,
2.5 mmol/l dithiothreitol, 2.5 mmol/l EDTA, 5 mmol/l MgCl2, 0.25 mg/ml
poly(dI–dC)poly(dI–dC) and 20% glycerol. After 10 min, the labelled
oligonucleotide (4 × 105 c.p.m.) was added and the mixture was
incu-bated for 20 min at room temperature in a final volume of 10 μl. When
indicated, controls for specific or non-specific competitions were
per-formed using unlabelled NF-κB which was added 20 min before the
incubation with the labelled oligonucleotide. Immediately after binding, the nucleoprotein–oligonucleotide complexes were separated from unbound oligonucleotide by electrophoresis on a non-denaturing 4% acrylamide gel at 100 V for 4 h using 0.5 mol/l Tris–borate–EDTA
(TBE) buffer. The gel was then dried and exposed overnight at –80°C to
×-ray films (BioMax; Eastman Kodak, Rochester, NY, USA).
Transfections and luciferase assays
Transfections were performed in triplicate in 24-well plates using Plus and LipofectAMINE Reagents (Invitrogen) as described by the
manu-facturer. Cells were transiently co-transfected with 0.5 μg of human
MIF promoter construct in the pGL3 luciferase reporter vector
(pGL3-MIF) and 1 μg of human IκB dominant-negative cDNA (S32A/S36A)
in the pUSE vector (pUSE-IκB) (Upstate, Charlottesville, VA, USA),
or with the appropriate control vectors pGL3 and pUSE. Cells were then washed with PBS and incubated in the culture medium contain-ing 10% FBS overnight before they were stimulated with 1 ng/ml TNF-α for 24 h. Cell extracts were assayed for firefly and renilla luci-ferase activities using the dual luciluci-ferase reporter assay system (Promega) as instructed by the manufacturer.
Statistical analysis
Data were expressed as mean ± SEM. An unpaired t-test was used
for comparing two means, and one-way ANOVA followed by the Dunnett’s test was performed for multiple comparisons using Graph-Pad Software, Prism 3.0 (GraphGraph-Pad Software, San Diego, CA, USA). Differences were considered as statistically significant whenever a
P-value <0.05 occurred.
Results
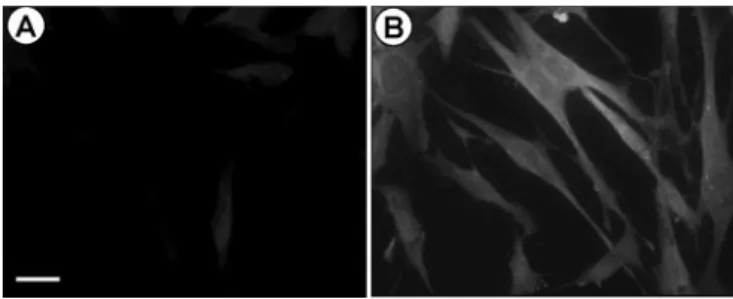
Analysis of MIF expression in endometrial stromal cells in response to TNF-α was first examined by immunocytofluores-cence using anti-MIF antibody. Only a weak staining was observed in cells incubated with the culture medium alone with-out stimulus (Figure 1A). However, cell incubation with TNF-α (1 ng/ml) for 24 h markedly increased MIF immunostaining (Fig-ure 1B). Staining was virtually absent in cells incubated with goat IgGs instead of anti-MIF antibody or with anti-MIF antibody pre-absorbed with an excess rhMIF (2 μg/ml) (data not shown).
MIF secretion by endometrial stromal cells in response to TNF-α was then quantified by ELISA. As shown in Figure 2, TNF-α induced a dose- and time-dependent increase in MIF con-centration in the culture medium. There was no significant increase in MIF secretion in response to TNF-α after 2 h of stim-ulation. Treatment with 0.1 ng/ml resulted in a progressive increase in MIF secretion, although with no statistical signifi-cance. MIF secretion was statistically significant after 6, 12 and 24 h of stimulation with 1 and 10 ng/ml TNF-α (P < 0.05), and appeared to reach a plateau after 6 h of treatment, whereas higher TNF-α concentration (100 ng/ml) induced a statistically signific-ant increase in MIF secretion after 24 h of stimulation (P < 0.05). The effects of TNF-α on MIF gene expression in endome-trial stromal cells were further analysed by RT–PCR. As shown in Figure 3, endometrial stromal cells express detectable levels of MIF mRNA in culture without stimuli. However, add-ing TNF-α (0–100 ng/ml) to the culture medium for 24 h con-siderably increased MIF mRNA levels in a dose-dependent manner. Quantitative analysis of MIF mRNA expression using real-time PCR confirmed these data and further showed a sig-nificant increase in MIF mRNA levels in endometrial stromal cells in response to 1 (P < 0.01) and 10 (P < 0.05) ng/ml TNF-α.
