HAL Id: hal-01208120
https://hal.archives-ouvertes.fr/hal-01208120
Submitted on 27 May 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Cardiac effects of long-term active immunization with
the second extracellular loop of human ß1- and/or
ß3-adrenoceptors in Lewis rats
E Montaudon, Laurence Dubreil, Valerie Lalanne, M. Vermot Des Roches,
Gilles Toumaniantz, Marion Fusellier, Jean-Claude Desfontis, Lionel
Martignat, Mohamed Yassine Mallem
To cite this version:
E Montaudon, Laurence Dubreil, Valerie Lalanne, M. Vermot Des Roches, Gilles Toumaniantz, et
al..
Cardiac effects of long-term active immunization with the second extracellular loop of
hu-man ß1- and/or ß3-adrenoceptors in Lewis rats. Pharmacological Research, 2015, 100, pp.210-219.
�10.1016/j.phrs.2015.08.006�. �hal-01208120�
See discussions, stats, and author profiles for this publication at:
http://www.researchgate.net/publication/281082314
Cardiac effects of long-term active
immunization with the second extracellular
loop of human β1- and/or β3-adrenoceptors in
Lewis rats
ARTICLE
in
PHARMACOLOGICAL RESEARCH · AUGUST 2015
Impact Factor: 4.41 · DOI: 10.1016/j.phrs.2015.08.006 · Source: PubMed READS13
9 AUTHORS
, INCLUDING:
Valerie Lalanne
École Nationale Vétérinaire, Agroalimentair…
7
PUBLICATIONS
81
CITATIONS
SEE PROFILE
Marion Fusellier
École Nationale Vétérinaire, Agroalimentair…
20
PUBLICATIONS
68
CITATIONS
SEE PROFILE
Desfontis Jean-Claude
École Nationale Vétérinaire, Agroalimentair…
50
PUBLICATIONS
218
CITATIONS
SEE PROFILE Available from: Elodie Montaudon Retrieved on: 01 October 2015
ContentslistsavailableatScienceDirect
Pharmacological
Research
jo u r n al hom e p ag e :w w w . e l s e v i e r . c o m / lo c a t e / y p h r s
Cardiac
effects
of
long-term
active
immunization
with
the
second
extracellular
loop
of
human
!
1
-
and/or
!
3
-adrenoceptors
in
Lewis
rats
E.
Montaudon
a,
L.
Dubreil
b,
V.
Lalanne
a,
M.
Vermot
Des
Roches
a,
G.
Toumaniantz
c,
M.
Fusellier
d,
J.-C.
Desfontis
a,
L.
Martignat
e,
M.Y.
Mallem
a,∗aLUNAMUniversity,Oniris,UPSP5304ofAnimalPathophysiologyandFunctionalPharmacology,AtlanpôleLaChantrerie,BP40706,44307Nantes,France
bLUNAMUniversity,Oniris,INRAUMRU703,PanTHER,AtlanpôleLaChantrerie,BP40706,44307Nantes,France
cLUNAMUniversity,INSERM,UMR1087/CNRS6291,InstitutduThorax,NantesF44007France
dLUNAMUniversity,Oniris,INSERMUMRS791,LIOAD,AtlanpôleLaChantrerie,BP40706,44307Nantes,France
eLUNAMUniversity,Oniris,UPSPSSBR,AtlanpôleLaChantrerie,BP40706,44307Nantes,France
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received6March2015
Receivedinrevisedform28June2015
Accepted7August2015
Availableonline11August2015
Keywords: Auto-antibodies !-Adrenoceptor Cardiaccontractility Dilatedcardiomyopathy Rats
a
b
s
t
r
a
c
t
!1-and!3-adrenoceptor(AR)auto-antibodiesweredetectedinpatientswithdilatedcardiomyopathy.
Manystudieshaveshownthat!1-ARauto-antibodieswithpartialagonist-likeeffectplayanimportant
roleinthepathogenesisofthisdisease.Moreover,arecentstudycarriedoutinourlaboratoryhasshown that!3-ARantibodies(!3-ABs),producedinrats,wereabletoreducecardiomyocytecontractilityvia
!3-ARactivation.Theaimsofthisstudywere(1)toinvestigate,inisolatedcardiomyocytesfromrabbit,
theroleofGiproteinsinthe!3-ABs-inducedcardiacnegativeinotropy,(2)todeterminewhether!3-ABs
mayexhibit!3-ARantagonisticpropertywhichischaracteristicofpartialagonists,and(3)todetermine
whetherlong-termactiveimmunizationproducingboth!1-ABsand/or!3-ABsleadstothedevelopment
ofcardiacdysfunctioninLewisrats.
Lewisratswereimmunizedfor6monthswithpeptidicsequencescorrespondingtothesecond extra-cellularloopofhuman!3-ARand/or!1-AR.Agonisticeffectof!3-ABswasevaluatedonelectrically
field-stimulatedisolatedcardiomyocytesfromadultrabbitbymeasuringthecellshortening. Echocar-diographyandexvivoisolatedperfusedheartstudieswereconductedonimmunizedrats.Finally,!-AR expressionwasquantifiedbyimmunofluorescenceandRT-qPCR.
SR58611A(10nM),apreferential!3-ARagonist,andpurified!3-ABs(25"g/ml)inducedadecrease
incellshortening(−39.71±4.9%(n=10)and−17.06±3.9%(n=10)respectively).Thiseffectwas signif-icantlyinhibitedwhenthecardiomyocyteswerepreincubatedwithpertussistoxin(0.3"g/ml),aGi
proteininhibitor(p<0.05).Inaddition,SR58611A-mediatednegativeinotropiceffectwasdecreased when cardiomyocytes were preincubated with !3-ABs (p<0.0001). Echocardiography revealed a
decreaseinthefractionalshorteningandejectionfractioninratsimmunizedagainst!1-ARandboth
!1-and!3-AR.However,thestudyonisolatedheartshowedadecreaseoftheisoproterenol-induced
lusitropicandinotropiceffectsinthe3groupsofimmunizedrats.Thesesystolicanddiastolicdysfunctions arecorrelatedwithadecreaseintheexpressionof!1-ARsandanincreaseof!3-ARsinratsimmunized
againstthe!1-ARandanincreaseofboth!3-ARand!1-ARinratsimmunizedagainstthe!3-AR.Forthe
firsttime,theseresultsshowedthat!3-ABshada!3-ARpartialagonist-likeactivitywhichmightplaya
roleinthepathogenesisofcardiacdysfunction.
©2015ElsevierLtd.Allrightsreserved.
Abbreviations: AB,antibody; AR,adrenoceptor;AAB, autoantibody;cAMP,
cyclicadenosinemonophosphate;DCM,dilatedcardiomyopathy;DP,developed
pressure;dP/dt,timederivativesofpressure;EDD,leftventricularend-diastolic
diameter; EDV, left ventricular end-diastolic volume; EF, ejection fraction;
ELISA, enzyme-linked immunosorbent assay; eNOS, endothelial nitric oxide
synthase;ESD,leftventricularend-systolicdiameter;ESV,leftventricular
end-systolic volume; FS, fractional shortening; IgG, immunoglobulin; MFI, mean
fluorescence intensity; NO, nitric oxide; OD, optical densities; PTX,
pertus-sistoxin;SR58611A,
[(RS)-N-[(25)-7-ethoxycarbonylmethoxy-1,2,3,4-tetrahydro-napht-2-yl]-(2R)-2(3-chlorophenyl)-2hydroethanaminehydrochloride].
∗ Correspondingauthor.
E-mailaddress:yassine.mallem@oniris-nantes.fr(M.Y.Mallem).
http://dx.doi.org/10.1016/j.phrs.2015.08.006
E.Montaudonetal./PharmacologicalResearch100(2015)210–219
1. Introduction
Idiopathicdilatedcardiomyopathy(DCM) isoneof themain causeofsevereheartfailureinyoungadults.In60–70%ofcases,the etiologyremainspoorlyunderstoodandgrowingevidencessuggest thattheimmunitysystemmayplayakeyroleinthisdisease[1].