Figure 1. TNF-α increased MIF expression as detected by immuno-cytofluorescence. Human endometrial stromal cell cultured to conflu-ence in chamber were incubated for 24 h in culture medium without
stimuli (A) or with 1 ng/ml TNF-α (B). Cells were fixed and MIF
protein was visualized with goat anti-hMIF and FITC-anti-goat IgGs.
Transcription factor NF-κB plays a major role in the regula-tion of the expression of multiple genes that control the immune system, growth and inflammation (Makarov et al., 1997). NF-κB appeared to play an important role in mediating TNF-α effects in a variety of cell types (Makarov et al., 1997). However, it is still unknown whether NF-κB is involved in MIF gene transcription. Our data showed that curcumin
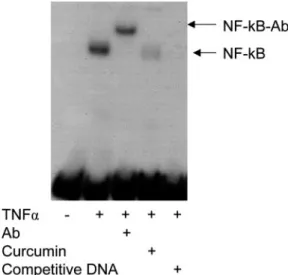
(10−8 mol/l), an NF-κB inhibitor (Xu et al., 1997; Bharti et al., 2003), significantly decreased the ability of TNF-α to induce MIF protein secretion (P < 0.05) and mRNA synthesis (P < 0.01) (Figure 4). Analysis of nuclear proteins showed that TNF-α induced expression of the functional p50 component of NF-κB in endometrial stromal cells, as indicated by binding of radiolabelled p50-specific NF-κB oligonucleotide and by super-shifting of this complex with an antibody specific to NF-κB p50. The TNF-α-induced NF-NF-κB p50 band was reduced or eliminated by curcumin (10−8 mol/l) or an excess of unlabelled oligonucleotide (Figure 5). Phosphorylation of the NF-κB inhibitor, IκB, inactivates IκB, and allows NF-κB to translo-cate into the cell nucleus where it triggers synthesis of inflam-matory cytokines (Makarov et al., 1997). As shown in Figure 6, pIκB was increased after exposure to TNF-α (1 ng/ml), and the increase was inhibited by curcumin (10−8 mol/l) as detected by western blot.
To further determine whether NF-κB can be involved in MIF gene transcription, the transcriptional activity of the MIF promoter–luciferase construct was evaluated after co-transfection with the pUSE-IκB dominant-negative construct in the presence and absence of TNF-α. The pUSE-IκB dominant-negative vec-tor expresses a mutated non-phosphorylatable, non-degradable IκB. The results showed that the activity of the MIF promoter Figure 2. TNF-α induced a dose- and time-dependent increase in
MIF protein secretion in the culture medium as analysed by ELISA.
The bars represent means ± SEM; *P < 0.05 versus control (culture
medium without stimuli for each incubation time), as analysed by the Dunnett’s test. 2h 6h 12h 24h TNFα (ng/ml) 0.1 + + + + 1 + + + + 10 + + + + 100 + + + + 0 500 1000 1500 2000 2500 MIF (pg/ml) * * * * * * *
Figure 3. TNF-α increased MIF mRNA steady-state levels in
cul-tured human endometrial stromal cells. Cells were treated with TNF-α
(0, 0.1, 1, 10 and 100 ng/ml) for 24 h. Total RNA was extracted and reverse transcribed, then MIF and GAPDH (internal control) cDNAs were amplified by PCR as described in Material and methods. (A) Analysis of amplified cDNAs by electrophoresis and ethidium bromide staining; (B) quantitative real-time PCR analysis. MIF mRNA levels were expressed as MIF to GAPDH mRNA ratio. The
bars represent means ± SEM; *P < 0.05, **P < 0.01 versus control
(culture medium without stimuli), as analysed by the Dunnett’s test.