!1-Adrenoceptor (AR) auto-antibodies (AABs) were first
detected by ELISA (enzyme-linked immunosorbent) in 26 to 60% of patients with DCM [2,3]. These !1-AABs are directed
againstthesecondextracellularloopofhuman!1-AR[4].Invitro
and in vivo studies have shown that !1-AABs induce
posi-tive inotropicand chronotropic effects viathe!1-AR/adenylate
cyclase/cAMP-dependentproteinkinase-Apathway[5–8]. Accord-ingtoMagnussonetal.[7],these!1-AABssharesomepropertiesof
partialagonists.Theycanactasanagonistandactivateweaklythe !1-ARs.Onthecontrary,theycanactasanantagonistofthe!1-ARs
andblockthemwhenthecatecholaminelevelsarehigh. Further-more, rator rabbit immunizationwiththesecondextracellular loop of !1-ARhasbeenreported tobeable toinduce
myocar-dial dysfunctionthatmay leadtoventricledilatation similarto thatobservedinpatients[9,10].Thiseffectisconsideredtoresult fromlong-termoverstimulationofthe!1-ARsbythe!1-AABs.In
agreementwiththoseobservations,clinicalstudieshavealsofound that!1-AABssuppressionbyimmunoabsorptionimprovescardiac
functionofpatientswithDCM[11,12],strengtheningthe possibil-itythat!1-AABsplayanimportantpathophysiologicalroleinthe
developmentofthisdisease.
Morerecently,circulating!3-AABsdirectedagainstthesecond
extracellularloopofthe!3-AR,havealsobeendetectedin30%of
serafrompatientswithheartfailure[13].Arecentstudycarried outinourlaboratoryhasshownthat!3-ARantibodies(!3-ABs),
producedinrats,wereabletoreducecardiomyocytecontractility via!3-ARactivation[14].Nevertheless,veryfewstudieshavebeen
donetocharacterizetheseauto-antibodiesandtoevaluatetheir involvementinDCM.
In additionto!1-AR, !3-ARis alsoexpressedinthehuman
andanimalventriclemyocardium.Itsactivationwasdescribedto induceanegativeinotropiceffectthatinvolvedGiprotein/nitric
oxide(NO)pathway[15].Severalstudies,conductedinfailingor non-failing myocardiumhavereportedthat the!1-ARsand !3
-ARsarecross-regulatedbyinteractivecompensatorymechanisms [16–19].Theopposed changesinthe!1-ARand !3-AR-induced
myocardial contraction in response to adrenergic stimulation seemstoplayaroleintheworseningoftheheartcontractility. To the best of our knowledge, it is not known whether simi-lar regulationmay occurin response to !1-AABsand !3-AABs
that could possesssympathomimetic-like properties.Therefore, themainobjectiveofthisstudywastodeterminewhether long-termactiveimmunizationproducingboth!1-and/or!3-ABsleads
tothedevelopmentofcardiacdysfunctioninLewisrats.Moreover, consideringthe!3-ARs-mediatedpertussistoxin(PTX)-sensitive
effectintheheart[20]andthe!3-ARagonist-likeactivityofthe
!3-ABs[14],wefirstinvestigated,inisolatedcardiomyocytesfrom
rabbit,theroleofGiproteinsinthe!3-ABs-inducedcardiac
nega-tiveinotropyandwhether!3-ABsmayexhibit!3-ARantagonistic
property. 2. Methods 2.1. Animals
Whole experimental project was validated by local ethics committeeforanimalexperimentation(N◦CEEA.2012.76)and
con-ducted in accordance with“The guidefor the care and use of laboratoryanimals”publishedbytheNationalInstituteofHealth
(NIHpublication,eightedition,2010).Nineweek-oldmaleLewis rats from Janvier Labs (Le Genest Saint Isle, France) and New Zealand rabbits(2 kilograms) from Hypharm (Roussay, France) wereusedforthisstudy.Theywerehousedatconstant temper-ature(22±2◦C)andsubjectedtoacycleofdark/light12:12h,with
standardchowanddrinkingwaterprovidedadlibitum. 2.2. Immunizationprotocol
Ratswereimmunizedbysubcutaneousinjectionsofan anti-gendissolvedin1mlofasolutioncontainingNa2CO3(0.1M)and
!-mercapto-ethanol1%,conjugatedwithFreund’sadjuvant(V/V) monthlyfor6months.Forthat,ratswererandomlydividedinto 4groups.Thefirstgroup(n=10)wasimmunizedwiththeantigen correspondingtothepeptidicsequenceofthesecondextracellular loop of human !1-AR (residues 197–222:
H-W-W-R-A-E-S-D-E-A-R-R-C-Y-N-D-P-K-C-C-D-F-V-T-N-R;1mg/ml)synthesizedby GeneCust (Dudelange, Luxembourg). The second group (n=10) was immunized with the antigen corresponding to the pep-tidicsequenceofthesecondextracellularloopofhuman!3-AR
(residues 176–200: R-V-G-A-D-A-E-A-Q-E-C-H-S-N-P-R-C-C-S-F-A-S-N-M-P; 2mg/ml) (GeneCust, Dudelange, Luxembourg). The thirdgroup(n=10)wasimmunizedwithboth !1-AR(1mg/ml)
and!3-AR(2mg/ml)peptidesandthelastgroup(n=10)
(adjuvant-treated)receivedonlysalineconjugatedwithFreund’sadjuvant (0.5ml).
2.3. ˇ1-andˇ3-adrenergicreceptorantibodiesdetectionand
purification
Serawerecollectedbeforethefirstimmunizationandafter2, 4and6monthsofimmunization.Theevolutionof!1-and!3-AB
titerswasfollowedbypeptide-basedELISA.
For that,!1-ARand !3-AR peptides(10"g/ml)used forthe
immunization were dissolved in BIC buffer (Na2CO3 0.1mol/l;
NaHCO30.1mol/l;indistilledwater;pH9.6)andcoatedona
96-wellmicroplate(polyNUNC,Denmark)overnightat4◦C.Serum
dilutions(100"l)from1:400to1:51,200inPBS-Tween80-NaCl 0.5mol/l, wereusedtoreactwiththepeptidesfor 1hat37◦C.
Afterwashing3timeswithPBS-Tween80,100"lofdonkey anti-ratimmunoglobulin(IgG)antibodyconjugatedwithhorseradish peroxidase (1:50,000 dilution in PBS-Tween 80-NaCl 0.5mol/l) (Jackson ImmunoResearch, USA) were added to the wells and incubated 1h at 37◦C. After 3 washings, 100"l of 3,3$, 5,5$
-tetramethylbenzidine(Sigma–Aldrich)wereincubatedat37◦Cto
detecttheboundantibodies.Thereactionwasstoppedafter20min ofincubationbyadding50"lofsulphuricacid(0.1mol/l).Optical densities(OD)werereadat450nminamicroplatereader(TriStar, BertholdTechnologies,BadWildbad,Germany).Theantibodytiters weredefinedbytheODvalues.
IgGfractionswerepurifiedfromseracollectedafter6monthsof immunizationusingtheProteusProteinGkit(AbDSerotec,Colmar, France)incompliancewiththemanufacturer’sinstructions. Puri-fiedantibodyconcentrationsweredeterminedbythebicinchoninic acidproteinassay(Uptima,Interchim,Montluc¸on,France). 2.4. FunctionalcharacterizationofIgGscontainingˇ3-adrenergic
receptorantibodies
Thefunctionalityofpurified!3-ABswasevaluatedon
ventric-ularcardiomyocytesisolatedinhealthyrabbit,whichisknownto expressfunctional!3-ARs[21].Briefly,rabbitswereanesthetized
withpentobarbitone(54mg/kgIV) andheparinized (2500IU/kg IV).Cardiomyocyteswereisolatedbyperfusion(7ml/min)ofheart mountedona Langendorff apparatuswith1mg/ml collagenase typeII(Worthington,Lakewood,NI,USA)and0.04mg/mlprotease
E.Montaudonetal./PharmacologicalResearch100(2015)210–219
typeXIV(Sigma–Aldrich,France)(30"MCaCl2)for20min.After
gentlemanualstirring,cardiaccellswereprogressivelyexposed toincreasingCa2+concentrationsin100%O2aeratedTyrode
solu-tion(NaCl137mM;KCl5.4mM;MgCl21.2mM;Na2HPO41.2mM;
HEPES20mM;d-glucose10mM;pH7.4).Cardiomyocyteswere platedonpoly-l-lysine-coated35mmculturedishesandperfused ataflowrateof3–4ml/minwithTyrodesolution(1.8mMCaCl2)
at37◦C.Cellswerestimulatedbyanelectricfield(1Hz)at9Vand
cellshorteningwasrecordedusingavideo-imagingsystem (Coy-oteBayInstruments,Manchester,NH,USA).Imaginganalysiswas performedusingMatroxInspectorsoftware(CoyoteBay Instru-ments,Manchester,NH,USA).Myocytesselectedfordataanalysis presentedclearstriation,rod-shapedformanda stablediastolic lengthatbaseline.Tenconsecutiveheartcontractionswereused fortheanalysis.