Figure 4. Curcumin suppressed TNF-α-induced increase in MIF expression in endometrial stromal cells. Confluent cultures of endometrial stromal cells were preincubated for 1 h with or without
curcumin (10 nmol/l); TNF-α (1 ng/ml) was then added and the cells
were incubated for further 24 h. (A) MIF concentration in the culture supernatant as measured by ELISA; (B) MIF mRNA levels as
evalu-ated by real-time PCR. The bars represent means ± SEM.
*Signifi-cant stimulation of MIF secretion and mRNA synthesis by TNF-α as
analysed by the unpaired t-test (P < 0.05); †P < 0.05 and ††P < 0.01,
significant inhibition of TNF-α-induced MIF secretion and mRNA
synthesis in the presence of curcumin, as analysed by the unpaired t-test.
A
B
0 1000 2000 TNFα Curcumin MIF (pg/ml) - + - + - - + + † 0 1 2 TNFα Curcumin - + - + - - + +Relative MIF mRNA
levels
(MIF/GAPDH ratio) ††
* *
was significantly stimulated with TNF-α (P < 0.05). Furthermore, cell co-transfection with the pUSE-IκB dominant-negative vector resulted in a significant inhibition of the TNF-α-induced MIF promoter activity (Figure 7).
Discussion
These results indicate, for the first time, that TNF-α up-regu-lates MIF gene expression and protein synthesis in human endometrial stromal cells and that this effect is mediated by activation of NF-κB. In quiescent cells, NF-κB is bound to IκB in the cytoplasm. Upon stimulation by various agents
such as TNF-α, IκB undergoes phosphorylation to form p-IκB, which subsequently undergoes ubiquitylation and degrada-tion. This process allows NF-κB to enter the nucleus, where it activates cytokines, adhesion molecules, cyclooxygenase and other target genes (Makarov et al., 1997). Several compounds are now used as NF-κB inhibitors with different levels of effects. Our data showed that curcumin depressed the ability of TNF-α to increase MIF secretion and inhibited pIκB phos-phorylation and NF-κB translocation into the nucleus. Fur-thermore, co-transfection of endometrial cells with MIF promoter and IκB dominant-negative vectors resulted in a sig-nificant inhibition of the TNF-α-induced activation of the MIF promoter. These data suggest that TNF-α activates MIF gene transcription in human endometrial stromal cells, and provide further evidence on the ability of TNF-α to up-regulate MIF expression via NF-κB.
It is now quite well-known that TNF-α is a pluripotent cell mediator having proinflammatory, growth/apoptotic and ang-iogenic effects and a wide spectrum of cell targets (Feldmann and Maini, 1999). TNF-α has been detected in various repro-ductive organs including the ovaries (Roby et al., 1990), the oviduct (Hunt, 1993), the endometrium and preimplantation embryos (Zolti et al., 1991; Hunt et al., 1992; Philippeaux and Piguet, 1993; Sharkey et al., 1995; Tabibzadeh et al., 1995; Figure 5. TNF-α induced nuclear translocation of NF-κB as
ana-lysed by electrophoretic mobility shift assay (EMSA). Confluent cell
cultures were exposed for 6 h to medium alone, medium with TNF-α
(1 ng/ml) or medium with TNF-α (1 ng/ml) following 1 h
preincuba-tion with curcumin (10−8 mol/l). Nuclear protein extracts (5 μg) were
incubated with radiolabelled NF-κB oligonucleotide. The resulting
complexes were separated on 5% non-denaturing polyacrylamide gel.
To test for specificity of NF-κB binding, supershift analysis with
antibody against the p50 subunit of NF-κB (NF-κB-Ab) and
competi-tion experiments with unlabelled oligonucleotide were conducted.
Figure 6. TNF-α induced IκB phosphorylation in human endome-trial stromal cells, as detected by western blot. Confluent cell cultures
were exposed to medium without stimuli, 1 ng/ml TNF-α or 1 ng/ml
TNF-α for 15 min following 1 h preincubation with 10−8 mol/l curcu-min. Twenty micrograms of total proteins were separated by electro-phoresis on a 10% gradient polyacrylamide gel and immunoblotted
using mouse monoclonal anti-pIκB antibodies and HRP-anti-mouse
IgGs (pIκBa, 43 kDa). Tubulin levels are shown to demonstrate equal
protein loading.
Figure 7. IκB dominant-negative inhibited TNF-α-induced MIF promoter activity. Human endometrial stromal cells were transiently
co-transfected with pGL3-MIF and pUSE-IκB, or with the
appropri-ate control vectors pGL3 and pUSE. The transfected cells were then
incubated for 24 h with and without TNF-α (1 ng/ml) before being
harvested. Cell lysates were analysed for firefly and renilla luciferase activities as described in Materials and methods. Data were expressed as precentage of control which represents MIF promoter activity
without co-expression of dominant-negative IκB and stimulation with
TNF-α (cells co-transfected with pGL3-MIF and the pUSE vector
and incubated with the culture medium). The bars represent means ±
SEM. *Significant increase in MIF promoter activity in response to
TNF-α as compared to the culture medium (P < 0.05); †significant
decrease in the TNF-α-induced MIF promoter activity following
co-transfection with dominant-negative IκB (P < 0.05), as analysed by
the unpaired t-test.
pGL3-M
IF/pUSE/TNF 0
pGL3-M
IF/pUSE/TNF 1
pGL3/-MIF/pUSE-IkB/TNF 0 pGL3-M
IF/pUSE-IkB/TNF 1 pGL3/pUSE/TNF 0 pGL3/pUSE/TNF 1
pGL3/pUSE-IkB/TNF 0 pGL3/pUSE-IkB/TNF 1 0 100 200 300 400 * Luciferase activity (% control) †
Chegini et al., 1999; von Wolff et al., 1999), thus implicating TNF-α in reproduction.
Several studies have shown the menstrual cycle-dependent production of TNF-α in the human endometrium, its expres-sion in different cell types and the presence of TNF receptors (Hunt et al., 1992; Philippeaux and Piguet, 1993; Tabibzadeh
et al., 1995; Chegini et al., 1999; von Wolff et al., 1999).
Despite discrepancies in the cyclic expression patterns, these studies suggest a local role for TNF-α in a variety of endome-trial functions. This is not surprising, since the human endometrium represents one of the most dynamic tissues dur-ing the reproductive age, and cytokines appear to have a cru-cial role in the regulation of the cyclic events of cell proliferation, remodelling, angiogenesis, apoptosis/necrosis, bleeding and menstrual shedding occurring in this tissue (Tabibzadeh, 1996; 1998; Lebovic et al., 2000; von Wolff et al., 2000; Bigonnesse et al., 2001; Boucher et al., 2001; Critchley
et al., 2001). Thus, our study showing an up-regulation of MIF
expression by TNF-α in human endometrial cells identifies one of the potential mechanisms underlying MIF expression in the human endometrium, and points to a plausible interaction between these two multifunctional cytokines the local expres-sion and biological properties of which are of particular rele-vance for the physiology of a tissue like the human endometrium.
MIF was first reported in 1932 and was described as a cytokine produced by T lymphocytes that inhibited the random migration of microphages during delayed hypersensitivity (David, 1966; Bloom and Bennett, 1970). It is now well docu-mented that MIF is a major multifunctional proinflammatory cytokine that activates a number of immunocompetent cells (Metz and Bucala, 1997; Calandra and Roger, 2003; Nishihira
et al., 2003) and overrides the anti-inflammatory effects of
glu-cocorticoids (Calandra and Bucala, 1997; Kitaichi et al., 2000). New evidence, however, showed a wider spectrum of action for MIF and an involvement in angiogenesis and tissue remod-elling (Metz and Bucala, 1997; Nishihira, 1998; Calandra and Roger, 2003; Nishihira et al., 2003). For instance, MIF was shown to promote directly endothelial cell proliferation in vitro (Chesney et al., 1999; Ogawa et al., 2000; Yang et al., 2000), to stimulate angiogenesis in vivo (Chesney et al., 1999; Nishihira
et al., 2003) and to induce the synthesis and the secretion of
matrix metalloproteinases in different cell types (Meyer-Siegler, 2000; Onodera et al., 2000), including endometrial cells accord-ing to our recent data (A.Akoum and M.J.Therriault, unpub-lished data). Accordingly, it is quite possible that local induction and production of MIF may contribute to active tissue remod-elling and angiogenesis that take place in the endometrial tissue and are required for the tissue’s growth and regeneration, as well as to the inflammatory-like process of leukocyte inva-sion and menstrual breakdown occurring at the end of the cycle in the absence of implantation (Critchley et al., 2001).