2.5. Echocardiography
At6monthsofimmunization,ratswerelightlyanaesthetized with1–1.5%isofluraneuntiltheheartratestabilizedto350–400 beats per minute. Transthoracic echocardiographywas realized usingahighfrequencyultrasoundimagingsystem(MyLab70XVG, Esaote,Indianapolis,IN)withalinear18Mhztransducer.Thefocal length used was adjusted around 10mm. To identify whether the immunization leads to the development of functional and structuralcardiacdysfunctions,fractionshortening(FS),ejection fraction(EF),leftventricularend-systolicdiameter(ESD),left ven-tricularend-diastolicdiameter(EDD),leftventricularend-systolic volume (ESV) and left ventricular end-diastolic volume (EDV) weredeterminedusing M-Moderecordingsin theshort axisof the heart. ESV and EDV were calculated using the monoplane area-lengthmethod:V=8×A2/3!L.FSandEF wererespectively
calculated using theformulas FS=[(EDD−ESD)/EDD]×100and EF=[(EDV−ESV)/EDV]×100.Threeconsecutiveheartcycleswere measuredandtheaverageusedforanalysis.
2.6. Exvivocardiacfunction
At 6 months of immunization, half of immunized rats were anaesthetized by intraperitoneal injection of pentobarbi-tone (54mg/kg) and heparinized (2500IU/kg) (n=5, for each group). The heart was immediately removed and placed into ice-cold Krebs–Henseleit buffer (NaCl 118.3mM; KCl 4.7mM; MgSO4,7H2O 1.2mM; KH2PO4 1.2mM; NaHCO3 20mM; EDTA
0.016mM;Glucose 11.1mM;CaCl2 2.5mM; pH7.4). The aorta
wasrapidly cannulatedand theheart retrogradely perfused by a non-recirculating-Langendorfftechniqueat aconstant flowof 10ml/min in continuously gassed (95%O2/5%CO2) prewarmed
(37◦C)KHbuffer. Todetermineleftventricularfunction,alatex
balloonwasinsertedintotheleftventriclethroughthemitralvalve andfilleduntiltheleftventricularend-diastolicpressurereached avalueof5mmHg.Anequilibrationperiodofatleast30minwas requiredtoensurethestabilityofrecordedparametersbeforeany additionofmolecule.Foreachimmunizedrat,systolic,diastolic, developedpressuresandtheheartratewererecordedinitiallyand aftertheadditionof(–)-isoproterenolhydrochloride(100nM).Left ventriculardevelopedpressure(DP)wasdeterminedasthe differ-encebetweenleftventricularsystolicpressureandleftventricular end-diastolicpressure.Timederivativesofpressurewere calcu-latedelectronicallyduringcontraction(dP/dTmax)andrelaxation (dP/dTmin)usinga Powerlab8/30Data Acquisitionsystemand analyzedbyLabChart®Prosoftware(V7,ADInstruments,France).
2.7. RealtimequantitativeRT-PCR
Theotherhalfofimmunizedrats(n=5,foreachgroups)were usedtoperformrealtimeRT-qPCRtoevaluatetheeffectof immu-nizationsonthemRNAlevelsof!1-AR,!2-ARand!3-ARinthe
heart.Briefly,heartfragmentswererapidlyfrozeninliquid nitro-genandstoredat−80◦C.Frozensamplesweredissolvedin1.5ml
ofTRIzol®Reagentandgroundtopowderinapotter,accordingto
manufacturer’sinstructions(LifeTechnologies,France).TotalRNA wereobtainedafterchloroformextractionandisopropanol precip-itationandresuspendedin50"lofRNase-freewater,heatedat 58◦Candstoredat−20◦C.ThetotalRNAintegritywascheckedby
agarosegeleletrophoresis.TotalRNAconcentrations,260/280and 260/230ratiosweredeterminedbyspectrophotometry(Nanodrop 2000,ThermoScientific,France).
ToremoveanycontaminatinggenomicDNA,aDNasedigestion step(DNaseIrecomb,RocheDiagnosticsFrance,France)was car-riedoutaccordingtomanufacturer’sinstructions.DNA-freetotal RNAwererecoveredin20"lofRNase-freewater.Absenceof con-taminating DNA fragments was again verified with a sensitive GAPDHPCR(primersinTable1)andwithagarosegel electrophore-sis.DNA-freetotalRNAconcentrationsandDOratioswerethen determinedbyspectrophotometry.
Each sample underwent the following reverse transcription step:1"gofDNA-freetotalRNAwasusedin20"ltotalofmix solutionbasedontheSuperscriptIVReverse-Transcriptasekit(Life Technologies,France),withboth1"lofRandomPrimers(3"g/"l) and1"lofOligo(dT)20Primer(50"M)forRT.RetrotranscribedRNA
efficiencywasfurthervalidatedwithanewGAPDHPCRandwith agarosegelelectrophoresis.
RealtimePCRreactionsweresetin15"lwith5XHOTFIREPol®
EvaGreen® qPCR Mix (high ROX) (Solis BioDyne, Riia, Estonia),
includingeither5"lofdilutedcDNA(1:6)and1"lofeachprimers (10"M).Afteraninitialdenaturation/DNApolymeraseactivation step of 15min at 95◦C, the PCR forty cycles consisted of 10s
at95◦Cdenaturationstepandof1minat60◦C(forADRB-1and
ADRB-2primers)or61◦C(forADRB-3andeNosprimers)
hybridiza-tion/elongationstep.Meltingcurveswereproducedtoconfirmthe presenceofasinglegenespecificpeakandtheabsenceofprimer dimers.Thequantificationofgeneexpressionwasbasedonthe 2−""Ctmethod[27].Briefly,themeanCtvalueofthecontrol
house-keepinggene!-actinwassubtractedfrommeanthresholdcycle valuesofduplicatesforeachinterestmRNA.Thedifferenceinthe mean"Ctvaluesbetweenadjuvant-treatedratsand!-immunized ratsallowsthecalculationofrelativelevelsofinducedorrepressed expressionoftheinterestgene.
2.8. Immunofluorescenceandquantificationofˇ-adrenergic receptorexpression
RatheartusedfortheRT-qPCRwerealsousedtoquantify!-AR expressionin theheart (n=5,foreach groups).Forthat, imme-diately aftertheexcision,heartwastransversallysectionedand themiddlesectionwaspost-fixedbyincubationin4% phosphate-bufferedparaformaldehyde(pH7.4)for4hat4◦C.After3washings
withPBSsolution,itwascryoprotectedbyovernightincubationin PBScontaining20%sucrose.Then,heartsectionwasembeddedin transversepositioninTissueTekOCTmediumandimmediately frozenbyimmersioninliquidisopentane.Frozensections(8"m thick) forimmunohistochemicalanalysiswerethawedand sub-mergedfor5mininacetoneat4◦Candnonspecificantigenbinding
wasblockedbyincubatingsectionsfor1hinaPBSsolution con-taining 0.3%Triton100Xand 2%bovineserumalbumin(Sigma) followingby1hat37◦Cincubationintheblockingbufferwitha
rabbitpolyclonalantibodyagainst!1-AR(1:50,SantaCruz
E.Montaudonetal./PharmacologicalResearch100(2015)210–219
Table1
Oligonucleotideprimersusedinthestudy.Oligonucleotidesequenceorientationsareindicatedasforward(Fw)andreverse(rv).
Primername Accession num-ber/publication Sequence(5$–3$) Ampliconsize(bp) ADRB-1-Fw ADRB-1-Rv NM012701 [22] CTGCTACAACGACCCCAAGTG AACACCCGGAGGTACACGAA 120 ADRB-2-Fw ADRB-2-Rv NM012492 Ourdesign CTCCTTAACTGGTTGGGCTATG TCCCATAGGTTTTCGAAGAAGA 127 ADRB-3-Fw ADRB-3-Rv NM013108 [23,24] GCCGAGACTACAGACCATAACCA CATTACGAGGAGTCCCACTACCA 79 eNos-Fw eNos-Rv NM 021838 [25] AGCTGGATGAAGCCCGGTGAC CCTCGTGGTAGCGTTGCTGA 60 B-Act-Fw B-Act-Rv NM007393 Ourdesign TTGCTGACAGGATGCAGAAG GTACTTGCGCTCAGGAGGAG 86 GAPDH-Fw GAPDH-Rv AF106860 [26] ACTGGCGTCTTCACCACCATGGAGAAGGCT CTCCTTGGAGGCCATGTAGGCCATGAGGTC 720
(1:100,SantaCruzBiotechnologyInc.,CA,USA)for1hat37◦C.After
beingwashedinPBS,thesectionswereincubatedwiththe sec-ondaryantibody,AlexaFluor555-conjuguateddonkeyanti-rabbit (1:300,LifeTechnologies,SaintAubin,France)ordonkeyanti-goat (1:300,LifeTechnologies,SaintAubin,France)during1hatroom temperature.Afterbeingwashed,thesectionswerestainedwith nucleardyeDRAQ5(1/1000,BioStatus,Shepshed,UnitedKingtom) and mountedin Mowiol(Calbiochem,SanDiego,CA,USA). The immunolabeled sectionswerescannedseriallyusingthehelium neonlaser(543nm)toobserveAlexafluor555(!-AR immunola-bellings)andwithaheliumneonlaser(633nm)toobserveDRAQ5 signals(nuclei).Eachimagewasrecordedinaseparatedchannel (channelredforAlexafluor555andchannelblueforDRAQ5)and overlayed.Acquisitionswereperformedbyusingaconfocal micro-scope(Nikon,C1,Champigny-sur-Marne,France).Imageanalysis wereperformedtoevaluate!-ARexpressionlevelintheheart ven-tricleofimmunizedratsbyusingFijisoftware.MeanFluorescence Intensity(MFI)valuesfor!-ARimmunolabellingineachcondition wereacquiredfrom5differentfieldsofimmunolabeledventricule heartsectionsbysectionwith5ratsbyconditions.Finally, analy-seswereperformedatleaston550±100cardiacfibersbyanimal. Thesamethresholdwasusedtomeasurethesumintensity fluo-rescenceof!-ARimmunolabellingineachsectionandtheMFIwas reportedtototalareaofanalyzedsection.
2.9. Drugs
(–)-Isoproterenol hydrochloride, L-748337 hydrate, pertussis toxin from Bordetella pertussis were obtained from Sigma–Aldrich (France). SR58611A [(RS)-N-[(25)-7-ethoxycarbonylmethoxy-1,2,3,4-tetrahydro-napht-2-yl]-(2R)-2(3-chlorophenyl)-2hydroethanamine
hydrochloride] was agenerous giftfrom Sanofigroup (France). Alldrugswerepreparedindistilledwaterwiththeexceptionof SR58611A that wasdissolvedin ethanol. Sodiumpentobarbital solution was purchased from CEVA Santé Animale (Libourne, France)andheparinechoayfromSanofiAventis(Paris,France). 2.10. Statisticalanalysis
Resultswereexpressedasa mean±SEMwherenrepresents thenumberofratsorrabbitsusedforthestudy.Statistical differ-encesincomparisonwiththeadjuvant-treatedratswereevaluated usingunpairedStudent’sttestwithPrism®softwareV.5.The
con-ditionsforthet-testweretestedwithaKolmogorov–Smirnov’test fortheGaussiandistributionandwithanF-testforthe homogene-ityofvariances.Fortheexvivostudy,differencesweredetermined usingnonparametricMann-Whitneytestandforthe
immunoflu-orescenceanalysisusingaunivariateStudent’sttest(Rsoftware V3.0.1).Avalueforp<0.05wasconsideredstatisticallysignificant. 3. Results
3.1. ˇ–adrenoceptorantibodyproductionandcharacterization !1-ABsand!3-ABsweregeneratedbyimmunizingratsfor6
monthsandtheirrespectiveimmunoreactivitiesweredetermined byELISA.Twomonthsafterthefirstimmunization,weobserveda highproductionofspecific!1-ABsinratsimmunizedwith!1-ARor
!1-and!3-ARpeptides(Fig.1).Afterthe6monthsof
immuniza-tion,!1-ABtiterswerestillhighandstablecomparedtothelow
levelofproductioninantibodytitersin!3-AR-immunizedratsand
adjuvant-treatedrats(ODat6months:2.09±0.24and2.30±0.21 for!1-ARand!1-and!3-AR-immunizedratsrespectivelyversus
0.07±0.005and0.08±0.03foradjuvant-treatedratsand!3
-AR-immunizedratsrespectively).Inthesameway,weobservedahigh increaseof!3-ABtitersinboth!3-AR-immunizedratsand!1-and
!3-AR-immunizedratsafter6monthsofimmunizationcompared
withthe!1-AR-immunizedratsandadjuvant-treatedrats(ODat
6months:1.37±0.26and2.28±0.18for!3-ARand!1-and!3
-AR-immunizedratsrespectivelyversus0.07±0.006and0.14±0.02 foradjuvant-treatedratsand!1-AR-immunizedratsrespectively)
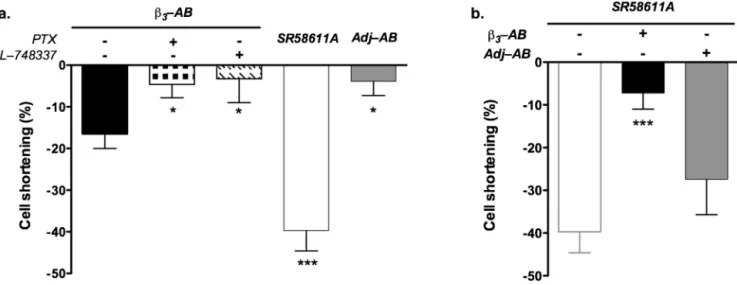
(Fig.1).Thedatahighlightedantibodyspecificityfortheirantigen andthelackofcross-reactivitybetweenthetwosubtypesof!-AR. 3.2. Negativeinotropiceffectofˇ3-adrenoceptorantibody
Todetermine whether!3-ABs exhibit an agonistic effect as
itwasalreadydescribed for!1-ABs [8],wemeasuredtheeffect
of!3-ABperfusiononelectricallyfield-stimulated cell
shorten-ing.!3-ABsinducedadecreaseofcellshortening(−17.06±3.9%,
n=10)andthis effectwassignificantly inhibitedinpresence of PTX(0.3"g/ml), a Gi proteininhibitor, and L-748337 (1"M), a
selective!3-ARantagonist(#<0.05)(Fig.2a).SR58611A(10nM),a
preferential!3-ARagonistproducedalsoasignificantdecreaseof
cellshorteningwhichwasstrongerthanthatinducedby!3-ABs
(−39.71±4.9%,n=10 and −3.88±3.4%,n=7, respectively).This negativeinotropiceffectwasnotobservedwhencardiomyocytes wereperfusedwiththeantibodiesfromadjuvant-treatedrats.
Then,assumingthat!3-ABsbehavedaspartial!3-ARagonists,
wetestedtheirability toexhibit !3-ARantagonisticproperties.
Incubationofrabbitcardiomyocytesfor2hwith!3-ABs(25"g/ml)
significantly reduced the SR58611A-induced negative inotropy (−7.22±3.8%,n=4)incomparisonwithantibodiesfrom ajduvant-treatedrats(−27.47±8.23%,n=7)(Fig.2b).
E.Montaudonetal./PharmacologicalResearch100(2015)210–219
Fig.1. Curvesshowingtheevolutionof!1-adrenoceptor(!1-AR)(a)and!3-adrenoceptor(!3-AR)(b)antibodytitersdeterminedbyELISAatdilution1/1600,inseraof
adjuvant-treatedrats(emptycircles,n=10),!1-AR-immunizedrats(emptysquares,n=10),!3-AR-immunizedrats(fullcircles,n=10)and!1-ARand!3-AR-immunized
rats(fullsquares,n=10).Antibodytitersaredefinedasmean±SEMofopticaldensityvalues,***p<0.0001versusadjuvant-treatedgroupdeterminedbyunpairedStudent’s
t-testatthedifferenttimes.