The findings of the present study may have a particular rele-vance for the understanding of endometriosis, a common gyne-cological disorder where an endometrial-like tissue creates lesions outside the uterus (Sampson, 1927; Taylor, 2003). Endometriosis is recognized as an inflammatory-like disorder; a chronic immunoinflammatory process has often been described
in the peritoneal cavity of endometriosis patients (Halme et al., 1987; Taylor, 2003; Wu and Ho, 2003). Peritoneal fluid from patients with endometriosis was shown to contain high levels of leukocytes, particularly macrophages, and inflammatory cytokines. These cytokines could then contribute to the develop-ment of peritoneal endometriosis by promoting endometrial cell adhesion, invasion and proliferation (Hammond et al., 1993; Zhang et al., 1993; Barcz et al., 2000; Wu and Ho, 2003).
Several studies have shown a significant role of TNF-α in endometriosis pathophysiology. Elevated concentrations of TNF-α were found in the peritoneal fluid of women with endometriosis (Rana et al., 1996; Barcz et al., 2000; Iwabe et al., 2000; Bedaiwy et al., 2002; Bullimore, 2003; Darai et al., 2003). TNF-α appeared to stimulate metalloproteinase expression (Sillem
et al., 2001), to favour cell adhesion to mesothelial cells (Zhang et al., 1993) and to play a considerable role in
endometriosis-associated immunoinflammatory changes (Sakamoto et al., 2003). Interestingly, TNF-α appeared to enhance eutopic and ectopic endometrial cell growth (Iwabe et al., 2000; Braun et al., 2002; Sakamoto et al., 2003), but failed to do so in endometrial cells of controls without endometriosis (Braun et al., 2002). This is suggestive of an endometriosis-related intrinsic difference in endometrial cell responsiveness to TNF-α, and in keeping with our previous data showing an increased secretion of monocyte chemotactic protein-1 (MCP-1) in endometrial epi-thelial cells of women with endometriosis exposed to TNF-α in
vitro (Akoum et al., 1995). The effect of TNF-α on MIF
secre-tion by eutopic as well as ectopic endometrial cells of women with endometriosis remains to be elucidated, and it is still to be determined whether these cells had a modified responsiveness to TNF-α with regard to MIF secretion. Nevertheless, our data showing a marked stimulatory effect of TNF-α on endometrial stromal cell secretion of MIF, suggest that peritoneal fluid TNF-α may interact with retrograde endometrial tissue and activate NF-κB, thereby stimulating MIF secretion and exacer-bating inflammation. This is all the more plausible since our previous studies showed a marked increase in MIF expression in ectopic endometrial tissue and the peritoneal fluid of endometriosis patients and a relationship with the disease stage (Kats et al., 2002a; b).
In conclusion, our study provides for the first time evidence that TNF-α increases MIF expression in human endometrial stromal cells and that such an effect is mediated by NF-κB. This, owing to the properties of these two major and multifunc-tional cytokines, may play an important role in the cyclic changes occurring in the human endometrial tissue and poten-tially contribute to endometriosis pathophysiolgy.
Acknowledgements
This work is supported by grant MOP-77737 to A.A. from the Canadian Institutes of Health Research. Dr T. Roger’s contribution in this work was supported by grants from the Leenaards Foundation and the Swiss National Science Foundation (3100-066972-01). The authors wish to thank Drs François Belhumeur, Jean Blanchet, Marc Bureau, Simon Carrier, Elphège Cyr, Marlène Daris, Jean-Louis Dubé, Jean-Yves Fontaine, Céline Huot, Pierre Huot, Johanne Hurtubise Rodolphe Maheux, Jacques Mailloux and Marc Villeneuve for patient evalua-tion and providing endometrial biopsies, Madeleine Desaulniers,
Monique Longpré, Johanne Pelletier, Sylvie Pleau and Marie-Josée Therriault for technical assistance, and Dr RM Brenner, Oregon National Primate Research Center, OR, USA, for critical reading of the manuscript’s discussion. W.-G.C. holds a Wyeth-Ayerst CIHR/ Rx&D Fellowship; A.A. is a ‘Chercheur Boursier National’ of the Fonds de la Recherche en Santé du Québec (FRSQ).