Fig.2. Effectsof!3-adrenoceptor(!3-AR)antibodiesonrabbitisolatedcardiomyocytecontractility.(a)Negativeinotropiceffectof!3-ARantibodies(25"g/ml)without
(blackbar,n=11)orwithpreincubation(2h)inthepresenceofpertussistoxin(0.3"g/ml)(n=4)orL-748337(1"M)(n=4),SR58611A(10nM)(whitebar,nn=10)and
adjuvantantibodies(25"g/ml)(greybar,n=7).(b)NegativeinotropiceffectofSR58611A(10nM)withpreincubation(2h)inthepresenceof!3-ARantibodies(25"g/ml)
(blackbar,n=6)oradjuvantantibodies(25"g/ml)(greybar,n=7).Valueswererepresentedasmean±SEM,*p<0.05,***p<0.0001versuscellsthatwerenotpreincubated,
determinedbyunpairedStudent’st-test.
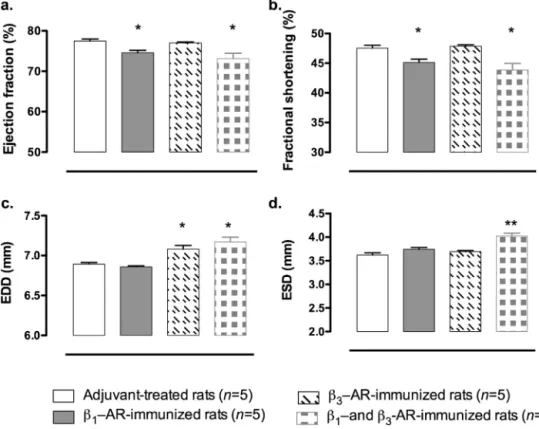
3.3. Influenceofimmunizationsoncardiacfunction—invivo study
Toevaluatewhetherthedifferentimmunizationswereableto impactthecardiaccontractility,weperformedechocardiography. DataindicatedthatEFandFSweredecreasedinratsimmunized during 6 months with!1-AR (EF: −3.7% and FS: −5.1% versus
adjuvant-treated rats)or both !1/3-AR peptides (EF:−5.6% and
FS: −7.8% versus adjuvant-treated rats) compared to adjuvant-treatedrats(EF:77.50±0.47;FS:47.53±0.48)(Fig.3).TheEDDand ESDwerenotalteredbytheimmunizationin!1-AR-immunized
rats while in!1/3-AR-immunizedrats both EDDand ESD were
increased(EDD:+4.02%andESD:+11.13%versusadjuvant-treated rats)(Fig.3).Incontrast,immunizationwithonly!3-ARpeptide
didnotaltertheEFandFSbutincreasedtheEDD(+2.73%versus adjuvant-treatedrats)(Fig.3c).Moreover,systolicbloodpressure wasnotmodifiedduringthe6monthsofimmunizationinthe4 groups ofrats(datanotshown)suggestingthat theseobserved effectsoncardiaccontractilitywerenotduetochangesinarterial pressureanddidnotimpactbloodpressure.
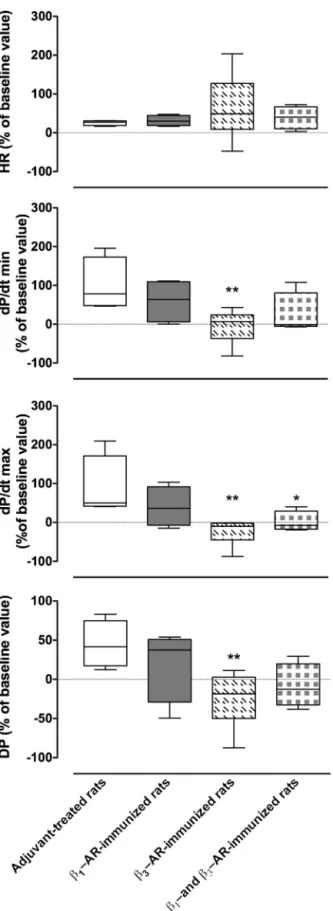
3.4. Influenceofimmunizationsoncardiacfunction—exvivo study
The influence of chronic immunization on left ventricular contractilitywasalsoevaluatedexvivousinga non-recirculating-Langendorfftechnique.Foreachgroupofrats,systolic,diastolic, developed pressures and the heart rate were recorded under
E.Montaudonetal./PharmacologicalResearch100(2015)210–219
Fig.3. Influenceofimmunizationwithpeptidescorrespondingtothesecondextracellularloopof!1-adrenoceptoror!3-adrenoceptororboth!1-and!3-adrenoceptorand
withadjuvantoncardiacfunction.(a)Ejectionfraction(EF),(b)fractionalshortening(FS),(c)leftventricularend-diastolicdiameter(EDD),(d)leftventricularend-systolic
diameter(ESD).Valueswererepresentedasmean±SEM,*p<0.05,**p<0.001versusadjuvant-treatedrats,n=5ratsforeachgroups.
basalconditionsandaftertheadditionofisoproterenol(100nM). The data showed that isoproterenol increased the heart rate, the maximal and minimal first derivative of change in pres-sureovertime(dP/dt(max/min))andthedevelopedpressureof heartsfromadjuvant-treatedrats.Theseeffectsweredecreased inheartsfromratsimmunizedwith!1-ARor!3-ARor!1/3-AR
peptides (Fig.4).Given that heart contractilityat baseline was not altered by immunizations(data not shown),these findings revealedthatimmunizationswitha!-ARpeptide(!1-ARand/or
!3-AR)decreasedtheisoproterenol-inducedlusitropy(dP/dtmin)
andtheinotropyparameters(dP/dtmaxanddevelopedpressure). We showedthat!1-ARand !3-AR-immunizationshadasimilar
outcomeonheartfunction.
3.5. Influenceofimmunizationsonˇ-adrenoceptormRNAand expression
RT-qPCRwerecarriedouttodeterminewhetherchronic immu-nizations modifiedcardiac mRNAlevel of!-ARs.Thedata have shown that !1-AR-immunization induced a slight increase of
!1-AR transcripts compared to adjuvant-treated rats whereas
!2-AR, !3-AR and eNOS (endothelial NO synthase) transcripts
seemed tonot be changed. A similar transcription profile was alsoobservedin!1/!3-AR-immunized.The!3-AR-immunization
induced a slight increase of !1-AR, !3-AR transcripts but no
modification of the !2-AR and eNOS mRNA levels (Fig. 5). To
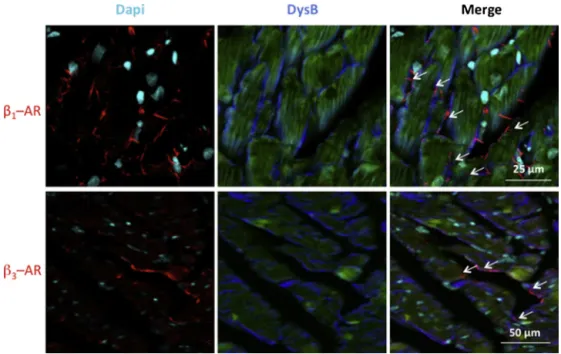
determine theexpression profileof the !1-ARand !3-AR
pro-teins on heart, immunofluorescence labeling were carried out. First, themembranelocalizationof!–ARs wascheckedby per-forming a co-labeling of !-AR/Dystrophin B to confirm the antibodyspecificity.Theco-labelingresultsshowedthat!1-ARs
and !3-ARs were expressed at the cell membrane in heart of
adjuvant-treatedrats(Fig.6).Thedataonexpression!1-ARand
!3-AR profile were generally in accordance with the
observa-tionsonmRNAlevels(Fig.7).The!1-AR-immunizationreduced
!1-AR expression (−11.89±9.74%; n=5) but increased that of
!3-ARs(+51.42±15.93%;n=5)whereasthe!3-AR-immunization
increasedboth !1-ARand !3-ARexpressions (+89.06±20.69%;
n=5and123.60±28.04%;n=5,respectively). 4. Discussion
Thepresentstudyclearlydemonstratedthat!3-ABs purified
from!3-AR-immunizedratsinduceda!3-ARpartialagonist-like
activityonisolatedrabbitcardiomyocytes.Inaddition,byexvivo approach,werevealedthatratimmunizationsfor6months pro-ducingfunctional!1-ABsand/or!3-ABsledtothedevelopmentof
bothsystolicanddiastolicdysfunctions.