References
Akoum A, Lemay A, Brunet C and Hebert J (1995) Secretion of monocyte chemo-tactic protein-1 by cytokine-stimulated endometrial cells of women with endome-triosis. Le groupe d’investigation en gynecologie. Fertil Steril 63,322–328. Barcz E, Kaminski P and Marianowski L (2000) Role of cytokines in
patho-genesis of endometriosis. Med Sci Monit 6,1042–1046.
Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR and Agarwal A (2002) Prediction of endometriosis with serum and perito-neal fluid markers: a prospective controlled trial. Hum Reprod 17,426–431. Bergqvist A, Nejaty H, Froysa B, Bruse C, Carlberg M, Sjoblom P and Soder O
(2000) Production of interleukins 1beta, 6 and 8 and tumor necrosis factor alpha in separated and cultured endometrial and endometriotic stromal and epithelial cells. Gynecol Obstet Invest 50,1–6.
Bergqvist A, Bruse C, Carlberg M and Carlstrom K (2001) Interleukin 1beta, interleukin-6, and tumor necrosis factor-alpha in endometriotic tissue and in endometrium. Fertil Steril 75,489–495.
Bharti AC, Donato N, Singh S and Aggarwal BB (2003) Curcumin (diferuloyl-methane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to sup-pression of proliferation and induction of apoptosis. Blood 101,1053–1062. Bigonnesse F, Labelle Y and Akoum A (2001) Triphasic expression of interleukin-1
receptor type I in human endometrium throughout the menstrual cycle of fertile women and women with unexplained infertility. Fertil Steril 75,79–87. Bloom BR and Bennett B (1970) Relation of the migration inhibitory factor
(MIF) to delayed-type hypersensitivity reactions. Ann N Y Acad Sci 169,258–265.
Boeuf P, Vigan-Womas I, Jublot D, Loizon S, Barale JC, Akanmori BD, Mercereau-Puijalon O and Behr C (2005) CyProQuant-PCR: a real time RT–PCR technique for profiling human cytokines, based on external RNA standards, readily automatable for clinical use. BMC Immunol 6,5. Boucher A, Mourad W, Mailloux J, Lemay A and Akoum A (2000) Ovarian
hormones modulate monocyte chemotactic protein-1 expression in endome-trial cells of women with endometriosis. Mol Hum Reprod 6,618–626. Boucher A, Kharfi A, Al-Akoum M, Bossu P and Akoum A (2001)
Cycle-dependent expression of interleukin-1 receptor type II in the human endometrium. Biol Reprod 65,890–898.
Braun DP, Ding J and Dmowski WP (2002) Peritoneal fluid-mediated enhancement of eutopic and ectopic endometrial cell proliferation is dependent on tumor necrosis factor-alpha in women with endometriosis. Fertil Steril 78,727–732.
Bullimore DW (2003) Endometriosis is sustained by tumour necrosis factor-alpha. Med Hypotheses 60,84–88.
Calandra T and Bucala R (1997) Macrophage migration inhibitory factor (MIF): a glucocorticoid counter-regulator within the immune system. Crit Rev Immunol 17,77–88.
Calandra T and Roger T (2003) Macrophage migration inhibitory factor: a reg-ulator of innate immunity. Nat Rev Immunol 3,791–800.
Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A and Bucala R (1995) MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377,68–71.
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N and Williamson B (1975) An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA 72,3666–3670.
Chegini N, Dou Q and Williams RS (1999) An inverse relation between the expression of tumor necrosis factor alpha (TNF-alpha) and TNF-alpha receptor in human endometrium. Am J Reprod Immunol 42,297–302. Chesney J, Metz C, Bacher M, Peng T, Meinhardt A and Bucala R (1999) An
essential role for macrophage migration inhibitory factor (MIF) in angiogen-esis and the growth of a murine lymphoma. Mol Med 5,181–191.
Critchley HO, Kelly RW, Brenner RM and Baird DT (2001) The endocrinol-ogy of menstruation—a role for the immune system. Clin Endocrinol (Oxf) 55,701–710.
D’Acquisto F, May MJ and Ghosh S (2002) Inhibition of nuclear factor kappa β (NF-β): an emerging theme in anti-inflammatory therapies. Mol Intervent 2,22–35.
Darai E, Detchev R, Hugol D and Quang NT (2003) Serum and cyst fluid lev-els of interleukin (IL)-6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum Reprod 18,1681–1685.
David JR (1966) Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci USA 56,72–77.
Feldmann M and Maini RN (1999) The role of cytokines in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 38 Suppl 2,3–7.
Ghosh S and Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109 (Suppl), S81–S96.
Giacomucci E, Bulletti C, Polli V and Flamigni C (1994) Immunologically mediated abortion (IMA). A minireview. Ann N Y Acad Sci 734,235–236. Halme J, Becker S and Haskill S (1987) Altered maturation and function of
peritoneal macrophages: possible role in pathogenesis of endometriosis. Am J Obstet Gynecol 156,783–789.
Hammond MG, Oh ST, Anners J, Surrey ES and Halme J (1993) The effect of growth factors on the proliferation of human endometrial stromal cells in culture. Am J Obstet Gynecol 168,1131–1136; discussion 1136–1138. Hazout A (1995) Tumor necrosis factor and underlying infection. Contracept
Fertil Sex 23,631–634.
Hunt JS (1993) Expression and regulation of the tumour necrosis factor-alpha gene in the female reproductive tract. Reprod Fertil Dev 5,141–153. Hunt JS, Chen HL, Hu XL and Tabibzadeh S (1992) Tumor necrosis
factor-alpha messenger ribonucleic acid and protein in human endometrium. Biol Reprod 47,141–147.
Iwabe T, Harada T, Tsudo T, Nagano Y, Yoshida S, Tanikawa M and Terakawa N (2000) Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endocrinol Metab 85,824–829.
Kats R, Al-Akoum M, Guay S, Metz C and Akoum A (2005) Cycle-dependent expression of macrophage migration inhibitory factor in the human endometrium. Hum Reprod. First published Aug 5, 2005: 10.1093/humrep/ dei234.
Kats R, Collette T, Metz CN and Akoum A (2002a) Marked elevation of mac-rophage migration inhibitory factor in the peritoneal fluid of women with endometriosis. Fertil Steril 78,69–76.
Kats R, Metz CN and Akoum A (2002b) Macrophage migration inhibitory fac-tor is markedly expressed in active and early-stage endometriotic lesions. J Clin Endocrinol Metab 87,883–889.
Kitaichi N, Kotake S, Mizue Y, Sasamoto Y, Goda C, Iwabuchi K, Onoe K, Matsuda H and Nishihira J (2000) High-dose corticosteroid administration induces increase of serum macrophage migration inhibitory factor in patients with Vogt-Koyanagi-Harada’s disease. Microbiol Immunol 44,1075–1077. Lebovic DI, Shifren JL, Ryan IP, Mueller MD, Korn AP, Darney PD and
Taylor RN (2000) Ovarian steroid and cytokine modulation of human endometrial angiogenesis. Hum Reprod 15 (Suppl 3),67–77.
Makarov SS, Johnston WN, Olsen JC, Watson JM, Mondal K, Rinehart C and Haskill JS (1997) NF-kappa B as a target for anti-inflammatory gene ther-apy: suppression of inflammatory responses in monocytic and stromal cells by stable gene transfer of I kappa B alpha cDNA. Gene Ther 4,846–852. Metz CN and Bucala R (1997) Role of macrophage migration inhibitory factor
in the regulation of the immune response. Adv Immunol 66,197–223. Meyer-Siegler K (2000) Macrophage migration inhibitory factor increases
MMP-2 activity in DU-145 prostate cells. Cytokine 12,914–921.
Nishihira J (1998) Novel pathophysiological aspects of macrophage migration inhibitory factor. Int J Mol Med 2,17–28.
Nishihira J, Ishibashi T, Fukushima T, Sun B, Sato Y and Todo S (2003) Mac-rophage migration inhibitory factor (MIF): Its potential role in tumor growth and tumor-associated angiogenesis. Ann N Y Acad Sci 995,171–182. Ogawa H, Nishihira J, Sato Y, Kondo M, Takahashi N, Oshima T and Todo S
(2000) An antibody for macrophage migration inhibitory factor suppresses tumour growth and inhibits tumour-associated angiogenesis. Cytokine 12,309–314.