Inthiswork,peptide-basedELISAmethodhasallowedtotest thespecificityofthe!3-ABsforthesecondextracellularloopofthe
!3-AR.Immunizedratshaveproducedhighconcentrationof!1-AR
and!3-ARIgG-likeABs.Inaddition,!3-ABsdidnotcross-reactwith
!1-ARsthatshareahighdegreeofstructuralandbiochemical
simi-laritieswith!3-ARs.AccordingtoBornholzetal.[2],!1-AABsfrom
patientswithDCMpreferentiallyrecognizeanative!1-AR
confor-mation.Therefore,thepeptide-basedELISAmethodseemsnottobe themostappropriatemethodtodetecthuman!1-AABs.However,
inthispresentwork,theuseofpeptidestodetectantibodies gen-eratedbyimmunizationinratswasnotanissuebecauseweused thesamepeptidesforboththeimmunizationandthedetectionof antibodies.
Wepreviouslyshowedthat!1-ABsproducedbyimmunization
ofratswith!1-ARpeptideinducedapositiveinotropiceffect[8].
Inordertotestwhether!3-ABspossessalsobiologicalactivity,
weperformedinotropic studyoncardiomyocytesisolatedfrom adulthealthyrabbit.The!3-ABs-inducedpertussistoxin-sensitive
effectstronglysuggeststhatthe!3-ABsareendowedwithnegative
E.Montaudonetal./PharmacologicalResearch100(2015)210–219
Fig.4.Cardiacresponsestoisoproterenol(100nM)inadjuvant-treatedrats(n=5)
andratsimmunizedwithpeptidescorrespondingtothesecondextracellularloopof
!1-adrenoceptor(AR)(n=5)or!3-AR(n=5)orboth!1-ARand!3-AR(n=5).Heart
rate(HR),timederivativesofpressureduringcontraction(dP/dtmax)and
relax-ation(dP/dtmin)anddevelopedpressure(DP)wererepresentedasmean±SEMof
percentageincreasefrombaselinewithsetat100%,*p<0.05versusadjuvant-treated
rats,determinedbynonparametricMann–Whitneytest.
Fig.5.ComparativeanalysisofthemRNAlevelsof!1-adrenoceptor(AR),!2-AR,!3
-ARandendothelialnitricoxidesynthase(eNOS)inheartfromadjuvant-treatedrats
(greybars),!1-AR-immunizedrats(blackbars),!3-AR-immunizedrats(whitebars)
andboth!1-and!3-AR-immunizedrats(strippedbars).Valueswereexpressedas
2−""Ctusing!-actinasahousekeepinggeneandwererepresentedasmean±SEM. pathway.Inaddition,thedecreaseofSR58611-inducednegative inotropyaftertheincubationofcardiomyocyteinpresenceof!3
-ABsindicatesthatthe!3-ABscanalsoantagonizethe!3-ARs.Taken
together,theseresultsareinfavorofapartialagonist-likeactivity ofthe!3-ABsonheart.
Theimmunizationprotocolusedtoproducespecificantibodies behaving likeAABs has beenalready applied tostudy the car-diostimulating effects of !1-AABs in rat and rabbit [8,28]. Our
findingsagreewitharecentstudyshowingthat!3-AABspurified
frompatientswithDCMinducednegativeinotropicandnegative chronotropiceffectsonratneonatalcardiomyocytes[29]. There-fore,itmightbearguedthat!3-ABsaswellas!1-ABswouldnot
E.Montaudonetal./PharmacologicalResearch100(2015)210–219
Fig.6. Immunofluorescentco-labelingof!1-adrenoceptors(ARs)or!3-ARsanddystrophinBinheartfromadjuvant-treatedrats.
Fig.7. Influenceofimmunizationwithpeptidescorrespondingtothesecondextracellularloopof!1-adrenoceptor(AR)(n=5)or!3-AR(n=5)on!1-AR(whitebars)and!3
-AR(blackbars)expressionsinheartcomparedtoadjuvant-treatedrats.Valueswererepresentedasmean±SEMofpercentageincreaseoffluorescencefromadjuvant-treated
E.Montaudonetal./PharmacologicalResearch100(2015)210–219
bejustabiomarker,asstatedbyMiaoetal.[30],butwouldhavea functionalrolewhichneedsmorethoroughinvestigation.
Toevaluatewhethercirculating!1-ABsand!3-ABshaving
ago-nisticpropertieswereabletoimpactthemyocardialfunction,rats wereimmunizedduring6monthswith!1-ARand/or!3-AR
pep-tides.
Asitwasalreadydescribed[31,32],ourresultsshowedaslight but a significantdecrease of fractional shortening and ejection fraction in rats immunized 6 months with the !1-AR peptide
without modifications of systolic arterialpressure, assessed by tailcuffmethod.Theseobservationswereconcordantwith pre-viousstudiesshowing thatleft ventricledysfunctions appeared after6monthsofimmunizationagainst!1-AR[9,10].However,
inourstudy,theinabilityof!1-ABstoinducethedevelopment
ofleftventricledilatation(i.e.,nomodificationofend-systolicand -diastolic diameters) is likely linked to thefact that 6 months ofimmunizationisnotsufficienttoobservenoticeablealteration inmyocardialcontractility.Inaccordancewithechocardiographic results,ourexvivofindingsalsorevealedbothsystolicand dias-tolic dysfunctions in rats immunized with the !1-AR or both
!1-and !3-ARpeptides.Thesedysfunctionswerecharacterized
by a reduction of isoproterenol-induced inotropy and lusitropy that likely involved an impairment of global !-AR reactivity (i.e., modification in !-AR expression or !-ARs-linked mecha-nisms). In linewiththis hypothesis, thequantification of !-AR expression by immunofluorescence revealed a downregulation of!1-ARsandanup-regulationof!3-ARsin!1-AR-immunized
rats when compared with theadjuvant-treated rats in the left ventricle. Thispatternof regulationwasalready reportedtobe involved in the alteration of myocardial contractility and was similar to that observed in patients with DCM [16,17]. Sur-prisingly, the !1-AR mRNA was not decreased and the !3-AR
mRNAwasnotsignificantlyincreasedin!1-AR-immunizedrats.
The reason of this discrepancy is not readily apparent in our study,butthechangeinthepost-transcriptionalorinthe post-translational mechanisms could explain the lack of correlation betweenmRNAandproteinexpressionswhichremainstobe elu-cidated.
Onepossibleexplanation toaccountforthecross-regulation of the !1-ARsand the!3-ARs in ourstudy is that !1-ABs, by
theirchronic!1-ARagonisticactionfollowingimmunization,could
desensitize !1-ARsand could upregulate!3-ARs asa
compen-satorymechanism.Somestudieshavedescribedthelossof!1-ARs
inresponseto!1-AABs[33]ortheexistenceofcross-regulation
between!1-ARsand!3-ARsintheheartwhen!1-ARsare
chron-ically stimulated[34].Theupregulationof!3-ARsisconsidered
usefulinthefirststageoftheheartfailurebecauseofitsprotective effectbyantagonizingthe!1-ARoverstimulation.
Similarlyto!1-AR-immunizedrats,exvivostudyhasrevealed
thedevelopmentofbothdiastolicandsystolicdysfunctionsin!3
-AR-immunizedrats,which werecharacterizedbya decreaseof isoproterenol-inducedinotropyandlusitropy.Thereby,weshowed in this study that theproduction of cardio-stimulatory !1-ABs
or cardio-inhibitory !3-ABs byimmunizing ratswith !1-ARor
!3-ARpeptidehad asimilaroutcomeonheartfunction.In rats
immunizedwith!3-AR peptide,theimmunofluorescenceassay
andRT–qPCRhavehighlightedanupregulationof!3-AR
expres-sionandmRNAintheleftventricle.Thus,itisnotunreasonable topostulatethatisoproterenol-mediatednegativeinotropiceffect via!3-AR activationcouldhave contributedtotheimpairment
ofcardiacfunctionin!3-AR-immunizedrats.Ideallytheeffetof
isoproterenol in thepresence of !3-AR antagonist shouldhave
beenperformedtoaccuratelyconfirmtheroleofthe!3-ARsin
theimpairmentofisoproterenol-inducedinotropy.Inourstudy, sinceeNOShasbeenshowntoplayaroleinthe!3-ARs-mediated
negativeinotropy[15],wetestedthehypothesisthatitsincreased
expressionthrougha largerproductionofNOcouldexplainthe depressedcontractilityobservedin!3-AR-immunizedrats.
Nev-ertheless,thelackofchangeofeNOSmRNAintheseratsargues against a roleof theGi protein/NO pathwaytoaccountfor the
reduced responsetoisoproterenolandsupportstheviewofthe existenceofalternativemechanisms(i.e.,reducedcAMPlevelvia Giproteins)thatneedfurtherinvestigation.
Althoughthepresentstudyhasnotspecificallyaddressedthe potential mechanism involved in the upregulation of ventricu-lar !3-ARs, sustainedactivation of!3-ARsby !3-ABsfollowing
long-term immunization could be considered as one possible explanationofthispatternofregulation.Inaccordancewiththis contention, recent studies have shown that stimulation of the !3-ARsinducedanincrease ofthe!3-ARexpression[35,36].In
addition,itwasshownthatanupregulationof!3-ARswasable
toaltercontractileresponsetoisoproterenolinmice[37]andin humanfailingmyocardium[16].Nevertheless,thosefindingsdo notagreewiththepreviousreport[29,38]showingtheprotective role of!3-AABsagainstcardiacdysfunction,anddonotsupport
the pointof viewthat !3-ARupregulation mayberegarded as
cardioprotectivemechanismthatmaybedevelopedtoprevent car-diomyocytedamage.
More interestingly,thedysfunctionsobservedexvivo inrats immunizedagainstthe!3-ARwerenotdetectedbyour
echocardio-graphicmeasurements.Weshowedthat6monthsofimmunization with!3-ARpeptidedidnotmodifythefractionalshorteningand
ejection fraction. The lack of correlation between ex vivo and echocardiographic parameters has already been reported [39]. In our study,thereason of this discrepancy is notclear, butit may be explained by theintervention of compensatory neuro-hormonalmechanismsthatmightoperateinvivothrough!1-ARs
to maintainmyocardial contractility.This emphasizesthe need totakeintoaccountthephysiologicalcontextintheinterpreting and theevaluationof leftventricularfunction.Moreover,based on our results, we cannot rule out the hypothesis that under invivoconditions,circulating!3-ABscouldhaveexhibiteda!3
-antagonisticpropertyinresponsetoendogenouscatecholamines that mightbe responsible for undetectablecardiac dysfunction implying!3-ARcomponent.However,whetherprolonged
immu-nizationperiodwith!3-ARpeptidecouldinduceimpairmentof
echocardiographic contractile parameters remains to be deter-mined.
Therearesomelimitationsofthisstudy.First,concerningthe functional study of !3-ABs, despite that rabbit cardiomyocytes
constitute a relevant approach to assess in vitro effects of !3
-ABs, translation toinvivoor totheclinicalsetting shouldonly madewithcaution.The!3-ABsproducedbyimmunizationinrats
couldhaveadifferentactionthanthatof!3-AABsfrompatients
withDCMwhich needsfurtherinvestigationinthefuture. Sec-ond,concerningtheinfluenceofimmunization,theimmunization time of6monthshasbeenselectedbecauseZuoetal.[9] have reportedthattheleftventriculardilationappearedfrom5months of immunization with!1-AR peptide.However, in viewof our
results,itseemsthattheputativeeffectof!3-ABsoncardiac
func-tionisamoreslowlyprocessandhence,theimmunizationtime would need tobeincreased in thefuture. Third,in contrastto the exvivo experiments,ourechocardiographicapproach failed tofindanyevidenceofcardiacdysfunctionin!3-AR-immunized
rats.Thissuggeststhatmeasurementsoffractionalshorteningand ejection fractionwould not bealways suitablefor the evaluat-ingofheartcontractilityunderbasalinvivoconditions.Therefore, future experiments using exogenous inotropic agents (i.e., iso-proterenol) willberequireedtoaccurately quantifythechange in the myocardial contractility that may occur in immunized rats.
E.Montaudonetal./PharmacologicalResearch100(2015)210–219
5. Conclusions
Theresultsofthepresentstudyshowedforthefirsttimethat !3-ABs induceda!3-ARpartialagonist-likeactivityinvolvinga
roleofGiproteinsinisolatedrabbitcardiomyocytes.Inaddition,
weshowthatimmunizationsfor6monthsproducingfunctional !1-ABsor!3-ABsledtothedevelopmentofsystolicanddiastolic
dysfunctionsinheartbyremodelingthe!1-AR/!3-ARratiointhe
leftventricle. Conflictsofinterest
None.
Acknowledgements
Theauthorswould liketothankChantalThorinforher help concerningthestatisticalanalysisandMireilleLedevinandSonia Becavinforthetechnicalassistance.
References
[1]G.Wallukat,I.Schimke,Agonisticautoantibodiesdirectedagainst
G-protein-coupledreceptorsandtheirrelationshiptocardiovascular
diseases,Semin.Immunopathol.36(2014)351–363.
[2]B.Bornholz,S.Weidtkamp-Peters,S.Schmitmeier,C.A.M.Seidel,L.R.Herda,
S.B.Felix,H.Lemoine,J.Hescheler,F.Nguemo,C.Schäfer,M.O.Christensen,C.
Mielke,F.Boege,Impactofhumanautoantiblodieson!1-adrenergicreceptor
conformation,activity,andinternalization,Cardiovasc.Res.97(2013)
472–480.
[3]J.M.Lappé,C.M.Pelfrey,W.W.H.Tang,Recentinsightsintotheroleof
autoimmunityinidiopathicdilatedcardiomyopathy,J.Card.Fail.14(2008)
521–530.
[4]R.Mobini,M.Fu,G.Wallukat,Y.Magnusson,A.Hjalmarson,J.A.Hoebeke,
monoclonalantibodydirectedagainstanautoimmuneepitopeonthehuman
beta1-adrenergicreceptorrecognizedinidiopathicdilatedcardiomyopathy,
Hybridoma19(2000)35–42.
[5]G.Wallukat,A.Kayser,A.Wollenberger,The!1-adrenoceptorasantigen:
functionalaspects,Eur.HeartJ.16(1995)85–88.
[6]A.Staudt,R.Mobini,M.Fu,Y.Grosse,V.Stangl,A.Thiele,G.Baumann,S.B.
Felix,Beta(1)-adrenoceptorantibodiesinducepositiveinotropicresponsein
isolatedcardiomyocytes,Eur.J.Pharmacol.423(2001)115–119.
[7]Y.Magnusson,G.Wallukat,F.Waagstein,F.Hjalmarson,J.Hoebeke,
Autoimmunityinidiopathicdilatedcardiomyopathy:characterizationof
antibodiesagaisntthebeta1-adrenoceptorwithpositivechronotropiceffect,
Circulation89(1994)2760–2767.
[8]M.A.Abdelkrim,M.Y.Mallem,G.Chatagnon,M.Gogny,J.C.Desfontis,J.
Noireaud,Autoantibodiesagainst!(1)-adrenoceptordonotaffectthe
low-affinitystate!(1)-adrenoceptor-mediatedinotropyinrat
cardiomyocytes,Can.J.Physiol.Pharmacol.90(2012)308–316.
[9]L.Zuo,H.Bao,J.Tian,X.Wang,S.Zhang,Z.He,L.Yan,R.Zhao,X.L.Ma,H.Liu,
Long-termactiveimmunizationwithasyntheticpeptidecorrespondingto
thesecondextracellularloopofbeta1-adrenoceptorinducesboth
morphologicalandfunctionalcardiomyopathicchangesinrats,Int.J.Cardiol.
149(2001)89–94.
[10]R.Jahns,V.Boivin,L.Hein,S.Triebel,C.E.Angermann,G.Ertl,M.J.Lohse,Direct
evidencefora!1-adrenergicreceptor-directedautoimmuneattackasacause
ofidiopathicdilatedcardiomyopathy,J.Clin.Invest.113(2004)1419–1429.
[11]W.V.Dörffel,G.Wallukat,G.Baumann,S.B.Felix,Immunoabsorptionin
dilatedcardiomyopathy,Ther.Apher.4(2000)235–238.
[12]S.B.Felix,A.Staudt,W.V.Dörffel,V.Stangl,K.Merkel,M.Pohl,W.D.Döcke,S.
Morgera,H.H.Neumaver,K.D.Wernecke,G.Wallukat,K.Stangl,G.Baumann,
Hemodynamiceffectsofimmunoabsorptionandsubsequent
immunoglubulinsubstitutionindilatedcardiomyopathy:threemonths
resultsfromarandomizedstudy,J.Am.Coll.Cardiol.35(2000)1590–1598.