Onodera S, Kaneda K, Mizue Y, Koyama Y, Fujinaga M and Nishihira J (2000) Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J Biol Chem 275,444–450.
Philippeaux MM and Piguet PF (1993) Expression of tumor necrosis factor-alpha and its mRNA in the endometrial mucosa during the menstrual cycle. Am J Pathol 143,480–486.
Rana N, Braun DP, House R, Gebel H, Rotman C and Dmowski WP (1996) Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril 65,925–930.
Roby KF, Weed J, Lyles R and Terranova PF (1990) Immunological evidence for a human ovarian tumor necrosis factor-alpha. J Clin Endocrinol Metab 71,1096–1102.
Sakamoto Y, Harada T, Horie S, Iba Y, Taniguchi F, Yoshida S, Iwabe T and Terakawa N (2003) Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa B activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. J Clin Endocrinol Metab 88,730–735.
Sampson J (1927) Peritoneal endometriosis due to menstrual dissemination of endometrial tisue into the peritoneal cavity. Am J Obstet Gynecol 14,422–469.
Sharkey AM, Dellow K, Blayney M, Macnamee M, Charnock-Jones S and Smith SK (1995) Stage-specific expression of cytokine and receptor mes-senger ribonucleic acids in human preimplantation embryos. Biol Reprod 53,974–981.
Sillem M, Prifti S, Koch A, Neher M, Jauckus J and Runnebaum B (2001) Regulation of matrix metalloproteinases and their inhibitors in uterine endometrial cells of patients with and without endometriosis. Eur J Obstet Gynecol Reprod Biol 95,167–174.
Tabibzadeh S (1996) The signals and molecular pathways involved in human menstruation, a unique process of tissue destruction and remodelling. Mol Hum Reprod 2,77–92.
Tabibzadeh S (1998) Molecular control of the implantation window. Hum Reprod Update 4,465–471.
Tabibzadeh S, Zupi E, Babaknia A, Liu R, Marconi D and Romanini C (1995) Site and menstrual cycle-dependent expression of proteins of the tumour necrosis factor (TNF) receptor family, and BCL-2 oncoprotein and phase-specific production of TNF alpha in human endometrium. Hum Reprod 10,277–286.
Taylor MM (2003) Endometriosis—a missed malady. AORN J 77,298,301– 309,312–313; quiz 314–316.
von Wolff M, Classen-Linke I, Heid D, Krusche CA, Beier-Hellwig K, Karl C and Beier HM (1999) Tumour necrosis factor-alpha (TNF-alpha) in human endometrium and uterine secretion: an evaluation by immunohistochemis-try, ELISA and semiquantitative RT–PCR. Mol Hum Reprod 5,146–152. von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W and Tabibzadeh S
(2000) Regulated expression of cytokines in human endometrium through-out the menstrual cycle: dysregulation in habitual abortion. Mol Hum Reprod 6,627–634.
Wu MY and Ho HN (2003) The role of cytokines in endometriosis. Am J Reprod Immunol 49,285–296.
Xu YX, Pindolia KR, Janakiraman N, Chapman RA and Gautam SC (1997) Curcumin inhibits IL1 alpha and TNF-alpha induction of AP-1 and NF-κB DNA-binding activity in bone marrow stromal cells. Hematopathol Mol Hematol 11,49–62.
Yang Y, Degranpre P, Kharfi A and Akoum A (2000) Identification of macro-phage migration inhibitory factor as a potent endothelial cell growth-promoting agent released by ectopic human endometrial cells. J Clin Endocrinol Metab 85,4721–4727.
Zhang RJ, Wild RA and Ojago JM (1993) Effect of tumor necrosis factor-alpha on adhesion of human endometrial stromal cells to peritoneal mes-othelial cells: an in vitro system. Fertil Steril 59,1196–1201.
Zolti M, Ben-Rafael Z, Meirom R, Shemesh M, Bider D, Mashiach S and Apte RN (1991) Cytokine involvement in oocytes and early embryos. Fertil Steril 56,265–272.
Submitted on November 12, 2004; resubmitted on August 2, 2005; accepted on August 22, 2005