[13]M.X.Li,X.L.Wang,J.N.Tang,X.J.Liu,J.Tian,L.Yan,H.R.Liu,Distributionand
propertyofanti-beta3-adrenoceptorautoantibodyinpatientswithheart
failure,ZhonghuaXinXueGuanBingZaZhi33(2005)1114–1118.
[14]E.Montaudon,M.A.Abdelkrim,J.C.Desfonstis,Y.Mallem,Cardiovascular
effectsofbeta1andbeta3-adrenergicreceptorautoantibodiesinLewisrat,
Ann.Cardiol.Angeiol.63(2014)128–134.
[15]C.Gauthier,V.Leblais,L.Kobzik,J.N.Trochu,N.Khandoudi,A.Bril,J.L.
Balligand,H.LeMarec,Thenegativeinotropiceffectofbeta3-adrenoceptor
stimulationismediatedbyanitricoxidesynthasepathwayinhuman
ventricle,J.Clin.Invest.102(1998)1377–1384.
[16]S.Moniotte,L.Kobzik,O.Feron,J.N.Trochu,C.Gauthier,J.L.Balligand,
Upregulationofbeta(3)-adrenoceptorsandalteredcontractileresponseto
inotropicaminesinhumanfailingmyocardium,Circulation103(2001)
1649–1655.
[17]Q.Zhao,F.Zeng,J.B.Liu,Y.He,B.Li,Z.F.Jiang,T.G.Wu,L.X.Wang,
Upregulationof!3-adrenergicreceptorexpressionintheatriumofratswith
chronicheartfailure,J.Cardiovasc.Pharmacol.Ther.18(2013)133–137.
[18]U.Mackiewicz,E.Klemenska,A.Beresewicz,Beta-adrenergicreceptorsin
normalandfailingheart,Kardiol.Pol.65(2007)294–302.
[19]T.A.Kohout,H.Takaoka,P.H.McDonald,S.J.Perry,L.Mao,R.J.Lefkowitz,H.A.
Rockman,Augmentationofcardiaccontractilitymediatedbythehuman
beta(3)-adrenergicreceptoroverexpressedintheheartsoftransgenicmice,
Circulation104(2001)2485–2491.
[20]C.Gauthier,G.Tavernier,F.Charpentier,D.Langin,H.LeMarec,Functional
beta3-adrenoceptorinthehumanheart,J.Clin.Invest.98(1996)556–562.
[21]L.Audigane,B.G.Kerfant,A.ElHarchi,I.Lorenzen-Schmidt,G.Toumaniantz,
A.Cantereau,D.Potreau,F.Charpentier,J.Noireaud,C.Gauthier,Rabbit,a
relevantmodelforthestudyofcardiacbeta3-adrenoceptors,Exp.Physiol.94
(2009)400–411.
[22]Z.Anna,S.Angela,B.Barbara,R.Jana,B.Tamara,V.Csilla,D.Victor,M.Oleksiy,
T.Narcisa,Heart-protectiveeffectofn-3PUFAdemonstratedinaratmodelof
diabeticcardiomyopathy,Mol.Cell.Biochem.389(1–2)(2014)219–227.
[23]S.Treskatsch,A.Feldheiser,A.T.Rosin,M.Sifringer,H.Habazettl,S.A.Mousa,
M.Shakibaei,M.Schäfer,C.D.Spies,Amodifiedapproachtoinduce
predictablecongestiveheartfailurebyvolumeoverloadinrats,PLoSOne9
(2014)e87531.
[24]Y.Hatakeyama,Y.Sakata,S.Takakura,T.Manda,S.Mutoh,Acuteandchronic
effectsofFR-149175,abeta3-adrenergicreceptoragonist,onenergy
expenditureinZuckerfattyrats,Am.J.Physiol.Regul.Integr.Comp.Physiol.
287(2004)R336–R341.
[25]K.Takemori,E.Yamamoto,H.Ito,T.Kometani,Prophylacticeffectsofelastin
peptidederivedfromthebulbusarteriosusoffishonvasculardysfunctionin
spontaneouslyhypertensiverats,LifeSci.120(2015)48–53.
[26]Y.Rautureau,G.Toumaniantz,S.Serpillon,P.Jourdon,J.N.Trochu,C.Gauthier,
Beta3-adrenoceptorinrataorta:molecularandbiochemicalcharacterization
andsignallingpathway,Br.J.Pharmacol.137(2002)153–161.
[27]K.J.Livak,T.D.Schmittgen,Analysisofrelativegeneexpressiondatausing
real-timequantitativePCRandthe2(-DeltaDeltaC(T))method,Methods25
(2001)402–408.
[28]Y.Gao,H.R.Liu,R.R.Zhao,J.M.Zhi,Autoantibodyagainstcardiac
beta1-adrenoceptorinducesapoptosisinculturedneonatalrat
cardiomyocytes,Acta.Biochim.Biophys.Sin.38(2006)443–449.
[29]J.Wang,M.Li,X.Ma,K.Bai,L.Wang,Z.Yan,T.Lv,Z.Zhao,R.Zhao,H.Liu,
Autoantibodiesagainstthe!3-adrenoceptorprotectfromcardiacdysfunction
inaratmodelofpressureoverload,PloSOne8(2013)e78207.
[30]G.Miao,Z.Chen,X.Fang,M.Liu,G.Hao,H.An,Z.Zhang,L.Lu,J.Zhang,L.
Zhang,Relationshipbetweentheautoantibodyandexpressionof
!3-adrenoceptorinlungandheart,PloSOne8(2013)e68747.
[31]X.Hao,S.Li,H.Liu,B.Wu,Immunizationwithbeta(1)-adrenoreceptor
peptideinducescardiomyopathy-likechangesinrabbithearts,Chin.Med.J.
115(2002)170–174.
[32]L.Buvall,M.S.Täng,A.Isic,B.Andersson,M.Fu,Antibodiesagainstthe
beta1-adrenergicreceptorinduceprogressinedevelopmentof
cardiomyopathy,J.Mol.Cell.Cardiol.42(2007)1001–1007.
[33]C.J.Limas,I.F.Goldenberg,C.Limas,Effectofantireceptorantibodiesindilated
cardiomyoapthyonthecyclingofcardiacbetareceptors,Am.HeartJ.122
(1991)108–114.
[34]C.Ufer,R.Germack,Cross-regulationbetweenbeta1-andbeta
3-adrenoceptorsfollowingchronicbeta-adrenergicstimulationinneonatal
ratcardiomyocytes,Br.J.Pharmacol.158(2009)300–313.
[35]Z.Zhang,L.Ding,Z.Jin,G.Gao,H.Li,L.Zhang,X.Lu,L.Hu,B.Lu,X.Yu,T.Hu,
Nebivololprotectsagainstmyocardialinfarctioninjuryviastimulationofbeta
3-adrenergicreceptorsandnitricoxidesignaling,PloSOne9(2014)
e98179.
[36]X.Niu,L.Zhao,X.Li,Y.Xue,B.Wang,Z.Lv,J.Chen,D.Sun,Q.Zheng,
!3-adrenoreceptorstimulationprotectsagainstmyocardialinfarctioninjury
viaeNOSandnNOSactivation,PloSOne9(2014)e98713.
[37]G.Tavernier,G.Toumaniantz,M.Erfanian,M.F.Heymann,K.Laurent,D.
Langin,C.Gauthier,Beta3-adrenergicstimulationproducesadecreaseof
cardiaccontractilityexvivoinmiceoverexpressingthehuman
beta3-adrenergicreceptor,Cardiovasc.Res.59(2003)288–296.
[38]J.Wang,X.Ma,Y.Zhang,H.Wang,J.Yang,J.Dong,J.Wang,Y.Yang,B.Li,
Cardiacprotectiveeffectoftheautoantibodyagainst!3-adrenoceptorinrats
withexperimentalheartfailure,ZhonghuaXinXueGuanBingZaZhi42
(2014)424–427.
[39]N.A.Franken,J.A.Camps,F.J.vanRavels,A.vanderLaarse,E.K.Pauwels,J.
Wondergem,Comparisonofinvivocardiacfunctionwithexvivocardiac
performanceoftheratheartafterthoracicirradiation,Br.J.Radiol.70(1997)